Abstract
Introduction:
Continuous video EEG (cEEG) monitoring is the recommended gold standard of care for at risk neonates but is not available in many NICU’s. In order to conduct a randomized treatment trial of levetiracetam for the first line treatment of neonatal seizures (the NEOLEV2 trial), we developed a monitoring infrastructure at five NICU’s, implementing recent technological advancements to provide cEEG monitoring and real time response to seizure detection. Here we report on the feasibility of providing this level of care.
Methods:
25 key informant interviews were conducted with study neurologists, neonatologists, coordinators and EEG technicians from the commercial EEG monitoring company Corticare. A general inductive approach was used to analyse these qualitative data.
Results:
A robust infrastructure for cEEG monitoring, remote review and real time seizure detection was established at all sites. At the time of this survey 260 babies had been recruited and monitored for 2–6 days. EEG technician review by the commercial EEG monitoring company was reassuring to families and neonatologists and led to earlier detection of seizures but did not reduce work load for neurologists.
Neurologists found the automated neonatal seizure detector algorithm provided by the EEG software company Persyst useful but the accuracy of the algorithm was not such that it could be used without review by human expert.
Placement of EEG electrodes to initiate monitoring, especially after hours remains problematic.
Conclusions:
Technological advancements have made it possible to provide at risk neonates with cEEG monitoring, real time detection of and response to seizures. However, this standard of care remains unfeasible in usual clinical practice. Chief obstacles remain starting a recording and resourcing the real time specialist review of suspect seizures.
Background:
Better treatments for neonatal seizures are urgently needed1. Efforts to develop better treatments are hindered by the need for systematic continuous video EEG (cEEG) monitoring in any drug trial. cEEG is the recommended gold standard of care2 but is not available in many NICU’s because of resource constraints.
In order to conduct the NEOLEV2 trial (NCT01720667), we developed an infrastructure for neonatal cEEG monitoring at 5 hospitals, utilizing remote review of cEEG monitoring, a commercial EEG monitoring company (Corticare) and automated neonatal seizure detection software to assist in early seizure detection. The 5 participating hospitals were Rady’s Children’s Hospital of San Diego, Sharp Mary Birch Hospital for Women and Newborns, San Diego, UC San Diego Medical Center, Hillcrest, UCSF Benioff Children’s Hospital in Oakland and Auckland Hospital in Auckland, New Zealand. At the time of this study 260 neonates had undergone cEEG monitoring for periods of 2–6 days. Longer periods of monitoring were required for neonates with hypoxic ischemic encephalopathy undergoing 72 hours of hypothermia and an additional subsequent rewarming period.
Recent research demonstrates that early detection and treatment of seizures leads to better response to treatment 3. In addition, we know that an increased seizure burden is associated with worse neurological outcome 4–6. The same infrastructure for early seizure detection and treatment is therefore desirable for standard clinical care, outside of a research protocol.
To assess the feasibility of providing this level of care in usual clinical practice, we report here on the challenges encountered during the NEOLEV2 study related to monitoring. We report the findings of key informant interviews conducted with study neonatologists, neurologists, NICN nurses, coordinators and EEG technicians from the commercial EEG monitoring company.
EEG Monitoring System for NEOLEV2 Study: Figure 1.
Figure 1.
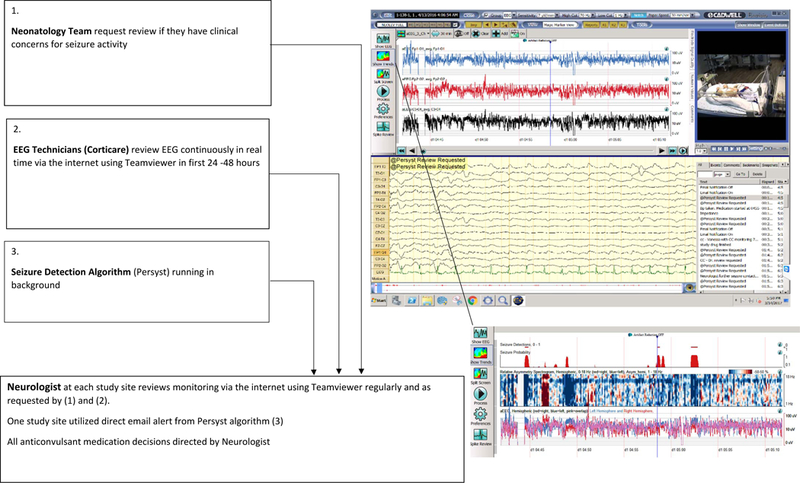
EEG Monitoring System used for the NEOLEV2 Study
Recordings were made on study-dedicated Cadwell EEG machines which displayed 3 channel aEEG trends, video and full montage1 raw EEG at the bedside.
The majority of EEG recordings were started after hours. Where EEG technicians were not available, monitoring was started by respiratory therapists and neonatal intensive care unit nurses with special training in the care of infants with neurological problems (NICN nurses) utilizing the Jordan elasticated EEG lead placement device.
The automated neonatal seizure detector algorithm ran on all acquisition stations during recording 7–9.
In the first 24 to 48 hours EEG was continuously reviewed via the internet using TeamViewer software by EEG technicians from the commercial EEG monitoring company. Study neurologists at each site reviewed the record regularly and whenever contacted by the commercial EEG monitoring company for suspected seizure. From Oct 2015 the automated neonatal seizure detector algorithm was systematically utilized in real time review as a research tool to aid seizure detection. Direct email alert generated by the seizure detector algorithm was available to study neurologists if they chose to use this function. All treatment decisions were made by study neurologists who could also access and review the live EEG remotely via the internet.
Method:
Key informant interviews were conducted by phone with research team members at all study sites. Study neurologists (9), study neonatologists (11), site principal investigators, site coordinators, research nurses and a respiratory therapist were interviewed. EEG technicians from the commercial EEG monitoring company (6) who had monitored frequently for the study completed a written questionnaire sent by email.
Questions were developed and phone interviews were conducted by the first author (CS), a neurologist and site principle investigator for the study. Question are included as appendix 1. Topics explored included:
cEEG monitoring practice prior to the study
difficulties encountered implementing the NEOLEV2 monitoring strategy
differences between EEG monitoring provided for study patients and patients not in the study
problems encountered related to EEG monitoring during the study
impressions of the commercial EEG monitoring company Corticare
impressions of the automated neonatal seizure detection algorithm provided by the EEG software company Persyst
the feasibility of offering a real time response to cEEG monitoring for all at risk neonates and suggestions as to how this could be improved
who should be responsible for interpretation of cEEG in the NICU
and level of interest in training in the interpretation of neonatal EEG
Field notes were made during each interview and summarized. A general inductive approach was used to analyse this qualitative data. Summarized results were sent back to research team members who had been interviewed and feedback was invited.
Results:
1. Technical problems encountered:
An infrastructure and practice for remote review of cEEG was operational at 2 of 5 sites prior to commencing the study and had to be developed at the other sites. In 4 hospitals internet connectivity was initially restricted to a subset of NICU bed spaces, necessitating disruptive moves of critically sick infants in order to conduct remote EEG review. Over the course of the study all 5 sites developed the capacity to monitor from all bed spaces. Three of 5 sites used Ethernet and the hospital server system as the conduit for remote review by the end of the study. One site now uses a 4G wireless conduit to the hospital server. At the fifth site where a hospital wireless network was the only available access for this research, internet speeds were marginal for high speed review and review was compromised by frequent disconnection problems.
During the time period that the study was conducted, clinical practice evolved so that long term cEEG became routine at 4 of 5 sites outside the study. At the fifth site, Brainz monitoring (four lead aEEG with raw trace EEG without video reviewed by neonatologists) remains the standard of clinical care outside the study.
The Cadwell EEG machines, Easy III and Persyst software and TeamViewer review system generally proved robust and secure for EEG acquisition, display and remote review.
On one occasion monitoring was interrupted briefly when TeamViewer shut down their software globally to patch a software vulnerability. Early difficulties were encountered with incompatibility of versions of TeamViewer between acquisition and reader stations.
Infrequently disruptions in monitoring were caused by EEG machine problems. Such problems were usually fixed by a simple reboot and restart. On two occasions a Windows software update led to a disconnection of the EEG amplifier. EEG monitoring was interrupted for days until technical assistance could be obtained to diagnose the problem and reconnect the amplifier. At three sites monitoring was shut down temporarily when the hard drive for the EEG machine ran out of memory.
Each disruption in monitoring was considerably problematic. Once a new clinical service is offered, even on a research basis, any disruption in the smooth running of that service quickly becomes unacceptable.
2. Starting a recording:
Networks of NICN nurses or respiratory therapists were available to start an EEG recording after hours at 3 sites prior to the commencement of the study. These networks became stronger during the study, limiting the potential delays caused by needing to call an EEG technician to start a recording after hours. At one study site the site principle investigator applied leads and started recordings for 18 months before transferring this duty to a study nurse. Efforts are ongoing to train a group of nurses in this skill set. At the fifth site there remains no capability for full montage EEG monitoring after hours, limiting recruitment to the study. At that site Brainz monitors are applied after hours, until EEG technicians are available to start a full montage video EEG recording.
At all sites the process of starting an EEG recording remains a significant resource commitment. NICN nurses and respiratory therapists estimate it takes 30 minutes to an hour for an experienced technician to start a recording. Furthermore, at least some leads will need to be reapplied or fixed every 4 hours on average. Reapplication was required less frequently at one site where needle electrodes are used.
Having circumvented involvement of EEG technicians in starting an EEG recording we inadvertently relinquished their technical support for problem shooting software and equipment failures and archiving of EEG recordings. EEG technicians were involved in the archiving of the research EEG’s at only 2 study sites, and in maintaining the electrodes during working hours at 3 study sites. Elsewhere nurses maintained the electrodes throughout the monitoring period and site principle investigators assumed responsibility for archiving EEG records. NICN nurses who have taken on the responsibility of starting the recording reported frustration at expectations that they would be able to solve technical issues, when these are well outside their brief.
3. Utility of real time remote review by EEG technicians from the commercial EEG monitoring company Corticare:
All Neonatologists surveyed (n=11) felt that the commercial EEG monitoring company added value, and a layer of security for the patient. Study staff reported that having an EEG technician reviewing the EEG continuously was reassuring and attractive to families and helped with recruitment to the research study.
Eight of 9 neurologists reported personal experience of monitoring by the commercial EEG monitoring company leading to earlier detection of seizures, especially overnight.
Neurologists reported that they tended to check the record less often if they knew the commercial EEG monitoring company was monitoring, which reduced their work load. Conversely, being called for technical issues or for false positive seizure detections increased demands on their time. Overall, 4 of 9 study neurologists felt that having the commercial EEG monitoring company monitoring a study patient reduced their own work in monitoring. Four neurologists felt their overall work load was unchanged. One neurologist felt having the involvement of the commercial EEG monitoring company increased his work load in monitoring. Four neurologists spontaneously commented that performance of the commercial EEG monitoring company was generally positive but very operator dependent; most technicians were proficient but some less familiar with neonatal EEG.
Errors in communication occurred when monitoring technicians typed information onto the computer screen which should have been reported by phone call to study personnel. A technician unfamiliar with NICU monitoring on one occasion instructed NICU staff not to touch the neonate undergoing monitoring as this was generating artefact. The commercial EEG monitoring company was very responsive to all feedback and communication became more streamlined as the study progressed.
Technicians from the commercial EEG monitoring company themselves, when surveyed about their work on the NEOLEV2 study reported difficulties when reviewing remotely trying to read EEG that was contaminated with artefact from loose electrodes and being unable to remedy this artefact. Technicians also reported frustration with poor camera image due to lighting or camera placement. Technicians from the commercial EEG monitoring company were unable to monitor study subjects when the EEG was being reviewed by other study personnel. The technicians dealt patiently with repeated internet disconnection problems with one study site, where at times reconnection was required every 15 minutes and sometimes more frequently. Technicians also reported it was difficult contacting a patient’s bedside nurse with the anonymized dataset given to them, an issue that would only occur within a research setting.
4. Utility of the automated neonatal seizure detector algorithm provided by the Persyst EEG software company:
All study neurologists found the automated neonatal seizure detector algorithm a useful tool for seizure detection. Many commented that the algorithm demonstrated fairly high sensitivity but that false positives (for example from patting artefact) were common. The accuracy of the algorithm was not such that it could be relied upon or used without review by an electroencephalographer. An instant messaging alert function was used by only 2 of 9 study neurologists, others finding disruption from false positive alerts too disruptive to other work commitments and sleep.
5. Resources for real time interpretation:
Study neurologists were asked if they considered it feasible as they were currently resourced to provide real time review and response to cEEG for at risk neonates outside of the research study and as part of routine clinical care.
None felt that they were adequately resourced to provide this and several stated that they were already under resourced clinically.
It should be emphasized that at 4 of the 5 sites a clinical practice of cEEG monitoring for all at risk neonates has evolved in recent years that is not dissimilar to what was provided within the NEOLEV2 study. EEG monitoring was usually reviewed at least 12 hourly at all sites, increasing, for seizing or very high risk subject to as frequently as every hour during the day. Review of the EEG overnight remains infrequent at all sites. At the hospitals with the best resources, this constituted the main difference between monitoring as it happened within the research study and how it is provided for all at risk neonates.
6. Solutions suggested by study neurologists and neonatologists that could increase the clinical feasibility of a real time response to neonatal seizure detection:
Study neurologist and neonatologists were asked what could improve the feasibility of a real time response to neonatal seizure detection. Suggestions included better automated seizure detection, and a larger contingent of electroencephalographers. It was considered that a separated on call roster would be required, such that the neurologist on duty to review and respond to neonatal (and other) EEG monitoring was not also on duty for general paediatric neurology, for neurologists to sustain this level of care.
Better training of EEG technicians and access to in-hospital EEG technicians who could both review studies and troubleshoot leads and equipment was suggested.
All neurologists were supportive of the idea that EEG technicians, neonatologists, and or NICN nurses with the appropriate comprehensive training and oversight by a neurophysiologist might be appropriate first line personnel for neonatal cEEG review.
Most surveyed neonatologists, NICN nurses and EEG technicians expressed interest in obtaining comprehensive training and certification in neonatal seizure detection.
Discussion:
There have been multiple recent technological advances in cEEG monitoring. These include smaller more affordable video EEG machines, improved internet speed and security, software advances for remote review and for automated seizure detection and lead placement devices to assist non EEG technicians in starting EEG acquisition. Continuous EEG review by EEG technicians is a commercially available service. As we embarked on the NEOLEV2 study we felt optimistic that these advances, meant that we would be able to demonstrate within our study that it was now feasible to provide the gold standard of cEEG monitoring for all at risk neonates with rapid detection and a real time response to seizures. While our experience has demonstrated that this is now technically feasible, the surveyed responses from our dedicated study personnel show that it is still not practical to provide this level of care clinically for all at risk neonates in most NICU’s.
The two most significant factors limiting the provision of this level of care were the difficulties surrounding the starting of a recording after hours and resourcing the physician review of suspect seizures in real time after hours. In addition, while continuous EEG review by EEG technicians is available, it is not funded in most hospitals. These factors are part of a much greater problem of insufficient resources for NICU cEEG monitoring in general.
It has been recognized for some time that because neonatal seizures are so frequently entirely subclinical, cEEG monitoring of at risk neonates is required for their reliable detection 10–13. The more recent dramatic upsurge in clinical demand for NICU cEEG monitoring reflects the increasing evidence that neonatal seizures are damaging 4,14,15–17. Although a causal link between seizures and brain injury in human subjects has not been proven it is reasonable to hope that early detection and treatment of seizures may reduce brain injury. Long term neurodevelopmental outcomes following neonatal seizures are poor 18–20. Preventing permanent neurodevelopmental disability in even a small percentage of these children would not only improve quality of life for these children and their families, but would greatly reduce the health care costs associated with caring for a child with permanent neurodevelopmental disability. The dramatic increase in the amount of neonatal cEEG monitoring performed in many hospitals has often occurred without the increased resource required to sustain it. Similar resource issues have been faced by adult and pediatric ICU’s charged with the provision of cEEG monitoring for their patients 21–24. Ability to respond to the new demand for ICU cEEG monitoring is hindered by a reimbursement system that only partly compensates for the increased costs associated with acquiring, reviewing and responding to ICU cEEG monitoring as compared with non ICU video EEG monitoring 23. Non-ICU cEEG monitoring, for example cEEG for epilepsy diagnosis or epilepsy surgery assessment, can be set up during working hours and in many cases can be reviewed electively and less frequently by interpreting neurophysiologists.
Solutions to these funding and resource issues are urgently required if we are to sustainably provide the gold standard of care with cEEG monitoring for at risk neonates, and through early detection and treatment of seizures improve their neurodevelopmental outcomes.
Acknowledgements:
This work was funded by a grant from the Orphan Products Division of the FDA #1R01FD004147
Appendix. Interview questions
Questionnaire for Site PI
In the set up for this study did you need to establish a high speed internet capability for remote review of EEG at your study site, or was that already in place at your institution?
If not already in place, how many months (approximately) did it take to establish this capability?
How does your system work? Wifi? Through hospital intranet?
Has your system worked well? Have there been problems with internet connectivity during monitoring that you are aware of?
Questionnaire for Study Neurologists
Did you use the Persyst software algorithm during monitoring, and if so what were your impressions of it?
Did you use the email alert function, and if so what did you think of it? If you did not use the email alert function, why did you not use it?
Did having Corticare EEG technicians monitoring the study reduce your work in monitoring study babies?
Did having Corticare EEG technicians monitoring the study lead to earlier pick up of seizures?
Do you have any other comments about Corticare?
In your view, as you are currently resourced, is it reasonable/ feasible/sustainable to ask you to provide the service of real time review of continuous video EEG monitoring for at risk neonates, outside of the research study and as a part of routine clinical care?
If not, how could that be changed?
If a neonate is undergoing cEEG outside of the research study, how frequently would you usually review their monitoring?
How commonly would neonatologists and nicn nurses ask for review inbetween times?
Would you support a model of nicu EEG monitoring where neonatologists were provided with quite comprehensive ( 20h-80h) training and assessment in neonatal seizure recognition, and were then the first line personnel for nicu EEG review, with scheduled regular review and quality control from neurologists/neurophysiologists?
What problems did you encounter, if any, related to EEG monitoring for NEOLEV2?
(Problems with obtaining EEG set up after hours? Problems with internet connectivity? Problems with the EEG machine? Problems with interactions with interpreting Neurologist? Problems with Teamviewer? Problems with Windows?)
Additional comments:
Exit Questionnaire for Study Neonatologists
We are conducting exit questionnaires with study personnel to assess the true feasibility of providing the EEG monitoring service that has been a part of NEOLEV2 research study, as part of routine clinical care.
At your hospital, if a neonate was a part of NEOLEV2, did that lead to a different level of intensity of EEG monitoring for that patient?
What problems did you encounter, if any, related to EEG monitoring for NEOLEV2?
(Problems with obtaining EEG set up after hours? Problems with internet connectivity? Problems with Corticare? Problems with the EEG machine? Problems with interactions with interpreting Neurologist? Problems with Teamviewer? Problems with Windows? )
Good things about Corticare? Did Corticare add value?
In other centers and at this center in previous eras, there was more involvement of Neonatologists in the interpretation of limited montage aEEG or CFM. In NEOLEV2 we shifted towards a Neurologist as first line physician interpreting the bedside EEG monitoring. This may not prove to be a practical means of providing real time interpretation of NICU EEG monitoring to all at risk neonates given resource constraints.
With ongoing support, and access to review from a Neurologist when needed, would you personally be interested in investing time in learning how to interpret neonatal EEG?
Neonatal EEG review is not rocket science but nor is it something which can be learnt in a short time period. We are considering seeking funding to convert our marked video EEG database into a training tool, which could generate a personalized certificate of competency at completion of the training.
Would you invest 20 hours in modular training to develop competency in neonatal EEG review?
Would you invest 40 hours in training? Would you invest 60 hours? Would you invest 80 hours?
Additional Comments:
Exit Questionnaire for Study Site Coordinators/ NICN Nurse specialist/ Respiratory Therapists
We are conducting exit questionnaires with study personnel to assess the true feasibility of providing the EEG monitoring service that has been a part of NEOLEV2 research study, as part of routine clinical care.
At your hospital, if a neonate was a part of NEOLEV2, did that lead to a different level of intensity of EEG monitoring for that patient?
What problems did you encounter, if any, related to EEG monitoring for NEOLEV2?
Problems with obtaining EEG set up after hours? Problems with internet? Problems with Corticare?
Good things about Corticare? Did Corticare add value?
Problems with the EEG machine? Problems with contacting the interpreting Neurologist? Other?
Neonatal EEG review is not rocket science but nor is it something which can be learnt in a short time period. We are considering seeking funding to convert our marked video EEG database into a training tool, which could generate a personalized certificate of competency at completion of the training.
Would you invest 20 hours in modular training to develop competency in neonatal EEG review?
Would you invest 40 hours in training? Would you invest 60 hours? Would you invest 80 hours?
Additional Comments?
Exit Questionnaire for Corticare Technicians (completed by email)
We are conducting exit questionnaires with study personnel to assess the true feasibility of providing the EEG monitoring service that has been a part of NEOLEV2 research study, as part of routine clinical care.
How many times did you monitor a NEOLEV2 study baby? (approximately)
What problems did you encounter, if any, related to EEG monitoring for NEOLEV2?
Problems with internet?
Problems with the EEG machine?
Problems with contacting the interpreting Neurologist?
Other? How does monitoring a continuous EEG recording via the internet compare with monitoring from the bedside from your perspective?
Neonatal EEG review is not rocket science but nor is it something which can be learnt in a short time period. We are considering seeking funding to convert our marked video EEG database into a training tool, which could generate a personalized certificate of competency at completion of the training.
Would you invest 20 hours in modular training to develop competency in neonatal EEG review?
Would you invest 40 hours? Would you invest 60 hours? Would you invest 80 hours?
Additional Comments?
Footnotes
Disclosure of conflicts of interest and funding sources
The NEOLEV2 study was funded by the FDA 1 RO1FD004147–01A1
The Persyst EEG software company have worked closely with the authors on the NEOLEV2 study and have provided their software to the researchers free of charge but have had no input into this feasibility study or this manuscript.
The Corticare commercial EEG monitoring company have worked closely with the authors on the NEOLEV2 study on a commercial basis. They have had no input into the writing of this manuscript.
This manuscript discusses use of the automated neonatal seizure detection algorithm created by the Persyst EEG software company, which is not yet FDA approved for commercial use.
This paper was presented as an abstract at the Pediatric Academic Society Meeting, San Francisco, May 2017
A 10–20 International placement montage modified for neonates was used recording from 10 scalp electrodes at FP1, FP2, FZ, C3 CZ C4, T3,T4, O1 and O2.
Contributor Information
CM Sharpe, Paediatric Neurology, Auckland District Health Board, Auckland.
SL Davis, Paediatric Neurology, Auckland District Health Board, Auckland.
GE Reiner, Department of Neurosciences, University of California, San Diego; Rady Children’s Hospital San Diego.
LI Lee, Department of Neurosciences, University of California, San Diego.
JJ Gold, Department of Neurosciences, University of California, San Diego; Rady Children’s Hospital San Diego.
M Nespeca, Department of Neurosciences, University of California, San Diego; Rady Children’s Hospital San Diego.
SG Wang, Department of Neurosciences, University of California, San Diego; Rady Children’s Hospital San Diego.
P Joe, Department of Neonatology, UCSF Benioff Children’s Hospital.
R Kuperman, Department of Pediatric Neurology, UCSF Benioff Children’s Hospital.
M Gardner, Department of Pediatric Neurology, UCSF Benioff Children’s Hospital.
J Honold, Department of Pediatrics, University of California, San Diego; Rady Children Hospital San Diego.
B Lane, Department of Pediatrics, University of California, San Diego; Rady Children’s Hospital San Diego.
EM Knodel, Division of Neonatology, Rady Children’s Hospital, San Diego.
DL Rowe, Faculty of Medical and Health Sciences, University of Auckland..
MR Battin, Neonatology, Auckland District Health Board.
R Bridge, Division of Neonatology, Department of Pediatrics, University of California, San Diego.
J Goodmar, Division of Neonatology, Department of Pediatrics, University of California, San Diego.
B Castro, Division of Neonatology, Department of Pediatrics, University of California, San Diego.
M Rasmussen, San Diego Neonatology Inc. Sharp Mary Birch Hospital for Women and Newborns, San Diego.
K Arnell, Neonatal Research Institute, Sharp Mary Birch Hospital for Women and Newborns, San Diego.
MJ Harbert, Department of Neurosciences, University of California, San Diego, Sharp Mary Birch Hospital for Women and Newborns, San Diego.
RH Haas, Departments of Pediatrics and Neurosciences, University of California, San Diego, Rady Children’s Hospital San Diego.
References
- 1.Sankar R, Painter MJ. Neonatal seizures: after all these years we still love what doesn’t work. Neurology 2005;64:776–7. [DOI] [PubMed] [Google Scholar]
- 2.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol 2011;28:611–7. [DOI] [PubMed] [Google Scholar]
- 3.Williams RP, Banwell B, Berg RA, et al. Impact of an ICU EEG monitoring pathway on timeliness of therapeutic intervention and electrographic seizure termination. Epilepsia 2016;57:786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr 2009;155:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne ET, Hahn CD. Continuous electroencephalography for seizures and status epilepticus. Curr Opin Pediatr 2014;26:675–81. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SB, Scheuer ML, Emerson RG, Gabor AJ. Seizure detection: evaluation of the Reveal algorithm. Clin Neurophysiol 2004;115:2280–91. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SB. A neural network method for automatic and incremental learning applied to patient-dependent seizure detection. Clin Neurophysiol 2005;116:1785–95. [DOI] [PubMed] [Google Scholar]
- 9.Wilson SB. Algorithm architectures for patient dependent seizure detection. Clin Neurophysiol 2006;117:1204–16. [DOI] [PubMed] [Google Scholar]
- 10.Scher MS, Alvin J, Gaus L, Minnigh B, Painter MJ. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol 2003;28:277–80. [DOI] [PubMed] [Google Scholar]
- 11.Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia 1988;29:256–61. [DOI] [PubMed] [Google Scholar]
- 12.Murray DM, Boylan GB, Ali I, Ryan CA, Murphy BP, Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed 2008;93:F187–91. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence R, Mathur A, Nguyen The Tich S, Zempel J, Inder T. A pilot study of continuous limited-channel aEEG in term infants with encephalopathy. J Pediatr 2009;154:835–41 e1. [DOI] [PubMed] [Google Scholar]
- 14.Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology 2002;58:542–8. [DOI] [PubMed] [Google Scholar]
- 15.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 2000;55:506–13. [DOI] [PubMed] [Google Scholar]
- 16.Toet MC, Groenendaal F, Osredkar D, van Huffelen AC, de Vries LS. Postneonatal epilepsy following amplitude-integrated EEG-detected neonatal seizures. Pediatr Neurol 2005;32:241–7. [DOI] [PubMed] [Google Scholar]
- 17.van Rooij LG, Toet MC, van Huffelen AC, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics 2010;125:e358–66. [DOI] [PubMed] [Google Scholar]
- 18.Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology 2007;69:1816–22. [DOI] [PubMed] [Google Scholar]
- 19.Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol 2009;36:901–14, vii-viii. [DOI] [PubMed] [Google Scholar]
- 20.Holmes GL, Ben-Ari Y. Seizures in the developing brain: perhaps not so benign after all. Neuron 1998;21:1231–4. [DOI] [PubMed] [Google Scholar]
- 21.Young GB. Continuous EEG monitoring in the ICU: challenges and opportunities. Can J Neurol Sci 2009;36 Suppl 2: S89–91. [PubMed] [Google Scholar]
- 22.Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG monitoring: current resources and practice in the United States and Canada. J Clin Neurophysiol 2013;30:156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ney JP, van der Goes DN, Nuwer MR, Nelson L, Eccher MA. Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005–2009. Neurology 2013;81:2002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vespa PM, Nenov V, Nuwer MR. Continuous EEG monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol 1999;16:1–13. [DOI] [PubMed] [Google Scholar]


