Significance
Despite their dominant role as electrophiles in biosynthetic pathways promoted by enzymes, organic phosphates are rarely employed in laboratory synthesis, because phosphates are generally very poor leaving groups. Here we report that a bis-thiourea catalyst promotes β-selective glycosylation reactions of phosphate donors under mild, neutral conditions. The catalytic method enables glycosylation of peptides, complex natural products, and pharmaceutical agents bearing a variety of reactive functionalities, thereby representing an important step toward enabling convenient access to glycosylated chemical matter of interest to synthetic chemists and chemical biologists.
Keywords: glycosylation, organocatalysis, H bonding, phosphate
Abstract
Glycosyl phosphates are shown to be activated to stereospecific nucleophilic substitution reactions by precisely tailored bis-thiourea catalysts. Enhanced reactivity and scope is observed with phosphate relative to chloride leaving groups. Stronger binding (Km) to the H-bond donor and enhanced reactivity of the complex (kcat) enables efficient catalysis with broad functional group compatibility under mild, neutral conditions.
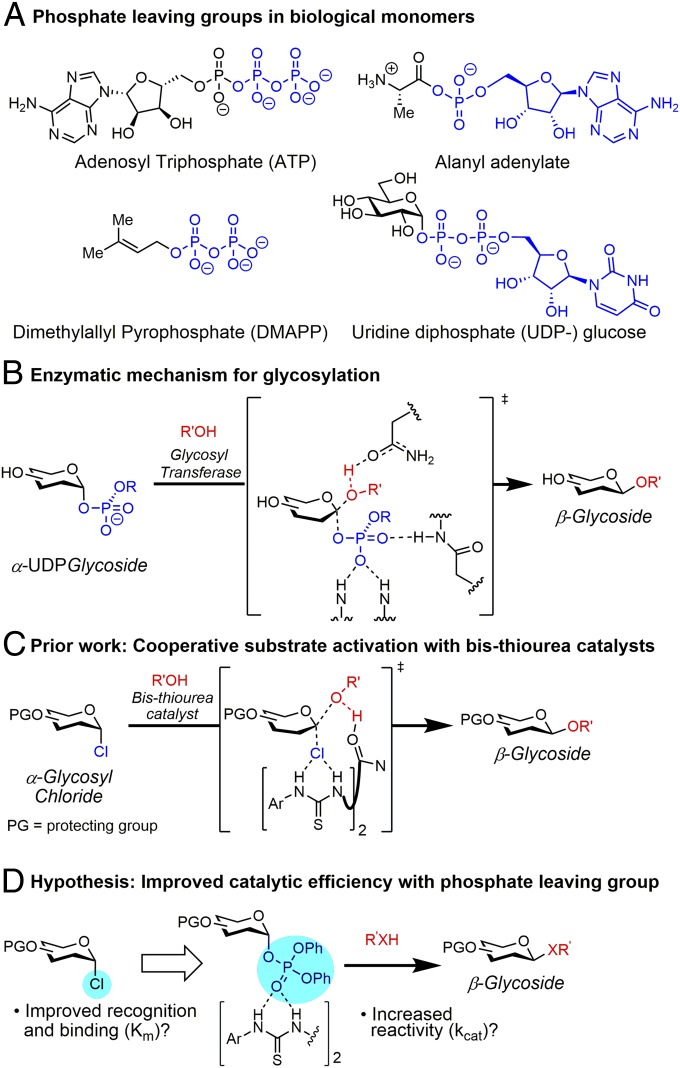
Phosphates are nature’s leaving group of choice in the biosynthesis of nearly all biological polymers, including oligonucleotides, proteins, terpenes, and carbohydrates (Fig. 1A). In rather stark contrast, phosphates are very rarely employed as leaving groups in nonbiological synthesis; synthetic chemists typically resort to more intrinsically reactive leaving groups such as halides and sulphinates (1). In a classic analysis, Westheimer (2) posited that nature evolved to use phosphates as a result of their kinetic stability and strong Lewis basic character, enabling specific enzymatic recognition. In principle, these advantages could translate to abiotic chemistry, as well, if methods could be devised for activating phosphates under mild conditions to promote nucleophilic substitution reactions. Instead, existing synthetic methods using phosphates (3) rely on highly Lewis acidic, stoichiometric activating agents that are poorly functional group-tolerant (4). Herein we report the catalytic activation of phosphate leaving groups with bis-thiourea catalysts to effect stereoselective glycosylation reactions. The neutral hydrogen-bond donor catalyst promotes coupling reactions between glycosyl phosphates and complex partners including glycosides, peptides, and highly functionalized natural products.
Fig. 1.
(A) Examples of phosphate leaving groups found in nature. (B) Invertive glycosylation mechanism utilized by glycosyl transferase enzymes. (C) Previously reported work catalyzing stereospecific glycosylation reactions via dual-activation of glycosyl chloride donors and alcohol acceptors with bis-thioureas. (D) Improving catalytic efficiency through tighter binding and increased reactivity of phosphate leaving group activated by hydrogen-bond-donor catalyst.
The use of hydrogen-bond donor organocatalysts in glycosylation reactions has been reported by several groups (5–12). While such systems can provide stable, mild, and user-friendly alternatives to strongly Lewis acidic and oxidizing reagents typical of chemical glycosylation, the attainment of useful reactivity and stereoselectivity with functionally complex coupling partners has proven elusive. We reported recently that precisely linked bis-thiourea derivatives catalyze stereospecific nucleophilic substitution reactions of glycosyl chlorides (13). On the basis of experimental and computational modeling studies, the mode of catalysis was proposed to involve simultaneous activation of both the donor and acceptor reacting partners through general acid and general base activation (14), in a manner loosely reminiscent of the mechanisms by which glycosyl transferase enzymes have been postulated to function (Fig. 1B) (15). However, in subsequent efforts to apply and extend the synthetic methodology to targets of interest for biological studies, we have observed that severe rate suppression occurs with functionally complex coupling partners, thereby imposing severe limitations to reaction scope. Kinetic analyses reveal that complex nucleophiles bearing multiple Lewis basic groups in fact function as catalyst inhibitors, presumably due to unproductive association of the key H-bond donor motifs (SI Appendix, Fig. S5). We hypothesized that catalytic efficiency was limited by the low Lewis basicity of the chloride leaving group, and that leaving groups with higher affinity toward the thiourea moiety might enable superior functional group compatibility in glycosylation reactions with complex coupling partners (Fig. 1C).
Here we report that precisely linked bis-thiourea catalysts activate glycosyl phosphates and promote β-selective glycosylation reactions. Engagement of phosphates as leaving groups results in significant improvements in reactivity and scope compared with previous systems, enabling efficient conjugation of saccharides to a variety of functionally complex coupling partners.
Results and Discussion
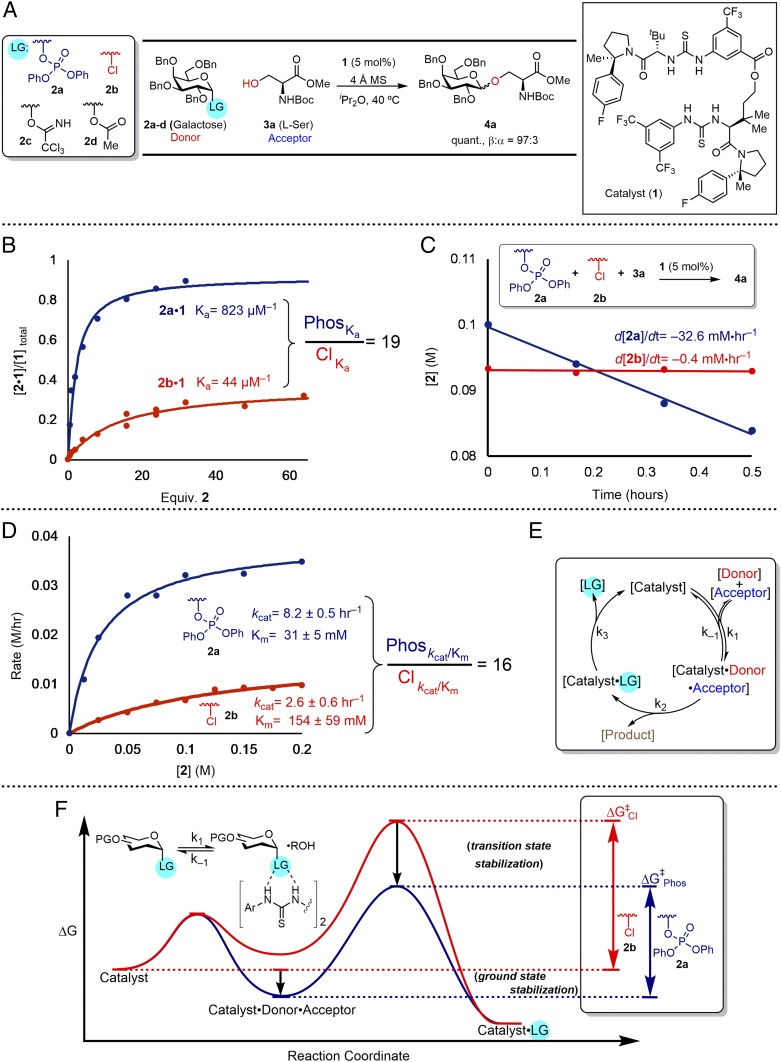
Inspired by nature’s selection of phosphate leaving groups, we hypothesized that our synthetic hydrogen-bond donor catalysts would be well suited to bind an organic phosphate leaving group due to its high Lewis basicity (16). We compared the binding of galactosyl phosphate 2a to bis-thiourea catalyst 1 to that of the corresponding glycosyl chloride 2b (Fig. 2B). The phosphate was observed to bind 19 times more strongly than the chloride.
Fig. 2.
(A) Model system for leaving group evaluation. (B) Binding of galactosyl phosphate 2a and galactosyl chloride 2b to bis-thiourea catalyst 1 as quantified by 19F NMR. The curves correspond to 1:1 binding fits. (C) Competition rate kinetics between 2a and 2b measured by 1H NMR. (D) Michaelis−Menten initial rate analysis determined by HPLC. (E) Proposed catalytic cycle. (F) Reaction coordinate diagram for phosphate and chloride leaving groups in model system.
While the binding study supported the straightforward idea that more Lewis basic leaving groups could result in increased concentration of the catalyst–donor complex, it was much less apparent whether this enhanced affinity would result in more facile H-bond-donor promoted nucleophilic substitution reactions, or whether catalyst turnover would be attainable. To address these questions, we evaluated a model glycosylation reaction between the galactosyl phosphate 2a and the protected serine derivative 3a as the acceptor. This substrate combination was poorly reactive in couplings with glycosyl chlorides in the previously reported system, providing 16% conversion (75:25 β:α) with 10 mol % catalyst after 14 h (SI Appendix, Scheme S2). In the presence of bis-thiourea 1, the glycosylation product was generated in quantitative yield and with high β selectivity (97:3 β:α). In a broad survey of other potential galactosyl donors, including the acetate 2c and trichloroacetimidate 2d, phosphate 2a proved to be uniquely reactive under the catalytic conditions (SI Appendix, Scheme S4).
A systematic evaluation of monomeric and dimeric hydrogen-bond donor derivatives revealed that linked bis-thiourea and bis-urea catalysts were the only effective catalysts for the model reaction (SI Appendix, Scheme S2). No reactivity was observed with macrocyclic bis-thiourea catalysts of varying size, in direct contrast to prior studies using glycosyl chlorides as donors. The relative and absolute stereochemistry and the linker length of bis-thiourea 1 were all found to be crucial parameters for effective catalysis.
We sought to establish whether the diphenylphosphoric acid generated as a stoichiometric by-product has any effect on the glycosylation reaction. In prior work by Schmidt and coworkers (6, 17–19), Fairbanks and coworkers (20), Toshima and coworkers (21), Nagorny and coworkers (22, 23), Galan and coworkers (24), Bennett and coworkers (25), and others (26, 27), phosphoric acids have been shown to be competent catalytic activators in glycosylation reactions involving different types of donors. However, in the model system, addition of exogenous diphenylphosphoric acid was shown to have no detectable effect on either reaction rate or α/β selectivity. Analysis of reaction mixtures by NMR revealed no measurable concentration of the acid by-product in solution, suggesting that insolubility is most likely responsible for the absence of any catalytic or inhibitory activity in the thiourea-promoted glycosylation. This effect is solvent-dependent, and optimal anomeric selectivity and reactivity are obtained with nonpolar solvents such as cyclohexane and ethereal solvents (SI Appendix, Table S11). Notably, when the reaction is performed in a solvent in which the phosphoric acid by-product is soluble (e.g., dichloromethane), the hydrogen-bond donor catalyzed pathway is suppressed almost completely.
Independent and competitive rate data for glycosyl donors 2a and 2b were generated to evaluate the effect of improved leaving group recognition on the catalytic reaction. In reactions with serine 3a as the nucleophile, the reactivity of chloride 2b was found to be severely inhibited (from 4.0 mM⋅h−1 to 0.4 mM⋅h−1) by the presence of equimolar concentrations of the phosphate 2a, while the reactivity of 2a was unaffected (32 mM⋅h−1) by the presence of the chloride (Fig. 2C). Michaelis−Menten kinetic analyses of reactions catalyzed by 1 revealed both lower Michaelis constant (Km) and a faster maximum rate (kcat) for the glycosyl phosphate relative to the chloride (Fig. 2D). Thus, the phosphate not only binds more tightly to the catalyst but also is more labile toward substitution when bound, resulting in a 16-fold improvement in catalytic efficiency (kcat/Km) throughout the entire course of reaction (Fig. 2F). Saturation kinetics are observed with respect to both donor (2a or 2b, Fig. 2D) and serine acceptor (SI Appendix, Figs. S10 and S13), consistent with rate-determining reaction of a ternary catalyst–donor–acceptor complex to form product (Fig. 2E).
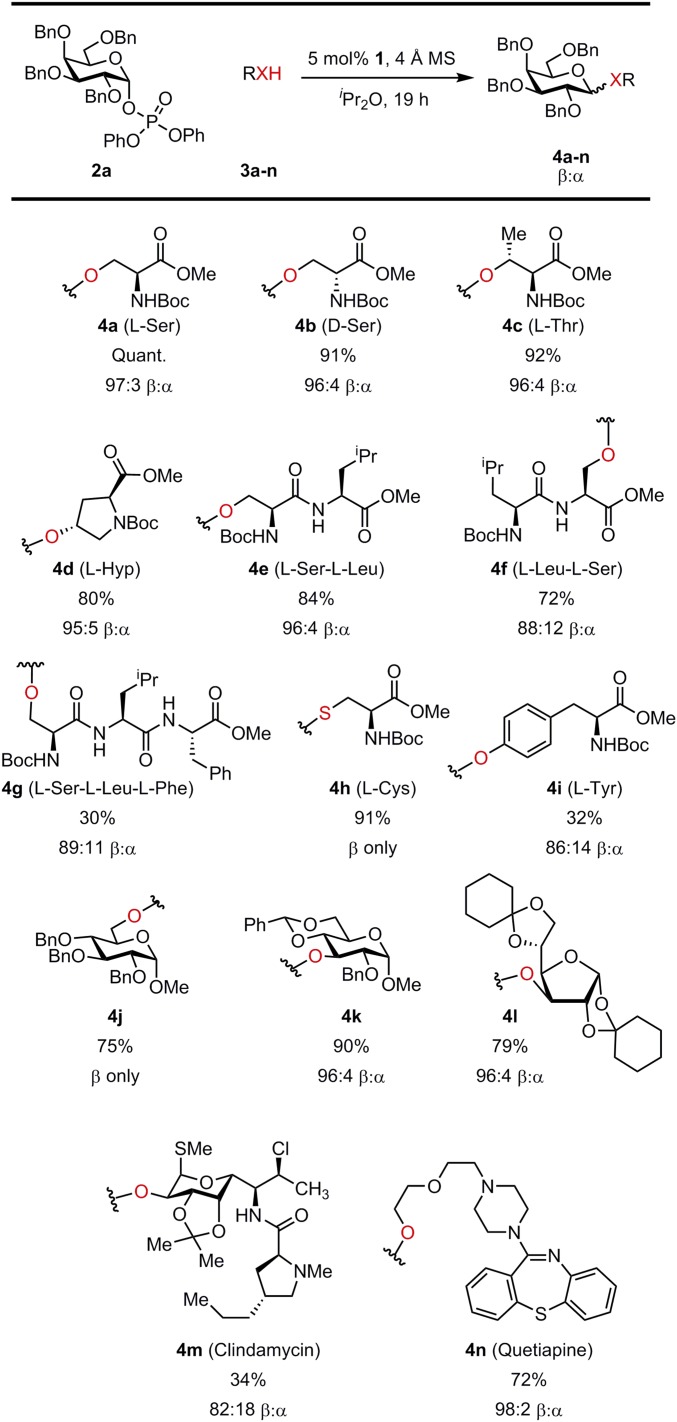
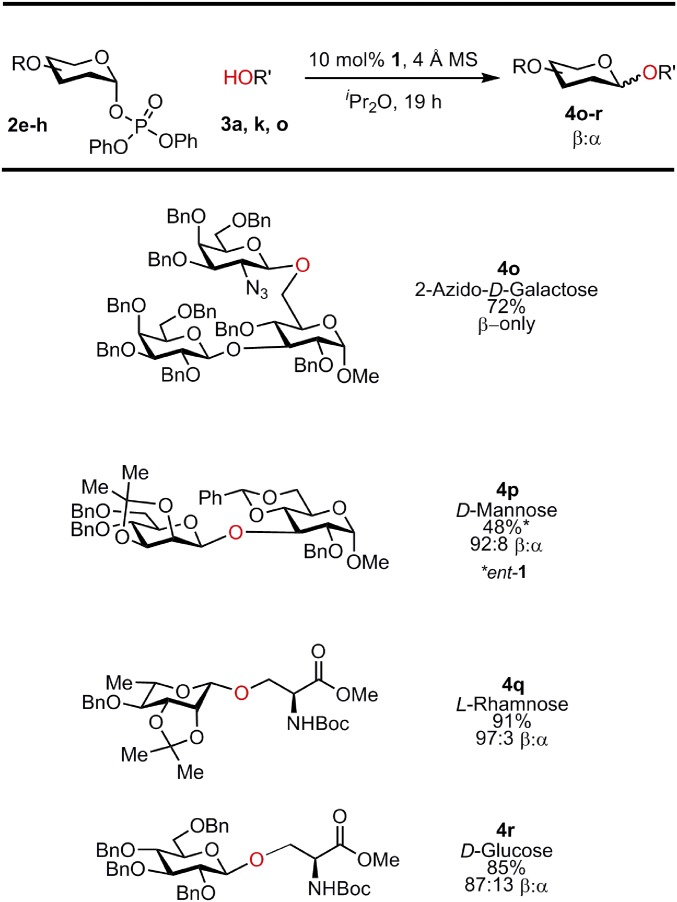
The improved binding and reactivity of glycosyl phosphate donors relative to chloride prompted us to evaluate the scope of the catalytic reaction with acceptors containing multiple Lewis basic sites. The model reaction in Fig. 2A could now be carried out efficiently using 1 mol % of bis-thiourea 1 and 1.2 equivalents of acceptor 3a in 12 h to afford 4a in 94% NMR yield (>95% conversion) and a β:α selectivity of 93:7. Excellent reactivity and β:α selectivity were observed with simple hydroxylic amino acids, including serine, threonine, and hydroxyproline (Fig. 3). Dipeptides l-Ser-l-Leu and l-Leu-l-Ser as well as the tripeptide l-Ser-l-Leu-l-Phe were engaged effectively as acceptors, although the limited solubility of the tripeptide under the reaction conditions resulted in decreased reactivity. Galactosylation of compounds bearing basic nitrogen functionality such as clindamycin and quetiapine was also achieved, suggesting that elaboration of medicinally active small molecules may be possible in a general manner. Phenols and thiols were also engaged effectively as acceptors, as illustrated in the β-selective galactosylations of both tyrosine and cysteine. However, while good scope was thus observed with protic nucleophiles, carbon-centered nucleophiles such as allyltrimethylsilane proved unreactive under the catalytic conditions (although primary and secondary amino acceptors afforded the β-product selectively under catalytic conditions, background reactivity was often competitive as well as β-selective). These observations are consistent with catalysis involving both anion abstraction from the electrophile and general base activation of the protic nucleophile (Fig. 1B), although substantiation of that hypothesis will require fuller mechanistic characterization. Initial efforts to expand the scope of the catalytic reaction to different phosphate donors are outlined in Fig. 4. Efficient reaction between 2-azido-galactosyl phosphate 2e and a disaccharide acceptor was promoted by 1 to afford the corresponding β-trisaccharide 4o, exclusively. Construction of cis-1,2 β-linkages was achieved, albeit with lower catalytic efficiency, as illustrated in the generation of β−mannosylation and β−rhamnosylation products 4p and 4q. While glycosylation reactions with bis-thiourea 1 were noted above to be insensitive to the absolute stereochemistry of the acceptors (e.g., 4a and 4b; Fig. 3), matching of the stereochemical features of the glycosyl donor and catalyst proved important. Thus, β−rhamnosylation was achieved with 1, while effective reactions with the pseudoenantiomeric mannosyl phosphate required use of ent-1.
Fig. 3.
Acceptor scope for galactose phosphate donor.
Fig. 4.
Donor scope with diphenylphosphate leaving group.
We have demonstrated in this study that precisely tailored neutral H-bond donor catalysts can activate organic phosphates as leaving groups, allowing stereospecific glycosylation reactions of functionally rich coupling partners under mild and neutral conditions. The catalytic method allows glycosylation of a wide range of functionally complex acceptors with good scope and under mild, neutral conditions. We propose that this approach represents an important advance in glycosylation methodology. Prior advances in nonenzymatic glycosylation have provided enabling tools for selective glycosidic bond construction, but these approaches typically require strongly oxidizing and Lewis acidic promotors that can be incompatible with sensitive substrates. Methods engaging glycosyl phosphates, for example, require stoichiometric trimethylsilyltriflate under cryogenic temperatures (3, 4). Other leaving groups are susceptible to competing pathways: Thioglycosides undergo aglycone transfer (29), and imidates are prone to rearrangement to the acetamide (28). Strong Lewis acid activation conditions are also known to promote poorly regioselective glycosylations of phenols, yielding mixtures of O- and C-glycosylated products (29). As a result of these limitations, reaction conditions and substrates often need to be tailored for specific reactions.
Similarly, enzymatic methods have been applied with exceptional success in the laboratory synthesis of complex polysaccharides, but there are important limitations to that approach that are avoided with the system described here. For example, access to the UDP-glycosyl donors can be limited, and the requisite glycosyltransferases are often difficult to obtain, unstable, narrow in substrate scope, challenging to manipulate, and/or prone to inhibition by the by-product nucleotide leaving group (30).
Ultimately, we anticipate that the successful development of this methodology may facilitate the synthesis and production of biomedically relevant glycans, glycopeptides, glycoproteins, glycolipids, and microbial polysaccharides and glycoconjugates.
Materials and Methods
General Procedure for Glycosylation Reaction Catalyzed by Catalyst 1.
To a flame-dry 5-mL round-bottom flask with a stir bar was charged 0.250 mmol of glycosyl donor, 250 mg of flame-dry 4-Å molecular sieves, 0.0125 mmol of catalyst, and 0.500 mmol of acceptor; 2.50 mL of diisopropyl ether was added to the reaction mixture open to air, the flask was closed with a plastic cap, and parafilm was used to seal the cap tightly. The mixture was then heated with efficient stirring at 40 °C in an oil bath for 19 h, over which time the mixture thickened. After 19 h, the mixture was cooled to room temperature, and 100 µL of aliquot was diluted with diethyl ether and filtered to remove the molecular sieves. This solution was concentrated, and 1H NMR analysis of the crude mixture was used to determine the anomeric selectivity. The mixture was then chromatographed directly with 50 g of SiO2 using an diethyl ether:hexanes gradient.
Hazard Note.
Diisopropyl ether is known to form peroxides and should be handled with appropriate care. Reaction outcomes with alternative solvents are described in SI Appendix, Table S11.
Supplementary Material
Acknowledgments
We thank Benjamin J. Levin (Harvard University) and Dr. Steven M. Banik for helpful discussions. We acknowledge support from the National Institutes of Health through the Common Fund Glycoscience Program U01 GM116249 and GM043214, and from a National Science Foundation predoctoral fellowship (S.M.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811186116/-/DCSupplemental.
References
- 1.Smith MB, March J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. John Wiley; New York: 2006. [Google Scholar]
- 2.Westheimer FH. Why nature chose phosphates. Science. 1987;235:1173–1178. doi: 10.1126/science.2434996. [DOI] [PubMed] [Google Scholar]
- 3.Plante OJ, Palmacci ER, Andrade RB, Seeberger PH. Oligosaccharide synthesis with glycosyl phosphate and dithiophosphate triesters as glycosylating agents. J Am Chem Soc. 2001;123:9545–9554. doi: 10.1021/ja016227r. [DOI] [PubMed] [Google Scholar]
- 4.Demchenko AV, editor. Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance. Wiley-VCH; Hoboken, NJ: 2008. [Google Scholar]
- 5.Reisman SE, Doyle AG, Jacobsen EN. Enantioselective thiourea-catalyzed additions to oxocarbenium ions. J Am Chem Soc. 2008;130:7198–7199. doi: 10.1021/ja801514m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geng Y, et al. Cooperative catalysis in glycosidation reactions with O-glycosyl trichloroacetimidates as glycosyl donors. Angew Chem Int Ed Engl. 2013;52:10089–10092. doi: 10.1002/anie.201302158. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Wu X, Xiong DC, Ye XS. Stereoselective Koenigs-Knorr glycosylation catalyzed by urea. Angew Chem Int Ed Engl. 2016;55:8041–8044. doi: 10.1002/anie.201600142. [DOI] [PubMed] [Google Scholar]
- 8.Medina S, et al. Stereoselective glycosylation of 2-nitrogalactals catalyzed by a bifunctional organocatalyst. Org Lett. 2016;18:4222–4225. doi: 10.1021/acs.orglett.6b01962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura T, Eto T, Takahashi D, Toshima K. Stereocontrolled photoinduced glycosylation using an aryl thiourea as an organo photoacid. Org Lett. 2016;18:3190–3193. doi: 10.1021/acs.orglett.6b01404. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto Y, Tanikawa S, Saito R, Sasaki K. β-Stereoselective mannosylation using 2,6-lactones. J Am Chem Soc. 2016;138:14840–14843. doi: 10.1021/jacs.6b08874. [DOI] [PubMed] [Google Scholar]
- 11.Peng P, Geng Y, Göttker-Schnetmann I, Schmidt RR. 2-Nitro-thioglycosides: α- and β-selective generation and their potential as β-selective glycosyl donors. Org Lett. 2015;17:1421–1424. doi: 10.1021/acs.orglett.5b00295. [DOI] [PubMed] [Google Scholar]
- 12.Balmond EI, Coe DM, Galan MC, McGarrigle EM. α-Selective organocatalytic synthesis of 2-deoxygalactosides. Angew Chem Int Ed Engl. 2012;51:9152–9155. doi: 10.1002/anie.201204505. [DOI] [PubMed] [Google Scholar]
- 13.Park Y, et al. Macrocyclic bis-thioureas catalyze stereospecific glycosylation reactions. Science. 2017;355:162–166. doi: 10.1126/science.aal1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwan EE, Park Y, Besser HA, Anderson TL, Jacobsen EN. Sensitive and accurate 13C kinetic isotope effect measurements enabled by polarization transfer. J Am Chem Soc. 2017;139:43–46. doi: 10.1021/jacs.6b10621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: Structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521–555. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- 16.Hunter CA. Quantifying intermolecular interactions: Guidelines for the molecular recognition toolbox. Angew Chem Int Ed Engl. 2004;43:5310–5324. doi: 10.1002/anie.200301739. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt RR, Wegmann B, Jung KH. Glycosyl imidates, 47. Stereospecific synthesis of α- and β-L-fucopyranosyl phosphates and of GDP-fucose via trichloroacetimidate. Liebigs Ann Chem. 1991;1991:121–124. [Google Scholar]
- 18.Schmidt RR, Stumpp M, Michel J. α- and β-D-glucopyranosyl phosphates from O-α-D-glucoypyranosyl trichloroacetimidates. Tet Lett. 1982;23:405–408. [Google Scholar]
- 19.Schmidt RR, Stumpp M. Glycosylimidate, 10. Glycosylphosphate aus glycosyl(trichloracetimidaten) Liebigs Ann Chem. 1984;1984:680–691. [Google Scholar]
- 20.Cox DJ, Smith MD, Fairbanks AJ. Glycosylation catalyzed by a chiral Brønsted acid. Org Lett. 2010;12:1452–1455. doi: 10.1021/ol1001895. [DOI] [PubMed] [Google Scholar]
- 21.Kimura T, Sekine M, Takahashi D, Toshima K. Chiral Brønsted acid mediated glycosylation with recognition of alcohol chirality. Angew Chem Int Ed Engl. 2013;52:12131–12134. doi: 10.1002/anie.201304830. [DOI] [PubMed] [Google Scholar]
- 22.Tay JH, et al. Regiodivergent glycosylations of 6-deoxy-erythronolide B and Oleandomycin-derived macrolactones enabled by chiral acid catalysis. J Am Chem Soc. 2017;139:8570–8578. doi: 10.1021/jacs.7b03198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Borovika A, Khomutnyk Y, Nagorny P. Chiral phosphoric acid-catalyzed desymmetrizative glycosylation of 2-deoxystreptamine and its application to aminoglycoside synthesis. Chem Commun (Camb) 2017;53:8976–8979. doi: 10.1039/c7cc05052f. [DOI] [PubMed] [Google Scholar]
- 24.Palo-Nieto C, Sau A, Williams R, Galan MC. Cooperative Brønsted acid-type organocatalysis for the stereoselective synthesis of deoxyglycosides. J Org Chem. 2017;82:407–414. doi: 10.1021/acs.joc.6b02498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu D, Sarrafpour S, Guo W, Goulart B, Bennett CS. Matched/mismatched interactions in chiral Brønsted acid-catalyzed glycosylation reactions with 2-deoxy-sugar trichloroacetimidate donors. J Carbohydr Chem. 2014;33:423–434. [Google Scholar]
- 26.Williams R, Galan MC. Recent advances in organocatalytic glycosylations. Eur J Org Chem. 2017;42:6247–6264. [Google Scholar]
- 27.Wang HY, Blaszczyk SA, Xiao G, Tang W. Chiral reagents in glycosylation and modification of carbohydrates. Chem Soc Rev. 2018;47:681–701. doi: 10.1039/c7cs00432j. [DOI] [PubMed] [Google Scholar]
- 28.Christensen HM, Oscarson S, Jensen HH. Common side reactions of the glycosyl donor in chemical glycosylation. Carbohydr Res. 2015;408:51–95. doi: 10.1016/j.carres.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsson M, Malmberg J, Ellervik U. Aromatic O-glycosylation. Carbohydr Res. 2006;341:1266–1281. doi: 10.1016/j.carres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Schmaltz RM, Hanson SR, Wong C-H. Enzymes in the synthesis of glycoconjugates. Chem Rev. 2011;111:4259–4307. doi: 10.1021/cr200113w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.