Abstract
OBJECTIVE
Age-related muscle atrophy of the laryngeal muscles contributes to presbyphonia. Remodeling of the neuromuscular junction is one aspect underlying age-related muscle atrophy. Although muscle disuse has been shown to exacerbate age-related neuromuscular changes in the limb muscles, it is unknown if reduced vocal use has a similar effect in the laryngeal muscles. The objective of this study was to examine the use of social isolation as a novel method to reduce vocal use in old rats, and to investigate the impact of that reduced vocal use on ultrasonic vocalization acoustics and neuromuscular junction morphology in the thyroarytenoid muscle.
STUDY DESIGN
Animal group comparison.
METHODS
Old F344/BN rats (31 months of age) were socially isolated (n = 8) or communally housed (n = 8) for 8 weeks. Effect of housing condition on ultrasonic vocalization acoustics was assessed by calculating the changes in vocalization fundamental frequency and amplitude from baseline to 8 weeks. Neuromuscular junction morphology was measured in the lateral and medial portions of the thyroarytenoid muscle at the conclusion of the experiment.
RESULTS
Vocalization amplitude decreased by a mean of −4.4 dB (SD, 4.49) after social isolation, whereas amplitude increased by a mean of 5.7 dB (SD, 5.07) in the communally housed rats (p = 0.002). There was no significant difference in the change in fundamental frequency between groups. Furthermore, there were no group differences in any measure of neuromuscular junction morphology.
CONCLUSIONS
These results suggest that neuromuscular junctions in the thyroarytenoid muscle of old rats are unaffected by 8 weeks of social isolation, despite functional changes in vocalizations.
Keywords: Larynx, voice/dysphonia, ultrasonic vocalizations, neurolaryngology, neuromuscular junction, thyroarytenoid, ultrasonic vocalizations
Introduction
Voice disorders are common in older adults, negatively affect quality of life, and can lead to social withdrawal, thereby increasing risks of both physical and mental health problems.1–3 While the etiology of presbyphonia is multifactorial, age-related laryngeal muscle atrophy (sarcopenia) is implicated as a primary cause.4 Sarcopenic changes in the limb musculature are preceded by alterations in neuromuscular junction (NMJ) morphology.5 Likewise, the thyroarytenoid (TA) muscle undergoes atrophic changes in advanced age accompanied by alterations of NMJ morphology.6–8 Additionally, functional age-related vocalization deficits, such as a decrease in the amplitude of the ultrasonic vocalizations (USVs) of old rats, have been associated with increased NMJ motor endplate dispersion in a normally aging rat model.9 Thus, changes in NMJ morphology are related to structural and functional sarcopenic changes.
Decreased activity in aged limb muscles increases the rate of atrophy and, in contrast to the effects of age alone, causes expansion of both the pre-synaptic nerve terminal and the post-synaptic motor endplate of the NMJ.10,11 Social isolation and loneliness are common in older adults, particular in advanced age (80+), and may lead to decreased voice use.12,13 The effect of social isolation and decreased voice use on the voice and its underlying neuromuscular mechanisms, however, is unknown.
Rats communicate affective state with one another using USVs.14 Additionally, rat USVs are produced and modulated using laryngeal muscle contraction, similar to human vocalizations.15 Therefore, increasing or decreasing USV production over time can be used to study the neuromuscular effects of decreased or increased use of the laryngeal muscles, as has been previously demonstrated.9,16 In this study we used social isolation to reduce vocal use in old rats. We hypothesized that social isolation would be associated with both functional and neuromuscular decline in laryngeal muscles. Specifically, we tested this hypothesis by examining vocal function (amplitude and fundamental frequency of USVs) and NMJ morphology in the TA muscles of old rats that were either socially isolated or communally housed.
Materials and methods
Animals
The animal use protocol for this study was approved by the Animal Care and Use Committee of the University of Illinois at Urbana-Champaign. A total of 16 Fischer 344/Brown Norway male rats were obtained from the National Institute on Aging animal colony, where they had been housed in groups of 2 males. Rats were 31 months old at the conclusion of the study, representing advanced senescence.17 Upon arrival, rats were evenly and randomly assigned (8 per group) to either a single-housed social isolation group or a paired-housed control group. Each group was maintained in this housing condition for 8 weeks. This duration was based on findings of NMJ adaptations in the limb disuse literature.10 Water and food were provided ad libitum.
USV recording
A 43 cm diameter chamber with wire-mesh walls, a translucent plexiglass floor, and an open top was used for USV recording (Dodecagon Hub, Coulbourn Instruments, Whitehall, PA). The chamber was placed on an infrared LED panel with an infrared video camera (SSC-M383, Sony) suspended above. The video signal was recorded for later analysis of the rat’s position relative to each microphone during the USV recording sessions (Viewer, BIOBSERVE, St. Augustin, Germany). All rats were familiarized to the recording chamber by placing them individually in the chamber for 10 minutes daily for 1 week before the recording session.
Four ultrasonic microphones (CMI6, Avisoft Bioacoustics, Germany) were evenly spaced at 90-degree intervals around the perimeter of the recording chamber. This was done to allow for full acoustic coverage of the chamber to improve the signal-to-noise ratio of captured USVs at any orientation of the rat. Signals from the 4 microphones were each digitized with a 250-kHz sampling rate and 16-bit depth (UltraSoundGate 416, Avisoft Bioacoustics, Germany) and simultaneously recorded in an uncompressed 4-channel wav file (RECORDER, Avisoft Bioacoustics, Germany). This bit depth provided an approximate 96 dB dynamic range (from −96 to 0 dB), where 0 dB represented the maximum amplitude the recording system was capable of capturing and −96 dB represented the minimum detectable amplitude. The recording level of each of the 4 microphones was calibrated using a 50-kHz pure tone generated by a synthesizer (RX6 Multi-Function Processor, Tucker-Davis Technologies, Alachua, FL) and played back through an electrostatic speaker (ES1, Tucker-Davis Technologies). Microphones were not calibrated to a known intensity and, therefore, the amplitude measurements from each microphone were relatively equal, but not absolute.
USVs were elicited and recorded at baseline and after 8 weeks of either standard paired housing or social isolation using an existing mating paradigm.18 In brief, a female rat in estrus was placed in the recording chamber with the male rat. After the male expressed interest in the female through sniffing, chasing, and attempts at mounting, the female rat was removed. This elicited USVs from the male rat. This process was repeated as many times as was necessary to record a minimum of 20 USVs from each male rat. All male rats were sexually naïve prior to the experiment.
Over the course of 8 weeks, the number of USVs from each cage during a 24-hour period was counted to confirm the socially isolated rats were vocalizing less than the socially housed rats. To achieve this, each cage was placed in an acoustic isolation chamber and acoustically monitored for 24 hours. Although it would have been preferable to count the number of USVs from each individual rat in the socially housed cages, it was not possible to distinguish which animal was vocalizing during the monitoring. Cages were selected at random twice throughout the 8 weeks with a minimum of 2 weeks between each monitoring session.
USV analysis
Baseline and post-experimental USVs were manually labeled and segmented from background noise on a spectrogram of the 4-channel audio files and automatically analyzed for mean amplitude and mean fundamental frequency (SASLab Pro, Avisoft Bioacoustics, Berlin, Germany). To account for the highly-directional cardioid polar pattern of the ultrasonic microphones, the video tracking data was analyzed to determine which of the four microphones the rat was facing during each USV production. The microphone channel corresponding to the smallest mouth-to-microphone orientation angle, as automatically calculated by the video tracking software, was selected for further statistical analysis.
A previous study showed there was no difference in USV amplitude loss between USV categories (complex vs. simple).9 Therefore, USVs were not categorized and all USVs were averaged for each rat to calculate mean amplitude and fundamental frequency at baseline and at post-experimental time points. Statistical analyses were conducted on the average change in mean amplitude and fundamental frequency from baseline to post-experiment.
NMJ Analysis
At the completion of the study, rats were euthanized, whole larynges were dissected and fixed for 1 hour in 4% formaldehyde, cryoprotected overnight in a 20% sucrose / 5% glycerol solution in phosphate-buffered saline, then flash frozen in isopentane chilled to just above its freezing point. NMJs in the medial and lateral portions of the TA were labeled using immunohistochemistry procedures previously established.9
NMJs were imaged using a spectral confocal microscope (LSM 710 LSO, Zeiss). Individual NMJ images were automatically analyzed for quantitative morphologic measurements using an existing custom algorithm in ImageJ.9,19 Taking advantage of the 3D information provided by confocal imaging, the motor endplate stack was rotated in both the x and y planes until the maximum area was found. This rotated position was considered to be an estimated en face position (Figure 1). The same x and y degrees of rotation were used to rotate the nerve terminal stack.
Fig. 1.
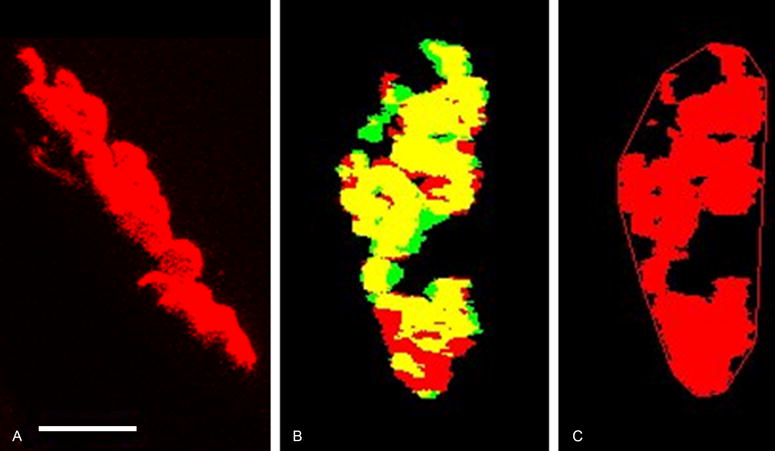
Maximum z-projections of a representative confocal image stack demonstrating the morphological measurements of the NMJ with the pre-synaptic nerve terminal (green), motor endplate (red) and areas of synaptic overlap (yellow) (color online). A) The motor endplate in the orientation as collected at the microscope. The white scale bar is equal to 10 microns and is the same scale in each panel. B) Synaptic overlap after rotation to the standardized en face orientation. C) Convex hull around the motor endplate used to calculate endplate dispersion (proportion of unlabeled area to labeled motor endplate area within the convex hull).
Pre-synaptic nerve terminal area and post-synaptic motor endplate area were measured on z-projections from this estimated en face position. The overlap between the pre-synaptic nerve terminal z-projection area and post-synaptic motor endplate z-projection area of each NMJ was assessed as the percentage of motor endplate labeled area that aligned with labeled nerve terminal area (maximum of 100%). Dispersion of the motor endplate was calculated as the percentage of the area of the labeled motor endplate relative to the total area taken up by the motor endplate, as measured by a convex hull around the labeled area. The greater the amount of unlabeled space between individual acetylcholine receptor clusters, the greater the dispersion ratio.
Statistical analysis
All statistical analyses were completed using R (R Core Team, 2013). The Welch Two Sample t-test was used to compare groups on normally distributed variables with homogeneous variances. Wilcoxon rank sum tests were used to compare groups on all other variables. Hedge’s g effect sizes were calculated for variables with a significant difference between experimental groups to explore the magnitude of that difference.20
Results
Two rats in the social isolation group did not vocalize during the post testing, despite repeated attempts across several days. Therefore, a change in fundamental frequency and amplitude could not be calculated for these rats resulting in 6 rats in the social isolation group and 8 in the control group in the final analyses of USV acoustics. All 8 rats in each group were used for NMJ morphology analysis.
Monitoring
Rats in the social isolation group produced a median of 11 USVs (range = 5 to 24 USVs) during the 24-hour acoustic monitoring sessions. The pairs of rats in the control group produced a median of 69 USVs (range = 39 to 100) during the monitoring sessions.
Acoustic analyses of USVs
USV acoustic results are presented as the average change from baseline to post-experiment. Therefore, a positive value indicates the measurement increased from baseline to post-experiment, while a negative value indicates the opposite. The USV amplitude of the social isolation group decreased after 8 weeks of social isolation with a mean change of −4.4 dB (SD, 4.49 dB). In contrast, the control group had a mean increase in USV amplitude of 5.7 dB (SD, 5.07 dB) after 8 weeks of social housing (Figure 2), a significantly different change in amplitude than the social isolation group (t = 3.94, df = 12, p = 0.002). Hedge’s effect size (g) was 2.0 (95% CI, 0.38 to 3.54), suggesting a large difference between the social isolation and control groups in the amount of amplitude change.
Fig. 2.
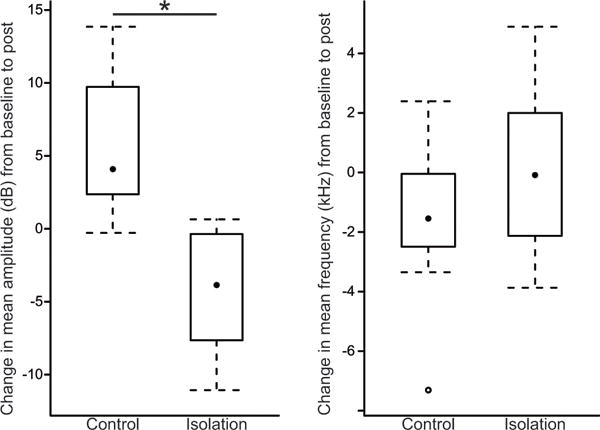
Box and whisker plots showing the change in USV acoustic parameters (amplitude and frequency) from baseline to after 8 weeks of either standard paired housing (Control) or social isolation (Isolation). The median is indicated with a black dot, the inter-quartile range is contained within the box, and the whiskers extend to the last observation within 1.5 times the inter-quartile range. The single outlier is indicated by an open circle. On the y-axis 0 (dB or kHz) means no change from baseline to post-experiment, a positive value indicates an increase from baseline to post-experiment, and a negative value means the measurement decreased from baseline to post-experiment. Change in amplitude was significantly lower in the social isolation group compared to the socially-housed control group (* indicates p < .05).
There was no significant difference in the change in mean frequency between the social isolation and control groups (t = −1.08, df = 10, p = 0.3) with mean changes of 0.1 kHz (SD, 3.1 kHz) and −1.6 kHz (SD, 2.9 kHz), respectively (Figure 2).
NMJ Morphology
Analysis of NMJs failed to reveal any significant effect of social isolation on any measure of NMJ morphology in either the lateral or medial portion of the TA (Table 1). Nerve terminal area was the same between experimental groups in both the lateral TA (t = 1.2, df = 13, p = 0.2) and the medial TA (t = −0.9, df = 14, p = 0.4). The same was true of motor endplate area in the lateral TA (t = 0.009, df = 14, p = 1.0) and the medial TA (t = −0.8, df = 14, p = 0.4). Likewise, there were no significant differences in synaptic overlap between experimental groups in either the lateral TA (W = 44, p = 0.23) or the medial TA (t = 0.41, df = 13, p = 0.69). Endplate dispersion ratio was not significantly different between groups in either the lateral TA, (t = 0.19, df =12, p = 0.8) or the medial TA (t = 0.35, df = 14, p = 0.7).
Table 1.
No significant differences between control (n=8) and isolation (n=8) groups were found in any measures of neuromuscular junction morphology in either the lateral or the medial thyroarytenoid, indicating there was no effect of social isolation. Data shown as mean (SD).
| Lateral Thyroarytenoid | Medial Thyroarytenoid | |||
|---|---|---|---|---|
| Control | Isolation | Control | Isolation | |
| Nerve Terminal Area (μm2) | 112 (51.7) | 83 (42.3) | 78 (27.8) | 92 (33.4) |
| Endplate Area (μm2) | 150 (55.2) | 149 (61.8) | 78 (28.3) | 90 (27.6) |
| Overlap (%) | 48 (27) | 34 (8) | 66 (21) | 62 (16) |
| Dispersion Ratio (%) | 29 (8) | 28 (5) | 28 (7) | 27 (6) |
Discussion
The purpose of this study was to determine the effects of social isolation on USV acoustics and NMJ morphology in the TA muscle of old, male rats. The results showed that 8 weeks of social isolation statistically decreased vocalization rate, decreased USV amplitude, but did not affect USV frequency. Furthermore, no difference in NMJ morphology was found between socially isolated and control groups.
Rats that were socially isolated for 8 weeks had a decrease in mean USV amplitude, which was significantly different from the increase in USV amplitude observed in the control group. In contrast, social isolation did not appear to influence USV frequency. This is consistent with results from our previous study of vocalization training in which social isolation was used to improve the response to training.9 In the current study, social isolation was the only variable between groups and, therefore, is implicated as the cause of the decrease in USV amplitude in the social isolation group.
Social isolation reduced vocal use, as confirmed by our monitoring recording sessions. However, the overall number of vocalizations in both the social isolation and communally housed groups was very low. Additionally, rats in both groups probably activated their laryngeal muscles equally during other laryngeal activities, such as swallowing and respiration.21,22 Water and food were both provided ad libitum to all animals and, therefore, it is likely the average number of swallows was similar between groups. Therefore, it is unlikely there was any meaningful difference in laryngeal muscle activity between the two groups over the 8 weeks. . This conclusion is consistent with the lack of significant differences in any measure of NMJ morphology between the social isolation and control groups in either the lateral or the medial portions of the TA.
There are several possible explanations for why USV amplitude decreased after 8 weeks of social isolation but that NMJ morphology remained intact. For example, it is very possible that a physiological mechanism other than the larynx was responsible for the decrease in amplitude, such as a decline in respiratory function. In humans, subglottal pressure positively correlates with vocalization amplitude. Additionally, in a mechanical model of an ultrasonic whistle, air pressure correlated to sound pressure level (amplitude) independent of fundamental frequency.23 However, the relationship between USV amplitude and subglottal pressure has not been directly investigated in vivo.15 Further investigation of how age-related respiratory changes affect vocalization is warranted.
The relatively low number and type of vocalizations produced by both the social isolation and control groups is consistent with previous studies of spontaneous USV production. In contrast, in elicitation paradigms, such as amphetamine administration, hundreds of USVs can be produced per hour in response to high stimulation, although very few USVs are produced during control conditions.24 The low number of spontaneous USVs found in the current study is consistent with the findings of Opiol, et al.25 who also found a low number of USVs during 24-hour isolation periods in rats that did not interact with research staff. Both in their study and in the current study, short, flat USVs were the primary type of spontaneous vocalizations observed.25 The TA muscle contracts with a lower intensity in these short USVs compared with the frequency-modulated USVs.15,26 Therefore, these short, unmodulated, infrequent spontaneous vocalizations may not play a large role in the maintenance of the TA.
Another possible explanation for the decrease in USV amplitude is that there may have been adaptations of other laryngeal muscles unexamined in this study. For example, the cricothyroid muscle is known to activate during USV frequency modulation.15 Additionally, the rat larynx contains the alar-cricoarytenoid (ACA) muscle, a muscle not found in the human larynx.27,28 The ACA is a paired muscle that attaches anteriorly to the alar cartilage, a wing-shaped cartilage (also not found in the human larynx) connected to the base of the epiglottis just superior to the anterior commissure of the vocal folds. The ACA courses posteriorly from the alar cartilage, attaching to both the arytenoid and the cricoid. Little is known about the function of this muscle, possibly because of its lack of a human homologue. The position and attachments of the ACA, however, make it a likely candidate to shape the glottal opening during USV production through adduction and depression of the epiglottis. It is also possible the ACA contributes directly to the fundamental frequency or the resonance characteristics of the USV whistle.
Lastly, USV amplitude may have decreased not because of any physiological reason, but because social isolation may have reduced motivation to engage in social encounters. Although this is the first study to investigate the effects of long-term social isolation on USV production in aged rats, there is evidence that social isolation of male pups decreases emission of 50-kHz USVs in response to a female encounter later in adulthood.29 Although the number of USVs was not a primary outcome of our study, we did find that two socially-isolated animals would not vocalize after repeated female introductions over several days. It is possible this reduced social response was also responsible for the reduced USV amplitude and there was no change in the larynx itself.
Conclusion
The clinical implication of this work is that relatively short periods of decreased vocal use (not complete disuse) may not have a negative impact on underlying laryngeal neuromuscular mechanisms in older adults and, therefore, may not exacerbate the effects of sarcopenia in the larynx. The effects of chronic social isolation and decreased voice use, however, are unknown. Furthermore, a limitation of the current study is that young animals were not included and the response of young laryngeal muscles to decreased voice remains unexplored. Although there was a statistically lower number of USVs produced in social isolation, the overall small number of USVs in both the socially-isolated and the communally-housed groups indicates this model may not meaningfully reduce laryngeal muscle activity. However, in light of previous work showing that USV training can affect NMJ morphology, social isolation may be an effective way to examine detraining effects on laryngeal neuromuscular mechanisms.
Acknowledgments
This research was supported by grant K23DC014517 from the National Institute on Deafness and other Communication Disorders of the National Institutes of Health. Imaging was completed at the microscopy core of the Institute for Genomic Biology at the University of Illinois at Urbana-Champaign. Thank you to the student research assistants who helped complete the data collection and analysis: Emily Itoku, Scott Neunaber, and Bethany Newkirk.
Footnotes
The author has no conflicts of interest to declare
LEVEL OF EVIDENCE
NA: Animal studies and basic research
References
- 1.Roy N, Stemple J, Merrill RM, Thomas L. Epidemiology of voice disorders in the elderly: preliminary findings. Laryngoscope. 2007;117(4):628–633. doi: 10.1097/MLG.0b013e3180306da1. [DOI] [PubMed] [Google Scholar]
- 2.Golub JS, Chen PH, Otto KJ, Hapner E, Johns MM., 3rd Prevalence of perceived dysphonia in a geriatric population. J Am Geriatr Soc. 2006;54(11):1736–1739. doi: 10.1111/j.1532-5415.2006.00915.x. [DOI] [PubMed] [Google Scholar]
- 3.Tomaka J, Thompson S, Palacios R. The relation of social isolation, loneliness, and social support to disease outcomes among the elderly. J Aging Health. 2006;18(3):359–384. doi: 10.1177/0898264305280993. [DOI] [PubMed] [Google Scholar]
- 4.Kendall K. Presbyphonia: a review. Curr Opin Otolaryngol Head Neck Surg. 2007;15(3):137–140. doi: 10.1097/MOO.0b013e328166794f. [DOI] [PubMed] [Google Scholar]
- 5.Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45(5):389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor NP, Suzuki T, Lee K, Sewall GK, Heisey DM. Neuromuscular junction changes in aged rat thyroarytenoid muscle. Ann Otol Rhinol Laryngol. 2002;111(7 Pt 1):579–586. doi: 10.1177/000348940211100703. [DOI] [PubMed] [Google Scholar]
- 7.McMullen CA, Andrade FH. Functional and morphological evidence of age-related denervation in rat laryngeal muscles. J Gerontol A Biol Sci Med Sci. 2009;64(4):435–442. doi: 10.1093/gerona/gln074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato T, Tauchi H. Age changes in human vocal muscle. Mech Ageing Dev. 1982;18(1):67–74. doi: 10.1016/0047-6374(82)90031-8. [DOI] [PubMed] [Google Scholar]
- 9.Johnson AM, Ciucci MR, Connor NP. Vocal training mitigates age-related changes within the vocal mechanism in old rats. J Gerontol A Biol Sci Med Sci. 2013;68(12):1458–1468. doi: 10.1093/gerona/glt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deschenes MR, Wilson MH. Age-related differences in synaptic plasticity following muscle unloading. J Neurobiol. 2003;57(3):246–256. doi: 10.1002/neu.10271. [DOI] [PubMed] [Google Scholar]
- 11.Deschenes MR, Sherman EG, Glass EK. The effects of pre-habilitative conditioning on unloading-induced adaptations in young and aged neuromuscular systems. Exp Gerontol. 2012;47(9):687–694. doi: 10.1016/j.exger.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dykstra PA. Older adult loneliness: myths and realities. Eur J Ageing. 2009;6(2):91–100. doi: 10.1007/s10433-009-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastian RW, Thomas JP. Talkativeness and Vocal Loudness: Do They Correlate With Laryngeal Pathology? A Study of the Vocal Overdoer/Underdoer Continuum. Paper presented at: 29th Annual Symposium: Care of the Professional Voice. 2000 [Google Scholar]
- 14.Brudzynski SM. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol. 2013;23(3):310–317. doi: 10.1016/j.conb.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Riede T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J Neurophysiol. 2011;106(5):2580–2592. doi: 10.1152/jn.00478.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenell C, Johnson AM. Sexual dimorphism in laryngeal muscle fibers and ultrasonic vocalizations in the adult rat. Laryngoscope. 2017;127(8):E270–E276. doi: 10.1002/lary.26561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth Curves and Survival Characteristics of the Animals Used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 18.Johnson AM, Doll EJ, Grant LM, Ringel L, Shier JN, Ciucci MR. Targeted training of ultrasonic vocalizations in aged and Parkinsonian rats. J Vis Exp. 2011;(54) doi: 10.3791/2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- 20.effsize: Efficient Effect Size Computation. R package version 0.5.4. 2015 [computer program] [Google Scholar]
- 21.McCulloch TM, Perlman AL, Palmer PM, Van Daele DJ. Laryngeal activity during swallow, phonation, and the Valsalva maneuver: an electromyographic analysis. Laryngoscope. 1996;106(11):1351–1358. doi: 10.1097/00005537-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Fregosi RF, Ludlow CL. Activation of upper airway muscles during breathing and swallowing. J Appl Physiol (1985) 2014;116(3):291–301. doi: 10.1152/japplphysiol.00670.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchins DA, Jones HW, Vermeulen PJ. The Modulated Ultrasonic Whistle as an Acoustic Source for Modeling. J Acoust Soc Am. 1983;73(1):110–115. [Google Scholar]
- 24.Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115(4):940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- 25.Opiol H, Pavlovski I, Michalik M, Mistlberger RE. Ultrasonic vocalizations in rats anticipating circadian feeding schedules. Behav Brain Res. 2015;284:42–50. doi: 10.1016/j.bbr.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Riede T. Rat ultrasonic vocalization shows features of a modular behavior. J Neurosci. 2014;34(20):6874–6878. doi: 10.1523/JNEUROSCI.0262-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inagi K, Schultz E, Ford CN. An anatomic study of the rat larynx: establishing the rat model for neuromuscular function. Otolaryngol Head Neck Surg. 1998;118(1):74–81. doi: 10.1016/S0194-5998(98)70378-X. [DOI] [PubMed] [Google Scholar]
- 28.Alli O, Berzofsky C, Sharma S, Pitman MJ. Development of the rat larynx: a histological study. Laryngoscope. 2013;123(12):3093–3098. doi: 10.1002/lary.24145. [DOI] [PubMed] [Google Scholar]
- 29.Inagaki H, Mori Y. The critical point at which post-weaning individual housing conditions affect the emission of 22-kHz calls in male rats. J Vet Med Sci. 2013;75(4):527–529. doi: 10.1292/jvms.12-0388. [DOI] [PubMed] [Google Scholar]


