Abstract
Objectives
To evaluate the prognostic value of the ratio between tricuspid annular plane systolic excursion (TAPSE)-pulmonary artery systolic pressure (PASP) as a determinant of right ventricular to pulmonary artery (RVPA) coupling in patients undergoing transcatheter aortic valve replacement (TAVI).
Background
RV function and pulmonary hypertension (PH) are both prognostically important in patients receiving TAVI. RV-PA coupling has been shown to be prognostic important in patients with heart failure but not previously evaluated in TAVI patients.
Methods
Consecutive patients with severe aortic stenosis who received TAVI from July 2011 through January 2016 and with comprehensive baseline echocardiogram were included. All individual echocardiographic images and Doppler data were independently reviewed and blinded to the clinical information and outcomes. Cox models quantified the effect of TAPSE/PASP quartiles on subsequent all-cause mortality while adjusting for confounders.
Results
A total of 457 patients were included with mean age of 82.8±7.2 years, left ventricular ejection fraction (LVEF) 54%±13%, PASP 44±17 mm Hg. TAPSE/PASP quartiles showed a dose-response relationship with survival. This remained significant (HR for lowest quartile vs highest quartile=2.21, 95% CI 1.07 to 4.57, p=0.03) after adjusting for age, atrial fibrillation, LVEF, stroke volume index, Society of Thoracic Surgeons Predicted Risk of Mortality.
Conclusion
Baseline TAPSE/PASP ratio is associated with all-cause mortality in TAVI patients as it evaluates RV systolic performance at a given degree of afterload. Incorporation of right-side unit into the risk stratification may improve optimal selection of patients for TAVI.
INTRODUCTION
Over the past decade, transcatheter aortic valve replacement (TAVI) has been established as an effective alternative to traditional surgical aortic valve replacement in patients with symptomatic aortic stenosis with high and intermediate surgical risk. Despite the exponential growth of this developing technology, mid-term mortality remains high with studies demonstrating >30% mortality at 2 years in high-risk cohorts.1 Preoperative risk assessment for patients undergoing TAVI becomes increasingly valuable as this approach has now being expanded into the low-risk patient population.2 In this regard, right-sided ventricular dysfunction and pulmonary hypertension (PH) have both been shown to have negative prognostic value in patients undergoing TAVI.3,4 However, most of the studies have evaluated those two components in isolation.
A better approach would be to evaluate the right ventricular to pulmonary artery (RV-PA) coupling, which integrates the RV systolic performance at a given degree of afterload. Until recently, this would require invasive haemodynamics evaluation. However, validation of a non-invasive ratio between tricuspid annular plane systolic excursion (TAPSE)-pulmonary artery systolic pressure (PASP) has been shown to accurately provide similar results, which are prognostically significant in heart failure populations.5,6
We therefore sought to evaluate the prognostic value of baseline TAPSE/PASP ratio in patients undergoing TAVI and to test whether RV-PA coupling would have a superior prognostic value than either parameter alone.
METHODS
Study design
We conducted a retrospective cohort analysis of patients who underwent TAVI at the University of Pittsburgh Medical Center, a large tertiary care centre, between July 2011 and January 2016. Since our purpose was to evaluate the prognostic value of TAPSE/PASP ratio in patients with native severe aortic stenosis (AS) undergoing TAVI, we excluded patients a) whose baseline echocardiogram was incomplete or unavailable for review, b) who had undergone valve-in-valve procedures and c) who had uninterpretable tricuspid regurgitation (TR) jet signal to estimate PASP.
Patients underwent comprehensive clinical evaluation by the designated Heart Team and deemed appropriate to undergo TAVI as suggested by the guidelines.2,7 Clinical, laboratorial and procedural data were collected. Patients were clinically followed up in our programme at 1, 6, 12 months post-TAVI, and annually thereafter. Primary outcome was all-cause mortality, which was obtained through querying the social security death index (using the updated Social Security Administration Death Master file, for which our healthcare system is exempt from the 3-year delay period by the Social Security Administration). The study was approved by the University of Pittsburgh Institutional Review Board Committee with a waiver of individual consent.
Study procedures (echocardiogram and TAVI procedure)
All included patients undergoing TAVI evaluation underwent comprehensive transthoracic echocardiogram (TTE) using commercial systems (Philips Medical Systems, Bothell, Washington, USA, Siemens Medical Solution, Malvern, Pennsylvania, USA or General Electric, Milwaukee, Wisconsin, USA). All baseline TTE studies and images were systematically and independently reviewed as per guidelines8–10 by a single level III trained cardiologist with 6 years of experience (JLC), who was blinded to the clinical information and outcomes. TAPSE was measured in the apical four-chamber view as the longitudinal systolic excursion of the tricuspid annulus. Three consecutive heart cycles were recorded and averaged for patients in sinus rhythm, whereas five cardiac cycles were averaged for those in atrial fibrillation. PASP was calculated using the maximal tricuspid regurgitant jet velocity obtained from continuous wave Doppler and integrated into the modified Bernoulli equation plus the estimated right atrial pressure (from the inferior vena cava size and variability with respiration). Right atrial pressure was estimated from the inferior vena cava size (normal ≤2.1 cm) and variability with respiration (>50% diameter change with inspiration), according to the guidelines.8
Lastly, all patients underwent TAVI procedure receiving either balloon-expandable or self-expanding bioprosthesis. Study procedural details are available in online supplemental document.
Statistical analysis
Patients were divided into four groups by quartile of TAPSE/PASP ratio. Continuous variables with symmetric distributions are presented as mean±SD for normally distributed variables and tested using analysis of variance; non-normally distributed variables are presented as median (IQR) and tested with non-parametric Kruskal-Wallis tests. Categorical variables are presented as n (%) and tested using χ2 tests. Kaplan-Meier analyses and Cox proportional-hazards regression models were used to assess the relationship between TAPSE/PASP ratio and mortality after TAVI. We report the results for TAPSE/PASP ratio in an unadjusted model as well as a model adjusting for Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM), a comprehensive risk prediction score that incorporates over 40 preoperative variables (see online supplemental document), to determine whether TAPSE/PASP ratio remains independently associated with mortality. We do not adjust for variables that are included in the STS-PROM score (such as age, left ventricular ejection fraction (LVEF), mitral regurgitation, tricuspid regurgitation, etc) as the inclusion of STS-PROM takes those factors into account and adding them would result in multicollinearity. We also tested the effects of all other echocardiographic parameters, which are not included in the STS-PROM score; none of them was significantly associated with mortality in a model that already included STS-PROM and TAPSE/PASP, nor was any of them significant confounders of the TAPSE/PASP effect on mortality (ie, none changed the TAPSE/PASP effect size by >10% when included in the model). Continuous net reclassification improvement (NRI) was calculated11 to compare the relative quality of the prediction models for TAPSE/PASP ratio versus either TAPSE or PASP alone over a ‘null’ model with STS-PROM alone. The proportional-hazards assumption was tested by looking at time-dependent covariates (time×covariate interaction variables) to determine whether the main effects varied over time. Statistical analyses were performed using SAS V.9.4 (SAS Institute, Cary, North Carolina, USA).
RESULTS
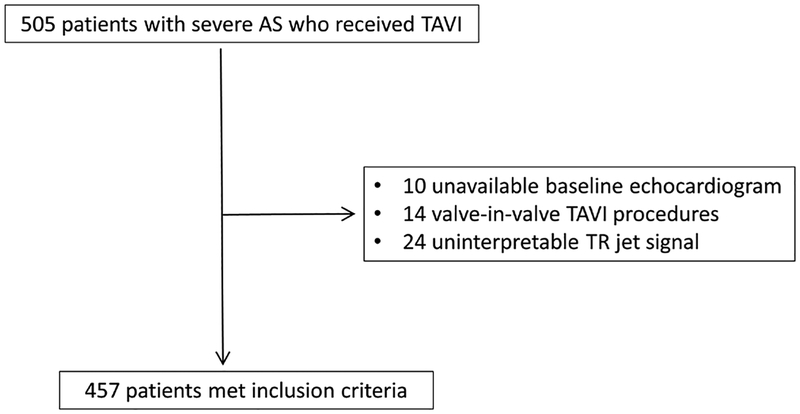
Of the identified 505 patients with severe symptomatic AS who underwent TAVI during the study period, 457 patients met the inclusion criteria. Figure 1 displays the study workflow. Baseline demographic characteristics (table 1) included a median age of 84 years (52–97 years), female sex in 222 patients (49%), New York Heart Association functional class III/IV in 444 (97.2%), and Society of Thoracic Surgeons predicted risk of mortality of 7.8% (1.0%–38.0%). This cohort was then divided into quartiles based on TAPSE/PASP ratios. The higher TAPSE/PASP ratio would imply adequate RV-PA coupling with preserved RV function and/or low PASP.
Figure 1.
Study population and workflow. TAVI, transcatheter aortic valve implantation; TR, tricuspid regurgitation.
Table 1.
Baseline characteristics of patients by TAPSE/PASP quartiles
| Total (n=457) | TAPSE/PASP <0.029 cm/mm Hg (n=112) | TAPSE/PASP 0.029–0.043 cm/mm Hg (n=114) | TAPSE/PASP 0.043–0.059 cm/mm Hg (n=113) | TAPSE/PASP >0.059 cm/mm Hg (n=118) | P values | |
|---|---|---|---|---|---|---|
| Age (years) | 84.0 (52.0–97.0) | 84.0 (52.0–95.0) | 84.5 (62.0–95.0) | 85.0 (59.0–96.0) | 84.0 (56.0–97.0) | 0.945 |
| Male gender | 235 (51.4%) | 67 (59.8%) | 53 (46.5%) | 52 (46.0%) | 63 (53.4%) | 0.123 |
| BMI (kg/m2) | 26.0 (14.2–56.4) | 26.5 (15.0–50.3) | 25.5 (17.4–56.4) | 25.9 (14.2–40.7) | 26.2 (14.3–52.5) | 0.444 |
| Current smoker | 36 (7.9%) | 5 (4.5%) | 10 (8.8%) | 9 (8.0%) | 12 (10.2%) | 0.427 |
| Diabetes | 177 (38.7%) | 44 (39.3%) | 48 (42.1%) | 43 (38.1%) | 42 (35.6%) | 0.784 |
| Dyslipidemia | 351 (76.8%) | 84 (75.0%) | 90 (78.9%) | 87 (77.0%) | 90 (76.3%) | 0.915 |
| Hypertension | 408 (89.3%) | 103 (92.0%) | 103 (90.4%) | 98 (86.7%) | 104 (88.1%) | 0.591 |
| Previous CABG | 141 (30.9%) | 46 (41.1%) | 42 (36.8%) | 34 (30.1%) | 19 (16.1%) | 0.0002 |
| Previous valve surgery | 26 (5.7%) | 6 (5.4%) | 11 (9.6%) | 6 (5.3%) | 3 (2.5%) | 0.135 |
| Previous MI | 171 (37.4%) | 43 (38.4%) | 48 (42.1%) | 43 (38.1%) | 37 (31.4%) | 0.394 |
| Prior CHF | 349 (76.4%) | 85 (75.9%) | 88 (77.2%) | 87 (77.0%) | 89 (75.4%) | 0.987 |
| STS-PROM (%) | 7.8 (1.0–38.0) | 8.9 (1.1–24.7) | 9.3 (1.8–28.4) | 7.5 (2.0–38.0) | 6.1 (1.0–22.2) | <0.0001 |
| Chronic lung disease | 182 (39.8%) | 40 (35.7%) | 57 (50.0%) | 44 (38.9%) | 41 (34.7%) | 0.072 |
| Atrial fibrillation/flutter | 206 (45.1%) | 81 (72.3%) | 59 (51.8%) | 30 (26.5%) | 36 (30.5%) | <0.0001 |
BMI, body mass index; CABG, coronary artery bypass graft; MI, myocardial infarction; CHF, congestive heart failure; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TAPSE/PASP, tricuspid annular plane systolic excursion/pulmonary artery systolic pressure.
Among the baseline demographic characteristics, patients in the higher TAPSE/PASP quartile had lower STS-PROM score and less commonly undergone coronary artery bypass graft (CABG). The remaining demographics were not different between groups.
Baseline echocardiographic characteristics are shown in table 2. When comparing TAPSE/PASP quartiles, there were statistically significant differences across the groups in such a way that those with lower TAPSE/PASP (worse RV-PA coupling) had more left-sided chamber remodelling, lower LVEF, stroke volume index and more commonly ≥ moderate MR and TR.
Table 2.
Baseline echocardiographic characteristics of patients by TAPSE/PASP quartiles
| Total (n=457) | TAPSE/PASP <0.029 cm/mm Hg (n=112) | TAPSE/PASP 0.029–0.043 cm/mm Hg (n=114) | TAPSE/PASP 0.043–0.059 cm/mm Hg (n=113) | TAPSE/PASP >0.059 cm/mm Hg (n=118) | P values | |
|---|---|---|---|---|---|---|
| LVEDD (cm) | 4.57±0.82 | 4.67±0.84 | 4.67±0.88 | 4.45±0.78 | 4.47±0.76 | 0.056 |
| LVESD (cm) | 3.30±0.99 | 3.54±1.04 | 3.42±1.06 | 3.09±0.95 | 3.15±0.85 | 0.001 |
| LAVI (mL/m2) | 49.2±17.4 | 55.9±20.4 | 52.6±18.0 | 46.9±14.8 | 42.1±12.6 | <0.0001 |
| LVMI (g/m2) | 158±43.7 | 157±37.3 | 165±53.1 | 156±40.5 | 155±42.0 | 0.328 |
| LVEF (%) | 53.9±13.4 | 48.6±15.8 | 52.7±12.8 | 57.6±11.3 | 56.4±11.7 | <0.0001 |
| SVI (mL/m2) | 35.8±11.0 | 30.3±9.31 | 35.0±13.3 | 38.3±9.28 | 39.4±9.38 | <0.0001 |
| AV mean gradient (mm Hg) | 48.0±15.4 | 44.6±15.6 | 47.4±16.7 | 50.2±14.5 | 49.6±14.4 | 0.025 |
| AVA (cm2) | 0.63±0.18 | 0.58±0.17 | 0.60±0.17 | 0.65±0.20 | 0.69±0.17 | <0.0001 |
| AVAi (cm2/m2) | 0.35±0.10 | 0.31±0.09 | 0.33±0.10 | 0.36±0.09 | 0.37±0.10 | <0.0001 |
| Lateral E/e′ | 22.7±11.4 | 22.4±11.6 | 24.7±12.6 | 21.4±10.1 | 22.4±11.4 | 0.526 |
| Septal E/e′ | 29.7±13.4 | 32.9±14.7 | 30.0±13.2 | 27.1±12.0 | 27.6±12.8 | 0.146 |
| Qualitative RV systolic function | <0.0001 | |||||
| Normal | 403 (88.2%) | 71 (63.4%) | 104 (91.2%) | 112 (99.1%) | 116 (98.3%) | |
| Mild decrease | 26 (5.7%) | 20 (17.9%) | 4 (3.5%) | 1 (0.9%) | 1 (0.8%) | |
| Moderate decrease | 20 (4.4%) | 18 (16.1%) | 2 (1.8%) | 0 (0.0%) | 0 (0.0%) | |
| Severe decrease | 1 (0.2%) | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| TAPSE (cm) | 1.89±1.11 | 1.27±0.34 | 1.74±0.37 | 1.96±0.33 | 2.54±1.91 | <0.0001 |
| TAPSE<1.6 cm (%) | 139 (30.4%) | 90 (80.4%) | 36 (31.6%) | 11 (9.7%) | 2 (1.7%) | <0.0001 |
| PASP (mm Hg) | 44.1±16.8 | 61.1±16.9 | 48.7±11.6 | 38.7±7.87 | 28.9±8.92 | <0.0001 |
| ≥Moderate MR | 55 (12.0%) | 20 (17.9%) | 23 (20.2%) | 5 (4.4%) | 7 (5.9%) | 0.0001 |
| ≥Moderate TR | 70 (15.3%) | 43 (38.4%) | 22 (19.3%) | 5 (4.4%) | 0 (0.0%) | <0.0001 |
AVA, aortic valve area; AVAi, aortic valve area index; E/e’, ratio of early diastolic filling/tissue Doppler velocity annulus; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MR, mitral regurgitation; SVI, stroke volume index; TAPSE, tricuspid annular plane systolic excursion; PASP, pulmonary artery systolic pressure; TR, tricuspid regurgitation.
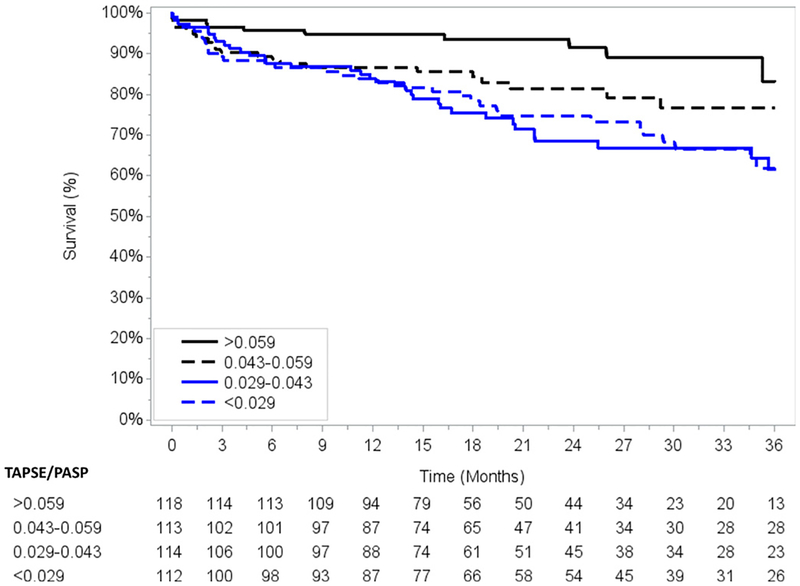
All patients received successful TAVI with either balloon-expandable or self-expanding bioprosthesis. Arterial access was via a transfemoral approach in 337 patients (73.6%) and via left subclavian in 94 (20.6%) (see online supplemental table 1). Over a mean follow-up of 23±14 months, there were 103 deaths. TAPSE/PASP either as a continuous variable (HR 0.88 per 0.01 increase, 95% CI 0.80 to 0.97, p=0.010) or as quartiles showed a significant dose-response relationship with survival (figure 2). This remained significant after adjusting for parameters significantly associated with mortality (p<0.05) in the univariate analysis (see online supplemental table 2 and 3). Furthermore, TAPSE/PASP ratio remained associated with mortality after adjustment for many other prognostically relevant parameters in the TAVI literature such as age, atrial fibrillation, LVEF, stroke volume index and comprehensive STS-PROM score (table 3). Additional sensitivity analysis model (total n=347), removing patients with baseline ≥moderate MR and/or ≥moderate TR, showed that the lowest quartile of TAPSE/PASP ratio (<0.029 cm/mm Hg) was still associated with mortality, despite adjustment for comprehensive STS-PROM (HR 2.34, 95% CI 1.09 to 5.00, p=0.028) (see online supplemental table 4). There was an association between TAPSE/PASP and mortality for both males and females (see online supplemental table 5A,B, figure S1 and S2). However, choosing gender-specific thresholds of TAPSE/PASP ratio will require a follow-up study with larger cohort and number of events for further validation. Sensitivity analysis, including only patients without prior CABG (n=316), showed that TAPSE/PASP ratio was strongly associated with all-cause mortality even after multivariate adjustment of other prognostically important covariates. (see online supplemental table 6, figure S3). Univariate Cox proportional hazard analysis for all clinical and echocardiographic parameters is presented in online supplemental table 7.
Figure 2.
Survival curves demonstrating TAPSE/PASP ratio and all-cause mortality after TAVI. PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion; TAVI, transcatheter aortic valve implantation.
Table 3.
Association between baseline TAPSE/PASP ratio and all-cause mortality
| Unadjusted | Adjusted for age, atrial fibrillation, LVEF, SVI and STS-PROM | |||
|---|---|---|---|---|
| TAPSE/PASP (vs ≥0.059 cm/mm Hg) | HR (95% CI) | P values | HR (95% CI) | P values |
| <0.029 cm/mm Hg | 2.90 (1.47 to 5.75) | 0.002 | 2.21 (1.07 to 4.57) | 0.033 |
| 0.029–0.043 cm/mm Hg | 3.13 (1.59 to 6.17) | 0.001 | 2.52 (1.25 to 5.10) | 0.010 |
| 0.043–0.059 cm/mm Hg | 2.16 (1.06 to 4.42) | 0.034 | 2.16 (1.04 to 4.47) | 0.038 |
LVEF, left ventricular ejection fraction; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; SVI, stroke volume index; TAPSE/PASP, tricuspid annular plane systolic excursion/pulmonary artery systolic pressure.
Comparison of NRI showed that TAPSE/PASP ratio (NRI vs null model with STS-PROM: 0.49) had a better risk stratification of all-cause mortality than either measurement by itself. (PASP NRI vs null model with STS-PROM=0.21; TAPSE NRI vs null model with STS-PROM=0.20). Lastly, a subset of 27 patients were randomly chosen to study reproducibility of the performed measurements. The intraclass correlation coefficient (ICC) for intraobserver reliability for TAPSE, PASP and TAPSE/PASP ratio were 0.98, 0.99 and 0.98, respectively. The ICC for interobserver reliability for TAPSE, PASP and TAPSE/PASP ratio were 0.91, 0.90 and 0.92, respectively. Together these findings suggest good reproducibility of those measurements.
DISCUSSION
In the current study, we evaluated the prognostic value of TAPSE/PASP ratio, as a simple, non-invasive metric assessment of RV-PA coupling in patients who received TAVI. Our findings support that RV-PA coupling is strongly associated with all-cause mortality and its evaluation may provide superior preoperative risk assessment when compared with traditional risk stratification using STS-PROM and/or either measure alone.
Baseline pulmonary hypertension is common in patients undergoing TAVI and associated with adverse outcomes.12 Equally important is the evaluation and quantification of RV systolic function, although results have been controversial with some studies showing association with adverse outcomes,4,13–15 whereas others have not.16,17 Since the RV systolic performance is tightly correlated with the afterload,18 perhaps more important than the quantification of each parameter in isolation, might be the combined evaluation of the RV-PA coupling which integrates the performance of the right-side unit3.
Until recently, such evaluation would typically require invasive haemodynamics with the analysis of pressure-volume loops. Simon and colleagues have shown that RV-PA coupling can stratify prognosis in patients with pulmonary arterial hyper-tension19 using a simple ratio of RV stroke volume/RV end-systolic volume using either gated cardiac computed tomography angiography or cardiac magnetic resonance. Guazzi et al have validated a simple non-invasive approach using the TAPSE/PASP ratio derived from echocardiography in a large cohort of patients with heart failure and preserved ejection fraction. There was a good correlation with invasive haemodynamics (r=0.69, p0.0001), but more importantly the ratio was shown to be associated with functional capacity and outcomes in these patients.5
Building on their prior work, we have shown that baseline evaluation of baseline RV-PA coupling using TAPSE/PASP ratio is feasible using echocardiography, provided dedicated imaging of the RV is systematically obtained. Importantly, this ratio is capable to risk stratify patients who received TAVI in a dose-response manner in such way that those with worse uncoupling were found to have the highest mortality. TAPSE/PASP ratio may be equally relevant and applicable to patients undergoing transcatheter mitral valve procedures or open valve surgery but this will need further validation studies.
It is important to highlight that those with worse RV-PA uncoupling also had higher burden of comorbidities such as higher STS-PROM, concomitant ≥moderate valvular regurgitation (mitral, tricuspid), lower LVEF and LV stroke volume index. Despite comprehensive adjustment of those comorbidities, which have been shown to also increase mortality in TAVI, RV-PA coupling remained independently associated with the outcomes.
RV myocardial contractility is intimately correlated with the afterload, and the ability of the RV to increase its contractility in response to increased afterload might be an important determinant of the observed outcomes. Thus, we believe that TAPSE/PASP evaluation prior to TAVI provides a different and more integrative approach in the understanding of the health of the right-side unit3 and therefore should be incorporated into the shared decision-making process.
LIMITATIONS
Our study has several limitations. First, although this a large and contemporary cohort of TAVI patients, it represents an observational and retrospective study from a single high-volume tertiary care centre, whose generalisability could be limited. Second, RV systolic function is much more complex than what gets measured by TAPSE. Given the unique geometry and contractility pattern of the RV, three-dimensional echocardiographic would be required, but unfortunately not available for those patients. Nonetheless, societal guidelines recommend use of TAPSE for the quantification of RV function given its association with outcomes,9 while understanding its limitations in patients post-sternotomy.16 Third, accuracy of Doppler estimation of PASP in this group of patients, in comparison to gold-standard right-heart catheterisation, is not optimal as previously demonstrated by our group.12 Nonetheless, TTE still used as the screening method for evaluation of pulmonary hyper-tension. Fourth, the clinical implications of RV-PA coupling into other important post-TAVI metrics such as quality of life, functional capacity and hospital readmissions will need to be equally evaluated in future studies. Fifth, our group has recently demonstrated that baseline pulmonary hypertension severity and its short-term changes after TAVI are equally associated with all-cause mortality.20 So whether changes in TAPSE/PASP ratio after TAVI would be equally associated with all-cause mortality, and whether evaluation of TAPSE/PASP reserve by dobutamine or exercise may improve the clinical prediction of outcomes, will require future studies. Lastly, while it is possible that cause of death may be related to factors other than pulmonary hypertension and RV dysfunction, we believe cardiac causes are likely in our cohort based on the limited documentation available describing circumstances of death. Adjudication for the cause of death is challenging, controversial and biased on available reporting and documentation, whereas all‐cause mortality is objective and inherently relevant.21 Furthermore, there appears to be a relationship between our proposed TAPSE/PASP ratio and all-cause mortality in this cohort despite adjusting for other known risk factors for this population such as age, atrial fibrillation, LVEF, stroke volume index and STS-PROM.
CONCLUSIONS
Evaluation of the right-side unit using the integrated, non-invasive TAPSE/PASP ratio allows for quantification of the RV-PA coupling, which is strongly associated with post-TAVI survival. Based on our data, we believe that quantitative assessment of RV-PA coupling should be included in the risk stratification of patients being considered for TAVI as it may identify those who are at increased risk, with superior risk classification than traditional STS-PROM or either parameter alone. Our findings would require confirmation and validation by other groups. In addition, whether changes of RV-PA coupling after TAVI would translate into improved outcomes will need to be tested prospectively.
Supplementary Material
Key questions.
What is already known on this subject?
► Right ventricular (RV) function and pulmonary hypertension (PH) are both prognostically important in patients receiving transcatheter aortic valve replacement (TAVI).
What might this study add?
► Non-invasive RV to pulmonary artery coupling is strongly associated with all-cause mortality in patients who received TAVI, and provides better risk stratification than either RV function or PH severity alone.
How might this impact on clinical practice?
► Incorporation of right-side unit into the risk stratification provides a strategy that may further improve selection of patients for TAVI.
Acknowledgments
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer JLC and TGG received investigator-initiated grant support from Medtronic Inc.
Footnotes
Competing interests None declared.
Patient consent Not required.
Ethics approval University of Pittsburgh Institutional Review Board Committee.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686–95. [DOI] [PubMed] [Google Scholar]
- 2.Otto CM, Kumbhani DJ, Alexander KP, et al. ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults With Aortic Stenosis: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. Journal of the American College of Cardiology 2017;2017:1313–46. [DOI] [PubMed] [Google Scholar]
- 3.Cavalcante JL, Simon MA, Chan SY. Comprehensive Right-Sided Assessment for Transcatheter Aortic Valve Replacement Risk Stratification: Time for a Change. J Am Soc Echocardiogr 2017;30:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz LA, Rozenbaum Z, Ghantous E, et al. Impact of Right Ventricular Dysfunction and Tricuspid Regurgitation on Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement. J Am Soc Echocardiogr 2017;30:36–46. [DOI] [PubMed] [Google Scholar]
- 5.Guazzi M, Dixon D, Labate V, et al. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure With Preserved Ejection Fraction: Stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc Imaging 2017;10:1211–21. [DOI] [PubMed] [Google Scholar]
- 6.Hussain I, Mohammed SF, Forfia PR, et al. Impaired Right Ventricular-Pulmonary Arterial Coupling and Effect of Sildenafil in Heart Failure With Preserved Ejection Fraction: An Ancillary Analysis From the Phosphodiesterase-5 Inhibition to Improve Clinical Status And Exercise Capacity in Diastolic Heart Failure (RELAX) Trial. Circ Heart Fail 2016;9:e002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2014;2014:2438–88. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. [DOI] [PubMed] [Google Scholar]
- 9.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner H, Hung J, Bermejo J, et al. American Society of E and European Association of E. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1–23. quiz 101–2. [DOI] [PubMed] [Google Scholar]
- 11.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelkarim I, Althouse AD, Thoma FW, et al. The Importance of Invasive Hemodynamics for Pulmonary Hypertension Screening in TAVR Patients. J Am Coll Cardiol 2017;70:510–1. [DOI] [PubMed] [Google Scholar]
- 13.Généreux P, Pibarot P, Redfors B, et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J 2017;38:3351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalcante JL, Rijal S, Althouse AD, et al. Right Ventricular Function and Prognosis in Patients with Low-Flow, Low-Gradient Severe Aortic Stenosis. J Am Soc Echocardiogr 2016;29:325–33. [DOI] [PubMed] [Google Scholar]
- 15.Griese DP, Kerber S, Barth S, et al. Impact of right and left ventricular systolic dysfunction on perioperative outcome and long-term survival after transcatheter aortic valve replacement. J Interv Cardiol 2017;30:217–25. [DOI] [PubMed] [Google Scholar]
- 16.Tamborini G, Muratori M, Brusoni D, et al. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr 2009;10:630–4. [DOI] [PubMed] [Google Scholar]
- 17.Lindman BR, Maniar HS, Jaber WA, et al. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: insights from the Placement of Aortic Transcatheter Valves II inoperable cohort. Circ Cardiovasc Interv 2015;8:e002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013;62:D22–33. [DOI] [PubMed] [Google Scholar]
- 19.Vanderpool RR, Pinsky MR, Naeije R, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 2015;101:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masri A, Abdelkarim I, Sharbaugh MS, et al. Outcomes of persistent pulmonary hypertension following transcatheter aortic valve replacement. Heart 2018;104:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauer MS, Blackstone EH, Young JB, et al. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol 1999;34:618–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.