Abstract
Introduction:
Paper spray mass spectrometry has provided a rapid, quantitative ambient ionization method for xenobiotic and biomolecule analysis. As an alternative to traditional sample preparation and chromatography, paper spray demonstrates the sampling ionization of a wide range of molecules and significant sensitivity from complex biofluids. The amenability of paper spray with dried blood spots and other sampling types shows strong potential for rapid, point-of-care (POC) analysis without time-consuming separation procedures.
Areas Covered:
This special report summarizes the current state and advances in paper spray mass spectrometry that relate to its applicability for clinical analysis. It also provides our perspectives on the future development of paper spray mass spectrometry and its potential roles in clinical settings.
Expert Commentary:
Paper spray has provided the fundamental aspects of ambient ionization needed for implementation at the POC. With further clinical management and standardization, paper spray has the potential to replace traditional complex analysis procedure for rapid quantitative detection of illicit drugs, therapeutic drugs and metabolites. Surface and substrate modifications also offer significant improvement in desorption and ionization efficiencies, resulting in enhanced sensitivity. Comprehensive analysis of metabolites and lipids will further extend the implementation of paper spray ionization mass spectrometry into clinical applications.
Keywords: Paper spray, ambient ionization, mass spectrometry, clinical analysis, quantitation
1. Introduction
Mass spectrometry (MS) has become one of the most important tools for biomedical analysis, especially for mixture analysis in complicated matrices. Among the extensive toolbox that accompanies mass spectrometry, ambient ionization has been significant in supplanting time consuming analytical procedures and developing MS-based point-of-care (POC) methods in clinical applications[1]. Paper spray (PS) is a representative ambient ionization method that has shown potential as a versatile direct analysis method for raw biofluid samples with significant quantitative power[2]. Due to its simplicity and efficacy, PS-MS has garnered significant interest. Numerous papers have published various modifications, methods and potential applications that demonstrate the rapid, quantitative power of PS-MS through targeted analysis of various molecules, including illicit drugs, therapeutic drugs, metabolites, lipids and proteins in biomedical samples[3–5]. PS-MS also represents a promising MS-based POC method, that requires minimal sample handling, little biohazard and chemical waste while remaining inexpensive[1,3]. The wide ionization capability of paper spray in tandem with MS, or ideally miniature MS, provides immense potential in translating it to a powerful clinical tool.
2. Methodology
The typical analytical method by PS-MS requires no sample preparation, where an aliquot of raw sample (< 5 µL) is directly deposited onto the substrate, dried and analyzed by MS as seen in Figure 1. By drying the sample, the complex matrices in the raw sample are bound to the porous substrate, minimizing matrix effects and preventing clogging. Solvent and high voltages are applied to the substrate for analyte extraction, transportation and ionization. Electrospray-like ionization occurs at the pre-formed macroscopically sharp tip, producing gas phase ions[6]. Without the need for sample preparation, analysis by PS-MS is simple and quick (less than a minute) while retaining significant specificity, sensitivity, and quantitative power for clinical applications[7].
Figure 1.
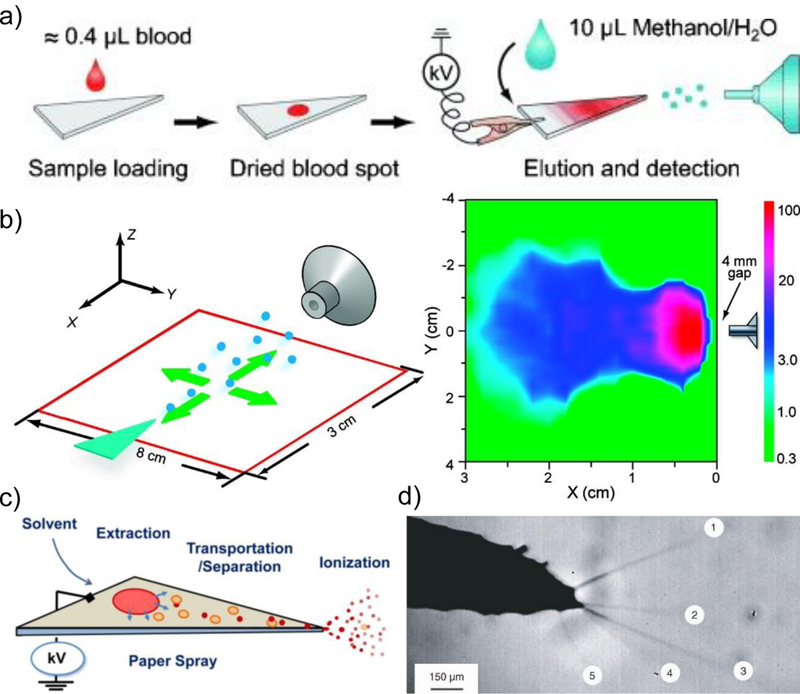
(a) Workflow for traditional PS-MS analysis [2]. (b) Spatial characterization of the paper spray intensity in relation to mass spectrometry inlet [5]. (c) Hypothesized three steps for paper spray ionization process [9]. (d) Multiple Taylor cone formation from paper spray ionization [3].
3. Paper spray mass spectrometry in clinical applications
3.1. Analysis of drugs of abuse
PS-MS has been well developed across numerous studies for the quantitation of drugs of abuse in dried blood spots (DBS) and urine samples. Although different analytes have reported varying limits of detection (LODs) (summarized in Table 1), most reported PS-MS methods were sensitive enough to quantify low amounts of drugs of abuse in physiological environments. Common drugs of abuse such as cocaine, methamphetamine and psychoactive substances like fentanyls have been quantified by PS-MS at ng/mL or even sub-ng/mL levels in biofluids, which provide insights into not only clinical applications, but also forensics[8–10]. Beside biofluid analysis, there may be demand on analyzing drugs of abuse on other kinds of matrices; for example, synthetic cannabinoids on a solid surface[11]. LODs of 2 ng were reported for six different synthetic cannabinoids by direct sampling by paper spray. The nature of paper substrates also makes it possible to combine fingerprint collecting with PS-MS analysis. An interesting work was presented for fast analysis of cocaine and its metabolites on fingerprints[12]. PS-MS was applied to a total of 239 fingerprint samples collected on triangle papers, yielding a 98.7% detection rate. Further visualization tests of fingerprint based on ridge characteristics can be used to identify the source of samples.
Table 1.
List of therapeutic and illicit drugs that have been analyzed by paper spray mass spectrometry.
| Analyte | Analyte Class | Molecular Weight (Da) | logP* | LOD/LOQ (ng/mL) | Reference |
|---|---|---|---|---|---|
| Acetaminophen | Pharmaceutical | 151.16 | 0.5 | 250 | [15] |
| Amitriptyline | Pharmaceutical | 277.41 | 4.92 | 1 | [6,7,15,16] |
| Amphetamine | Illicit | 135.21 | 1.76 | 1 | [30] |
| Anabasine | Pharmaceutical | 308.37 | 3.57 | 0.1 | [9] |
| Atenolol | Pharmaceutical | 266.34 | 0.57 | 50 | [5] |
| Benzethonium | Pharma | 412.637 | 3.13 | 0.02 | [15,20] |
| Benzoylecgonine | Metabolite | 289.33 | 1.71 | 1 | [11] |
| Buprenorphine | Illicit | 467.64 | 4.98 | 1.6 | [11] |
| Citalopram | Pharmaceutical | 324.39 | 3.5 | 0.1 | [15,16] |
| Cocaine | Illicit | 303.12 | 2.3 | 0.05 | [11,16] |
| Cotinine | Metabolite | 176.219 | 0.07 | 3,2,5 | [9] |
| Cyclophosphamide | Pharmaceutical | 261 | 0.8 | 11 | [8] |
| Cyclosporine | Pharmaceutical | 1202.63 | 3.64 | 5 | [14] |
| Dextromorphan | Pharmaceutical | 271.4 | 3.6 | 0.6 | [15] |
| Docetaxel | Pharmaceutical | 807.88 | 2.4 | 13 | [8] |
| Heroin | Illicit | 369.41 | 1.58 | 125 | [5,11] |
| Hydralazine | Pharmaceutical | 160.176 | 1 | 16 | [9] |
| Ibuprofen | Pharmaceutical | 206.28 | 3.97 | 500 | [15] |
| Imatinib | Pharmaceutical | 493.6 | 3 | 9 | [2,8,39] |
| Irinotecan | Pharmaceutical | 586.67 | 3.2 | 13 | [8] |
| Lidocaine | Pharmaceutical | 234.34 | 2.44 | 4 | [16,17] |
| MDMA | Illicit | 193.24 | 1.65 | 0.04 | [30] |
| Methamphetamine | Illicit | 149.23 | 2.07 | 0.3 | [11,30] |
| Morphine | Pharmaceutical/Illicit | 285.34 | 0.89 | 12 | [11,30] |
| Nicotine | Toxic Alkaloid | 162.23 | 1.17 | 1 | [9] |
| Oxycodone | Illicit | 315.36 | 0.3 | 16 | [11] |
| Paclitaxel | Pharmaceutical | 853.9 | 3 | 12,15 | [8,15] |
| Pazopanib | Pharmaceutical | 437.52 | 3.59 | 0.5 | [8] |
| Proguanil | Pharmaceutical | 253.73 | 2.53 | 0.08 | [15] |
| Simvastatin | Pharmaceutical | 418.57 | 4.68 | 50 | [15] |
| Sirolimus | Pharmaceutical | 914.19 | 4.3 | 0.5 | [14] |
| Sitamaquine | Pharmaceutical | 343.51 | 5.11 | 5 | [15] |
| Sunitinib | Pharmaceutical | 398.47 | 5.2 | 0.1 | [20] |
| Tacrolimus | Pharmaceutical | 804.01 | 3.3 | 0.2 | [18] |
| Tamoxifen | Pharmaceutical | 371.51 | 7.1 | 8 | [8] |
| Telmisartan | Pharmaceutical | 514.62 | 7.7 | 0.3 | [15] |
| THC | Illicit | 314.46 | 5.65 | 4 | [30] |
| Topotecan | Pharmaceutical | 421.45 | 0.8 | 17 | [8] |
| Verapamil | Pharmaceutical | 454.6 | 3.79 | 3, 0.75, 0.1 | [15–17] |
LogP values were found from PubChem
3.2. Therapeutic drug monitoring
The initial application of PS-MS started with the analysis of different therapeutic drugs. As an early demonstration, PS-MS was used to analyze imatinib in blood samples, showing a wide linear quantitation range which covered the entire effective therapeutic range [2]. Good quantitative performances have also been observed on PS-MS based analysis of therapeutic drugs such as verapamil, amitriptyline, and sirolimus[13–16]. For drugs with narrow therapeutic ranges or wide variability in adsorption among individuals, pharmacokinetic studies are critical. The use of chromatography for these studies is time-consuming, leading to drug dosing approximations and potential exposure to side effects[13,17]. Several studies have shown the development of pharmacokinetic curves through PS-MS, as seen in Figure 2[18,19]. Pharmacokinetic parameters can be obtained quickly after blood collection, minimizing drug degradation during blood storage.
Figure 2.
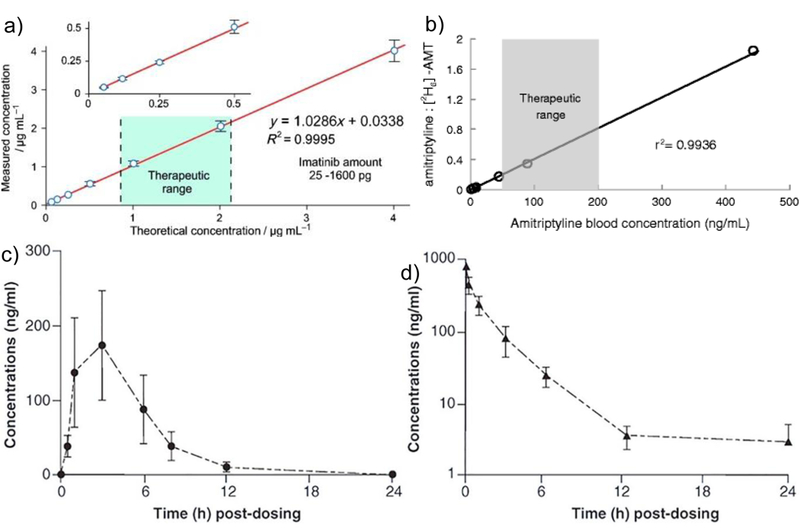
(a) Quantitation curve for Imatinib with identified therapeutic range drawn by PS-MS [2]. (b) Quantitation curve for amitriptyline spiked in blood, drawn by PS-MS. Pharmacokinetic curves drawn by PS-MS: (c) oral administration of (15 mg/kg) and (d) IV administration (5 mg/kg) of sunitinib in mice.
3.3. Metabolite analysis
Metabolite analysis is important for the study of unique chemical information that is specific to cellular processes. PS-MS also found its role in metabolite analysis. For example, by well-developed DBS-based PS-MS, underivatized acylcarnitines were quantified in serum and blood samples[20]. The identification of elevated levels of specific acylcarnitines has been proven as significant biomarkers for fatty acid oxidation disorders. The application of PS-MS takes advantage of DBS in newborn screening, enabling rapid, quantitative screening of newborns for metabolic disorders. Environmental carcinogenic chemicals such as polycyclic aromatic hydrocarbon quinones that are also present in biological samples have also been analyzed by PS-MS[21]. One of the challenges of this class of metabolites is its poor ionization efficiency, resulting in a required derivatization for analysis. Reactive paper spray was developed for this purpose, which enables the simultaneous detection and ionization of target compounds on paper substrates. For the determination of quinones in urine, serum, and cell culture medium, LODs as low as 0.04 ng were obtained by reactive PS-MS.
Tissue samples have also been analyzed directly by PS-MS for metabolite analysis. For clinical analysis, it can be combined with clinical practices such as biopsy[22]. In this work, tissue samples from biopsy were deposited onto the paper substrates, following with solvent application and MS analysis. Lipids of different classes were observed, and the lipid profiles were used to differentiate human prostate tumor and normal tissue. This has potential applications for a surgery room, where biopsy punch analysis can be done at POC for tumor identification. Similar with tissue samples, microorganisms can be deposited directly onto the paper substrate for PS-MS analysis[23,24]. This method has been reported to discriminate Gram positive/negative bacteria through bacteria-specific phospholipids. Along with numerical data fusion, six bacterial colonies could be differentiated, which provided the basis for rapid identification in bacteria.
3.4. Protein analysis
Since large molecules are apt to bind onto the cellulose paper substrate, PS-MS has largely been considered to be inapplicable for protein analysis. However, efforts were still made for protein analysis due to the unique advantages of PS-MS. Through standard PS-MS methods with proper solvents (Whatman Grade 1, methanol/water (1:1, v/v), standard solution), angiotensin I and cytochrome C were observed at a concentration of 8 µg/mL[2]. As an example a mixture analysis, ionization was achieved for noncovalent protein complexes in human blood detritus with removed plasma[25]. Several noncovalent protein complexes were characterized by ion mobility spectroscopy, including TTR tetramer, dfAT dodecamer, conA tetramer, SAP pentamer, and human hemoglobin tetramer. Further characterization of intact proteins in gel was done by modifying carbon nanotubes which increased the surface area, pore diameter and conductivity of the paper substrate[26]. This allows for an electrophoresis-like behavior in the paper where proteins are extracted and migrated to the tip for ionization. Cytochrome C, lysozyme, and myoglobin were successfully separated in-gel and analyzed by PS-MS in ambient conditions. Improvements in analyzing tryptic digests and protein identifications of native proteins, noncovalent complexes and organometallic complexes were also achieved by using wooden substrates[27]. Due to the porous and hydrophilic nature of the wood substrate, protein and tryptic digests were able to effectively adhere to the substrate and subsequently ionized. This method was also used to analyze initial ionization of undigested protein (~ 10 s) and then a transition to sustained ionization of tryptic peptides, which is hypothesized to occur due to differences in surface activity.
4. Advances in paper spray
The performance for PS-MS analysis of targeted analytes is affected by various factors that influence extraction, transportation and ionization of analytes, such as the solvent or substrate. An appropriately selected solvent aids in analyte desorption and Taylor cone formation. Otherwise, suppressed ionization may occur[28]. Similarly, different substrates are suitable for different applications. A study reported that printing paper was found to be amenable for paper spray, where its smaller pore size allows for immediate analysis of drugs of abuse in raw blood while lowering background signals [29]. By altering these factors, several paper-spray derivates were developed, as well as several new protocol developments for implementation into clinical practices.
4.1. Modifications to paper
One of the biggest recent advancements to paper spray is the introduction of materials onto the paper substrates. In order to improve extraction and ionization of targeted analytes, various materials such as polystyrene microspheres, graphene or carbon nanotubes have shown to be bound to the substrate as seen in Figure 3. By coating the paper with polystyrene microspheres, sensitivity was found to increase 10 to 546-fold for the analysis of various therapeutic drugs[30]. Mesoporous graphene coatings have also been used to improve separation and elution efficiency through highly specific surface areas and increased electrical conductivity; linear dynamic ranges are expanded 10-fold in comparison to unmodified paper spray with a low limit of quantitation as low as 1 pg/mL[31]. Another study used a molecularly imprinted polymer substrate to analyze cocaine in oral fluids, obtaining a LOQ of 1 ng/mL and less than 15% inter/intra-day precision and accuracy[32]. Further modifications to the substrate include developing a hydrophobic layer on paper spray to perform rapid liquid-liquid extraction from a sample droplet[33]. The addition of an organic solvent helps selectively extract several illicit drugs in different biofluids, improving sensitivity and quantitative accuracy to parts-per-trillion levels. Combinations of several modifications have been studied, where an aptamer-modified carbon nanotube paper spray was used to improve detection of methamphetamine [34]. Carbon nanotubes have shown improved ionization due to generation of high electric fields[35], while aptamer-modifications have demonstrated selective extraction in complex matrices[36,37]. The combination of both modifications showed improved sensitivity and selectivity, reporting LODs of 0.45 ng/mL in plasma and saliva.
Figure 3.
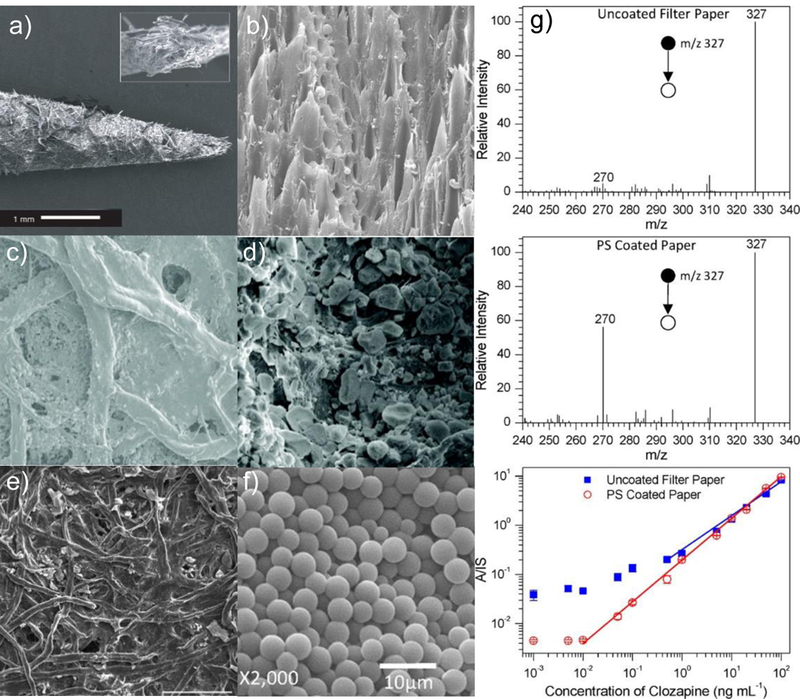
SEM surface images of paper spray and ps-modifications. (a) Unmodified paper spray [3]. (b) Wooden tip spray [27]. (c) Silica coated paper (d). Close-up of silica coated paper [15] (e) Graphene coated paper [31]. (f) Polystyrene microspheres. (g) Comparison of PS-MS/MS of clozapine with uncoated paper and PS-coated paper with polystyrene microspheres. Quantitation curves are compared for coated and uncoated PS, showing improved detection limits for PS coated paper[30].
4.2. Method development for clinical practices
With the development of more sensitive, application-specific PS-MS methods, attention has begun to shift towards the adoption of PS for clinical applications. The amenability of PS-MS for DBS analysis enables the utilization of sampling protocols that involve minimally or non-invasive biofluid collection[38]. After appropriate training, finger prick blood can be directly drawn onto the paper and analyzed by healthcare professionals. For urine sampling, a disposable pipette can be provided to draw a consistent volume of urine sample and then analyzed. Although PS-MS analysis has not been involved in real clinical studies yet, a significant effort has been focused on quantifying single target analyte and identifying quantitative linearity throughout the target drug therapeutic range. Several studies reported the simultaneous detection of eight drugs in whole blood by PS-MS, with LODs well under physiological relevance[39,40]. The PS-MS assay for drugs of abuse in this study was developed by following SWGTOX standard practices for method validation in forensic toxicology and achieved quantification in less than two minutes per sample. The first application of PS-MS in a clinical laboratory was reported for therapeutic drug monitoring of tacrolimus, an immunosuppressant with a narrow therapeutic drug range, which was cross-validated by FDA approved immunoassays and LC-MS/MS assays[17]. The PS-MS method showed a significant correlation in accuracy to both the immunoassay and LC-MS/MS method by pure tacrolimus solution.
5. Conclusion
PS-MS has been shown to be amenable for analysis of drugs and other compounds in biomedical samples. By binding different materials or functional polymers onto the paper surface, the capabilities of paper spray have expanded, allowing for metabolite profiling or biomarker discovery. Furthermore, PS-MS studies on multiplexing protocol development and real clinical samples provide the validation necessary for clinical applications. Paper spray’s simplicity and versatility have great potential for MS-based analysis at the point of care without compromising significant levels of sensitivity and LODs.
6. Expert commentary
Since its inception, paper spray has been one of the most widely-used ambient ionization methods. Its simplicity, ease-of-use and amenability to analyzing complex biofluids have made it an ideal candidate as a significant ionization alternative. Xenobiotic analysis has been well-characterized with numerous studies showing significant levels of sensitivity (as low as sub-ppt levels) for quantitation and targeted analysis[10,12,41]. Similarly, recent advancements in analyzing endogenous biomolecules, which traditionally had been challenging for PS-MS, have reported preliminary investigations quantifying significant metabolites and lipid profiling of tissue samples[26]. Developing a comprehensive PS-MS system that can be operated in a clinical setting could be greatly advantageous, providing patients with inexpensive, rapid biofluid diagnostics and monitoring. Despite its potential, PS-MS is still a relatively young analytical method; several issues still remain to be addressed before use in clinical applications.
One of the biggest challenges of translating PS-MS from research applications to clinical applications is its standardization. Although numerous studies have shown significant quantitative power by paper spray, the reported experimental conditions differ and result in different sensitivities and LODs as seen in Table 1. Factors such as substrate type, tip quality, distance to MS inlet and differences in sample matrices can affect analyte recovery and subsequently, quantitation[5,10]. Fortunately, strategies have been pursued to calibrate PS-MS and improve its reproducibility, such as development of simple protocols, disposable paper spray cartridges, and automated paper spray devices[40–44]. Another key strategy for accurate quantitation is the use of internal standards (IS) through simple procedures. Several methods have been proposed for the incorporation of IS such as preprinting onto the paper substrate and spiking into clinical samples[45]. However, these methods rely upon standardized routine use of IS and may still require validation of quantitative accuracy. Another potential option without using IS is the monitoring of metabolite or lipid profiles for certain diseases such as prostate cancer [22,46]. Recent advancements in improving extraction and ionization efficiencies through substrate modifications show strong potential in developing metabolite quantitative protocols for disease diagnosis. Even so, standardizing experimental protocols through either quantitation with IS or metabolomic profiling is crucial for clinical applications.
Even with standardized protocols, care must also be taken to objectively characterize the practicality of PS-MS implementation and identify its role in the overall clinical framework. In a direct comparison to LC-MS, as summarized in Table 2, several limitations are highlighted. Studies have shown that PS can be applied as an alternative to chromatographic separation in analytical laboratories for traditional xenobiotic and biomolecule analysis; several of which have been demonstrated as shown in Figure 4. Yet, PS cannot analyze some compounds that require chromatographic or chiral separations, nor is it suitable for comprehensive analysis of a broad range of chemicals with high sensitivity. PS was not developed to supplant LC-MS, but rather to be utilized as a targeted investigatory tool for POC routine sampling to eliminate unnecessary testing. For these applications, one must consider the practicality of a MS-based POC method. Although PS fulfills POC criteria such as costs, ease-of-use, and portability, conventional MS instrumentation are unfeasible in a clinical setting and therefore require cheaper alternatives like miniature mass spectrometry systems. Numerous studies have hinted at a combination of PS with portable/miniature mass spectrometers, but these technologies still need to undergo significant clinical studies to validate its overall robustness and evaluate its practicality in a clinical setting[1,6,38]. To become an ideal POC methodology for routine sampling, PS-MS will need to address concerns related to standardization, costs and necessary training. Novel POC methods have been constantly introduced for disease screening or routine tests by varying extraction and detection methods. The criteria such as analytical performance, costs, ease-of-use, and integration are all decisive factors. The MS-based clinical detection provides a significant advantage over other technologies in its sensitivity and amenability to analyze various molecules, while PS will continue to emerge as the ideal complementary tool for targeted clinical analysis of both xenobiotics and endogenous biomolecules.
Table 2.
Criteria Comparison between PS-MS and LC-MS/MS
| Criteria | Paper Spray (PS) | LC-MS/MS |
|---|---|---|
| Extraction Method | None | liquid extraction or solid-phase extraction |
| Extraction Selectivity | Minimal; Improvements in substrate modifications could improve selectivity | Highly selective; pre-existing methods for hard to separate compounds like isomers, chiral compounds, etc. |
| Sample Preparation | None | Manual preparation |
| Sample Volume | < 10 uL | > 10 uL |
| Analysis Time | Short ( < 1 min) | Long (~ 30 min) |
| Cost per Analysis | Cheap | Expensive |
| Reusability | Disposable Cartridges | Expensive Instruments |
| Compatibility to current MS Devices | Compatible | Compatible |
| Sensitivity | Appropriate for targeted analysis | High |
| Analytical Range | Low-ppb to ppm (Improvements to ppt) |
Sub-ppt to ppm |
| Easy-to-Use | Yes | No (Requires trained professionals) |
| Solvent Use | Minimal | Large Volume |
| In Situ Analysis | Yes | No |
Figure 4.
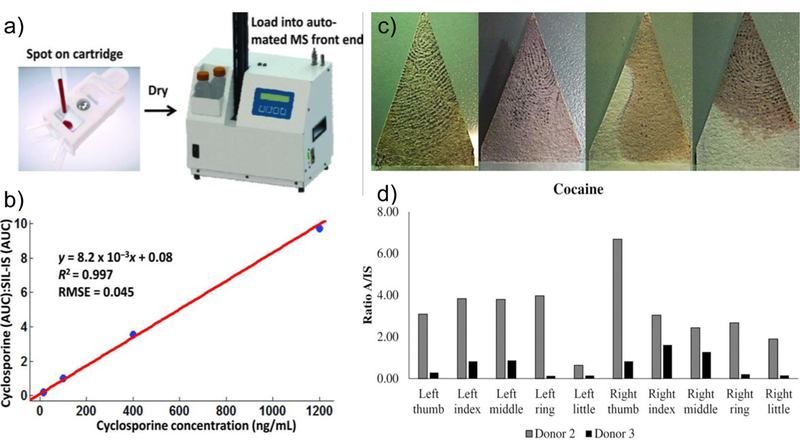
(a) Rapid measurement of cyclosporine by automated PS-MS instrument. (b) Quantification was done by AUC method for each analyte, normalized by pre-spotted internal standard [13]. (c) Coupling of cocaine testing by PS-MS with silver-nitrate based fingerprints collecting. (d) Analyte to internal standard ratios for every finger of two participants that were seeking treatments at a rehabilitation center [12].
7. Five-year view
We envision that PS-MS will be readily introduced into clinical applications, as progress in protocol development and technological advancements have diminished the variability and improved reproducibility. This can also be observed in several settings, including analytical laboratories and healthcare clinics. Currently, further development and modification of PS-MS method is still necessary. In order to improve the ionization of a wider range of compounds, we propose to further develop new substrates or modify the paper substrates with functional materials. Cartridge development will critical in bridging PS-MS into clinically-relevant analytical techniques, which requires a fast, high-throughput, reproducible, and easy-to-operate analytical method by non-professionals. Integrating MS-based immunoassays and other more selective methods would help improve the overall selectivity of paper spray and improve its value as a convenient ionization technique that can overcome the more expensive requirements of chromatography.
In terms of clinical applications, we envision a more comprehensive analysis to be reported. In addition to the current range of drugs that have been analyzed, more attention should be invested in metabolites such as amino acids, small organic acids and lipids, which have been proven to have good ionization efficiencies by paper spray. PS-MS based shotgun metabolomics and lipidomics may have a high impact on biomarker discovery and disease differentiation. The utilization of paper spray allows for rapid screening and verification of potential metabolites, and can be used further downstream for clinical diagnostics, once verified. PS-MS may also find its role in batch analysis of clinical samples. It is very possible that PS-MS could take over large-scale quantitation of therapeutic drugs for pre-clinical and therapeutic drug monitoring of new drugs entering the market, which has been predominately done through LC-MS. Last but not the least, both POC and in-field clinical analyses show strong promise for the adoption of paper spray coupled with miniature mass spectrometry systems.
Key Issues.
Mass spectrometry is a powerful tool for clinical analysis due to its capability in the analysis of complex samples and great quantitative performance, even in low concentrations. However, traditional mass spectrometry analysis usually requires rigorous sample preparation or purification.
Paper spray is a sample preparation-free sampling ionization method, which has been well-characterized in drugs of abuse and therapeutic drugs but lacks standardization for clinical applications.
Modification of paper substrates can improve the extraction, desorption and ionization efficiencies, thus improving sensitivity for PS-MS analysis of drugs or other molecules
Large molecules such as proteins are apt to bind with the cellulose paper substrate, calling for further improvements to be done before application of PS-MS for protein analysis.
Acknowledgments
Funding
The work included in this paper done by the authors was supported by grants from the National Natural Science Foundation of China [Project 21627807] and National Institutes of Health [Project 1R01AI122298 and R01AI122932].
Footnotes
Declaration of interest
Z. Ouyang is the founder of PURSPEC Technologies Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Papers of special note have been highlighted:
of interest (•)
of considerable interest (••) to readers.
References
- 1.Ferreira CR, Yannell KE, Jarmusch AK, Pirro V, Ouyang Z, Graham Cooks R. Ambient ionization mass spectrometry for point-of-care diagnostics and other clinical measurements. Clinical Chemistry 2016. [DOI] [PMC free article] [PubMed]
- 2.Wang H, Liu J, Cooks RG, Ouyang Z. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angew Chemie 2010;122(5):889–92.•• First report of paper spray mass spectrometry method.
- 3.Lin C-H, Liao W-C, Chen H-K, Kuo T-Y. Paper spray-MS for bioanalysis. Bioanalysis 2014;6(2):199–208. [DOI] [PubMed] [Google Scholar]
- 4.Manicke NE, Bills BJ, Zhang C. Analysis of biofluids by paper spray MS: advances and challenges. Bioanalysis 2016;8(6):589–606. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Wang H, Manicke NE, Lin J, Cooks RG. Application of Paper Spray Ionization Development, Characterization, and Application of Paper Spray Ionization. Anal Chem 2010;82(6):2463–71. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q, Wang H, Maas JD, Chappell WJ, Manicke NE, Cooks RG, Ouyang Z. Paper spray ionization devices for direct, biomedical analysis using mass spectrometry. Int J Mass Spectrom 2012;312:201–7.•• Development and validation of paper spray cartridges.
- 7.Li L, Chen T-C, Ren Y, Hendricks PI, Cooks RG, Ouyang Z. Mini 12, Miniature Mass Spectrometer for Clinical and Other Applications—Introduction and Characterization. Anal Chem 2014;86(6):2909–16.•• Paper spray coupled miniature mass spectrometer for biological analysis.
- 8.Su Y, Wang H, Liu J, Wei P, Cooks RG, Ouyang Z. Quantitative paper spray mass spectrometry analysis of drugs of abuse. Analyst 2013;138(16):4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy JH, Palaty J, Gill CG, Wiseman JM. Rapid analysis of fentanyls and other novel psychoactive substances in substance use disorder patient urine using paper spray mass spectrometry. Rapid Commun Mass Spectrom 2018;32(15):1280–6. [DOI] [PubMed] [Google Scholar]
- 10.Vandergrift GW, Hessels AJ, Palaty J, Krogh ET, Gill CG. Paper spray mass spectrometry for the direct, semi-quantitative measurement of fentanyl and norfentanyl in complex matrices. Clin Biochem 2018;54:106–11. [DOI] [PubMed] [Google Scholar]
- 11.Ma Q, Bai H, Li W, Wang C, Cooks RG, Ouyang Z. Rapid analysis of synthetic cannabinoids using a miniature mass spectrometer with ambient ionization capability. Talanta 2015;142:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa C, Webb R, Palitsin V, Ismail M, De Puit M, Atkinson S, Bailey MJ. Rapid, secure drug testing using fingerprint development and paper spray mass spectrometry. Clin Chem 2017;63(11):1745–52. [DOI] [PubMed] [Google Scholar]
- 13.Shi RZ, El Gierari ETM, Faix JD, Manicke NE. Rapid measurement of cyclosporine and sirolimus in whole blood by paper spray-tandem mass spectrometry. Clin Chem 2016;62(1):295–7. [DOI] [PubMed] [Google Scholar]
- 14.Manicke NE, Abu-Rabie P, Spooner N, Ouyang Z, Cooks RG. Quantitative analysis of therapeutic drugs in dried blood spot samples by paper spray mass spectrometry: An avenue to therapeutic drug monitoring. J Am Soc Mass Spectrom 2011;22(9):1501–7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Xu W, Manicke NE, Cooks RG, Ouyang Z. Silica coated paper substrate for paper-spray analysis of therapeutic drugs in dried blood spots. Anal Chem 2012;84(2):931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooks RG, Manicke NE, Dill AL, Ifa DR, Eberlin LS, Costa AB, Wang H, Huang G, Ouyang Z. New ionization methods and miniature mass spectrometers for biomedicine: DESI imaging for cancer diagnostics and paper spray ionization for therapeutic drug monitoring. Faraday Discuss 2011;149:247–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi R-Z, El Gierari ETM, Manicke NE, Faix JD. Rapid measurement of tacrolimus in whole blood by paper spray-tandem mass spectrometry (PS-MS/MS). Clin Chim Acta 2015;441:99–104.•• First report of eveluation of paper spray mass spectrometry for clinical practices.
- 18.Shah VP, Midha KK, Findlay JWA, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A. Bioanalytical Method Validation - A Revisit with a Decade of Progress. Pharm Res 2000;17(12):1551–7. [DOI] [PubMed] [Google Scholar]
- 19.Takyi-Williams J, Dong X, Gong H, Wang Y, Jian W, Liu C-F, Tang K. Application of paper spray–MS in PK studies using sunitinib and benzethonium as model compounds. Bioanalysis 2015;7(4):413–23. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Manicke NE, Wang H, Petucci C, Cooks RG, Ouyang Z. Direct and quantitative analysis of underivatized acylcarnitines in serum and whole blood using paper spray mass spectrometry. Anal Bioanal Chem 2012;404(5):1389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, Pei J, Huang G. Reactive paper spray mass spectrometry for in situ identification of quinones. Rapid Commun Mass Spectrom 2014;29(1):100–6. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Manicke NE, Yang Q, Zheng L, Shi R, Cooks RG, Ouyang Z. Direct analysis of biological tissue by paper spray mass spectrometry. Anal Chem 2011;83(4):1197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamid AM, Jarmusch AK, Pirro V, Pincus DH, Clay BG, Gervasi G, Cooks RG. Rapid discrimination of bacteria by paper spray mass spectrometry. Anal Chem 2014;86(15):7500–7. [DOI] [PubMed] [Google Scholar]
- 24.Pulliam CJ, Wei P, Snyder DT, Wang X, Ouyang Z, Pielak RM, Graham Cooks R. Rapid discrimination of bacteria using a miniature mass spectrometer. Analyst 2016; 141(5):1633–6. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Ju Y, Huang C, Wysocki VH. Paper spray ionization of noncovalent protein complexes. Anal Chem 2014;86(3):1342–6. [DOI] [PubMed] [Google Scholar]
- 26.Han F, Yang Y, Ouyang J, Na N. Direct analysis of in-gel proteins by carbon nanotubes-modified paper spray ambient mass spectrometry. Analyst 2015;140(3):710–5. [DOI] [PubMed] [Google Scholar]
- 27.Hu B, So PK, Chen H, Yao ZP. Electrospray ionization using wooden tips. Anal Chem 2011;83(21):8201–7. [DOI] [PubMed] [Google Scholar]
- 28.Ren Y, Wang H, Liu J, Zhang Z, McLuckey MN, Ouyang Z. Analysis of biological samples using paper spray mass spectrometry: An investigation of impacts by the substrates, solvents and elution methods. Chromatographia 2013;76(19–20):1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Ren Y, McLuckey MN, Manicke NE, Park J, Zheng L, Shi R, Graham Cooks R, Ouyang Z. Direct quantitative analysis of nicotine alkaloids from biofluid samples using paper spray mass spectrometry. Anal Chem 2013;85(23):11540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang T, Zheng Y, Wang X, Austin DE, Zhang Z. Sub-ppt Mass Spectrometric Detection of Therapeutic Drugs in Complex Biological Matrixes Using Polystyrene-Microsphere-Coated Paper Spray. Anal Chem 2017;89(15):7988–95. [DOI] [PubMed] [Google Scholar]
- 31.Ji J, Nie L, Liao L, Du R, Liu B, Yang P. Ambient ionization based on mesoporous graphene coated paper for therapeutic drug monitoring. J Chromatogr B 2016;1015–1016:142–9. [DOI] [PubMed]
- 32.Tavares LS, Carvalho TC, Romão W, Vaz BG, Chaves AR. Paper Spray Tandem Mass Spectrometry Based on Molecularly Imprinted Polymer Substrate for Cocaine Analysis in Oral Fluid. J Am Soc Mass Spectrom 2018;29(3):566–72. [DOI] [PubMed] [Google Scholar]
- 33.Damon DE, Davis KM, Moreira CR, Capone P, Cruttenden R, Badu-Tawiah AK. Direct Biofluid Analysis Using Hydrophobic Paper Spray Mass Spectrometry. Anal Chem 2016;88(3):1878–84. [DOI] [PubMed] [Google Scholar]
- 34.Zargar T, Khayamian T, Jafari MT. Aptamer-modified carbon nanomaterial based sorption coupled to paper spray ion mobility spectrometry for highly sensitive and selective determination of methamphetamine. Microchim Acta 2018;185(2):103. [DOI] [PubMed] [Google Scholar]
- 35.Narayanan R, Sarkar D, Cooks RG, Pradeep T. Molecular ionization from carbon nanotube paper. Angew Chemie - Int Ed 2014;53(23):5936–40.• First report of Modification of paper spray by carbon nanomaterials.
- 36.Hashemian Z, Khayamian T, Saraji M. Anticodeine aptamer immobilized on a Whatman cellulose paper for thin-film microextraction of codeine from urine followed by electrospray ionization ion mobility spectrometry. Anal Bioanal Chem 2015;407(6):1615–23. [DOI] [PubMed] [Google Scholar]
- 37.Zargar T, Khayamian T, Jafari MT. Immobilized aptamer paper spray ionization source for ion mobility spectrometry. J Pharm Biomed Anal 2017;132:232–7. [DOI] [PubMed] [Google Scholar]
- 38.Wilhelm AJ, Jeroen, Den Burger CG, Swart EL Therapeutic Drug Monitoring by Dried Blood Spot: Progress to Date and Future Directions 2014 [DOI] [PMC free article] [PubMed]
- 39.Espy RD, Teunissen SF, Manicke NE, Ren Y, Ouyang Z, Van Asten A, Cooks RG. Paper spray and extraction spray mass spectrometry for the direct and simultaneous quantification of eight drugs of abuse in whole blood. Anal Chem 2014;86(15):7712–8. [DOI] [PubMed] [Google Scholar]
- 40.Teunissen SF, Fedick PW, Berendsen BJA, Nielen MWF, Eberlin MN, Graham Cooks R, van Asten AC. Novel Selectivity-Based Forensic Toxicological Validation of a Paper Spray Mass Spectrometry Method for the Quantitative Determination of Eight Amphetamines in Whole Blood. J Am Soc Mass Spectrom 2017;28(12):2665–76. [DOI] [PubMed] [Google Scholar]
- 41.Keating JE, Minges JT, Randell SH, Glish GL. Paper spray mass spectrometry for high-throughput quantification of nicotine and cotinine. Anal Methods 2018;10(1):46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salentijn GI, Oleschuk RD, Verpoorte E. 3D-Printed Paper Spray Ionization Cartridge with Integrated Desolvation Feature and Ion Optics. Anal Chem 2017. ;89(21):11419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damon DE, Maher YS, Yin M, Jjunju FPM, Young IS, Taylor S, Maher S, Badu-Tawiah AK. 2D wax-printed paper substrates with extended solvent supply capabilities allow enhanced ion signal in paper spray ionization. Analyst 2016;141(12):3866–73. [DOI] [PubMed] [Google Scholar]
- 44.Shen L, Zhang J, Yang Q, Manicke NE, Ouyang Z. High throughput paper spray mass spectrometry analysis. Clin Chim Acta 2013;420:28–33. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Cooks RG, Ouyang Z. Enabling quantitative analysis in ambient ionization mass spectrometry: Internal standard coated capillary samplers. Anal Chem 2013;85(12):5632–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banerjee S, Zare RN, Tibshirani RJ, Kunder CA, Nolley R, Fan R, Brooks JD, Sonn GA. Diagnosis of prostate cancer by desorption electrospray ionization mass spectrometric imaging of small metabolites and lipids. Proc Natl Acad Sci U S A 2017;114(13):3334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X, Liu J, Cooks RG, Ouyang Z. Development of miniature mass spectrometry systems for bioanalysis outside the conventional laboratories. Bioanalysis 2014;6(11):1497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Espy RD, Manicke NE, Ouyang Z, Cooks RG. Rapid analysis of whole blood by paper spray mass spectrometry for point-of-care therapeutic drug monitoring. Analyst 2012;137(10):2344. [DOI] [PubMed] [Google Scholar]


