Abstract
Background:
Although rodent TBI studies provide valuable information regarding the effects of injury and recovery, an animal model with neuroanatomical characteristics closer to humans may provide a more meaningful basis for clinical translation. The ferret has a high white/gray matter ratio, gyrencephalic neocortex, and ventral hippocampal location. Furthermore, ferrets are amenable to behavioral training, have a body size compatible with pre-clinical MRI, and are cost-effective.
New methods:
We optimized the surgical procedure for controlled cortical impact (CCI) using 9 adult male ferrets. We used subject-specific brain/skull morphometric data from anatomical MRIs to overcome across-subject variability for lesion placement. We also reflected the temporalis muscle, closed the craniotomy, and used antibiotics. We then gathered MRI, behavioral, and immunohistochemical data from 6 additional animals using the optimized surgical protocol: 1 control, 3 mild, and 1 severely injured animals (surviving one week) and 1 moderately injured animal surviving sixteen weeks.
Results:
The optimized surgical protocol resulted in consistent injury placement. Astrocytic reactivity increased with injury severity showing progressively greater numbers of astrocytes within the white matter. The density and morphological changes of microglia amplified with injury severity or time after injury. Motor and cognitive impairments scaled with injury severity.
Comparison with existing method(s):
The optimized surgical methods differ from those used in the rodent, and are integral to success using a ferret model.
Conclusions:
We optimized ferret CCI surgery for consistent injury placement. The ferret is an excellent animal model to investigate pathophysiological and behavioral changes associated with TBI.
Keywords: Surgery, Behavior, Immunohistochemistry, Landmarks, Microglia, Astrocytes, MRI, Craniotomy, TBI, CCI, Temporalis, Antibiotics, Reproducibility, White matter, Cognitive, Motor, Impairment, Acute, Chronic, Inflammation
1. Introduction
Each year an estimated 1.7 million people sustain a traumatic brain injury (TBI) (Faul et al., 2010). Many of those affected are young and the resulting cognitive, physical, and behavioral impairments impede functioning in work and leisure activities for many years (Faul et al., 2010; Ponsford et al., 2014).
Mice and rats have been used for decades to study basic mechanisms of pathophysiology following TBI and to develop therapeutics. The translation of basic research findings into beneficial clinical outcomes in TBI patients however, is challenging (Morales et al., 2005; Statler et al., 2001; Stein 2015; Xiong et al., 2013). One obstacle may be the structural differences between the brains of rodents and humans (Defelipe, 2011; Duhaime, 2006; Gennarelli, 1994; Johnson et al., 2015; Mychasiuk et al., 2016). The human cortex contains extensive sulci and gyri and substantial white matter, whereas the rodent brain is lissencephalic with a low volume of white matter. Since TBI produces damage that reflects cranial and cerebral geometry, and often affects white matter in humans, this is a significant distinction (Johnson et al., 2013). The location of the rodent hippocampus differs as well, as it is situated in a superior position making it susceptible to specific types of experimental brain injury, whereas the ferret hippocampus is located primarily in the temporal lobe, as it is in the human.
A number of larger animals have brains with features similar to humans such sulci and gyri in the cerebral cortex and large amounts of white matter. Swine, sheep, and non-human primates are used to study TBI, but not as frequently as rodents because they present complications related to housing, cost, and/or ethical concerns. In addition, they require greater monitoring during the injury and related surgery. Conducting an MRI requires using human equipment due to their larger size; immunohistochemical markers established in rodents require further refinement to be effective, and behavioral tests have not been fully developed and standardized (Manley et al., 2006). Nevertheless, a translationally relevant animal model would be extremely valuable for studying the complex pathogenesis of TBI. In order to address this need, we propose that the ferret (Mustela putorius furo), as a small, gyrencephalic mammal, may be a more relevant and well-suited model to study TBI.
The ferret is used in numerous fields of anatomic and physiologic research because it possesses many features that are similar to humans. Examples include immune and respiratory systems, auditory systems, cerebrovascular research, endocrine system, and gastric anatomy (Atkinson et al., 1989; Bakthavatchalu et al., 2016; Cabot and Fox, 1990; Gold et al., 2015; Oh and Hurt, 2016). Ferrets are advantageous for many practical reasons such as a comparatively low cost, sociability to allow group housing thereby minimizing space needs, and body dimensions with a slender torso that allows use of specialized pre-clinical MRI scanners for in vivo imaging. Ferrets are also amenable to many types of behavioral testing (e.g., Christensson and Garwicz, 2005; Gold et al., 2015; Haddad et al., 1976; Rabe et al., 1985; Zhou et al., 2016). For a full review of various issues to consider when choosing the ferret as a laboratory animal see Ball (2006). The recent publication of the ferret genome increases the usefulness of this animal and demonstrates that less genetic divergence occurs between humans and ferrets than between humans and mice (Peng et al., 2014).
The ferret was one of the first animals used to develop the controlled cortical impact (CCI) technique for TBI research (Lighthall, 1988; Lighthall et al., 1990; see Osier and Dixon, 2016 for a review). The CCI technique uses a pneumatically or electromagnetically driven metal rod to directly strike the surgically exposed dura, causing brain injury. The benefit of this model is the level of control of the impact − velocity, impact depth, angle, and duration. In the ferret, the impactor strikes a gyrus, and the biomechanical forces would likely be transmitted throughout the brain in a similar way as in the human brain, which also has sulci and gyri. The increased amount of white matter in the ferret compared to the rodent increases the likelihood of diffuse axonal injury. While the CCI technique was used initially with the ferret, the resulting pathology and functional impairments were not fully characterized at that time and the ferret has not been used for CCI study since. Several current brain injury studies using ferrets investigate the effect of blast, primarily to examine the threshold for intracranial hemorrhage, cardiorespiratory instability and overall survival risk assessment but did not study the specific effects on the brain (Rafaels et al., 2012; Rafaels et al., 2016). The ferret is also being used in brain imaging studies (Feng et al., 2013; Sawada et al., 2013). The ferret is becoming recognized as a model to investigate multiple aspects of brain function and the effects of injury. Recently, the ferret was proposed to study perinatal brain injury (Empie et al., 2015). The information presented here provides evidence that ferrets are important and highly useful to characterize neuropathological changes following brain injury.
Our goal was to optimize surgical procedures to perform controlled cortical impact in the ferret. We also provide preliminary evidence that the injury characteristics and consequent functional impairment exhibit elements similar to the human condition through imaging, histology, and behavioral testing in animals at short and long term time-points.
2. Materials and methods
2.1. Animals and housing
All animal procedures were conducted with the approval of the Uniformed Services University of Health Sciences Institutional Animal Care and Use Committee and in accordance with the animal care guidelines issued by the National Institutes of Health. Adult male ferrets (Mustela putorius furo), 5–9 months of age, weighing 1.3–2.1 kg, were purchased from Marshall Bioresources (North Rose, NY, USA) and housed 2 per cage prior to surgery (modified rabbit cage by Lenderking Caging Products, Millersville, MD) and 1 ferret per cage after surgery. Ferrets had ad libitum access to food and water before and during experimental procedures and were on a 12-h light/12-h dark cycle with the room temperature range of 61–72 °F (16–22 °C) and humidity of 30–70%. All animals survived for 1 week after the injury except 1 animal that survived 16 weeks. A total of 15 ferrets were used: nine to optimize the surgery, five additional animals using the optimized surgical protocol with varying severities of injury, and one control animal. The naïve control did not receive anesthesia and was employed for comparison with MRI, immunohistochemistry and behavioral results (see Table 1). Also note that the ferrets used for optimizing the surgery had a range of injuries from mild to severe.
Table 1.
Animal Usage. Nine ferrets were used for surgical optimization using different controlled cortical impact (CCI) parameters in the ranges shown. The remaining 6 animals were used to investigate markers of injury using magnetic resonance imaging (MRI), behavioral testing, and immunohistochemistry.
| InjuryType | # MaleFerrets | CCI Parameters | MRI | Behavior | Immuno-histochemistry | Survival Time |
|---|---|---|---|---|---|---|
| Surgical optimization: mild-severe | 9 | Velocity: 3–5 m/s Depth: 1–4 mm |
x | - | - | 1 Week |
| Mild | 1 | Velocity: 3 m/s Depth: 1 mm |
- | x | x | 1 Week |
| Mild | 2 | Velocity: 4 m/s Depth: 1 mm |
x | - | - | 1 Week |
| Moderate | 1 | Velocity: 5 m/s Depth: 2 mm |
x | - | x | 16 Weeks |
| Severe | 1 | Velocity: 5 m/s Depth: 4 mm |
x | x | x | 1 Week |
| Naive Control | 1 | N/A | x | x | x | 1 Week |
| TOTAL: | 15 |
2.2. Navigation to the target brain site
The target brain site was the primary somatosensory cortex of the left hemisphere (McLaughlin et al., 1998). This region can be identified as the dimple in the posterior sigmoid gyrus, located between the coronal and cruciate sulci and anterior to the ansinate sulcus (see Fig. 1A). We used a skull landmark previously identified by Lawes and Andrews (1987) to navigate to the target brain site. This landmark is the junction of the supraorbital crests, which we will refer to as the anterior landmark (Fig. 1B). In the animals studied earlier in this set of experiments, the anterior landmark was identified visually during surgery and pre-determined coordinates measured on the skull to determine the craniotomy site. In the later studied animals, a baseline structural MRI identified the anterior landmark on the skull as well as the target brain site, which then determined the anterior-posterior (A-P) and medial-lateral (M-L) coordinates (see Fig. 1C). These coordinates were then used during stereotaxic surgery after visual identification of the anterior landmark on the skull.
Fig. 1.
Navigation to target brain site. (A) The target brain site is shown as a green dot with the nearby sulci identified. The skull landmarks are shown in (B) including the anterior landmark at the junction of the supraorbital crests. The posterior landmark is also indicated. (C) The brain sulci are identified on the MRI reconstruction to locate the target brain site and calculate the distance from the anterior skull landmark. These coordinates are then used during the surgery to determine the craniotomy position.
2.3. Optimized surgical procedure
The surgical procedure used aseptic technique in a dedicated surgical suite. Ferrets were food deprived 1 h prior to intubation. We placed each animal in an induction chamber infused with 4–5% isoflurane mixed with 100% oxygen until unresponsive to toe-pinch. Each ferret was intubated with a 2–3 mm endotracheal tube and connected to a non-rebreathing circuit with manual ventilation, if needed. Anesthesia was maintained using 2–3% isoflurane with 100% oxygen at a flow of 1–1.5 liters per minute. Artificial tears ointment was applied to both eyes and the antibiotic Cefazolin injected intramuscularly at 20 mg/kg at least 10 min prior to first incision. Each ferret was monitored for O2 saturation (optimal: 100%), end-tidal CO2 concentration (optimal range: 35–45 mmHg), heart rate (optimal range: 200–400 bpm), respiratory rate (optimal range: 30–40 bpm), and rectal body temperature (optimal range: 36.7–40 °C). Thermoregulation was maintained with a Bair Hugger warming system. The surgical site was shaved with electric clippers and scrubbed alternating with chlorahexidine and 70% alcohol. The head of each ferret was placed in a digital rat stereotaxic frame (Stoelting Co., Wood Dale, IL) in the prone position and secured with blunt ear bars using a cat adaptor plate with palate and eye bars. The animal was appropriately draped and the surgical site had a final prep with 70% isopropyl alcohol and 2% Beta-dine. The skin incision was made at midline (approximately 5 cm). The anterior skull landmark (i.e., the junction of the supraorbital crests) was located and marked. The left temporalis muscle and epicranial aponeurosis were detached from the sagittal crest and reflected laterally using blunt hook elastic retractors to expose the skull. The coordinates determined from the MRI were then measured from the anterior landmark and indicated on the skull with a surgical marker. Under an operating microscope (Zeiss OPMI 1), a 4–5 mm diameter craniotomy was performed using a handheld microdrill, first using a friction grip crosscut taper fissure bur (iM3, 702) and then a smaller 701 bur as the skull thinned. The surface of the skull was cooled with periodic application of room temperature saline. The 3.0-mm-diameter impactor was then angled so the flat face of the impactor tip was parallel to the dural surface (20–25 ° from vertical). The impactor tip was lowered until it touched the brain and the impact induced using the electromagnetically controlled stereotaxic impactor (Impact One Stereotaxic Impactor, Leica Microsystems, Buffalo Grove, IL) with piston velocity set at 3.0–5.0 m/s, depth of penetration of 1.0–4.0 mm and dwell time of 100 ms. Bleeding was controlled throughout the procedure with surgical sponges. Following impact, absorbable gelatin sponge (Ethicon, REF 1972, Somerville, NJ) was immersed in sterile saline and placed over the exposed brain, then the surrounding skull was dried and scored with the scalpel to optimize dental acrylic adhesion. Dental acrylic (Duz-All, Coralite Dental Products, Skokie, IL) was mixed with sterile saline, placed over the gelatin sponge and the surrounding skull and allowed to harden for 10 min. The temporalis muscle was returned to its original position by laying it over the dental acrylic and sutured to its corresponding muscle on the opposite side using 4–0 PDS monofilament, incorporating muscle fascia. The skin was closed using an intradermal pattern with 4–0 PDS.
During post-surgical recovery, the ferret received buprenorphine intramuscularly (0.01–0.03 mg/kg) for pain management. When ambulatory, each animal returned to its housing cage and received a blanket for warmth. A second dose of buprenorphine was given intramuscularly following behavioral testing at 6 h post-injury. Amoxicillin trihydrate/clavulanate potassium (Clavamox, Zoetis, Kalamazoo, MI) was given orally twice a day for 3–5 days post-surgery (1 ml; 62.5 mg/kg). The surgical incision was monitored twice a day for 1 week; the control animal received no surgery, anesthesia, antibiotics or pain medicine.
2.4. Markers of injury
Following surgical optimization, ferrets were investigated using behavioral testing (n = 3), MRI (n = 6) and immunohistochemistry (n = 4) to add supplementary information after CCI (see Table 1). In vivo MRI scans were collected prior to the injury and 1 day post-injury (DPI). For this assessment, three animals received a mild injury (CCI velocity = 3.0 m/s (1) or 4.0 m/s, depth = 1.0 mm (2)) and one animal received a more severe injury (velocity = 5.0 m/s, depth = 4.0 mm) and survived for 7 days (short-term). In addition, we evaluated one animal that received a slightly more moderate injury (velocity = 5.0 m/s, depth = 2.0 mm) and survived 16 weeks (long term survival). We also included a control animal. We performed behavioral testing at 6 h post injury (HPI), 1 DPI, 3 DPI and 7 DPI in the control, one mild, and the severely injured animals.
2.4.1. MRI
2.4.1.1. Skull length and target site variability analysis.
Pre-surgical T2W MRI scans measured the distance between the anterior and posterior skull landmarks as well as the coordinates for the target CCI brain site with reference to the anterior landmark in uninjured brains. Using ITKsnap software tools, brain and skull regions were segmented and their 3D rendered surfaces used to identify and record the coordinates for the cranial and cortical landmarks. The distances between the landmarks were calculated and plotted. Linear regression analyses between skull length and ferret weight and age were also performed.
2.4.1.2. In vivo ferret MRI.
Ferrets were imaged using a Bruker 7T MRI system with an 8.6-cm quadrature volume coil for transmit and receive and ParaVision 5.1 software. A Rapid Acquisition with Relaxation Enhancement pulse sequence was used to acquire 2D sagittal structural MRI scans with the following parameters: TE/TR = 20 ms/8 s, nex = 2, 1 repetition, FOV = 60 mm × 70 mm, matrix = 140 × 120, slices = 40, slice thickness = 0.5 mm. The multi-echo T2W images were used to create T2 maps for each brain using the Carr-Purcell-Meiboom-Gill method implemented in custom Matlab software (R2016a; Natick, MA).
2.4.1.3. Lesion mask analysis of in-vivo MRI.
The location and extent of damaged tissue was identified and compared using in-vivo T2W MRI scans and quantitative maps obtained at 1 DPI in six injured ferrets used for surgical optimization. The target brain site was the same across animals, but the CCI parameters varied (velocity: 3–5 m/s; depth: 1–4 mm). Each MRI scan from the injured brains was transformed into a common template space by diffeomorphic registration to an in-vivo ferret brain MRI template (Hutchinson et al., 2017) using the ANTs software package (Avants et al., 2008). The resulting transformations were also applied to the T2 maps, which were used to classify lesioned tissue based on the increased T2 values in injured tissue. For each T2 map in template space, a lesion mask was generated using active contour semi-automatic methods in the ITKsnap software package (Yushkevich et al., 2006).
Individual regions of interest (ROI) masks were summed over all brain volumes to generate a ROI overlay map, which reports the number of brains with T2-based lesion at each voxel in template space. This approach combines information across all six brains to report the anatomical variability and extent of the T2 lesion at 1 DPI. In addition to the summative ROI overlay map, a second type of ROI overlay map was generated with distinguishable ROIs (by color coding) for two ferret brains that received a CCI with the same parameters (velocity: 4 m/s; depth: 1 mm) using the optimized surgical procedures to show the coincidence of the T2 lesions in these brains.
2.4.2. Behavioral testing
The controlled cortical impact technique causes a focal injury at the impact site, but further widespread subtle damage can also be detected in the rodent (Dixon et al., 1991; Hall et al., 2008). With more severe injuries, not only does more extensive cortical damage occur with concomitant connections to other brain regions, but the likelihood of distant injury increases. We targeted the somatosensory cortex because of our extensive knowledge of the organization and cytoarchitecture of that region and its proximity to the motor cortex (e.g. McLaughlin et al., 1998; Noctor et al., 1997; Poluch and Juliano, 2015). In the current study we chose commonly used rodent behavioral tests for ease of execution and interpretation. They included open field (which measures activity level and physical impairments), gait, adhesive detection, and novel object recognition (NOR) as a cognitive/memory test. Our aim was to determine the usefulness of these tests in a ferret model of TBI. Impaired sensation (the primary function of our target brain site) could be expected to affect adhesive detection as well as gait and locomotion results as these tests are more sensorimotor in nature rather than purely motor. Behavioral testing was performed in 3 animals (1 control, 1 mild, 1 severe). Animals were acclimated to testing devices prior to the testing sessions. Open field, gait, adhesive removal and novel object recognition were tested pre-injury, 1 DPI, 3 DPI, and 7 DPI, with gait and open field also tested at 6 HPI.
2.4.2.1. Open field test.
We used the rat version of the open field test (100 cm × 100 cm, Stoelting) with the opaque walls extended to 63.5 cm by securing pieces of opaque plastic behind the existing walls. Ferrets were acclimated to the open field 3 times prior to testing because they were initially animated in the open field apparatus, but became calmer with repeated exposure. Using Any-maze software (Stoelting Co., Wood Dale, IL) and a video camera, we recorded for 10 min/session and measured the total distance traveled, the average and maximum speeds, and the number of times the animals reared.
2.4.2.2. Gait.
Each paw was dipped into a different color water-based non-toxic, tempera poster paint (Palmer Paints) and then the animal was placed onto a narrow path covered in paper (45.7 cm wide, 2.13 m long). Ferrets acclimated to the paint application until able to walk calmly down the paper length. For each testing session, the animal received 3 trials. Within each trial, 3 samples were measured to determine stride length (measured independently for each paw: right front, right back, left front, left back).
2.4.2.3. Novel object recognition test.
This test was performed immediately after the open field test using dog toys. For the familiarization phase, two identical objects were placed in opposite corners of the open field apparatus and the ferret was allowed to investigate them for 10 min. The ferret was then returned to his home cage for 1 h. In the test phase, a novel object was substituted for one of the original objects. The activity of the ferret when placed into the open field apparatus was recorded for 10 min using the Anymaze software and video camera, with active interest in each object (sniffing, biting, investigating) indicated by keystroke by the investigator (the software automatic detection was not used because it falsely reported interaction when the ferret simply stood or lay near the object). Different objects were used for each time-point, with the same objects used across animals. The retention index was calculated using data from the test phase for each animal at each time-point: Retention Index = time spent investigating the novel object/(time spent investigating the novel object + time spent investigating the original object) *100.
2.4.2.4. Adhesive tape detection test.
A 5 cm piece of tape (Fisher-brand self-sticking labeling tape, cat# 1590110R) was placed on the foot and digit pads of the back right leg. Tape detection (shake of the leg, or interacting with the tape by sniffing/biting) within a 3 min period was noted.
2.4.3. Tissue processing
Each animal was deeply anesthetized by isoflurane inhalation (5% in oxygen) and given an IP injection of Euthasol (0.22 ml/kg). Upon cessation of reflexes, ferrets were transcardially perfused with 1 l of ice-cold phosphate buffered saline (PBS) (pH 7.4) containing 53.1 mg of heparin (Sigma-Aldrich, St. Louis, MO) followed by 1 l of 4% paraformaldehyde solution in PBS (Santa Cruz Biotechnology, Santa Cruz, CA). The brains were post-fixed in 4% paraformaldehyde for 8–10 days, and then transferred to a storage solution containing 0.03% sodium azide in PBS (PBS-NaN3). After ex vivo MRI scanning, the brains were cut at 50-μm thick coronal sections using a vibratome (Leica VT1000; Leica) and stored in PBSNaN3 at 4 °C until processed with the subsequent DAB chromogen or immunofluorescence staining.
2.4.4. Immunohistochemistry
Immunohistochemistry revealed microglial activation (ionized calcium-binding adapter molecule 1: IBA-1) and astrocytic reactivity (glial fibrillary acidic protein: GFAP) after brain injury. Sections in the injury epicenter (anatomically matched with the greatest abnormality on MRI) were selected for histological examination. To reveal DAB reaction product, after antigen retrieval with 1X Citrate buffer (Thermo Scientific, Waltham, MA) for 20 min in a 80 °C water bath, free-floating sections were rinsed 4 times (10 min each) with PBS and incubated at room temperature for 5 min with BLOXALL (Vector Laboratories, Burlingame, CA) to inhibit endogenous peroxidase activity. The sections were then rinsed with PBS and incubated in blocking buffer solution (0.1% Triton™ X-100, 3% normal goat serum in PBS) for 2 h at room temperature, followed by an overnight incubation at 4 °C with anti-rabbit IBA-1 antibody (1:1000; Wako, 019–19741; Antibody Registry: AB 839504). After washes in PBS, the sections were incubated for 1 h at room temperature in anti-rabbit biotinylated secondary antibody (Vector Laboratories, PK-4001) diluted 1:500 in blocking buffer. The sections were rinsed in PBS and subsequently incubated in a solution containing Vectastain ABC Reagent (Vector Laboratories) at room temperature for 1 h. Following PBS washes, the reaction was developed using DAB (3,3′-diaminobenzidine, Vector Laboratories, SK-4100) as a chromogen for approximately 2–5 min. At the end of the DAB incubations, the sections were rinsed in PBS, mounted onto plus-coated slides, air dried and dehydrated through an ascending ethanol series, cleared with xylene, and coverslipped using DPX mounting medium (Sigma-Aldrich). Photomicrographs were captured by a NanoZoomer 2.0-RS Digital slide scanner (Hamamatsu Photonics K.K., Bridgewater, NJ).
For immunofluorescence labeling, selected sections were subjected to antigen retrieval and incubation in blocking buffer as described above, then incubated overnight at 4 °C with a primary antibody against GFAP (1:500; Abcam, ab4674; Antibody Registry: AB 304558). After three PBS washes, astrocytic staining was revealed by 2 h incubation at RT with the appropriate Alexa 488 conjugated secondary antibody (Invitrogen, Carlsbad, CA) diluted 1:500 in blocking buffer. Following 3 more washes in PBS, the sections were mounted onto slides and coverslipped with Mowiol 4–88 mounting medium (Polysciences, Warminster, PA). Fluorescent labeled brain sections were analyzed and imaged using a Zeiss Axiovert 200 microscope equipped with an Apotome and Axio-vision 4.7 Zen software (Carl Zeiss Microscopy, Jena, Germany). Higher magnification images were acquired using a Zeiss 710 confocal laser scanning microscope (Carl Zeiss Microscopy).
3. Results
3.1. Cranial landmark and target brain site variability
We used the baseline anatomical MRI scan to identify an anterior (junction of supraorbital crests) and posterior (junction of occipital crests) landmark to measure skull length and calculate the coordinates to the target brain site. Skull length variability was considerable ranging from 32.04 to 40.61 mm across animals with mean = 35.45 +/− 2.69 mm (see Fig. 2A). For the animals measured here (n = 11), we found that the target brain site A-P coordinate varied from 10.01 to 15.13 mm relative to the anterior landmark (mean = 13.05 +/− 1.59) and the M-L coordinate measured from the midline ranged from 4.75 to 6.75 mm with mean = 5.70 +/− 0.61 mm (Fig. 2B). Skull length was not predicted by weight (R2 = 0.1952; y = 0.0344x + 0.4336) or age (R2 = 0.0995; y = −0.0578x + 9.0246), corroborating previous reports in ferrets (Lawes and Andrew, 1987; He et al., 2002). The MRI driven coordinates originating from the anterior landmark were used in the optimal surgery protocol to overcome skull length variability.
Fig. 2.
Anatomical variability considerations on method of navigation to brain site. The anterior coordinate (junction of supraorbital crests) and posterior coordinate (junction of occipital crests) were identified on the baseline anatomical MRI for each ferret and used for measurements of skull length and anterior and posterior distances to the target brain site. Eleven ferrets received the pre-surgical MRI scan. (A). Skull length variability boxplot showing the interquartile range with the ends of the whiskers designating the maximum and minimum skull length across ferrets. The line in the box represents the median, while the x represents the mean. Individual data points are shown as black circles. (B) Skull length correlation with age or weight and the linear regression equation; R2 shown on the plot. Neither of these variables were highly correlated with skull length. (C) The variability of the target site coordinates (A-P and M-L) originating from the anterior landmark plotted for each animal. Considerable spread in the A-P coordinate occurs across animals, which relates to the variability in skull length.
3.2. Lesion location reproducibility and scaling effects
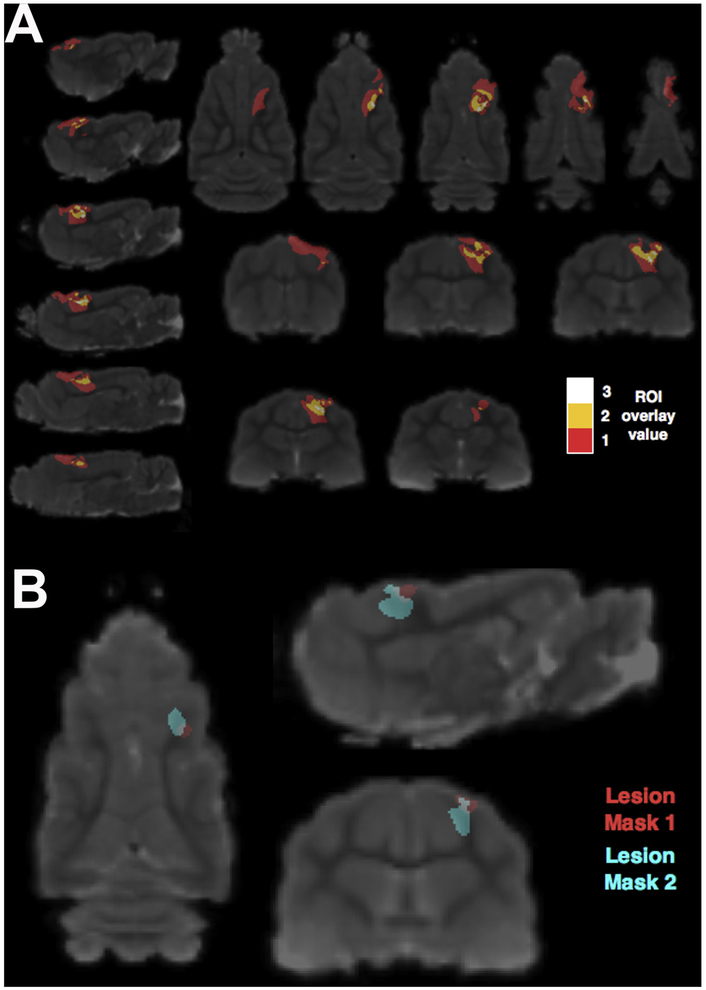
At 1 DPI we used in vivo T2 imaging to determine the reproducibility of injury placement across animals as well as injury severity scaling effects for 6 animals utilized for the surgical optimization portion of this study (Fig. 3A). All 6 animals exhibited injury in the posterior sigmoid gyrus of the somatosensory cortex, which was the target brain site, with damage extending into the underlying white matter. In the white matter closest to the impact site, we observed the greatest overlap of lesioned tissue across brains (white, 3 brains damaged/voxel). Tissue damage further from the impact site relates to more severe CCI parameters and varies spatially (red, 1 brain damaged/voxel) showing damage extending substantially in the rostral direction. Fig. 3B demonstrates that two injuries with the same CCI parameters performed with the optimized surgical procedures overlap considerably but show T2 changes of differing extent at 1 day after an injury.
Fig. 3.
MRI based visualization of lesion extent across ferrets. (A) An ROI overlay map is shown in MRI template space at several levels in three orthogonal planes where the color indicates the number of animals (out of 6) with a detectable T2 hyper intense lesion in a particular voxel at 1 day post injury. The animals included here were part of the surgical optimization study and used varying CCI (controlled cortical impact) parameters from mild to severe (velocity: 3–5 m/s, depth: 1–4 mm). (B) T2 hyper intense lesions from two ferrets studied with the optimized surgical procedures determined in this study and with the same CCI parameters (velocity: 4 m/s, depth: 1 mm, dwell time: 100 ms, impactor size: 3 mm) targeting the dimple in the posterior sigmoid gyrus are shown in MRI template space to demonstrate similarities and differences in lesion extent and spatial localization.
3.3. Importance of sealing the craniotomy
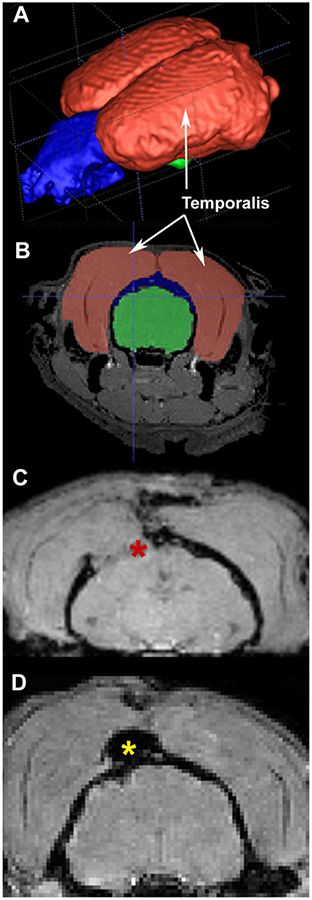
In the ferret, the temporalis muscle is extensive (Fig. 4 A − MRI reconstruction of muscle (red), skull (blue) and brain (green) and B − coronal view). When the craniotomy was left unsealed, an in vivo MRI at 1 DPI revealed that the temporalis muscle invaded the opening, potentially putting pressure on the underlying brain and causing further damage (Fig. 4C). Our subsequent application of gelfoam and dental acrylic to seal the craniotomy prevented the temporalis from coming into contact with the brain (Fig. 4D).
Fig. 4.
Importance of sealing the craniotomy. (A) MRI reconstruction of the temporalis muscle (muscle in shown in red, skull in blue and brain in green). (B) The temporalis muscle position relative to the brain in coronal view. (C) in vivo MRI at 1 day post-injury where the craniotomy was not closed and gravity caused the temporalis muscle to invade the craniotomy and put pressure on the underlying brain tissue (red asterisk). (D) The muscle was prevented from entering the craniotomy in an animal when gelfoam was inserted into the craniotomy and dental acrylic (yellow asterisk) applied over it and allowed to dry for 10 min prior to closure.
3.4. Indications of histological markers of injury (1WPI and 16 WPI)
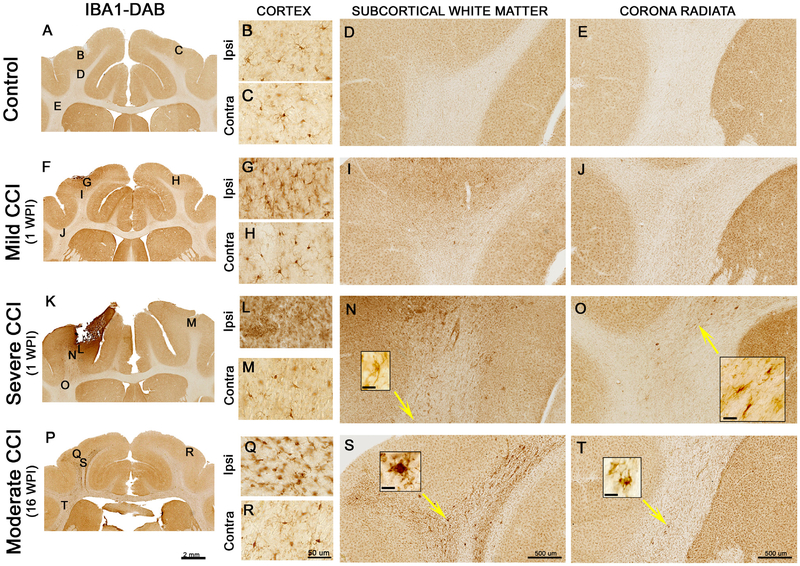
We evaluated the location and morphology of microglia and astrocytes as markers of cerebral inflammation. These results are from a single animal in each group and provide support for a scaled effect of the injury. IBA-1 is a microglia/macrophage-specific calcium binding protein with roles in actin-bundling, membrane ruffling and phagocytosis (Ohsawa et al., 2004). IBA-1 immunostaining in a control animal demonstrated homogeneous staining of non-reactive microglial cell bodies with multiple, fine processes throughout the neocortex, with few IBA-1+ cells in the white matter (Fig. 5A–E). In the animals that underwent CCI, IBA-1 immunostaining in the non-injured, contralateral hemisphere was similar to that of control animals (Fig. 5H, M, R). After a mild injury with short term survival (1 WPI), the density and cell size of reactive microglia in the neocortex is increased with a slight increase in the underlying white matter (Fig. 5G & I). After a severe injury (4-mm depth) with short term survival, a cavity formed at the cortical impact site, accompanied by the activation and recruitment of microglia exhibiting morphological features devoid of processes (resembling amoeboid microglia), which indicates phagocytic activity (Fig. 5L). In the severely injured animal, IBA-1 immunoreactive cells are visible in the subcortical white matter extending through the corona radiata superior to the internal capsule (Fig. 5N and O). In a ferret with a moderate injury and long term survival (16 WPI), the pattern of IBA-1 immunoreactivity shows highly ramified, short, thick, hypertrophic microglial processes in the neocortex (Fig. 5Q). In the subcortical white matter a dramatic increase of microglial reactive cells extends subcortically (Fig. 5S and T). Higher power views of abnormally appearing microglia can be seen in Fig. 5N, O, S, and T. These initial results demonstrate an injury effect that enhances with time.
Fig. 5.
Microglia immunoreactivity after controlled cortical impact in the ferret. The top row (A-E) shows IBA-1 immunoreactivity in an uninjured, control ferret. The 2nd and 3rd rows show IBA-1 immunoreactivity after short term survival (1 week post injury − 1WPI) in a mild (F-J) and a severe (K-O) injured animal. The final row shows the response after long term survival (16 WPI) with a moderate injury (P-T). In the mild, short term condition, the density of microglia increases in the ipsilateral (G), but not the contralateral cortex (H). The density of immunoreactivity in the subcortical white matter (I) and corona radiata just superior to the internal capsule (J) also increases. After a severe injury with short term survival, the ipsilateral cortex (L) is damaged leading to a dramatic increase in the number of activated microglial cells. The density of microglial staining increases in the subcortical white matter (N) and the corona radiata (O). The insets shown in (N) and (O) are a higher power views of cells in the vicinity of the yellow arrow tips (N inset scale bar = 10 μm, O inset scale bar = 50 μm). After long term survival with a moderate injury, there is an increase in IBA-1 immunoreactivity in both the ipsilateral cortex (Q) and in the white matter (S and T). In the ipsilateral cortex, microglia had shorter, thicker processes or none at all (Q). At this later time point, we also observed clustering of microglia in the white matter regions investigated (S and T). The insets shown in (S) and (T) show higher power views of cells in the vicinity of the yellow arrow tips (S inset scale bar = 25 μm, T inset scale bar = 25 μm). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
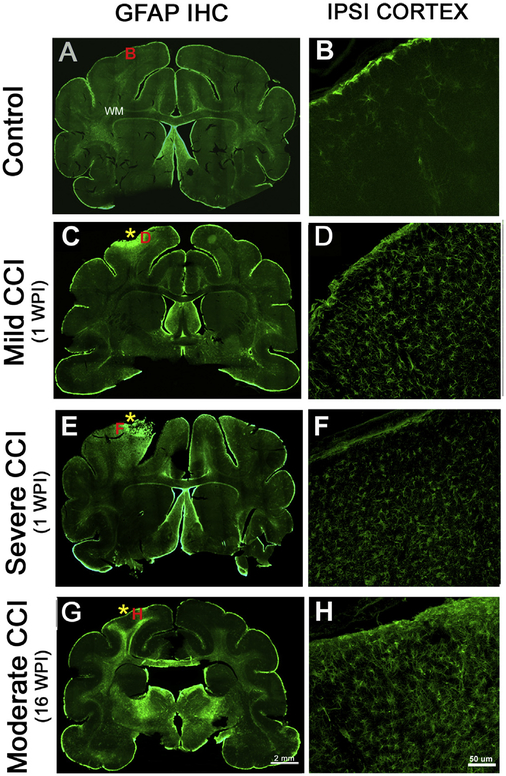
GFAP label in the control brain occurs primarily in scattered astrocytes residing in the white matter and along the pial surface (Fig. 6A–B), with sparse GFAP+ cells in the gray matter (Fig. 6A). After a mild injury with short term survival (1 WPI), reactive astrocytes are found primarily in the vicinity of the injury within the cerebral cortex (Fig. 6C–D). An increase in GFAP reactivity can be seen in the white matter underlying the injured cerebral cortex (Fig. 6C). After a severe injury, reactive cells also appear in the injured cortex and are more evident in the underlying white matter and surrounding cortical regions (Fig. 6E–F). The astrocytes increase in complexity and show thicker processes. Long term survival time (16 WPI) after a moderate injury results in increased astrocytic immunoreactivity in the region of the injury, which reaches extensively into the subcortical white matter (Fig. 6G–H). The astrocytes also demonstrate substantial increase in morphologic complexity.
Fig. 6.
Astrogliosis after controlled cortical impact in the ferret. (A-B) The control animal shows limited GFAP reactivity in the cerebral cortex, with slight immunore-activity extending into the pia and surrounding blood vessels. The white matter also shows typical immunoreactivity against GFAP. (C-D) At 1 week survival after a mild injury there was a substantial increase in astrogliosis in the cerebral cortex, while the underlying white matter shows little change from the control. The location of the impact site is indicated with an asterisk. (E-F) A more severe injury caused an increase in the GFAP-expressing astrocytes in the cortex at the site of the impact and surrounding cortex and also shows increased reactivity in the white matter. (G-H) After the moderate injury with a long term survival of 16 WPI, an increase in astrocytes was observed in the subcortical white matter extending deep into the brain and also along the corpus callosum. The location of the matching higher magnification images is shown with a corresponding red letter (B, D, F, H). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Functional markers of injury
3.5.1. Open field test
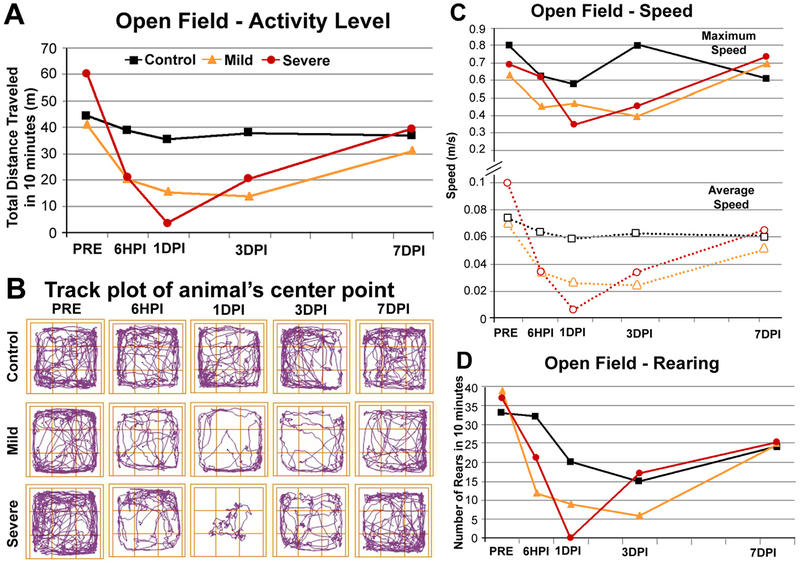
We used the Open Field test to evaluate motor behavior and overall activity level (Fig. 7A–D). The control animal showed little change in activity level over the times of test administration. Activity level decreased after both mild and severe injury in a scaled fashion. We saw effects at 6 HPI for both animals. The effect continued for 1DPI and 3DPI and mostly resolved at 7DPI, which is observed in the activity level graph (Fig. 7A). The track plot (Fig. 7B) clearly displays the decreased distance traveled after injury and at 1DPI, and shows that the severe animal did not move far from the center of the field where he was initially placed. Both the average and maximum speeds were affected after brain injury with the greatest effects observed during the same times (6 HPI, 1 DPI, and 3 DPI) with a return to control level by 7 DPI (Fig. 7C). Rearing activity was impaired in a scaled fashion in the mild injury (maximal reduction at 3 DPI) and severe injury (maximal reduction at 1 DPI) and returned to near control level by 7 DPI (Fig. 7D).
Fig. 7.
Behavioral results − open field. (A) Activity levels of a control animal, an animal with a mild TBI and an animal with a severe TBI within the open field for 10 min. Impairment in distance traveled is seen in the TBI animals at 6 HPI, 1 DPI and 3 DPI. The impairment scaled with injury severity. (B) Track plots of the animal’s center point in the open field arena. At 1DPI, the animal with a mild TBI showed less activity than prior to the injury but covered all areas of the open field. The severely injured animal at 1 DPI moved very little from his original placement in the center of the open field. (C) Maximum speed (solid lines) and average speed (dotted lines) were both affected by TBI, the animal with severe injury shows greater impairment at 1 DPI. (D) Number of rears during the open field test. Note that the number decreases in the control animal with habituation to the open field. Nonetheless, the animals with TBI displayed even less rearing activity than the controls at 6 HPI and 1 DPI. The effect scaled with injury severity.
3.5.2. Gait
Gait impairment involving a foot drag of the right hind limb was only observed at 6 h post severe injury (Fig. 8A). No gross impairments in stride length were observed across paws/time-points/injury levels. The control animal increased in stride length after the initial testing period. Both of the injured animals also increased in stride length across paws with time after injury, with no time-point exceeding the stride length values observed in the control animal.
Fig. 8.
Behavioral results − gait and NOR (Novel Object Recognition). (A) Paint pattern of paw prints during walking for the animal with a severe TBI at 6 HPI, note that the right back paw in green is dragging. (B) Novel object recognition test design and objects. The retention index indicates how much of the total investigation time was spent with the novel object. In the control and mildly injured animals, at all time-points after injury − the animal spent more than 50% of the time with the novel object. Note that in the severely injured animal at 1 DPI and 3 DPI, more time was spent with the original object (bars are less than 50%), which may indicate a memory impairment. The numbers above each marker indicate the total amount of time spent (s; seconds) investigating either object.
3.5.3. Novel object recognition test
The NOR test procedure and objects used are displayed in Fig. 8B. A retention index greater than 50 indicates the animal spent more time with the novel object, and less than 50 means the animal spent more time with the original object. In this study, only the animal with the severe injury had a retention index less than 50, which occurred at 1 DPI and 3 DPI, suggesting a memory impairment. By 7 DPI, the animal was performing normally (Fig. 8B).
3.5.4. Adhesive tape detection test
All animals detected the tape on the back right paw at all testing sessions. Only 1 animal removed the tape in one of the testing sessions.
4. Discussion
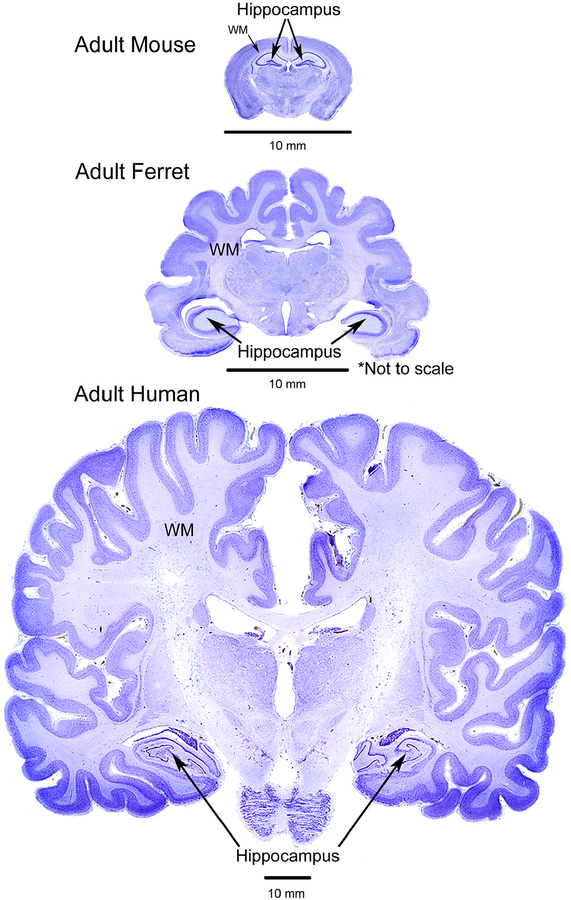
Rodent models are prevalent in traumatic brain injury research, but disparities in brain anatomy may limit the translational effectiveness of the results. As a small mammal with a brain containing many characteristics similar to that of a human, the ferret is an important model to study TBI (see Fig. 9 for a comparison between mouse, ferret and human brains and Table 2 for a list of reasons to use a ferret model for TBI studies). The weight of a male ferret ranges from 1 to 2 kg, a female, 600–900 g, while the body length (nose to tip of tail) ranges from 44 to 46 cm (Fox, 1988). The size makes them very reasonable to use in a laboratory, even for longitudinal studies.
Fig. 9.
Comparative anatomy. Comparison of the brains of the adult mouse, ferret and human (not to scale − see scale bars) showing coronal slices stained with cresyl violet stain. Note the location of the hippocampus in the ferret is similar to the location in the human. In addition, the amount of white matter (WM) is much greater in the ferret than the mouse. The gyral folds in the ferret are also obvious, displaying greater similarity to the human than the mouse. Human brain slice adapted with permission from http://www.brains.rad.msu.edu, supported by the US National Science Foundation and the National Institutes of Health. Brain images were modified in color, brightness and contrast for optimal comparison purposes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Merits of a ferret model of traumatic brain injury.
| Merits of a Ferret Model of TBI |
|---|
|
We developed a surgical method for delivering a controlled cortical impact in the ferret and discovered several key surgical optimizations: i) reflection of the temporalis muscle versus other displacement techniques, ii) using an anatomical MRI to dictate coordinates for navigation to the target brain site, iii) the importance of antibiotics, iv) and the value of skull closure after impact to avoid the temporalis muscle putting pressure on the brain. Following surgical optimization, markers of injury were investigated with immunohistochemistry and behavior to support the surgical observations, which indicated a scaling effect of severity and time after injury.
4.1. Surgical optimization
4.1.1. Reflection of temporalis muscle
The temporalis muscle closes the jaw and in the ferret attaches to the skull at the sagittal crest and occipital crest as well as along the frontoparietal area of the skull (see Fig. 1B and Fig. 4A and B). During surgical optimization, we attempted several techniques to expose the skull over our region of interest (ROI). These included a) cutting along the muscle fibers directly above the ROI and using metal retractors to displace it anteriorly and posteriorly and b) using blunt forceps to separate the muscle fibers above the ROI and then retracting the muscle. Both of these methods required the muscle fibers to stretch substantially in order to expose the skull and clear away the muscle to be able to drill the craniotomy. Using a third method, c) we cut across the muscle fibers and retracted medially and laterally. All of these methods resulted in slow recovery lasting 2–3 days. Finally, d) we detached the muscle from the midline ridge and reflected laterally using blunt hook elastic retractors. On closure, we sutured the muscle to the corresponding muscle on the contralateral side. Using this method the animals were alert and active after surgery and we did not observe any problems with eating, consistent with reports by Lawes and Andrews (1987). Other studies report removing the entire muscle without eating difficulties (Dobbins et al., 2007).
4.1.2. Navigation to the target brain site
Bregma, the intersection of the coronal and sagittal skull sutures, is a common landmark for stereotaxic surgery in rodents. In ferrets, however, the sutures of the skull are only visible in young animals and as the animal ages, boney ridges form that obscure the sutures and form the sagittal, supraorbital and occipital crests, which give origin to the temporalis muscle (Lawes and Andrews, 1987). Lawes and Andrews (1987) studied a number of external skull landmarks for their predictive ability to locate internal landmarks, which they used as a reasonable indicator of brain morphology. They determined that the supraorbital crest was the most accurate predictor for structures rostral to the interaural line, and that the occipital crest was the most surgically practical predictor for intracranial structures caudal to the interaural line. In this study, we used the junction of the supraorbital crests for our anterior skull landmark and the junction of the occipital crests as our posterior landmark. The ferret skull displays substantial variability in length, which affects the ability to accurately target identical surgical sites across animals (He et al., 2002; Lawes and Andrews, 1987). Our results confirm the variability in skull length across ferrets. We noticed early in the surgical optimization that even when using the anterior landmark with a pre-determined set of coordinates, we could not consistently locate the craniotomy over the target brain site. We began creating larger craniotomies to ensure the exposure of the target brain site. However, the intact dura, blood vessels and the restricted cranial window view made it difficult to visually distinguish the target brain site. The anatomical variability across animals spurred us to use the baseline anatomical MRI to measure the distance from the anterior landmark to the target brain site on a subject-specific basis. This allowed us to consistently create a small craniotomy − only slightly larger than our 3-mm-diameter impactor tip. This surgery is possible without a baseline MRI, but may require a larger craniotomy to consistently reveal the target brain site across animals.
4.1.3. Injury site reproducibility and scaling effects
Fig. 3A and B demonstrates reproducibility in the location of the injury when using the subject-specific anatomical MRI driven coordinates (Table 1). In Fig. 3A, the injury extent varied across animals with several CCI parameters, but all animals showed injury in the target gyrus (as measured by hyperintensity on the T2 MRI at 1DPI). The greatest overlap of injury across animals occurred in the white matter near the impact site. As the injury severity increased, the spatial location and extent of the damage became more variable. Our observation showing larger regions of injured white matter in this model makes it valuable to mimic human injury. The images in Fig. 3B also show that although the CCI targeted the same area in the brain and the CCI parameters were held constant, the extent of the damage is variable. It is not clear why this occurs, although it possibly represents an individual response to injury, which may be stronger in the ferret than the rat or mouse.
4.1.4. Importance of antibiotics
Our results reveal that ferrets are more susceptible to post-surgical infection than rodents. Early in the surgical optimization we did not use antibiotics. We found variability in recovery from the surgery, however, with some animals being lethargic after the procedure exhibiting a recovery time of 2–3 days. Although we conducted aseptic surgery, a subset of animals developed infection at the surgical site and as a result, we added antibiotics to the surgical protocol. From that point on, the animals recovered excellently, being alert and active soon after waking from anesthesia.
4.1.5. Skull closure after impact
In rodent CCI surgeries, often the craniotomy is left open and the skin sutured closed above it. This is in part due to the fact that the injured brain may display edema that extends into the craniotomy; replacing the skull piece requires pushing the brain into the cranium, causing additional injury. In the initial ferret surgeries, while we did not observe brain swelling with the impact, we did not close the craniotomy. At the 1 DPI MRI we observed the temporalis muscle encroaching into the craniotomy and touching the brain, potentially causing damage. As a result, we decided to close the craniotomy with dental acrylic. To protect the brain under the craniotomy we first placed absorbable gelfoam on the cortical site and then applied dental acrylic over the gelfoam and surrounding skull. Fig. 4 demonstrates that after sealing the craniotomy, the temporalis muscle does not impact the brain.
4.2. Pilot study of injury markers
4.2.1. Histological markers of injury
Our preliminary characterization of glial immunoreactivity after CCI in ferrets demonstrates that reactive gliosis exacerbates with increased injury intensity. Our CCI parameters ranged from mild to severe, and in each category we detected changes in inflammatory markers after a single TBI in the ferret. At 1 week after the mild injury, astrocytes and activated microglia occur primarily in the gray matter near the impact site. After a severe injury, the reactivity in the white matter is more substantial and extends to a greater depth. At 16 weeks after a moderate injury, the glial response remained strong and extensive, but progressed from the injured cortex to the underlying white matter and continued into the corona radiata superior to the internal capsule and corpus callosum. Glial reactivity is a major component of human brain injury pathology, persisting for years after a single injury event, particularly in white matter regions (Johnson et al., 2013; Smith et al., 2013; for a review see: Kou and VandeVord, 2014). White matter inflammation is also a long-lasting consequence of TBI in mice (Loane et al., 2014; Winston et al., 2016). Our pilot evaluation of a chronic injury suggests that a similar glial activation pattern is prominent in subcortical white matter regions of the ferret, which highlights the utility of this animal model in TBI research. Further studies are necessary to elucidate the underlying mechanisms leading to TBI-induced chronic glial activation and its relationship to behavioral impairments. An initial study following acute CCI in the ferret using the surgical methods defined here describes markers of MRI and DTI that are sensitive to tissue glial abnormalities acutely after injury (Hutchinson et al., 2016).
4.2.2. Functional markers of injury
We evaluated a variety of behaviors after TBI in the ferret. Similar to previous researchers, we found ferrets to be amenable to behavioral testing. Specifically, other researchers investigated spatial maze learning, delayed response, visual discrimination learning, shock avoidance learning, ambulation, spontaneous alternation, as well as T-maze, Lashley III maze, reversal, visual discrimination, auditory gap-detection and open field locomotion (Christensson and Garwicz 2005; Gold et al., 2015; Haddad et al., 1976; Rabe et al., 1985; Zhou et al., 2016). In this study, we tested a variety of behaviors (locomotion, anxiety, memory, gait, adhesive detection) in a naïve control and in ferrets that received either a mild and severe brain injury. We chose behavioral tests primarily developed and used in rodents, which we easily modified to be effective in the ferret. This strategy allowed us to compare behavioral changes with those observed in rodents after CCI and also added to our knowledge about ferret behavior, as limited functional testing of the ferret after brain injury exists. In particular, we were interested in whether these tests could be accomplished in the ferret, if impairments occur after CCI, and if behavioral scaling occurs with injury severity as has been observed in rodent CCI models (Fox et al., 1998).
In the open field test, for the total distance traveled analysis, we observed an impairment and a scaling effect that suggests the deficit is not simply due to the experience of surgery. Other animal TBI studies report decreased exploratory behavior after injury (Lesniak et al., 2016; Mychasiuk et al., 2015; O’Connor et al., 2003) whereas hyperactivity also occurs (Hsieh et al., 2014; Kimbler et al., 2012; Tucker et al., 2016). Accounts in human TBI cite slower walking speeds, reduced stride and increased time on both limbs (Esquenazi et al., 2017; Williams et al., 2009). In the more severely impaired animal we visually observed a foot drag contralateral to the injury, which was replicated in the gait footprint analysis, but only early after injury. The fact that it was only observed in the contralateral hind limb suggests it is related to the injury and not due to general surgery or anesthesia effects. In the severe injury, the damaged area extends into other brain regions (motor cortex just anterior to the somatosensory cortex) which most likely is the reason why the animal exhibited early foot drag (McLaughlin et al., 1998). Although we did not impact the motor cortex directly, the somatosensory cortex is intimately connected with the motor cortex, which could easily show impairment after the injury elicited here.
We also observed impairment in the NOR after the severe injury, suggesting that the ferret did not remember the familiar object. Memory impairment after TBI is widely reported (Draper and Ponsford, 2008; Scheid et al., 2006). Severely injured TBI patients specifically have difficulty with visual memory both early (median: 4 months) and late (median: 2 years) after injury (Shum et al., 2000). Rodent models of brain injury including fluid percussion injury, weight drop and CCI, report impairment on the NOR early (hours, days) or later (1 month) after injury (Eakin et al., 2014; Munyon et al., 2014; Zhao et al., 2012). In this study, we used a 1 h break and only saw an impairment in the most severely injured animal similar to the severity discrimination observed by Zhao et al., 2012. Increasing the time to 24 h as described by Eakin and colleagues (2014) may provide greater sensitivity to test memory impairment.
In summary, we carried out a battery of behavioral tests in ferrets and observed functional impairments after CCI; the impairments were greater in the more severely affected animal, signifying a scaling effect. Although we observed a mild motor impairment with either injury severity, we only observed cognitive impairment in the more severely affected animal. Most of the deficits resolved during the 7 day testing period regardless of injury severity or testing modality. Following traumatic brain injury in humans, a complex sequelae of behavioral outcomes have been observed including motor impairments and cognitive deficits (Benedictus et al., 2010). In humans, additional symptoms manifest long after the acute symptoms resolve (Thomas et al., 2015). Most patients with mild TBI improve acutely (within weeks), with 5–20% experiencing chronic impairments (Mott et al., 2012). Although the animals in this study recovered from deficits within a week, it would be possible to increase the difficulty of the tests to allow assessment over a longer period of time. One possibility is to increase the time between familiarization and testing in the NOR task. The gait task could be made more difficult with hidden obstacles along the path; in a human chronic TBI study, participants had difficulty adapting to novel walking environments but general locomotion was fine (Vasudevan et al., 2014). The adhesive tape test could use tape of different sizes/stickiness. Other possibilities include using the five-choice serial-reaction time task (5-CSRTT), which is a test of attentional performance and vigilance and requires a testing chamber with indicator lights and nose-poke apertures (Rodriguiz and Wetsel, 2006; Robbins et al., 1993). Another difficult task is the Go-NoGo task, a nonspatial recognition memory test, which assesses impulsivity and attention (Eagle and Robbins, 2003). Nevertheless, observations in the present study suggest a range of behavioral impairments result following CCI in the ferret that modulate with time after injury. Our data provide a solid basis to proceed with larger TBI studies using the ferret.
4.3. Disadvantages to this model
Compared to rodents, the CCI surgery is more involved requiring a sterile surgery suite and substantial monitoring. Ferret experiments can be limited by small sample sizes due to the effort required for surgery and behavioral testing, limited caging capacity, and higher costs than rodents. Studies are also inhibited by the availability of genetically modified mutants. Although genetically modified ferrets exist, to date they are limited and difficult to obtain or produce (Keiser and Engelhardt, 2011; Kou et al., 2015; Sun et al., 2014).
Studies in female ferrets are hindered by estrus-related health problems. Adult female ferrets that are not bred or spayed will remain in heat with high estrogen levels, which can suppress bone marrow function (Fox, 1988). Females can receive an ovo-hysterectomy or hormonal therapy, with either option likely affecting the response to brain injury. Some human and animal TBI studies show that females respond differently than males to brain injury, although other experiments indicate that little differences in behavioral tests occur in male and female mice (Roof and Hall, 2000a, 2000b; Suzuki et al., 2004; Tucker et al., 2016; Tucker et al., 2017; Velosky et al., 2017).
In addition, we cannot guarantee that a highly controlled injury with consistent results reflects the heterogeneous human TBI population. Comparisons with other models of injury continues to be necessary as well as greater delineation of human TBI creates sub-populations where this type of model will correspond more closely and the results translate more directly.
4.4. Conclusions
This study optimized the controlled cortical impact technique in the ferret as a model of TBI. We describe surgical methods that differ from those used in the rodent, which are integral to success using a ferret model. The ferret brain, with its high white/gray matter ratio and convoluted cortex is more similar to human brain morphology than rodents and will be specifically effective in studies of white matter pathophysiology following traumatic brain injury.
HIGHLIGHTS.
Subject-specific MRI-guided coordinates overcame anatomical variability.
The temporalis muscle should be detached from the midline and reflected.
The craniotomy should be closed to avoid the temporalis muscle pushing on the brain.
This optimized surgical procedure created scaled injury in a reproducible location.
Behavior and astrocyte/microglial responses were scaled to injury severity.
Acknowledgements
This research was supported by the Congressionally Directed Medical Research Programs CDMRP) (award numbersW81XWH-13–2–0019 and W81XWH-13–2–0018). The authors thank the Center for Neuroscience and Regenerative Medicine (CNRM) for core facility support enabling this work, specifically the translational imaging facility with Asamoah Bosomtwi and Alexandru Korotcov. The authors also thank the Laboratory of Animal Medicine staff for assistance during the surgery and care of the animals: Dennis Aguilar, Kayln Alloway, Cassie Barnett, Andrew Brown, Marla Brunell, Amanda Christy, Clayton Gerrian, Althea Jamerson, Branden Maxwell, Herlyth Pemberton, Stephen Petroski, David Smith, and Robert Wolfe. We also thank Branden Maxwell for document editing.
Abbreviations:
- CCI
controlled cortical impact
- DPI
days post injury
- GFAP
glial fibrillary acidic protein
- HPI
hours post injury
- IBA-1
ionized calcium-binding adapter molecule 1
- MRI
magnetic resonance imaging
- NOR
novel object recognition
- PBS
phosphate-buffered saline
- ROI
region of interest
- TBI
traumatic brain injury
- WPI
weeks post injury
References
- Atkinson CS, Press GA, Lyden P, Katz B, 1989. The ferret as an animal model in cerebrovascular research. Stroke 20 (August (8)), 1085–1088, PMID: . [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC, 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal 12 (1), 26–41, 10.1016/j.media.2007.06.004, PMID: PMCID: PMC2276735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakthavatchalu V, Muthupalani S, Marini RP, 2016. Fox JG endocrinopathy and aging in ferrets. Vet. Pathol 53 (March (2)), 349–365, 10.1177/0300985815623621, PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball RS, 2006. Issues to consider for preparing ferrets as research subjects in the laboratory. ILAR J. 47 (4), 348–357, PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedictus MR, Spikman JM, van der Naalt J, 2010. Cognitive and behavioral impairment in traumatic brain injury related to outcome and return to work. Arch. Phys. Med. Rehabil 91 (September (9)), 1436–1441, 10.1016/j.apmr.2010.06.019, PMID: . [DOI] [PubMed] [Google Scholar]
- Cabot EB, Fox JG, 1990. Bile reflux and the gastric mucosa: an experimental ferret model. J. Invest. Surg 3 (2), 177–189, PMID: . [DOI] [PubMed] [Google Scholar]
- Christensson M, Garwicz M, 2005. Ontogenesis of within-session locomotor habituation in the open field. Neuroreport 16 (August (12)), 1319–1323, PMID: . [DOI] [PubMed] [Google Scholar]
- Defelipe J, 2011. The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Front. Neuroanat 16 (May (5)), 29, 10.3389/fnana.2011.00029, eCollection 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL, 1991. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39 (October (3)), 253–262, PMID: . [DOI] [PubMed] [Google Scholar]
- Dobbins HD, Marvit P, Ji Y, Depireux DA, 2007. Long-termally recording with a multi-electrode array device in the auditory cortex of an awake ferret. J. Neurosci. Methods 161 (March (1)), 101–111, 10.1016/j.jneumeth.2006.10.013, Epub 2006 Nov 28. PMID: PMCID: PMC1955228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper K, Ponsford J, 2008. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology 22, 618–625. [DOI] [PubMed] [Google Scholar]
- Duhaime AC, 2006. Large animal models of traumatic injury to the immature brain. Dev. Neurosci 28 (4–5), 380–387, 10.1159/000094164, PMID: . [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW, 2003. Inhibitory control in rats performing a stop-signal reaction time task: effects of lesions of the medial striatum and d-amphetamine. Behav. Neurosci 117, 1302. [DOI] [PubMed] [Google Scholar]
- Eakin K, Baratz-Goldstein R, Pick CG, Zindel O, Balaban CD, Hoffer ME, Lockwood M, Miller J, Hoffer BJ, 2014. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS ONE 9 (4), 10.1371/journal.pone.0090617, p. e90617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empie K, Rangarajan V, Juul SE, 2015. Is the ferret a suitable species for studying perinatal brain injury. Int. J. Dev. Neurosci 45, 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquenazi A, Lee S, Wikoff A, Packel A, Toczylowski T2, Feeley J, A Comparison of Locomotor Therapy Interventions: Partial Body Weight-Supported Treadmill, Lokomat, and G-EO Training in People With Traumatic Brain Injury. PM R. 2017. January 16 pii: S1934–1482(17)30030–8. doi: 10.1016/j.pmrj.2016.12.010. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG, 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta (GA). [Google Scholar]
- Feng Y, Clayton EH, Chang Y, Okamoto RJ, Bayly PV, 2013. Viscoelastic properties of the ferret brain measured in vivo at multiple frequencies by magnetic resonance elastography. J. Biomech 46 (5), 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JG, 1988. Biology and diseases of the ferret. Lea & Febiger, Malvern. [Google Scholar]
- Gennarelli TA, 1994. Animate models of human head injury. J. Neurotrauma 11 (August (4)), 357–368, 10.1089/neu.1994.11.357, Review. PMID: . [DOI] [PubMed] [Google Scholar]
- Gold JR, Nodal FR, Peters F, King AJ, Bajo VM, 2015. Auditory gap-in-noise detection behavior in ferrets and humans. Behav. Neurosci 129 (August (4)), 473–490, 10.1037/bne0000065, Epub 2015 Jun 8. PMID: PMCID: PMC4516322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Rabe A, Dumas R, Lazar JW, 1976. Position reversal deficit in young ferrets. Dev. Psychobiol 9 (July (4)), 311–344, 10.1002/dev.420090403, PMID: . [DOI] [PubMed] [Google Scholar]
- Hall ED, Bryant YD, Cho W, Sullivan PG, 2008. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma 25 (March (3)), 235–247, 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- He T, Friede H, Kiliaridis S, 2002. Macroscopic and roentgenographic anatomy of the skull of the ferret (Mustela putorius furo). Lab. Anim 36 (January (1)), 86–96, PMID: . [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Niemi EC, Wang SH, Lee CC, Bingham D, Zhang J, Cozen ML, Charo I, Huang EJ, Liu J, Nakamura MC, 2014. J. Neurotrauma 31 (20), 1677–1688, 10.1089/neu.2013.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson EB, Schwerin SC, Radomski KL, Irfanoglu MO, Juliano SL, Pierpaoli CM, 2016. Quantitative MRI and DTI abnormalities during the acute period following CCI in the ferret. Shock 46 (September (3 Suppl 1)), 167–176, 10.1097/shk.0000000000000659, PMID: PMCID: PMC4978600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson EB, Schwerin SC, Radomski KL, Sadeghi N, Jenkins J, Komlosh ME, Irfanoglu MO, Juliano SL, Pierpaoli C, 2017. Population based MRI and DTI templates of the adult ferret brain and tools for voxelwise analysis. Neuroimage 152 (March (16)), 575–589, 10.1016/j.neuroimage.2017.03.009 [Epub ahead of print] PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W, 2013. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42, 10.1093/brain/aws322, PMID: PMCID: PMC3562078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Meaney DF, Cullen DK, Smith DH, 2015. Animal models of traumatic brain injury. Handb. Clin. Neurol 127, 115–128, 10.1016/B978-0-444-52892-6, 00008–8 PMCID: PMC4946951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser NW, Engelhardt JF, 2011. New animal models of cystic fibrosis: what are they teaching us? Curr. Opin. Pulm. Med 17 (November (6)), 478–483, 10.1097/MCP.0b013e32834b14c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbler DE, Shields J, Yanasak N, Vender JR, Dhandapani KM, 2012. Activation of × 7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One 7 (7), e41229, 10.1371/journal.pone.0041229, Published online 2012 Jul 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou Z, VandeVord PJ, 2014. Traumatic white matter injury and glial activation: from basic science to clinics. Glia 62, 1831–1855, 10.1002/glia.22690, PMID: . [DOI] [PubMed] [Google Scholar]
- Kou Z, Wu Q, Kou X, Yin C, Wang H, Zuo Z, Zhuo Y, Chen A, Gao S, Wang X, 2015. CRISPR/Cas9-mediated genome engineering of the ferret. Cell Res. 25 (December (12)), 1352–1372, 10.1038/cr.2015.130, Epub 2015 Nov 13. PMID: PMCID: PMC4670990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawes INC, Andrews PLR, 1987. Variation of the ferret skull (Mustela putorius furo L.) in relation to stereotaxic landmarks. J. Anat 154, 157–171, PMID: PMCID: PMC1261843. [PMC free article] [PubMed] [Google Scholar]
- Lesniak A, Pick CG, Misicka A, Lipkowski AW, Sacharczuk M, 2016. Biphalin protects against cognitive deficits in a mouse model of mild traumatic brain injury (mTBI). Neuropharmacology 101 (February), 506–518, 10.1016/j.neuropharm.2015.10.014, Epub 2015 Oct 22. PMID: . [DOI] [PubMed] [Google Scholar]
- Lighthall JW, Goshgarian HG, Pinderski CR, 1990. Characterization of axonal injury produced by controlled cortical impact. J. Neurotrauma 7 (2), 65–76, 10.1089/neu.1990.7.65, Summer PMID: . [DOI] [PubMed] [Google Scholar]
- Lighthall JW, 1988. Controlled cortical impact: a new experimental brain injury model. J. Neurotrauma 5 (1), 1–15, 10.1089/neu.1988.5.1, PMID: . [DOI] [PubMed] [Google Scholar]
- Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI, 2014. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol 73, 14–29, 10.1097/NEN.0000000000000021, PMID: PMCID: PMC4267248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, Rosenthal G, Lam M, Morabito D, Yan D, Derugin N, Bollen A, Knudson MM, Panter SS, 2006. Controlled cortical impact in swine: pathophysiology and biomechanics. J. Neurotrauma 23 (February (2)), 128–139, 10.1089/neu.2006.23.128, PMID: . [DOI] [PubMed] [Google Scholar]
- McLaughlin DF, Sonty RV, Juliano SL, 1998. Organization of the forepaw representation in ferret somatosensory cortex. Somatosens. Mot. Res 15 (4), 253–268, PMID: . [DOI] [PubMed] [Google Scholar]
- Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, Longhi L, Laurer H, Maegele M, Neugebauer E, Graham DI, Stocchetti N, McIntosh TK, 2005. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience 136 (4), 971–989, 10.1016/j.neuroscience.2005.08.030, Epub 2005 Oct 20. PMID: . [DOI] [PubMed] [Google Scholar]
- Mott TF, McConnon ML, Rieger BP, 2012. Subacute to chronic mild traumatic brain injury. Am. Fam. Physician 86 (December (11)), 1045–1051, 1. [PubMed] [Google Scholar]
- Munyon C, Eakin KC, Sweet JA, Miller JP, 2014. Decreased bursting and novel object-specific cell firing in the hippocampus after mild traumatic brain injury. Brain Res. 1582, 220–226. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Esser MJ, 2015. A mild traumatic brain injury (mTBI) induces secondary attention-deficit hyperactivity disorder-like symptomology in young rats. Behav. Brain Res 286 (June (1)), 285–292, 10.1016/j.bbr.2015.03.010, Epub 2015 Mar 11. PMID: . [DOI] [PubMed] [Google Scholar]
- Noctor SC, Scholnicoff NJ, Juliano SL, 1997. Histogenesis of ferret somatosensory cortex. J. Comp. Neurol 387 (October (2)), 179–193, PMID: . [PubMed] [Google Scholar]
- O’Connor C, Heath DL, Cernak I, Nimmo AJ, Vink R, 2003. Effects of daily versus weekly testing and pre-training on the assessment of neurologic impairment following diffuse traumatic brain injury in rats. J. Neurotrauma 20 (October (10)), 985–993. [DOI] [PubMed] [Google Scholar]
- Oh DY, Hurt AC, 2016. Using the ferret as an animal model for investigating influenza antiviral effectiveness. Front. Microbiol 4 (February (7)), 10.3389/fmicb.2016.00080, 80. eCollection PMCID: PMC4740393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Sasaki Y, Kohsaka S, 2004. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J. Neurochem 88 (February (4)), 844–856, PMID: . [DOI] [PubMed] [Google Scholar]
- Osier ND, Dixon CE, 2016. The controlled cortical impact model: applications, considerations for researchers, and future directions. Front. Neurol 17 (August (7)), 134, 10.3389/fneur.2016.00134, Collection 2016. PMID: PMCID: PMC4987613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Alföldi J, Gori K, Eisfeld AJ, Tyler SR, Tisoncik-Go J, Brawand D, Law GL, Skunca N7, Hatta M, Gasper DJ, Kelly SM, Chang J, Thomas MJ, Johnson J, Berlin AM, Lara M6, Russell P, Swofford R, Turner-Maier J, Young S, Hourlier T, Aken B, Searle S, Sun X, Yi Y, Suresh M, Tumpey TM, Siepel A, Wisely SM, Dessimoz C, Kawaoka Y, Birren BW, Lindblad-Toh K, Di Palma F, Engelhardt JF, Palermo RE, Katze MG, 2014. The draft genome sequence of the ferret (Mustela putorius furo) facilitates study of human respiratory disease. Nat. Biotechnol 32 (December (12)), 1250–1255, 10.1038/nbt.3079, Epub 2014 Nov 17. PMID: PMCID: PMC4262547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluch S, Juliano SL, 2015. Fine-tuning of neurogenesis is essential for the evolutionary expansion of the cerebral cortex. Cereb. Cortex 25 (Februry (2)), 346–364, 10.1093/cercor/bht232, Epub 2013 Aug 22. PMID: PMCID: PMC4351427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsford JL, Downing MG, Olver J, Ponsford M, Acher R, Carty M, Spitz G, 2014. Longitudinal follow-up of patients with traumatic brain injury: outcome at two, five, and ten years post-injury. J. Neurotrauma 31 (January (1)), 64–77, 10.1089/neu.2013.2997, PMID: . [DOI] [PubMed] [Google Scholar]
- Rabe A, Haddad R, Dumas R, 1985. Behavior and neurobehavioral teratology using the ferret. Lab. Anim. Sci 35 (June (3)), 256–267, PMID: . [PubMed] [Google Scholar]
- Rafaels KA, Bass CR, Panzer MB, Salzar RS, Woods WA, Feldman SH, Walilko T, Kent RW, Capehart BP, Foster JB, Derkunt B, Toman A, 2012. Brain injury risk from primary blast. J Trauma Short-term Care Surg. 73 (October (4)), 895–901, 10.1097/TA.0b013e31825a760e, PMID: . [DOI] [PubMed] [Google Scholar]
- Rafaels K, DiLeonardi A, Bass C, 2016. Understanding the brain injury mechanisms of primary blast exposure. In: International Research Council on Biomechanics of Injury (IRCOBI) Conference, Malaga, Spain, September 14–16, IRC-16–60. [Google Scholar]
- Robbins TW, Muir JL, Killcross AS, Pretsell D, 1993. In: Sahgal A (Ed.), Methods for Assessing Attention and Stimulus Control in the Rat Behavioral Neuroscience: A Practical Approach. I Oxford University Press, New York, p. 13. [Google Scholar]
- Rodriguiz RM, Wetsel WC, 2006. Assessments of cognitive deficits in mutant mice In: Levin ED, Buccafusco JJ (Eds.), Animal Models of Cognitive Impairment. CRC Press/Taylor & Francis, Boca Raton (FL). [PubMed] [Google Scholar]
- Roof RL, Hall ED, 2000a. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J. Neurotrauma 17 (December (12)), 1155–1169. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED, 2000b. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma 17 (May (5)), 367–388. [DOI] [PubMed] [Google Scholar]
- Sawada K, Horiuchi-Hirose M, Saito S, Aoki I, 2013. MRI-based morphometric characterizations of sexual dimorphism of the cerebrum of ferrets (Mustela putorius). Neuroimage 83, 294–306. [DOI] [PubMed] [Google Scholar]
- Scheid R, Walther KR, Guthke T, Preul C, von Cramon DY, 2006. Cognitive sequelae of diffuse axonal injury. Arch. Neurol 63, 418–424. [DOI] [PubMed] [Google Scholar]
- Shum DH, Harris D, O’Gorman JG, 2000. Effects of severe traumatic brain injury on visual memory. J. Clin. Exp. Neuropsychol 22, 25–39. [DOI] [PubMed] [Google Scholar]
- Smith C, Gentleman SM, Leclercq PD, Murray LS, Griffin WST, Graham DI, Nicoll JAR, 2013. The neuroinflammatory response in humans after traumatic brain injury. Neuropathol. Appl. Neurobiol 39, 654–666, 10.1111/nan.12008, PMID: PMCID: PMC3833642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statler KD, Jenkins LW, Dixon CE, Clark RS, Marion DW, Kochanek PM, 2001. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. J. Neurotrauma 18 (November (1)), 1195–1206, 10.1089/089771501317095232, Review. PMID: . [DOI] [PubMed] [Google Scholar]
- Stein DG, 2015. Embracing failure: what the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 29 (11), 1259–1272, Epub 2015 Aug 14. Review. PMID: PMCID: PMC4667711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Yan Z, Liu X, Olivier AK, Engelhardt JF, 2014. In: Fox JG, Marini RP (Eds.), Genetic Engineering in the Ferret, in Biology and Diseases of the Ferret, 3 John Wiley & Sons, Inc., Ames, USA, p. ch28, 10.1002/9781118782699. [DOI] [Google Scholar]
- Suzuki T, Bramlett HM, Ruenes G, Dietrich WD, 2004. The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J. Neurotrauma 21 (July (7)), 842–853. [DOI] [PubMed] [Google Scholar]
- Tucker LB, Fu AH, McCabe JT, 2016. Performance of male and female C57BL/6J mice on motor and cognitive tasks commonly used in pre-clinical traumatic brain injury research. J. Neurotrauma 33 (May 9 (1)), 880–894, 10.1089/neu.2015.3977, Epub 2015 Aug 12. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LB, Burke JF, Fu AH, McCabe JT, 2017. Neuropsychiatric symptom modeling in male and female C57BL/6J mice after experimental traumatic brain injury. J. Neurotrauma 34 (February (15) (4)), 890–905, 10.1089/neu.2016.4508, Epub 2016 Jun 7. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan EV1, Glass RN, Packel AT, 2014. Effects of traumatic brain injury on locomotor adaptation. J. Neurol. Phys. Ther 38 (July (3)), 172–182, 10.1097/NPT.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Velosky AG, Tucker LB, Fu AH, Liu J, McCabe JT, 2017. Cognitive performance of male and female C57BL/6J mice after repetitive concussive brain injuries. Behav. Brain Res 324 (May (1)), 115–124, 10.1016/j.bbr.2017.02.017, Epub 2017 Feb 15. PMID: . [DOI] [PubMed] [Google Scholar]
- Williams G, Morris ME, Schache A, McCrory PR, 2009. Incidence of gait abnormalities after traumatic brain injury. Arch. Phys. Med. Rehabil 90, 587–593. [DOI] [PubMed] [Google Scholar]
- Winston CN, Noel A, Neustadtl A, Parsadanian M, Barton DJ, Chellappa D, Wilkins TE, Alikhani AD, Zapple DN, Villapol S, Planel E, Burns MP, 2016. Dendritic spine loss and chronic white matter inflammation in a mouse model of highly repetitive head trauma. Am. J. Pathol 186, 552–567, 10.1016/j.ajpath.2015.11.006, PMID: PMCID: PMC4816714 [Available on 2017–03–01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2013. Animal models of traumatic brain injury. Nat. Rev. Neurosci 14 (February (2)), 128–142, 10.1038/nrn3407, Review. PMID: PMCID: PMC3951995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, 2006. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage, 10.1016/j.neuroimage.2006.01.015, PMID: . [DOI] [PubMed] [Google Scholar]
- Zhao Z, Loane DJ, Murray II MG, Stoica BA, Faden AI, 2012. Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J. Neurotrauma 29 (October (15)), 2475–2489, 10.1089/neu.2012.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZC, Yu C, Sellers KK, Fröhlich F, 2016. Dorso-Lateral frontal cortex of the ferret encodes perceptual difficulty during visual discrimination. Sci. Rep 30 (March (6)), 23568, 10.1038/srep23568, PMID: PMCID: PMC4812342. [DOI] [PMC free article] [PubMed] [Google Scholar]