Abstract
ABSTRACT: We report a rare case of seronegative autoimmune pancreatitis (AIP) that presented as a pancreatic focal lesion and was considered to be pancreatic cancer based on the clinical presentation and imaging findings. The endoscopic ultrasound-guided biopsies of the pancreatic mass revealed no malignant cells and the pancreatic swelling had become diffuse on repeat imaging. AIP was suspected and a trial of steroids was considered as a diagnostic and therapeutic method. The patient responded dramatically to corticosteroid treatment with resolution of symptoms and normal imagining and laboratory parameters. This case highlights the challenge in the diagnostic approach of a pancreatic mass.
Keywords: pancreatic cancer, autoimmune pancreatitis, endoscopic ultrasound, endoscopic ultrasound-guided fine-needle aspiration
Introduction
Autoimmune pancreatitis (AIP) is a rare form of chronic pancreatitis and comprises two entities, with significant differences regarding their natural history, clinical presentation and histopathological patterns [1,2]. Type 1 AIP has been described as a pancreatic manifestation of immunoglobulin G4 (IgG4)-related disease and is associated with a high serum level of IgG4 [3]. Type 2 AIP is a pancreatic specific disorder not associated with IgG4 [4].
The definitive diagnosis of AIP is challenging, as this disorder may present as a pancreatic mass mimicking pancreatic cancer. Misdiagnosis can have severe consequences such as delaying or losing the opportunity for potential curative surgery in case of pancreatic malignancy or performing pancreatic surgery for benign disease, with high risk of related-morbidity and mortality.
Herein, we report the case of a seronegative AIP presented as a pancreatic focal lesion, referred from the community to our tertiary referral cancer center as it was considered to be pancreatic cancer based on the clinical presentation and imaging findings. This case highlights the challenge in the diagnostic approach of a pancreatic mass, particularly in distinguishing benign from malignant disease.
Case Report
A 67-year-old gentleman with a medical history of bladder cancer 7 years prior to his admission, treated with surgical resection followed by systemic chemotherapy, presented with a fairly constant 2/10 in intensity epigastric pain, acholic stools and steatorrhea. The lab workup at that time revealed an increased lipase level of 3800U/L (normal range: 23-300U/L). Serum carbohydrate antigen (CA19-9) was slightly elevated (68U/mL, normal range: 3-300U/L). Computed tomography (CT) scan revealed a pancreatic head mass measuring 2.3 by 2.2cm associated with mild peripancreatic fat stranding (Figure 1A).
Figure 1.
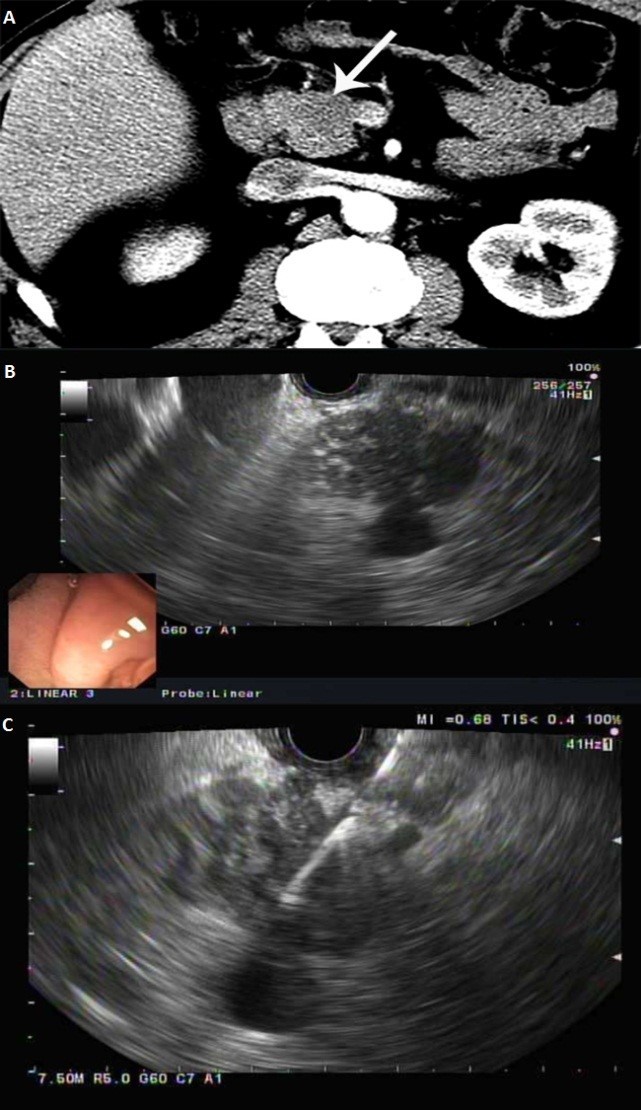
A. Axial contrast enhanced CT showing a hypoattenuating mass in the pancreatic head (arrow) abutting the SMV; B. Endoscopic ultrasound showing a hypoechoic, heterogenous pancreatic mass, with loss of interface with superior mesenteric vein; C. Endoscopic ultrasound-guided fine needle aspiration of the pancreatic mass using a 25 gauge needle
The differential diagnosis at the time included a tumor, either adenocarcinoma or neuroendocrine, or less likely a focal episode of pancreatitis causing fibrosis. A follow-up MRI of the abdomen confirmed a 2.4x2.1cm ill-defined mass in the pancreatic head, showing a mildly decreased T1/mildly increased T2 signal, with post-contrast imaging showing progressive enhancement and mild pancreatic ductal dilatation, most concerning for an adenocarcinoma. There was no dilation of the common bile duct nor encasement of the surrounding vascular structures. The patient was then referred to MD Anderson for diagnostic work-up of the pancreatic head lesion.
In order to clarify the nature of the referred lesion, endoscopic ultrasound (EUS) was performed, which revealed a 3.8 by 2.3cm mass in the pancreatic head with evidence of superior mesenteric vein abutment (Figure 1B).
EUS-guided fine needle aspiration was further performed (Figure 1C) and pathology revealed no malignant cells. Given the absence of malignancy on initial EUS-guided biopsy, repeat EUS/CT was recommended for close interim follow-up after 6 weeks.
The follow-up CT images (Figure 2A) showed diffuse pancreatic enlargement with diminished visualization of pancreatic duct, peripancreatic inflammatory changes, central biliary dilation, and narrowing of the common bile duct at the level of the pancreatic head. Repeat EUS identified a hypoechoic mass in the pancreatic head, with irregular margins, surrounding pancreatic head with changes of chronic pancreatitis. The body and tail of the pancreas were also grossly abnormal with echogenic septations and scattered echogenic foci. The main pancreatic duct was minimally dilated in the body. EUS-guided FNA and core biopsy were performed using a 22 gauge Cook Procore needle. Cytopathology report revealed no malignancy and the immunoperoxidase stain for IgG4 was negative. Serum immunological tests revealed a normal level of IgG4. The constellation of findings was most consistent with IgG4-seronegative autoimmune pancreatitis and steroid therapy was recommended. Prior to the initiation of steroids, the patient presented again to the Emergency Department with mild abdominal pain and jaundice. The patient’s bilirubin was found to be 5.7mg/dl (normal range: 0.2-1.3mg/dl). Laboratory examinations revealed elevated serum levels of aspartate aminotransferase (123U/l, normal range: 15-46U/l) and alanine aminotransferase (170 U/l., normal range: 7-56U/l). Repeat imaging revealed edematous pancreas along with diffuse thickening of the common bile duct and increased extra and intrahepatic biliary dilatation. The findings were likely related to autoimmune pancreatitis that had progressed to autoimmune cholangitis (Figure 2C).
Figure 2.
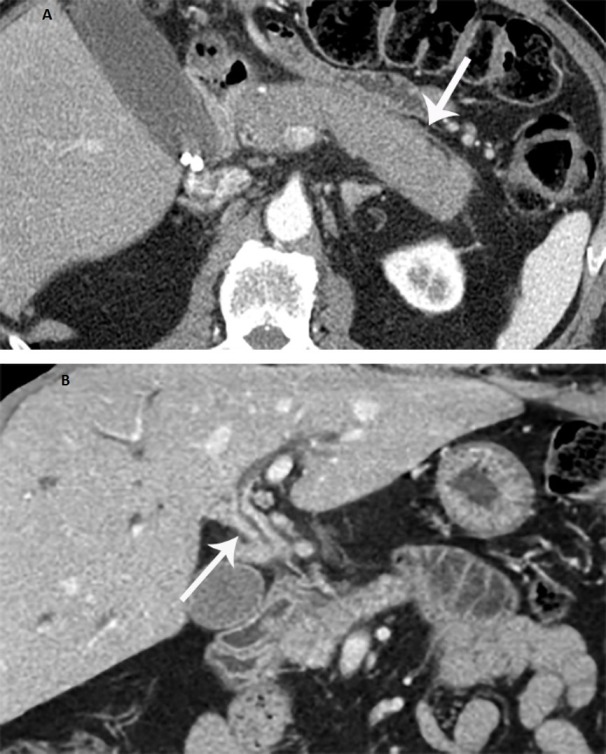
A. Axial CT showing diffuse enlargement of the pancreas due to autoimmune pancreatitis with peripancreatic inflammatory changes (arrow); B. Coronal post-contrast CT scan shows diffuse thickening of the common bile duct (arrow) due to autoimmune cholangiopathy
The patient was started on high-dose steroids, 30 mg of prednisone daily. Repeat imaging one month later revealed complete resolution of the patient’s pancreatitis and cholangitis, with normal appearance of the pancreas and biliary system (Figure 3).
Figure 3.
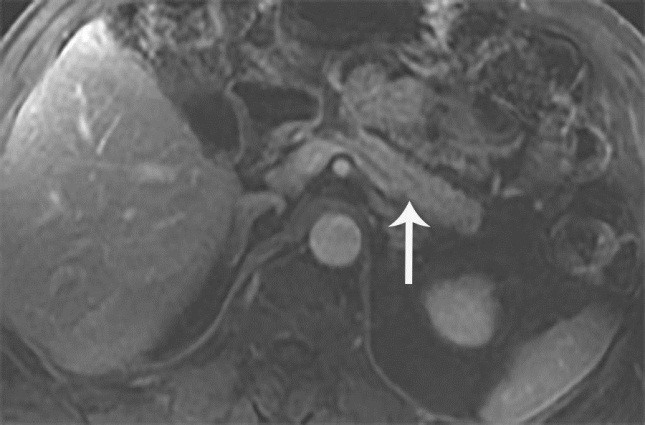
MRI T1 post contrast T1 weighted sequence showing normal pancreas (arrow)
Laboratory parameters were within normal limits and the patient was asymptomatic. The good response to steroid therapy supported the diagnosis of autoimmune pancreatocholangitis. Following cessation of corticosteroid use the patient had recurrent episodes of pancreatitis and cholangitis, with abdominal pain, increased liver function test, lipase and amylase, requiring repeated courses of prednisone to resolve.
Since the patient was having recurrent episodes of AIP, maintenance therapy was considered, for long-term remission and to decrease the risk of chronic biliary obstruction and secondary biliary cirrhosis. Consequently, the patient was offer the possibility to be maintained on low-dose prednisone for a couple of years or to start immunomodulation. The patient chose low-dose corticotherapy, and he is currently on 5 mg prednisone. His imaging and laboratory parameters are under control and the patient is asymptomatic for nearly one year.
Discussion
A form of idiopathic chronic pancreatitis associated with hyperglobulinemia was first reported by Sarles et al. [5] in 1961. The term “autoimmune pancreatitis” was introduced by Yoshida et al. [6] in 1995 to describe a steroid-responsive disease associated with autoimmune features. Initially considered a rare clinical entity isolated mostly to Japan, the incidence of AIP ranges between 1.86 and 6.6% of all cases of chronic pancreatitis [7].
Diagnosis of AIP can be challenging as it is a great masquerader. In the last decade, several diagnostic criteria have been proposed, including the Japanese diagnostic criteria, the Korean diagnostic criteria, the Mayo Clinic’s HISORt criteria, or more recently International Consensus Diagnostic Criteria from International Association of Pancreatology [2,8,9].
The HISORt criteria take into account histology (lymphoplasmacytic infiltrate), imaging (diffuse or focal pancreatic enlargement), serology (elevated serum level of IgG4), other organ involvement (biliary strictures), and response to steroid therapy [10].
Some cases of AIP present as focal enlargements of the pancreas. Although the ability of the clinicians to diagnose AIP has greatly improved due to increased awareness of the disease and proposed diagnostic criteria, differentiating mass-forming AIP from pancreatic cancer remains a challenge. We report the case of a solid pancreatic mass suspected to be pancreatic cancer based on the imaging findings and clinical presentation to increase awareness of AIP and to highlight the the challenges in the diagnostic approach of a pancreatic mass.
In this case, AIP progressed to autoimmune cholangitis. The involvement of the biliary tract has been previously described and addressed as autoimmune pancreatocholangitis [11,12].
Esposito et al. [11] suggested that AIP-associated biliary tract involvement should not be regarded as an extrapancreatic manifestation of AIP, but rather as a subtype of a disease, which affects the pancreatic duct and may extend to the biliary system.
AIP can be difficult to distinguish from pancreatic cancer as the clinical presentation and radiographic features are often similar. In this case, the radiographic features of the pancreatic mass combined with the initial clinical presentation were concerning for pancreatic cancer.
Abdominal pain and obstructive jaundice secondary to pancreatic enlargement represents a well-known diagnostic problem, and a reliable distinction between cancer and chronic pancreatitis remains difficult without histological examination. In our case, the biopsy of the pancreatic mass revealed no malignant cells and the pancreatic swelling had become diffuse on repeat imaging. In cases of pancreatic head cancer, obstruction of the pancreatic duct can also cause pancreatitis and enlargement of the pancreatic body or tail.
Given the repeat negative EUS-guided FNA and core biopsies, the diagnosis of malignancy was considered less likely in this case, though not completely ruled out.
Other alternative diagnosis was sought, such as seronegative AIP, and a trial of steroids was considered to be a reasonable intervention at this point, as a diagnostic and therapeutic method.
The patient responded to corticosteroid treatment with improvement of symptoms and resolution of the pancreatic mass, dilated intrahepatic and extrahepatic bile ducts.
Of note, the sensitivity of serum IgG4 in diagnosing type I AIP ranges from 53% to 95%, so having a negative IgG4 level does not exclude the disease [13,14].
‘‘Seronegative AIP’’ has been described in a study by Ghazale et al [15] where 24% of patients with AIP had a normal IgG4 level. IgG4 can also be elevated in other conditions including pancreatic cancer in which a mild elevation in IgG4 can occur [7]; therefore, this test may not always be specific for differentiating AIP from pancreatic cancer [16].
Unlike other forms of pancreatitis, AIP is highly responsive to steroid therapy [3,17].
If this disease is suspected, a ‘steroid trial’ might be used. However, patients with pancreatic cancer can also experience a lessening of their symptoms; therefore, this ‘trial’ should be used only by patients with tumor-negative biopsies [18].
The diagnosis of AIP should be reconsidered in patients who do not respond to steroids. Relapse is common following cessation of corticosteroid use [19].
The patient presented in this case took repeated courses of corticosteroid with recurrence of symptoms on cessation of corticosteroid treatment.
Since AIP responds dramatically to steroid treatment, a correct diagnosis of the disease is important to avoid surgery. On the other hand, in the presence of a resectable pancreatic mass, which may actually be a pancreatic adenocarcinoma, a misdiagnosis of AIP can lead to a delay in treatment for pancreatic cancer.
Consequently, even in the case of a pancreatic lesion described by the imaging studies as most probably malignant, in the right clinical setting a possibility of a rare disease such as AIP should be taken into consideration if biopsies of the pancreatic lesion are benign, as this could change the treatment and prognosis dramatically.
Conclusion
In conclusion, this report describes a rare case of seronegative AIP with associated biliary tract involvement, highlighting the challenge in the diagnostic approach of a pancreatic mass.
Although the diffuse form of AIP can be easily distinguished from pancreatic cancer on imaging, differentiating focal AIP from pancreatic malignancy is challenging.
Making the correct diagnosis and differentiating AIP from pancreatic cancer is important as their treatment and prognosis are vastly different.
Acknowledgment
This work was supported by a grant of Ministry of Research and Innovation, CNCS - UEFISCDI, project number PN-III-P4-ID-PCE-2016-0561, within PNCDI III.
Glossary
| Abbreviation | Expansion |
|---|---|
| AIP | Autoimmune pancreatitis |
| CT | Computed tomography |
| EUS | Endoscopic ultrasound |
| EUS-FNA | Endoscopic ultrasound-guided fine needle aspiration |
| IgG4 | Immunoglobulin G4 |
| MRI | Magnetic Resonance Imaging |
| PC | Pancreatic Cancer |
References
- 1.Klöppel G, Detlefsen S, Chari ST, Longnecker DS, Zamboni G. Autoimmune pancreatitis: the clinicopathological characteristics of the subtype with granulocytic epithelial lesions. Journal of gastroenterology. 2010;45(8):787–793. doi: 10.1007/s00535-010-0265-x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Chari S, Smyrk TC, Deshpande V, Klöppel G, Kojima M, Liu X, Longnecker DS, Mino-Kenudson M, Notohara K. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas. 2011;40(8):1172–1179. doi: 10.1097/MPA.0b013e318233bec5. [DOI] [PubMed] [Google Scholar]
- 3.Hart PA, Zen Y, Chari ST. Reviews in basic and clinical gastroenterology and hepatology. Gastroenterology. 2015;149:39–51. doi: 10.1053/j.gastro.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T, Tabata T, Hara S, Kuruma S, Chiba K, Kanno A, Masamune A, Shimosegawa T. Recent advances in autoimmune pancreatitis. Frontiers in physiology. 2012;3:374–374. doi: 10.3389/fphys.2012.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarles H, Sarles J-C, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas—an autonomous pancreatic disease. The American journal of digestive diseases. 1961;6(7):688–698. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida K, Toki F, Takeuchi T, Watanabe S-I, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Digestive diseases and sciences. 1995;40(7):1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 7.Robison LS, Canon CL, Varadarajulu S, Eloubeidi MA, Vickers S, Wilcox CM. Autoimmune pancreatitis mimicking pancreatic cancer. Journal of hepato-biliary-pancreatic sciences. 2011;18(2):162–169. doi: 10.1007/s00534-010-0321-1. [DOI] [PubMed] [Google Scholar]
- 8.Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim M-H, Klöppel G, Lerch MM, Löhr M. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40(3):352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 9.Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clinical Gastroenterology and Hepatology. 2006;4(8):1010–1016. doi: 10.1016/j.cgh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Vijayakumar A, Vijayakumar A. Imaging of focal autoimmune pancreatitis and differentiating it from pancreatic cancer. ISRN Radiol. 2013;2013:569489–569489. doi: 10.5402/2013/569489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esposito I, Born D, Bergmann F, Longerich T, Welsch T, Giese NA, Büchler MW, Kleeff J, Friess H, Schirmacher P. Autoimmune pancreatocholangitis, non-autoimmune pancreatitis and primary sclerosing cholangitis: a comparative morphological and immunological analysis. PloS one. 2008;3(7):e2539–e2539. doi: 10.1371/journal.pone.0002539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Buuren, Vleggaar FP, Willemien Erkelens, Zondervan PE, Lesterhuis W, Van Eijck, Puylaert JB, Van Der. Autoimmune pancreatocholangitis: a series of ten patients. Scandinavian Journal of Gastroenterology. 2006;41(sup243):70–78. doi: 10.1080/00365520600664326. [DOI] [PubMed] [Google Scholar]
- 13.Onweni C, Balagoni H, Treece JM, Addo Yobo, Patel A, Phemister J, Srinath M, Young MF. Autoimmune Pancreatitis Type 2: Case Report. Journal of investigative medicine high impact case reports. 2017;5(4):2324709617734245–2324709617734245. doi: 10.1177/2324709617734245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pieringer H, Parzer I, Wöhrer A, Reis P, Oppl B, Zwerina J. IgG4-related disease: an orphan disease with many faces. Orphanet journal of rare diseases. 2014;9(1):110–110. doi: 10.1186/s13023-014-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. The American journal of gastroenterology. 2007;102(8):1646–1646. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 16.Raina A, Krasinskas AM, Greer JB, Lamb J, Fink E, Moser AJ, Zeh III, Slivka A, Whitcomb DC. Serum immunoglobulin G fraction 4 levels in pancreatic cancer: elevations not associated with autoimmune pancreatitis. Archives of pathology & laboratory medicine. 2008;132(1):48–53. doi: 10.5858/2008-132-48-SIGFLI. [DOI] [PubMed] [Google Scholar]
- 17.Martins C, Lago P, Sousa P, Araújo T, Davide J, Castro-Poças F, Pedroto I. Type 2 Autoimmune Pancreatitis: A Challenge in the Differential Diagnosis of a Pancreatic Mass. GE-Portuguese Journal of Gastroenterology. 2017;24(6):296–300. doi: 10.1159/000461589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dede K, Salamon F, Taller A, Teknos D, Bursics A. Autoimmune pancreatitis mimicking pancreatic tumor. J Surg Case Rep. 2012;2012(11):rjs012–rjs012. doi: 10.1093/jscr/rjs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139(1):140–148. doi: 10.1053/j.gastro.2010.03.054. [DOI] [PubMed] [Google Scholar]


