Abstract
Essential oils (EOs) obtained from aerial parts of Pogostemon deccanensis were analyzed for GC–MS profiling, and evaluated for antioxidant, anti-inflammatory, and anti-proliferative activities. GC–MS analysis revealed a total of 47 constituents, establishing the EOs rich in sesquiterpene with > 20 sesquiterpenes constituting around 77% of the total EO yield. Major constituents included Curzerene (Benzofuran, 6-ethenyl-4,5,6,7-tetrahydro-3,6-dimethyl-5-isopropenyl-, trans-) (26.39%) and epi-Cadinol (22.68%), Ethanone, 1-(2,4,6-trihydroxyphenyl) (6.83%, Acetophenones), and Boldenone (3.47%, anabolic steroid). EOs found to be rich in phytochemicals attributed for antioxidant potentials of aromatic/medicinal plants, viz., flavonoids (2.71 µg quercetin equivalents g−1 EO), total phenols (3.94 µg gallic acid equivalents (GAE) g−1 EO), carotenoids (14.3 µg β-carotene equivalents g−1 EO), and ascorbic acid (2.21 µg ascorbic acid equivalents g−1 EO). P. deccanensis EOs exhibited striking antioxidant activities assessed by wide range of assays including ferric reducing antioxidant potential (FRAP, 255.3 GAE at 2 µg mL−1 EO), total antioxidant activity (TAA, 264.3 GAE at 2 µg ml−1) of EO, DPPH (65% inhibition at 2 µg mL−1), and OH (58% inhibition at 2 µg mL−1) scavenging. Interestingly, EOs showed considerably higher anti-lipid peroxidation activity than the standard antioxidant molecule ascorbic acid, with 50% protection by 1.29 µg mL−1 EO against 20.0 µg mL−1 standard. EOs showed strong anti-inflammatory activity with 50% inhibition at 1.95 µg mL−1 EO. The anti-proliferative activity of EOs was tested against mouse cancer cell line and the EOs proved a potent anti-proliferative agent with only 2.1% cell survival at 2 µg mL−1 EO, whereas the EOs were largely non-toxic-to-normal (non-cancerous) cells with approximately 80% cell survival at the 2 µg mL−1 EOs. This being the first attempt of phytochemical profiling and wide array of biological activities of P. deccanensis EOs holds significance as the striking activities were observed at very low concentrations, in some cases at lower than the commercial standards, and has, therefore, great potential for pharmaceutical or commercial exploration.
Keywords: Antioxidants, Anti-inflammatory activity, Anti-proliferative activity, Cytotoxicity, Essential oils
Introduction
Aromatic plants are traditionally used for culinary and medicinal purposes. According to the International Organization of Standardization (ISO), an essential oil (EO) is a volatile product, extracted from aromatic plants, mostly by steam distillation process (da Silva Ramos et al. 2017). This concentrated mixture of volatile aroma compounds principally contains mono-/ses-quiterpenoids, benzoids, phenylpropenoids, etc., and interacts differentially with the other plants and animals, including humans (Buchbauer 2010). As a complex mixture of spectrum of natural compounds with versatile organic structures, EOs are known for their striking medicinal properties (Mohammadhosseini and Nekoei 2014). Along with their therapeutic applications, EOs are also heavily used in cosmetic products as well as food-flavoring agents (Adorjan and Buchbauer 2010). Noteworthy potencies of EOs include anti-septic, anti-inflammatory, anti-microbial, anti-fungal, and antioxidant (Burt 2004; Bakkali et al. 2008; Tajkarimi et al. 2010; Amorati et al. 2013).
The antioxidants are important molecules, and provide protection against the oxidation of essential cellular elements during the stress or disease condition (Kumar et al. 2013a, b). These compounds possess the capability to slow down or impede the free-radical mediated oxidation of an oxidizable material, used at the low concentrations (< 1%) with respect to the volume of material to protect (Amorati et al. 2013). The commercial need of the antioxidants’ supply is often fulfilled by synthetic antioxidants such as butylhydroxytoluene (BHT) or butylated hydroxyanisol (BHA); however, they are assumed to be possibly injurious to human health (Lanigan et al. 2002; EFSA 2012). The exploration of non-toxic, natural antioxidants has led to the abundant studies on antioxidant potency of EOs in recent years (Amorati et al. 2013). The connotations of diverse phyto-compounds present in EOs with differential antioxidant potential deliver overall greater antioxidant activity than that of the individual components (Marin et al. 2016). The abilities of EOs in diffusing across the microbial cell membrane combining with their antioxidant properties make them plausible replacement for synthetic antibiotics, as well. The EO constituents such as thymol, carvone, carvacrol, caryophyllene, and limonene often get attributed for these properties (Okoh et al. 2016).
The Lamiaceae family is one of the most promising plant families with capabilities to synthesize EOs, and hence, it plays vital role in various economic zones (e.g., cosmetics, pharmaceuticals, perfumery and food, etc.). Members from this family have been associated with medicinally important activities such as antiflu, antiemetic, carminative, insecticidal, anti-bacterial, anti-parasitic activities, as well as their use in treatment against fever, headache, and burns (da Silva Ramos et al. 2017). Numerous members from the different genus of Lamiaceae have been investigated for their EOs, including Thymus zygis, Thymus mastichina (Ballester-Costa et al. 2017), Thymus vulgaris (Bozin et al. 2006; Ballester-Costa et al. 2017), Thymus pectinatus (Vardar-Unlu et al. 2003), Ocimum basilicum, Origanum vulgare (Bozin et al. 2006), Satureja thymbra, S. parnassica (Fitsiou et al. 2016), Monarda didyma (Ricci et al. 2017), Clinopodium gilliesii (Barbieri et al. 2016), Mentha pulegium, and M. rotundifolia (Brahmi et al. 2016).
The genus Pogostemon (Lamiaceae) is scattered in Asia, Africa, and Australia, and South and South-east Asian regions account for higher diversity and endemism in this genus. Globally, the genus has been documented with 97 species from which 54 species are existent in India (Murugan and Livingstone 2010a). Amongst most of the discovered species, P. cablin is heavily explored for its EO; typically referred as patchouli EO (Suganya et al. 2015). Patchouli EO is FDA-approved substance as natural food additive which is industrially important due to its characteristic pleasant and long-lasting woody, earthy, and camphoraceous odor (FDA 2002, Deguerry et al. 2006; Donelian et al. 2009). Other species of the genus including P. plectranthoides, P. heyneanus, and P. benghalensis are also considered as a source of patchouli EO (Suganya et al. 2015; Murugan and Livingstone 2010b).
P. deccanensis (formerly Eusteralis deccanensis) is a plant with phytochemical potential possessing patchoulenechemotype (Thoppil 2000). P. deccanensis is a small marshy herb with reduced spine-like leaves, showing dark violet inflorescence. The plant is documented in Western Ghats of the southern Indian region (Shinoj et al. 2016). Though there is a serious dearth of investigations aimed at its phytochemical exploration; though a previous GC analysis of the volatile oil from this plant revealed the presence of sesquiterpene alcohol (delta-cadinol), two oxygenated monoterpenes (geranyl acetate and neryl acetate) and three sesquiterpenes (beta-caryophyllene, beta-bourbonene, and beta-elemene) (Thoppil 2000).
There are some reports on the composition and phytochemical potential of the essential oils from genus Pogostemon, especially on P. cablin (Dechayont et al. 2017; Wei and Shibamoto 2007). But till now, there is no information available on antioxidant activities and phytochemical constituents of EO from P. deccanensis. The current work was, therefore, first attempt aimed to chemically and biologically investigate the EO isolated from P. deccanensis including (i) phytochemical profiling of the EO by GC–MS, (ii) their antioxidant potentials using various standard methods, (iii) their anti-inflammatory activities, and (iv) anti-proliferative activities against cancerous cells.
Materials and methods
Plant material and isolation of essential oils
The plant material from P. deccanensis growing in forest region of Bhandardara, Maharashtra, India, was collected, and samples were authenticated at Anantrao Pawar College, Pune (Specimen Voucher No. APCP/32/2017-18). The aerial parts (stem, leaves, and flowers) were air dried at room temperature supplemented with ventilation. Completely dried samples (500 g) were then subjected to hydro-distillation using Clevenger-type apparatus. The isolated EO was then stored at 4 °C in sealed glass vials.
Chromatographic analysis (GC–MS) of essential oils
The chromatographic separation and subsequent analysis of the separated components of EO was achieved by gas chromatography–mass spectrometry (GC–MS) using 7890B GC system Agilent Technologies coupled with Agilent 5977A Mass Selective Detector (MSD) system. The capillary column (GsBP-5MS, General Separation Technologies, Newark, DE) with dimensions 30 m × 0.32 mm id × 0.25 µm film thickness was employed with Helium as a carrier gas (flow rate: 1.4 ml per min). For the total run time, the temperature program was set initially at 60 °C for 1 min, followed by heating at the rate of 3 °C per min to 246 °C. The Mass Selective Detector was equipped with splitless injector (at 75 eV, 250 °C). Quantitative estimation of the isolated components was performed using percentage peak area integrated by the analysis program, and the identification of individual compounds was done by equating the retention time (RT) and fragmentation pattern with the spectral data obtained from NIST 2011 and Wiley-10th edition mass spectral libraries and literature survey.
Quantification of antioxidants and assessing antioxidant potentials of essential oils
The known quantity of the isolated EO was dissolved in 0.5% dimethyl sulphoxide (DMSO) and the dissolved EO mixture was used for estimation of total flavonoid content, total phenols, total ascorbic content, and total carotenoid content using spectrophotometric assays (Shimadzu UV-1800, Japan).
Total flavonoid content
Total flavonoid content was measured according to Marinova et al. (2005); briefly, the EO (4 µL) was mixed with distilled water to make up the volume as 2 mL, followed by addition of 0.3 mL of 0.5% NaNO2 and 10% AlCl3. After 6 min incubation, 2 mL of NaOH was added. The absorbance was recorded at 510 nm and total flavonoid content was determined as µg quercetin equivalents g−1 EO.
Total phenols
Estimation of total phenols was done using Folin–Ciocalteu (FC) method (Gul et al. 2011). Briefly, 2 ml distilled water containing 4 µL of EO mixture was allowed reacting with 0.5 mL of FC reagent. After 3 min incubation at room temperature, 2 mL Na2CO3 was added in the reaction followed by incubation in boiling water bath for 1 min. The absorbance was recorded at 765 nm once the mixture cooled. Standard Gallic acid curved was used to quantify total phenols, expressed as µg Gallic acid equivalents g−1 (GAE) EO.
Total carotenoid content
To the EO mixture, equal volume of 10% methanolic KOH and diethyl ether was added to isolate carotenoids. The reaction mixture was then washed with 5% ice cold saline and then dried on anhydrous Na2SO4 for 2 h. The filtrate was collected and the absorbance was measured at 450 nm. The concentration of carotenoids was determined from standard β-carotene graph and expressed in µg β-carotene equivalents g−1 EO (Jensen 1978).
Total ascorbic acid
Total ascorbic acid content was determined using 2, 4-dinitrophenylhydrazine (DNPH) method as described by Kapur et al. (2012). Briefly, 2 mL of acidic DNPH reagent and 1 drop of 10% thiourea was added to EO mixture, and the mixture was incubated in boiling water bat for 15 min. After cooling, 5 µL of 80% (v/v) sulfuric acid was added to mixture at 0 °C on ice bath. The absorbance was then recorded at 521 nm and ascorbic acid was estimated using standard ascorbic acid curve.
Free-radical scavenging screening
Varying concentrations of EO (0.5, 1.0, 1.5, and 2.0 µg ml−1) in DMSO were prepared and antioxidant activity was estimated using FRAP, total antioxidant activity (TAA), DPPH radical scavenging activity, hydroxyl radical scavenging activity, and ABTS radical scavenging assays.
Ferric reducing antioxidant power (FRAP)
The antioxidant capacity of EO was measured spectrophotometrically by the FRAP method (Benzie and Strain 1999) which involves the reduction of TPTZ (Fe3+-tetra (2-pyridyl) pyrazine) complex to Fe2+-tetrepyridyltriazine by the electron-donating compounds present in EO at low pH. The acidic TPTZ reagent-containing 20 mM FeCl3.6H2O was prepared in 300 mM acetate buffer. To 1 mL of this reagent, 500 µL of various essential oil concentration as well as standard was added and incubated at 37 °C for half an hour. The absorbance of these samples was measured at 593 nm and the reducing potential was expressed in GAE.
Total antioxidant activity (TAA)
The TAA of EO was measured as reduction of Mo (VI) to Mo (V) by EO, which results in the formation of green-color phosphate/ Mo (V) in acidic conditions (Prieto et al. 1999). Various concentrations of EO were allowed to react with 1 mL of phosphomolybdenum reagent. The mixture was then incubated in boiling water bath at for 90 min. After samples attaining the room temperature, the absorbance was measured at 695 nm and TAA was expressed in terms of GAE.
DPPH radical scavenging activity
The scavenging effect of EO on stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals was performed as mentioned by Braca et al. (2002). The methanolic solution of DPPH (0.05 mM) was added to various EO concentrations and incubated for 5 min. The absorbance of the mixture was then measured at 517 nm. The scavenging potential was expressed using the formula:
Percent inhibition of DPPH radical = [(absorbance control – absorbance test)/absorbance control] × 100.
Hydroxyl radical scavenging activity
The scavenging potential of EO against hydroxyl radicals generated by Fenton’s reaction is assessed as per the method by Kunchandy and Rao (1990). The reaction mixture was prepared by adding deoxyribose (2.8 mM in KH2PO4–KOH buffer, pH 7.4), FeCl3 (0.1 mM), EDTA (0.1 mM), Ascorbic acid (0.1 mM), H2O2 (1 mM), and various EO concentrations. The reaction mixture was incubated at 37 °C for 1 h and degradation of deoxyribose was measured using TBA (thiobarbituric acid) assay by adding 1 mL of each TBA (1%) and TCA (trichloroacetic acid, 2.8%) with incubation at 100 °C for 20 min. The absorbance of the reaction mixture was recorded at 532 nm.
Determination of inhibition of lipid peroxidation
The anti-lipid peroxidation potential of EO was evaluated using the method given by Halliwell and Gutteridge (1989) with slight modifications. The freshly prepared goat liver homogenate (10% (w/v), in cold phosphate buffer saline, pH 7.4) was filtered to get clear solution. Three milliliters of this filtered homogenate was then allowed to react with different concentrations of EO and lipid peroxidation was initiated by adding 15 mM FeSO4 (100 µL). After 30 min incubation, 100 µL of this mixture was added to 1.5 mL TCA (10%). TBA (0.67% in 50% acetic acid, 1.5 mL) was added after 10 min and the reaction was allowed for 30 min in boiling water bath. The intensity of development of orange–pink complex was recorded at 535 nm. The results were expressed by the percent inhibition of lipid peroxidation.
Anti-inflammatory activity
Anti-inflammatory in vitro activity was evaluated using HRBC membrane stabilization method (Emamuzo et al. 2010). Blood sample collected from healthy volunteers was mixed with equal volume of sterilized Alsevers solution and centrifuged at 3000 rpm to separate packed cell volume (PCV). The separated PCV was washed with iso-saline solution and a 10% (v/v) suspension was prepared in iso-saline. Different EO concentrations were separately mixed with 1 mL of phosphate buffer, 2 mL of hypo-saline, and 0.5 mL of HRBC suspension. The assay mixtures were incubated at 37 °C for 30 min and centrifuged at 3000 rpm. The isolated hemoglobin content was estimated by a spectrophotometrically (560 nm). The percentage hemolysis was estimated by assuming the hemolysis produced in the control as 100%, which was calculated as: percentage protection = 100 − (ODsample/ODcontrol) × 100.
Anti-proliferative activity against cancer cells
The extracted EO was tested for anti-proliferative activity/cytotoxicity against mouse cancer cell line, B16F1 (procured from National Centre for Cell Science, Pune, India), as described previously by Costa-Lotufo et al. (2005). The cell line was maintained as monolayer in Minimal Essential Medium (MEM) supplemented with heat-inactivated Fetal Bovine Serum (FBS, 10%), penicillin (10 U mL−1), and streptomycin (100 µg mL−1). Cells were seeded into 96-well plate (5000 cells per well) and allowed proliferate and adhere overnight at 37 °C in 5% CO2 incubator. The following day, proliferated cells were treated with varied concentrations (0, 0.25, 0.5, and 2 µg mL−1) of EO 24 h and further incubated for 48 h in the absence of EO. The survival rate of the cells was assessed using MTT assay by decanting the utilized medium and adding 50 µL of MTT solution (1 mg mL−1, in MEM without phenol red). After 4 h of incubation at 37 °C, the formed formazan crystals were solubilized in 50 µl of isopropanol and absorbance was recorded at 570 nm using 630 nm as reference filter. Untreated cells were considered as 100% survival for determination of survival rate of the cells in the presence of various concentrations of EO.
Cytotoxicity against normal non-cancerous cells
The normal human peripheral blood mononuclear cells (PBMCs) were isolated from whole blood obtained from healthy volunteers for cytotoxicity studies of EO against non-cancerous cells (Jenny et al. 2011). Blood sample (heparinized) was diluted (1:1) with sterile phosphate buffer saline (PBS) supplemented with fetal bovine serum (2%) and PBMCs were isolated using ficoll density gradient (HiSep™ LSM 1077, Himedia, India). The isolated PBMCs were subsequently washed with PBS supplemented with fetal bovine serum (5%) and used for cytotoxicity analysis. The isolated PBMCs were seeded in 96-well plate (106 cells per well) and allowed to proliferate for 24 h in medium (RPMI-1640, supplemented with 20% fetal bovine serum, 2 mM glutamine) with or without concentrations of EO (0.125, 0.25, 0.5, 1.0, and 2.0 µg ml−1). The 0% viability control (negative control) was set using 0.5% Triton X-100, whereas phytohemagglutinin (PHA) (5 µg ml−1)-stimulated cells served as positive control. Cells were incubated at 37 °C in 5% CO2. After completion of incubation period, cells were allowed to react with MTT (10 µl, prepared as 10 mg ml−1 in PBS). After 4 h of incubation at 37 °C, each well was added with 50 µl of DMSO for the solubilization of formazan crystal and absorbance was recorded at 570 nm using 630 nm as reference filter. Untreated cells were considered as 100% survival for the determination of survival rate of the cells in the presence of various concentrations of EO.
Statistical analyses
The results are presented as means of three replicates ± standard error. The means were compared using Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05 using MSTAT-C statistical software. The graphs were plotted using Microcal Origin 6.0 software.
Results and discussion
Chemical composition of essential oils
The EO yield from the aerial parts of P. deccanensis was 0.3%. The chemical composition along with the respective percentage in the EO is represented in Table 1. The GC–MS profiling resulted in the identification of 47 different constituents representing 98.71% of the oil. The extracted EO was rich in sesquiterpene as out of total identified components more than 20 components belongs to the class of sesquiterpenes which accounts approximately 77% of the total EO yield. The most predominant components include curzerene (benzofuran, 6-ethenyl-4,5,6,7-tetrahydro-3,6-dimethyl-5-isopropenyl-, trans-) (26.39%), a sesquiterpene, and epi-cadinol (22.68%) which represents sesquiterpenoid alcohol. Certain other sesquiterpenes include α-Caryophyllene (0.79%), β-Caryophyllene (3.17%), α-Cubebene (1.29%), β-Elemene (3.06%), Valencene (1.04%), γ-Cadinene (3.53%), α-Guaiene (2.95%), etc. The EO also showed the presence of some monoterpenes including α-Terpinyl acetate, Terpinolene; (−)-Camphor (bicyclic monoterpene ketone); Thymol (monoterpene phenol); (−)-α-Terpineol (monoterpene alcohol). However, compared to the portion of sesquiterpenes, monoterpenes shared much lesser proportion of the total EO. Furthermore, very less amounts of Eugenol (phenylpropenes), Aspidinol (phloroglucinol), and β–Linalool (terpene alcohol) were detected. Other detected components include Ethanone, 1-(2,4,6-trihydroxyphenyl) (6.83%, Acetophenones), Boldenone (3.47%, anabolic steroid), and (−)-Spathulenol (2.52%, tricyclic sesquiterpene alcohol). To the best of our knowledge, this is the first report of the phytochemical profiling of the EO from the aerial parts of P. deccanensis.
Table 1.
GC–MS-based phytochemical profiling of essential oils from Pogostemon deccanensis
| Peak | R. T. (min) | Compound | (%) |
|---|---|---|---|
| 1 | 4.848 | α-Terpinyl acetate | 0.68 |
| 2 | 5.213 | Cyclooctene, 1,2-dimethyl- | 0.04 |
| 3 | 5.339 | Terpinolene | 0.09 |
| 4 | 5.552 | β-Linalool | 0.29 |
| 5 | 5.711 | (−)-Camphor | 0.12 |
| 6 | 6.086 | 4-Terpineol | 0.03 |
| 7 | 6.211 | Thymol | 0.03 |
| 8 | 3.307 | (−)-α-Terpineol | 0.03 |
| 9 | 6.372 | Methyl salicylate | 0.10 |
| 10 | 6.512 | 4,7-Dimethylbenzofuran | 0.05 |
| 11 | 7.207 | 4,7,7-Trimethylbicyclo[4.1.0]heptan-3-one | 0.18 |
| 12 | 7.611 | Cyclohexene, 2-ethenyl-1,3,3-trimethyl- | 0.03 |
| 13 | 7.891 | Edulan I, dihydro- | 0.03 |
| 14 | 8.382 | Ascaridole epoxide | 0.04 |
| 15 | 8.742 | Elixene | 0.12 |
| 16 | 8.885 | α-Cubebene | 1.29 |
| 17 | 8.918 | Eugenol | 0.20 |
| 18 | 9.427 | Copaene | 0.57 |
| 19 | 9.670 | Valencene | 1.04 |
| 20 | 9.759 | β-Cubebene | 0.47 |
| 21 | 9.948 | Benzoic acid, 2,4,6-trimethoxy- | 0.28 |
| 22 | 10.279 | β-Caryophyllene | 3.17 |
| 23 | 10.378 | Bergamotol, Z-à-trans- | 0.56 |
| 24 | 10.474 | β-Elemene | 3.06 |
| 25 | 11.612 | α-Caryophyllene | 0.79 |
| 26 | 11.055 | Alloaromadendrene | 0.67 |
| 27 | 11.434 | α-Cubebene | 1.36 |
| 28 | 11.832 | Benzofuran, 6-ethenyl-4,5,6,7-tetrahydro-3,6-dimethyl-5-isopropenyl-, trans- | 26.39 |
| 29 | 11.972 | Ledene oxide-(II) | 0.44 |
| 30 | 12.110 | γ-Cadinene | 3.53 |
| 31 | 12.244 | α-Guaiene | 2.95 |
| 32 | 12.512 | β-Guaiene | 0.23 |
| 33 | 12.677 | α-Muurolene | 0.56 |
| 34 | 12.830 | Elemol | 0.39 |
| 35 | 12.923 | γ-Elemene | 0.75 |
| 36 | 13.381 | (−)-Spathulenol | 2.52 |
| 37 | 13.434 | Caryophyllene oxide | 2.17 |
| 38 | 13.886 | 7-Oxabicyclo[4.1.0]heptane, 2,2,6-trimethyl-1-(3-methyl-1,3-butadienyl | 2.99 |
| 39 | 14.113 | Cubenol | 3.63 |
| 40 | 14.334 | α-Guaiene | 1.11 |
| 41 | 14.808 | Epi-cadinol | 22.68 |
| 42 | 14.949 | Murolan-3,9(11)-diene-10-peroxy | 0.83 |
| 43 | 15.468 | Isoaromadendrene epoxide | 0.59 |
| 44 | 16.054 | Boldenone | 3.47 |
| 45 | 16.340 | Ethanone, 1-(2,4,6-trihydroxyphenyl)- | 6.83 |
| 46 | 17.131 | 3-Oxo-androsta-1,4-dien-17á-spiro-2′-3′-oxo-oxetane | 0.30 |
| 47 | 18.672 | Aspidinol | 1.05 |
| Total identified | 98.71 | ||
Earlier, volatile-oil GC analysis of this plant revealed the presence of δ-cadinol (sesquiterpene alcohol), geranyl acetate, neryl acetate (monoterpenoids), β-caryophyllene, β-bourbonene, and β-elemene (sesquiterpenes) (Thoppil 2000). Our results also confirmed the presence of some of the components including cadinol, caryophyllene, and elemene. Suganya et al. (2015) reported the phytochemical composition of EO from P. plectranthoides, and comparison of their findings with the present results confirms the presence of many common entities between these two species. Some prominent ones include linalool, terpineol, cubebene, elemene, caryophyllene, humulene, alloaromadendrene, cadinene, spathulenol, and cadinol. Some other members from the family Lamiaceae have also shown similar phytochemical nature of their respective EOs. Leaves of two Algerian Lamiaceae plants, Rosmarinus tournefortii and Callosobruchus maculatus, have been screened for their EO composition (Menaceur et al. 2016). The investigation confirms the presence of terpineol, copaene, caryophyllene, humulene, and cadinene in the EOs from the mentioned plants; which are also reported by us. Mamadalieva et al. (2017) have screened EOs from three Uzbek Scutellaria species (Lamiaceae) (Scutellaria immaculate, S. ramosissima, and S. schachristanica) for their composition and antioxidant activities. The resulted chemical constituents share similar phytochemical entities with our findings.
Collectively, the GC–MS profiling confirms the large portion of sesquiterpenes along with the presence of monoterpenes in EO of P. deccanensis. Chemical compounds from the family of sesquiterpenes (C15) are well known from their antioxidant properties (Gonzalez-Burgos and Gomez-Serranillos 2012). Correspondingly, high radical scavenging is also attributed to the amount of monoterpenes (Smeriglio et al. 2017). Hence, the phytochemical profile of the EO can be positively correlated with high antioxidant and radical scavenging potentials.
Quantification of antioxidants
Antioxidants are the elements proficient in competing with the other oxidizable substrates at moderately low amount and delay/prevent the oxidation of these substrates (Bayala et al. 2014). The presence of phenolics, ascorbic acid, carotenoids, and flavonoids, which are known strong antioxidants, accomplish the resilient antioxidant status of the EO. The quantified data for the aggregates of antioxidants from EO of P. deccanensis are presented in Table 2. The majority of the potent antioxidants are usually with at least one aromatic ring and hydroxyl group, such as phenols and flavonoids. The analysis showed 3.94 ± 0.10 µg GAE g−1 EO of total phenols and 2.71 ± 0.09 µg QE g−1 EO of flavonoids. The analysis also showed the presence of 14.28 ± 0.54 µg BCE g−1 EO of carotenoids as well as 2.21 ± 0.11 µg AAE g−1 EO of ascorbic acid. In the mixture of these complex chemical entities, it is predicted that an antioxidant arrangement is produced that may act by numerous mechanisms providing an effective defense machinery for free-radical scavenging (da Silva Ramos et al. 2017). Plants with higher polyphenols content exhibit the higher antioxidant potential (Kumar et al. 2013a, b). Hence, the antioxidant quantitation positively correlates with higher antioxidant potentials of P. deccanensis EOs.
Table 2.
Flavonoids, total phenols, carotenoids, and ascorbic acid contents of EO from P. deccanensis
| Phytochemicals | Amount |
|---|---|
| Flavonoids (µg QE g−1 EO) | 2.71 ± 0.09 |
| Total phenols (µg GAE g−1 EO) | 3.94 ± 0.10 |
| Carotenoids (µg BCE g−1 EO) | 14.3 ± 0.54 |
| Ascorbic acid (µg AAE g−1 EO) | 2.21 ± 0.11 |
Values are expressed as average of three replicates ± standard error (SE)
QE quercetin equivalents, GAE gallic acid equivalents, BCE β-carotene equivalents, AAE ascorbic acid equivalents
Free-radical scavenging screening
Generation of reactive oxygen species (ROS) is an indication of unfavorable conditions (e.g., stress, infection, or nutrient deficiency) for the organism. The ultimate consequence of the higher degree of ROS generation is high cellular oxidative damage. Therefore, antioxidants are usually considered as crucial players in maintaining organism’s good health due to their enormous potential to overcome oxidative injuries as well as to modulate biological pathways and membrane functionality (Smeriglio et al. 2017). As plant-based natural antioxidants are preferred over their synthetic counterparts due to their less/no side effects, research in the stream of antioxidants intensely advocates the exploration of phyto-molecules (Kumar et al. 2013a, b). Hence, the present study was aimed to decipher the radical scavenging potential of the EOs from P. deccanensis. The complexity and diversity of the antioxidant mechanisms urges the multi-methodic assessment for determination and understanding of overall antioxidant capability (Marin et al. 2016). Thus, the screening of free-radical scavenging was performed via FRAP, TAA, DPPH radical scavenging activity, and hydroxyl radical scavenging activity assays.
The results of FRAP and TAA are summarized in Table 3. Highest FRAP and TAA activities were observed at concentration of 2.0 µg mL−1 of EO (252.28 ± 1.89 and 264.33 ± 2.91 GAE, respectively). Concentration-dependent increase in activity was observed in both the scavenging assays. However, the extent of increase in the activity with concentration was higher in case of TAA as compared to FRAP activity; as at highest concentration of EO (2.0 µg ml−1), 1.2-fold increment in FRAP activity was observable compared to 2.25-fold increment in TAA activity at the same concentration of EO.
Table 3.
Ferric reducing antioxidant potential (FRAP) and total antioxidant activity (TAA) of EO from Pogostemon deccanensis
| EO (µg ml−1) | FRAP of EO (GAE) | TAA of EO (GAE) |
|---|---|---|
| 0.5 | 205.3 ± 0.35a | 117.8 ± 1.29a |
| 1.0 | 225.7 ± 1.72b | 160.9 ± 1.96b |
| 1.5 | 247.6 ± 1.43c | 254.0 ± 1.93c |
| 2.0 | 255.3 ± 1.89d | 264.3 ± 2.91d |
Values are expressed as average of three replicates ± Standard Error (SE). The mean within the column followed by different superscript letters indicated significant difference according to Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05
GAE gallic acid equivalents
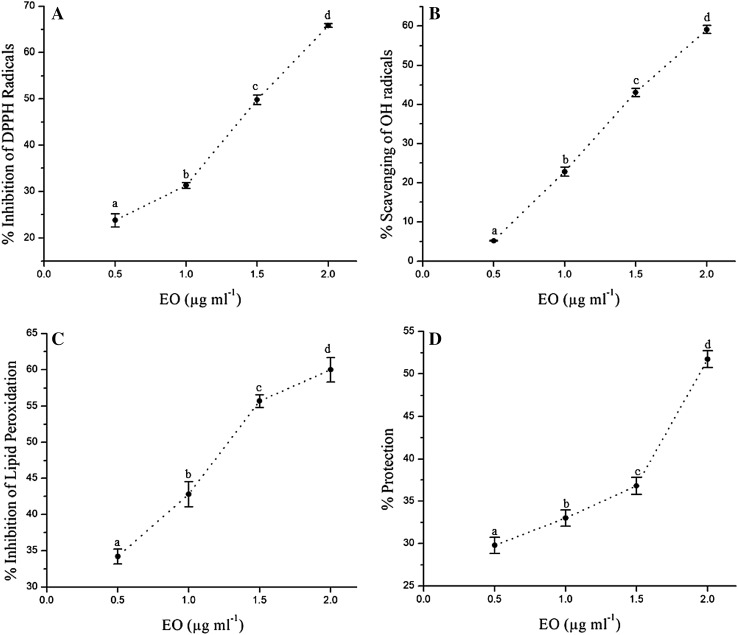
Along with FRAP and TAA, radical scavenging was also assessed in terms of DPPH and OH˙ scavenging (Fig. 1). Pronounced DPPH radical scavenging potential of EO was observed as even 0.5 µg mL−1 concentration was also able to depict 23.79% inhibition. The highest DPPH scavenging was observed at 2 µg mL−1 EO with 65.8% inhibition. The similar trend was observed in case of OH˙ scavenging activity, where higher EO concentration showed more OH˙ scavenging (59.1%). The EO showed notably higher DPPH and OH˙ scavenging potentials than the standard antioxidant molecule (ascorbic acid) verified with similar concentration of EO (IC50 of EO and ascorbic acid: 1.50 µg mL−1 and 21.05 µg mL−1, respectively, for DPPH scavenging; and 1.72 µg mL−1 and 31.50 µg mL−1, respectively, for OH˙ scavenging). The antioxidant potential of EO from Lamiaceae and non-Lamiaceae members have been studied widely, including Thymus zygis, T. mastichina, T. capitatus, T. vulgaris (Ballester-Costa et al. 2017), Salvia hypoleuca (Habib et al. 2016), Dennettia tripetala (Okoh et al. 2016), Curcuma angustifolia (Jena et al. 2017), etc. According to the eco-geographical status and stage of maturity, the qualitative and quantitative variation in components of the EO has been reported leading to the differential antioxidant capacities. Curzerene, a major sesquiterpenoid, is considered as an excellent antioxidant, predominant in Curcuma species (Zhao et al. 2010). On the other hand, many experiments have proved the active participation of some phytochemicals including caryophyllene, elemene, and cadinolin higher antioxidant status (Zhang et al. 2017; Wang et al. 2017a). Therefore, a stronger antioxidants potency and radical scavenging rate of the P. deccanensis EO can be ascribed to the presence of curzerene, caryophyllene, elemene along with small fractions of known antioxidants such as thymol and eugenol, as well as higher phenolics.
Fig. 1.
a DPPH radical scavenging activity. b Hydroxyl radical scavenging activity. c Inhibition of lipid peroxidation and d in vitro anti-inflammatory activity of EO from P. deccanensis. Each value represents the mean of three replications ± Standard Error (SE). Each point on the line with different letters shows significant difference at P ≤ 0.05 according to Duncan’s Multiple Range test
Anti-lipid peroxidation and anti-inflammatory activities
There are chances that, if an EO is a strong radical-scavenger, it could also possess anti-inflammatory properties as one of the responses of inflammatory pathways is oxidative burst occurring in various kinds of cells (Migueal 2010). The present study uses the HRBC method for evaluation of in vitro anti-inflammatory activity due to analogs nature of RBC membrane to lysosomal membrane. Total percent inhibition indicates the level of anti-inflammation by EO, which was observed to be gradually increasing in accordance with EO concentration. 1.95 µg mL−1 EO induced 50% inhibition (Fig. 1). Several studies have highlighted the β-caryophyllene and β-caryophyllene oxide as possessing chemo-preventive properties, including anti-inflammatory effects (Di Giacomo et al. 2017). Both the compounds are present in the EO isolated from P. deccanensis. On the other hand, prevention of lipid peroxidation was measured using liver homogenate. The IC50 values for EO and standard antioxidant (ascorbic acid) differ drastically with EO showing ~ 15 times less required concentration for 50% protection (IC50 of EO: 1.29 µg ml−1 and that of the ascorbic acid: 20.0 µg ml−1). Patil et al. (2010) have also evaluated the anti-lipid peroxidation potential of EO from Ageratum conyzoides using the similar approach, where they found a considerably higher activity of EO to prevent lipid peroxidation. The presence of terpinen-4-ol, linalool, and thymol in EO have been correlated to the higher antioxidant activity in five EO-bearing spice plants; evaluated through thiobarbituric acid reactive species method (Viuda-Martos et al. 2010). Caryophyllene is also proved to be possessing good lipid peroxidation prevention capacity (Patil et al. 2010). Hence, we may confirm the high lipid peroxidation prevention potential positively may be attributed to the presence of the mentioned phyto-entities in EO of P. deccanensis.
Anti-proliferative activity against cancerous cells
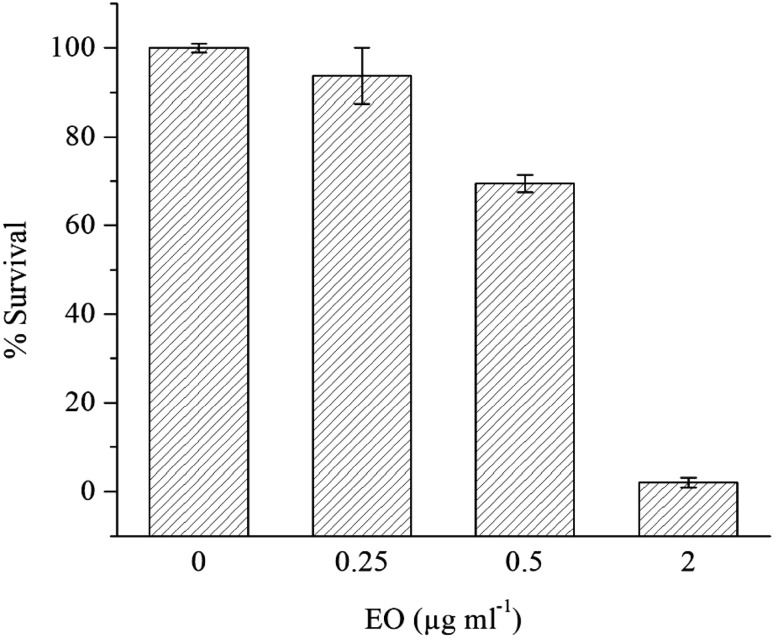
The isolated EO was assayed for its anti-proliferative potential against mouse cancer cell line (B16F1; adherent mouse-skin melanoma cell line) to scientifically validate the traditional claims of this plant being anti-cancerous. Lower concentration of EO (0.25 µg mL−1) was less lethal as survival rate of the plated cells was 93.7%, considering the 100% survival in the untreated cells. The EO at 2 µg mL−1 concentration showed highest anti-proliferative activity against B16F1 cells as the survival rate observed was only 2.1% (Fig. 2). Curzerene, which is present in high amounts in EO, has been reported for anti-proliferative activities against SPC-A1 human lung carcinoma (IC50: 47 µM L−1) (Wang et al. 2017b). Besides, EO also showed the presence of α-caryophyllene (0.79%) as well as β-caryophyllene (3.17%). Surprisingly, Legault and Pichette (2007) have proven the potentiating effect of β-caryophyllene on anti-cancer activity of α-humulene which is also known as α-caryophyllene. Furthermore, β-caryophyllene and β-caryophyllene oxide are also known for their anti-proliferative chemo-preventive nature (Di Giacomo et al. 2017). Therefore, the strong anti-proliferative properties of the isolated EO can be clearly attributed to the predominance of discussed anti-proliferative/anti-cancer agents in the EO of P. deccanensis.
Fig. 2.
Anti-proliferative activity of EO from P. deccanensis against cancerous cells (B16F1). Each bar represents the mean of three replications ± Standard Error (SE). Each bar value shows significant difference at P ≤ 0.05 according to Duncan’s Multiple Range test
Cytotoxicity of essential oils against non-cancerous cells
The non-cytotoxic nature of the plant products is an essential requirement for therapeutic applications in treating cancer (Langhasova et al. 2014). The EOs from plants including Aloysia citriodora (Oukerrou et al. 2017), Annona vepretorum (Bomfim et al. 2016), and Myrica rubra (Langhasova et al. 2014) have displayed anti-proliferative potentials against cancerous cell lines, and, at the same time, have unveiled their non-toxic nature against normal non-cancerous cells. The current evaluation of P. deccanensis EO has also shown the similar anti-cancer potential. The cytotoxicity studies of P. deccanensis EO revealed non-toxic nature against normal human cells (PBMCs). High cell viability was recorded under all the assessed concentrations (0.125, 0.25, 0.5, 1.0, 2.0 µg mL−1) of EO, with 79.97% viability at the highest EO concentration (2 µg mL−1) (Table 4).
Table 4.
Cytotoxic activity of EO from Pogostemon deccanensis against normal human peripheral blood mononuclear cells (PBMCs)
| EO (µg ml−1) | Cell survival (% of control) |
|---|---|
| 0.000 | 100.00 ± 0.0f |
| 0.125 | 96.77 ± 0.13e |
| 0.250 | 92.47 ± 0.27d |
| 0.500 | 84.81 ± 0.19c |
| 1.000 | 81.85 ± 0.31b |
| 2.000 | 79.97 ± 0.29a |
Values are expressed as average of three replicates ± Standard Error (SE). The mean within the column followed by different superscript letters indicated significant difference according to Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05
Conclusion
The present study was first attempt to characterize the phytochemical constituents of essential oils extracted from P. deccanensis besides determining their antioxidant, anti-inflammatory, and anti-proliferative activities using the standard methods. It can be concluded from the results that P. deccanensis essential oil is a rich source of antioxidants; possesses potent antioxidant, anti-inflammatory, and anti-proliferative activities; has the potential to be investigated further and used for pharmaceutical purposes. The non-cytotoxic nature of the EOs holds significance for their further therapeutic explorations.
Acknowledgements
The authors would like to acknowledge the use of facilities created under FIST program of Department of Science and Technology (DST), Government of India and Star Status from the Department of Biotechnology (DBT), Government of India to Modern College, Ganeshkhind, Pune.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Adorjan B, Buchbauer G. Biological properties of essential oils: an updated review. Flavour Fragr J. 2010;25:407–426. doi: 10.1002/ffj.2024. [DOI] [Google Scholar]
- Amorati R, Foti MC, Valgimigli L. Antioxidant activity of essential oils. J Agric Food Chem. 2013;61:10835–10847. doi: 10.1021/jf403496k. [DOI] [PubMed] [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Ballester-costa C, Sendra E, Viuda-martos M. Assessment of antioxidant and antibacterial properties on meat homogenates of essential oils obtained from four Thymus species achieved from organic growth. Foods. 2017;6:59. doi: 10.3390/foods6080059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri N, Costamagna M, Gilabert M, Perotti M, Schuff C, Isla MI, Benavente A. Antioxidant activity and chemical composition of essential oils of three aromatic plants from La Rioja province. Pharm Biol. 2016;54:168–173. doi: 10.3109/13880209.2015.1028077. [DOI] [PubMed] [Google Scholar]
- Bayala B, Bassole IH, Gnoula C, Nebie R, Yonli A, Morel L, Figueredo G, Nikiema JB, Lobaccaro JM, Simpore J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS One. 2014;9:e92122. doi: 10.1371/journal.pone.0092122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/S0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- Bomfim LM, Menezes LR, Rodrigues AC, Dias RB, Gurgel Rocha CA, Soares MB, Neto AF, Nascimento MP, Campos AF, Silva LC, Costa EV, Bezerra DP. Antitumour activity of the micro encapsulation of Annona vepretorum essential oil. Basic Clin Pharmacol Toxicol. 2016;118:208–213. doi: 10.1111/bcpt.12488. [DOI] [PubMed] [Google Scholar]
- Bozin B, Mimica-Dukic N, Simin N, Anackov G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem. 2006;54:1822–1828. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002;79:379–381. doi: 10.1016/S0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Brahmi F, Abdenour A, Bruno M, Silvia P, Alessandra P, Danilo F, Drifa YG, Fahmi EM, Khodir M, Mohamed C. Chemical composition and in vitro antimicrobial, insecticidal and antioxidant activities of the essential oils of Mentha pulegium L. and Mentha rotundifolia (L.) Huds growing in Algeria. Ind Crops Prod. 2016;88:96–105. doi: 10.1016/j.indcrop.2016.03.002. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Costa-Lotufo LV, Khan MT, Ather A, Wilke DV, Jimenez PC, Pessoa C, de Moraes ME, de Moraes MO. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J Ethnopharmacol. 2005;99:21–30. doi: 10.1016/j.jep.2005.01.041. [DOI] [PubMed] [Google Scholar]
- da Silva Ramos R, Rodrigues ABL, Farias ALF, Simoes RC, Pinheiro MT, dos Anjos Ferreira RM, Barbosa LMC, Souto RNP, Fernandes JB, da Silva Santos L, da Silva de Almeida SSM Chemical composition and in vitro antioxidant, cytotoxic, antimicrobial, and larvicidal activities of the essential oil of Mentha piperita L. (Lamiaceae) Sci World J. 2017 doi: 10.1155/2017/4927214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechayont B, Ruamdee P, Poonnaimuang S, Mokmued K, Chunthorng-Orn J. Antioxidant and antimicrobial activities of Pogostemon cablin (Blanco) Benth. J Bot. 2017;2017:1–6. doi: 10.1155/2017/8310275. [DOI] [Google Scholar]
- Deguerry F, Pastore L, Wu S, Clark A, Chappell J, Schalk M. The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch Biochem Biophys. 2006;454:123–136. doi: 10.1016/j.abb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Di Giacomo S, Di Sotto A, Mazzanti G, Wink M. Chemosensitizing properties of β-caryophyllene and β-caryophyllene oxide in combination with doxorubicin in human cancer cells. Anticancer Res. 2017;37:1191–1196. doi: 10.21873/anticanres.11433. [DOI] [PubMed] [Google Scholar]
- Donelian A, Carlson LHC, Lopes TJ, Machado RAF. Comparison of extraction of patchouli (Pogostemon cablin) essential oil with supercritical CO2 and by steam distillation. J Supercrit Fluids. 2009;48:15–20. doi: 10.1016/j.supflu.2008.09.020. [DOI] [Google Scholar]
- EFSA ANS Scientific Opinion on the re-evaluation of butylated hydroxytoluene BHT (E 321) as a food additive. EFSA J. 2012;10:2588. doi: 10.2903/j.efsa.2012.2588. [DOI] [Google Scholar]
- Emamuzo ED, Miniakiri SI, Tedwin EJ, Ufouma O, Lucky M. Analgesic and anti-inflammatory activities of the ethanol extract of the leaves of Helianthus Annus in Wistar rats. Asian Pac J Trop Med. 2010;3:341–347. doi: 10.1016/S1995-7645(10)60083-1. [DOI] [Google Scholar]
- Federal Regulations Code (2002) Food and drugs administration, from the US Government Printing Office via GPO Access [CITE: 21CFR172. 510] USA 3:49–52
- Fitsiou E, Anestopoulos I, Chlichlia K, Galanis A, Kourkoutas I, Panayiotidis MI, Pappa A. Antioxidant and antiproliferative properties of the essential oils of Satureja thymbra and Satureja parnassica and their major constituents. Anticancer Res. 2016;36:5757–5763. doi: 10.21873/anticanres.11159. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos E, Gomez-Serranillos MP. Terpene compounds in nature: a review of their potential antioxidant activity. Curr Med Chem. 2012;19:5319–41. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- Gul MZ, Bhakshu LM, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA. Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using in vitro assays. BMC Complement Altern Med. 2011;11:64. doi: 10.1186/1472-6882-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib KD. Chemical composition and antioxidant activity of essential oil from Salvia hypoleuca at different growth stages. Nusant Biosci. 2016;8:145–149. doi: 10.13057/nusbiosci/n080203. [DOI] [Google Scholar]
- Halliwell B, Gutteridge JMC. Protection against lipid peroxidation, in free radicals in biology and medicine. Tokyo: Japan Scientific Societies Press; 1989. [Google Scholar]
- Jena S, Ray A, Banerjee A, Sahoo A, Nasim N, Sahoo S, Kar B, Patnaik J, Panda PC, Nayak S. Chemical composition and antioxidant activity of essential oil from leaves and rhizomes of Curcuma angustifolia Roxb. Nat Prod Res. 2017;31:2188–2191. doi: 10.1080/14786419.2017.1278600. [DOI] [PubMed] [Google Scholar]
- Jenny M, Klieber M, Zaknun D, Schroecksnadel S, Kurz K, Ledochowski M, Schennach H, Fuchs D. inflammatory properties of compounds employing peripheral blood mononuclear cells freshly isolated from healthy donors. Inflamm Res. 2011;60:127–135. doi: 10.1007/s00011-010-0244-y. [DOI] [PubMed] [Google Scholar]
- Jensen A. In: Chlorophyll and carotenoids, in handbook of physiochemical and biochemical methods. Hallebust JA, Craigie JS, editors. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- Kapur A, Hasković A, Čopra-Janićijević A, Klepo L, Topčagić A, Tahirović I, Sofić E. Spectrophotometric analysis of total ascorbic acid contetnt in various fruits and vegetables. Bull Chem Technol Bosnia Herzegovina. 2012;38:39–42. [Google Scholar]
- Kumar V, Lemos M, Sharma M, Shriram V. Antioxidant and DNA damage protecting activities of Eulophia nuda Lindl. Free Rad Antiox. 2013;3:55–60. doi: 10.1016/j.fra.2013.07.001. [DOI] [Google Scholar]
- Kumar V, Sharma M, Lemos M, Shriram V. Efficacy of Helicteres isora L. against free radicals, lipid peroxidation, protein oxidation and DNA damage. J Pharm Res. 2013;6:620–625. doi: 10.1016/j.jopr.2013.05.017. [DOI] [Google Scholar]
- Kunchandy E, Rao MNA. Oxygen radical scavenging activity of curcumin. Int J Pharm. 1990;58:237–240. doi: 10.1016/0378-5173(90)90201-E. [DOI] [Google Scholar]
- Langhasova L, Hanusova V, Rezek J, Stohanslova B, Ambroz M, Kralova V, Vanek T, Lou JD, Yun ZL, Yang J, Skalova L. Essential oil from Myrica rubra leaves inhibits cancer cell proliferation and induces apoptosis in several human intestinal lines. Ind Crops Prod. 2014;59:20–26. doi: 10.1016/j.indcrop.2014.04.018. [DOI] [Google Scholar]
- Lanigan RS, Yamarik TA. Final report on the safety assessment of BHT(1) Int J Toxicol. 2002;2:19–94. doi: 10.1080/10915810290096513. [DOI] [PubMed] [Google Scholar]
- Legault J, Pichette A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol. 2007;59:1643–1647. doi: 10.1211/jpp.59.12.0005. [DOI] [PubMed] [Google Scholar]
- Mamadalieva NZ, Sharopov F, Satyal P, Azimova SS, Wink M. Composition of the essential oils of three Uzbek Scutellaria species (Lamiaceae) and their antioxidant activities. Nat Prod Res. 2017;31:1172–1176. doi: 10.1080/14786419.2016.1222383. [DOI] [PubMed] [Google Scholar]
- Marín I, Sayas-Barberá E, Viuda-Martos M, Navarro C, Sendra E. Chemical composition, antioxidant and antimicrobial activity of essential oils from organic fennel, parsley, and lavender from Spain. Foods. 2016;5:18. doi: 10.3390/foods5010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in bulgarian fruits and vegetables. J Univ Chem Technol Metall. 2005;40:255–260. [Google Scholar]
- Menaceur F, Hazzit M, Mouhouche F, Mohammedi H, Baaliouamer A, Benchabane A. Phytochemical screening and biological activities of essential oils from leaves of two algerian Lamiaceae plants on Callosobruchus maculatus (Fabricius, 1775) J Essent Oil-Bear Plants. 2016;19:806–819. doi: 10.1080/0972060X.2016.1160799. [DOI] [Google Scholar]
- Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadhosseini M, Nekoei M. Chemical compositions of the essential oils and volatile compounds from the aerial parts of Ferula ovina using hydrodistillation, MAHD, SFME and HS-SPME methods. J Essent Oil Bear Plants. 2014;17:747–757. doi: 10.1080/0972060X.2014.884951. [DOI] [Google Scholar]
- Murugan R, Livingstone C. Origin of the name “patchouli” and its history. Curr Sci. 2010;99:1274–1276. [Google Scholar]
- Murugan R, Livingstone C. Pogostemon raghavendranii (Lamiaceae), a new species from Anamalai hills, India. Rheedea. 2010;20:21–24. [Google Scholar]
- Okoh SO, Iweriegbor BC, Okoh OO, Nwodo UU, Okoh AI. Bactericidal and antioxidant properties of essential oils from the fruits Dennettia tripetala G. Baker. BMC Complement Altern Med. 2016;16:486. doi: 10.1186/s12906-016-1459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oukerrou MA, Tilaoui M, Mouse HA, Leouifoudi I, Jaafari A, Zyad A. Chemical composition and cytotoxic and antibacterial activities of the essential oil of Aloysia citriodora palau grown in Morocco. Adv Pharmacol Sci. 2017;2017:7801924. doi: 10.1155/2017/7801924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil RP, Nimbalkar MS, Jadhav UU, Dawkar VV, Govindwar SP. Antiaflatoxigenic and antioxidant activity of an essential oil from Ageratum conyzoides L. J Sci Food Agric. 2010;90:608–614. doi: 10.1002/jsfa.3857. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Ricci D, Epifano F, Fraternale D. The essential oil of Monarda didyma L. (Lamiaceae) exerts phytotoxic activity in vitro against various weed seeds. Molecules. 2017;22:222. doi: 10.3390/molecules22020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoj K, Vimal KP, Sunojkumar P. A checklist of the genus Pogostemon Desf. in Southern Western Ghats. S Indian J Biol Sci. 2016;2:46. doi: 10.22205/sijbs/2016/v2/i1/100343. [DOI] [Google Scholar]
- Smeriglio A, Denaro M, Barreca D, Calderaro A, Bisignano C, Ginestra G, Bellocco E, Trombetta D. In vitro evaluation of the antioxidant, cytoprotective, and antimicrobial properties of essential oil from Pistacia vera L. variety Bronte Hull. Int J Mol Sci. 2017;18:1212. doi: 10.3390/ijms18061212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganya P, Jeyaprakash K, Mallavarapu GR, Murugan R. Comparison of the chemical composition, tyrosinase inhibitory and anti-inflammatory activities of the essential oils of Pogostemon plectranthoides from India. Ind Crops Prod. 2015;69:300–307. doi: 10.1016/j.indcrop.2015.02.045. [DOI] [Google Scholar]
- Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- Thoppil JE. Essential oil analysis of Eusteralis deccanensis Panigrahi (Lamiaceae) Boll Chim Farm. 2000;139:194–195. [PubMed] [Google Scholar]
- Vardar-Ünlü G, Candan F, Sökmen A, Daferera D, Polissiou M, Sökmen M, Dönmez E, Tepe B. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae) J Agric Food Chem. 2003;51:63–67. doi: 10.1021/jf025753e. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M, Ruiz Navajas Y, Sánchez Zapata E, Fernández-López J, Pérez-Álvarez JA. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr J. 2010;25:13–19. doi: 10.1002/ffj.1951. [DOI] [Google Scholar]
- Wang L, Wu Y, Huang T, Shi K, Wu Z. Chemical compositions, antioxidant and antimicrobial activities of essential oils of Psidium guajava L. Leaves from different geographic regions in China. Chem Biodivers. 2017;14:e1700114. doi: 10.1002/cbdv.201700114. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Guo J, Wang Q, Zhu S, Gao S, Yang C, Wei M, Pan X, Zhu W, Ding D. Cytotoxic and antitumor effects of curzerene from Curcuma longa. Planta Med. 2017;83:23–9. doi: 10.1055/s-0042-107083. [DOI] [PubMed] [Google Scholar]
- Wei A, Shibamoto T. Antioxidant activities and volatile constituents of various essential oils. J Agric Food Chem. 2007;55:1737–1742. doi: 10.1021/jf062959x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang Z, Chen D, Huang Z, Li Y, Lan X, Su P, Pan W, Zhou W, Zheng X, Du Z. Variation on composition and bioactivity of essential oils of four common curcuma herbs. Chem Biodivers. 2017;14:e1700280. doi: 10.1002/cbdv.201700280. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang JS, Yang B, Lv GP, Li SP. Free radical scavenging activity and characterization of sesquiterpenoids in four species of Curcuma Using a TLC Bioautography assay and GC–MS analysis. Molecules. 2010;15:7547–7557. doi: 10.3390/molecules15117547. [DOI] [PMC free article] [PubMed] [Google Scholar]