Abstract
Purpose
Osteogenesis imperfecta (OI) predisposes to recurrent fractures, bone deformities, and short stature. There is a lack of large-scale systematic studies that have investigated growth parameters in OI.
Methods
Using data from the Linked Clinical Research Centers, we compared height, growth velocity, weight, and body mass index (BMI) in 552 individuals with OI. Height, weight, and BMI were plotted on the Centers for Disease Control normative curves.
Results
In children, the median Z-scores for height in OI types I, III, and IV were −0.66, −6.91, and −2.79, respectively. Growth velocity was diminished in OI types III and IV. The median Z-score for weight in children with OI type III was −4.55. The median Z-scores for BMI in children with OI types I, III and IV were 0.10, 0.91 and 0.67, respectively. Generalized linear model analyses demonstrated height Z-score positively correlated with severity of OI subtype (p<0.001), age, bisphosphonate use, and rodding (p<0.05).
Conclusions
From the largest cohort of individuals with OI, we provide median values for height, weight, and BMI Z-scores which can aid in evaluation of overall growth in the clinic setting. This study is an important first step in the generation of OI-specific growth curves.
Keywords: Osteogenesis Imperfecta, natural history study, growth, height, weight
INTRODUCTION
Osteogenesis imperfecta (OI), a group of Mendelian disorders of connective tissue, predisposes to recurrent fractures, bone deformities, and short stature1. Nearly 90% of individuals with OI have pathogenic variants in COL1A1 and COL1A2 that encode for the α1 and α2 chains of type I collagen, a major protein of the bone matrix2. Over the past decade, discovery of numerous genes as causes for OI has underscored the genetic heterogeneity of this disorder. However, the Sillence classification, which was proposed well before the genetic basis of OI was known, continues to be used for management and counseling purposes3. Accordingly, the autosomal dominant, type I collagen related-OI is classified into non-deforming (type I), perinatally lethal (type II), progressively deforming (type III), and common variable (type IV) forms.
Short stature is a hallmark of the moderate-to-severe forms of OI. Previous studies have demonstrated that the mean birth length and weight in individuals with OI are below the normative data from the general population4,5. Whereas final height is significantly restricted in those with OI types III and IV, even those with milder forms of disease, i.e., type I, have reductions in the overall height as compared to the general population5–8. To date, only few studies have systematically analyzed the growth parameters in OI. Most reports are limited either by the size of the cohort or the lack of data on adults with OI5,6,8–13. In fact, there are only two studies outlining the growth parameters in both children and adults with OI7,8. The largest cohort with both children and adults with OI described to date (144 children and 199 adults) revealed that in addition to decreased height, obesity was common in OI7. Detailed assessments of anthropometric measures in OI can have both diagnostic and management implications. Appropriate characterization of the growth parameters can help identify affected individuals who are not meeting the expected growth patterns and institute lifestyle modifications for weight control.
In this report, we analyzed the cross-sectional growth parameters in a large cohort of individuals with OI from across North America (n=552; 408 children and 144 adults) who were enrolled in the observational study “Longitudinal Study of Osteogenesis Imperfecta” that was conducted by the OI Linked Clinical Research Centers (LCRC)14. The large sample size of the cohort allowed us to examine growth parameters in the various subtypes of OI and assess the potential correlations between height and the type of OI, surgical rodding, and bisphosphonate use.
MATERIALS AND METHODS
Study Population
The details about LCRC and the Longitudinal Study of Osteogenesis Imperfecta have been previously described14. The LCRC, comprised five clinical: Baylor College of Medicine (Houston, TX), Kennedy Krieger Institute (Baltimore, MD) with Nemours/Alfred I. DuPont Hospital for Children (Wilmington, DE), Oregon Health and Science University (Portland, OR), Shriners Hospital for Children (Chicago, IL), and Shriners Hospital for Children (Montreal, QC). The data collected from these clinical sites were coordinated and managed by the NIH Rare Disease Clinical Research Network’s (RDCRN) Data Management and Coordinating Center at the University of South Florida College of Medicine. The Collagen Diagnostic Laboratory at the University of Washington served as the center for molecular and biochemical analyses. The respective Institutional Review Boards of participating clinical sites approved the study. Informed consent was obtained from all subjects or their legal guardians.
At the clinical sites, demographic data, medical history, anthropometric measures of height, weight, and arm-span were collected and recorded in a uniform fashion by trained personnel. Height, weight, and arm-span were recorded as single measurements. The data were collected at every clinical site in accordance to the instructions outlined in the Manual of Operations and were reported using online case report forms.
Height, defined as the vertical distance between crown of head and soles of feet, was measured using a wall-mounted stadiometer and recorded to the nearest 0.1 cm. When participants could not stand, supine length was measured from the heels to the top of the head.
Weight was measured on an upright calibrated digital or beam scale to the nearest 0.1 kg. In individuals who were too young to stand alone, infant scale was used. When children could not stand on the scales, they were weighed while being held by a parent and weight of the child was calculated by subtracting the weight of the parent from the total weight. Body mass index (BMI) was calculated as weight in kg/ (height in m)2.
Arm span was measured as the distance from one furthermost fingertip to the other furthermost fingertip when the participant’s arms were stretched out horizontally using a non-stretching long measuring tape to the nearest 0.1 cm. Arm span was measured in a single measure and not in parts which were then added together.
Overall, 552 participants were enrolled; this included 244 with OI type I, 110 with OI type III, 150 with OI type IV, 15 with OI type V, 12 with OI type VI, 5 with OI type VII, and 16 with an unclassified type (Table 1). The classification of OI was based on clinical features outlined in the Manual of Operations; however, genotypic information was used to reclassify patients when available. Data collected for analyses included age at enrollment, sex, OI type, family history of OI, history of bisphosphonate use (yes or no), history of rodding (yes or no), self-reported parental height, subject height, weight, and arm span. To analyze height within particular genotypes, we classified the type I collagen mutations as glycine substitution mutations within the triple helix domain (n=160), loss of function (nonsense, deletion, frameshift or splicing; n=144) mutations, and non-glycine missense variations (n=9). Given the small number of individuals with OI types V, VI and, VII, they were excluded from further formal statistical evaluations. The height, weight, and BMI data presented here were collected at the initial enrollment visit.
Table 1.
Characteristics of individuals with OI enrolled in the LCRC
| OI I | OI III | OI IV | OI V | OI VI | OI VII | Unclassified | All | |
|---|---|---|---|---|---|---|---|---|
| Enrollment number | 244 | 110 | 150 | 15 | 12 | 5 | 16 | 552 |
| Male, n (%) | 113 (46.3) | 47 (42.7) | 66 (44) | 5 (33.3) | 7 (58.3) | 0 (0) | 7 (43.8) | 245 |
| Female, n (%) | 131 (53.7) | 63 (57.3) | 84 (56) | 10 (66.7) | 5 (41.7) | 5 (100) | 9 (56.3) | 307 |
| Median age in years at enrollment (range) | 13.5 (0–67) | 11.0 (0–54) | 11.6 (0–63) | 12.5 (0–35) | 9.9 (2–32) | 4.2 (0–20) | 23.6 (2–47) | 12.2 |
| Race, n (%) | ||||||||
| White | 227 (93) | 87 (79.1) | 127 (84.7) | 11 (73.3) | 8 (66.7) | 3 (60) | 12 (75) | 475 |
| Black | 7 (2.9) | 10 (9.1) | 14 (9.3) | 2 (13.3) | 0 (0) | 0 (0) | 2 (12.5) | 35 |
| Others | 10 (4.1) | 13 (11.8) | 9 (6) | 2 (13.3) | 4 (33.3) | 2 (40) | 2 (12.5) | 42 |
| Family history, n (%) | 153 (62.7) | 9 (8.2) | 44 (29.3) | 5 (33.3) | 1 (8.3) | 3 (60) | 7 (43.8) | 222 |
| Molecular testing done, n(%) | 154 (63) | 67 (61) | 109 (73) | 8 (53) | 4 (33) | 5 (100) | 347 | |
Statistical analysis
For participants below 20 years (OI type I, n=163; OI type III, n=83; OI type IV, n=123), the Centers for Disease Controls (CDC) growth curves were utilized to plot the height, weight, and BMI. The Z-scores were calculated using the L, M, S parameters based on the methodology described previously15. The Z-scores were computed using the AGD library in R. For comparison of Z-scores for height, weight, and BMI, between the OI types, Komogrov-Smirnov test was performed to evaluate for normal distribution; ANOVA or ANOVA on ranks were used as appropriate. When comparing Z-scores for height, weight, and BMI between OI types, we categorized individuals into age groups of 0–5 years, 5–10 years, 10–15 years, 15–20 years, and above 20 years. Z-scores for height and weight for individuals above 20 years of age were calculated using normative values from 20 year-old individuals. The differences in proportions of individuals below the 3rd percentile for height and weight between the OI types were analyzed using Fisher exact tests. For adult participants in the study (OI type I, n=81; OI type III, n=27 ; OI type IV, n=27) expected mid-parental height was calculated from self-reported parental heights and 5 cm was considered as one standard deviation from the mean as previously published 16. The differences in proportions of individuals with final heights below 2 standard deviations from the expected height were analyzed using Fisher’s exact test. The growth velocity between OI subtypes was assessed and the proportion of individuals falling outside of 2 standard deviations from the mean based on normative data were ascertained 17.
To further evaluate the potential effects of age, history of orthopedic rodding, history of bisphosphonate use, and sex on height, we performed generalized linear model (GLM) analyses using backward elimination strategy with the ‘step’ function in R. The height Z-score was the dependent variable while the independent categorical variables included OI type (type I, III, or IV), sex (male or female), history of rodding (yes or no), and history of bisphosphonate therapy (yes or no); age was included as a continuous numeric variable. P values were calculated using the likelihood ratio test and correlations present with p<0.05 were tested for evidence of interaction.
The arm span to height ratio between OI subtypes were compared by one-way ANOVA among the age groups <10 years, 10–20 years, and >20 years. These age categories were chosen based on previous normative data that show increase in arm span to height after 10 years of age 18.
RESULTS
The characteristics of individuals enrolled in the study are outlined in Table 1. The autosomal dominant forms of type I collagen-related OI accounted for over 90 percent of all enrolled individuals.
Height in OI
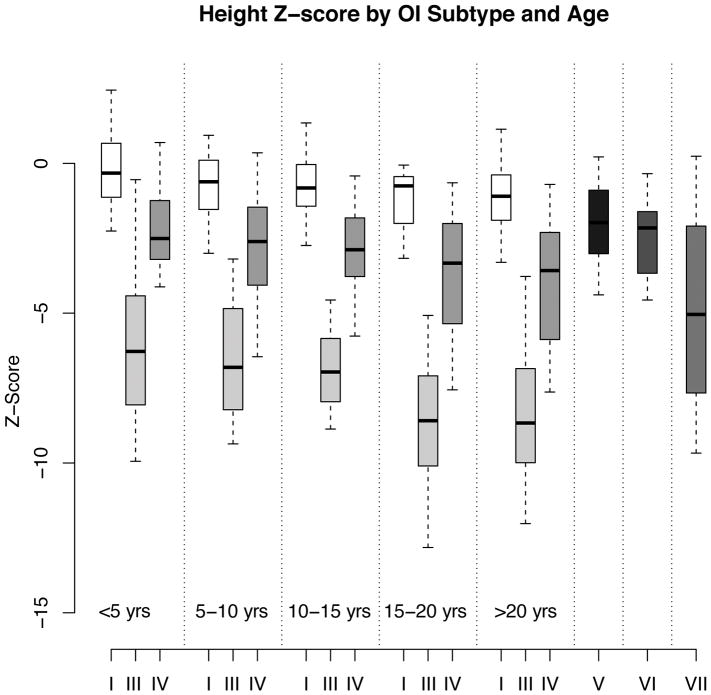
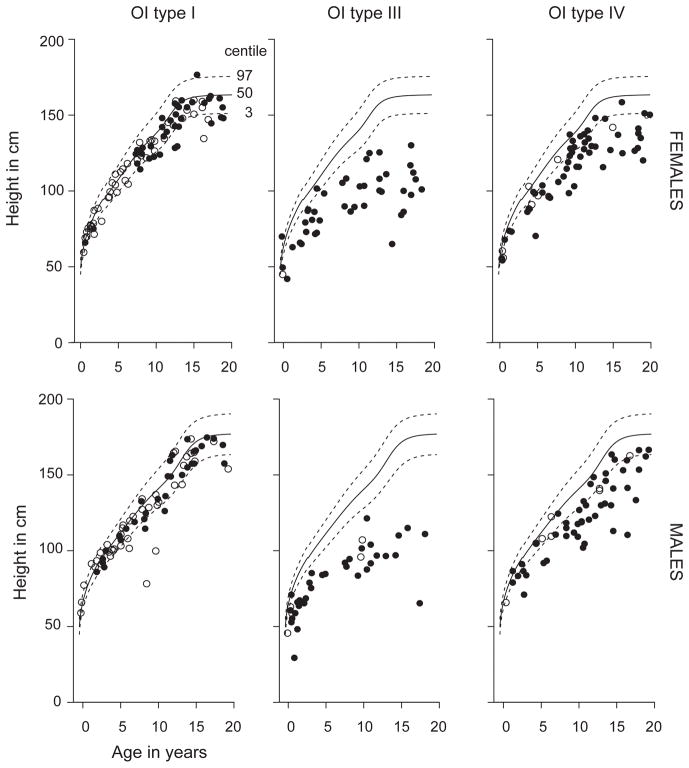
In the pediatric population (age < 20 years), the median (interquartile range or IQR) Z-scores for height in OI types I, III, IV, V, and VI were −0.66 (−1.43 to −0.02), −6.91 (−8.41 to −5.17), −2.79 (−3.95 to −1.69), −1.65 (−2.16 to −0.77), and −1.72 (−2.36 to −1.38), respectively (Figure 1). For the type I collagen-related OI, as expected, across all age groups, individuals with OI type III had diminished height compared to OI type I and OI type IV, and individuals with OI Type IV had decreased height compared to OI type I (p<0.05) (Figure 1). When plotted on the CDC growth curves, 9.5% of males and 18.1% of females with OI type I, 97.4% of males and 95.5% of females with OI type III, and 70.4% of males and 68.9% of females with OI type IV were below the third percentile for height (Figure 2). The odds ratio (95% confidence interval or CI) for being below the third percentile was 219 (50.5–953.4) for OI type III (p<0.001) and 11.7 (6.5–20.9) for OI type IV (p<0.001) as compared to type I. For OI type III the odds ratio for being below the third percentile was 18.76 (4.37–80.45) as compared to OI type IV (p<0.001).
Figure 1. Height Z-scores in OI.
Box plots showing the median and the IQR for height Z-scores in the various age groups in type I collagen-related OI demonstrate significantly lower Z-scores in individuals with OI type III and type IV as compared to OI type I. The number of participants in types V, VI, and VII were limited and the pooled data have been represented.
Figure 2.
Cross sectional height measurements for participants in the cohort plotted on the CDC growth curves. Each circle represents one participant. Open circles represent individuals who were naïve to bisphosphonate treatment while black circles represent individuals who have received bisphosphonate at some point in their life.
In adults with OI, the median (IQR) Z-scores for height were −1.10 (−1.90 to −0.38), −8.67 (−9.99 to −6.85) and −3.58 (−5.88 to −2.30), in OI types I, III, and IV, respectively. Pairwise comparisons revealed significant differences between OI subtypes (p<0.05). In adults without a family history of OI, we calculated the expected mid-parental height based on self-reported parental height. The final adult height was affected in all OI subtypes with 48% with OI type I, 100% with OI type III, and 58% with OI type IV below 2 standard deviations from the expected mid-parental height. Fisher’s exact test demonstrated a severe diminishing of final adult height for type III as compared to type IV OI (p <0.001) and type I OI (p < 0.01); however, no statistically significant difference was noted between OI types I and IV.
We correlated the height Z-scores to mutation type in individuals who had demonstrated pathogenic variants in either COL1A1 or COL1A2. For individuals with glycine substitution mutations, the median height Z-score (IQR) was −4.7 (−6.7 to −2.1), for individuals with other missense variants the median was −3.5 (−1.7 to −5.2) and for loss of function variants the median was −1.28 (−2.2 to −0.72). Height was significantly diminished in individuals with glycine substitutions compared to loss of function (p<0.001). Furthermore, we examined whether different glycine substitutions had an effect on height. Individuals with glycine-to-serine substitutions had a median height Z-score of −5.6 (−6.7 to −3.1), whereas the median heights in glycine-to-aspartate and glycine-to-another amino acid were −3.5 (−5.5 to −3.2) and −3.2 (−6.8 to −1.7), respectively. While the median was lower for glycine to serine substitutions, this difference did not reach statistical significance.
Growth Velocity in OI
We utilized the longitudinal aspect of the cohort to examine growth velocity in participants between the ages 3 and 16 years. We selected individuals that had at least two height measurements that were separated by 6 to 18 months. The velocity of growth was calculated as cm/year and the value obtained was plotted against normative data. In individuals with three or more measurements, the value between two consecutive measurements were considered as distinct data points and thus plotted separately. In total, there were 127 individuals with OI type I (n=61 male; n=66 female; n=259 growth velocity measurements), 46 individuals with OI type III (n=20 male; n=26 female, n=79 growth velocity measurements), and 76 individuals with OI type IV (n=37 male, n=39 female, n=148 growth velocity measurements) that were included in these analyses. In OI type I, 12.4% of growth velocities (32/259) were 2 standard deviations below the mean or lower for growth velocity whereas in OI types III and IV, this proportion was 51.9% (41/79), and 31.1% (46/148), respectively. This corresponds to increased odds ratio (95% CI) of having diminished growth velocity of 7.65 (4.30–13.61) in OI type III compared to type I, and of 3.20 (1.92–5.32) in OI type IV compared to type I (p<0.0001).
Weight in OI
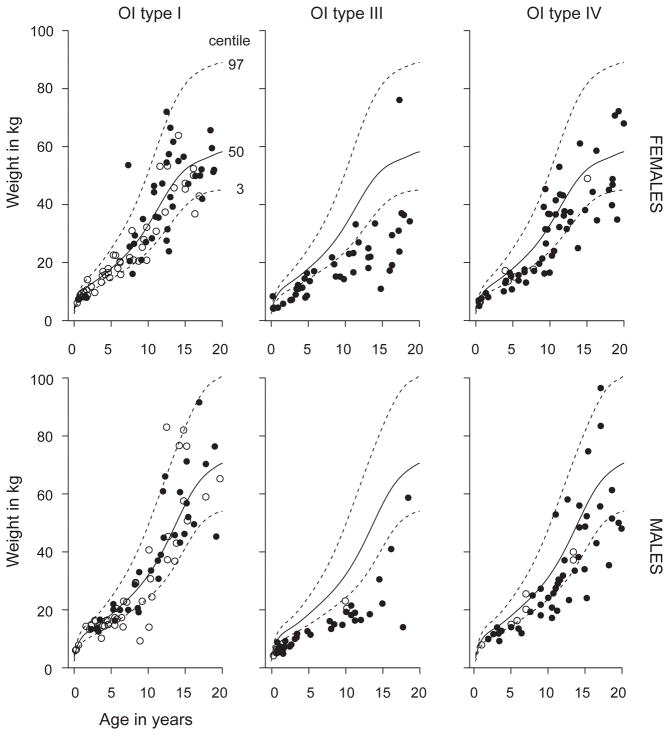
The median (IQR) Z-scores for weight in the pediatric population in OI types I, III, IV, V, and VI were −0.41(−1.14 to 0.56), −4.55 (−6.40 to −2.85), −1.55 (−2.37 to −0.31), −0.31(−1.81 to 0.32) and −0.99(−1.68 to 0.08), respectively (Supplementary Figure 1). ANOVA on ranks demonstrated significant differences between the OI subtypes across all age groups with weight in OI type III being significantly lower than in OI types I and IV (p<0.05). We observed significant differences for weight between OI type IV and OI type I only in age groups of 0–5 years and 10–15 years (p<0.05, Supplementary Figure 1). As in height, a similar relationship was seen for weight where 11.8% males and 16.9% of females with OI type I, 35.8% of males and 33.3% of females with OI Type IV, and 94.7% of males and 84.8% of females with OI type III were below the third percentile (p<0.001; Figure 3). The odds ratio (95% CI) for being below the third percentile was 43.20 (19.50–95.69) for OI type III (p<0.001) and 15.76 (7.32–33.95) for OI type IV (p<0.001) as compared to type I. For individuals with OI type III compared to OI type IV, the odds ratio for being below the third percentile was 2.74 (1.52–4.94) (p<0.005).
Figure 3.
Cross sectional weight measurements for participants in the cohort plotted on the CDC growth curves. Each circle represents one participant. Open circles represent individuals who have naïve to bisphosphonate treatment while black circles represent individuals who have received bisphosphonate at some point in their life.
BMI in OI
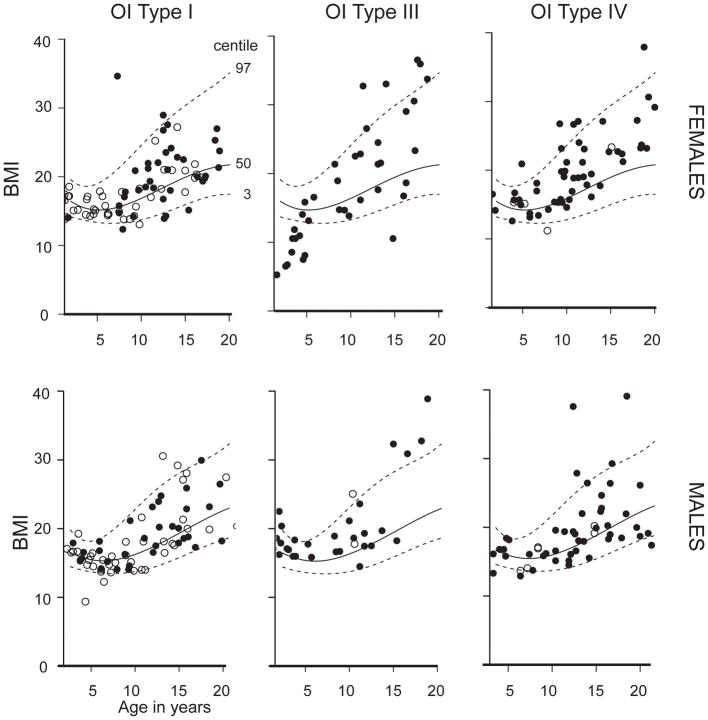
The median (IQT) of Z-scores for BMI in the pediatric population in OI types I, III, IV were 0.10 (−0.58 to 0.94), 0.91 (0.42 to 1.61) and 0.67 (−0.18 to 1.33) (Supplementary Figure 2). When BMI was calculated by using measured height, individuals with OI type III had increased BMI as compared to OI types I and IV in adults and in children in the age groups 5–10 years and 15–20 years (p<0.05; Figure 4 and Supplementary Figure 2).
Figure 4.
Cross sectional BMI for participants in the cohort plotted on the CDC growth curves. Open circles represent individuals who were naïve to bisphosphonate treatment while black circles represent individuals who have received bisphosphonate at some point in their life.
Arm span to height ratio in OI
The arm span to height ratio is an indicator of the relative severity of linear growth abnormalities of the axial and/or lower limb as compared to the upper limbs. The adult arm span is typically 5 cm greater in males and 2 cm greater in females as compared to height18. Arm span/height ratio changes with age wherein arm span is less than height in early childhood, equal to height by about 10 years in males and 12 years in females, and greater than height thereafter 18. Thus, we compared the arm span/height ratio in age groups of <10 years, 10–20 years, and >20 years. Only in the >20 years group, we observed significant increased arm span/height in OI types III and IV compared to type I (p<0.001 and p<0.05, respectively) (Supplementary Figure 3).
Potential correlates affecting height in OI
Previous studies have shown that individuals with severe forms of OI can have “flattening” of their height curves so that the height Z-scores worsen with age7,8. With the widespread use of bisphosphonates, the effect of the medications on linear growth has been assessed in short-term studies without conclusive answers19–23. In order to understand the correlations between some co-variates and the height Z-scores, we performed a GLM analyses. As expected, OI subtype had a strong correlation with the height Z-scores through all pediatric ages (p<0.001). Increasing age correlated with reduced height Z-scores (p<0.05). Independent correlations were also observed for history of rodding (p<0.01) and use of bisphosphonates (p<0.05), where both of these co-variates correlated with overall reduced Z-scores. However, there were significant interactions between the OI subtype, history of rodding and bisphosphonate use. Thus, we could not independently assess the effects of these covariates on height.
DISCUSSION
OI is a clinically heterogeneous disorder characterized by increased predisposition to recurrent fractures and bone deformities3. Whereas it is well recognized that growth deficiency can be found in patients with severe forms of OI, there is a lack of large-scale studies on growth parameters in various subtypes of OI. In this study, we have included the largest cohort of children and adults with OI from multiple clinical centers in North America.
The height measurements in this cohort are consistent with the expected phenotypes in OI and the growth patterns that were observed in other studies 3,5–8. Height in OI type III is more severely affected as compared to OI types I and IV. Whereas some individuals with OI type I and IV can have heights within the normal ranges, the overall adult height is affected in all subtypes. Even in OI type I, the mildest form, the final height in nearly half of individuals is 2 standard deviations below the expected mid-parental height. Given these distinct patterns of height, height Z-scores could be utilized for clinical classification of type I collagen-related OI.
The etiology for short stature in OI is multifactorial. Scoliosis, kyphosis, vertebral fractures, recurrent long bone fractures, and bone deformities are important contributing factors. The incidence of scoliosis and the rate of progression of curvature are significantly higher in the more severe forms of OI 24. In a retrospective study of over 300 children with OI, Anissipour and colleagues found that the rates of progression of scoliosis were 6°, 4°, and 1°, in OI types III, IV, and I, respectively. The spinal deformities in the severe forms of OI can lead to reduction in truncal height. Similarly, fractures and bowing of femur and tibia, fractures occurring through the growth plates, and abnormalities of the epiphyses in the severe forms of OI lead to decreased limb length and overall height. The varying severity of involvement of the axial and appendicular skeleton can be reflected by the arm span to height ratio. In this study, we observed that arm span to height ratio, was increased in adults with types III and IV OI as compared to type I. These results are consistent with previous observations by Lund and colleagues and Aglan and colleagues6,8. Collectively, these findings suggest that truncal height is relatively more reduced than the length of the long bones in the more severe forms of OI.
However, clinical experience and previous studies have demonstrated that the overall height can be affected even in individuals without significant bone deformities7,8. Thus, it has been hypothesized that the primary matrix and cellular abnormalities may have a role in the decreased growth rates in OI. Decreased responsiveness to growth hormone/insulin-like growth factor 1 has been suggested as one of the mechanisms in few studies25,26. More recently, it was discovered that excessive transforming growth factor-beta (TGF-β) signaling is an important driver of the bone and extra-skeletal abnormalities in moderate-to-severe forms of OI27. The collagen over modification in severe forms of OI affects the interaction of type I collagen with small leucine-rich proteoglycans that bind TGF-β and thus results in increased availability of the ligand 27–30. Increased TGF-β signaling in the growth plate could affect bone growth. In a mouse model of deficiency of E-selectin ligand, a negative regulator of TGF-β, Yang and colleagues demonstrated reduced chondrocyte proliferation and delayed terminal differentiation31. Thus, it is possible that increased TGF-β signaling could contribute to the abnormal growth in OI. While final adult height is affected across all types of OI, using the longitudinal nature of the data set, we also demonstrate that the growth velocity is affected. Current information on growth velocity in OI patients is rather limited, and studies that have addressed this have done in the context of therapy11,32,33. It is not known whether the decreased growth velocity in OI type III and IV is driven by the recurrent fractures or intrinsic abnormalities of the bone and cartilage.
Bisphosphonates are currently considered a standard-of-care and are widely used for the treatment of individuals with OI19–23,33–37. Whereas the effects of bisphosphonates on fracture risk in OI are difficult to address, the results have been inconclusive19–23,33–37. Few studies have addressed the effects of bisphosphonates on height in OI. Zeitlin and colleagues showed that 4 years of therapy with pamidronate increased height Z-scores in children with moderate-to-severe OI11. Similarly, it has also been shown that bisphosphonate therapy decreases the rate of progression of scoliosis in type III OI, if treatment is started before the age of six years24; however recent meta-analyses have not shown a significant effect of bisphosphonates on height38,39. In this study, we tried to find correlations between height Z-scores and the use of bisphosphonates. In our cohort, 30% of individuals with OI type I, 84% with OI type III, and 76% with OI type IV had received IV bisphosphonate at some time and 15% had received oral bisphosphonates in each subtype. However on GLM analyses, there was a significant interaction between the OI subtype and the use of bisphosphonates, and the proportion of individuals with severe OI who were naïve to bisphosphonates was very low precluding independent assessment of the effect of bisphosphonates on the height.
Weight in OI has not been studied as extensively as height. In this report, we observed that individuals with OI type III continue to fall below third percentile of the CDC growth curves across all age groups. These results are consistent with the previous publications that report underweight in individuals with severe OI6. However, in spite of the lower weight, calculated BMI in OI tends to be higher, especially in OI type III. Using peripheral quantitative computed tomography, Palomo and colleagues have shown that the fat cross sectional area at the forearm in individuals with OI is similar to the control population40. These data imply that “increased BMI” is a result of the small value of the denominator during calculation and may not portend increased metabolic risks. It may thus be more important and practical to monitor serial weight in OI, especially in adults. Future studies that systematically measure body composition in OI may be helpful in risk stratification and further management.
Disorder-specific growth charts can be very helpful in monitoring growth in the clinic. To our knowledge, the only growth charts that have been developed in OI are for OI type I 13. One challenge in creating standard growth charts for all subtypes of OI is the great variability in the clinical presentations. The experience gained for the studies in the OI LCRC was pivotal in establishing the Brittle Bone Disorders Consortium (BBDC). The data being collected in the BBDC would be helpful in generating OI-specific growth curves for all subtypes of OI.
In summary, our analyses of the largest cohort of individuals with OI demonstrates that individuals with severe forms of OI have reduced height as well as weight as compared to those with type I OI. This important study would be a significant first step in the construction of growth curves in this disorder.
Supplementary Material
Acknowledgments
This work was supported by the BBDC (1U54AR068069-0), a part of the NCATS’ RDCRN. BBDC is funded through a collaboration between the ORDR of NCATS, NIAMS, and NIDCR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The BBDC is also supported by the OI Foundation. The work was supported by The Clinical Translational Core of BCM IDDRC (1U54HD083092) from the Eunice Kennedy Shriver NICHD. SB was supported by Texas Department of Health Services. MJ and AT were supported by T32GM07526-40. This work was supported by the DDCF, Grant #2013095 (SCN).
The authors acknowledge the contributions of the Members of the Brittle Bone Disease Consortium: David R Eyre PhD, Department of Orthopedic and Sports Medicine, University of Washington, Seattle, WA, USA; Deborah Krakow MD, Department of Orthopedic Surgery, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA; Laura Tosi MD, Bone Health Program, Children’s National Health System, Washington, DC, USA; Cathleen L Raggio MD, Hospital for Special Surgery, New York, NY ; Eric S Orwoll MD, Department of Medicine, Division of Endocrinology, Oregon Health Sciences University, Portland, OR; and Eric T Rush MD, University of Nebraska Medical Center, Omaha, NE.
We acknowledge the clinical research teams: M. Mullins, A. Tran, S. Carter (BCM); V. Vensel, J. Christie, A. Hata (OHSU); M. Durigova (Shriner’s, Montreal); L. Davey (A.I. duPont Hospital).
References
- 1.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363(9418):1377–1385. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 2.Sykes B, Wordsworth P, Ogilvie D, Anderson J, Jones N. Osteogenesis imperfecta is linked to both type I collagen structural genes. Lancet. 1986;328(8498):69–72. doi: 10.1016/S0140-6736(86)91609-0. [DOI] [PubMed] [Google Scholar]
- 3.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellur S, Jain M, Cuthbertson D, et al. Cesarean delivery is not associated with decreased at-birth fracture rates in osteogenesis imperfecta. Genet Med. 2016;18(6):570–576. doi: 10.1038/gim.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vetter U, Pontz B, Zauner E, Brenner RE, Spranger J. Osteogenesis imperfecta: a clinical study of the first ten years of life. Calcif Tissue Int. 1992;50(1):36–41. doi: 10.1007/BF00297295. [DOI] [PubMed] [Google Scholar]
- 6.Aglan MS, Zaki ME, Hosny L, El-Houssini R, Oteify G, Temtamy SA. Anthropometric measurements in Egyptian patients with osteogenesis imperfecta. Am J Med Genet Part A. 2012;158 A(11):2714–2718. doi: 10.1002/ajmg.a.35529. [DOI] [PubMed] [Google Scholar]
- 7.Germain-Lee EL, Brennen FS, Stern D, et al. Cross-sectional and longitudinal growth patterns in osteogenesis imperfecta: implications for clinical care. Pediatr Res. 2015 doi: 10.1038/pr.2015.230. [DOI] [PubMed] [Google Scholar]
- 8.Lund aM, Müller J, Skovby F. Anthropometry of patients with osteogenesis imperfecta. Arch Dis Child. 1999;80(6):524–528. doi: 10.1136/adc.80.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelbert RH, Gerver WJ, Breslau-Siderius LJ, et al. Spinal complications in osteogenesis imperfecta: 47 patients 1–16 years of age. Acta Orthop Scand. 1998;69(3):283–286. doi: 10.3109/17453679809000931. [DOI] [PubMed] [Google Scholar]
- 10.Engelbert RH, Uiterwaal CS, Gerver WJ, Van Der Net JJ, Pruijs HE, Helders PJ. Osteogenesis imperfecta in childhood: Impairment and disability. A prospective study with 4-year follow-up. Arch Phys Med Rehabil. 2004;85(5):772–778. doi: 10.1016/j.apmr.2003.08.085. [DOI] [PubMed] [Google Scholar]
- 11.Zeitlin L, Rauch F, Plotkin H, Glorieux FH. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics. 2003;111(5 Pt 1):1030–1036. doi: 10.1542/peds.111.5.1030. [DOI] [PubMed] [Google Scholar]
- 12.Hamza RT, Abdelaziz TH, Elakkad M. Anthropometric and nutritional parameters in Egyptian children and adolescents with osteogenesis imperfecta. Horm Res Paediatr. 2015;83(5):311–320. doi: 10.1159/000374111. [DOI] [PubMed] [Google Scholar]
- 13.Graff K, Syczewska M. Developmental charts for children with osteogenesis imperfecta, type I (body height, body weight and BMI) Eur J Pediatr. 2017;176(3):311–316. doi: 10.1007/s00431-016-2839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RM, Nagamani SCS, Cuthbertson D, et al. A cross-sectional multicenter study of osteogenesis imperfecta in North America - results from the linked clinical research centers. Clin Genet. 2015;87(2):133–140. doi: 10.1111/cge.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: Methods and Development. 2002;11 doi:12043359. [PubMed] [Google Scholar]
- 16.Nwosu BU, Lee MM. Evaluation of short and tall stature in children. [Accessed September 22, 2017];Am Fam Physician. 2008 78(5):597–604. http://www.ncbi.nlm.nih.gov/pubmed/18788236. [PubMed] [Google Scholar]
- 17.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. [Accessed September 22, 2017];J Pediatr. 1985 107(3):317–329. doi: 10.1016/s0022-3476(85)80501-1. http://www.ncbi.nlm.nih.gov/pubmed/3875704. [DOI] [PubMed] [Google Scholar]
- 18.Belt-Niedbala BJ, Ekvall S, Cook CM, Oppenheimer S, Wessel J. Linear growth measurement: a comparison of single arm-lengths and arm-span. [Accessed September 22, 2017];Dev Med Child Neurol. 1986 28(3):319–324. doi: 10.1111/j.1469-8749.1986.tb03880.x. http://www.ncbi.nlm.nih.gov/pubmed/3721076. [DOI] [PubMed] [Google Scholar]
- 19.Bishop N, Adami S, Ahmed SF, et al. Risedronate in children with osteogenesis imperfecta: A randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9902):1424–1432. doi: 10.1016/S0140-6736(13)61091-0. [DOI] [PubMed] [Google Scholar]
- 20.Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the Treatment of Mild Pediatric Osteogenesis Imperfecta: A Randomized Placebo-Controlled Study. J Bone Miner Res. 2009;24(7):1282–1289. doi: 10.1359/jbmr.090213. [DOI] [PubMed] [Google Scholar]
- 21.Sakkers R, Kok D, Engelbert R, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: A 2-year randomised placebo-controlled study. Lancet. 2004;363(9419):1427–1431. doi: 10.1016/S0140-6736(04)16101-1. [DOI] [PubMed] [Google Scholar]
- 22.Seikaly MG, Kopanati S, Salhab N, et al. Impact of alendronate on quality of life in children with osteogenesis imperfecta. J Pediatr Orthop. 2005;25(6):786–791. doi: 10.1097/01.bpo.0000176162.78980.ed. [DOI] [PubMed] [Google Scholar]
- 23.Letocha AD, Cintas HL, Troendle JF, et al. Controlled Trial of Pamidronate in Children With Types III and IV Osteogenesis Imperfecta Confirms Vertebral Gains but Not Short-Term Functional Improvement. J Bone Miner Res. 2005;20(6):977–986. doi: 10.1359/JBMR.050109. [DOI] [PubMed] [Google Scholar]
- 24.Anissipour AK, Hammerberg KW, Caudill A, et al. Behavior of scoliosis during growth in children with osteogenesis imperfecta. J Bone Joint Surg Am. 2014;96(3):237–243. doi: 10.2106/JBJS.L.01596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marini JC, Bordenick S, Heavner G, Rose S, Chrousos GP. Evaluation of growth hormone axis and responsiveness to growth stimulation of short children with osteogenesis imperfecta. Am J Med Genet. 1993;45(2):261–264. doi: 10.1002/ajmg.1320450223. [DOI] [PubMed] [Google Scholar]
- 26.Marini JC, Bordenick S, Heavner G, et al. The growth hormone and somatomedin axis in short children with osteogenesis imperfecta. J Clin Endocrinol Metab. 1993;76(1):251–256. doi: 10.1210/jcem.76.1.8421094. [DOI] [PubMed] [Google Scholar]
- 27.Grafe I, Yang T, Alexander S, et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med. 2014;20(6):670–675. doi: 10.1038/nm.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keene DR, San Antonio JD, Mayne R, et al. Decorin binds near the C terminus of type I collagen. J Biol Chem. 2000;275(29):21801–21804. doi: 10.1074/jbc.C000278200. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi Y, Kodama Y, Matsumoto T. Bone matrix decorin binds transforming growth factor-beta and enhances its bioactivity. [Accessed September 23, 2017];J Biol Chem. 1994 269(51):32634–32638. http://www.ncbi.nlm.nih.gov/pubmed/7798269. [PubMed] [Google Scholar]
- 30.Markmann A, Hausser H, Schönherr E, Kresse H. Influence of decorin expression on transforming growth factor-beta-mediated collagen gel retraction and biglycan induction. [Accessed September 23, 2017];Matrix Biol. 2000 19(7):631–636. doi: 10.1016/s0945-053x(00)00097-4. http://www.ncbi.nlm.nih.gov/pubmed/11102752. [DOI] [PubMed] [Google Scholar]
- 31.Yang T, Grafe I, Bae Y, et al. E-selectin ligand 1 regulates bone remodeling by limiting bioactive TGF- in the bone microenvironment. Proc Natl Acad Sci. 2013;110(18):7336–7341. doi: 10.1073/pnas.1219748110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Land C, Rauch F, Munns CF, Sahebjam S, Glorieux FH. Vertebral morphometry in children and adolescents with osteogenesis imperfecta: Effect of intravenous pamidronate treatment. Bone. 2006;39(4):901–906. doi: 10.1016/j.bone.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339(14):947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 34.Dimeglio LA, Ford L, McClintock C, Peacock M. A comparison of oral and intravenous bisphosphonate therapy for children with osteogenesis imperfecta. [Accessed September 23, 2017];J Pediatr Endocrinol Metab. 2005 18(1):43–53. doi: 10.1515/jpem.2005.18.1.43. http://www.ncbi.nlm.nih.gov/pubmed/15679068. [DOI] [PubMed] [Google Scholar]
- 35.Gatti D, Antoniazzi F, Prizzi R, et al. Intravenous Neridronate in Children With Osteogenesis Imperfecta: A Randomized Controlled Study. J Bone Miner Res. 2004;20(5):758–763. doi: 10.1359/JBMR.041232. [DOI] [PubMed] [Google Scholar]
- 36.Barros ER, Saraiva GL, de Oliveira TP, Lazaretti-Castro M. Safety and efficacy of a 1-year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. [Accessed September 23, 2017];J Pediatr Endocrinol Metab. 2012 25(5–6):485–491. doi: 10.1515/jpem-2012-0016. http://www.ncbi.nlm.nih.gov/pubmed/22876543. [DOI] [PubMed] [Google Scholar]
- 37.Bishop N, Harrison R, Ahmed F, et al. A randomized, controlled dose-ranging study of risedronate in children with moderate and severe osteogenesis imperfecta. J Bone Miner Res. 2010;25(1):32–40. doi: 10.1359/jbmr.090712. [DOI] [PubMed] [Google Scholar]
- 38.Hald JD, Evangelou E, Langdahl BL, Ralston SH. Bisphosphonates for the prevention of fractures in osteogenesis imperfecta: meta-analysis of placebo-controlled trials. J Bone Miner Res. 2015;30(5):929–933. doi: 10.1002/jbmr.2410. [DOI] [PubMed] [Google Scholar]
- 39.Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. In: Basel D, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2014. p. CD005088. [DOI] [PubMed] [Google Scholar]
- 40.Palomo T, Glorieux FH, Schoenau E, Rauch F. Body Composition in Children and Adolescents with Osteogenesis Imperfecta. J Pediatr. 2016;169:232–237. doi: 10.1016/j.jpeds.2015.10.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.