Significance
Translocation of the nuclear factor (NF)-κB homolog Relish in response to microbial infection is a hallmark of the arthropod immune deficiency (IMD) pathway. Classically, transduction within the IMD molecular cascade is regulated by the inhibitor of apoptosis protein 2 (IAP2). In the Lyme disease Ixodes scapularis tick, X-linked inhibitor of apoptosis (XIAP) is considered a functionally analogous enzyme. Ticks lack homologs for some genes of the IMD network. As such, substrates of XIAP remain elusive. Here, we identify the molecule p47 as a binding partner of XIAP and Kenny (IKKγ/NEMO). We demonstrate that p47 induces a signaling cascade within the tick IMD pathway to limit microbial infection. This work emphasizes the importance of studying ticks to discover fundamental immunological processes.
Keywords: ticks, Lyme disease, rickettsial infections, IMD pathway, ubiquitin ligase
Abstract
The E3 ubiquitin ligase X-linked inhibitor of apoptosis (XIAP) acts as a molecular rheostat for the immune deficiency (IMD) pathway of the tick Ixodes scapularis. How XIAP activates the IMD pathway in response to microbial infection remains ill defined. Here, we identified the XIAP enzymatic substrate p47 as a positive regulator of the I. scapularis IMD network. XIAP polyubiquitylates p47 in a lysine 63-dependent manner and interacts with the p47 ubiquitin-like (UBX) module. p47 also binds to Kenny (IKKγ/NEMO), the regulatory subunit of the inhibitor of nuclear factor (NF)- κB kinase complex. Replacement of the amino acid lysine to arginine within the p47 linker region completely abrogated molecular interactions with Kenny. Furthermore, mitigation of p47 transcription levels through RNA interference in I. scapularis limited Kenny accumulation, reduced phosphorylation of IKKβ (IRD5), and impaired cleavage of the NF-κB molecule Relish. Accordingly, disruption of p47 expression increased microbial colonization by the Lyme disease spirochete Borrelia burgdorferi and the rickettsial agent Anaplasma phagocytophilum. Collectively, we highlight the importance of ticks for the elucidation of paradigms in arthropod immunology. Manipulating immune signaling cascades within I. scapularis may lead to innovative approaches to reducing the burden of tick-borne diseases.
Ancient and phylogenetically widespread nuclear factor (NF)-κB transcription factors induce changes in cellular physiology during microbial infection (1–9). In arthropods, the NF-κB homolog Relish is activated in response to signaling within the immune deficiency (IMD) pathway (10). Classically described in Drosophila melanogaster (hereafter referred to as Drosophila), the IMD pathway is analogous to the tumor necrosis factor receptor (TNFR) pathway. The IMD network is triggered by diaminopimelic (DAP) peptidoglycans (PGN) derived primarily from gram-negative bacteria (10–13). DAP-PGN binds transmembrane (14) or intracellular PGN-recognition proteins (PGRPs) (13, 15, 16). Biochemical relay is nucleated by a protein complex consisting of the central adaptor molecule Imd (17), Fas-associated death domain protein (FADD) (18), and Dredd (19, 20). Dredd cleaves Imd, which allows the E3 ubiquitin ligase inhibitor of apoptosis protein 2 (dIAP2) to ubiquitylate this molecule. Dredd then forms a scaffold composed of lysine (K)63-linked ubiquitin and enables docking of Kenny to the inhibitor of NF-κB kinase (IKK)β (21–26). Dredd also cleaves Relish, resulting in the nuclear translocation of the N-terminal fragment and expression of antimicrobial peptides (AMP) (27–30). Noncanonical activation of the IMD pathway occurs in response to certain viral infections (31–33). Three differing models implicated stimulator of IFN genes (STING) in antimicrobial responses mediated by Relish (34–37). In Ixodes scapularis ticks, lipid agonists activate the IMD signaling pathway (38). Altogether, these studies highlight the diversity of NF-κB signaling networks in arthropods.
Vector-borne infections comprise 17% of all infectious diseases (39). In the United States, the most prevalent vector-borne malady is Lyme disease (40), which is transmitted by the tick I. scapularis and caused by the spirochete Borrelia burgdorferi (41). I. scapularis transmits several other pathogens, including the rickettsial agent Anaplasma phagocytophilum (41). As the immune system impacts vector competence, or the arthropod’s ability to acquire, maintain, and transmit microbes to a susceptible host (42), vector immunology is of particular interest to basic scientists and clinicians. Biochemical pathways that govern vector competence in the tick are relatively unexplored compared with Drosophila (42, 43). The recent sequencing and annotation of the I. scapularis genome enabled in silico reconstruction of tick immune pathways. Notably, homologs of many upstream IMD pathway genes were absent. For instance, transmembrane PGRPs, the central adaptor Imd, and FADD were undetectable through bioinformatics analysis (43–47). Nonetheless, a functional IMD circuit was discovered in I. scapularis (38), underlining the unexplored diversity in arthropod immunity that may be leveraged to develop strategies to prevent vector-borne diseases (41, 43, 48).
The tick E3 ubiquitin ligase X-linked inhibitor of apoptosis (XIAP), together with the heterodimeric E2 conjugating enzyme complex Bendless/Ubiquitin-conjugating enzyme E2 variant 1A (Uev1a), positively regulates the IMD pathway through K63-dependent polyubiquitylation. Silencing xiap, relish, and other core members of the IMD pathway limited A. phagocytophilum and B. burgdorferi colonization of I. scapularis (38, 49). Two fundamental questions remain unanswered in the field of arthropod immunology. What are the substrates of XIAP? Do these ubiquitylated proteins impact IMD anti-pathogen defenses in ticks? Given the lasting relationship between ticks and bloodmeal-acquired microbes (50), we hypothesized that undiscovered biochemical immune cascades exist in I. scapularis. In this article, we report that in response to A. phagocytophilum and B. burgdorferi infection, I. scapularis XIAP ubiquitylates p47. P47 then activates the IMD pathway through the binding of Kenny (IKKγ/NEMO), the regulatory subunit of the IKK complex. Mitigation of p47 expression levels in I. scapularis limited Kenny accumulation, reduced phosphorylation of IKKβ (IRD5), and impaired cleavage of the NF-κB molecule Relish.
Results
I. scapularis XIAP Polyubiquitylates the Substrate p47 in a K63-Dependent Manner.
To find XIAP interacting partners, we incubated A. phagocytophilum-infected lysates derived from the tick ISE6 cell line with recombinant I. scapularis XIAP tagged with GST. We then coimmunoprecipitated XIAP-GST and identified binding molecules through mass spectrometry (SI Appendix, Fig. S1A). The resulting list was filtered to remove common “sticky” proteins and contaminants (e.g., keratin) (SI Appendix, Table S1). Each molecule was queried against Ubpred, an in silico ubiquitylation prediction algorithm (51). The top hit was annotated as a “protein tyrosine phosphatase.” Further analysis revealed similarity of this molecule to an evolutionarily conserved protein named p47. P47 was first identified as a cofactor of the p97 ATPase in mammals (52). P47 has also been implicated in cell cycle regulation, organelle membrane biogenesis (52–56), and autophagy in yeast (57). In humans, p47 negatively regulates NF-κB independently of p97 (58). Thus, we proceeded to investigate the role of I. scapularis p47 in the IMD biochemical cascade.
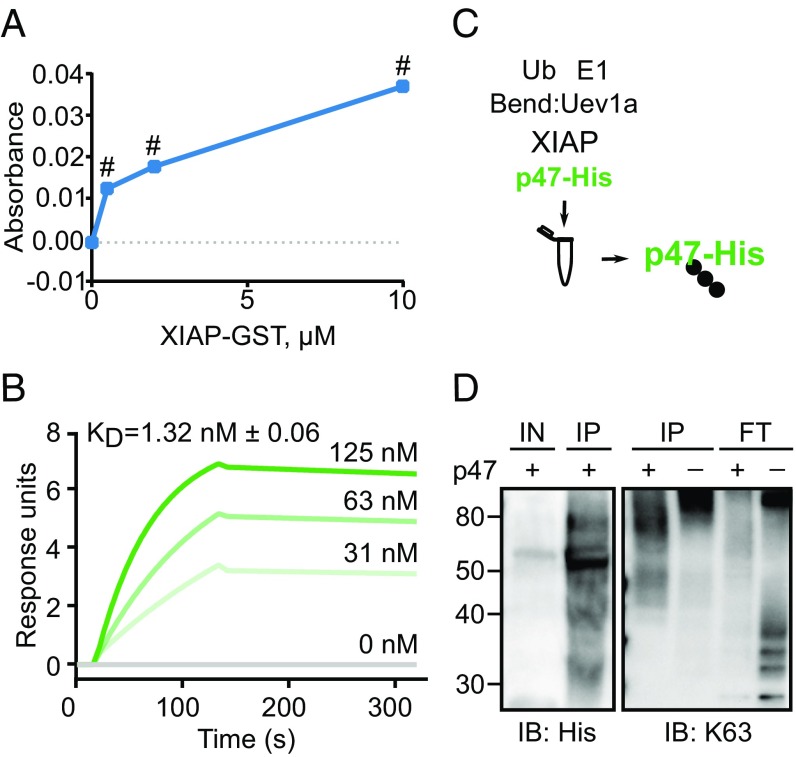
We expressed His-tagged I. scapularis p47 in Drosophila S2* cells. To confirm the interaction between XIAP and p47, we showed that these proteins interacted by performing an ELISA (Fig. 1A). Next, we performed surface plasmon resonance (SPR) and determined that XIAP-GST bound to p47-His with nanomolar affinity (Fig. 1B). We also leveraged an in vitro ubiquitylation approach to determine if XIAP ubiquitylated p47 (Fig. 1C). XIAP generated free polyubiquitin chains in the absence of substrate as previously reported (Fig. 1D, K63 blot, flow-through [FT], right-most lane) (38, 49). We isolated p47-His from the reaction (IP) and probed for the His tag and K63-dependent ubiquitylation (Fig. 1D). We detected a higher molecular weight after the reaction (Fig. 1D, His blot, compare input [IN] and IP p47-His), as well as a smear that overlapped with p47-His (Fig. 1D, K63 blot, left-most lane), indicating that this tick protein was ubiquitylated by XIAP in a K63-dependent manner. We also confirmed that XIAP did not ubiquitylate p47-His in a K48-dependent manner (SI Appendix, Fig. S1B). Overall, these data demonstrated that the tick proteins XIAP and p47 interact as an E3 ubiquitin ligase-substrate pair.
Fig. 1.
X-linked inhibitor of apoptosis (XIAP) binds and ubiquitylates p47 in a lysine (K)63-dependent manner. (A) 11.4 pmol of recombinant p47-His or BSA was incubated with increasing amounts of XIAP-GST in triplicate. The BSA background signal was subtracted from the p47-His signal. Means ± SEM are graphed. Statistical significance was evaluated by a one-way analysis of variance (ANOVA) followed by a Tukey post hoc test to determine which XIAP-GST concentrations were significantly different from baseline. (B) Surface plasmon resonance (SPR) demonstrated that recombinant XIAP-GST bound increasing amounts of recombinant p47-His with nanomolar affinity. (C) Analysis of in vitro ubiquitylation reactions showed that (D) XIAP ubiquitylated p47-His (Left) in a K63-dependent manner (Right). Due to limited material, panels are from different but representative experiments. Numeric labels indicate protein ladder molecular weights (kDa). Three independent experiments were performed for the ELISA and ubiquitylation assays. Bend, Bendless; E1, E1 activating enzyme; FT, flow-through; IB, immunoblot; IN, input; IP, immunoprecipitation; KD, dissociation coefficient; Ub, ubiquitin; Uev1a, ubiquitin-conjugating enzyme E2 variant 1A; #P < 0.0001.
The p47 Ubiquitin-Like (UBX) Domain Interacts with XIAP.
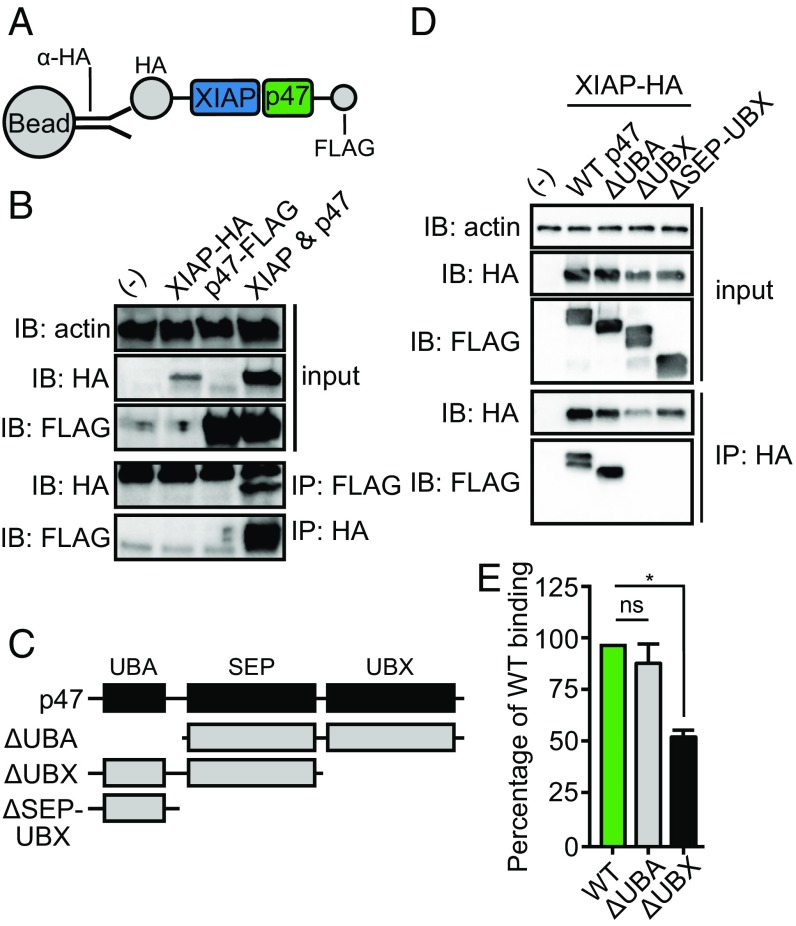
We next sought to interrogate the molecular interactions between p47 and XIAP in a physiological setting. As it is currently not feasible to ectopically express proteins in tick lines, we coexpressed and coimmunoprecipitated XIAP-hemagglutinin (HA) and p47-FLAG in human embryonic kidney (HEK) 293T cells (Fig. 2A). Consistent with the ELISA and SPR data in Fig. 1 A and B, we detected the HA tag in the FLAG pulldown, and vice versa, indicating binding of XIAP to p47 (Fig. 2B). Next, we generated p47-FLAG deletion constructs to investigate which domain of p47 was required for XIAP binding (Fig. 2C). Only p47-FLAG species containing UBX domains bound to XIAP-HA (i.e., wild-type [WT] p47-FLAG and Δubiquitin-binding domain [UBA] p47-FLAG) (Fig. 2D). To validate this result, we expressed recombinant ΔUBA p47-His and ΔUBX p47-His truncations in S2* cells and performed an ELISA-based binding assay. We demonstrated that binding of ΔUBX p47-His to XIAP-GST was reduced by ∼50% compared with WT or ΔUBA p47-His proteins (Fig. 2E). Collectively, these data suggested that the UBX domain mediates the interaction between p47 and XIAP.
Fig. 2.
The ubiquitin-like (UBX) domain of p47 is required for interaction with XIAP. (A and C) Recombinant tick xiap-hemagglutinin (HA) and p47-FLAG constructs were generated and transfected into human embryonic kidney (HEK) 293T cells to demonstrate that (B) p47-FLAG bound XIAP-HA (D) through the UBX domain. Western blots are representative of at least two independent experiments. (E) 50 ng wild-type (WT) recombinant p47-His, Δ ubiquitin-binding domain (UBA) p47-His, and ΔUBX p47-His were incubated in triplicate with 5 μM XIAP-GST in an ELISA-based binding experiment. The binding signal was normalized to WT p47-His binding (expressed as 100% binding) and is represented as an average of three technical replicates ± SEM. Statistical significance was evaluated by a one-way ANOVA followed by a Tukey post hoc test. Two independent experiments were performed. IB, immunoblot; IP, immunoprecipitation; ns, not significant; *P < 0.05; SEP, shp1, eyes closed, p47 domain; (-) mock transfection.
P47 Interacts with the NF-κB Regulatory Protein Kenny.
Activation of the evolutionarily conserved transcription factor NF-κB results in potent antimicrobial effects (59). The NF-κB signaling network relies on elaborate regulatory mechanisms to tune the magnitude and duration of immune responses (7). Shibata et al. (58) showed that p47 binding of polyubiquitylated NF-κB essential modulator (NEMO) negatively regulated NF-κB through degradation. In arthropods, NEMO (IKKγ) is also known as Kenny. Hence, we investigated whether the tick protein p47 interacted with Kenny as the I. scapularis IMD pathway is characterized by Relish (NF-κB) activation.
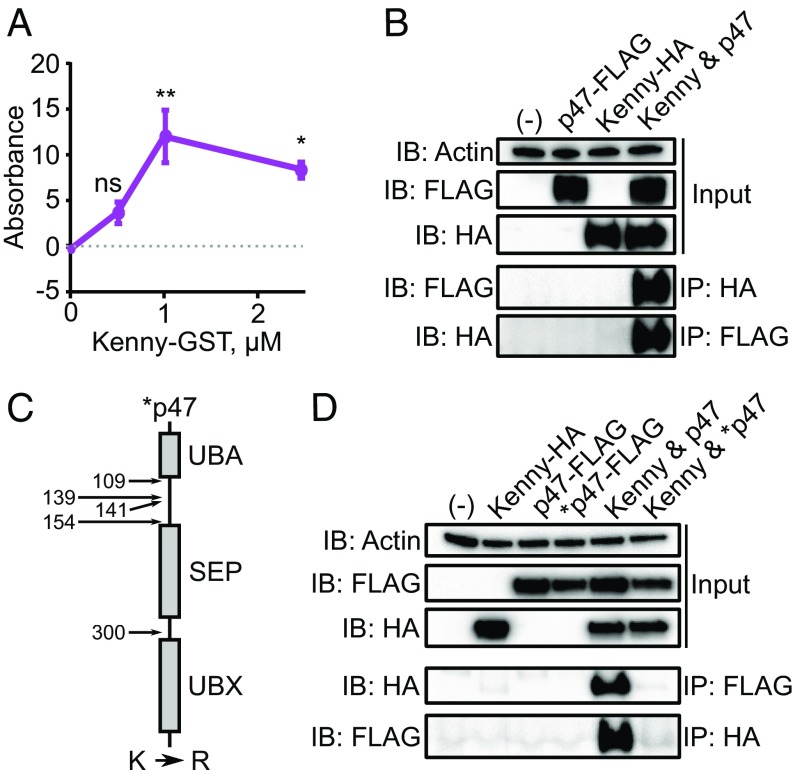
We expressed recombinant p47-His and Kenny-GST proteins and demonstrated binding through ELISA (Fig. 3A). To explore interactions between these two molecules in a physiological setting, we cotransfected p47-FLAG and kenny-HA constructs into HEK 293T cells. Consistent with Fig. 3A, we detected the HA tag in the p47-FLAG pulldown and vice versa, indicating that p47 and Kenny interacted within cells (Fig. 3B). In Fig. 1D, we demonstrated that p47 is a substrate of XIAP. Therefore, we hypothesized that if the ubiquitylation of p47 were to be prevented, the interaction between p47 and Kenny would be disrupted. Correspondingly, we identified five lysine residues that were (i) predicted to be ubiquitylated with high or medium confidence by Ubpred (51) and/or (ii) located within the putative regulatory linker region between the UBA and Shp1, Eyc, p47 (SEP) domains (60, 61).
Fig. 3.
The Ixodes scapularis protein p47 binds to Kenny. (A) 11.4 pmol recombinant p47-His or BSA was incubated with increasing amounts of Kenny-GST in triplicate. The BSA background signal was subtracted from the p47-His signal, and the normalized data were multiplied by 1,000 for convenient graphing. Means ± SEM are graphed. Statistical significance was evaluated by a one-way ANOVA followed by a Tukey post hoc test comparing each Kenny-GST concentration to baseline. (B) Kenny-HA bound p47-FLAG as demonstrated by coimmunoprecipitation. (C) The quintuple p47-FLAG mutant (*p47-FLAG) was generated by mutating lysine to arginine (R) residues at positions 109, 139, 141, 154, and 300. (D) The wild type, but not the p47-FLAG* interacted molecularly. All data are representative of two independent experiments. UBA, Δ ubiquitin-binding domain; UBX, ubiquitin-like domain; IB, immunoblot; IP, immunoprecipitation; HA, hemagglutinin; ns, not significant; *P < 0.05; **P < 0.005.
Thus, we replaced the lysine residues of p47 to arginine in the p47-FLAG construct (i.e., K»R quintuple point mutations) at positions 109, 139, 141, 154, and 300, generating a p47 mutant (henceforth named *p47) (Fig. 3C). Lysine to arginine replacements are typically made in ubiquitin biology because these positively charged amino acids impact protein-protein stability through the formation of electrostatic interactions (62). Our results demonstrated that without the lysine residues of p47, the mutant protein was unable to interact with Kenny (Fig. 3D). Taken together, these data suggested that the lysine residues at positions 109, 139, 141, 154, and 300 of p47 are required for binding to Kenny.
P47 Silencing Impairs Molecular Transduction in the I. scapularis IMD Pathway.
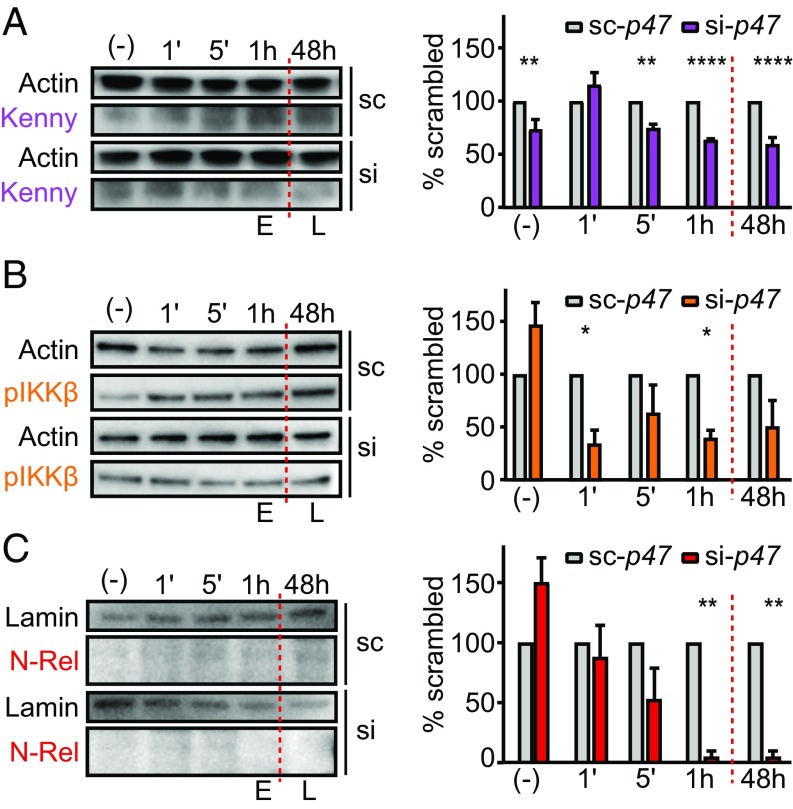
XIAP is the central regulator of the IMD pathway in I. scapularis (38). Furthermore, p47 bound to Kenny (Fig. 3), a classical member of the IMD network in arthropods. Therefore, we investigated whether p47 was required for IMD signaling in I. scapularis by assessing three critical events: (i) Kenny accumulation; (ii) IKKβ (IRD5) phosphorylation; and (iii) Relish cleavage (Fig. 4). We transfected small interfering RNA (siRNA) targeting p47 into the hemocyte-like I. scapularis IDE12 cell line and stimulated the IMD pathway with A. phagocytophilum. We monitored uninfected cells [“(-)”] and early (1, 5, and 60 min) and late (48 h) activation of the I. scapularis IMD pathway. These time points were chosen because independent laboratories have demonstrated Relish cleavage within minutes of microbial stimulation (22, 38). p47 silencing substantially reduced Kenny accumulation in the cytoplasm, with the effect beginning at 5 min and persisting for the duration of the experiment (Fig. 4A). Compared with the control treatment, p47-silenced cells also displayed reduced phosphorylation of IKKβ in the cytoplasm and impaired nuclear translocation of the N-terminal fragment of Relish (Fig. 4 B and C). Altogether, these data indicated that p47 promotes signal transduction within the I. scapularis IMD pathway.
Fig. 4.
P47 silencing impairs activation of the tick IMD pathway. Silencing p47 expression in I. scapularis IDE12 cells (A) diminished Kenny accumulation, (B) reduced IKKβ (also known as IRD5) phosphorylation, and (C) prevented Relish cleavage and nuclear translocation in response to A. phagocytophilum infection. Band intensity was normalized to the loading control (actin for IKKβ and Kenny and nuclear lamin for Relish). Normalized data for si-p47 (si) were divided by corresponding scrambled control values and expressed as a percentage of sc-p47 (sc) binding. The mean of at least three biological replicates ± SEM is graphed. Statistical significance was evaluated by a two-way ANOVA followed by Sidak post hoc testing. E, early; L, late; p, phosphorylated; (-) uninfected. *P < 0.05, **P < 0.005; ****P < 0.0001.
P47 Silencing Affects Microbial Infection by I. scapularis Ticks.
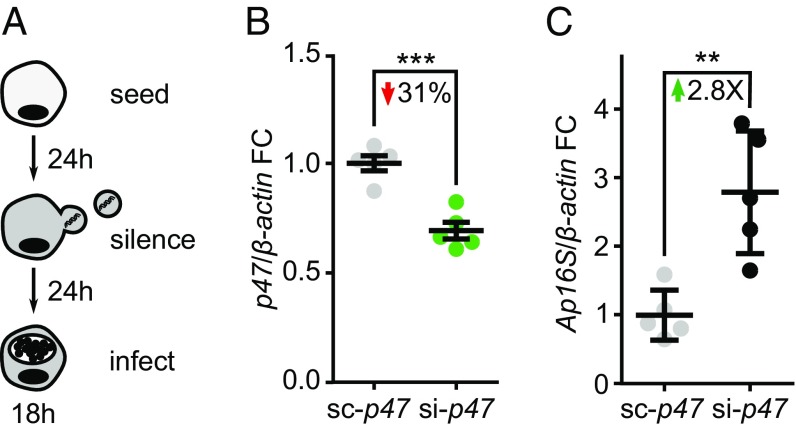
To determine whether p47 affects microbial infection, I. scapularis ISE6 cells were transfected with scrambled control (sc)-p47 or si-p47 siRNA and infected with A. phagocytophilum (Fig. 5A). We observed an increase of approximately threefold in A. phagocytophilum load for p47-silenced cells compared with control tick cells (Fig. 5 B and C). We then investigated the role of p47 during microbial infection of I. scapularis ticks. To date, in vivo studies in ticks rely on delivering siRNA into the midgut or salivary glands to modulate gene expression (63, 64). Complete genetic knockout within I. scapularis remains technically unfeasible (65). Therefore, changes in bacterial load between treatments are typically modest (38, 49).
Fig. 5.
P47 restricts A. phagocytophilum infection in I. scapularis cells. (A) 1 × 106 ISE6 cells were transfected with the small interfering RNAs (siRNAs) sc-p47 or si-p47. After 24 h, transfection reagents were removed, and cells were infected with A. phagocytophilum MOI 50. Cells were harvested after 18 h infection and stored in TRIzol to preserve RNA. (B) P47 expression and (C) A. phagocytophilum (Ap) 16S rRNA transcripts were quantified by qRT-PCR and normalized to I. scapularis β-actin before calculating fold change (FC). Results are represented as means ± SEM. A two-tailed Student’s t test was used to determine statistical significance. n = 5; **P < 0.01; ***P < 0.001.
Previously, we demonstrated the feasibility of manipulating the IMD pathway by microinjecting I. scapularis nymphs with siRNA constructs targeting relish, xiap, bendless, uev1a, and caspar. We showed differences in A. phagocytophilum and B. burgdorferi colonization (38). We confirmed these findings by silencing relish and observed a 17-fold increase in B. burgdorferi burden in vivo (SI Appendix, Fig. S2). In this study, I. scapularis nymphs were microinjected with si-p47 or sc-p47 siRNA. A subset of microinjected ticks was pooled and lysed to obtain protein extracts. The tick lysate was incubated with either XIAP-GST or GST alone to show successful p47 knockdown by ELISA (Fig. 6A). Single ticks did not yield sufficient material to detect p47 levels by Western blot. The remaining ticks fed to repletion on mice infected with either A. phagocytophilum or B. burgdorferi (Fig. 6B). No difference in weight was observed in p47-silenced versus the control treatment, indicating that p47 did not impact tick feeding (SI Appendix, Fig. S3). Engorged ticks were subjected to quantitative reverse transcription–PCR (qRT-PCR) to determine p47 expression and bacterial burden. Consistently, despite modest silencing (Fig. 6C), p47-deficient ticks acquired more A. phagocytophilum and B. burgdorferi compared with the control ticks (Fig. 6 D and E). Taken together, these data provided strong evidence that p47 positively regulates the tick IMD pathway during microbial infection.
Fig. 6.
Silencing p47 enhances microbial infection in I. scapularis nymphs. (A) 10 flat I. scapularis nymphs were microinjected with 10 ng si-p47 or sc-p47. Ticks were harvested 24 h postinjection, pooled, and subjected to protein extraction. Protein lysates were incubated with increasing amounts of XIAP-GST or GST in triplicate. Means ± SEM are graphed. (B) I. scapularis nymphs were silenced (20 ticks for either si-p47 or sc-p47 per experiment) and allowed to feed to repletion on infected mice. RNA was extracted from single ticks and subjected to qRT-PCR to determine (C) p47 expression and the burden of (D) A. phagocytophilum (Ap16S) or (E) Borrelia burgdorferi (recA). Three independent experiments for both B. burgdorferi and A. phagocytophilum were performed. Data were normalized to I. scapularis β-actin before calculating fold change (FC) and analyzed as one group for each condition (n = 22–35 ticks). (C–E) Individual tick samples as well as means ± SEM are graphed. Two-tailed Student’s t tests were performed to determine statistical significance. *P < 0.05; ***P < 0.001; recA, recombinase A.
Discussion
The I. scapularis IMD pathway is regulated by XIAP-mediated K63 ubiquitylation and restricts the colonization of two clinically relevant tick-borne microbes, B. burgdorferi and A. phagocytophilum (38, 44–49). To date, no XIAP substrates have been identified, leaving the underlying regulatory mechanisms of the tick IMD pathway unknown. In this report, we uncovered p47 as a substrate of XIAP. Our model suggests that, upon microbial infection, p47 is ubiquitylated in a K63-dependent manner (Fig. 1D). Ubiquitylated p47 binds to Kenny (Fig. 3), the regulatory subunit of the IKK complex. These findings provide strong evidence that the pairing of XIAP and p47 licenses Relish translocation to the nucleus (SI Appendix, Fig. S4).
Silencing p47 expression resulted in persistent defects in the biochemical relay of the IMD pathway (Fig. 4). We also observed increased bacterial burdens in vitro and in vivo (Figs. 5 and 6). However, we noticed that some Relish cleavage still occurred at 1 and 5 min poststimulation in p47-silenced cells (Fig. 4C). Additional regulation may explain the residual signaling within the I. scapularis IMD network. For example, the Drosophila HOIL-1L interacting protein (HOIP) ortholog linear ubiquitin E3 ligase (LUBEL) works in concert with dIAP2 to activate Relish through methionine 1-dependent ubiquitylation of Kenny (66). This is expected, as the transduction of NF-κB is tightly modulated through redundancy to prevent aberrant inflammation and limit fitness costs (7, 58).
The successful maintenance of a vector-borne microbe occurs in a tripartite system consisting of I. scapularis, the microbiome, and the mammalian host. As such, the evolutionary and ecological history of ticks is critical to understand arthropod immunity. As long-lived arthropods, ticks must maintain sufficient fitness while preventing excessive microbial colonization (67). Thus, the activation of the IMD pathway does not result in sterilizing immunity against A. phagocytophilum and B. burgdorferi infection. While we did not test whether A. phagocytophilum and B. burgdorferi directly interact with p47, we speculate that microbial effectors may facilitate immune evasion within I. scapularis.
We contemplate that activation of the IMD pathway during starvation limits microbial infection (42). Furthermore, physical changes to the tick midgut coinciding with the rapid proliferation of B. burgdorferi during feeding suggest an intriguing role for the IMD pathway (68, 69). Like Drosophila (70), the tick IMD network may synergize with other biochemical cascades maintaining the integrity of the midgut epithelial barrier. As only a few spirochetes can be detected in the hemolymph (69), the IMD pathway may regulate the dissemination of these bacteria into the salivary glands. Assessing time- and tissue-dependent activation of the tick IMD biochemical cascade will be critical to comprehend vector competence. However, a major impediment for these studies is the paucity of reagents and the lack of genetic tools for tick research.
Model organisms serve as powerful systems for dissecting the fundamentals of arthropod immunity (11). Nevertheless, evolutionarily distant species have evolved under unique environmental pressures and occupy distinct ecological niches. Thus, it is important to take the biology of each species into consideration when studying immunology (67). For instance, our results are discordant compared with findings observed for p47-mediated NF-κB regulation in humans. Although we do not know the reason for these incongruencies, we speculate that this discrepancy may be attributed to the variability of molecular circuits encountered throughout the animal kingdom. Indeed, vertebrates and arthropods diverged hundreds of millions of years ago and evolved independently since then (6). By studying ticks, one may exploit biological processes in arthropod immunity and aspire to reduce the public health burden of vector-borne diseases.
Materials and Methods
Detailed experimental procedures, and tables listing primers and antibodies are provided in the SI Appendix.
Animal Experimentation.
All mouse experiments were approved by the Institutional Biosafety (IBC, 00002247) and Animal Care and Use (IACUC, 0216015) committees at the University of Maryland School of Medicine and complied with National Institutes of Health (NIH) guidelines (Office of Laboratory Animal Welfare [OLAW] assurance numbers A3200-01, A323-01, A3270-1).
Plasmids.
GST-tagged recombinant proteins were generated by cloning bendless, xiap, and codon-optimized I. scapularis kenny into pGEX-6P-2 and expressing the constructs in Escherichia coli. Xiap and kenny were cloned into pCMV-HA for expression in HEK 293T cells, while codon-optimized p47 and associated mutants were cloned into pCMV-FLAG (HEK 293T expression) and pMT/V5/His for expression in S2* cells. All constructs were verified by sequencing.
Silencing Experiments.
ISE6 and IDE12 cells were transfected with sc-p47 or si-p47 constructs for 24 h before A. phagocytophilum infection (ISE6: multiplicity of infection [MOI] 50, IDE12: MOI 150) for the indicated timepoints. Cells were subsequently harvested and preserved for pathogen burden quantification by qRT-PCR (SI Appendix, Table S2) or IMD pathway activation by Western blotting (SI Appendix, Table S3). SiRNA and scrambled control (scRNA) constructs were microinjected into midguts of I. scapularis nymphs to silence p47 and relish expression. Ticks rested overnight and were placed on C57BL/6 mice infected with A. phagocytophilum HZ 7 d postinfection; or, alternatively, C3H/HeJ mice infected with B. burgdorferi B31 clone MSK5 10 d postinfection. Individual engorged ticks were collected after detachment (3 to 5 d postplacement), weighed, snap frozen, and homogenized. Gene expression and microbial burdens were determined by qRT-PCR.
IMD Pathway Activation Assay.
Cytoplasmic and nuclear proteins were extracted from p47-silenced and A. phagocytophilum-stimulated IDE12 cells. Western blots assessing IMD pathway activation were performed (SI Appendix, Table S3). ImageJ 1.8 was used to perform densitometry measurements (71). Profile plots of the bands of interest in each lane were drawn (Gel Analyzer function). For each target and timepoint, the targeted band signal was normalized to the corresponding loading control to correct for small differences in protein loading. The ratio of the siRNA to scRNA signal was determined by dividing siRNA relative intensity by scRNA relative intensity for each timepoint and multiplying by 100, thus fixing the scRNA value at 100%.
Ubiquitylation Assays.
Experimentation consisted of human Ube1, Uev1a, and ubiquitin proteins purchased from Boston Biochemical and recombinant I. scapularis Bendless, XIAP, and p47-His. Reactions were incubated at 37 °C for 1 h, stopped, and dialyzed against PBS. P47-His was isolated by incubating the reactions with nickel resin. The eluate and flow-through were dialyzed against PBS to remove imidazole and subjected to Western blotting to assess K63- and K48-specific ubiquitylation.
Statistical Analysis.
Sample sizes were chosen based on reports in the literature and previous experiments in the Pedra Laboratory (38). Three to five replicates were included for in vitro experiments. In vivo experiments were performed three times with 10–20 ticks each. Data from all in vivo experiments were combined into a single analysis for each pathogen. Data were checked for equal variances using the Brown-Forsythe test before pooling. Three independent experiments were performed for each I. scapularis targeted protein related to the IMD pathway. Means ± SE of mean (SEM) were graphed and analyzed with either an unpaired Student’s t test or one- or two-way analysis of variance (ANOVA) with post hoc testing. GraphPad Prism versions 6.0 and 7.0 were used to generate the graphs and perform the statistical calculations. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Neal Silverman (University of Massachusetts Medical School) for providing the Kenny antibody and the Drosophila S2* cell line; Greg A. Snyder and Eric J. Sundberg (University of Maryland School of Medicine) for early technical assistance; Robert Bloch and Yinghua Zhang (University of Maryland School of Medicine) for the surface plasmon resonance experimentation and expertise; David R. Goodlett and Young Ah Goo (University of Maryland School of Pharmacy) for mass spectrometry technical services; Jon Skare (Texas A&M Health Science Center) for providing the B. burgdorferi B31 strain, clone MSK5; and Ulrike G. Munderloh (University of Minnesota) for providing ISE6 and IDE12 tick cells and insightful feedback. This work was supported by NIH grant F31AI138440 (to E.E.M.C.) and grants P01AI138949, R01AI116523, and subcontract recipient for R01AI049424 (to J.H.F.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808905116/-/DCSupplemental.
References
- 1.Shinzato C, et al. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature. 2011;476:320–323. doi: 10.1038/nature10249. [DOI] [PubMed] [Google Scholar]
- 2.Meyer E, et al. Sequencing and de novo analysis of a coral larval transcriptome using 454 GSFlx. BMC Genomics. 2009;10:219. doi: 10.1186/1471-2164-10-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan JC, Kalaitzidis D, Gilmore TD, Finnerty JR. Rel homology domain-containing transcription factors in the cnidarian Nematostella vectensis. Dev Genes Evol. 2007;217:63–72. doi: 10.1007/s00427-006-0111-6. [DOI] [PubMed] [Google Scholar]
- 4.Sebé-Pedrós A, de Mendoza A, Lang BF, Degnan BM, Ruiz-Trillo I. Unexpected repertoire of metazoan transcription factors in the unicellular holozoan Capsaspora owczarzaki. Mol Biol Evol. 2011;28:1241–1254. doi: 10.1093/molbev/msq309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauthier M, Degnan BM. The transcription factor NF-kappaB in the demosponge Amphimedon queenslandica: Insights on the evolutionary origin of the Rel homology domain. Dev Genes Evol. 2008;218:23–32. doi: 10.1007/s00427-007-0197-5. [DOI] [PubMed] [Google Scholar]
- 6.Gilmore TD, Wolenski FS. NF-κB: Where did it come from and why? Immunol Rev. 2012;246:14–35. doi: 10.1111/j.1600-065X.2012.01096.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-κB: A blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernikova D, Motamedi S, Csürös M, Koonin EV, Rogozin IB. A late origin of the extant eukaryotic diversity: Divergence time estimates using rare genomic changes. Biol Direct. 2011;6:26. doi: 10.1186/1745-6150-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange C, et al. Defining the origins of the NOD-like receptor system at the base of animal evolution. Mol Biol Evol. 2011;28:1687–1702. doi: 10.1093/molbev/msq349. [DOI] [PubMed] [Google Scholar]
- 10.Lemaitre B, et al. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA. 1995;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster–From microbial recognition to whole-organism physiology. Nat Rev Immunol. 2014;14:796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myllymäki H, Valanne S, Rämet M. The Drosophila imd signaling pathway. J Immunol. 2014;192:3455–3462. doi: 10.4049/jimmunol.1303309. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko T, et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the Drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- 14.Iatsenko I, Kondo S, Mengin-Lecreulx D, Lemaitre B. PGRP-SD, an extracellular pattern-recognition receptor, enhances peptidoglycan-mediated activation of the Drosophila Imd pathway. Immunity. 2016;45:1013–1023. doi: 10.1016/j.immuni.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Paik D, et al. SLC46 family transporters facilitate cytosolic innate immune recognition of monomeric peptidoglycans. J Immunol. 2017;199:263–270. doi: 10.4049/jimmunol.1600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leulier F, et al. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat Immunol. 2003;4:478–484. doi: 10.1038/ni922. [DOI] [PubMed] [Google Scholar]
- 17.Georgel P, et al. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell. 2001;1:503–514. doi: 10.1016/s1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- 18.Leulier F, Vidal S, Saigo K, Ueda R, Lemaitre B. Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr Biol. 2002;12:996–1000. doi: 10.1016/s0960-9822(02)00873-4. [DOI] [PubMed] [Google Scholar]
- 19.Chen P, Rodriguez A, Erskine R, Thach T, Abrams JM. Dredd, a novel effector of the apoptosis activators reaper, grim, and hid in Drosophila. Dev Biol. 1998;201:202–216. doi: 10.1006/dbio.1998.9000. [DOI] [PubMed] [Google Scholar]
- 20.Leulier F, Rodriguez A, Khush RS, Abrams JM, Lemaitre B. The Drosophila caspase Dredd is required to resist gram-negative bacterial infection. EMBO Rep. 2000;1:353–358. doi: 10.1093/embo-reports/kvd073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meinander A, et al. Ubiquitylation of the initiator caspase DREDD is required for innate immune signalling. EMBO J. 2012;31:2770–2783. doi: 10.1038/emboj.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paquette N, et al. Caspase-mediated cleavage, IAP binding, and ubiquitination: Linking three mechanisms crucial for Drosophila NF-kappaB signaling. Mol Cell. 2010;37:172–182. doi: 10.1016/j.molcel.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, Wu LP, Anderson KV. The antibacterial arm of the drosophila innate immune response requires an IkappaB kinase. Genes Dev. 2001;15:104–110. doi: 10.1101/gad.856901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutschmann S, et al. Role of Drosophila IKK gamma in a toll-independent antibacterial immune response. Nat Immunol. 2000;1:342–347. doi: 10.1038/79801. [DOI] [PubMed] [Google Scholar]
- 25.Silverman N, et al. A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 2000;14:2461–2471. doi: 10.1101/gad.817800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleino A, et al. Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J. 2005;24:3423–3434. doi: 10.1038/sj.emboj.7600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoven S, et al. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci USA. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleino A, et al. Pirk is a negative regulator of the Drosophila Imd pathway. J Immunol. 2008;180:5413–5422. doi: 10.4049/jimmunol.180.8.5413. [DOI] [PubMed] [Google Scholar]
- 29.Ertürk-Hasdemir D, et al. Two roles for the Drosophila IKK complex in the activation of Relish and the induction of antimicrobial peptide genes. Proc Natl Acad Sci USA. 2009;106:9779–9784. doi: 10.1073/pnas.0812022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stöven S, Ando I, Kadalayil L, Engström Y, Hultmark D. Activation of the Drosophila NF-kappaB factor Relish by rapid endoproteolytic cleavage. EMBO Rep. 2000;1:347–352. doi: 10.1093/embo-reports/kvd072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dostert C, et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- 32.Lamiable O, et al. Cytokine Diedel and a viral homologue suppress the IMD pathway in Drosophila. Proc Natl Acad Sci USA. 2016;113:698–703. doi: 10.1073/pnas.1516122113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa A, Jan E, Sarnow P, Schneider D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS One. 2009;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goto A, et al. The kinase IKKbeta regulates a STING- and NF-κB-dependent antiviral response pathway in Drosophila. Immunity. 2018;49:225–234.e4. doi: 10.1016/j.immuni.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hua X, et al. Stimulator of interferon genes (STING) provides insect antiviral immunity by promoting Dredd caspase-mediated NF-κB activation. J Biol Chem. 2018;293:11878–11890. doi: 10.1074/jbc.RA117.000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, et al. Inflammation-induced, STING-dependent autophagy restricts Zika virus infection in the Drosophila brain. Cell Host Microbe. 2018;24:57–68.e3. doi: 10.1016/j.chom.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin M, Hiroyasu A, Guzman RM, Roberts SA, Goodman AG. Analysis of Drosophila STING reveals an evolutionarily conserved antimicrobial function. Cell Rep. 2018;23:3537–3550.e6. doi: 10.1016/j.celrep.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw DK, et al. Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat Commun. 2017;8:14401. doi: 10.1038/ncomms14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization 2017 Vector-borne diseases. Available at www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. Accessed May 23, 2018.
- 40.Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2015;29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Nelder MP, et al. Human pathogens associated with the blacklegged tick Ixodes scapularis: A systematic review. Parasit Vectors. 2016;9:265. doi: 10.1186/s13071-016-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de la Fuente J, et al. Tick-pathogen interactions and vector competence: Identification of molecular drivers for tick-borne diseases. Front Cell Infect Microbiol. 2017;7:114. doi: 10.3389/fcimb.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliva Chávez AS, Shaw DK, Munderloh UG, Pedra JH. Tick humoral responses: Marching to the beat of a different drummer. Front Microbiol. 2017;8:223. doi: 10.3389/fmicb.2017.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bechsgaard J, et al. Comparative genomic study of arachnid immune systems indicates loss of βGRPs and the IMD pathway. J Evol Biol. 2015;29:277–291. doi: 10.1111/jeb.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosa RD, et al. Exploring the immune signalling pathway-related genes of the cattle tick Rhipicephalus microplus: From molecular characterization to transcriptional profile upon microbial challenge. Dev Comp Immunol. 2016;59:1–14. doi: 10.1016/j.dci.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Palmer WJ, Jiggins FM. Comparative genomics reveals the origins and diversity of arthropod immune systems. Mol Biol Evol. 2015;32:2111–2129. doi: 10.1093/molbev/msv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith AA, Pal U. Immunity-related genes in Ixodes scapularis–Perspectives from genome information. Front Cell Infect Microbiol. 2014;4:116. doi: 10.3389/fcimb.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gulia-Nuss M, et al. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat Commun. 2016;7:10507. doi: 10.1038/ncomms10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Severo MS, et al. The E3 ubiquitin ligase XIAP restricts Anaplasma phagocytophilum colonization of Ixodes scapularis ticks. J Infect Dis. 2013;208:1830–1840. doi: 10.1093/infdis/jit380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rehm P, et al. Dating the arthropod tree based on large-scale transcriptome data. Mol Phylogenet Evol. 2011;61:880–887. doi: 10.1016/j.ympev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Radivojac P, et al. Identification, analysis, and prediction of protein ubiquitination sites. Proteins. 2010;78:365–380. doi: 10.1002/prot.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kondo H, et al. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–78. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Guha S, Volkert FC. The Saccharomyces SHP1 gene, which encodes a regulator of phosphoprotein phosphatase 1 with differential effects on glycogen metabolism, meiotic differentiation, and mitotic cell cycle progression. Mol Cell Biol. 1995;15:2037–2050. doi: 10.1128/mcb.15.4.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng YL, Chen RH. The AAA-ATPase Cdc48 and cofactor Shp1 promote chromosome bi-orientation by balancing Aurora B activity. J Cell Sci. 2010;123:2025–2034. doi: 10.1242/jcs.066043. [DOI] [PubMed] [Google Scholar]
- 55.Uchiyama K, Kondo H. p97/p47-mediated biogenesis of Golgi and ER. J Biochem. 2005;137:115–119. doi: 10.1093/jb/mvi028. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Satoh A, Warren G, Meyer HH. VCIP135 acts as a deubiquitinating enzyme during p97-p47-mediated reassembly of mitotic Golgi fragments. J Cell Biol. 2004;164:973–978, and correction (2004) 166:433. doi: 10.1083/jcb.200401010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krick R, et al. Cdc48/p97 and Shp1/p47 regulate autophagosome biogenesis in concert with ubiquitin-like Atg8. J Cell Biol. 2010;190:965–973. doi: 10.1083/jcb.201002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibata Y, et al. p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO. Nat Commun. 2012;3:1061. doi: 10.1038/ncomms2068. [DOI] [PubMed] [Google Scholar]
- 59.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Yuan X, et al. Structure, dynamics and interactions of p47, a major adaptor of the AAA ATPase, p97. EMBO J. 2004;23:1463–1473. doi: 10.1038/sj.emboj.7600152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uchiyama K, et al. The localization and phosphorylation of p47 are important for Golgi disassembly-assembly during the cell cycle. J Cell Biol. 2003;161:1067–1079. doi: 10.1083/jcb.200303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armstrong CT, Mason PE, Anderson JL, Dempsey CE. Arginine side chain interactions and the role of arginine as a gating charge carrier in voltage sensitive ion channels. Sci Rep. 2016;6:21759. doi: 10.1038/srep21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narasimhan S, et al. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc Natl Acad Sci USA. 2004;101:1141–1146. doi: 10.1073/pnas.0307669100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de la Fuente J, Kocan KM, Almazán C, Blouin EF. RNA interference for the study and genetic manipulation of ticks. Trends Parasitol. 2007;23:427–433. doi: 10.1016/j.pt.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Chmelař J, et al. Sialomes and mialomes: A systems-biology view of tick tissues and tick-host interactions. Trends Parasitol. 2016;32:242–254. doi: 10.1016/j.pt.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aalto AL, et al. M1-linked ubiquitination by LUBEL is required for inflammatory responses to oral infection in Drosophila. Cell Death Differ. July 19, 2018 doi: 10.1038/s41418-018-0164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shaw DK, et al. Vector immunity and evolutionary ecology: The harmonious dissonance. Trends Immunol. 2018;39:862–873. doi: 10.1016/j.it.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Silva AM, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 69.Dunham-Ems SM, et al. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest. 2009;119:3652–3665. doi: 10.1172/JCI39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhai Z, Boquete JP, Lemaitre B. Cell-specific Imd-NF-κB responses enable simultaneous antibacterial immunity and intestinal epithelial cell shedding upon bacterial infection. Immunity. 2018;48:897–910.e7. doi: 10.1016/j.immuni.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 71.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.