Abstract
Objectives
Laryngeal function requires neuromuscular activation of the intrinsic laryngeal muscles (ILMs). Rapid activation of the ILMs occurs in cough, laughter, and voice-unvoiced-voiced segments in speech and singing. Abnormal activation is observed in hyperfunctional disorders such as vocal tremor and dystonia. In this study we evaluate the dynamics of ILM contraction.
Study/Design
Basic science study in an in vivo canine model.
Methods
The following ILMs were stimulated: thyroarytenoid (TA), lateral cricoarytenoid/interarytenoid (LCA/IA), cricothyroid (CT), all laryngeal adductors (LCA/IA/TA), and the posterior cricoarytenoid (PCA). Neuromuscular stimulation was performed via the respective nerves at current levels needed to achieve maximum vocal fold posture change. Muscle contraction and posture changes were recorded with high speed video (HSV). HSV frames were then analyzed to measure response times required from the onset of muscle contraction to the time the vocal folds achieved maximum posture change.
Results
In all muscles the onset of posture change occurred within 10–12 milliseconds after neuromuscular stimulation. The average times (± standard deviation) to achieve final posture were as follows: TA 34.5 ± 6 ms (N = 15), LCA/IA 55 ± 12 ms (N =14), RLN 43 ± 8 ms (N = 18), CT 100.8 ± 17 ms (N = 26), PCA 91.2 ± 8 ms (N =3). Data distribution appeared normal.
Conclusions
Results showed a difference in muscle activation time between different ILMs consistent with reported differences in muscle fiber composition. These data also provide an estimate of the limits of laryngeal contraction frequency in physiologic and pathologic laryngeal states.
Keywords: Intrinsic laryngeal muscles, in vivo canine, muscle activation times, prephonatory posture
INTRODUCTION
Activation of the intrinsic laryngeal muscles (ILMs) is essential in nearly all laryngeal functions including voice production, airway protection, respiration, and an effective cough reflex. Many neurolaryngologic pathologies such as vocal tremor, spasmodic dysphonia, and laryngospasm result in hyperfunctional movements of the laryngeal muscles. Thus, measurement of ILM dynamics is useful to understand neuromuscular function and the limitations in normal physiology as well as pathologic states with laryngeal hyperfunction.
The ILMs consist of four paired muscles and one unpaired muscle. Each muscle has a unique trajectory and role in shaping the glottis for various laryngeal functions1,2. Previous investigations of ILM function have primarily focused on their role in voice production and in the control of laryngeal posture, but the dynamics of their movement has received little attention. Laryngeal muscle dynamics reflect their muscle fiber composition, which is reported as a percentage of type 1 (slow) and type 2 (fast) fibers, Table 1.
Table 1.
Muscle fiber type composition for each intrinsic laryngeal muscle.
| Muscle | Animal | Type 1 (%) | Type 2 (%) | Reference No. |
|---|---|---|---|---|
| PCA | Human | 52–67 | 37–48 | 25–27 |
| Vertical Belly | 61 | 36 – 44.7 | 8, 24 | |
| Horizontal Belly | 75 | 23 – 28.3 | 8, 24 | |
| TA | Human | 26–53.3 | 46.6–65 | 6, 23–24, 26 |
| Canine | 5–11.36 | 95 | 22, 27 | |
| Feline | 9.2 | 90.8 | 30 | |
| CT | Human | 38.3–47 | 61.6 | 6, 25–26 |
| Canine | 40–45 | 60 | 22 | |
| Feline | 44.6 | 55.4 | 30 | |
| LCA | Human | 41.6 | 58.3 | 26 |
| Canine | 16.3 | 83.7 | 27–29 | |
| Feline | 11.4 | 88.6 | 30 |
Laryngeal adductor muscles include the paired thyroarytenoid (TA) and lateral cricoarytenoid (LCA) and the unpaired interarytenoid (IA). The TA moves the vocal process medially and inferiorly during vocal fold adduction3. The TA is composed primarily of fast-twitch Type II fibers. The fast isoforms (Type IIA and IIX) compose 53.8% of the TA4. In our previous investigation on ILM function using an in vivo canine neuromuscular model, onset of TA activation was noted as an inferomedial bulging of the vocal fold around 14 ms after nerve stimulation. The mid-membranous region was near fully adducted by 22 ms, and the entire membranous fold adducted by 47 ms 5. The LCA is composed primarily of Type IIB fibers6. Much like the TA, the LCA exhibits high succinic dehydrogenase and low NADH2 tetrazolium reductase, suggesting greater fatigue resistance than limb muscle phenotypes6. LCA/IA activation was noted to begin adducting the posterosuperior vocal fold by 16 to 22 ms and achieved maximal posture change by 47 ms 5.
The paired posterior cricoarytenoid (PCA) muscle abducts the vocal fold by moving the vocal process laterally and superiorly7. In the human PCA, the vertical belly is composed of 61% Type I (slow-twitch) and 36% Type II (fast-twitch) fibers, compared to 75% and 23% respectively in the horizontal belly8. The slight difference in fiber composition is consistent with the sphincter function of the vertical belly in phonation and swallowing, which requires a relatively short response time, and the more dominant role in respiration of the horizontal belly, which is active during inspiration8. During in vivo neuromuscular stimulation of a canine larynx, the PCA has a slow time course of activation, with changes first appreciable by 16–33 ms and maximum posture change achieved by 100 ms 5. The cricothyroid (CT) muscle is composed of two bellies, the pars recta and pars oblique. Together these two components pull the anterior part of the cricoid cartilage backward and upward to lengthen vocal fold, constrict the glottis, and dilate the pharynx3,9–11. If the vocal folds are already adducted, the CT can be activated to increase vocal fold tension leading to increased pitch3,6. In ex vivo human larynges, the CT is composed of fast MyHC isoforms (i.e. IIA, IIB, and IIX), and Type I and beta slow fibers4,6. Changes in glottal posture from CT activation in the in vivo canine model are first appreciable by 16–33 ms with maximum posture change by 100 ms 5.
It is generally understood that the TA and the LCA muscles are the fastest while the CT and PCA are the slowest ILMs. However, the variability of ILM dynamics between different larynges is unknown. Thus, we investigated ILM dynamics across 26 unique vocal folds (left and right) from 15 canine subjects. We evaluated the activation times for TA, LCA/IA, CT, PCA, and the combined laryngeal adductors (‘RLN’, TA/LCA/IA). While we expect to find the TA and LCA/IA to be faster muscles compared to the PCA and CT within larynges, we hypothesize that there will be some variability of muscle dynamics between larynges that reflects the reported variability in fiber type ratios among ILMs. We also seek to better understand the biomechanics and limits of laryngeal muscle activation as it pertains to both normal and pathologic laryngeal function.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committee at the University of California, Los Angeles. An in vivo canine selective laryngeal activation model was utilized as described previously12,13. Data was collected from 15 Mongrel canines prospectively. All ILMs from each larynx could not be evaluated because each canine experiment had a unique focus on a subset of muscles. As such, the number of unique vocal folds analyzed for each muscle varied. There were 15 unique vocal folds for TA, 14 for LCA, 18 for the combined adductors (RLN), 26 for CT and 3 for PCA.
Surgical exposure of the larynges and laryngeal nerves was performed as previously described12,13. Surgery was performed by the same surgeon in all cases12,13. In brief, under general endotracheal anesthesia a vertical midline incision was made in the neck to expose the larynx and trachea. A low tracheostomy was then performed and used for ventilation. The recurrent laryngeal nerve (RLN) was identified in the neck, followed distally, and the nerve branches to the TA, LCA/IA, and PCA were identified. The external branch of the superior laryngeal nerve (SLN) was identified close to its insertion to the CT muscle. An infrahyoid pharyngotomy was performed and the larynx was exteriorized in the neck for high-speed video recording. A supraglottic laryngectomy was also performed to fully expose and optimize the superior view of the true vocal folds. India ink was used to mark 0.5 mm fleshpoints on the superior surface of the vocal folds in 3 locations; the vocal process, mid-membranous vocal fold, and just posterior to the anterior commissure.
ILMs were activated as follows: to activate the TA, the TA branch was divided from the RLN and the distal stump (TA branch) stimulated using a cuff electrode. The LCA/IA was stimulated together because the IA branch is small and exposing it can damage surrounding muscles ad nerves. To stimulate the LCA/IA, the TA and PCA branches were divided and the intact RLN stimulated. To simulate all adductors (TA/LCA/IA), the PCA branch was divided and the RLN was stimulated. The location of RLN stimulation in each experiment was consistent at about 2 cm caudal to the cricopharyngeus muscle. To stimulate the CT muscle, the external branch of the superior laryngeal nerve (eSLN) was stimulated. The internal branch of the SLN was always divided during the larynx exteriorization. The location of eSLN stimulation was also consistent in all experiments at about 2 cm from the insertion of the nerve to the CT muscle. To stimulate the PCA muscle the RLN branch to the PCA was identified and the distal stump (PCA branch) was stimulated.
Neuromuscular stimulation of each ILM was performed for 1,500 milliseconds with uniform 0.1 millisecond cathodic pulses at 100 Hz. The lowest stimulation level that achieved the maximal posture change was selected for analysis of muscle dynamics for this study. This stimulation level was determined by gradually increasing the stimulation current from threshold muscle activation, where just a hint of muscle movement was seen, to maximal activation, where no additional posture change to stimulation was seen. Each 1500 ms pulse train was followed by a 3.5 second pause to allow for muscle recovery. From our previous experience, we needed about 8–10 steps of increasing stimulation current to reach the level that led to maximal posture change. Vocal fold motion and deformation during neuromuscular stimulation was captured with a high-speed digital camera at 3,000 frames per second at a resolution of 512 × 512 pixels (Phantom v210, Vision Research Inc., Wayne, NJ).
Movement of the India ink landmark was used to assess the onset and completion of all visible vocal fold motion and posture change from muscle activation. Onset time for measurement of muscle dynamics was marked at the first visible sign of movement of the ink mark. The ink mark at the vocal process was tracked for assessment of PCA, LCA/IA, all adductors (RLN), and CT muscle dynamics, and the mark at the mid-membranous vocal fold was tracked for TA muscle dynamics. Measurement of the duration of postural change was assessed manually using the Phantom Camera Control Application software (PCC 1.3, Vision Research Inc.), which allows frame-by-frame analysis of landmark trajectory and measurement of distance traveled. The muscle activation time, defined as the time required for displacement from onset of movement to final maximum posture, was calculated based on the number of video frames needed to travel this distance (three video frames per millisecond), Figure 1.
Figure 1. Experimental glottal view of vocal fold adduction.
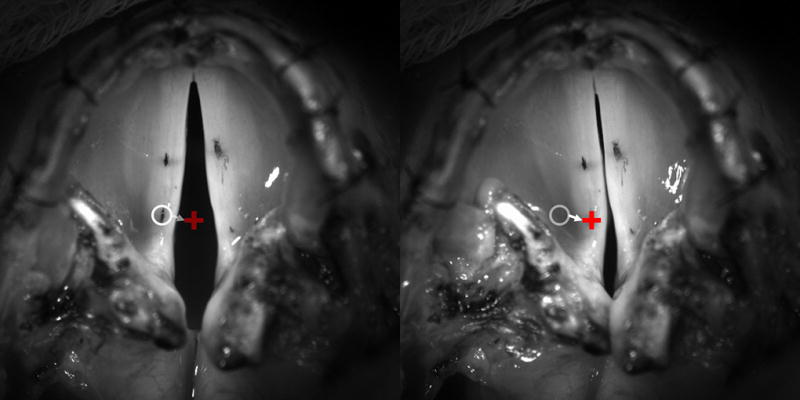
Superior view of glottis with India ink fleshpoints seen on the left and right mid and posterior vocal folds. Left frame is resting posture just at the onset of left LCA stimulation. Right frame is final fully activated posture showing full adduction of the left vocal fold at the vocal process. Open white circle denotes point of reference along the posterior vocal fold at rest just before motion onset. White arrow denotes trajectory of vocal fold adduction. Red ‘+’ denotes final adducted posture. The time it takes to go from the white circle to the red ‘+’ is taken as the activation time.
Statistical analysis included one-way ANOVA and post-hoc Least Significant Differences (LSD) tests to compare across ILMs, Students t-test to compare left and right sides for a given ILM, measurement of kurtosis, and the nonparametric one-sample Kolmogorov-Smirnov (KS) test to evaluate the distribution of activation times for each ILM. This was performed using IBM SPSS Statistics, Version 24.
RESULTS
In all muscles the onset of posture change (vocal fold movement) consistently occurred within 10–12 milliseconds after the start of neuromuscular stimulation. Table 2 details the muscle activation times for all larynges for each muscle group. Bolded numbers represent muscles from the left side of the larynx while non-bolded numbers represent the right side for a given ILM. The average muscle activation times (± standard deviation) to achieve final pre-phonatory posture were as follows: TA 34.5 ± 6 ms, LCA/IA 55 ± 12 ms, RLN 43 ± 8 ms, CT 100.8 ± 17 ms, and PCA 91.2 ± 8 ms. Figure 2 plots the muscle activation times for all larynges for each muscle group to provide an idea of the data distribution. Furthermore, the Kurtosis was computed for each muscle group to get an idea of normalcy of the data distribution. The kurtosis was near zero for TA, LCA/IA, and CT while the RLN demonstrated a more positive Kurtosis and thus a more peaked distribution (RLN kurtosis of 2.47). Kolmogorov-Smirnov testing further demonstrated that all ILMs exhibited a uniform distribution with the exception of the combined adductors. Students t-tests were performed to compare left versus right sided muscles for each ILM. Left versus right TA, LCA/IA, and RLN were not significantly different while left versus right sided CT muscle activation times differed significantly (p = 0.012). One-way ANOVA confirmed a significant intergroup difference among ILMs with post-hoc LSD tests demonstrating a significant difference of the means of all ILMs except when comparing the CT and PCA muscle groups (p = 0.20).
Table 2.
Average time from onset of vocal fold movement to achieving final posture.
| N | TA | LCA/IA | RLN | CT | PCA |
|---|---|---|---|---|---|
| 1 | 23.7 | 43 | 42 | 85.7 | 90 |
| 2 | 44.3 | 70 | 41 | 96 | 83.7 |
| 3 | 30.7 | 59.3 | 34 | 97 | 100 |
| 4 | 36 | 61 | 34 | 100 | |
| 5 | 33.7 | 62.7 | 43 | 103.3 | |
| 6 | 35.3 | 45.7 | 32.7 | 114.7 | |
| 7 | 29.3 | 69 | 49 | 85 | |
| 8 | 31 | 32.7 | 42.7 | 98.3 | |
| 9 | 33.3 | 58.7 | 42.3 | 113 | |
| 10 | 32 | 63.3 | 42 | 76.3 | |
| 11 | 32 | 37.7 | 47.7 | 100 | |
| 12 | 42 | 46.3 | 37.3 | 65.7 | |
| 13 | 43.7 | 59.7 | 37.7 | 91 | |
| 14 | 31 | 61.3 | 41.7 | 76.7 | |
| 15 | 39.7 | 42.3 | 120 | ||
| 16 | 44 | 94.7 | |||
| 17 | 64 | 121 | |||
| 18 | 55.7 | 113.3 | |||
| 19 | 129.7 | ||||
| 20 | 74.7 | ||||
| 21 | 115.7 | ||||
| 22 | 115.7 | ||||
| 23 | 82.7 | ||||
| 24 | 110.7 | ||||
| 25 | 122.7 | ||||
| 26 | 116.3 | ||||
|
| |||||
| Average (ms) | 34.5 | 55 | 43 | 100.8 | 91.2 |
| SD | 6 | 12 | 8 | 17 | 8 |
Bold numbers represent left sided muscles, non-bold numbers represent the right side. All times are in milliseconds.
CT, cricothyroid; IA, Interarytenoid; LCA, lateral cricoarytenoid; ms, millisecond; PCA, posterior cricoarytenoid; RLN, recurrent laryngeal nerve; SD, standard deviation; TA, thyroarytenoid.
Figure 2. Intrinsic laryngeal muscle activation times.
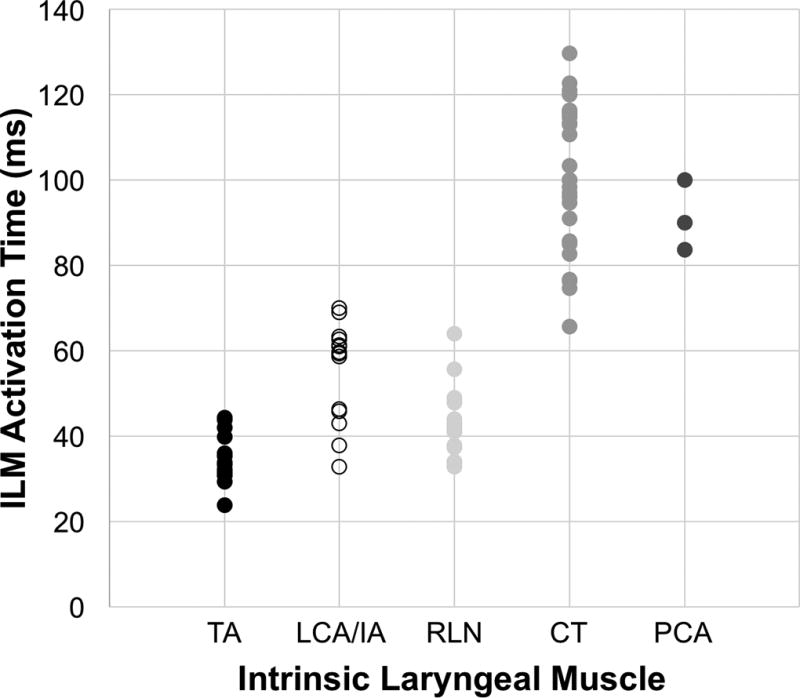
Activation times plotted for each muscle for each subject provides an idea of the data distribution. Post-hoc Least Significant Difference (LSD) test demonstrated significant differences of the means of all ILMs except when comparing CT and PCA muscle groups (p = 0.20).
DISCUSSION
In this study, we evaluated ILM dynamics over a large number of canine larynges. As expected, the TA and LCA/IA had fast activation times of around 50 ms or less, while the CT and PCA had activation times of around 100 ms. This is also consistent with our previous findings from ILM activation in an in vivo canine hemilarynx model5,14. As detailed in Table 1 these stark differences in activation times are related to differences in muscle fiber type composition with the laryngeal adductors having a greater percentage of type 2 (fast) fibers. We now demonstrate that there is also interlaryngeal variation within each muscle, as illustrated in Figure 2. This is consistent with fiber type ratio variations within muscle groups between larynges as well. The phonetogram, or voice range profile (VRP), of human individuals may vary greatly depending upon age, sex, time of day, experience, respiratory volume, mouth opening, medical treatment, and many other potential independent variables15,16. Variations in individual muscle activation times, as we capture here, may further contribute to variability in voice production across subjects.
Many laryngeal tasks require rapid adduction/abduction of ILMs. These include glottal stops (e.g. uh-oh!), voice-unvoiced-voiced segments in speech, laughing, and coughing. Neurologic vocal dysfunctions such as vocal tremor also manifest as rapid rhythmic activation of the ILMs. Laughter is a series of repeated vocalizations during exhalation, requiring rapid adduction followed by relaxation of the vocal folds. Interestingly, the typical frequency of both vocal tremor and laughter is about 5–6 Hz17–19. In the present study, we find that the average combined activation times of all laryngeal adductors (44 ms) and the PCA (91 ms) is 131 ms. If we consider the TA (35 ms) and the PCA, this cumulative activation time is 127 ms on average, or about 8 Hz and well within the findings in tremor and laughter. One could even surmise that the limits for the frequency of an adduction/abduction cycle on average is about 8 Hz. Note that this estimation of 8 Hz is based on one cycle of full adduction and full abduction, which is not often found in most laryngeal tasks except cough. Thus the possible frequency for tasks requiring limited excursion of adduction/abduction could be significantly higher.
However, there are always additional biomechanical constraints for a given muscle motion task beyond muscular contraction. In voice and speech production, the physiologic constraints for vocal fold motion include the time to initiate joint motion from rest, directional constraints of joint mobility, and tissue viscoelasticity as the entire vocal fold needs to move regardless of the ILM activated. Vocal fold motion also requires neuromuscular coordination of agonist-antagonist muscles; i.e. when adductor muscles contract the abductor muscle has to relax and stretch, and vice versa. For a given vocalization task, stabilizing the pre-phonatory posture to ensure the correct posture before phonation also requires time. Finally, muscles have to adjust posture along a continuum (one posture is the direct product of the preceding posture) and cannot change position in a discontinuous fashion. Literature on appendicular skeletal muscle tells us that muscle performance may be tied to pre-activation length and/or joint angle as has been shown with the hamstring muscles20,21.
In addition, control of the laryngeal muscles is complex. The primary motor neurons controlling the intrinsic laryngeal muscles are located in the nucleus ambiguous (NA). The NA receives both excitatory and inhibitory input from the brainstem central pattern generators controlling respiration, cough and swallowing. For volitional movement, such as for phonation, there is direct innervation from the cerebral cortex. For emotional vocalizations, there are additional connections from the limbic system. Therefore, despite the rapidity for which the laryngeal adductors and abductors can contract in relative isolation, the maximum frequency at which these motions can be coordinated is limited by these biomechanical and neurophysiologic constraints.
CONCLUSION
The intrinsic laryngeal muscles have unique muscle fiber type compositions which contribute to their dynamics. The laryngeal adductors have more type 2 (fast) fibers and demonstrated rapid activation times from onset of movement to final posture of around 50 ms or less in our study. The CT and PCA have proportionally more type 1 (slow) muscle fibers, consistent with the slower activation times of around 100 ms. Activation times for each ILM maintained a uniform distribution across all 26 unique vocal folds. These activation times would theoretically allow for more rapid laryngeal posture changes than what is actually observed in vocal tremor or laughing. This discrepancy is most likely related to biomechanical and neurophysiologic constraints. Future studies aimed to address these questions are warranted.
Acknowledgments
This study was supported by grants R01DC011299 and R01DC011300 from the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST: None
Presented as a podium presentation at the 2018 Triological Society Combined Sections Meeting, Scottsdale, Arizona, USA on January 18–20, 2018. Manuscript submission #286.
References
- 1.Shiba TL, Chhetri DK. Dynamics of phonatory posturing at phonation onset. The Laryngoscope. 2016;126:1837–1843. doi: 10.1002/lary.25816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chhetri DK, Neubauer J. Differential roles for the thyroarytenoid and lateral cricoarytenoid muscles in phonation. The Laryngoscope. 2015;125:2772–2777. doi: 10.1002/lary.25480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludlow CL. Laryngeal Reflexes: Physiology, Technique, and Clinical Use. J Clin Neurophysiol. 2015;32:284–293. doi: 10.1097/WNP.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toniolo L, Macchi V, Porzionato A, et al. Myosin heavy chain isoforms in human laryngeal muscles: an expression study based on gel electrophoresis. International journal of molecular medicine. 2008;22:375–379. [PubMed] [Google Scholar]
- 5.Vahabzadeh-Hagh AM, Zhang Z, Chhetri DK. Quantitative Evaluation of the In Vivo Vocal Fold Medial Surface Shape. Journal of voice : official journal of the Voice Foundation. 2017;31:513.e515–513.e523. doi: 10.1016/j.jvoice.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoh JF. Laryngeal muscle fibre types. Acta Physiol Scand. 2005;183:133–149. doi: 10.1111/j.1365-201X.2004.01402.x. [DOI] [PubMed] [Google Scholar]
- 7.Chhetri DK, Neubauer J, Sofer E. Posterior cricoarytenoid muscle dynamics in canines and humans. The Laryngoscope. 2014;124:2363–2367. doi: 10.1002/lary.24742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asanau A, Timoshenko AP, Prades JM, Galusca B, Martin C, Feasson L. Posterior cricoarytenoid bellies: relationship between their function and histology. Journal of voice : official journal of the Voice Foundation. 2011;25:e67–73. doi: 10.1016/j.jvoice.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Hong KH, Kim HK, Kim YH. The role of the pars recta and pars oblique of cricothyroid muscle in speech production. Journal of voice : official journal of the Voice Foundation. 2001;15:512–518. doi: 10.1016/S0892-1997(01)00051-0. [DOI] [PubMed] [Google Scholar]
- 10.Alipour F, Titze I. Active and passive characteristics of the canine cricothyroid muscles. Journal of voice : official journal of the Voice Foundation. 1999;13:1–10. doi: 10.1016/s0892-1997(99)80056-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z. Mechanics of human voice production and control. J Acoust Soc Am. 2016;140:2614. doi: 10.1121/1.4964509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chhetri DK, Neubauer J, Bergeron JL, Sofer E, Peng KA, Jamal N. Effects of asymmetric superior laryngeal nerve stimulation on glottic posture, acoustics, vibration. The Laryngoscope. 2013;123:3110–3116. doi: 10.1002/lary.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chhetri DK, Neubauer J, Berry DA. Graded activation of the intrinsic laryngeal muscles for vocal fold posturing. J Acoust Soc Am. 2010;127:El127–133. doi: 10.1121/1.3310274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vahabzadeh-Hagh AM, Zhang Z, Chhetri DK. Three-dimensional posture changes of the vocal fold from paired intrinsic laryngeal muscles. The Laryngoscope. 2017;127:656–664. doi: 10.1002/lary.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman RF. Sources of variation in phonetograms. Journal of voice : official journal of the Voice Foundation. 1993;7:1–14. doi: 10.1016/s0892-1997(05)80107-9. [DOI] [PubMed] [Google Scholar]
- 16.Printz T, Sorensen JR, Godballe C, Grontved AM. Test-Retest Reliability of the Dual-Microphone Voice Range Profile. Journal of voice : official journal of the Voice Foundation. 2017 doi: 10.1016/j.jvoice.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Sulica L, Louis ED. Clinical characteristics of essential voice tremor: a study of 34 cases. The Laryngoscope. 2010;120:516–528. doi: 10.1002/lary.20702. [DOI] [PubMed] [Google Scholar]
- 18.Kimaid PA, Quagliato EM, Crespo AN, Wolf A, Viana MA, Resende LA. Laryngeal electromyography in movement disorders: preliminary data. Arquivos de neuro-psiquiatria. 2004;62:741–744. doi: 10.1590/s0004-282x2004000400034. [DOI] [PubMed] [Google Scholar]
- 19.Lester RA, Barkmeier-Kraemer J, Story BH. Physiologic and acoustic patterns of essential vocal tremor. Journal of voice : official journal of the Voice Foundation. 2013;27:422–432. doi: 10.1016/j.jvoice.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Kirk EA, Rice CL. Contractile function and motor unit firing rates of the human hamstrings. Journal of neurophysiology. 2017;117:243–250. doi: 10.1152/jn.00620.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onishi H, Yagi R, Oyama M, Akasaka K, Ihashi K, Handa Y. EMG-angle relationship of the hamstring muscles during maximum knee flexion. Journal of electromyography and kinesiology : official journal of the International Society of Electrophysiological Kinesiology. 2002;12:399–406. doi: 10.1016/s1050-6411(02)00033-0. [DOI] [PubMed] [Google Scholar]
- 22.Braund KG, Steiss JE, Marshall AE, Mehta JR, Amling KA. Morphologic and morphometric studies of the intrinsic laryngeal muscles in clinically normal adult dogs. American journal of veterinary research. 1988;49:2105–2110. [PubMed] [Google Scholar]
- 23.Happak W, Zrunek M, Pechmann U, Streinzer W. Comparative histochemistry of human and sheep laryngeal muscles. Acta oto-laryngologica. 1989;107:283–288. doi: 10.3109/00016488909127510. [DOI] [PubMed] [Google Scholar]
- 24.Horton MJ, Rosen C, Close JM, Sciote JJ. Quantification of myosin heavy chain RNA in human laryngeal muscles: differential expression in the vertical and horizontal posterior cricoarytenoid and thyroarytenoid. The Laryngoscope. 2008;118:472–477. doi: 10.1097/MLG.0b013e31815c1a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascarello F, Veggetti A. A comparative histochemical study of intrinsic laryngeal muscles of ungulates and carnivores. Basic and applied histochemistry. 1979;23:103–125. [PubMed] [Google Scholar]
- 26.Rosenfield DB, Miller RH, Sessions RB, Patten BM. Morphologic and histochemical characteristics of laryngeal muscle. Archives of otolaryngology (Chicago, Ill : 1960) 1982;108:662–666. doi: 10.1001/archotol.1982.00790580056018. [DOI] [PubMed] [Google Scholar]
- 27.Wu YZ, Baker MJ, Crumley RL, Blanks RH, Caiozzo VJ. A new concept in laryngeal muscle: multiple myosin isoform types in single muscle fibers of the lateral cricoarytenoid. Otolaryngol Head Neck Surg. 1998;118:86–94. doi: 10.1016/S0194-5998(98)70380-8. [DOI] [PubMed] [Google Scholar]
- 28.Wu YZ, Crumley RL, Armstrong WB, Caiozzo VJ. New perspectives about human laryngeal muscle: single-fiber analyses and interspecies comparisons. Archives of otolaryngology--head & neck surgery. 2000;126:857–864. doi: 10.1001/archotol.126.7.857. [DOI] [PubMed] [Google Scholar]
- 29.Wu YZ, Crumley RL, Caiozzo VJ. Are hybrid fibers a common motif of canine laryngeal muscles? Single-fiber analyses of myosin heavy-chain isoform composition. Archives of otolaryngology--head & neck surgery. 2000;126:865–873. doi: 10.1001/archotol.126.7.865. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama T, Nonaka S, Mori S. Histochemical properties of intrinsic laryngeal muscles in cats. Journal of the autonomic nervous system. 1995;56:50–60. doi: 10.1016/0165-1838(95)00064-6. [DOI] [PubMed] [Google Scholar]


