Abstract
The epithelial growth factor receptor (EGFR) is involved in various stages of cancer growth such as tumor initiation, angiogenesis and metastasis, being an attractive target for oncogenic therapy. The present study aims to evaluate the EGFR expression in 54 cases of serous and mucinous ovarian borderline tumors and carcinomas. EGFR expression was present in more than half of the investigated tumors, more frequently in carcinomas than in borderline tumors, especially in the serous type. The highest values of the final staining score (FSS) were observed only in serous carcinomas in the advanced stages of the disease. As a result of frequent expression of EGFR in ovarian tumors, it is necessary to monitor EGFR inhibitors for ovarian cancer therapy, but probably after establishing more rigorous selection and stratification criteria for patients.
Keywords: malignant ovarian tumors , EGFR
Introduction
For many years, the efforts to identify the prognostic factors of ovarian carcinomas focused on molecular markers that were most often immunohistochemically investigated. Until now, there are no defined clinical or molecular markers that can help to identify borderline tumors or ovarian carcinomas with increased risk for aggressive behavior.
The effect of the epithelial growth factor receptor (EGFR) as a tyrosine kinases receptor is considered a target for cancer immunotherapy. EGFR is one of the four members of the human epidermal growth factor that has been shown to have physiological activity but also and oncogenic roles in some malignancies [1].
Because EGFR is involved in various stages of cancer growth, such as tumor initiation, angiogenesis and metastasis, it is an attractive target for oncogenic therapy [2,3]. In addition, EGFR participates by various pathways as a proto-oncogene in several types of cancers such as gastrointestinal, oral and breast ones [4].
The present study aims to evaluate the expression of EGFR in ovarian serous and mucinous borderline tumors and carcinomas.
Material and methods
The study included 54 malignant ovarian tumors represented by borderline tumors and carcinomas, of which 41 corresponded to the serous subtype and 13 were mucinous tumors.
The analyzed cases were represented by pacients from the Surgery and Gynaecology Clinics of the Emergency County Hospital Craiova.
Surgical excision specimens were fixed in 10% buffered formalin, then processed by the classic histopathological technique and stained with Hematoxylin-Eosin. The classification of lesions was made according to the literature recommendations [5].
Subsequently, the immunoreactions were performed on serial sections using the monoclonal mouse antihuman EGFR antibody (Dako, Redox, Romania), clone E30, in dilution 1:1000 and without antigen retrieval pretreatment. The immunohistochemical reactions were done using a system based on polymerization-based amplification (Histophine polymer-Horseradish Peroxidase, Nichirei, Japan, ready to use, code 414151F). To visualize the reactions the DAB (3,3'-diaminobenzidine) cromogen was used (code 3467, Dako), and for validating the reactions were used positive (oral mucosa) and negative external controls (by omitting the primary antibody).
The semiquantitative assessment of EGFR expression was done through a scoring system that was independently assigned by two specialists (CE and AS) based on the intensity of the staining and the percentage of positive cells [6].
The intensity score was noted as follows: 1 (weak intensity), 2 (moderate intensity), and 3 (strong intensity), and the cutoff value for positivity reactions was set at 5%.
The percentage of cells labelled was scored by 1 (6-25% positive cells), 2 (26-50% positive cells), 3 (51-75% positive cells) or 4 (>75% positive cells). The final staining score (FSS) was calculated by multiplying the intensity and labeled cells scores.
The study was approved by the local ethical committee (no.195/24.10.2017), and written informed consent was obtained from all the patients.
Results
The study included 54 malignant ovarian tumors of which 41 corresponded to the serous subtype and 13 were mucinous tumors (Table 1).
Table 1.
Distribution of the investigated cases
| Stage/Tumor | Serous tumors | Mucinous tumors | ||
| Borderline | Carcinomas | Borderline | Carcinomas | |
| Stage I | 14 | 0 | 6 | 7 |
| Stage II | 0 | 3 | 0 | 0 |
| Stage III | 0 | 24 | 0 | 0 |
The 41 serous malignant tumors included 14 borderline tumors and 27 carcinomas, of which three of low-grade and 24 of high-grade. The mucinous malignant tumors corresponded in six cases to borderline tumors and in seven cases to low grade carcinomas.
EGFR expression analysis revealed the positivity reaction in 28 cases (51.8%), distributed across all the tumor groups analyzed (Table 2). For serous tumors, EGFR positivity was observed in a small number of borderline tumors compared to carcinomas, respectively in three versus 21 cases. Negative cases corresponded to both tumor types.
Table 2.
Distribution of EGFR positive cases
| Tumor type/No. cases | Serous tumors | Mucinous tumors | ||
| Borderline | Carcinomas | Borderline | Carcinomas | |
| Number of positive cases/ Total number of cases | 3/14 | 21/27 | 1/6 | 3/7 |
| % | 21.4% | 77.7% | 16.6 | 42.8 |
For mucinous tumors, the number of EGFR positive-cases was lower, also lower for borderline tumors compared to carcinomas, respectively one case of borderline tumor and three cases of mucinous carcinomas.
Referring to EGFR FSS score, we found that the highest mean values were found in high-grade serous carcinomas (Table 3).
Table 3.
Distribution of tumors depending on EGFR FSS values
| Tumor type / stage (SC) | Serous tumors | Mucinous tumors | |||
| Borderline tumors | Low-grade carcinoma | High-grade carcinoma | Borderline tumors | Low-grade carcinoma | |
| Stage I | 2 | 2 | 0 | 2 | 6 |
| Stage II | 0 | 0 | 1 | 0 | 0 |
| StageIII | 0 | 0 | 11.2 | 0 | 0 |
We found the presence of EGFR immunostaining in all analyzed tumors, borderline or carcinomas but with varying incidence.
In all EGFR-positive tumors the expression was cytoplasmic and membranous, sometimes with a clearly granular pattern at the cytoplasmic level.
In serous borderline tumors, EGFR was expressed in less than 25% of tumor cells with moderate intensity, the mean immunostaining score being 2 (Fig. 1).
Figure 1.
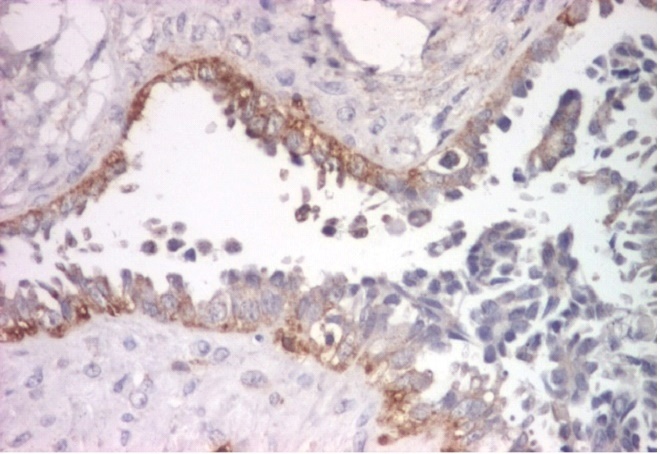
Serous borderline tumor, EGFR immunoexpression, x100
Similar aspects were also present in low grade serous carcinomas, EGFR expression being identified in a single case, with a reduced intensity and in less than 25% of the tumor cells (Fig. 2).
Figure 2.
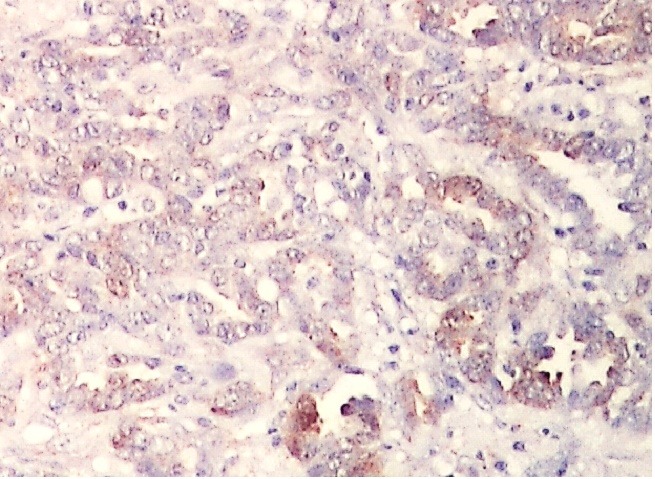
Low grade serous carcinoma, EGFR immunoexpression, x100
In contrast, in high-grade serous carcinoma we frequently observed diffuse immunostaining (score 4) and with often increase intensity (score 3) with a mean FSS value of 11,2 (Fig. 3).
Figure 3.
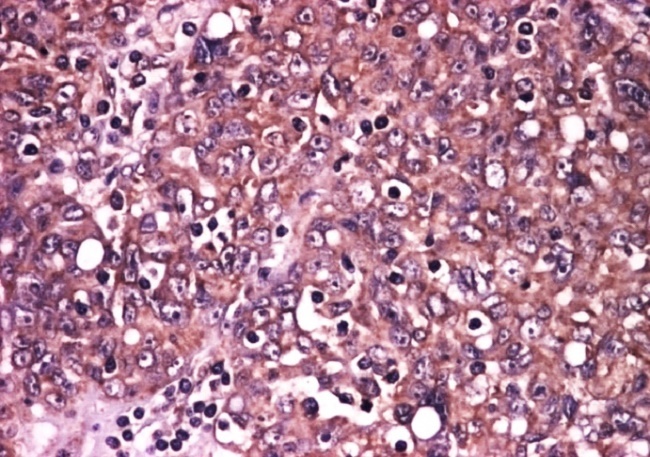
High grade serous carcinoma, EGFR immunoexpression, x100
For mucinous borderline tumors, EGFR expression was identified in a single case, with immunostaining also in less than 25% of tumors cells and moderate intensity, similar to that observed in benign tumors areas (Fig. 4).
Figure 4.
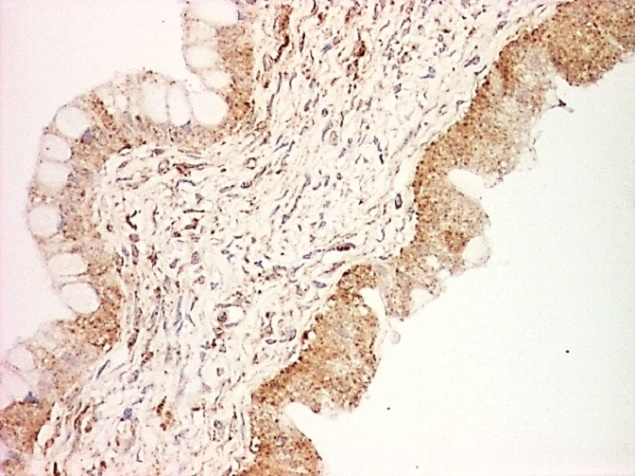
Borderline mucinous tumor, FR immunoexpression, x100
Mucinous carcinomas expressed EGFR in less than half of the tumors cells with moderate intensity, with a mean immunostaining score of 6 (Fig. 5).
Figure 5.
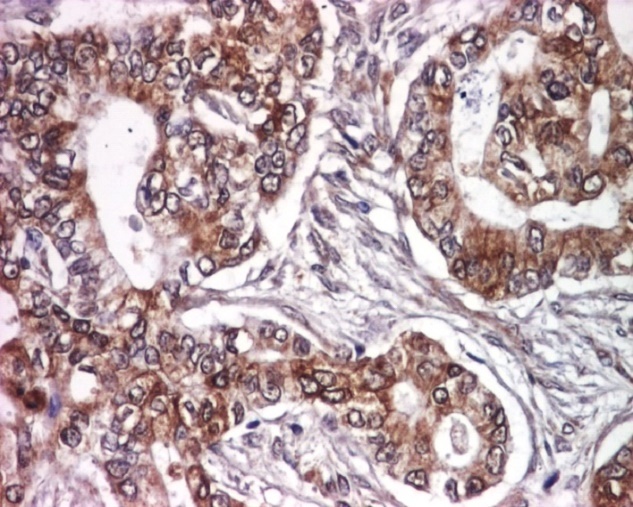
Mucinous carcinoma, EGFR immunoexpression, x100
Discussions
The ErbB family of receptor tyrosine kinases play a role in the tumorigenesis of many types of solid tumors and comprises epidermal growth factor (EGF) receptor 1 (EGFR, HER1/ErbB1), EGFR2/ErbB2 (HER2/neu), HER3/ErbB3 and HER4/ErbB4 [7].
The abnormal activation of these receptors has been associated with various pathological processes, including malignant transformation [8], the four EGF receptors playing a key role in promoting carcinogenesis through proliferation, survival, migration, adhesion and cell differentiation.
EGFR is a transmembrane receptor with tyrosine kinase activity, involved in cell growth and proliferation [9]. EGFR plays a role in cell survival, epithelial-mesenchymal transition, angiogenesis and metastasis [10]. The EGFR amplification, mutations and overexpression have been extensively and variably reported in ovarian carcinoma [11].
Normally, the ovarian epithelium expresses poorly EGFR, unlike ovarian carcinomas in which overexpression of EGFR varies in different studies between 4-100% of cases [12,13]. In this study we observed the EGFR expression in half of the analyzed cases.
The expression was cytoplasmic in both types of malignant serous tumors, borderline and carcinomas, but with different incidence, respectively three cases (21.4%) and 21 cases (87.5%). One study reported the protein expression in a significantly higher proportion in carcinomas compared with borderline tumors, respectively 69% versus 18% [14].
Chen et al. indicated that the EGFR cytoplasmic positivity in both ovarian borderline tumors and carcinomas was significantly higher than in normal ovarian tissue and benign tumors [15]. Similarly, Nielsen et al reported that 67% of ovarian borderline tumors and 62% of ovarian carcinomas revealed cytoplasmic positivity [16]. Instead, Fujiwara et al reported the lack of positivity in borderline tumors, while 39.3% of serous carcinomas showed cytoplasmic staining [17]. Also, Brustmann et al. reported EGFR negativity in benign and borderline serous tumors compared with the positivity (64%) of serous carcinomas [18].
The analysis of EGFR expression for borderline tumors and serous carcinomas analyzed in correlation with stage and tumor grade indicated the highest values for advanced stage serous carcinomas. EGFR is disturbed in ovarian cancer, and increased expression is associated with a low survival rate [19,20,21]. In a study of 50 ovarian serous carcinomas, EGFR positivity was reported in 64% of cases, the results being correlated with tumor grade and survival [18]. Several studies have concluded that EGFR expression and tumor grade are independent parameters [12,13]. An extensive study which analyzed the expression of EGFR and p53 proposed to stratify them in three groups: low risk (well-differentiated tumors, negative for p53 and EGFR), intermediate risk (well differentiated tumors, p53/EGFR positive or poorly differentiated p53 and p53/EGFR negative) and high risk group (poorly differentiated tumors and p53/EGFR positive) [22]. The significance of EGFR immunoexpression is controversial regarding malignant serous ovarian tumors [18]. Although the constant correlation of EGFR expression with the aggressiveness of the disease has not been demonstrated, it still appears to be associated with poor prognosis and weaker therapeutic response [12,23,24].
For mucinous tumors, in this study we also observed EGFR cytoplasmic expression in both types of malignant tumors, borderline and carcinomas, but for a small number of cases, respectively for only one case in borderline tumors (16.6%) and for three cases in mucinous carcinomas (48.2%). Data from the literature report similar aspects, with EGF and EGFR expression being significantly higher in mucinous carcinomas than in mucinous cystadenomas or mucinous borderline tumors [25].
The analysis of EGFR expression in relation to the stage and tumor grade for the investigated serous carcinomas indicated the presence of high immunostaining scores in the advanced stages of the disease. A recent study on ovarian carcinomas found for non-serous neoplasia the correlation of EGFR overexpression with tumor grade and stage [26]. Although the prognostic significance of EGFR has been extensively studied in ovarian cancer, it still remains unclear. Several studies that have been concerned about the relationship between EGFR overexpression and clinical pathological characteristics, evaluation of chemotherapy response and survival rates indicated conflicting results [27,28]. Very few reports included borderline tumors and, in particular, have investigated a small number of cases or have studied either only the protein expression or only mutations. These results suggest that the EGFR immunostaining can not be a useful prognostic biomarker for ovarian cancer patients [29].
As a consequence of frequent EGFR expression in ovarian carcinomas, this has been a subject of investigation for the therapies with monoclonal antibodies and small molecule tyrosine kinase inhibitors, exploring it as a potential therapeutic agent. Thus, as a result of frequent EGFR expression in ovarian tumors [30] and the ability of this pathway to induce tumor cells proliferation and dissemination, follow-up of EGFR inhibitors is required for ovarian cancer therapy, but the results observed in clinical trials conducted so far are quite weak [31,32,33], which indicates the need for better methods of patients selection and stratification.
Conclusions
EGFR expression was present in more than half of the investigated tumors, more frequently in carcinomas than in borderline tumors, especially in the serous type. The highest values of the immunostaining score were observed only in advanced stage serous carcinomas. As a result of frequent EGFR expression in ovarian tumors, follow-up of EGFR inhibitors is required for ovarian cancer therapy, but probably after establishing more rigorous selection and stratification criteria for patients.
References
- 1.Bull Phelps, Schorge JO, Peyton MJ, Shigematsu H, Xiang LL, Miller DS, Lea JS. Implications of EGFR inhibition in ovarian cancer cell proliferation. Gynecol Oncol. 2008;109(3):411–417. doi: 10.1016/j.ygyno.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 2.Noske A, Schwabe M, Weichert W, Darb-Esfahani S, Buckendahl AC, Sehouli J, Braicu EI, Budczies J, Dietel M, Denkert C. An intracellular targeted antibody detects EGFR as an independent prognostic factor in ovarian carcinomas. BMC Cancer. 2011;11:294–294. doi: 10.1186/1471-2407-11-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landen CN, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26(6):995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K, Babic I, Nathanson D, Akhavan D, Guo D, Gini B, Dang J, Zhu S, Yang H, De Jesus, Amzajerdi AN, Zhang Y, Dibble CC, Dan H, Rinkenbaugh A, Yong WH, Vinters HV, Gera JF, Cavenee WK, Cloughesy TF, Manning BD, Baldwin AS, Mischel PS. Oncogenic EGFR signaling activates an mTORC2-NF-k B pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1(6):524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seidman JD, Bell DA, Crum CP, Gilks CB, Kurman RJ, Levine DA, Lonacre TA, Pasini B, Riva C, Sherman ME, Shih IM, Singer G, Soslow R, Vang R. Serous cystadenoma, adenofibroma and surface papilloma. In: Kurman RJ, Carcangiu ML, Harrington CS, Young RH, editors. WHO Classification of tumors of female reproductive organs. Lyon: Iarc Press; 2014. pp. 17–18. [Google Scholar]
- 6.Wang N, Dong CR, Jiang R, Tang C, Yang L, Jiang QF, Chen GG, Liu ZM. Overexpression of HIF-1a, metallothionein and SLUG is associated with high TNM stage and lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2013;7(1):322–330. [PMC free article] [PubMed] [Google Scholar]
- 7.Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer. 2013;13(9):663–673. doi: 10.1038/nrc3559. [DOI] [PubMed] [Google Scholar]
- 8.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 9.Yewale C, Baradia D, Vhora I, Patil S, Misra A. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials. 2013;34(34):8690–8707. doi: 10.1016/j.biomaterials.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 10.Casanova ML, Larcher F, Casanova B, Murillas R, Fernández-Aceñero MJ, Villanueva C, Martínez-Palacio J, Ullrich A, Conti CJ, Jorcano JL. A critical role for ras-mediated, epidermal growth factor receptor-dependent angiogenesis in mouse skin carcinogenesis. Cancer Res. 2002;62(12):3402–3407. [PubMed] [Google Scholar]
- 11.Siwak DR, Carey M, Hennessy BT, Nguyen CT, McGahren Murray, Nolden L, Mills GB. Targeting the epidermal growth factor receptor in epithelial ovarian cancer: current knowledge and future challenges. J Oncol. 2010;2010:568938–568938. doi: 10.1155/2010/568938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyes HD, Thiel KW, Carlson MJ, Meng X, Yang S, Stephan JM, Leslie KK. Comprehensive profiling of EGFR/HER receptors for personalized treatment of gynecologic cancers. Mol Diagn Ther. 2014;18(2):137–151. doi: 10.1007/s40291-013-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirte HW. Profile of erlotinib and its potential in the treatment of advanced ovarian carcinoma. Onco Targets Ther. 2013;6:427–435. doi: 10.2147/OTT.S30373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Haaften-Day, Russell P, Boyer CM, Kerns BJ, Wiener JR, Jensen DN, Bast RC, Hacker NF. Expression of cell regulatory proteins in ovarian borderline tumors. Cancer. 1996;77(10):2092–2098. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2092::AID-CNCR19>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 15.Chen AP, Zhang J, Liu H, Zhao SP, Dai SZ, Sun XL. Association of EGFR expression with angiogenesis and chemoresistance in ovarian carcinoma. Zhonghua Zhong Liu Za Zhi. 2009;31(1):48–52. [PubMed] [Google Scholar]
- 16.Nielsen JS, Jakobsen E, Hølund B, Bertelsen K, Jakobsen A. Prognostic significance of p53, Her-2, and EGFR overexpression in borderline and epithelial ovarian cancer. Int J Gynecol Cancer. 2004;14(6):1086–1096. doi: 10.1111/j.1048-891X.2004.14606.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara S, Terai Y, Kawaguchi H, Takai M, Yoo S, Tanaka T, Tsunetoh S, Sasaki H, Kanemura M, Tanabe A, Yamashita Y, Ohmichi M. GPR30 regulates the EGFR-Akt cascade and predicts lower survival in patients with ovarian cancer. J Ovarian Res. 2012;5(1):35–35. doi: 10.1186/1757-2215-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brustmann H. Epidermal growth factor receptor expression in serous ovarian carcinoma: an immunohistochemical study with galectin-3 and cyclin D1 and outcome. Int J Gynecol Pathol. 2008;27(3):380–389. doi: 10.1097/PGP.0b013e31815d060d. [DOI] [PubMed] [Google Scholar]
- 19.Gui T, Shen K. The epidermal growth factor receptor as a therapeutic target in epithelial ovarian cancer. Cancer Epidemiol. 2012;36(5):490–496. doi: 10.1016/j.canep.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Marinas MC, Mogos G, Ciurea R, Mogos DG. EGFR, HER2÷neu and Ki67 immunoexpression in serous ovarian tumors. Rom J Morphol Embryol. 2012;53(3):563–567. [PubMed] [Google Scholar]
- 21.Li D, Bi FF, Cao JM, Cao C, Li CY, Yang Q. Effect of BRCA1 on epidermal growth factor receptor in ovarian cancer. J Exp Clin Cancer Res. 2013;32:102–102. doi: 10.1186/1756-9966-32-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skírnisdóttir I1, Seidal T, Sorbe B. A new prognostic model comprising p53, EGFR, and tumor grade in early stage epithelial ovarian carcinoma and avoiding the problem of inaccurate surgical staging. Int J Gynecol Cancer. 2004;14(2):259–270. doi: 10.1111/j.1048-891X.2004.014209.x. [DOI] [PubMed] [Google Scholar]
- 23.Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta. 2008;1785(2):232–265. doi: 10.1016/j.bbcan.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Tas F, Karabulut S, Serilmez M, Ciftci R, Duranyildiz D. Increased serum level of epidermal growth factor receptor (EGFR) is associated with poor progression-free survival in patients with epithelial ovarian cancer. Cancer Chemother Pharmacol. 2014;73(3):631–637. doi: 10.1007/s00280-014-2396-x. [DOI] [PubMed] [Google Scholar]
- 25.Niikura H, Sasano H, Sato S, Yajima A. Expression of epidermal growth factor-related proteins and epidermal growth factor receptor in common epithelial ovarian tumors. Int J Gynecol Pathol. 1997;16(1):60–68. doi: 10.1097/00004347-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Alshenawy HA. Immunohistochemical expression of epidermal growth factor receptor, E-cadherin, and matrix metalloproteinase-9 in ovarian epithelial cancer and relation to patient deaths. Ann Diagn Pathol. 2010;14(6):387–395. doi: 10.1016/j.anndiagpath.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri-Broët S, Hardy-Bessard AC, Le Tourneau, Paraiso D, Levrel O, Leduc B, Bain S, Orfeuvre H, Audouin J, Pujade-Lauraine E. HER-2 overexpression is an independent marker of poor prognosis of advanced primary ovarian carcinoma: a multicenter study of the GINECO group. Ann Oncol. 2004;15(1):104–112. doi: 10.1093/annonc/mdh021. [DOI] [PubMed] [Google Scholar]
- 28.Psyrri A, Kassar M, Yu Z, Bamias A, Weinberger PM, Markakis S, Kowalski D, Camp RL, Rimm DL, Dimopoulos MA. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res. 2005;11(24 Pt 1):8637–8643. doi: 10.1158/1078-0432.CCR-05-1436. [DOI] [PubMed] [Google Scholar]
- 29.Mehner C, Oberg AL, Goergen KM, Kalli KR, Maurer MJ, Nassar A, Goode EL, Keeney GL, Jatoi A, Radisky DC, Radisky ES. EGFR as a prognostic biomarker and therapeutic target in ovarian cancer: evaluation of patient cohort and literature review. Genes Cancer. 2017;8(5-6):589–599. doi: 10.18632/genesandcancer.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teplinsky E, Muggia F. EGFR and HER2: is there a role in ovarian cancer? Transl Cancer Res. 2015;4(1):107–117. [Google Scholar]
- 31.Posadas EM, Liel MS, Kwitkowski V, Minasian L, Godwin AK, Hussain MM, Espina V, Wood BJ, Steinberg SM, Kohn EC. A phase II and pharmacodynamic study of gefitinib in patients with refractory or recurrent epithelial ovarian cancer. Cancer. 2007;109(7):1323–1330. doi: 10.1002/cncr.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon AN, Finkler N, Edwards RP, Garcia AA, Crozier M, Irwin DH, Barrett E. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int J Gynecol Cancer. 2005;15(5):785–792. doi: 10.1111/j.1525-1438.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 33.Schilder RJ, Sill MW, Chen X, Darcy KM, Decesare SL, Lewandowski G, Lee RB, Arciero CA, Wu H, Godwin AK. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clinical Cancer Research. 2005;11(15):5539–5548. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]


