Abstract
Purpose:
Dissolution behavior of dry powder inhaler (DPI) antibiotic formulations in the airways may affect their efficacy especially for poorly-soluble antibiotics such as azithromycin. The main objective of this study was to understand the effects of surface composition on the dissolution of spray dried azithromycin powders by itself and in combination with colistin.
Methods:
Composite formulations of azithromycin (a poorly water-soluble molecule) and colistin (a water-soluble molecule) were produced by spray drying. The resultant formulations were characterized for particle size, morphology, surface composition, solid-state properties, solubility and dissolution.
Results:
The results demonstrate that surfaces composition has critical impacts on the dissolution of composite formulations. Colistin was shown to increase the solubility of azithromycin. For composite formulations with no surface colistin, azithromycin released at a similar dissolution rate as the spray-dried azithromycin alone. An increase in surface colistin concentration was shown to accelerate the dissolution of azithromycin. The dissolution of colistin from the composite formulations was significantly slower than the spray-dried pure colistin. In addition, FTIR spectrum showed intermolecular interactions between azithromycin and colistin in the composite formulations, which could contribute to the enhanced solubility and dissolution of azithromycin.
Conclusions:
Our study provides fundamental understanding of the effects of surface concentration of colistin on azithromycin dissolution of co-spray-dried composite powder formulations.
Keywords: Dry powder inhaler, aerosol performance, spray drying, poorly water soluble drug, solubility, dissolution, azithromycin, colistin
INTRODUCTION
Lower respiratory tract infections (or lung infections) are common and associated with high mortality and morbidity (1, 2). Excessive airway inflammation and damage of lung function are the primary causes of death from respiratory tract infections (3). Infections can also cause the worsening of lung function in respiratory tract disorders such as cystic fibrosis (CF), bronchiectasis and chronic obstructive pulmonary disease (COPD) (4, 5). For some antibiotics such as colistin, traditional oral/parenteral antibiotic therapies are not effective against such infections as only a very small proportion of the administrated drug reaches the site of infection i.e., the deep lungs (6). Exposure to such sub-optimal concentrations of antibiotics increases the risk of emergence of resistance; while administration of higher doses via the systemic routes may lead to severe adverse effects (7, 8).
Inhalation enables delivery of drugs directly to the lungs, which can achieve high drug concentrations in the airways at relatively low dose (9, 10). Inhaled colistin has been shown to be effective against respiratory tract infections (11). However, there has been a rise in the incidence of colistin-resistant Gram-negative infections (12, 13). Additionally, colistin exhibits limited effectiveness against the biofilm mucoid planktonic bacterial cells (14, 15). Thus, there is a need to develop novel colistin inhalation therapies to address challenges in bacterial biofilm and emerging resistance.
Combination therapy could be a practical approach to target the resistant strains and biofilms. Azithromycin is a promising candidate for combination therapy with colistin as it exerts an excellent antibacterial action (16). Macrolide antibiotics can also disrupt the protective biofilm integrity (17, 18) and reduce mucus secretion and sputum viscoelasticity (19). Clinical studies have shown that azithromycin therapy can facilitate significant improvements in the lung function attributable to its anti-inflammatory action (20, 21). Combinations of azithromycin and colistin have also been shown to exhibit synergistic antibacterial action against some multidrug resistant (MDR) Gram-negative bacterial strains (22, 23). Thus, azithromycin could be a suitable candidate for combination with colistin for improved inhalation antibiotic therapy.
Dry powder inhalers (DPIs) are becoming more popular than their liquid counterparts due to better chemical stability, greater portability, and ease of use (24–27). Since only the dissolved fraction of antibiotics is typically available to exert therapeutic action, it is vital that the constituting drugs dissolve after deposition in the lungs for optimal efficacy (28). Importantly, drug dissolution in the airway is affected by several factors including the low volume of epithelial lining fluid (10–20 mL/100 m2) (29) and the presence of mucus especially in the diseased state. Moreover, the time available for the particles to dissolve is limited as undissolved particles can be cleared by pulmonary clearance mechanisms (30). For instance, azithromycin binds to the 50S ribosomal subunit of susceptible microorganisms and interferes with bacterial protein synthesis (31). The deposited azithromycin particles should dissolve and produce relatively high drug concentrations at the infection sites so that drugs can enter the bacterial cells. However, azithromycin is a poorly water-soluble drug (~0.2 mg/mL) (32), and its slow dissolution could be a limiting factor in achieving optimal antimicrobial activities for pulmonary delivery.
Our early study has shown that spray drying azithromycin with L-leucine improved the solubility and dissolution of azithromycin DPI formulations due to the molecular interactions and low pH microenvironment (33). Recently, the combination of two or more active components (typically referred as co-amorphous drug delivery systems) have been shown to effectively improve the solubility and dissolution of poorly water-soluble drugs (34). These studies have indicated that the dissolution of poorly water-soluble drug depends on the solubility of co-former drug, where the co-former with higher solubility allows poorly soluble drug to dissolve faster (34). It has been shown that colistin is a water-soluble molecule with surfactant-like character, which may increase the solubility of azithromycin (35). Our previous study also showed that when colistin was co-spray-dried with hydrophobic azithromycin, azithromycin tends to enrich on the composite particle surface, which prevented moisture-induced agglomeration of colistin at elevated humidity (36). However, there may be a concern that surface enrichment of hydrophobic azithromycin may retard the wetting and hence the dissolution of colistin. Thus, the main objective is to understand the effects of surface composition on the dissolution of poorly water-soluble azithromycin.
In this study, azithromycin and colistin were spray-dried to produce combination powder formulations for inhalation. The resultant formulations were characterized for aerodynamic particle size distribution, morphology, solid-state character, moisture sorption behavior and in-vitro aerosolization. The surface composition was characterized by X-ray photoelectron spectroscopy (XPS). The equilibrium solubility and in-vitro dissolution were determined to understand the effects of surface composition on the solubility and dissolution of the composite powder formulations.
MATERIALS AND METHODS
Materials
Azithromycin dihydrate and colistin sulphate were purchased from BetaPharma (Shanghai) Co. Ltd (Wujiang City, JiangSu Province, China). Acetonitrile (HPLC grade) was purchased from Merck (Fair Lawn, New Jersey, USA). Potassium dihydrogen phosphate, sodium hydroxide, sodium sulfate, methanol are all from Fischer Scientific (Fair Lawn, New Jersey, USA) and ethanol is from Decon Laboratories, King of Prussia, PA, USA.
Spray drying
Dry powder formulations were produced by employing a Büchi 290 spray dryer (Büchi Labortechnik AG, Falwil, Switzerland). Briefly, azithromycin and colistin were weighed and dissolved separately in equal volumes of ethanol and water, respectively. After obtaining a clear solution, the colistin and azithromycin solutions were combined and mixed. To produce composite powders, the solutions consisting of colistin and azithromycin with varying molar composition (1:4, 1:2, 1:1 and 2:1, as shown in Table 1) were spray-dried. The spray-drying conditions were as follows: inlet temperature 80 °C; outlet temperature 48 ± 2 °C; aspirator 35 m3/h; atomizer setting 700 L/h; feed rate 2 mL/min (36). The spray-dried product was stored in a desiccator with silica gel at 20 ± 3 °C. The total solid load of the spray feed solutions was kept constant (10 mg/mL) amongst different formulations (36).
Table 1:
Compositions of spray-dried formulations.
| Formulations | Colistin (% w/w) | Azithromycin (% w/w) |
|---|---|---|
| Azithromycin-SD | 0 | 100 |
| Colistin-Azithromycin_1–4 | 28.8 | 71.2 |
| Colistin-Azithromycin_1–2 | 44.7 | 55.3 |
| Colistin-Azithromycin_1–1 | 61.8 | 38.2 |
| Colistin-Azithromycin_2–1 | 73.2 | 26.8 |
| Colistin-SD | 100 | 0 |
Scanning electron microscopy (SEM)
A field emission scanning electron microscope (NOVA nanoSEM, FEI Company, Hillsboro, Oregon, USA) was used to examine the morphology of spray-dried particles. Briefly, adhesive carbon tape was placed on the stainless-steel stub and then a small amount of spray-dried powder was scattered over it and the excess powder was removed with the help of pressurized air. The stubs were then coated with platinum using a sputter coater (208 HR, Cressington Sputter Coater, England, UK) at 40 mA for 1 min, which corresponds to coating thickness of approximately 10 – 30 nm. The particles mounted on the coated stubs were visualized under the SEM and the images were captured at an acceleration voltage of 5 kV.
Particle size distribution
Particle size was measured using the scanning electron microscopy images (as described above) with the help of ImageJ software (37, 38). Martin’s diameter of ~150 randomly selected particles was determined in a fixed downwards direction from three different SEM images and D10, D50 and D90 were calculated from the size distribution data.
Specific surface area
The specific surface area of the powder formulations was determined following the Brunauer–Emmett–Teller (BET) method of nitrogen adsorption/desorption under 77 K using a Micromeritics TriStar II 3020 apparatus (Norcross, GA). Before each BET measurement, the sample powders were degassed for at least 4 h using a FlowPrep 060 degasser (Micromeritics, Norcross, GA).
X-ray powder diffraction (XPRD)
The crystallinity of the selected powder formulations was determined by X-ray diffraction with a Rigaku Smartlab™ diffractometer (Rigaku Americas, Texas, USA). The instrument was equipped with a Cu-Kα radiation source and a highly sensitive D/tex ultra-detector. The powders were placed on glass sample holders and x-ray diffraction was acquired from 5 to 40° 2θ at a scan speed of 5°/min and a step size of 0.02°. The voltage and current used were 40 kV and 44 mA, respectively.
Water vapor sorption isotherm
A dynamic vapor sorption (DVS-Intrinsic, Surface Measurement Systems Ltd., London, UK) was used to evaluate the moisture sorption behavior. Each formulation (15 ± 5 mg) was placed on the pan, which was equilibrated to 0% relative humidity (RH) to provide a baseline. The experimental cycle consisted of a sorption cycle followed by a desorption cycle. The sorption cycle involved equilibrating powders to RH ranging from 0 to 90% at 10% RH increments; while desorption cycle involved equilibrating the resultant powders to RH decreasing from 90 to 0% at 10% RH. Powders were considered to reach equilibrium, when the change in mass with respect to time i.e., dm/dt was less than 0.002% per minute.
Attenuated total reflectance Fourier-transform infrared spectroscopy (FTIR) spectroscopy
The powder formulations were analyzed using an FTIR with attenuated total reflectance (ATR) (Cary 600 series FTIR spectrometer, Agilent Technologies, Santa Clara, CA, USA). A small amount of powder was carefully placed onto the ATR crystal, and the pressure valve was used to improve the uniformity of contact between the sample and the ATR crystal of the instrument. Samples were analysed at a resolution of 1 cm−1 with 128 numbers of scan in the range on 400–4000 cm−1. The peaks of spectra were analyzed using ResolutionPro (Version 5.2.0, Agilent Technologies, Santa Clara, CA, USA) with sensitivity of 8.
X-ray photoelectron spectroscopy (XPS)
Surface concentrations of colistin and azithromycin were determined using X-ray photoelectron spectroscopy (XPS) (AXIS Ultra DLD spectrometer, Kratos Analytical Inc., Manchester, UK) with monochromic Al Kα radiation (1486.6 eV) at pass energy (PE) of 20 and 160 eV for high-resolution and survey spectra, respectively. A commercial Kratos charge neutralizer was used to avoid non-homogeneous electric charge and to achieve better resolution. Typical instrument resolution for PE of 20 eV is ~0.35 eV. Binding energy (BE) values refer to the Fermi edge and the energy scale was calibrated using Au 4f7/2 at 84.0 eV and Cu 2p3/2 at 932.67 eV. Powder samples were scattered on a double-sided sticking Cu tape mounted on a stainless-steel sample holder bar. The C-C component of the C 1s peak was set to a binding energy of 284.8 eV to correct for charge on each sample and the data were analyzed. Curve-fitting was performed following a Shirley background subtraction using model peaks obtained from pure compounds. Atomic concentrations of the element in the near-surface region were estimated after a Shirley background subtraction considering the corresponding Scofield atomic sensitivity factors and inelastic mean free path (IMFP) of photoelectrons using standard procedures in the CasaXPS software assuming homogeneous mixture of the elements within the information depth (~10 nm).
Drug quantification
Concentrations of azithromycin and colistin were determined using a validated HPLC method (36). The HPLC system consisted of G1311C (1260 Quat Pump VL) pump, G1330B (1290 Thermostate) thermostate, G1329B (1260 ALS) autosampler, G1316A (1260 TCC) thermostated column compartment, G1314F (1260 VWD) variable wavelength detector, and an Agilant Eclipse Plus, 5 μm C18 150 × 4.60 mm column (all from Agilant, Waldbronn, Germany).
For azithromycin, the mobile phase consisted of 20 mM potassium dihydrogen phosphate (adjusted to pH 7 with 10 % w/v sodium hydroxide) (A) and methanol (B). The isocratic elution program used for azithromycin detection was 20% A and 80% B v/v for 15 min at the flow rate of 1.0 mL/min with retention time at ~10 min at 210 nm. The calibration curve prepared using water and ethanol (1:1 v/v) as the solvent system in the concentration range of 0.5 – 0.01 mg/mL was linear (r 2 > 0.999). For colistin, the mobile phase consisted of 30 mM sodium sulfate (adjusted to pH 2.5 with H3PO4) (A) and acetonitrile (B). The isocratic elution program used for colistin detection was 76% A and 24% B v/v for 7 min at the flow rate of 1.0 mL/min. Colistin peaks were observed at ~3 and ~5 min (for colistin A and colistin B) and a calibration curve in the range of 0.5 – 0.01 mg/mL was linear (r2 > 0.999).
Equilibrium solubility
Equilibrium azithromycin solubility of the raw azithromycin, the spray-dried azithromycin, physical mixtures (at the same molar composition as spray-dried formulations in Table 1) of azithromycin and colistin as well as the spray-dried composite formulations was determined (33). Briefly, an excess of azithromycin (i.e., 5 mg/mL equivalent of azithromycin of each formulation) was added to 5 mL of phosphate buffer saline (PBS, pH 7.4) maintained at room temperature (22 ± 2 °C) and 37 ±1 °C. The resultant suspensions were constantly stirred at 500 rpm (VWR International, Arlington Heights, IL, USA) with the help of a magnetic bar (12 mm diameter and 5 mm diameter, VWR International, Arlington Heights, IL, USA). After 24 h the suspensions were filtered using a 0.45 µm nylon syringe filter (VWR International, Arlington Heights, IL, USA) and the concentrations of azithromycin and colistin were determined by the validated HPLC method.
In-vitro aerosol efficiency
In-vitro aerosol performance was measured by a next-generation impactor (NGI, Apparatus X, USP X, Copley, Nottingham, UK) with a USP induction port. The collection plates of all the stages were coated with silicone oil before each testing to minimize particle bouncing. Powder weighing 10 ± 2 mg was filled (unless stated otherwise) in the capsules (size 3 hydroxypropyl methylcellulose capsules, Qualicaps, Whitsett, NC, USA). Since the optimal dose for each formulation should be determined by future animal and clinical studies, a fixed dose was used here to evaluate interfacial effects on the In-vitro aerosol efficiency of the composite formulations.
The resultant capsules were used in a low-resistance RS01 DPI device (similar design to Osmohaler, Plastiape S.p.A., Osnago, Italy), with 4 L of air passing through the inhaler at an airflow of 100 L/min for 2.4 s, with a pressure drop of approximately 4 kPa (39). Drugs retained in the capsule, inhaler device, USP throat and Stage 1–8 of the impactor were collected using 20 mL of a co-solvent consisting of 1:1 v/v of 20 mM potassium dihydrogen phosphate (adjusted to pH 5 with 10 % w/v sodium hydroxide) and methanol. Fine particle fraction (FPF) was calculated as the fraction of drugs with an aerodynamic diameter < 5 μm over the recovered dose.
In-vitro dissolution studies
The dissolution kinetics of selected powder formulations were investigated using two different methods: beaker methods and Franz diffusion cell using the protocol described previously with minor modifications (33, 40). The dissolution of powder was carried out using aerosolized particles with aerodynamic diameter smaller than 5 µm (41). Briefly, a filter disk (Whatman® Grade 2, pore size 5 μm, GE Healthcare, Parramatta, Australia) was placed under the nozzle of Stage 4 of NGI to collect the aerosolized particles.
Phosphate buffer saline (20 mL at pH 7.4) has been used widely as dissolution media in inhalation studies consistent with the existing literature (41, 42). An aliquot of 0.2 mL sample was drawn at predetermined time intervals (2, 5, 10, 15, 20, 30, 45, 60, 90, 120, 150 and 180 min), which was replaced with 0.2 mL of fresh medium immediately after sample collection. Total amount of drug loaded on the filter disc was determining by adding 5 mL ethanol at the end of dissolution studies to dissolve all solids. Drug released was calculated by dividing the amount of drug released by the total amount of drug deposited on the filter disk.
In the Franz diffusion method, Franz cell (V6B, PermeGear Inc., Hellertown, PA, USA) reservoirs were filled with 20 mL PBS (pH 7.4) maintained at 37 ± 1°C. The dissolution media was stirred constantly at a pre-set fixed speed of 600 rpm (6-station Franz Cell stirrer, PermeGear Inc., Hellertown, PA, USA) with the help of a magnetic bar (12.5 mm diameter and 3 mm wide). The aerosol powder collected on the filter disk from NGI was placed on the top of the Franz cells while ensuring that the air bubble is not trapped underneath, and samples were collected at predetermined time intervals.
In the beaker method, the filter disk was gently placed in a 100 mL jacketed beaker (4.7 mm internal diameter × 8.5 cm high, Vineland, NJ, USA) with 20 mL PBS (pH 7.4) maintained at 37 ± 1 °C. The dissolution media was constantly stirred at a fixed speed of 500 rpm with a magnetic stirrer (VWR International, Arlington Heights, IL, USA) and a magnetic bar (12 mm diameter and 5 mm diameter, VWR International, Arlington Heights, IL, USA).
Statistical analysis
Results are expressed as mean ± standard deviation (SD) and the statistical analysis was performed via one-way analysis of variance (ANOVA) with Tukey–Kramer post-hoc tests using a GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
RESULTS
SEM
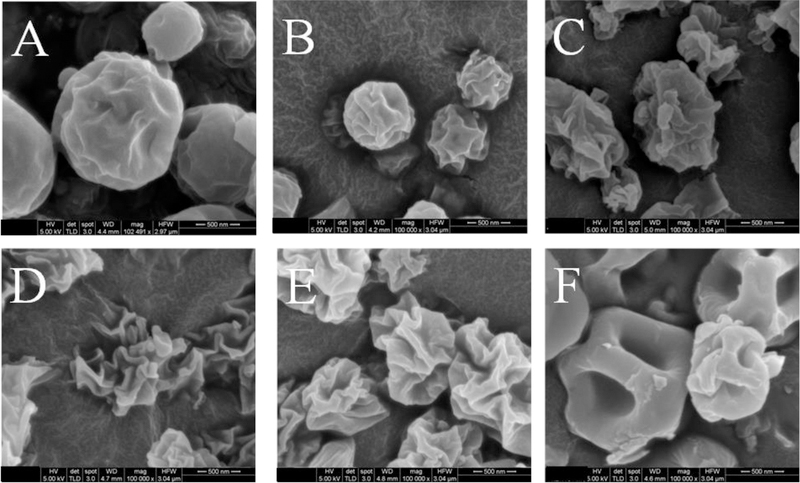
Figure 1 shows the representative SEM images of the spray-dried formulations. Azithromycin-SD appeared as near-spherical particles with slightly rough surfaces. The SEM image of Colistin-SD showed spherical dimpled, or occasionally smooth but hollow particles. Interestingly, morphology and surface roughness of the composite formulations was different considerably from both Colistin-SD and Azithromycin-SD. Apparently, the surface roughness increased with increasing colistin feed concentration up to the 1:1 ratio. The surfaces of Colistin-Azithromycin_2–1 particles (Figure 1E) appeared to be less wrinkled when compared with Colistin-Azithromycin_1–1 (Figure 1D). A previous study has shown that the surface roughness decreased at higher colistin to azithromycin ratio when colistin concentration in the formulations exceeds a threshold (36).
Figure 1:
Representative scanning electron microscopy images of Azithromycin-SD (A), Colistin-Azithromycin_1–4 (B), Colistin-Azithromycin_1–2 (C), Colistin-Azithromycin_1–1 (D), Colistin-Azithromycin_2–1 (E) and Colistin-SD (F). Scale bars represent 500 nm.
Particle sizing and specific surface area
Table 2 shows the particle sizes of spray-dried formulations. Our results indicated that physical diameters of majority of the formulations were < 4 µm. Azithromycin-SD has the lowest D50 of 0.9 ± 0.1 µm; while Colistin-Azithromycin_1–1 has the largest D50 of 2.0 ± 0.4 µm.
Table 2:
Particle size and specific surface area resultsof the spray-dried powder formulations. The data are presented as mean ± SD (n=3).
| Formulations | Particle Size (µm) |
Specific Surface Area (m2/g) |
||
|---|---|---|---|---|
| D10 | D50 | D90 | ||
| Azithromycin-SD | 0.6 ± 0.1 | 0.9 ± 0.1 | 1.4 ± 0.2 | 6.13 ± 0.06 |
| Colistin-Azithromycin_1–4 | 0.6 ± 0.1 | 1.0 ± 0.1 | 1.5 ± 0.1 | 8.57 ± 0.33 |
| Colistin-Azithromycin_1–2 | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.9 ± 0.2 | 9.78 ± 0.18 |
| Colistin-Azithromycin_1–1 | 1.5 ± 0.5 | 2.0 ± 0.4 | 3.4 ± 0.8 | 19.56 ± 0.10 |
| Colistin-Azithromycin_2–1 | 0.9 ± 0.1 | 1.5 ± 0.2 | 2.3 ± 0.0 | 11.64 ± 0.06 |
| Colistin-SD | 0.9 ± 0.1 | 1.4 ± 0.1 | 2.0 ± 0.2 | 8.37 ± 0.66 |
Both spray dried pure azithromycin and colistin formulations have relatively lower specific surface area compared to those composite formulations. The Colistin-Azithromycin_1–1 formulation has the highest specific surface area of 19.56 ± 0.10, which is in agreement with the most corrugated morphology shown in the SEM images.
XPS
Table 3 compares the theoretical formulation concentrations of colistin and azithromycin in the composite formulation with their surface concentrations as determined by XPS. The results show that colistin was not detected at the surfaces of Colistin-Azithromycin_1–4 and Colistin-Azithromycin_1–2. The surfaces of Colistin-Azithromycin_1–1 and Colistin-Azithromycin_2–1 showed some colistin but azithromycin was dominant at the surfaces of all the composite formulations. The surface concentrations of azithromycin were higher than its theoretical formulation compositions indicating azithromycin enrichment at the surfaces of composite particles, which are in agreement with our previous findings (36).
Table 3:
Theoretical formulation and surface compositions of the selected spray-dried formulations as measured by XPS.
| Formulations | % Theoretical Composition | % Surface Composition | ||
|---|---|---|---|---|
| Colistin | Azithromycin | Colistin | Azithromycin | |
| Colistin-Azithromycin_1–4 | 25.5 | 74.5 | 0.0 | 100.0 |
| Colistin-Azithromycin_1–2 | 40.6 | 59.4 | 0.0 | 100.0 |
| Colistin-Azithromycin_1–1 | 57.8 | 42.2 | 8.4 | 91.6 |
| Colistin-Azithromycin_2–1 | 73.2 | 26.8 | 27.5 | 72.5 |
XPRD
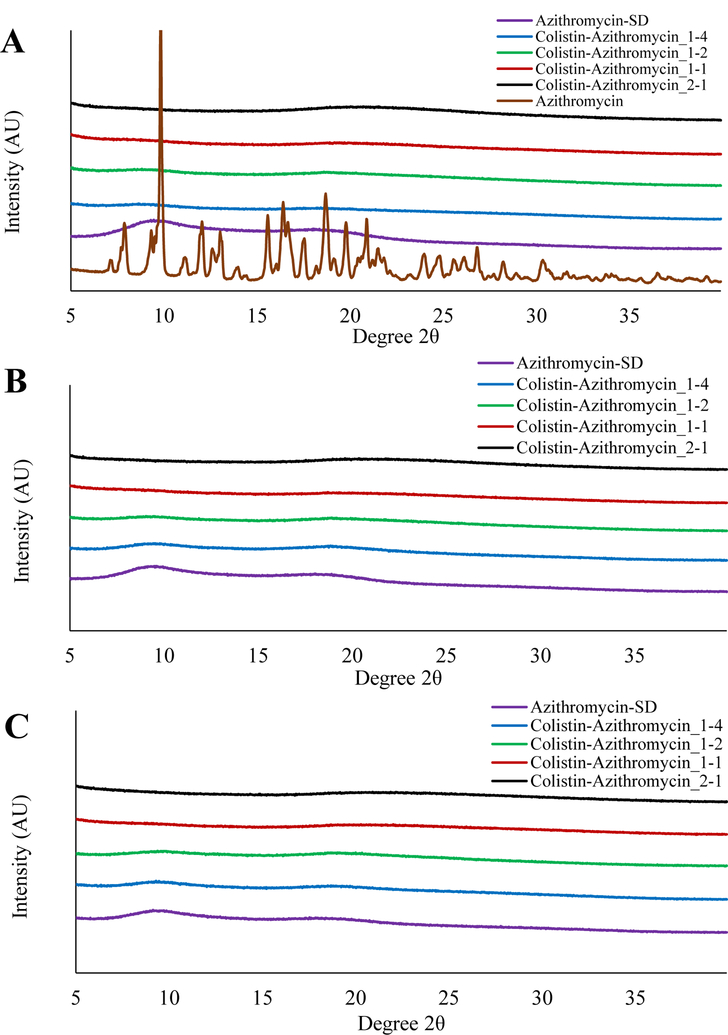
XPRD was used to evaluate the solid-state properties of the samples (Figure 2). The XPRD diffractogram of raw azithromycin showed sharp peaks indicating crystallinity (Figure 2A). In contrast, PXRD diffractogram of Azithromycin-SD showed the amorphous halos indicating the lack of long-range molecular order. In the spray drying process here, the drying rate is very rapid and hence the time for solutes to re-arrange into a crystal lattice is not sufficient, and such conditions typically leading to formation of amorphous solids.
Figure 2:
X-ray diffraction patterns of raw Azithromycin and freshly spray-dried formulations (A), spray-dried formulations stored at 55% RH for 3 months (B), and spray-dried formulations stored at 75% RH for 3 months (C).
All composite formulations showed no sharp peaks indicating their amorphous nature. Studies have shown that the surface enrichment of poorly water-soluble molecules in the spray drying may be a result of their crystallization, as crystals exhibit slower diffusion, then these molecules accumulate at the surfaces (43, 44). The XPRD pattern clearly indicates that surface enrichment of azithromycin was not a result of its crystallization. We also investigated the physical stability of the spray-dried formulations stored at 55% RH and 75% RH for 3 months (Figure 2B and 2C); the formulations showed no sharp peaks indicating absence of moisture-induced crystallization. This highlights the robust physical stability of our spray-dried formulations.
Water vapor sorption isotherm
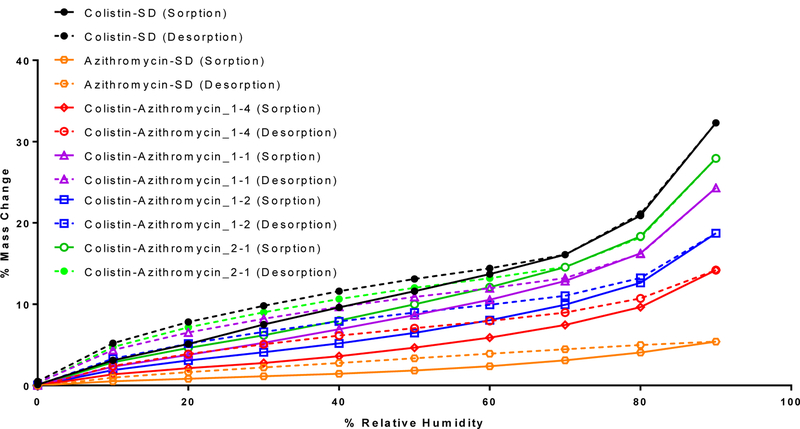
As indicated by the water sorption data, Colistin-SD absorbed substantial amounts water at high relative humidity (Figure 3). In contrast, Azithromycin-SD absorbed relatively smaller amount of water (~5% w/w) compared with the other spray dried formulations. The amount of water uptake increased with increasing proportion of colistin in the composite formulations. The water uptake in the composite formulations was also completely reversible indicating the absorbed water did not result crystallization. This agrees with our XPRD data, which indicated no change in solid-state properties of the formulation following storage at the elevated humidity.
Figure 3:
Dynamic water sorption behavior of the spray-dried formulations. The solid and dotted lines indicate changes in mass in sorption and desorption cycles, respectively.
Attenuated Total Reflectance–FTIR spectroscopy
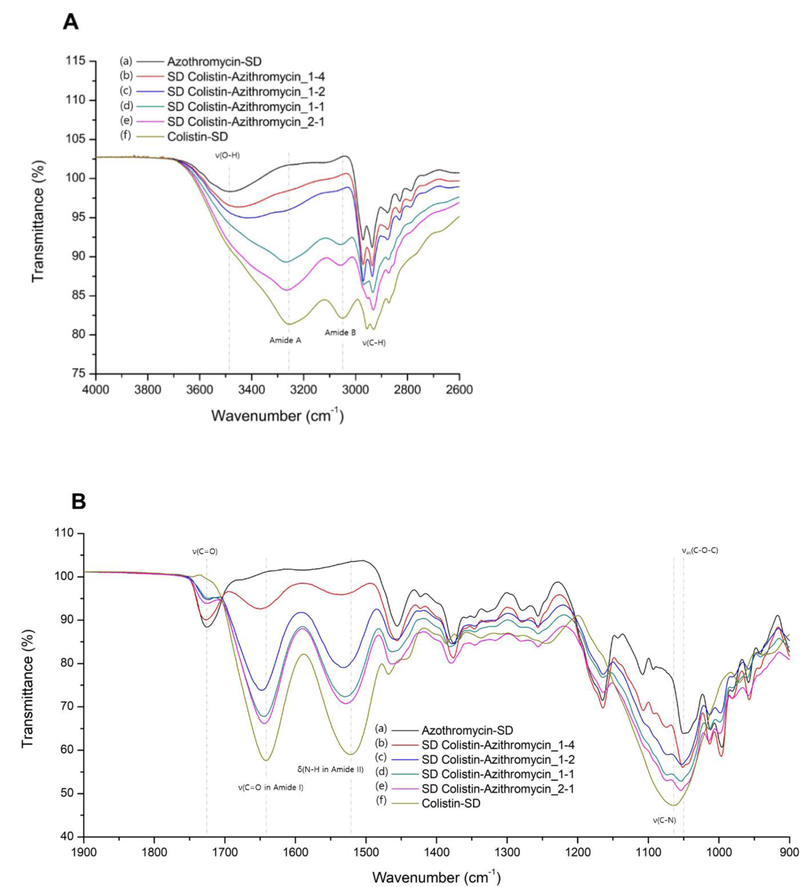
FTIR spectroscopy was used to identify potential chemical interactions between colistin and azithromycin in the composite formulations (Figure 4). The two spectral regions between 900 – 1900 cm−1 and 2600 – 4000 cm−1 were chosen for the analysis. The spray dried azithromycin showed distinguishable characteristic peaks at 3480, 1726 and 1050 cm−1 attributable to the O-H stretching, lactone carbonyl C=O stretching and asymmetric C-O-C stretching, respectively (45). FTIR spectra of colistin showed peaks at 3257 cm−1 and 3048 cm−1 corresponding to the characteristic of amide A and B, respectively, due to N–H stretch in resonance with amide II overtone. In addition, the distinguishable peaks from the stretching vibrations of amide I C=O stretching, amide II N-H bending, and C-N stretching were observed at 1642, 1523 and 1064 cm−1, respectively (46). Various C–H stretching vibrations of azithromycin and colistin were observed at around 2900 cm−1. In the composite formulations of azithromycin and colistin, there were remarkable changes in the IR spectra compared to each spray dried pure azithromycin and colistin. The IR peaks of Colistin-Azithromycin_1–4 that correspond to the amide I C=O stretching and amide II N-H bending of colistin were shifted to higher wavenumbers of 1651 and 1537 cm−1, respectively, with decreased intensity (Table 4). It can be seen that the degree of change in these two peaks becomes larger as the ratio of azithromycin to colistin increases. Correspondingly, other two peaks of amide A and B were also shifted to higher wavenumber, and almost disappeared in the spectrum of Colistin-Azithromycin_1–4. In addition, the stretching vibration peak of C-N in the amide structure of colistin was changed from 1064 cm−1 to 1074 cm−1. The changes in peaks corresponding to azithromycin, were also observed very remarkably (Table 4). In particular, the peak corresponding to the stretching vibrations of O-H in Colistin-Azithromycin_1–4 was shifted from 3480 cm−1 to 3457 cm−1 with increased intensity and broadening of peak. This change was more pronounced as the ratio of colistin to azithromycin increased and this peak was not detected in the spectrum of Colistin-Azithromycin_1–1. In addition, the azithromycin C-O-C stretching vibrations of Colistin-Azithromycin_2–1 was shifted from 1050 and 1054 cm−1. In contrast, there was no change in the stretching vibration peak of C=O.
Figure 4:
Infrared spectra of spray-dried formulations at specific spectral ranges of (A) 2600 – 4000 cm−1 and (B) 900 – 1900 cm−1 ; (a) Azithromycin-SD, (b) Colistin-Azithromycin_1–4, (c) Colistin-Azithromycin_1–2, (d) Colistin-Azithromycin_1–1, (e) Colistin-Azithromycin_2–1, (f) Colistin-SD (ν: stretching, νas: asymmetric stretching, δ: bending).
Table 4:
FTIR band assignments for the spray dried formulations.
| Band assignment | Wavenumber (cm−1) | |||||
|---|---|---|---|---|---|---|
| Azithromycin | Azithromycin : Colistin | Colistin | ||||
| 4:1 | 2:1 | 1:1 | 1:2 | |||
| O-H stretching | 3480 | 3457 | 3423 | |||
| Amide A | 3268 | 3264 | 3257 | |||
| Amide B | 3058 | 3057 | 3048 | |||
| C-H stretching | 2971 | 2971 | 2970 | 2968 | ||
| 2953 | 2956 | |||||
| 2878 | 2877 | 2878 | 2874 | 2872 | 2872 | |
| C=O stretching | 1726 | 1727 | 1727 | 1726 | 1726 | |
| Amide I C=O stretching | 1651 | 1647 | 1644 | 1644 | 1642 | |
| Amide II N-H bending | 1537 | 1531 | 1530 | 1528 | 1523 | |
| C-N stretching | 1074 | 1073 | 1073 | 1073 | 1064 | |
| Asymmetric C-O-C stretching |
1050 | 1052 | 1053 | 1054 | 1054 | |
In-vitro aerosolization efficiency
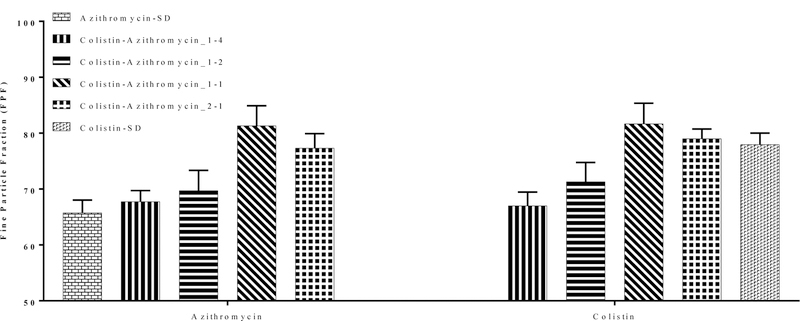
Figure 5 shows the FPF of the spray-dried formulations. Our results showed that Azithromycin-SD had a relatively high FPF of 65.8 ± 2.2 %; while Colistin-SD had a substantially higher FPF 78.0 ± 2.1 % as compared with Azithromycin-SD (Figure 5). Composite formulations i.e., Colistin-Azithromycin_1–4 and Colistin-Azithromycin_1–2 showed no change in the FPF when compared with Azithromycin-SD. However, the FPF of Colistin-Azithromycin_1–1 and Colistin-Azithromycin_2–1 was substantially higher when compared with Azithromycin-SD suggesting that colistin could improve the aerosolization of azithromycin. This may be attributed to an increase in the particle surface roughness of composite formulations. A change in surface composition as well as surface roughness could lower inter-particulate cohesive forces (47, 48). The FPF of Colistin-Azithromycin_1–1 and Colistin-Azithromycin_2–1 was comparable to that of Colistin-SD.
Figure 5:
Fine particle fraction (FPF) of selected spray-dried formulations. The data are presented as mean ± SD (n=4).
Equilibrium solubility
Figure 6 shows the solubility of azithromycin as a function of colistin concentration. The results clearly indicated that the azithromycin solubility (raw or spray-dried) was low. The solubility of azithromycin in physical mixture increased linearly as a function of colistin concentration (Figure 6B). This is in line with previous findings that colistin can substantially increase the solubility of azithromycin (35). The solubility of azithromycin in the spray-dried formulation increased with an increase in the colistin concentration (Figure 6A). We proposed that azithromycin being dominant molecule at the surface of composite particle governs the dissolution of particles. For instance, when azithromycin solubility is higher, more colistin can release into the solution. This effect was not observed in physical mixture as colistin and azithromycin exist as separate particles. Further studies are warranted to confirm this hypothesis.
Figure 6:
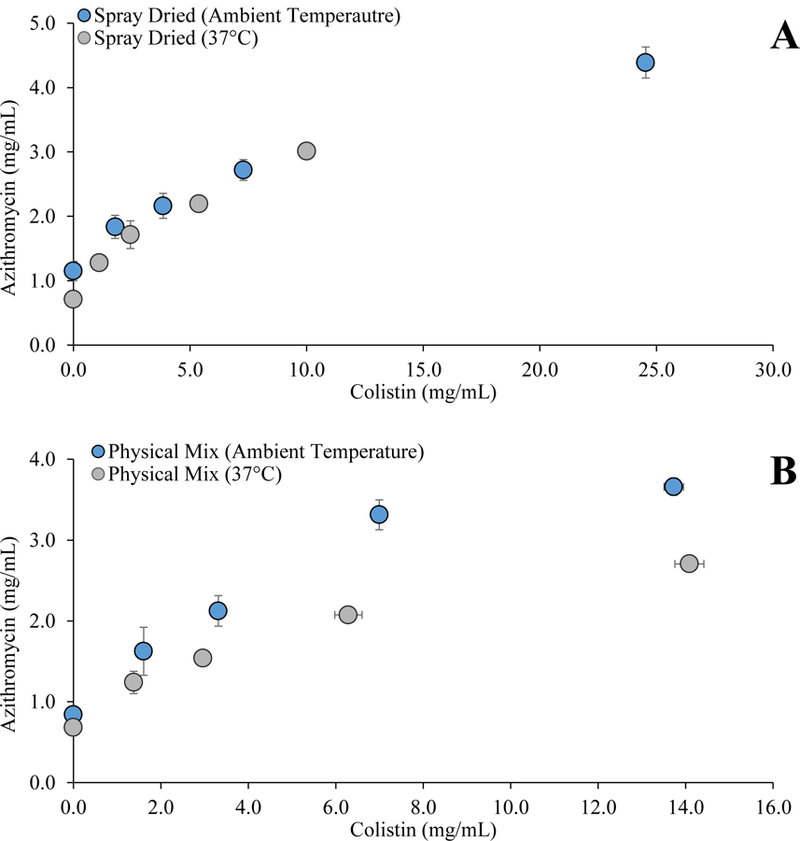
Azithromycin solubility as a function of colistin concentration in PBS (pH 7.4) for (A) spray-dried formulations and (B) physical mixtures determined at ambient temperature and 37 C. The data are presented as mean ± SD (n=4).
In-vitro dissolution studies
Franz diffusion method
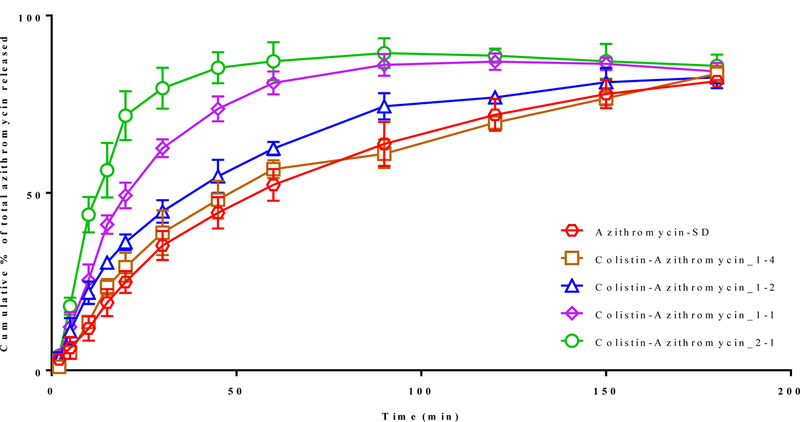
Figure 7 shows the dissolution of azithromycin from the spray-dried formulations. The Franz dissolution studies indicated that ~70 % of azithromycin dissolved after 120 minutes and the maximum azithromycin released was ~80% after 180 min for Azithromycin-SD. The dissolution rate of azithromycin in Colistin-Azithromycin_1–4 and Colistin-Azithromycin_1–2 was similar to that of Azithromycin-SD; the surfaces of these two composite formulations showed no colistin. In contrast, the formulations (Colistin-Azithromycin_1–1 and Colistin-Azithromycin_2–1), which showed colistin at the particle surface, had a much faster azithromycin dissolution when compared with Azithromycin-SD. The dissolution rate of Colistin-Azithromycin_2–1 was faster when compared with Colistin-Azithromycin_1–1, which likely be attributed to an increase in surface colistin concentration.
Figure 7:
The release of azithromycin cumulative % of total from selected spray-dried formulations as a function of time (min) as determined using the Franz diffusion method. The data are presented as mean ± SD (n=4).
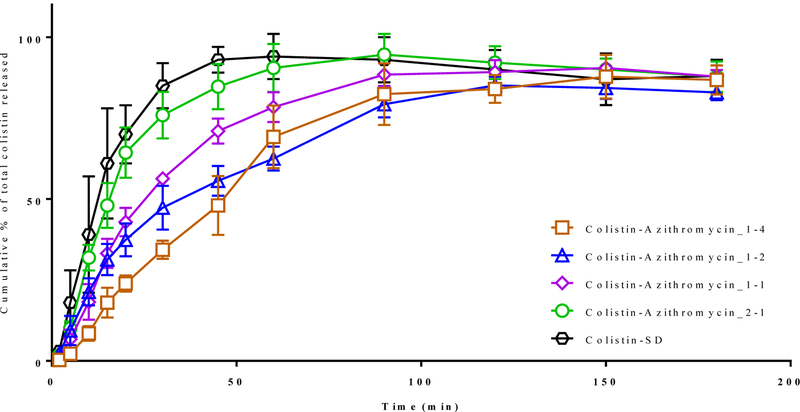
We also analyzed the dissolution kinetics of colistin in these samples (Figure 8). The results indicated that Colistin-SD showed a rapid release with >80 % released within 30 min. The rate of colistin release for Colistin-Azithromycin_2–1 and Colistin-SD was similar. A significant drop in the dissolution of colistin was observed with increasing proportion of azithromycin in the composite formulations. This is most likely attributed to the enrichment of azithromycin at the surface of composite formulations, which can substantially hinder the release of colistin.
Figure 8:
The release of colistin cumulative % of total from selected spray-dried formulations as a function of time (min) as determined using the Franz diffusion method. The data are presented as mean ± SD (n=4).
Beaker method
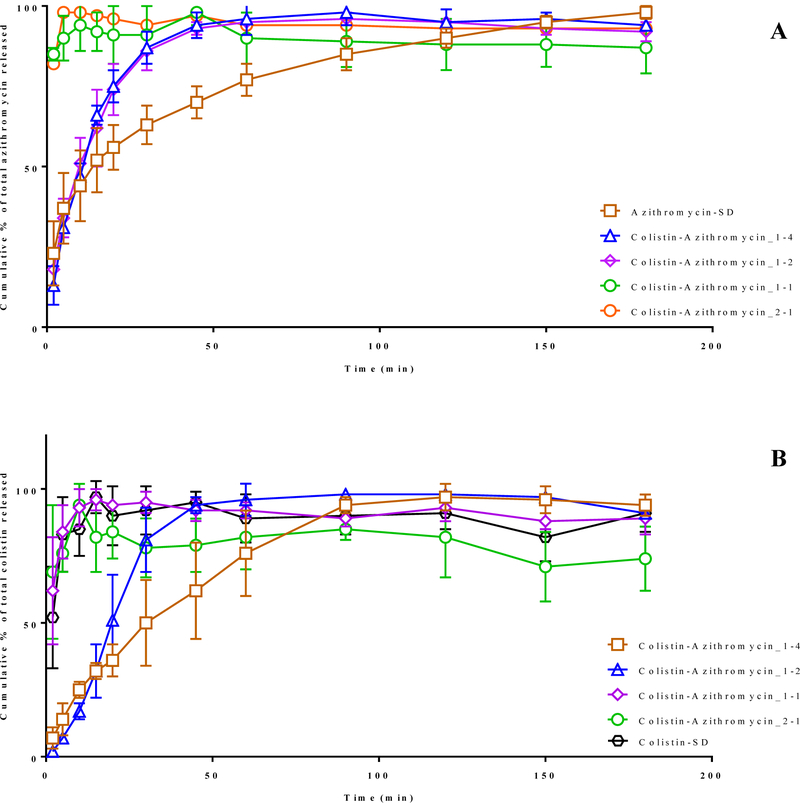
In case of Azithromycin-SD, ~ 70% of azithromycin was dissolved in 30 min (Figure 9A). Colistin-Azithromycin_1–4 and Colistin-Azithromycin_1–2 showed similar azithromycin release profiles as compared with Azithromycin-SD, which is consistent with the Franz cell data. In contrast, Colistin-Azithromycin_1–1 and Colistin-Azithromycin_2–1 showed faster azithromycin release with > 80% being released within first 5 min.
Figure 9:
The release of azithromycin (A) and colistin (B) cumulative % of total from selected spray-dried formulations as a function of time (min) as determined using the beaker method. The data are presented as mean ± SD (n=4).
We also investigated the dissolution of colistin in these formulations and compared it with Colistin-SD (Figure 9B). The results indicated that colistin showed a burst release behavior with >80% of drug was dissolved in the first 10 min. The similar burst release of colistin was also observed in case of Colistin-Azithromycin_1–1 and Colistin-Azithromycin_2–1 with >80 colistin released in first the 10 min. Interestingly, the rate of colistin release was slower in case of Colistin-Azithromycin_1–4 and Colistin-Azithromycin_1–2. These results agree with those dissolution data using the Franz diffusion method. The dissolution rate was higher in the beaker method when compared with the corresponding formulations in the Franz diffusion methods. This is attributed to direct contact of the powder with the solvent in the beaker method (40).
DISCUSSION
Inhalation of large doses can be accompanied with local adverse events, which may cause concerns in patient adherence (6). Therefore, it is recommended to minimize the use of excipient in powders for inhalation, especially in the case of high dose formulations. Co-spraying with colistin was shown to improve the aerosolization, solubility and dissolution of azithromycin in this study. Such formulation augmentation is of special interest in case of inhalable powders as this may obviate the need of significant excipients loading, thus reducing the overall powder load and improving patient tolerance and adherence (49). Another advantage of composite antibiotic DPI formulation over physical mixture is that the two or more drugs are contained within one particle and are more likely to deposit together at the site of infection. Thus, the potential synergistic effect of combination antibiotic is ensured to a greater degree, when compared with the corresponding physical mixture.
Studies have shown that the combination of azithromycin and colistin may exhibit synergistic activity against Gram-negative infections (50, 51). The anti-biofilm (17, 18), anti-inflammatory (20, 21) and muco-regulatory (19) effects of azithromycin could further complement the in vivo efficacy when co-administered with colistin. Spray drying is a popular strategy to produce combination antibiotic powder formulations for inhalation (52). The surface compositions may have heterogeneous distributions due to varying solubility and diffusion of the components (43, 44). Such heterogeneous surface distributions may have significant impact on dispersion behavior due to changes in particle density, morphology and surface energy, which have been investigated extensively.(48, 53–55) This study focuses on effects of such heterogeneous surface distributions on dissolution behavior of composite particles. Since wetting and dissolution of particles begin at the surface of the particles; the surface composition can have a dominating effect on the dissolution of the particle formulation (56). However, understanding of the effect of surface composition on the dissolution of spray-dried composite formulation is rarely reported. In this study, we investigated the effect of surface composition on the dissolution of colistin and azithromycin dry powder formulations.
In composite formulations, the dissolution rate of the poorly water-soluble component typically depends on the water solubility of the co-former (57, 58). Our results also showed that colistin (a highly water-soluble molecule) improved the dissolution of poorly water-soluble azithromycin; however, this increase was only shown for those formulations with the presence of surface colistin (i.e., Colistin-Azithromycin_1–1 and Colistin-Azithromycin_2–1). The rate of dissolution also increased with increasing colistin surface concentrations. In contrast, colistin release was slower in the formulations with high surface azithromycin (i.e., Colistin-Azithromycin_4–1 and Colistin-Azithromycin_1–2). Furthermore, there are not clear correlations between dissolution and particle size/specific surface area results in this study. These findings indicate that surface composition played a dominating role in governing the dissolution of azithromycin and colistin from the composite formulations. More hydrophilic colistin on the surface can improve the wettability of the particles in the aqueous media, thus enhance the dissolution.
In our previous study, we have shown azithromycin was enriched on the surface of co-spray dried particles with colistin (36). Such enrichment of azithromycin was likely due to the lower solubility in the spray drying feed solution as compared with colistin(43). Boraey et al. demonstrated such solubility of varying components in the feed solution had significant impact on surface composition and dispersion (54). Therefore, the solvent system for the feed solution is critical to determine the surface composition of co-spray dried particles.
Previous studies have shown that colistin is a surfactant-like molecule, which can improve the solubility of azithromycin (35, 59). Our results also indicated that the solubility of azithromycin increased with increasing colistin concentration in the co-sprayed formulations as well as in the physical mixture. This may be attributed to the nature of composite formulations, where the constituting components are imbedded/packed in a matrix-like system, thus the dissolution of one component is dependent on the dissolution of the secondary component. However, this phenomenon was not observed in case of physical mixture, where the dissolution of colistin was independent of the dissolution of azithromycin as well as temperature. These suggest the molecular interactions measured by FTIR between azithromycin and colistin in the composite particle play a critical role in the solubility.
It was proposed that colistin improves the solubility of azithromycin by forming micelles which follow a ‘closed association’ model (35). This means that a discrete (fixed) number of colistin monomers are required to form a micelle. If micelle formation was the only mechanism of increased dissolution, the number of colistin molecules will typically be higher than the number of azithromycin. Our studies showed that both drugs release in synchronized fashion i.e., one molecule of azithromycin was released for every single molecule of colistin in Colistin-Azithromycin_1–1 formulation (Appendix A1). This indicates that micelle formation may not be the primary and/or only mechanism of azithromycin dissolution enhancement, as indicated by the different solubility behavior between composite and physical mixture formulations.
Intermolecular interactions between the drugs are proposed to explain the improved solubility. The most notable peaks among the many changes of FTIR spectra are O-H stretching of azithromycin and Amide I C=O stretching of colistin. As mentioned above, the peak that corresponds to the amide I C=O stretching of colistin was shifted to higher wavenumbers with decreased intensity as the ratio of azithromycin to colistin increases. In addition, the peak due to the O-H stretching vibrations of azithromycin was shifted to lower wavenumber with increased intensity and broadening of peak as the ratio of colistin increased. This change in O-H peak can be attributed to the lengthening and weakening of O-H bond due to its attraction to hydrogen acceptor. This kind of hydrogen bond is called red-shifting hydrogen bond, which is very typical type of hydrogen bond (60). This FTIR pattern change is very similar to the result of previous literatures which showed the O-H of azithromycin is involved in hydrogen bond as a hydrogen donor (61). These spectral changes suggested that the O-H of azithromycin and Amide I C=O of colistin could participate in hydrogen bonding as hydrogen donor and acceptor, respectively. When the C=O group bonded to N-H is involved in hydrogen bond as a hydrogen acceptor, the amide II N-H bending can be shifted to higher wavenumber with decreased intensity even if hydrogen atom of N-H is not involved in an interaction with other hydrogen acceptor. This is called blue shifted bond which further substantiate the hydrogen bonding between azithromycin and colistin (54). It has been reported that the hydrogen bond can be formed between the carbonyl group of a water-soluble material and a hydrogen donor of a poorly water soluble material such as hydroxyl or amine group (62). The change in stretching band of axial C-O-C, which acts as a bridge connecting the lactone ring and the sugar substituents in chemical structure of azithromycin, indicates that the hydrogen bond between colistin and azithromycin influences the conformational change of azithromycin structure.
CONCLUSIONS
In this study, we have investigated the effects of surface composition and intermolecular interaction on the dissolution of individual drug from a range of colistin-azithromycin composite formulations. The results highlight that the surface composition of the composite particles and intermolecular interactions could substantially affect solubility and dissolution of the constituting components. The findings provide a new insight and greater fundamental understanding on the dissolution behavior and interfacial effects of composite formulations.
ACKNOWLEDGEMENTS AND DISCLOSURES
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI132681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Jian Li is an Australian NHMRC Senior Research Fellow. The authors are grateful for the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Future Industries Institute, University of South Australia. The authors are thankful for access to FTIR in Tonglei Li’s lab. Kind donations of RS01 DPI device from Plastiape S.p.A. and HPMC capsules from Qualicaps, Inc. are acknowledged.
Appendix
Appendix A1:
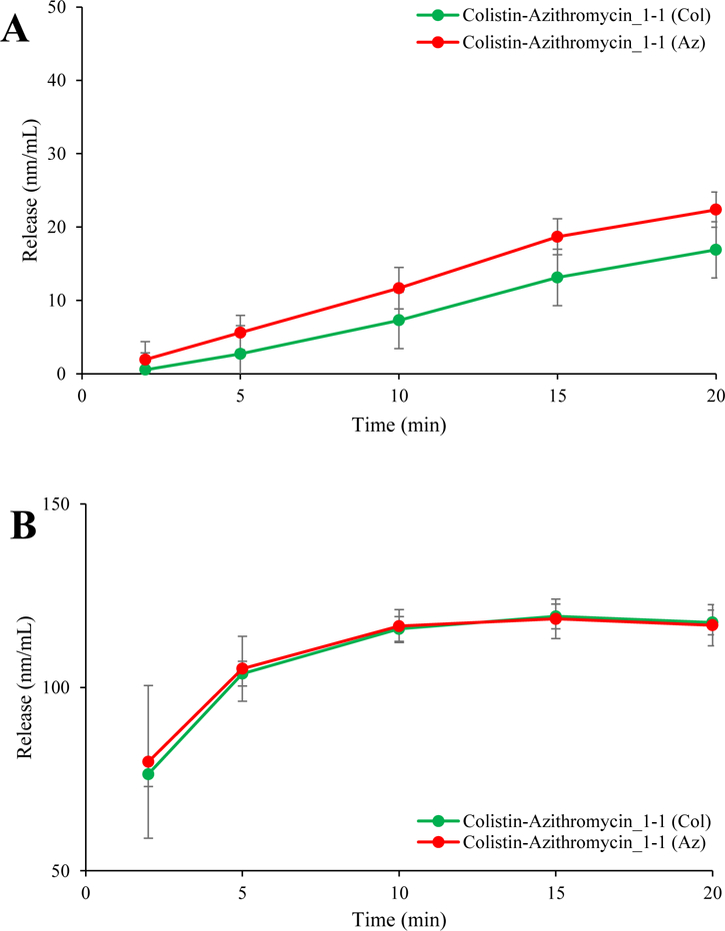
Dissolution rate of colistin and azithromycin (in nm) measured by the Franz diffusion method (A) or beaker method (B).
Appendix A2:
MMAD and GSD of spray dried formulations
| Formulations | MMAD (µm) | GSD (µm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Azithromycin | Colistin | Azithromycin | Colistin | |||||
| Azithromycin-SD | 1.5 | 0.06 | - | - | 2.2 | 0.01 | - | - |
| Colistin-Azithromycin_1–4 | 1.6 | 0.04 | 1.6 | 0.09 | 2.0 | 0.09 | 2.0 | 0.06 |
| Colistin-Azithromycin_1–2 | 1.8 | 0.14 | 1.9 | 0.14 | 1.8 | 0.06 | 1.9 | 0.10 |
| Colistin-Azithromycin_1–1 | 2.2 | 0.02 | 2.2 | 0.02 | 1.9 | 0.06 | 1.8 | 0.09 |
| Colistin-Azithromycin_2–1 | 1.5 | 0.01 | 1.6 | 0.03 | 2.0 | 0.15 | 2.1 | 0.15 |
| Colistin-SD | - | - | 1.7 | 0.16 | - | - | 3.1 | 0.22 |
REFERENCES
- 1.Mizgerd JP. Lung infection--a public health priority. PLoS medicine 2006;3(2):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. The 10 leading causes of death in the world, 2000 and 2012 whoint/mediacentre/factsheets/fs310/en/. May 2014.
- 3.Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. The American journal of medicine 2000;109(4):288–295. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine 2003;167(8):1090–1095. [DOI] [PubMed] [Google Scholar]
- 5.Patel I, Seemungal T, Wilks M, Lloyd-Owen S, Donaldson G, Wedzicha J. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002;57(9):759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velkov T, Abdul Rahim N, Zhou Q, Chan H-K, Li J. Inhaled anti-infective chemotherapy for respiratory tract infections: Successes, challenges and the road ahead. Advanced Drug Delivery Reviews 2015;85:65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traini D, Young PM. Delivery of antibiotics to the respiratory tract: an update. Expert Opinion on Drug Delivery 2009;6(9):897–905. [DOI] [PubMed] [Google Scholar]
- 8.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. Population Pharmacokinetics of Colistin Methanesulfonate and Formed Colistin in Critically Ill Patients from a Multicenter Study Provide Dosing Suggestions for Various Categories of Patients. Antimicrobial Agents and Chemotherapy 2011;55(7):3284–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yapa SWS, Li J, Porter CJH, Nation RL, Patel K, McIntosh MP. Population pharmacokinetics of colistin methanesulfonate in rats: Achieving sustained lung concentrations of colistin for targeting respiratory infections. Antimicrobial Agents and Chemotherapy 2013;57(10):5087–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cipolla D, Chan H-K. Inhaled antibiotics to treat lung infection. Pharmaceutical Patent Analyst 2013;2(5):647–663. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q, Girardi C, Zhang M, Bouhemad B, Louchahi K, Petitjean O, Wallet F, Becquemin M-H, Le Naour G, Marquette C-H, Rouby J-J. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Medicine 2010;36(7):1147–1155. [DOI] [PubMed] [Google Scholar]
- 12.Cai Y, Chai D, Wang R, Liang B, Bai N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. Journal of Antimicrobial Chemotherapy 2012;67(7):1607–1615. [DOI] [PubMed] [Google Scholar]
- 13.Paterson DL, Harris PNA. Colistin resistance: a major breach in our last line of defence. The Lancet Infectious Diseases 2016;16(2):132–133. [DOI] [PubMed] [Google Scholar]
- 14.Gurung J, Khyriem A, Banik A, Lyngdoh W, Choudhury B, Bhattacharyya P. Association of biofilm production with multidrug resistance among clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa from intensive care unit. Indian Journal of Critical Care Medicine 2013;17(4):214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HA, Ryu SY, Seo I, Suh SI, Suh MH, Baek WK. Biofilm Formation and Colistin Susceptibility of Acinetobacter baumannii Isolated from Korean Nosocomial Samples. Microbial drug resistance (Larchmont, NY) 2015;21(4):452–457. [DOI] [PubMed] [Google Scholar]
- 16.Imamura Y, Higashiyama Y, Tomono K, Izumikawa K, Yanagihara K, Ohno H, Miyazaki Y, Hirakata Y, Mizuta Y, Kadota J, Iglewski BH, Kohno S. Azithromycin exhibits bactericidal effects on Pseudomonas aeruginosa through interaction with the outer membrane. Antimicrob Agents Chemother 2005;49(4):1377–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favre-Bonté S, Köhler T, Van Delden C. Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. Journal of Antimicrobial Chemotherapy 2003;52(4):598–604. [DOI] [PubMed] [Google Scholar]
- 18.Gillis RJ, Iglewski BH. Azithromycin retards Pseudomonas aeruginosa biofilm formation. Journal of clinical microbiology 2004;42(12):5842–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotfried MH. Macrolides for the Treatment of Chronic Sinusitis, Asthma, and COPD. Chest 2004;125(2, Supplement):52S–61S. [DOI] [PubMed] [Google Scholar]
- 20.Molina SA, Hunt WR. Chapter 12-Cystic Fibrosis: An Overview of the Past, Present, and the Future A2-Sidhaye, Venkataramana K. In: Koval M, editor. Lung Epithelial Biology in the Pathogenesis of Pulmonary Disease Boston: Academic Press; 2017. p. 219–249. [Google Scholar]
- 21.Prescott WA, Johnson CE. Antiinflammatory Therapies for Cystic Fibrosis: Past, Present, and Future. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2005;25(4):555–573. [DOI] [PubMed] [Google Scholar]
- 22.Kumaraswamy M, Lin L, Olson J, Sun CF, Nonejuie P, Corriden R, Dohrmann S, Ali SR, Amaro D, Rohde M, Pogliano J, Sakoulas G, Nizet V. Standard susceptibility testing overlooks potent azithromycin activity and cationic peptide synergy against MDR Stenotrophomonas maltophilia. The Journal of antimicrobial chemotherapy 2016;71(5):1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timurkaynak F, Can F, Azap OK, Demirbilek M, Arslan H, Karaman SO. In vitro activities of non-traditional antimicrobials alone or in combination against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii isolated from intensive care units. International journal of antimicrobial agents 2006;27(3):224–228. [DOI] [PubMed] [Google Scholar]
- 24.Lin YW, Wong J, Qu L, Chan HK, Zhou QT. Powder production and particle engineering for dry powder inhaler formulations. Current pharmaceutical design 2015;21(27):3902–3916. [DOI] [PubMed] [Google Scholar]
- 25.Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respiratory care 2005;50(9):1209–1227. [PubMed] [Google Scholar]
- 26.Zhou Q, Tang P, Leung SSY, Chan JGY, Chan H-K. Emerging inhalation aerosol devices and strategies: Where are we headed? Advanced Drug Delivery Reviews 2014;75:3–17. [DOI] [PubMed] [Google Scholar]
- 27.Behara SRB, Worth Longest P, Farkas DR, Hindle M. Development and Comparison of New High-Efficiency Dry Powder Inhalers for Carrier-Free Formulations. Journal of Pharmaceutical Sciences 2014;103(2):465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warnken Z, Smyth HDC, Williams RO. Route-Specific Challenges in the Delivery of Poorly Water-Soluble Drugs. In: Williams RO, Watts AB, Miller DA, editors. Formulating Poorly Water Soluble Drugs, 2nd Edition; 2016. p. 1–39.
- 29.Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 1986;60. [DOI] [PubMed] [Google Scholar]
- 30.Mobley C, Hochhaus G. Methods used to assess pulmonary deposition and absorption of drugs. Drug Discovery Today 2001;6(7):367–375. [DOI] [PubMed] [Google Scholar]
- 31.Highlights of prescribing information for ZITHROMAX. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/050710s043,050711s040,050784s027lbl.pdf. Accessed 29 Oct 2018.
- 32.Aucamp M, Odendaal R, Liebenberg W, Hamman J. Amorphous azithromycin with improved aqueous solubility and intestinal membrane permeability. Drug Development and Industrial Pharmacy 2015;41(7):1100–1108. [DOI] [PubMed] [Google Scholar]
- 33.Mangal S, Nie H, Xu R, Guo R, Cavallaro A, Zemlyanov D, Zhou QT. Physico-Chemical Properties, Aerosolization and Dissolution of Co-Spray Dried Azithromycin Particles with L-Leucine for Inhalation. Pharmaceutical research 2018;35(2):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dengale SJ, Grohganz H, Rades T, Löbmann K. Recent advances in co-amorphous drug formulations. Advanced Drug Delivery Reviews 2016;100:116–125. [DOI] [PubMed] [Google Scholar]
- 35.Wallace SJ, Li J, Nation RL, Prankerd RJ, Velkov T, Boyd BJ. Self-Assembly Behavior of Colistin and Its Prodrug Colistin Methanesulfonate: Implications for Solution Stability and Solubilization. The Journal of Physical Chemistry B 2010;114(14):4836–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou QT, Loh ZH, Yu J, Sun SP, Gengenbach T, Denman JA, Li J, Chan HK. How Much Surface Coating of Hydrophobic Azithromycin Is Sufficient to Prevent Moisture-Induced Decrease in Aerosolisation of Hygroscopic Amorphous Colistin Powder? The AAPS journal 2016;18(5):1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan F, Bohr A, Maltesen MJ, Bjerregaard S, Foged C, Rantanen J, Yang M. Critical Solvent Properties Affecting the Particle Formation Process and Characteristics of Celecoxib-Loaded PLGA Microparticles via Spray-Drying. Pharmaceutical Research 2013;30(4):1065–1076. [DOI] [PubMed] [Google Scholar]
- 38.Shekunov BY, Chattopadhyay P, Tong HHY, Chow AHL. Particle Size Analysis in Pharmaceutics: Principles, Methods and Applications. Pharmaceutical Research 2007;24(2):203–227. [DOI] [PubMed] [Google Scholar]
- 39.Shetty N, Zeng L, Mangal S, Nie H, Rowles MR, Guo R, Han Y, Park JH, Zhou Q. Effects of Moisture-Induced Crystallization on the Aerosol Performance of Spray Dried Amorphous Ciprofloxacin Powder Formulations. Pharmaceutical Research 2018;35(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W, Zhou QT, Sun S-P, Denman JA, Gengenbach TR, Barraud N, Rice SA, Li J, Yang M, Chan H-K. Effects of Surface Composition on the Aerosolisation and Dissolution of Inhaled Antibiotic Combination Powders Consisting of Colistin and Rifampicin. The AAPS journal 2016;18(2):372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.May S, Jensen B, Wolkenhauer M, Schneider M, Lehr CM. Dissolution Techniques for In Vitro Testing of Dry Powders for Inhalation. Pharmaceutical Research 2012;29(8):2157–2166. [DOI] [PubMed] [Google Scholar]
- 42.Salama RO, Traini D, Chan H-K, Young PM. Preparation and characterisation of controlled release co-spray dried drug–polymer microparticles for inhalation 2: Evaluation of in vitro release profiling methodologies for controlled release respiratory aerosols. European Journal of Pharmaceutics and Biopharmaceutics 2008;70(1):145–152. [DOI] [PubMed] [Google Scholar]
- 43.Vehring R, Foss WR, Lechuga-Ballesteros D. Particle formation in spray drying. J Aerosol Sci 2007;38(7):728–746. [Google Scholar]
- 44.Vehring R Pharmaceutical particle engineering via spray drying. Pharm Res 2008;25(5):999–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaiswar DR, Jha D, Amin PD. Preparation and characterizations of stable amorphous solid solution of azithromycin by hot melt extrusion. Journal of Pharmaceutical Investigation 2016;46(7):655–668. [Google Scholar]
- 46.Freudenthal O, Quiles F, Francius G, Wojszko K, Gorczyca M, Korchowiec B, Rogalska E. Nanoscale investigation of the interaction of colistin with model phospholipid membranes by Langmuir technique, and combined infrared and force spectroscopies. Biochimica et biophysica acta 2016;1858(11):2592–2602. [DOI] [PubMed] [Google Scholar]
- 47.Chew NYK, Tang P, Chan HK, Raper JA. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm Res 2005;22(1):148–152. [DOI] [PubMed] [Google Scholar]
- 48.Lechuga-Ballesteros D, Charan C, Stults CLM, Stevenson CL, Miller DP, Vehring R, Tep V, Kuo M-C. Trileucine improves aerosol performance and stability of spray-dried powders for inhalation. J Pharm Sci 2008;97(1):287–302. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Q, Leung SSY, Tang P, Parumasivam T, Loh ZH, Chan H-K. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Advanced Drug Delivery Reviews 2015;85:83–99. [DOI] [PubMed] [Google Scholar]
- 50.Lin L, Nonejuie P, Munguia J, Hollands A, Olson J, Dam Q, Kumaraswamy M, Rivera H Jr, Corriden R, Rohde M, Hensler ME, Burkart MD, Pogliano J, Sakoulas G, Nizet V. Azithromycin Synergizes with Cationic Antimicrobial Peptides to Exert Bactericidal and Therapeutic Activity Against Highly Multidrug-Resistant Gram-Negative Bacterial Pathogens. EBioMedicine 2015;2(7):690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bae S, Kim M-C, Park S-J, Kim HS, Sung H, Kim M-N, Kim S-H, Lee S-O, Choi S-H, Woo JH, Kim YS, Chong YP. In vitro synergistic activity of antimicrobial agents in combination against clinical isolates of colistin-resistant Acinetobacter baumannii. Antimicrobial Agents and Chemotherapy 2016. [DOI] [PMC free article] [PubMed]
- 52.Chan JGY, Tyne AS, Pang A, Chan H-K, Young PM, Britton WJ, Duke CC, Traini D. A Rifapentine-Containing Inhaled Triple Antibiotic Formulation for Rapid Treatment of Tubercular Infection. Pharmaceutical Research 2014;31(5):1239–1253. [DOI] [PubMed] [Google Scholar]
- 53.Baldelli A, Vehring R. Analysis of cohesion forces between monodisperse microparticles with rough surfaces. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2016;506:179–189. [Google Scholar]
- 54.Boraey MA, Hoe S, Sharif H, Miller DP, Lechuga-Ballesteros D, Vehring R. Improvement of the dispersibility of spray-dried budesonide powders using leucine in an ethanol–water cosolvent system. Powder technology 2013;236:171–178. [Google Scholar]
- 55.Mangal S, Park H, Zeng L, Heidi HY, Lin Y-w, Velkov T, Denman JA, Zemlyanov D, Li J, Zhou QT. Composite particle formulations of colistin and meropenem with improved in-vitro bacterial killing and aerosolization for inhalation. International journal of pharmaceutics 2018;548(1):443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iranloye TA, Parrott EL. Effects of compression force, particle size, and lubricants on dissolution rate. Journal of Pharmaceutical Sciences 1978;67(4):535–539. [DOI] [PubMed] [Google Scholar]
- 57.Löbmann K, Laitinen R, Strachan C, Rades T, Grohganz H. Amino acids as co-amorphous stabilizers for poorly water-soluble drugs – Part 2: Molecular interactions. European Journal of Pharmaceutics and Biopharmaceutics 2013;85(3, Part B):882–888. [DOI] [PubMed] [Google Scholar]
- 58.Jensen KT, Blaabjerg LI, Lenz E, Bohr A, Grohganz H, Kleinebudde P, Rades T, Löbmann K. Preparation and characterization of spray-dried co-amorphous drug–amino acid salts. Journal of Pharmacy and Pharmacology 2016;68(5):615–624. [DOI] [PubMed] [Google Scholar]
- 59.Mestres C, Alsina MA, Busquets MA, Murányi I, Reig F. Interaction of colistin with lipids in liposomes and monolayers. International Journal of Pharmaceutics 1998;160(1):99–107. [Google Scholar]
- 60.Joseph J, Jemmis ED. Red-, blue-, or no-shift in hydrogen bonds: a unified explanation. Journal of the American Chemical Society 2007;129(15):4620–4632. [DOI] [PubMed] [Google Scholar]
- 61.Kauss T, Gaubert A, Boyer C, Ba BB, Manse M, Massip S, Léger J-M, Fawaz F, Lembege M, Boiron J-M. Pharmaceutical development and optimization of azithromycin suppository for paediatric use. International journal of pharmaceutics 2013;441(1–2):218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanaze FI, Kokkalou E, Niopas I, Georgarakis M, Stergiou A, Bikiaris D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: A comparative study. Journal of Applied Polymer Science 2006;102(1):460–471. [Google Scholar]