Abstract
The ability of gellan gum-immobilised cells of the heavy metal-tolerant bacterium Alcaligenes sp. AQ05-001 to utilise both heavy metal-free and heavy metal-polluted feathers (HMPFs) as substrates to produce keratinase enzyme was studied. Optimisation of the media pH, incubation temperature and immobilisation parameters (bead size, bead number, gellan gum concentration) was determined for the best possible production of keratinase using the one-factor-at-a-time technique. The results showed that the immobilised cells could tolerate a broader range of heavy metal concentrations and produced higher keratinase activity at a gellan gum concentration of 0.8% (w/v), a bead size of 3 mm, bead number of 250, pH of 8 and temperature of 30 °C. The entrapped bacterium was used repeatedly for ten cycles to produce keratinase using feathers polluted with 25 ppm of Co, Cu and Ag as substrates without the need for desorption. However, its inability to tolerate/utilise feathers polluted with Hg, Pb, and Zn above 5 ppm, and Ag and Cd above 10 ppm resulted in a considerable decrease in keratinase production. Furthermore, the immobilised cells could retain approximately 95% of their keratinase production capacity when 5 ppm of Co, Cu, and Ag, and 10 ppm of As and Cd were used to pollute feathers. When the feathers containing a mixture of Ag, Co, and Cu at 25 ppm each and Hg, Ni, Pb, and Zn at 5 ppm each were used as substrates, the immobilised cells maintained their operational stability and biological activity (keratinase production) at the end of 3rd and 4th cycles, respectively. The study indicates that HMPF can be effectively utilised as a substrate by the immobilised-cell system of Alcaligenes sp. AQ05-001 for the semi-continuous production of keratinase enzyme.
Keywords: Immobilisation, Gellan gum, Alcaligenes sp., Feather degradation, Heavy metals
Introduction
Advances in biotechnology have enabled scientists in the field of bioremediation and biotransformation to use a large amount of feather wastes generated from slaughterhouses for other purposes. Apart from their application as a source of important peptides in animal feed formulation and as a substrate for keratin-degrading microorganisms in the production of industrially important keratinase enzyme, they are now being used as cheap biosorption materials in the removal of heavy metals from polluted water (Al-Asheh et al. 2003; Gavrilescu 2004; Parrado et al. 2014). Bioremediation, an environmentally friendly technique where microorganisms are used in the degradation/transformation of feathers has replaced the conventional method of feather disposal that uses chemical, heat and land-filling methods that add to environmental pollution (Taskin et al. 2011; Lima de Silva et al. 2012; Al-Musallam et al. 2013; Mazotto et al. 2013).
Compared with other biosorption means of removing heavy metal from water, the use of raw and chemically treated feathers has been reported to possess a high capacity to remove different heavy metals from water surfaces (Al-Asheh et al. 2003; Eslahi et al. 2013). Thus, secondary feather waste laden with different amount of heavy metals is generated, unfortunately polluting the environment more than primary feathers due to the heavy metals they contain. The toxicity of heavy metals to many feather-degrading microbes (FDMs) has resulted in very slow or unsatisfactory degradation of heavy metal-polluted feathers (HMPFs). However, these HMPFs are often disposed using environmentally unfriendly methods, such as incineration and land filling, but no study, thus far, has attempted to search for microorganisms that possess two characteristics of heavy metal tolerance as well as keratin-degrading ability to degrade HMPFs in an eco-friendly way. Hence, we assume the combination of the ability of microbes to tolerate the toxicity of the adsorbed heavy metals and ability to carry out the keratinolytic process will be needed to degrade the large volume of HMPFs accumulated over time.
Previous studies have indicated that microbes resident in environments contaminated with heavy metals tend to adapt to the toxicity of heavy metals through the development or acquisition of a genetic system that will confer different mechanisms of resistance to heavy metal toxicity (Ng et al. 2012). In our fundamental effort to achieve excellent bioremediation of HMPFs, our laboratory has isolated many heavy metal-tolerant bacteria from heavy metal-rich soils where feathers are dumped, but only few could, in addition to tolerance to heavy metals, degrade feathers effectively. Free cells of two strains, Bacillus sp. Khayat and Alcaligenes sp. AQ05-001, showed excellent performance in degrading HMPFs (Yusuf et al. 2015, 2016). However, due to some limitations, such as a slow growth rate, low keratinase yield and poor feather degradation, encountered when free living cells of Alcaligenes sp. AQ05-001 were applied in biodegradation of HMPFs, an attempt was made to immobilise the whole cells of the bacteria to improve the process of keratinase production.
A promising immobilisation technique that has gained increasing attention recently is cell entrapment (Bibi et al. 2015; Ibrahim et al. 2016; Mohanty and Jena 2017; Karamba et al. 2017). Many immobilised FDMs have been successfully used in the production of keratinase that is highly necessary in the biodegradation and biotransformation of heavy metal-free feather wastes (Prakash et al. 2010; Shrinavas et al. 2012). Immobilised cells present specific advantages over free cells, such as (1) operational stability, (2) high cell retention capacity, (3) reusability, (4) high efficiency of catalysis (Adinarayana et al. 2005) and (5) use as a sorbent agent (Gavrilescu 2004). Several studies have examined using immobilisation carriers, such as agar, alginate with or without microbes to remove heavy metals, or dyes to degrade heavy metal-free feathers (HMFFs), but studies on the use of gellan gum as a carrier matrix remains inadequate despite their advantages over others. Some of its advantages include non-toxicity, a wide pH stability range (2–10), and high mechanical and thermal stability (Moslemy et al. 2003).
Thus, in this study, gellan gum was selected as a carrier for cell immobilisation, and a previously isolated heavy metal-tolerant feather-degrading strain was immobilised in gellan gum to utilise HMPFs as a substrate to produce keratinase. We aimed to investigate the following: (1) the preparation of gellan gum-immobilised Alcaligenes sp. AQ05-001; (2) optimisation of the conditions affecting the immobilised cells; (3) the capability of immobilised AQ05-001 to utilise HMPFs; and (4) the tolerance level of immobilised AQ05-001 to different concentrations of heavy metals.
Materials and methods
Chemicals and feathers
Gellan gum, heavy metal stock solutions and other analytical grade chemicals were obtained unless otherwise stated from Sigma-Aldrich Corporation, USA. In the current study, the azokeratin used for the keratinase assay was prepared in our laboratory from scissor-cut white chicken feathers according to the procedure described by Joshi et al. (2007). The azokeratin had been air dried and further cut into size ranging between 0.6 and 0.85 mm before use.
Heavy metal-free feathers were collected from local markets, farms and slaughterhouses in Selangor, Malaysia. All feathers were washed using detergent, rinsed off several times with distilled water and dried to constant weight in an oven at 50 °C (Fakhfakh-Zouari et al. 2010). Feathers of relatively equal sizes and weight were used.
Microorganisms and culture conditions
The heavy metal-tolerant feather-degrading bacterium Alcaligenes sp. AQ05-001 was isolated from feather dumpsites in Johor Bahru, Malaysia (Yusuf et al. 2016). Cell suspensions of Alcaligenes sp. AQ05-001 were diluted in sterile normal saline to an optical density of 1.00 at 600 nm and were used to inoculate 100 mL of feather meal broth (FMB) containing 10.0 g/L of feather, 0.5 g/L of NaCl, 0.7 g/L of K2HPO4, 1.4 g/L of KH2PO4, and 0.001 g/L of MgSO4·6H2O pH 7.5 as described by Tork et al. (2010). Incubation was carried out using an orbital shaker at 150 rpm at 25 °C. At the appropriate time interval, two flasks were withdrawn, and their contents were used for the keratinase and feather degradation assays. Crude keratinase was obtained by centrifuging 5 mL of the FMB at 10,000×g, 4 °C for 10 min. The supernatant was filtered through a 0.45-µm membrane and was stored at − 20 °C until ready for use in enzyme assays.
Immobilisation of the AQ05-001 strain on gellan gum
Two litres volume culture of Alcaligenes sp. AQ05-001 was created using nutrient broth (NB) in a flat-bottomed flask under forced aeration for 3 days at 25 °C. After incubation, the AQ05-001 cells in NB were harvested by centrifugation at 10,000×g, 4 °C for 10 min. The pellets obtained were washed three times with sterilised saline and were re-centrifuged to obtain the washed pellets.
The pellets of Alcaligenes sp. AQ05-001 were immobilised in gellan gum for use in the batch degradation of feathers and keratinase production, as described previously by Ahmad et al. (2012). Briefly, 0.75% (w/v) of gellan gum powder was suspended in 100 mL of deionised water and was heated to 75 °C on a hot plate to completely dissolve the gum. Thereafter, 0.06% (w/v) calcium chloride (CaCl2) (Fischer scientific) was added to the gum suspension and was allowed to slowly cool down to 45 °C. The pH of the Gum + CaCl2 suspension was adjusted to pH 7.0 using 0.1 M NaOH solutions. Next, 3.5 g (w/v) pellets of Alcaligenes sp. AQ05-001 were added to the gum + CaCl2 mixture and were continuously stirred until thoroughly mixed. Beads were formed using a peristaltic pump and then were dropped into canola oil containing 0.1% Span 80. The oil was carefully decanted into a separate container, and the beads were then added to 500 mL of 0.1% (w/v) CaCl2 and placed in a refrigerator for 2 h. The beads were then rinsed repeatedly with 0.1% (v/v) Tween 80 solution and stored in a chiller (4 °C) until ready for use.
Preparation of heavy metal-polluted feathers (HMPFs)
Different concentrations of nine different heavy metals such as arsenic (As), cadmium (Cd), cobalt (Co), copper (Cu), lead (Pb), mercury (Hg), nickel (Ni), silver (Ag) and zinc (Zn) were prepared from their 1,000 ppm stock solutions by diluting to an appropriate initial concentration in FMB prepared with deionised water (Kar and Misra 2004). The optimum pH for the absorption of each heavy metal by 0.5 g of feathers was first determined in 100 mL of FMB by comparing the final dry weight of feathers placed in FMB pH 7.5 with and without heavy metals after 36 h of constant shaking at 150 rpm and 25 °C. The pH was adjusted to between 4 and 10 using HCl (100 mmol/dm3) and NaOH (100 mmol/dm3) solutions, and care was taken not to exceed the precipitation pH of each metal. The HMPFs harvested were dried in an oven at 50 °C for 30 min, were adequately labelled and were stored in a refrigerator until further use. The analysis of different heavy metals adsorbed by the feather was further confirmed using atomic absorption spectrometry (AAS) after nitric acid digestion.
Batch experiments with immobilised cells
The ability of immobilised AQ05-001 to utilise HMFFs and HMPFs as substrates to produce keratinase was conducted as follows: 250 beads of gellan gum-immobilised AQ05-001 were added into a 250-mL Erlenmeyer flask containing 100 mL of FMB pH 7.5 containing 10% (w/v) HMFFs or HMPFs (with the appropriate heavy metal concentration). Control flasks contained the following: (1) HMFFs or HMPFs alone without bacteria/or with empty gellan gum beads; (2) HMFFs or HMPFs and 5% (v/v) free cells of the AQ05-001 strain. At the end of 36 h of incubation on a rotary shaker at 150 rpm, the feather-degrading ability (FDA) of free and immobilised AQ05-001 on HMFFs/HMPFs and keratinase activities (KAs) were determined and compared.
Optimisation of immobilised cell performance
To enhance FDA or utilisation of HMPFs as a substrate by immobilised AQ05-001, factors such as pH, temperature, gellan gum concentration, bead size and number that are known to affect keratinase production were optimised. The temperature (range 20–50 °C), pH (range 6.5–10), gellan gum concentration (range 0.65–0.9% w/v), bead size (range 1–6 mm in diameter) and bead number (range 50–400) were optimised using a one-factor-at-a-time approach (Ahmad et al. 2012). Optimisation experiments were also carried out in batches as described above.
Heavy metal tolerance studies
The influence of different heavy metal concentrations such as 0, 1, 5, 10, 15, 20, 25 and 30 ppm on the ability of the immobilised AQ05-001 to degrade feather and produce keratinase was studied. Additionally, the influence of the mixture of heavy metals to which the immobilised cells have a relatively similar tolerance level was determined. Control flasks were set up as previously described, and the tolerance level to each heavy metal at different concentrations was determined. The level of tolerance of the immobilised AQ05-001 to each heavy metal at a particular concentration was based on the percentage of feather degraded and amount of keratinase produced by the immobilised cells at the end of 36 h of incubation in an optimised FMB compared with the control flask experiment containing no heavy metal. The levels of non-tolerance (A), tolerance (T), and highly tolerance (H) are shown in Table 1.
Table 1.
Categorisation of tolerance level of immobilised AQ05-001 to different concentrations of different heavy metals
| Level of tolerance | Degradation (%) in comparison with control | Keratinase activity (U/mL) in comparison with control |
|---|---|---|
| A | ≤ 50 | ≤ 60 |
| T | 51–94 | 61–99 |
| H | 95–100 | 100 and above |
Repeated degradation of HMPFs
The repeated degradation of HMPFs through keratinase production by the same beads of immobilised AQ05-001 was also assessed in optimised FMB containing 10% (w/v) feathers polluted with different concentrations of heavy metals. The immobilised cells were placed into a 100 mL FMB containing 10% HMPFs and were incubated on a rotary shaker for 24 h. After 24 h of shaking, the FMB was decanted, and immobilised cells were washed with sterile water before being transferred to fresh FMB-containing 10% HMPFs.
Analytical analysis
At a defined time of cultivation, aliquots of culture broth were taken aseptically, and KA was determined using azokeratin as a substrate as described by Joshi et al. (2007). Feather degradations were quantified by subtracting the final weight of a feather in the test from the final weight of the feather from the control as described by Jeong et al. (2010) and Yusuf et al. (2016). Feather remnants at the end of the incubation period were filtered from the hydrolysates using a well-dried Whatman no. 1 filter paper. Bacteria and protein remaining on the feathers were removed by gentle washing with distilled water. The feathers were then dried in an oven at 60 °C to obtain a constant weight. Percentage of degradation was calculated using the equation:
where X = final weight of feather, Zx= final weight of filter paper, Y = initial weight of feather, and Zy= initial weight of filter paper.
Statistical analysis
A completely randomised design was used throughout this study. All experiments were run in triplicates. The means and standard deviations (SD) were then determined from the triplicate samples at each time to check for errors and variation among the triplicate samples, and the values are represented as the means ± SD. Differences in the rate of degradation and keratinase production under different conditions between the groups were analysed by Student’s t test and one-way analysis of variance (ANOVA) at the 95% confidence level using the Minitab (version 16) software package (State College, Pennsylvania). Tukey’s post hoc analysis after ANOVA was used to check the differences in keratinase production and feather degradation under the influence of different heavy metals.
Results and discussion
Comparing the feather-degrading ability (FDA) of free and immobilised cells of Alcaligenes sp. AQ05-001 on HMFFs and HMPFs
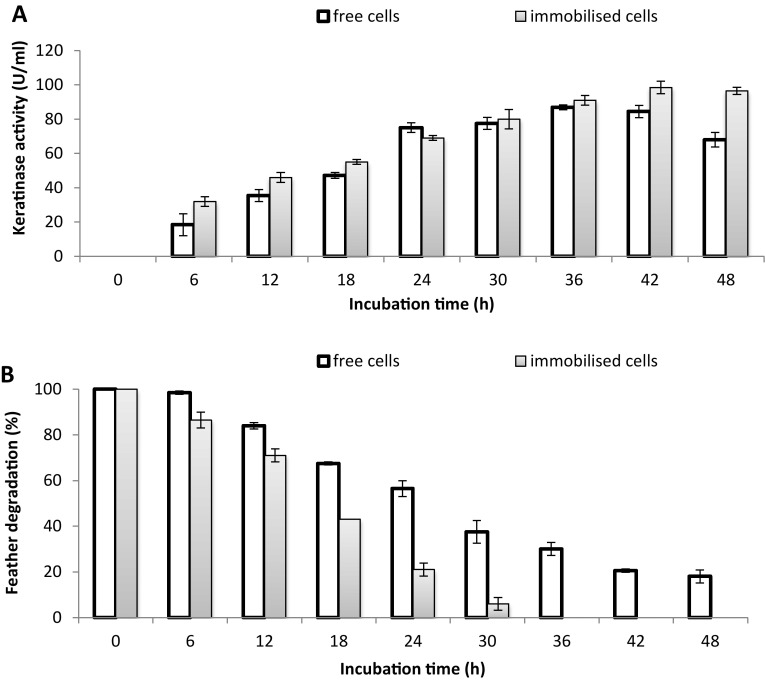
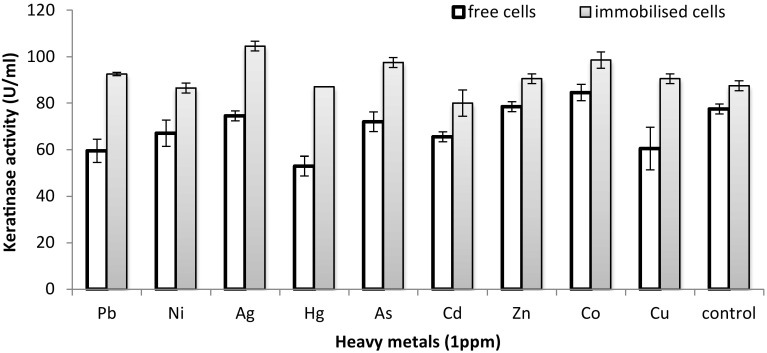
In our previous study (Yusuf et al. 2016), the isolation, selection and characterisation of the heavy metal-tolerant bacterium Alcaligenes sp. AQ05-001 was described. The present study extends the previous one, where free cells of Alcaligenes sp. AQ05-001 are used to degrade feathers polluted with a single heavy metal or a combination of heavy metals. However, in the present study, we focused on utilising both HMFFs and HMPFs as substrates for keratinase production by immobilised Alcaligenes sp. AQ05-001. Comparison of the FDA of free and immobilised cells of AQ05-001 on HMFFs showed that the immobilised cells degrade 10% (w/v) of HMFFs completely and produced a high level of keratinase (91 U/mL) at 36 h of incubation (Fig. 1a) compared with free cells that degrade approximately 70% of the feathers (Fig. 1b) and only produced 86.9 U/mL of keratinase at the end of 36 h of incubation. Although the number of cells in FMB containing free-living and immobilised AQ05-001 was relatively equal, there was a marked difference (p < 0.05) in terms of feather degradation between free and immobilised cells. The faster degradation of feathers by immobilised AQ05-001 may have resulted due to modification of key metabolic features of the entrapped cells, such as cell growth (Moslemy et al. 2002). The immobilised cells may generate concentration gradients that enable them to easily adapt to a new environment, thereby significantly reducing their lag phase. However, in free cells, there is a need for gradual adaptation to a new environment to build up a reasonable cell mass, possibly explaining the low degradation rate observed compared with immobilised cells (Wilson and Bradley 1997). However, in the presence of 1 ppm of different heavy metals in 100 mL of FMB, the KA and FDA of the immobilised AQ05-001 were markedly increased compared with those of free cells (Fig. 2).
Fig. 1.
Comparing a keratinolytic activity and b feather-degrading ability of free and immobilised cells of AQ05-001 in 100 mL basal FMB containing 10% (w/v) HMFFs. Data are the means ± standard deviations of three independent replicates
Fig. 2.
Comparing KA of free and immobilised AQ05-001 in 100 mL basal FMB polluted with 1 ppm of different heavy metals containing 10% (w/v) HMFFs at 36 h. Data are the means ± standard deviations of three independent replicates
This indicated that KA was affected to a varying level by different heavy metals at lower concentrations of 1 ppm and that immobilised cells can withstand different heavy metal toxicity to degrade feathers in the FMB. At this stage, it is unclear whether the gellan gum also possesses the ability to absorb heavy metals from the FMB that may have enabled the bacteria to degrade the feathers and produced high keratinase activity. It was reported that immobilising matrices, such as alginate, agar–agar and k-carrageen, can absorb certain heavy metals from aqueous solutions (Pires et al. 2011).
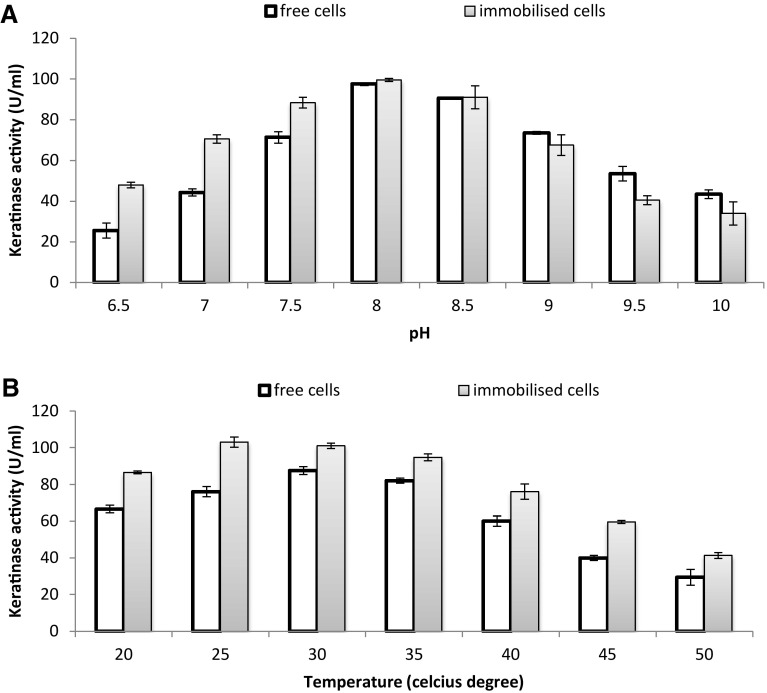
Optimisation of pH and incubation temperature were then carried out to enhance the KA and FDA of immobilised cells. The experiment was conducted compared with free cells. At an optimum medium pH of 8 and 30 °C incubation temperature, both free and immobilised cells of strain AQ05-001 showed improved KA and FDA, showing that the duo conditions favoured the bacterium in both forms at the end of 36 h (Fig. 3).
Fig. 3.
Optimisation of media a pH and b incubation temperature for free and immobilised cells. Data are the means ± standard deviations of three independent replicates
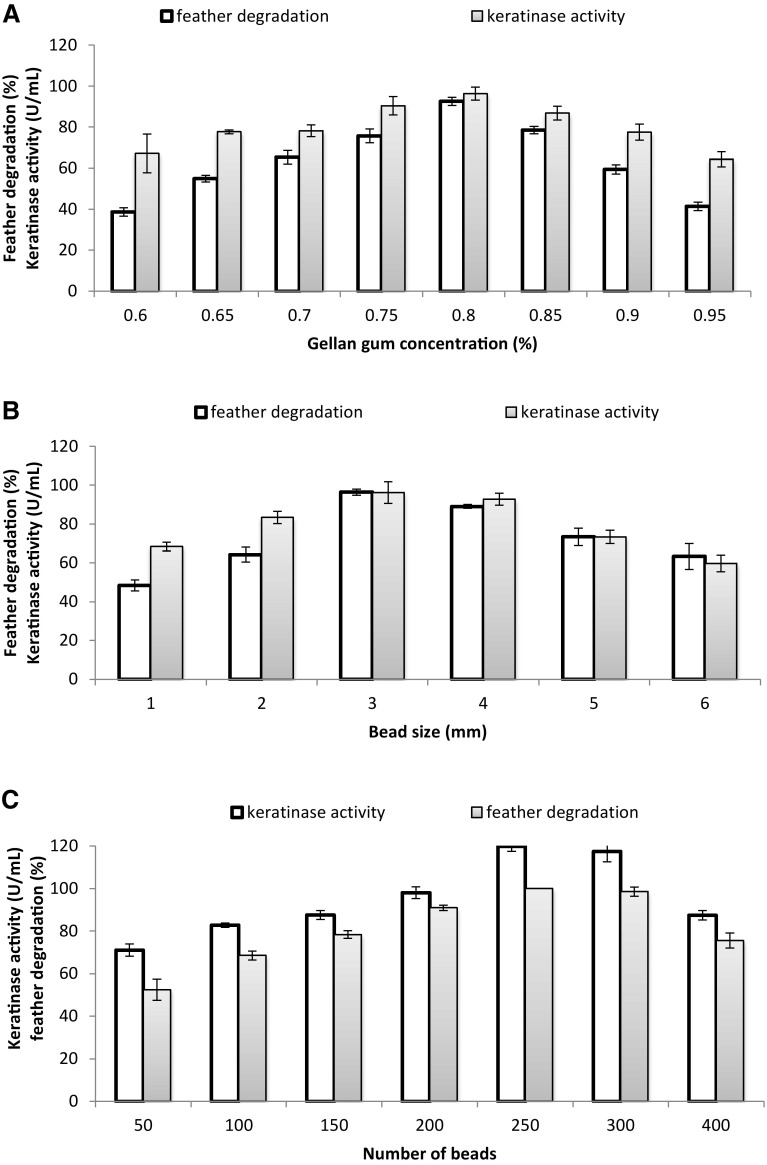
Optimisation of immobilised conditions
The concentration of gellan gum used in the preparation of immobilised cells as well as the number of beads and sizes were then optimised using the optimised FMB. An enhanced KA and a faster feather degradation rate were observed when 0.8% gellan gum, 3-mm-sized bead and 200 beads were used (Fig. 4a–c). The gum concentration of 0.8% (w/v) has improved the mechanical strength and pore size of the beads, in turn affecting the diffusion of the substrates in and out of the beads and affecting cell leakage. This is because, at this concentration of gellan gum, the beads could withstand shaking at a high speed (300 rpm) for 24 h, unlike, at 0.7%, where the beads disintegrated at the same speed in less than 12 h. Furthermore, the activity of immobilised cells was significantly (p < 0.05) decreased at gum concentrations under 0.7% and above 0.8% (Fig. 4a). This can be explained by a low gellan gum concentration resulting in fragile beads while a high concentration above 0.8% may block feather and keratinase diffusion in and out of the beads (Ahmad et al. 2012).
Fig. 4.
Optimisation of immobilisation conditions. a Optimisation gellan gum concentration, b optimisation of bead size and c optimisation of bead numbers. Data are the means ± standard deviations of three independent replicates
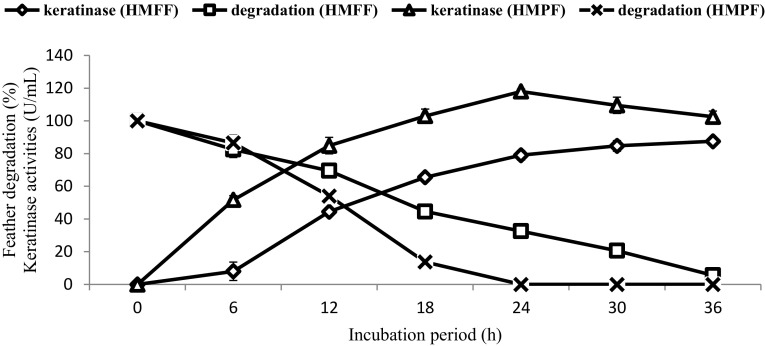
The effect of the numbers of 3-mm-sized beads on feather degradation and the keratinase yield by the immobilised bacteria showed that 250–300 beads resulted in faster degradation and higher KA (Fig. 4b, c). However, at lower and higher bead numbers, the FDA and KA were reduced. This may be due to the role that cell density plays in feather degradation. Low concentrations have the less bacterial mass to do the job, and a higher cell density leads to a greater demand for oxygen and nutrients (Ahmad et al. 2012). Under these conditions, complete degradation of 10% (w/v) of HMFFs and HMPFs (Ag) was completed at 24 and 36 h, respectively (Fig. 5). Interestingly, all the polluted feathers, including the highly recalcitrant rachises, were degraded with the highest keratinase activities of 118 ± 2.3 U/mL at 24 h in immobilised cells (Fig. 5).
Fig. 5.
Comparing keratinase production and degradation of 10% (w/v) of HHFFs and HMPFs containing Ag by gellan gum-immobilised cells of Alcaligenes sp. AQ05-001 under optimised conditions. Data are the means ± standard deviations of three independent replicates
Feather degradation is catalysed by extracellular keratinase, a proteinase enzyme induced by feather keratin. Enhancement of KA is believed to be caused by some heavy metals in FMB. However, the immobilisation tends to improve the stability of keratinase by retaining them in their natural surroundings during immobilisation and subsequent operations (Kar and Misra 2004). Furthermore, the gellan gum might have also played a role in protecting the bacterial cells and keratinase against the toxic effects of heavy metals that, in both cases, can further increases the degradation capability of immobilised cells.
Absorption of different heavy metals by feathers in FMB
Earlier literature has shown a relationship between the media pH and sorption of different heavy metals by different sorbent materials (Guo et al. 2010). In this study, the same feather has different absorption capacities for different heavy metals at different pH values. Feathers absorbed 5 ppm of As, Co, Cu, Pb, Ni, Ag and Zn from the optimised FMB more effectively between pH 7 and 8 (Tables 2, 3). This finding suggests that the sorption of these heavy metals is preferred at neutral to slight alkaline pH and agrees with the findings of Al-Asheh et al. (2003) that feather biosorption of Cu and Zn is highest in alkaline media. However, Cd and Hg were better absorbed between pH 4 and 6, which is acidic. It was interesting to observe that the colouration of some FMB occurred when some heavy metals were added and that disappeared after 18 h of shaking on an orbital shaker. However, the coloured FMB remained in the control flask containing no feathers, indicating progression in the absorption of the heavy metals by the feathers. No significance difference (p > 0.05) was found in the net gain in the weight of feathers placed in flasks containing different heavy metals between 18 and 24 h of shaking except for Cd and Ni.
Table 2.
Variation in net weight of 0.5 g feathers in 100 mL FMBs that contains 5 ppm of each heavy metal under different media pH
| Heavy metal (MM) | Net weight gain by 5% (w/v) of feather per 100 mL of FMB adjusted to different pH | |||||||
|---|---|---|---|---|---|---|---|---|
| 4.0 | 5.0 | 6.0 | 7.0 | 8.0 | 9.0 | 10.0 | 11.0 | |
| Ag (107.87) | 0.55 ± 1.4e | 0.6 ± 0.5e | 0.83 ± 0.9d | 2.64 ± 2.3b | 3.72 ± 1.1a | 2.74 ± 3.2b | 2.71 ± 0.0b | 1.61 ± 2.4c |
| As (74.922) | 0.01 ± 2.1d | 0.12 ± 2.9d | 0.68 ± 1.3c | 1.70 ± 2.0b | 1.94 ± 1.9a | 0.74 ± 4.2c | 0.73 ± 4.1c | 0.65 ± 0.0c |
| Cd (112.41) | 1.43 ± 3.1c | 2.98 ± 3.4a | 2.81 ± 1.9a | 1.83 ± 1.9b | 1.78 ± 0.4b | 0.86 ± 1.4d | 0.76 ± 2.7d | 0.48 ± 2.4e |
| Co (58.922) | 0.21 ± 0.4e | 0.39 ± 2.5de | 0.72 ± 3.5bc | 1.71 ± 3.0a | 1.95 ± 0.7a | 1.02 ± 0.9b | 0.69 ± 0.3c | 0.59 ± 1.7cd |
| Cu (63.546) | 0.45 ± 1.0d | 0.51 ± 1.5d | 1.68 ± 4.1b | 1.79 ± 2.8b | 2.73 ± 1.0a | 2.71 ± 0.1a | 0.70 ± 1.0c | 0.51 ± 1.3d |
| Hg (200.59) | 4.32 ± 0.3a | 4.81 ± 0.7b | 3.77 ± 2.5c | 2.81 ± 1.9d | 1.85 ± 2.5e | 1.35 ± 1.1f | 0.83 ± 0.6 g | 0.80 ± 1.2g |
| Ni (58.693) | 2.11 ± 1.4a | 2.21 ± 0.7a | 1.57 ± 0.1b | 1.09 ± 4.2c | 1.02 ± 3.8c | 0.70 ± 2.2d | 0.31 ± 0.5e | 0.07 ± 0.4f |
| Pb (207.2) | 2.26 ± 2.6d | 2.34 ± 1.3d | 3.72 ± 1.0b | 3.84 ± 3.2b | 4.36 ± 0.2a | 2.87 ± 2.2c | 1.37 ± 1.1e | 0.99 ± 1.0e |
| Zn (65.723) | 0.52 ± 3.1f | 1.45 ± 2.0e | 2.29 ± 2.4b | 2.62 ± 2.3a | 2.03 ± 2.3c | 1.72 ± 3.0d | 0.81 ± 1.0f | 0.70 ± 0.1f |
Results are expressed as mean ± SD. Mean values that do not share a letter within a row are significantly different (p < 0.05)
MM Molecular weight
Table 3.
Variation in rate of adsorption of heavy metals by 0.5 g feathers in 100 mL FMBs that contains 5 ppm (0.005 mg/mL) of each heavy metal under different media pH
| Heavy metals (initial concentrations 0.005 mg/mL) | Amount of heavy metal adsorbed (in mg/mL) by 5% (w/v) of feather per 100 mL of FMB adjusted to different pH | |||||||
|---|---|---|---|---|---|---|---|---|
| 4.0 | 5.0 | 6.0 | 7.0 | 8.0 | 9.0 | 10.0 | 11.0 | |
| Ag | 0.000 ± 0.0 | 0.001 ± 1.2 | 0.0017 ± 0.1 | 0.0026 ± 0.0 | 0.0032 ± 0.1 | 0.0024 ± 0.0 | 0.0013 ± 0.0 | 0.000 ± 0.0 |
| As | 0.000 ± 0.0 | 0.000 ± 0.0 | 0.0020 ± 0.0 | 0.0041 ± 0.0 | 0.0050 ± 0.1 | 0.0026 ± 1.2 | 0.0020 ± 1.1 | 0.001 ± 0.1 |
| Cd | 0.001 ± 1.4 | 0.0031 ± 0.2 | 0.0048 ± 1.2 | 0.0041 ± 0.0 | 0.0034 ± 0.0 | 0.0026 ± 0.1 | 0.0010 ± 0.0 | 0.000 ± 0.0 |
| Co | 0.0010 ± 0.0 | 0.0029 ± 0.5 | 0.0032 ± 1.0 | 0.0044 ± 0.0 | 0.0050 ± 0.0 | 0.0032 ± 0.0 | 0.0013 ± 0.2 | 0.000 ± 0.0 |
| Cu | 0.000 ± 0.0 | 0.0010 ± 0.0 | 0.0011 ± 0.1 | 0.0021 ± 0.2 | 0.0048 ± 1.0 | 0.0043 ± 0.0 | 0.0027 ± 0.1 | 0.0012 ± 0.0 |
| Hg | 0.0041 ± 0.1 | 0.005 ± 0.0 | 0.0037 ± 0.2 | 0.0028 ± 0.0 | 0.0023 ± 0.1 | 0.0018 ± 0.1 | 0.000 ± 0.0 | 0.000 ± 0.0 |
| Ni | 0.0034 ± 1.1 | 0.0043 ± 0.1 | 0.0038 ± 0.0 | 0.0031 ± 0.2 | 0.0023 ± 0.3 | 0.0018 ± 0.0 | 0.0001 ± 0.0 | 0.000 ± 0.0 |
| Pb | 0.0012 ± 0.1 | 0.0023 ± 0.0 | 0.0026 ± 0.0 | 0.0039 ± 0.2 | 0.0046 ± 0.0 | 0.0039 ± 1.3 | 0.0023 ± 0.0 | 0.0018 ± 0.0 |
| Zn | 0.0019 ± 0.1 | 0.0027 ± 0.0 | 0.0039 ± 0.4 | 0.0041 ± 1.0 | 0.0034 ± 0.3 | 0.0021 ± 0.0 | 0.0013 ± 0.1 | 0.0010 ± 0.0 |
Better absorption of Ag, Cd, Hg and Pb at their respective pH as shown in Table 2 may be due to their relatively high molecular weight compared with the others. A similar observation has been previously reported by Kar and Misra (2004). Although the mechanism of absorption in this study is not known, it was documented that heavy metal absorption by keratin-containing materials, such as feathers, may occur either by physiosorption, chemiosorption, or their combination (Kar and Misra 2004). While the former occurs through the trapping of metal ions into networks of porous fibres in the feather, the latter occurs by the binding of heavy metal ions to carboxylic-binding sites of the keratin-containing materials.
Degradation of HMPFs by immobilised cells
Approximately 10% (w/v) of dried HMPFs containing different concentrations of heavy metals were used as the source of substrate in the preparation of FMB. Approximately 250 beads of immobilised AQ05-001 were then introduced into 100 mL of FMB, and their ability to degrade the HMPFs was determined after 24 h of incubation. A control flask was setup containing 10% (w/v) HMFFs in 100 mL of FMB pH 8 and was inoculated with 250 beads of immobilised AQ05-001. The results obtained clearly indicated that immobilised AQ05-001 effectively degraded HMPFs completely to fragile fibres in most of the flasks, while HMPFs in FMB inoculated with a control strain remained intact after 36 h of incubation except in flasks containing HMFFs. Similarly, there was a high rise in keratinase activity in FMB containing HMPFs at varying concentrations compared with that in FMB containing HMFFs as shown in Table 4.
Table 4.
Keratinase activities and biodegradation of HMPF and HMFF containing different concentrations of heavy metals by immobilised cells of Alcaligenes sp. AQ05-001
| HMPFs type | Concentrations of heavy metals (ppm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 15 | 20 | 25 | 30 | ||||||||
| KA (U/mL) | FD (%) | KA (U/mL) | FD (%) | KA (U/mL) | FD (%) | KA (U/mL) | FD (%) | KA (U/mL) | FD (%) | KA (U/mL) | FD (%) | KA (U/mL) | FD (%) | |
| Hg | 98.6 ± 0.4c | 100 ± 0a | 96.9 ± 1.4e | 98 ± 0.2a | 15.4 ± 1.4g | 14.7 ± 3.1f | 1.9 ± 2.4h | 0 ± 0f | 0.1 ± 0h | 0 ± 0e | 0 ± 0d | 0 ± 0e | 0 ± 0e | 0 ± 0c |
| Zn | 101.3 ± 1.1c | 100 ± 0a | 100 ± 0e | 98 ± 0.4a | 33.1 ± 0.8f | 43.2 ± 1.5e | 11.4 ± 0.1g | 7 ± 0e | 0.4 ± 0h | 0 ± 0e | 0 ± 0d | 0 ± 0e | 0 ± 0e | 0 ± 0c |
| Pb | 100.3 ± 1.7c | 92 ± 0.6b | 72.8 ± 1.2f | 93 ± 1.8a | 29.3 ± 2.1f | 21 ± 0.9d | 17.6 ± 0.8f | 15 ± 0d | 9.5 ± 0.5g | 10 ± 0d | 2.4 ± 1.4d | 0 ± 0e | 0 ± 0e | 0 ± 0c |
| Ni | 103.1 ± 1.9c | 99 ± 0.1a | 67.3 ± 2.2 g | 78 ± 2.3b | 55.6 ± 0.5e | 56 ± 2.3c | 22.5 ± 1.2e | 32 ± 2.6c | 0 ± 0h | 10 ± 0d | 0 ± 0d | 0 ± 0e | 0 ± 0e | 0 ± 0c |
| As | 118.4 ± 0.5b | 99 ± 0.1a | 108 ± 1.8d | 97 ± 1.3a | 73.9 ± 0.8d | 81 ± 1.4b | 71.4 ± 2.5d | 78.6 ± 1.8b | 34.1 ± 2.1f | 17 ± 0.3c | 0.5 ± 0.1d | 0 ± 0e | 100 ± 0a | 0 ± 0c |
| Cd | 116.3 ± 0.8b | 100 ± 0a | 119 ± 0bc | 100 ± 0a | 112.7 ± 1.1c | 100 ± 0a | 103.9 ± 2.3c | 98.5 ± 1.7a | 43.2 ± 0.9e | 76 ± 0.4b | 53.2 ± 1.2c | 10 ± 0d | 0 ± 0e | 0 ± 0c |
| Co | 120.4 ± 0.4ab | 100 ± 0a | 119 ± 0bc | 100 ± 0a | 121.7 ± 1.0ab | 100 ± 0a | 120.2 ± 0.4a | 100 ± 0a | 114.3 ± 2.3c | 100 ± 0a | 90.6 ± 1.8b | 94 ± 1.2c | 90.4 ± 1.6c | 91 ± 0c |
| Cu | 119.1 ± 3.1ab | 100 ± 0a | 121 ± 1.3b | 100 ± 0a | 117.9 ± 0.8bc | 100 ± 0a | 115.5 ± 0.9b | 100 ± 0a | 83.1 ± 1.1d | 96 ± 1.0a | 54.3 ± 0.8c | 71.9 ± b | 49.3 ± 2.3d | 54 ± 1.2b |
| Ag | 122.7 ± 2.4a | 100 ± 0a | 129 ± 0.7a | `100 ± 0a | 123.8 ± 0.4a | 100 ± 0a | 116.9 ± 0.3b | 100 ± 0a | 121.3 ± 1.4a | 100 ± 0a | 118.4 ± 0.6a | 100 ± a | 108.8 ± 3.2b | 97 ± 1.1a |
| Control (HMFF) | 117.1 ± 0.4ab | 98.2 ± 1.3a | 117.3 ± 0.7c | 99.6 ± 0.5a | 117.6 ± 0.5bc | 100 ± 0.0a | 117.5 ± 0.8ab | 100 ± 0.0a | 117.9 ± 1.3b | 97.6 ± 1.1a | 117.6 ± 1.3a | 98.6 ± .08a | 118.2 ± 0.8a | 98.7 ± 0.8a |
Results are expressed as mean ± SD. Mean values that do not share a letter within a column are significantly different (p < 0.05)
KA Keratinase activities, FD feather degradation, ppm part per million
The FDA of the immobilised bacterium toward different HMPFs depends on the metal type and concentration. Ag, Co and Cu are highly tolerated (H) by the immobilised cells at a 5 ppm concentration. However, others including highly toxic Hg and Pb were rated T at 5 ppm each. Factors such as the nature and type of microbe, site and pollution level at the site of isolation were indicated as possible reasons for the varying level of resistance of different bacteria within the same species or genera to different heavy metals (Hassen et al. 1998). Although the strains of Alcaligenes are known to resist heavy metals and multiple antibiotics (Silver and Phung 1996), the strain is strange in the field of feather degradation and keratinase production.
Above 5 ppm, the immobilised cells could not tolerate the pollution stress of Hg and Pb, and a drastic drop in the tolerance level to A was recorded. By contrast, the immobilised AQ05-001 better tolerated Ag and Co pollution at concentrations of 20–30 ppm, with a feather degradation rate above 90%. An increase in the concentration of Cd and As from 10 to 15 ppm did not yield any significant difference in the FDA and KA by the immobilised cells. Our finding regarding the tolerance level of the bacterium to multiple metals, such as Ag, Co, Cu, Hg and Cd, are in line with previous finding where Gram-negative bacteria, such as Alcaligenes sp. AQ05-001, were reported to tolerate heavy metals more than Gram-positive bacteria (Lima de Silva et al. 2012).
Repeated use of immobilised AQ05-001 in the degradation of HMPFs
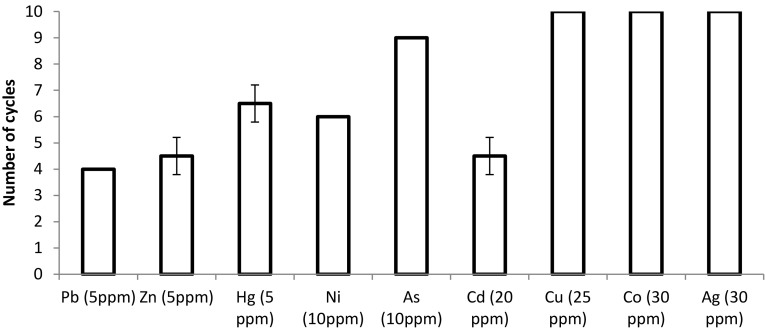
To investigate the ability of immobilised AQ05-001 in the continuous production of keratinase using HMPFs as a substrate, 250 beads were used in batch culture repeatedly with each cycle taking 24 h. The result showed that immobilised AQ05-001 could degrade more than 90% of HMPFs in most FMB for several cycles (Fig. 6). The rate of degradation of feathers laden with 5–30 ppm Ag, Co and Cu fluctuated between 91% and 98% in the first six cycles after which a slight decline to 78% was observed from eight to ten cycles. Achieving high FDA at early cycles indicates that immobilised cells adapted faster to the toxic environment. By contrast, in the presence of Pb, Zn and Hg, FDA was slower at the first and second cycles (54%) and then increased to 74–80% at the 4th and 6th cycles, suggesting that the immobilised cells were unstable at the initial stage of the incubation. From previous studies, the continuous use of plain immobilised gel or immobilised dead cells in the removal of heavy metals from aqueous solution will require desorption with mineral acid to prevent the beads from being saturated (Rani et al. 2010). However, in this study, immobilised AQ05-001 was used continuously for up to ten cycles in some cases to produce keratinase from feathers polluted with 20–30 ppm of Ag, Co and Cu without the need for desorption.
Fig. 6.
Repeated use of immobilised AQ05-001 in the degradation of 10% (w/v) of feathers laden with varying concentration of heavy metals. Data are the means ± standard deviations of three independent replicates
It is noteworthy that immobilised cells that failed to degrade HMPFs containing a higher concentration of heavy metals, such as Hg and Pb, were used in the degradation of feathers laden with other heavy metals for several cycles without desorption. However, this finding indicates that, at a higher concentration, the metal only affects the keratinolytic process temporarily and that the active participation of the immobilised cells in metal adsorption is minimal (Pandey et al. 2007). Immobilised cells of Synechococcus were used for only six cycles to adsorb Pb in a column that is lower than that with this bacterium (Korkmaz et al. 2004). The stability of beads in FMBs containing most types of HMPFs remained intact throughout the reuse experiment except for FMB containing feathers laden with Cd, Zn and Pb, where a decrease in the size of beads after the 4th cycle was noticed.
The strain may have possessed multiple resistances to many heavy metals because of changes that might have occurred in its physiological or molecular characteristics over a long time in the heavy metal-contaminated site where it was isolated (Lima de Silva et al. 2012). The multiple high-level heavy metal resistances by this strain were relatively similar to that reported in Alcaligenes eutrophus CH34, which is resistant to high levels of Cd, Co, Cr, Cu, Hg, Ni, Ti and Zn. Multiple resistances of strain CH34 to Co, Cd, Ni and Zn have been related to its ability to harbour two mega plasmids (pMOL28 and pMOL30) that carry the cnr operon and czc operons, respectively, which function based on an efflux mechanism (Collard et al. 1994).
Continuous dissolution of feathers polluted with certain heavy metals for up to ten cycles with a resultant yield of a high concentration of keratinase as observed in this study may be of industrial importance. The direct usefulness of the hydrolysates produced following the degradation of such HMPFs cannot be ascertained presently because the heavy metal remnant in the protein hydrolysates was not determined. However, through scale-up, the large tonnes of HMPFs generated when used to adsorb heavy metals from water can serve as a source of industrially valuable keratinase, especially when immobilised AQ05-001 is used.
Degradation of feathers polluted with mixture of heavy metals
Experiments were performed on two, three, four and five different combinations of heavy metals. The combination was based on the relative closeness of optimum sorption pH and tolerance level of individual heavy metals to immobilised cells. Immobilised AQ05-001 was then used to degrade feathers polluted with a mixture of heavy metals at different concentrations in batches, and aliquots from the resulting hydrolysates were analysed for keratinase activity (Table 5).
Table 5.
Average degradation of feathers laden with combination of heavy metals and number of times the beads were reused
| Heavy metal combinations | Concentration of each (ppm) | Average feather degradation (%) | Number of cycles | Tolerance level |
|---|---|---|---|---|
| As, Cd | 10 | 87 ± 1.5a | 7 | T |
| As, Cd | 15 | 72 ± 3.2b | 4 | T |
| Ag, Co, Cu | 10 | 100 ± 0a | 8 | H |
| Ag, Co, Cu | 20 | 100 ± 0a | 8 | H |
| Ag, Co, Cu | 25 | 55 ± 9.3b | 3 | T |
| Hg, Ni, Pb, Zn | 1 | 54 ± 2.9a | 6 | T |
| Hg, Ni, Pb, Zn | 5 | 32 ± 3.3b | 4 | A |
| As, Ag, Cd, Co, Cu | 5 | 95 ± 2.5 | 5 | H |
Results of feather degradation were expressed as mean ± SD. Mean values of similar heavy metal combinations that do not share the same superscript letter in the column of “average feather degradation” are significantly different (p < 0.05)
The results showed that immobilised AQ05-001 effectively degraded feathers polluted with different concentrations of the combinations of As/Cd, Ag/Co/Cu and As/Ag/Cd/Co/Cu with an average reduction in the feather mass from 87 to 100%. It can be inferred from these results that the immobilised cells still maintain their FDA and keratinolytic activity in the presence of high heavy metal pollution. Additionally, some immobilised cells were reused in a continuous batch for several cycles. Feathers polluted with 10 and 20 ppm each of Ag, Co and Cu were completely degraded in a continuous batch up to eight cycles without desorption. However, when the concentration was increased to 30 ppm each, making a total of 90 ppm, there was a drastic reduction in feather degradation to 55% even when the incubation period was extended to 36 h, but the beads were still used for three cycles to achieve an average of 40% degradation.
Conclusion
The ability of immobilised Alcaligenes sp. AQ05-001 to utilise feathers laden with different types and concentrations of a single heavy metals or a mixture of heavy metals, to produce a high amount of keratinase despite their cell or enzyme inhibition properties, indicates its potential usage in the production of an industrially important keratinase enzyme similar to that using unpolluted feathers.
Acknowledgements
This project was supported by a fund from The Ministry of Science, Technology and Innovation (MOSTI), Malaysia, under FRGS (02-02-13-1256FR) and Science Fund (02-01-04-SF1473SF); and from Universiti Putra Malaysia under Putra Grant (9601500).
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
References
- Adinarayana K, Jyothi B, Ellaiah P. Production of alkaline protease with immobilised cells of Bacillus subtilis PE-11 in various matrices by entrapment technique. AAPS Pharm Sci Tech. 2005;6:E391–E397. doi: 10.1208/pt060348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad SA, Shamaan NA, Arif NM, Koon GB, Shukor MY, Syed MA. Enhanced phenol degradation by immobilised Acinetobacter sp. strain AQ5NOL 1. World J Microbiol Biotechnol. 2012;28:347–352. doi: 10.1007/s11274-011-0826-z. [DOI] [PubMed] [Google Scholar]
- Al-Asheh S, Banat F, Al-Rousan D. Beneficial reuse of chicken feathers in removal of heavy metals from wastewater. J Clean Prod. 2003;11:321–326. doi: 10.1016/S0959-6526(02)00045-8. [DOI] [Google Scholar]
- Al-Musallam AA, Al-Ghrabally DH, Vadakkancheril N. Biodegradation of keratin in mineral-based feather medium by thermophilic strains of a new Coprinopsis sp. Intl Biodeter Biodegr. 2013;79:42–48. doi: 10.1016/j.ibiod.2012.11.011. [DOI] [Google Scholar]
- Bibi Z, Shahid F, Ul Qader SA, Aman A. Agar-agar entrapment increases the stability of endo-β-1,4-xylanase for repeated biodegradation of xylan. Intl J Biol Macromol. 2015;75:121–127. doi: 10.1016/j.ijbiomac.2014.12.051. [DOI] [PubMed] [Google Scholar]
- Collard JM, Corbisier P, Diels L, Dong Q, Jeanthon C, Mergeay M, Taghavi S, van der Lelle D, Wilmotte A, Wuertz S. Plasmids for heavy metal resistance in Alcaligenes eutrophus CH34: mechanisms and applications. FEMS Microbiol Rev. 1994;14:405–414. doi: 10.1111/j.1574-6976.1994.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Eslahi N, Dadashian F, Nejad NH. An investigation on keratin extraction from wool and feather waste by enzymatic hydrolysis. Prep Biochem Biotechnol. 2013;43:624–648. doi: 10.1080/10826068.2013.763826. [DOI] [PubMed] [Google Scholar]
- Fakhfakh-Zouari N, Haddar A, Hmidet N, Frikha F, Nasri M. Application of statistical experimental design for optimization of keratinases production by Bacillus pumilus A1 grown on chicken feather and some biochemical properties. Process Biochem. 2010;45:617–626. doi: 10.1016/j.procbio.2009.12.007. [DOI] [Google Scholar]
- Gavrilescu M. Removal of heavy metals from the environment by biosorption. Eng Life Sci. 2004;4:219–232. doi: 10.1002/elsc.200420026. [DOI] [Google Scholar]
- Guo H, Luo S, Chen L, Xiao X, Xi Q, Wei W, Zeng G, Liu C, Wan Y, Chen J, He J. Bioremediation of heavy metals by growing hyperaccumulaor endophytic bacterium Bacillus sp. L14. Bioresour Technol. 2010;101:8599–8605. doi: 10.1016/j.biortech.2010.06.085. [DOI] [PubMed] [Google Scholar]
- Hassen A, Saidi N, Cherif M, Boudabous A. Resistance of environmental bacteria to heavy metals. Bioresour Technol. 1998;64:7–15. doi: 10.1016/S0960-8524(97)00161-2. [DOI] [Google Scholar]
- Ibrahim S, Shukor MY, Syed MA, Johari WLW, Shamaan NA, Sabullah MK, Ahmad SA. Enhanced caffeine degradation by immobilised cells of Lefsonia sp. strain SIU. J Gen Appl Microbiol. 2016;62:18–24. doi: 10.2323/jgam.62.18. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Park KH, Oh DJ, Hwang DY, Kim HS, Lee CY, Son HJ. Keratinolytic enzyme-mediated biodegradation of recalcitrant feather by a newly isolated Xanthomonas sp. P5. Polym Degrad Stabil. 2010;95:1969–1977. doi: 10.1016/j.polymdegradstab.2010.07.020. [DOI] [Google Scholar]
- Joshi SG, Tejashwini MM, Revati N, sridevi R, Roma D. Isolation, identification and characterization of a feather degrading bacterium. Intl J Poult Sci. 2007;6:689–693. doi: 10.3923/ijps.2007.689.693. [DOI] [Google Scholar]
- Kar P, Misra M. Use of keratin fibre for separation of heavy metals from water. J Chem Technol Biotechnol. 2004;79:1313–1319. doi: 10.1002/jctb.1132. [DOI] [Google Scholar]
- Karamba KI, Ahmad SA, Zulkharnain A, Yasid NA, Khalid A, Shukor MY. Biodegradation of cyanide and evaluation of kinetic models by immobilized cells of Serratia marcescens strain AQ07. Int J Environ Sci Tech. 2017;14:1945–1958. doi: 10.1007/s13762-017-1287-1. [DOI] [Google Scholar]
- Korkmaz H, Hür H, Dincer S. Characterization of alkaline keratinase of Bacillus licheniformis strain HK-1 from poultry waste. Ann Microbiol. 2004;54:201–211. [Google Scholar]
- de LimaSilva AA, de Carvalho MAR, de Souza SAL, Dias PMT, da Silva Filho RG, de Meirelles Saramago CS, de Bento M, Hofer CAE. Heavy metal tolerance (Cr, Ag and Hg) in bacteria isolated from sewage. Braz J Microbiol. 2012;43:1620–1631. doi: 10.1590/S1517-83822012000400047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazotto AM, Couri S, Damaso MCT, Vermelho AB. Degradation of feather waste by Aspergillus niger keratinases: comparison of submerged and solid-state fermentation. Intl Biodeter Biodegr. 2013;85:189–195. doi: 10.1016/j.ibiod.2013.07.003. [DOI] [Google Scholar]
- Mohanty SS, Jena HM. Biodegradation of phenol by free and immobilized cells of a novel Pseudomonas sp. NBM11. Braz J Chem Engin. 2017;34:75–84. doi: 10.1590/0104-6632.20170341s20150388. [DOI] [Google Scholar]
- Moslemy P, Neufeld RJ, Guiot SR. Biodegradation of gasoline by gellan gum-encapsulated bacterial cells. Biotech Bioeng. 2002;80:175–184. doi: 10.1002/bit.10358. [DOI] [PubMed] [Google Scholar]
- Moslemy P, Neufeld RJ, Millette D, Guiot SR. Transport of gellan gum microbeads through sand: an experimental evaluation for encapsulated cell bioaugmentation. J Environ Manag. 2003;69:249–259. doi: 10.1016/j.jenvman.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ng SP, Polombo EA, Bhave M. The heavy metal tolerant soil bacterium Achromobacter sp. AO22 contains a unique copper homeostasis locus and two mer operons. J Microbiol Biotechnol. 2012;22:742–753. doi: 10.4014/jmb.1111.11042. [DOI] [PubMed] [Google Scholar]
- Pandey A, Bera D, Shukla A, Ray L. Studies on Cr (VI), Pb (II) and Cu (II) adsorption–desorption using calcium alginate as biopolymer. Chem Speciat Bioavail. 2007;19:17–24. doi: 10.3184/095422907X198031. [DOI] [Google Scholar]
- Parrado J, Rodriguez-Morgado B, Tejada M, Hernandez T, Garcia C. Proteomic analysis of enzyme production by Bacillus licheniformis using different feather wastes as the sole fermentation media. Enzyme Microb Technol. 2014;57:1–7. doi: 10.1016/j.enzmictec.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Pires C, Marques AP, Guerreiro A, Magan N, Castro PM. Removal of heavy metals using different polymer matrixes as support for bacterial immobilization. J Hazard Mater. 2011;191:277–286. doi: 10.1016/j.jhazmat.2011.04.079. [DOI] [PubMed] [Google Scholar]
- Prakash P, Jayalakshmi SK, Sreeramulu K. Production of keratinase by free and immobilised cells of Bacillus halodurans strain PPKS-2: partial characterization and its application in feather degradation and dehairing of the goat skin. Appl Biochem Biotechnol. 2010;160:1909–1920. doi: 10.1007/s12010-009-8702-0. [DOI] [PubMed] [Google Scholar]
- Rani MJ, Hemambika B, Hemapriya J, Kannan VR. Comparative assessment of heavy metal removal by immobilised and dead bacterial cells: a biosorption approach. Afr J Environ Sci Technol. 2010;4:77–83. [Google Scholar]
- Shrinivas D, Kumar R, Naik GR. Enhanced production of alkaline thermostable keratinolytic protease from calcium alginate immobilized cells of thermo alkalophilic Bacillus halodurans JB 99 exhibiting dehairing activity. J Ind Microbiol Biotech. 2012;39:93–98. doi: 10.1007/s10295-011-1003-y. [DOI] [PubMed] [Google Scholar]
- Silver S, Phung LT. Bacterial heavy metal resistance: new surprises. Ann Rev Microbiol. 1996;50:753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- Taskin M, Sisman T, Erdal S, Kurbanoglu EB. Use of waste chicken feathers as peptone for production of carotenoids in submerged culture of Rhodotorula glutinis MT-5. Eur Food Res Technol. 2011;233:657–665. doi: 10.1007/s00217-011-1561-2. [DOI] [Google Scholar]
- Tork S, Aly MM, Nawar L. Biochemical and molecular characterization of a new local keratinase producing Pseudomomanas sp., MS21. Asian J Biotechnol. 2010;2:1–13. doi: 10.3923/ajbkr.2010.1.13. [DOI] [Google Scholar]
- Wilson NG, Bradley G. A study of a bacterial immobilization substratum for use in the bioremediation of crude oil in a saltwater system. J Appl Microbiol. 1997;83:524–530. doi: 10.1046/j.1365-2672.1997.00352.x. [DOI] [Google Scholar]
- Yusuf I, Shukor M, Syed M, Yee P, Shamaan NA, Ahmad SA. Investigation of keratinase activity and feather degradation ability of immobilised Bacillus sp. khayat in the presence of heavy metals in a semi continuous. J Chem Pharm Sci. 2015;8:342–347. [Google Scholar]
- Yusuf I, Ahmad SA, Phang LY, Syed MA, Shamaan NA, Abdul Khalil K, Dahalan F, Shukor MY. Keratinase production and biodegradation of polluted secondary chicken feather wastes by a newly isolated multi heavy metal tolerant bacterium-Alcaligenes sp. AQ05-001. J Environ Manag. 2016;183:182–195. doi: 10.1016/j.jenvman.2016.08.059. [DOI] [PubMed] [Google Scholar]