Abstract
Introduction:
CHRFAM7A is a uniquely-human gene that encodes a human-specific variant of the alpha-7 nicotinic acetylcholine receptor (α7nAchR). While the homopentameric α7nAchR consists of 5 equal subunits, previous studies demonstrated that CHRFAM7A expression disrupts the formation of α7nAchR homopentamers. Here we use a rat neuronal cell line expressing CHRFAM7A and a transgenic mouse expressing CHRFAM7A to define the alpha-bungarotoxin (α-BTX) binding in vitro and in vivo.
Methods:
Rat PC12 cells were stably transfected with human CHRFAM7A. α-BTX, a protein that irreversibly binds the α7nAchR, was utilized to assess the capacity for CHRFAM7A to interfere with α 7AchR subunits using immunohistochemistry and flow cytometry. To evaluate the effects of CHRFAM7A on α7nAchR at the neuromuscular junction in vivo, transgenic mice were engineered to express the uniquely human gene CHRFAM7A under the control of the EF1-α promoter. Using this model, muscle was harvested and CHRFAM7A and CHRNA7 gene expression evaluated by PCR. Binding of α-BTX to the α7nAchR in muscle was compared in sibling-matched wild-type C57 mice by immunostaining the neuromuscular junction using α- BTX and neurofilament antibodies.
Results:
Expression of CHRFAM7A in transfected, but not vector cells, was confirmed by PCR and by immunoblotting using an antibody we raised to a peptide sequence unique to CHRFAM7A. CHRFAM7A decreased α-BTX binding as detected by immunohistochemistry and flow cytometry. In vivo, α-BTX co-stained with neurofilament at the neuromuscular junction in wild-type mice, however, α-BTX staining was decreased at the neuromuscular junction of CHRFAM7A transgenic mice.
Conclusion:
CHRFAM7A expression interferes with the binding of α7nAchR to α-BTX. Understanding the contribution of this uniquely human gene to human disease will be important in the identification of potential therapeutic targets.
Keywords: duplicated nicotinic acetylcholine receptor, CHRFAM7A, human-specific genes, α-bungarotoxin, transgenic mouse, neural α7nAchR
Introduction:
First discovered in the 1990s, the α7 subunit of the nicotinic Acetylcholine receptor (α7nAchR), encoded by the CHRNA7 gene on chromosome 15, is a homopentamer with both transmembrane and cytoplasmic domains [6]. The α7nAchR is widely expressed in the human nervous system and has been linked to neurocognitive function including memory and attention as well as neuropsychiatric disorders such as schizophrenia [5]. Therefore, the α7nAchR has long been considered a potential therapeutic target for neurologic disease.
Interestingly, humans express a variant of CHRNA7 called CHRFAM7A located 1.6Mb upstream of the CHRNA7 gene. Uniquely human CHRFAM7A is a partial duplication and rearrangement of CHRNA7 consisting of a duplication of exons 5–10 fused to 4 new exons duplicated from chromosome 3 and encoding 27 amino acids [13]. We have previously shown that CHRFAM7A is widely expressed and has its own promoter regulating expression of a functional open reading frame that produces a protein product [9]. Although CHRFAM7A expression has been associated with neurocognitive disorders including schizophrenia, psychosis, bipolar disorder, autism, and dementia [1, 3, 15, 21], the effects of CHRFAM7A expression on α7nAchR binding in the nervous system remains largely unknown.
Based on its similarity to the CHRNA7 gene, several investigators have demonstrated that CHRFAM7A alters the assembly and function of the α7nAchR complex by forming a heteropentamer of α7nAchR/CHRFAM7A subunits that alter ligand binding to the receptor [8, 19]. Here, we hypothesize that the expression of CHRFAM7A would alter binding of αBTX to the α7nAchR in a neural cell line in vitro and at a neuromuscular junction in vivo. Based on the human variability in CHRFAM7A expression [9], understanding the effects of CHRFAM7A expression on binding to the α7nAchR may have important effects on the response to therapeutics targeting the α7nAchR.
Material and Methods:
PC12 culture
PC12 cells were obtained from ATCC (Manassas, VA) and maintained at 37°C (5% CO2) in RPMI-1640 medium supplemented with 5% fetal bovine serum, 10% horse serum, 1% penicillin/streptomycin (Gibco). Medium was changed every 2–3 days.
PC12 transfection with CHRFAM7A vector
PC12 cell lines stably expressing CHRFAM7A gene or vector were created using Lenti-X Packaging Single Shots system according manufacture (Clontech). Briefly, 7μg of pLVX-IRES-ZsGreen1 lentiviral vector encoding CHRFAM7A gene or vector only was mixed with Lenti-X Packaging plasmids to transfect Lenti-X 293T cells in 10 cm plate. After 48 hours, lentiviral particles were collected and used immediately to infect PC12 cells for stable expression with ratio of 1 part of lentiviral medium to 3 parts of fresh culture medium. Three to four days after infection, the cells were sorted by fluorescence-activated cell sorting (FACS) for ZsGreen positive population (GFP+), and expanded in regular PC12 culture medium.
PCR for CHRNA7 and CHRFAM7A
RNA was prepared using Direct-zol RNA miniprep plus kit (Zymo Research, Irvine, CA) including DNase treatment. Total of 2 μg of total RNA was submitted for cDNA generation using the BioRad Reverse Transcriptase Kit according to manufacturer’s directions (Hercules, CA); and 1 μ1 of cDNA reaction product was used per reaction for PCR. Expression levels of CHRNA7 and CHRFAM7A mRNAs were measured by PCR (Bio-Rad iQ5, Hercules, CA); using the SYBR Green gene expression assays (OriGene, Rockville, MD). PCR rat CHRNA7 and rat GAPDH primers were obtained from Quantitect; CHRFAM7A specific primers were Forward 5’-ATAGCTGCAAACTGCGATA-3′, Reverse 5′- CAGCGTACATCGATGTAGCAG −3′. For PCR experiments, Platinum blue PCR super mix was used for 35 cycles; 94°C for 20 sec, 56.5°C for 30 sec, 72°C for 1 min. PCR of muscle for CHRNA7 and CHRFAM7A was performed as described above.
Immunoblotting
Total protein extracts were prepared using 10 × 106 cells. Briefly, PC12 cells were collected by centrifugation for 5 minutes at 1000 rpm in 4°C. The pellets were resuspended in 300 μl of Triton X-100-containing buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, protease and phosphatase inhibitors (Sigma-Aldrich Co.), 5 mM DTT and 0.2 mM PMSF) and sonicated at moderate speed on ice (three times for 20 seconds with 30 second intervals). The protein was extracted after 30 minutes incubation on ice by centrifugation at 13000 rpm for 15 minuntes. Cell protein concentrations were measured using the BCA method. Fifty μg of total PC12 protein extracts, and a standard molecular weight marker (New England Biolabs) were size-fractionated on 4–12% SDS-PAGE mini-gel and transferred to a PVDF membrane (Invitrogen, #LC2005). The membranes were incubated for 1 hour with blocking buffer (5% BSA in TBS with 0.1% Tween - TBST), following incubation with anti-CHRFAM7A (1μg/ml). For creation of a specific antibody for CHRFAM7A, antibodies to the 27 amino acid peptide that differentiates CHRFAM7A from CHRNA7 were raised in rabbits by contract with GenWay (San Diego, CA), affinity purified by the supplier, and titers measured by ELISA with the synthetic peptide. Specificity of the antibody for CHRFAM7A and its inability to cross-react with the α7nAchR was established by immunoblotting transfected cells, respectively. Anti-β-Actin was used as a loading control (dilution 1:1000, Cell Signaling Technology #8457). After being washed three times in TBST, the membranes were incubated for 1 hour using a secondary with a corresponding HRP-linked antibody. After three further washes in TBST and two washes in H2O, signals were developed using Super Signal West Femto (Pierce Biotechnology Inc) on CL-XPosure film (Thermo, Rockford, IL).
Immunohistochemistry
PC12 cells expressing CHRFAM7A or parental PC12 cells were plated on collagen 1 coated coverslips in 6 well at 5 × 104 cells per well. Following day live cells were directly stained with α-Bungarotoxin (α-BTX)-tetramethylrhodamine (Sigma, conc: 1μg/ml) in PBS for 30 minutes at RT in dark. After three washes in PBS for 5 minutes, exposure matched images (40x, 1/3 second exposure) were taken using FSX100 inverted microscope equipped with contrast optics (Olympus).
Measurement of Fluorescence
Changes in α-BTX binding levels were also confirmed by measuring fluorescence on a Fluostar Omega Fluorimeter using 544/ 590 excitation and emission filters. In this instance, 2×105 PC12 stably expressing vector GFP or CHRFAM7A cells were live-stained with 0 or 1μg/ml of α-BTX-tetramethylrhodamine (Sigma) in PBS for 30 minutes at RT in dark. After two washes in PBS, cells were transferred onto 96 well plate (Corning, CLS3915) for fluorescence measurement. Final fluorescence intensity was normalized by individual cell count.
Flow cytometry
For flow cytometry analysis, 5×105 of PC12-CHRFAM7A or vector GFP expressing cells were collected and washed once with PBS. Live cells were directly stained with Alexa Fluor® 647 α-BTX (ThermoFisher 1μg/ml) in PBS for 30 minutes at RT in dark. In some instances, cells were also co-treated with 0 or 50 μM nicotine in four replicates per condition for 30 minutes in incubator. The cells were washed three times with PBS and analyzed by flow cytometry. α-BTX binding intensity was quantified by using Flowjo software (TreeStar, Oregon) on the GFP positive cell population.
α-bungarotoxin binding at the neuromuscular junction
Transgenic mice were produced by contract with Cyagen Biosciences (Santa Clara, CA) with CHRFAM7A regulated by the EF1α promoter in a C57BL/6 mouse background. Each mouse was genotyped to confirm the presence of CHRFAM7A by the contractor and colonies established with breeding pairs at UC San Diego. Animal housing and experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee. Control mice used for experimentation were sibling-matched wild-type (WT) C57BL/6 mice.
Gastrocnemius muscle neuromuscular junction staining for α-BTX binding was performed as previously described[14, 23]. Briefly, mice were anesthetized and euthanized by cervical dislocation. Gastrocnemius muscle was gently dissected over ice immediately after autopsy and the tissue fixed in 4% paraformaldehyde for 20 minutes. The muscle tissue was then incubated with α-BTX conjugated to tetramethylrhodamine (TMR) (Sigma-Aldrich T0195 1 μg/mL). Tissues were then submerged in methanol at −20°C for 5 min and subsequently blocked with 1 mg/mL BSA, 0.1% triton in PBS for 1 hour. To help localize neuromuscular junctions, an anti-neurofilament primary (Rabbit; Sigma-Aldrich N4142 1:500) was used in an overnight incubation at room temperature. Tissues were then exposed to an AlexaFluor 488 conjugated secondary antibody (Rabbit; Invitrogen A11008 1:400) for 4 hours. All gastrocnemius muscle samples were mounted and affixed with SlowFade (Invitrogen S36936) for confocal imaging. All WT and CHRAFM7A images were exposure-matched. Confocal images were captured with an Olympus FluoView 1000 (ASW 1.7b) laser scanning confocal microscope equipped with a 20x/0.7NA dry objective lens on a BX61 microscope (Olympus).
Statistical Analysis
Values are expressed as the mean and standard deviation where n represents the number of animals in each experimental group. The statistical significance between groups was determined using a two-tailed student t test. A p value < 0.05 was considered statistically significant.
Results:
To evaluate the effects of CHRFAM7A on a7nAChR binding, we performed stable transfection of CHRFAM7A in rat neural cells that have no endogenous expression of this gene as it is unique to humans. Next, we used PCR to confirm that CHRFAM7A gene expression was present in transfected cells, with no CHRFAM7A expression seen in vector cells (Fig. 1A). Both vector and CHRFAM7A cells express endogenous CHRNA7, the gene that produces the α7nAChR (Fig 1B). We verified that insertion of the CHRFAM7A gene resulted in protein production by immunoblotting with an antibody raised to a peptide sequence unique to CHRFAM7A (Fig. 1C). As expected, no CHRFAM7A protein expression was seen in vector cells.
Figure 1:
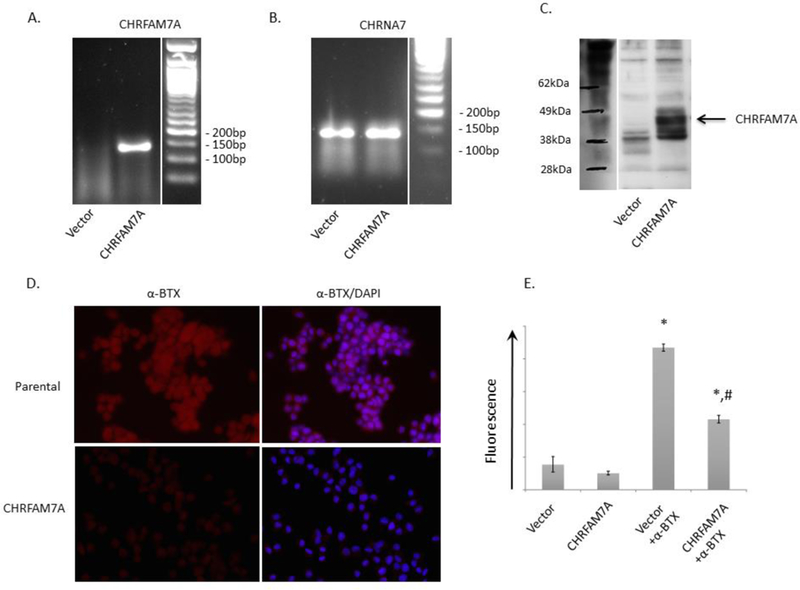
Forced expression of CHRFAM7A alters α-bungarotoxin (α-BTX) binding. Rat PC12 cells were transduced with CHRFAM7A to assess the effect of the uniquely human gene on ligand binding to the α7nAchR. A. PCR confirmed the presence of CHRFAM7A in transduced cells with no expression seen in vector PC12 cells. B. PCR demonstrated that CHRNA7, the α7nAchR gene, was expressed in both vector and CHRFAM7A transduced cells. C. Immunoblotting demonstrated that CHFAM7A transduced PC12 cells were functional and produced a protein with molecular weight consistent with CHRFAM7A. D. PC12 cells were stained with α-BTX (red) to assess binding to the α7nAchR. Cells were co-stained with DAPI (blue) to identify the cell nucleus. E. Fluorescence was measured using an Omega Fluorimeter to quantify changes in α-BTX binding between vector and CHRFAM7A transduced cells. Decreased staining for α-BTX was seen in CHRFAM7A transduced cells compared to vector. * p<0.0001 vs Vector, CHRFAM7A; # p<0.01 vs. Vector + α-BTX.
To visualize binding to the α7nAChR, cells were stained with α-BTX that binds α7nAChR with high specificity [12], and exposure-matched images were viewed by microscopy. Cells were also stained with DAPI to visualize the cell nucleus. Vector cells demonstrated uniform staining with α-BTX showing binding to α7nAChR (Fig. 1D, top row). Cells expressing CHRFAM7A, however, demonstrated decreased α-BTX staining compared to vector cells (Fig. 1D, bottom row). Fluorescence of cells stained with α-BTX was also directly measured in transfected cells to demonstrate that introduction of CHRFAM7A decreases α-BTX binding to the α7nAChR compared to vector cells (Fig. 1E).
To further quantify the effects of CHRFAM7A expression on α7nAChR subunits, α-BTX binding was measured using flow cytometry (Fig. 2A). Transfection of cells with CHRFAM7A decreased α-BTX binding compared to vector (Fig. 2B). Next, cells were pre-incubated with nicotine to compete for binding to the α7nAChR to assess the specificity of α-BTX binding in CHRFAM7A transduced cells. As expected, pre-incubation with nicotine prevented α-BTX binding in vector PC-12 cells. Nicotine also decreased α-BTX binding in CHRFAM7A transduced cells (Fig. 2C). This suggests that the expression of CHRFAM7A decreased the number of functional α7nACh receptors but did not completely abrogate α7nAChR ligand binding (Fig. 2D). These findings demonstrate that CHRFAM7A decreases ligand binding to the neural α7nAChR in vitro.
Figure 2:
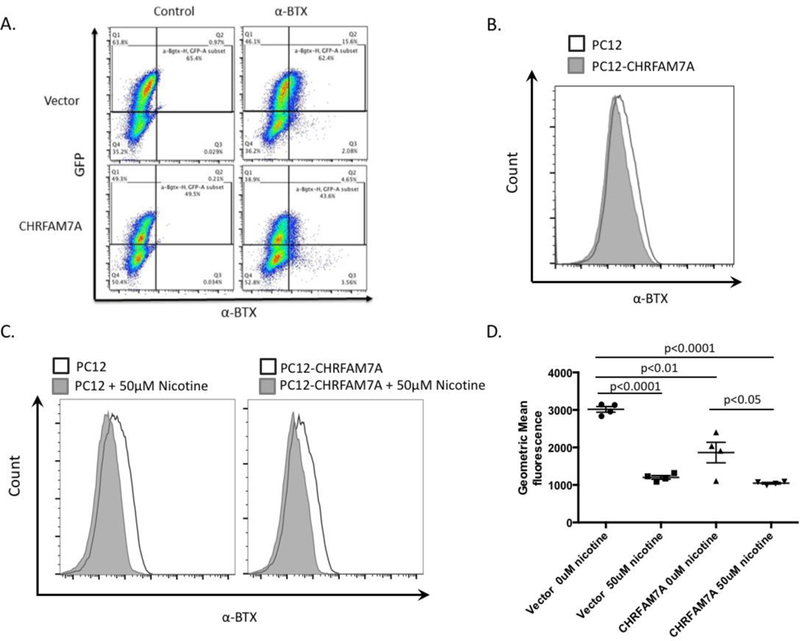
CHRFAM7A expression decreases ligand binding to the α7nAchR in PC12 cells. Rat PC12 cells were transduced with human CHRFAM7A to quantify changes in α-BTX binding using flow cytometry. A. Gating strategy to identify the GFP+CHRFAM7A+ population in vector and CHRFAM7A-transduced cells. B. Representative histogram demonstrating decreased α-BTX binding in CHRFAM7A transduced PC12 cells compared to vector. C. Cell preincubated with the α7nAchR ligand nicotine (50μM) to prevent α-BTX binding to functional α7nAch receptors. D. Quantification of all flow cytometry experiments showing that the introduction of uniquely human CHRFAM7A decreases α-BTX binding to PC12 cells. * p<0.05.
To evaluate the effects of CHRFAM7A expression on α7nAChR ligand binding in vivo, we created transgenic mice expressing uniquely human CHRFAM7A. Muscle was harvested from CHRFAM7A transgenic mice and WT siblings to confirm CHRFAM7A gene expression in transgenic but not WT animals (Fig. 3A). CHRNA7 gene expression was seen in both WT and CHRFAM7A transgenic animals (Fig. 3B). To evaluate binding to the α7nAChR at the neuromuscular junction, muscle was dissected and stained with α-BTX. Exposure matched images were viewed using confocal microscopy (Fig. 3C, red). Muscle tissue was also costained with a neurofilament specific antibody to visualize neurons and confirm their colocalization with α-BTX at the neuromuscular junction (Fig 3C, green). Sections from WT animals demonstrated staining with α-BTX that co-stained with neurofilament at the motor endplate. Muscle from CHRFAM7A transgenic mice, conversely, demonstrated decreased α-BTX binding at the neuromuscular junction compared to WT. These findings confirm that CHRFAM7A expression decreased α7nAChR ligand binding in vivo.
Figure 3:
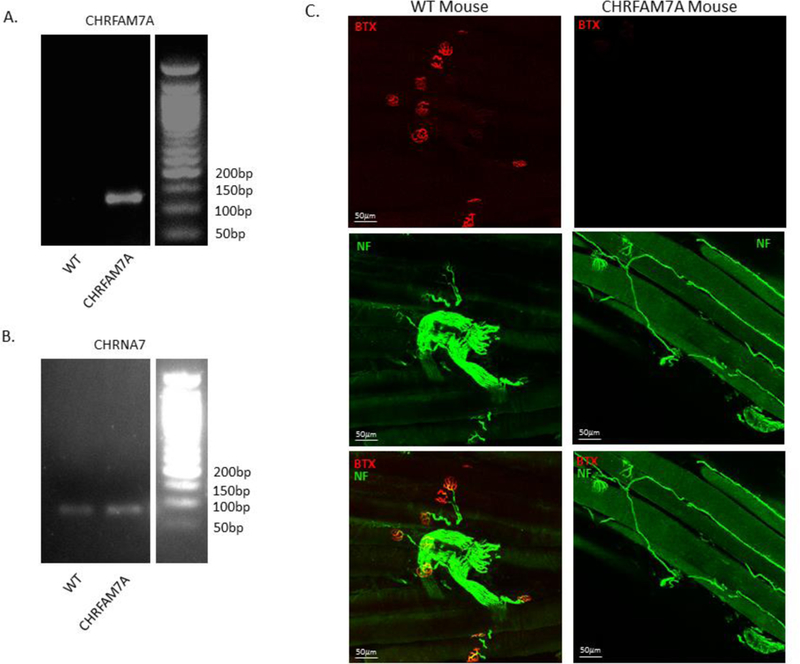
Binding to the neural a7nAchR is decreased in CHRFAM7A transgenic animals. Transgenic mice expressing the uniquely human gene CHRFAM7A were utilized to assess binding to the α7nAchR at the neuromuscular junction in muscle. A. PCR of muscle tissue demonstrates CHRFAM7A gene expression in transgenic mice. There was no expression of CHRFAM7A in WT mice. B. PCR of muscle tissue demonstrates CHRNA7, the α7nAchR gene, is present in the muscle of both WT and CHRFAM7A transgenic mice. C. Exposure matched confocal microscopy of muscle tissue stained for α-BTX (red) to identify staining to the α7nAchR and Neurofilament (green) to identify the neuromuscular junction. There is decreased α-BTX staining at the neuromuscular junction in CHRFAM7A transgenic mice compared to sham. Size bar = 50μm.
Discussion:
In humans, the α7nAChR is expressed both pre- and post-synaptically in neuronal and non-neuronal cells where it has been implicated in diverse physiologic functions ranging from cognitive function to the regulation of the inflammation response [4, 8, 10]. Uniquely human CHRFAM7A is a partial duplication and rearrangement of the CHRNA7 gene that encodes the α7nAChR. Previous data suggests that CHRFAM7A expression may alter the structure of the α7nAChR [16]. CHRFAM7A expression is highly variable across individuals suggesting that α7nAChR function may be variable as well [9]. Currently, the effects of CHFAM7A on α7nAChR ligand binding are poorly understood.
Previous studies have considered the potential of CHRFAM7A to alter α7nAChR function in heterologous systems [22], however, the effects of CHRFAM7A on α7nAChR ligand binding in a mammalian system that endogenously expresses CHRNA7 have previously been undefined. Here, we demonstrate that CHRFAM7A expression decreases α-BTX binding using both in vitro and in vivo models. These are the first studies to utilize a transgenic animal model to evaluate the effects of human CHRFAM7A when expressed in mouse.
Araud, et al. previously evaluated the ability of CHRFAM7A expression to alter α7nAChR channel function using a heterologous system utilizing oocytes harvested from Xenopus that underwent nuclear injection of both CHRNA7 and CHRFAM7A[2]. They performed voltage clamp experiments and found that calcium evoked current was decreased in cells injected with both CHRFAM7A and CHRNA7 compared to cells injected with CHRNA7 alone. Based on these results, the authors concluded that if CHRFAM7A is not a pseudogene, its expression could have dominant negative effects on α7nAChR function.
This study was followed shortly thereafter by de Lucas-Cerrilo, et al. who used a similar Xenopus oocyte model to evaluate α-BTX binding [11]. After injection of oocytes with α7nAChR mRNA alone or in combination with CHRFAM7A mRNA they found that α-BTX staining was decreased in cells expressing both α7nAChR and CHRFAM7A compared to α7nAChR alone and concluded that the presence of CHRFAM7A decreases the number of functional α7nAChR. More recently, studies evaluating the effect of CHRFAM7A on α7nAChR binding have demonstrated that while both CHRFAM7A and α7nAChR are expressed simultaneously, the physical interaction between the CHRFAM7A and α7nAChR subunits results in a heteropentamer that can be trapped in the endoplasmic reticulum and decreases the expression of functional α7nAChR homopentamers that can bind their ligand [18].
Lasala, et al. also demonstrated that CHRFAM7A expression alters the assembly of the α7nAChR homopentamer in mammalian BOSC-23 kidney cells [16]. Utilizing a human kidney cell line, they showed that CHRFAM7A expression resulted in heteromeric receptors containing combinations of α7 and CHRFAM7A subunits. Using patch clamp analysis to measure single channel function, they showed that at least two α7 subunits are required for channel function and that CHRFAM7A co-expression could decrease ligand binding in a subunit dependent fashion. Here, we demonstrate that CHRFAM7A expression alters binding to the α7nAChR in neuronal cells. a-BTX binding is decreased in cells expressing CHRFAM7A, however the ability to further limit α-BTX binding by pre-incubation with the α7nAChR ligand nicotine demonstrates that some α7nAChR binding in the presence of CHRFAM7A remains functional.
The α7nAChR is widely expressed in the brain in areas ranging from the cerebral cortex to the hippocampus to the dorsal motor nucleus of the vagus nerve [7]. Impaired function of the α7nAChR in the brain has also been linked to neurocognitive disorders including schizophrenia, depression, and autism spectrum disorders [17]. The ability of CHRFAM7A, which is also widely expressed in the brain to potentially alter α7nAChR function and contribute to these neurocognitive disorders is critical to understand the pathogenesis of these diseases [20]. Similarly, these findings could be critical in the development of therapeutics targeting the α7nAChR.
Conclusions:
We demonstrate that CHRFAM7A decreases ligand binding to the neuronal α7nAChR in vivo but further studies will be required to determine the biological activity of CHRFAM7A and its potential as a target for therapeutic interventions to modulate α7nAChR-mediated signaling. The availability of a transgenic mouse that expresses a gene that is normally found in humans but is absent from all other species, opens the possibility of studying its contribution to human mental health in ways that were not previously possible.
Key Highlights:
CHRFAM7A is a uniquely-human gene that encodes a human-specific variant of the alpha-7 nicotinic acetylcholine receptor (α7nAchR).
Forced expression of CHRFAM7A in an in vitro neuronal cell line decreases binding to the α7nAchR.
Alpha-bungarotoxin staining was decreased at the neuromuscular junction of CHRFAM7A transgenic mice, demonstrating that CHRFAM7A alters binding to the α7nAchR in vivo.
Acknowledgements:
The authors would like to thank Emelie Amburn and Ann-Marie Hageny for their expert technical assistance throughout the course of the work. We would also like to thank the laboratory of Dr. Eran Perlson, Tel Aviv University, for their assistance with developing our neuromuscular staining techniques.
Funding Sources:
This work was supported by the National Institutes for Health [1R01GM121530–01, TWC] and [5T32DC000028–27, T. Chan]; the American Surgical Association Foundation, Beverly, MA (TWC); and the Shock Society, Bethesda, MD (T. Chan).
Footnotes
Declarations of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Akbarian S, Kundakovic M, CHRNA7 and CHRFAM7A: psychosis and smoking? Blame the neighbors!, Am J Psychiatry 172 (2015) 1054–1056. [DOI] [PubMed] [Google Scholar]
- [2].Araud T, Graw S, Berger R, Lee M, Neveu E, Bertrand D, Leonard S, The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of alpha7*nAChR function, Biochem Pharmacol 82 (2011) 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bacchelli E, Battaglia A, Cameli C, Lomartire S, Tancredi R, Thomson S, Sutcliffe JS, Maestrini E, Analysis of CHRNA7 rare variants in autism spectrum disorder susceptibility, Am J Med Genet A 167A (2015) 715–723. [DOI] [PubMed] [Google Scholar]
- [4].Baird A, Coimbra R, Dang X, Eliceiri BP, Costantini TW, Up-regulation of the human-specific CHRFAM7A gene in inflammatory bowel disease, BBA Clin 5 (2016) 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bakanidze G, Roinishvili M, Chkonia E, Kitzrow W, Richter S, Neumann K, Herzog MH, Brand A, Puls I, Association of the Nicotinic Receptor alpha7 Subunit Gene (CHRNA7) with Schizophrenia and Visual Backward Masking, Front Psychiatry 4 (2013)133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bertrand D, Lee CH, Flood D, Marger F, Donnelly-Roberts D, Therapeutic Potential of alpha7 Nicotinic Acetylcholine Receptors, Pharmacol Rev 67 (2015) 1025–1073. [DOI] [PubMed] [Google Scholar]
- [7].Breese CR, Adams C, Logel J, Drebing C, Rollins Y, Barnhart M, Sullivan B, Demasters BK, Freedman R, Leonard S, Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain, J Comp Neurol 387 (1997) 385–398. [DOI] [PubMed] [Google Scholar]
- [8].Costantini TW, Dang X, Coimbra R, Eliceiri BP, Baird A, CHRFAM7A, a human- specific and partially duplicated alpha7-nicotinic acetylcholine receptor gene with the potential to specify a human-specific inflammatory response to injury, J Leukoc Biol 97 (2015) 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Costantini TW, Dang X, Yurchyshyna MV, Coimbra R, Eliceiri BP, Baird A, A Human-Specific alpha7-Nicotinic Acetylcholine Receptor Gene in Human Leukocytes: Identification, Regulation and the Consequences of CHRFAM7A Expression, Mol Med 21 (2015) 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dang X, Eliceiri BP, Baird A, Costantini TW, CHRFAM7A: a human-specific alpha7-nicotinic acetylcholine receptor gene shows differential responsiveness of human intestinal epithelial cells to LPS, FASEB J 29 (2015) 2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].de Lucas-Cerrillo AM, Maldifassi MC, Arnalich F, Renart J, Atienza G, Serantes R, Cruces J, Sanchez-Pacheco A, Andres-Mateos E, Montiel C, Function of partially duplicated human alpha77 nicotinic receptor subunit CHRFAM7A gene: potential implications for the cholinergic anti-inflammatory response, J Biol Chem 286 (2011) 594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fertuck HC, Woodward W, Salpeter MM, In vivo recovery of muscle contraction after alpha-bungarotoxin binding, J Cell Biol 66 (1975) 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gault J, Robinson M, Berger R, Drebing C, Logel J, Hopkins J, Moore T, Jacobs S, Meriwether J, Choi MJ, Kim EJ, Walton K, Buiting K, Davis A, Breese C, Freedman R, Leonard S, Genomic organization and partial duplication of the human alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7), Genomics 52 (1998) 173–185. [DOI] [PubMed] [Google Scholar]
- [14].Ionescu A, Zahavi EE, Gradus T, Ben-Yaakov K, Perlson E, Compartmental microfluidic system for studying muscle-neuron communication and neuromuscular junction maintenance, Eur J Cell Biol 95 (2016) 69–88. [DOI] [PubMed] [Google Scholar]
- [15].Kunii Y, Zhang W, Xu Q, Hyde TM, McFadden W, Shin JH, Deep-Soboslay A, Ye T, Li C, Kleinman JE, Wang KH, Lipska BK, CHRNA7 and CHRFAM7A mRNAs: co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders, Am J Psychiatry 172 (2015) 1122–1130. [DOI] [PubMed] [Google Scholar]
- [16].Lasala M, Corradi J, Bruzzone A, Esandi MDC, Bouzat C, A human-specific, truncated alpha7 nicotinic receptor subunit assembles with full-length alpha7 and forms functional receptors with different stoichiometries, J Biol Chem (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Levin ED, alpha7-Nicotinic receptors and cognition, Curr Drug Targets 13 (2012) 602–606. [DOI] [PubMed] [Google Scholar]
- [18].Maldifassi MC, Martin-Sanchez C, Atienza G, Cedillo JL, Arnalich F, Bordas A, Zafra F, Gimenez C, Extremera M, Renart J, Montiel C, Interaction of the alpha7- nicotinic subunit with its human-specific duplicated dupalpha7 isoform in mammalian cells: Relevance in human inflammatory responses, J Biol Chem (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sinkus M, Graw S, Freedman R, Ross R, Lester H, Leonard S, The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function, Neuropharmacology 96 (2015) 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sinkus ML, Graw S, Freedman R, Ross RG, Lester HA, Leonard S, The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function, Neuropharmacology 96 (2015) 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sinkus ML, Lee MJ, Gault J, Logel J, Short M, Freedman R, Christian SL, Lyon J, Leonard S, A 2-base pair deletion polymorphism in the partial duplication of the alpha7 nicotinic acetylcholine gene (CHRFAM7A) on chromosome 15q14 is associated with schizophrenia, Brain Res 1291 (2009) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang Y, Xiao C, Indersmitten T, Freedman R, Leonard S, Lester HA, The Duplicated alpha7 Subunits Assemble and Form Functional Nicotinic Receptors with the Full-length alpha7, J Biol Chem 289 (2014) 26451–26463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zahavi EE, Ionescu A, Gluska S, Gradus T, Ben-Yaakov K, Perlson E, A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions, J Cell Sci 128 (2015) 1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]


