During the last three decades, the development and commercialization of conjugate vaccines against Haemophilus influenzae type b (Hib), pneumococcus, and serogroups C, A, W, and Y of meningococcus contributed to the virtual elimination of bacterial meningitis caused by the bacteria included in the vaccines and to the prevention of diseases that used to cause more than a million deaths annually (1, 2). Despite the great impact on public health of these vaccines, our understanding of the way these vaccines work is still limited, and we have many unanswered questions. In PNAS, Sun et al. (3) report new mechanistic insights on conjugate vaccines.
History of Conjugate Vaccines
Conjugate vaccines have been developed to induce a robust immune response against bacterial capsular polysaccharides (CPSs). CPSs are long polymers composed of many repeating units of simple sugars and serve as a protective external layer for many bacteria. Depending on the chemical composition of the repeating unit (usually composed of one to seven monosaccharides). Bacteria can synthesize hundreds of chemically and immunologically different polysaccharides. Antibodies against the polysaccharides of many pathogenic bacteria, such as meningococcus, Hib, and pneumococcus, protect people from disease. Vaccines composed of purified polysaccharides against meningococcus and pneumococcus were developed in the 1970s. Unfortunately, those vaccines, while partially immunogenic in adults, were completely unable to induce an antibody response in infants and children, the population for whom the vaccines were mostly needed. The problem was solved in the 1980s when John Robbins and Rachel Schneerson at the National Institutes of Health in Bethesda, Maryland, and David Smith and Porter Anderson in Rochester, New York, independently figured out that, in 1929, it had been reported that bacterial CPSs become very immunogenic when covalently linked to a carrier protein (4, 5) and, thus, started working on a conjugate vaccine against Hib, which worked beautifully in infants and children. The Hib vaccine was licensed in 1990 in the United States, and John Robbins, Rachel Schneerson, Porter Anderson, and David Smith received the Albert Lasker Award for Clinical Research in 1996 for preventing meningitis in children (6). In parallel, conjugate vaccines were developed for meningococcus (7) and pneumococcus (8), and both were licensed in 1999 and 2000 in the United Kingdom and United States, respectively. Conjugate vaccines have been described also for group B Streptococcus, Shigella, Salmonella typhi, and Salmonella paratyphi. A S. typhi vaccine has been recently licensed in India and received World Health Organization prequalification (9).
Recently, metabolic engineering of bacteria allowed the construction of Escherichia coli strains that produce and export in the periplasm polysaccharides already linked to carrier proteins (10). Some of these so-called bioconjugates naturally produced in E. coli have already been successfully tested in several clinical trials. Bioconjugates represent a great simplification of the production process of conjugate vaccines, and they are expected to allow the production of large amounts of conjugates at a cost easier to afford for low-income countries.
Mechanistic Considerations for Conjugate Vaccines
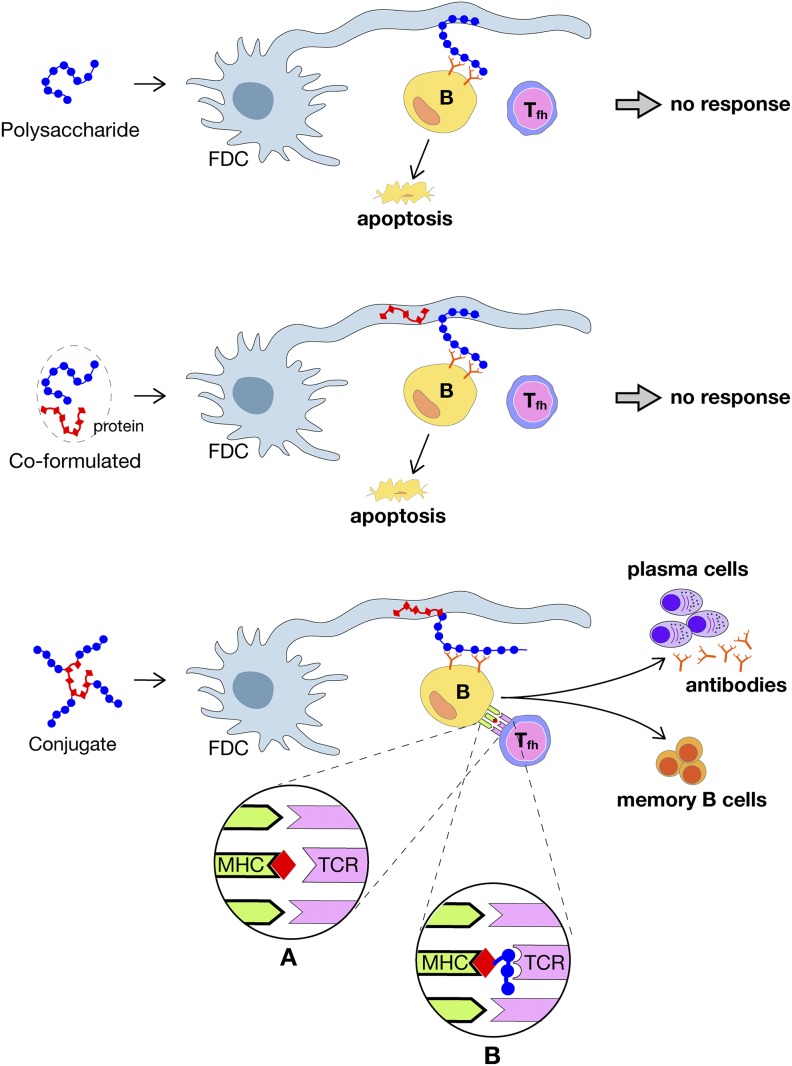
The present knowledge of the mechanism of action of conjugate vaccines has been recently reviewed in depth (11) and is schematically summarized in Fig. 1. Briefly, after immunization, polysaccharides or conjugate vaccines are taken up by dendritic cells and transported to lymph nodes where, to induce an immune response, they need to engage both B and T cells and start the formation of germinal centers (GCs). GCs are sites within lymph nodes and the spleen where mature B cells proliferate, differentiate, and mutate their antibody genes through somatic hypermutation. To form GCs, three main cells are necessary: the polysaccharide-specific B cells expressing the antibody on their surface as a receptor [B cell receptor (BCR)]; the follicular helper T (Tfh) cells, which recognize the protein carrier antigen presented on the surface of B cells; and follicular dendritic cells (FDCs), which contain and present the antigen to the B cells. The GC reaction produces higher-affinity antibodies and switches the class of antibodies (e.g., from IgM to IgG) during a normal immune response to an infection or after vaccination. The actions occur in spatially distinct regions of the GC called the light and dark zones. B cell selection and activation occur in the light zone, and the proliferation and mutation of the antibody genes occur in the dark zone. Usually, B cells bind and extract protein antigens from the FDCs in the light zone and then internalize the antigens into the endosome, process them into small peptides, and load the peptides into the cavity of the major histocompatibility complex (MHC), which exposes the peptide on the surface of the B cells so that it can be recognized by the receptor of Tfh cell. The activated Tfh cell then provides help to the B cell by direct cell–cell interaction and by secreting cytokines. B cells retrieve the antigen by applying tensile force so that the stronger the BCR’s affinity for the antigen is, the larger the amount of antigen retrieved will be and the more intense the help received from the Tfh cells will be. Tfh cells also sense the affinity for the antigen loaded in the MHC, and the higher the affinity is, the higher the intensity of help provided to the B cells in the light zone will be and, thus, the selected B cells will undergo more cycles of replication in the dark zone and will have a more efficient affinity maturation. Once activated, B cells enter the dark zone, where they multiply rapidly and express the activation-induced cytidine deaminase, which triggers the introduction of random mutations in the Ig gene encoding the variable region of the BCR, thus generating mutated BCRs. Mutated B cells with functional receptors reenter the light zone, where they retrieve antigen from the surface of FDCs, which they process and present to Tfh cells and restart the cycle of affinity maturation. B cells in the GC need to engage T cells to survive, and they undergo apoptosis unless they are positively selected by interacting with Tfh cells and antigen.
Fig. 1.
Interactions of polysaccharide and conjugate vaccines with FDC, B, and Tfh cells in the GCs. (Top) Polysaccharide alone. (Middle) Polysaccharide coformulated with protein. (Bottom) Conjugate vaccine. Circles A and B show the two mechanisms by which conjugate vaccines can engage the TCR. In circle A, the peptide is loaded into the MHC molecule and is the antigen recognized by the TCR. In circle B, the TCR recognizes the sugar linked to the peptide anchored in the MHC. From ref. 11. Modified with permission from AAAS.
In the case of polysaccharides, they can easily engage B cells. Polysaccharides (Fig. 1, Top) are bound by the specific antibodies present on the surface of B cells, extracted from the FDCs, and internalized into the endosome. However, once inside the cells, the reaction stops, because polysaccharides do not fit into the MHC cavity and cannot be exposed on the B cell surface to engage the receptor of Tfh cells. As a consequence, polysaccharides cannot engage T cells and cannot start the GC reaction that is necessary for survival, affinity maturation, and proliferation of polysaccharide-specific B cells; thus, in the absence of T cell interaction, B cells undergo apoptosis.
Fortunately, this deficiency can be overcome when polysaccharides are covalently conjugated to carrier proteins, because in this case, the B cells that recognize the polysaccharide on FDCs retrieve and internalize both the polysaccharide and the carrier protein that is covalently linked to it (Fig. 1, Bottom). The conjugate retrieved by this mechanism is then processed, and the peptides derived from the carrier proteins are loaded into the MHC cavity and presented to the T cells. Therefore, with conjugate vaccines, T cells are engaged; they provide the help necessary to start the GC reaction that leads to affinity maturation, proliferation, and the production of plasma cells (which produce polysaccharide-specific antibodies) and memory B cells.
During the last decade, several times the question has been asked whether the carrier protein needs to be covalently linked to the polysaccharide or whether formulations able to codeliver the polysaccharide and the protein to the same FDCs would be sufficient. So far, none of the attempts to make polysaccharides immunogenic without covalent linkage to a carrier protein has been consistently successful. A model that may explain why a covalent (or at least a very strong) linkage between polysaccharide and carrier protein is necessary is shown in Fig. 1, Middle. In this model, the B cell binds the polysaccharide via the BCR and extracts the antigen from the FDCs. When the carrier protein is covalently linked to the polysaccharide, the BCR retrieves the polysaccharide and the protein linked to it. Once it is internalized, the conjugate is processed, exposed on the MHC molecule, and recognized by the T cells. However, when the carrier protein is not covalently linked to the polysaccharide, even if present in the same FDC, the protein is not retrieved by the BCR and is not internalized and processed by the B cells, so it is not able to engage the T cells.
An additional reason why a covalent link between the polysaccharide and the protein may be necessary was proposed in 2011 by Avci et al. (the team of Kasper and coworkers) (12, 13). In their paper, they challenged the dogma that T cells recognize the peptides derived from the carrier protein (Fig. 1, circle A) and proposed that T cells actually recognize the sugar portion of the conjugates. In their model (Fig. 1, circle B), the role of the conjugate is to allow loading the sugars into the MHC molecule. Briefly, following processing of the conjugates, the glycopeptides containing the junctions between the carrier protein and the polysaccharide are loaded into the MHC carrying with them the attached sugars that now are available for recognition by the T cell receptor (TCR). To prove this hypothesis, Avci et al. (12) reported the isolation of two T cell clones that recognized the polysaccharide of serotype III group B Streptococcus. They named these clones carbohydrate-specific CD4+ T cells (Tcarbs). In reality, the two mechanisms described in Fig. 1 are likely to coexist. Unfortunately, 7 years after the first publication by Avci et al. (12), no additional evidence has been reported about the existence of Tcarbs and the second mechanism of antigen presentation. No more Tcarbs have been described, and structural studies showing the junction peptide linked to the MHC or TCR have not been reported; however, the induction of Tcarb-mediated immunity has been reported also for additional conjugate vaccines such as pneumococcus (14). The paper by Sun et al. (3) in PNAS reports instead that, although three of the four conjugate vaccines studied in the paper work via Tcarbs, the conjugate vaccine made from the group C polysaccharide of Neisseria meningitidis (MenC) does not work via the Tcarb mechanism because the polysaccharide (which is a polymer of sialic acid) is completely depolymerized in the endosome and no junctional glycopeptides are generated. This supports the fact that the two mechanisms of antigen presentation, peptide and peptide–Tcarb, usually coexist and that successful conjugate vaccines such as MenC do not need the Tcarb mechanism.
Conclusions
If the Tcarb hypothesis is the primary mechanism to engage T cells in conjugate vaccines, we should be able to improve their immunogenicity by increasing the number of covalent junctions between the protein and the polysaccharide. Testing this hypothesis can be an opportunity to optimize the novel chemical or biological conjugation technologies that have been recently described (10, 15). Lastly, the study by Sun et al. (3) was conducted by using, as a correlate of the Tcarb mechanism, the antibody response after priming with a polysaccharide conjugated with a protein and boosting with the same polysaccharide conjugated to a different protein. Although this correlate is likely to be correct, it would be important to nail down the presence of Tcarbs in a definitive way by isolating more Tcarbs and by determining the structure of the Tcarb bound to a glycopeptide.
Acknowledgments
We thank Giorgio Corsi for the artwork and Catherine Mallia for editorial assistance.
Footnotes
Conflict of interest statement: R.R. and E.D.G. are employees of the GlaxoSmithKline group of companies; P.C. is retired and a former employee of the GlaxoSmithKline group of companies.
See companion article on page 193.
References
- 1.Gilchrist SA, Nanni A, Levine O. Benefits and effectiveness of administering pneumococcal polysaccharide vaccine with seasonal influenza vaccine: An approach for policymakers. Am J Public Health. 2012;102:596–605. doi: 10.2105/AJPH.2011.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pichichero ME. Protein carriers of conjugate vaccines: Characteristics, development, and clinical trials. Hum Vaccin Immunother. 2013;9:2505–2523. doi: 10.4161/hv.26109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun X, Stefanetti G, Berti F, Kasper DL. Polysaccharide structure dictates mechanism of adaptive immune response to glycoconjugate vaccines. Proc Natl Acad Sci USA. 2019;116:193–198. doi: 10.1073/pnas.1816401115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate-proteins: II. Immunological specificity of synthetic sugar-protein antigens. J Exp Med. 1929;50:533–550. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goebel WF, Avery OT. Chemo-immunological studies on conjugated carbohydrate-proteins: I. The synthesis of p-aminophenol beta-glucoside, p-aminophenol beta-galactoside, and their coupling with serum globulin. J Exp Med. 1929;50:521–531. doi: 10.1084/jem.50.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albert and Mary Lasker Foundation (2018) 1996 Albert Lasker Clinical Medical Research Award: Vaccine for preventing meningitis in children. Available at www.laskerfoundation.org/awards/show/vaccine-for-preventing-meningitis-in-children/. Accessed December 12, 2018.
- 7.Costantino P, et al. Development and phase 1 clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine. 1992;10:691–698. doi: 10.1016/0264-410x(92)90091-w. [DOI] [PubMed] [Google Scholar]
- 8.Obaro SK. The new pneumococcal vaccine. Clin Microbiol Infect. 2002;8:623–633. doi: 10.1046/j.1469-0691.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 9.Jin C, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: A randomised controlled, phase 2b trial. Lancet. 2017;390:2472–2480. doi: 10.1016/S0140-6736(17)32149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ihssen J, et al. Production of glycoprotein vaccines in Escherichia coli. Microb Cell Fact. 2010;9:61. doi: 10.1186/1475-2859-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rappuoli R. Glycoconjugate vaccines: Principles and mechanisms. Sci Transl Med. 2018;10:eaat4615. doi: 10.1126/scitranslmed.aat4615. [DOI] [PubMed] [Google Scholar]
- 12.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med. 2011;17:1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rappuoli R, De Gregorio E. A sweet T cell response. Nat Med. 2011;17:1551–1552. doi: 10.1038/nm.2587. [DOI] [PubMed] [Google Scholar]
- 14.Middleton DR, Sun L, Paschall AV, Avci FY. T cell-mediated humoral immune responses to type 3 capsular polysaccharide of Streptococcus pneumoniae. J Immunol. 2017;199:598–603. doi: 10.4049/jimmunol.1700026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chennasamudram S, Vann WF. Conjugate vaccine production technology. In: Wen EP, Ellis R, Pujar NS, editors. Vaccine Development and Manufacturing. Wiley; Hoboken, NJ: 2014. pp. 217–235. [Google Scholar]