Significance
Due to difficulty in obtaining relevant samples and low levels of dengue virus (DENV) present in patients, it has been difficult to characterize the properties of virions circulating in humans. We characterized DENV1 directly from early acute primary dengue samples and from their cell culture isolates. We observed that virions in humans are more infectious and mature as compared with their cell culture isolates. DENV1 cell culture isolates were hypersensitive to neutralization by human antibodies. Cross-reactive fusion loop antibodies and heterotypic immune sera poorly neutralized plasma DENV1. Identical genome sequences showed that the phenotypic differences are due to the differences in maturation state. The observed structural variation is a significant advance applicable to understanding vaccine failure and designing future vaccine strategies.
Keywords: dengue, structure, maturation, antibody, neutralization
Abstract
The four dengue virus (DENV) serotypes are mosquito-borne flaviviruses of humans. The interactions between DENVs and the human host that lead to asymptomatic, mild, or severe disease are poorly understood, in part, because laboratory models are poor surrogates for human DENV disease. Virologists are interested in how the properties of DENVs replicating in people compare with virions propagated on laboratory cell lines, which are widely used for research and vaccine development. Using clinical samples from a DENV type 1 epidemic in Sri Lanka and new ultrasensitive assays, we compared the properties of DENVs in human plasma and after one passage on laboratory cell lines. DENVs in plasma were 50- to 700-fold more infectious than cell culture-grown viruses. DENVs produced by laboratory cell lines were structurally immature and hypersensitive to neutralization by human antibodies compared with DENVs circulating in people. Human plasma and cell culture-derived virions had identical genome sequences, indicating that these phenotypic differences were due to the mature state of plasma virions. Several dengue vaccines are under development. Recent studies indicate that vaccine-induced antibodies that neutralized DENVs in cell culture assays were not sufficient for protecting people from DENV infections. Our results about structural differences between DENVs produced in humans versus cell lines may be key to understanding vaccine failure and developing better models for vaccine evaluation.
Several hundred million people are infected each year by the four dengue virus (DENV) serotypes, which are mosquito-borne flaviviruses responsible for dengue fever (DF) and severe dengue hemorrhagic fever (DHF) (1). Infection with any DENV serotype confers lifelong immunity against the infecting serotype but not against heterologous serotypes (2). While the pathogenesis of severe dengue disease is incompletely understood, a key determinant is the ability of some antibodies to enhance the replication of DENVs in infants born to DENV-immune mothers and older individuals experiencing secondary DENV infections (3, 4). DENV-specific antibodies and T cells protect people from DENV infection and disease (reviewed in ref. 5). With existing laboratory models to study DENVs, it has been challenging to define interactions between the virus and human host that suppress or promote DENV replication in people. In this study, we explore whether structural differences between DENVs circulating in patients and virions produced under laboratory conditions impede current efforts to study dengue pathogenesis and develop vaccines.
The dengue virion contains two integral membrane proteins designated as envelope (E) and premembrane (prM). During virus secretion from infected cells, prM is cleaved to generate mature infectious virions, which have a smooth surface of 90 antiparallel E dimers that are tightly packed to form a protein coat with icosahedral symmetry (6). In laboratory cell lines commonly used to propagate DENVs, prM processing is inefficient and variable, and virions released from cells are a heterogeneous mixture of immature, partially mature, and fully mature virions containing variable amounts of unprocessed prM (7, 8). Recent studies have also established that the viral envelope is quite flexible in some laboratory strains of DENV. This phenomenon, termed virus breathing, is influenced by temperature and specific mutations on E protein (9, 10). Both the maturation state of DENVs and the flexibility of the envelope can have profound effects on the ability of mAbs and human immune sera to neutralize the virus (11–13).
While a rich body of work has emerged from these structure and function studies of DENVs used in the laboratory, no systematic studies have been done to compare the properties of DENVs propagated using cell lines and DENVs produced in infected people. This is a major gap in our knowledge, especially given recent reports from vaccine trials that many people were susceptible to DENV infections despite vaccines inducing antibodies that neutralized DENVs in cell culture assays (14). The goal of our study was to compare the structural and functional properties of dengue virions circulating in people and virions propagated using laboratory cell lines.
Here, we show that DENV1 circulating in patients is more infectious and mature than cell culture isolates. Cross-reactive dengue antibodies and heterotypic dengue immune sera poorly neutralize plasma DENV1 compared with cell culture-produced virus. Our results are directly relevant to evaluating vaccines in the current pipeline and for developing new vaccines.
Results
To study the properties of DENVs circulating in humans, we collected blood from patients suspected of having DF during a DENV1 epidemic in Sri Lanka in 2014. Samples collected during the febrile period (1–4 d after onset of fever) were tested by RT-PCR to detect acute DENV1 infections. Blood samples from six positive subjects who had acute infections were selected for the current study.
DENVs Circulating in Patients Are More Infectious than DENVs Produced in Cell Culture.
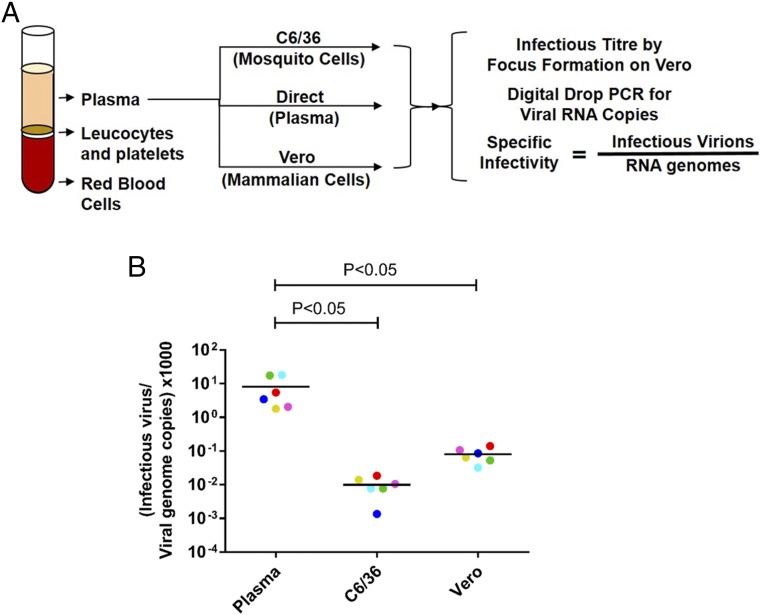
To compare the specific infectivity of DENV1 virions circulating in patients and virions released by laboratory cell lines, we infected Vero (mammalian) and C6/36 (mosquito) cells using plasma from each viremic subject (Fig. 1A). To calculate specific infectivity, we measured the number of infectious virions and the number of viral genome copies in each plasma sample and the corresponding clarified cell culture supernatants (SI Appendix, Table S1). The number of infectious virions was measured by focus assay (SI Appendix, Fig. S1), and the number of viral genomes was measured by digital drop RT-PCR. Viral-specific infectivity was expressed as the number of infectious virions per genome copy. The low-passage (P1) clinical isolates of DENV1 from Sri Lankan patients harvested from C6/36 and Vero cell culture media contained, on average, 2.0 × 105 and 1.6 × 104 viral genome copies per infectious virion (Fig. 1B and SI Appendix, Table S1). In contrast, DENV1 virions in human plasma were significantly more infectious and contained, on average, only 2.8 × 102 viral genomes per infectious unit (Fig. 1B and SI Appendix, Table S1). Thus, the plasma DENV1 virions were rendered 725-fold less infectious after a single passage on C6/36 insect cells and 56-fold less infectious after a single passage on Vero cells.
Fig. 1.
Specific infectivity of DENV1 virions produced in people and laboratory cell lines. (A) Plasma from six subjects experiencing primary DENV1 infections was used to infect C6/36 and Vero cells. Viral-specific infectivity in human plasma and cell culture supernatants (Vero and C6/36 cells) was estimated by dividing the number of infectious virions by the number of viral genomes in each sample. (B) Specific infectivity of DENV1 viruses in human plasma and respective cell culture isolates. Cell culture virions are color-coded to match their respective plasma virus sample. Infectious virus titers and viral genome copies were the results of average values from two technical replicates in a single experiment. Specific infectivity for each virus was calculated from the above-calculated average values. Lines indicate the mean. Two-way ANOVA with a Tukey’s multiple comparisons test was performed to compare specific infectivity differences among plasma DENV1 samples and their cell culture isolates.
DENVs Circulating in Patients Are More Mature than DENVs Produced in Cell Culture.
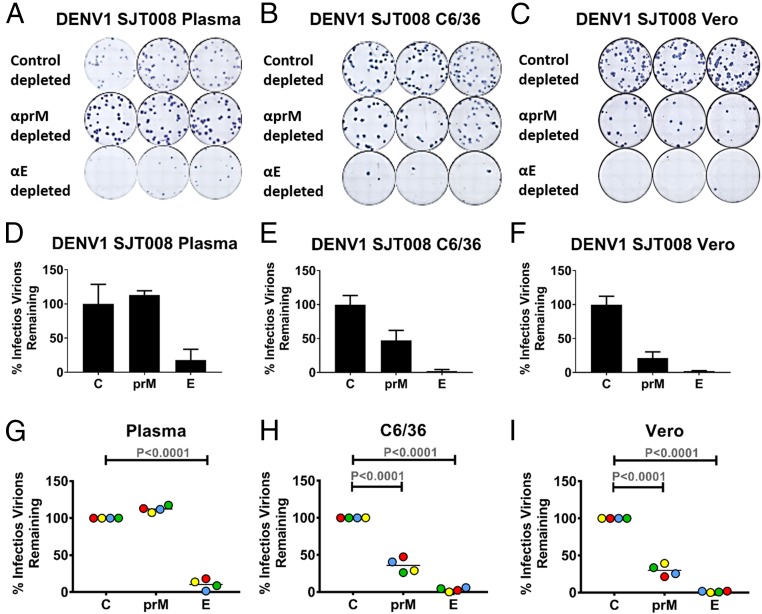
We next characterized the maturation state of DENV1 virions in human plasma and cell culture media using an antibody to detect unprocessed prM protein on virions. Blood samples from four subjects who had serological profiles indicative of primary DENV1 infections were selected for the rest of the study. We excluded people with secondary DENV1 infections because preexisting DENV-binding antibodies in the acute samples could interfere with the assays to characterize virions. To check maturation state, we incubated virus samples with magnetic beads containing mAbs that bound to prM protein (2H2) or DENV E protein (1M7) and then measured the fraction of infectious virions that were not removed by the mAb-coated beads. In theory, all virions in each specimen should be removed by beads containing the 1M7 E protein-specific mAb, whereas only prM containing partially and fully immature virions should be depleted with the 2H2 prM-specific mAb. This approach was first validated using virions of defined maturation state produced in the laboratory (SI Appendix, Fig. S2). DENV1 virions in human plasma were efficiently removed by the E-specific 1M7 mAb but not by the prM-specific 2H2 mAb (Fig. 2 and SI Appendix, Fig. S3). In contrast, the majority of P1 clinical isolates in C6/36 and Vero cell culture supernatants were removed by the 2H2 prM mAb (Fig. 2 and SI Appendix, Fig. S3). The prM antibodies bind to partially mature DENVs and enhance infection of Fc receptor-bearing cell lines (7). As an alternate method for detecting low levels of prM protein on virions, we performed prM antibody enhancement assays with human plasma and Vero cell-derived DENV1 samples (SI Appendix, Fig. S4). The prM antibodies readily enhanced Vero cell-derived virions but not human plasma-derived virions, confirming the absence of prM protein on plasma virions. We conclude that DENV1 virions circulating in patients are mature, whereas even a single passage in cell culture leads to the release of virions with prM protein on the surface indicative of incomplete intracellular processing.
Fig. 2.
Maturation state of DENV1 virions in human plasma and cell culture supernatant. DENV1 virions in clinical and cell culture samples were depleted using magnetic beads coated with E-specific mAb 1M7, prM-specific mAb 2H2, and DENV3 type-specific mAb 8A1 (control depletion). After removal of specific virus populations using mAb-coated magnetic beads, the remaining infectious virions were detected using a Vero cell-based infectious focus assay. Depletion of SJT008 DENV1 from human plasma (A and D) and corresponding P1 clinical isolates from C6/36 (B and E) and Vero (C and F) cells are shown. The percentage of infectious virions remaining after depletion was calculated from three technical replicates in a single experiment. Error bars indicate the range of the three technical replicates. Compiled depletion data for DENV1 from four plasma samples (SJT008, SJT003, SJT001, and SJT004) (G) and their isolates in C6/36 (H) and Vero (I) cells are shown. Samples are color-coded to match corresponding plasma samples. (D–I) All measurements are normalized to the corresponding control-depleted (C) sample. Two-way ANOVA was performed to compare depletion variations within plasma DENV1 samples and their clinical isolates in C6/36 and Vero cells. Lines indicate the mean.
Human Antibodies Directed to the Fusion Loop of E Protein Poorly Neutralize DENVs Derived from Patients Compared with DENVs Produced in Cell Culture.
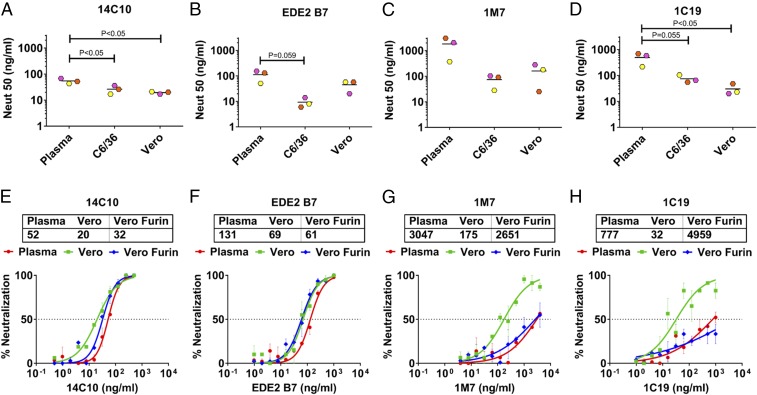
As the interaction of some antibodies with DENVs is strongly influenced by subtle changes in virion structure, including maturation state, we compared the ability of a panel of well-characterized human mAbs (SI Appendix, Table S2) to neutralize DENV1 virions circulating in people and produced by laboratory cell lines (15–18). The 14C10 mAb is a DENV1 type-specific and strongly neutralizing human mAb isolated from a DENV1 case, whereas EDE2 B7 is a DENV serotype cross-reactive and strongly neutralizing mAb isolated from a secondary DENV case (16, 18). The 14C10 and EDE2 B7 mAbs recognize quaternary-structure epitopes centered on domain 1 of E protein (EDI) and the hinge region between EDI and EDII, respectively (16, 18). Both 14C10 and EDE2 B7 effectively neutralized human plasma (EC50 = 43–154 ng/mL) and the corresponding C6/36 and Vero cell culture-derived DENV1 virions (Fig. 3 A and B). The C6/36 and Vero cell-derived viruses were neutralized two- to 20-fold more efficiently than plasma viruses by these human mAbs. Human mAbs 1M7 and 1C19 bind to highly conserved, simple epitopes near the fusion loop on EDII (17). These antibodies poorly neutralized plasma virions (EC50 ∼ 300–1,800 ng/mL) compared with cell culture-derived DENV1 virions (Fig. 3 C and D). We conclude that some human mAbs, especially those targeting conserved epitopes near the fusion loop on EDII, are effective at neutralizing cell culture-derived virions but not virions circulating in infected people. On the other hand, mAbs directed to quaternary epitopes near EDI efficiently neutralized both plasma and cell culture-derived DENV1 virions.
Fig. 3.
Neutralization ability of human mAbs against plasma and cell culture DENV1. (A–D) Various dilutions of human mAbs were used to neutralize DENV1 viruses from plasma and their C6/36 and Vero cell culture clinical isolates. Neutralization curves for human mAbs were plotted using GraphPad Prism (SI Appendix, Fig. S5). This figure shows the compiled neutralization data of human mAbs for DENV1 from three plasma samples (SJT008, SJT003, and SJT004) and their isolates in C6/36 and Vero cells. Lines indicate the mean. Two-way ANOVA with a Tukey’s multiple comparisons test was performed to compare the plasma DENV1 with C6/36 and Vero cell culture isolates. (E–H) Various dilutions of human mAbs were used to neutralize DENV1 viruses from plasma and their Vero and furin-overexpressing Vero (Vero furin) cell culture clinical isolates. Neutralization curves for human mAbs were plotted using GraphPad Prism. Each data point is the mean of percent neutralization for two technical replicates in a single experiment. Error bars indicate the range of two technical replicates.
Mature Virions Are Poorly Neutralized by Cross-Reactive Fusion Loop Antibodies.
Cell culture-derived virions may be more sensitive than plasma virions to fusion loop antibodies because the fusion loop is more exposed in immature cell culture virions compared with plasma virions. To test this hypothesis, we used the DENV1 clinical isolate from patient SJT008 to infect regular and furin protease-overexpressing Vero cells. The virions released from furin-overexpressing cells were more mature than virions released from regular Vero cells (SI Appendix, Fig. S6 A and B). Quaternary epitope human mAbs 14C10 and EDE2 B7 efficiently neutralized virions released by regular and furin-overexpressing Vero cells (Fig. 3 E and F). On the other hand, two human mAbs targeting conserved epitopes near the fusion loop efficiently neutralized virions released by regular Vero cells and failed to neutralize the mature virions released by the furin-overexpressing Vero cells, reverting the neutralization phenotype similar to that obtained from plasma virions (Fig. 3 G and H). These results support the hypothesis that DENVs produced on standard laboratory cells are hypersensitive to neutralization by some human cross-reactive antibodies because they are more immature compared with virions circulating in people.
Heterotypic DENV Human Immune Sera Poorly Neutralized Plasma DENV1.
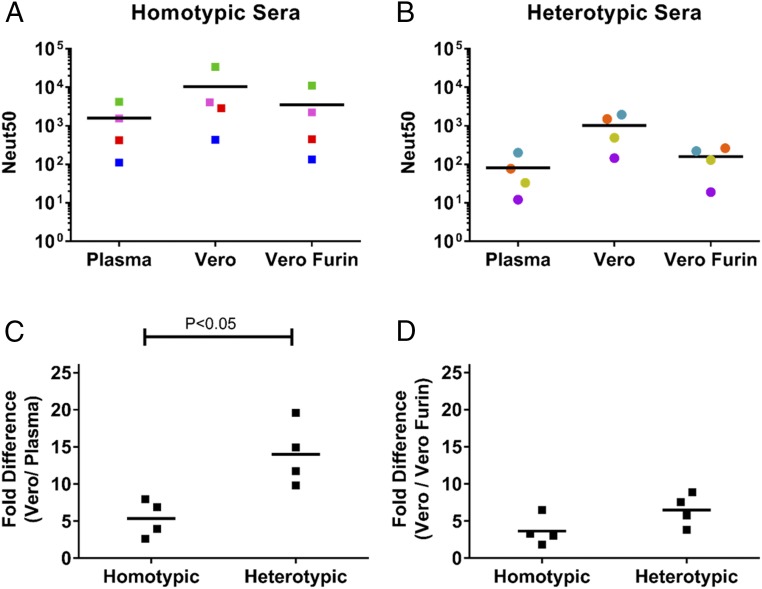
Next, we performed studies to compare the ability of human DENV immune sera to neutralize plasma and cell culture-derived virions. A panel of immune sera from people who had recovered from primary DENV1 infections (homotypic immune sera) or primary DENV2 or DENV3 infections (heterotypic immune sera) (SI Appendix, Table S3) was used for the experiments. The four homotypic immune sera strongly neutralized both the plasma [mean neutralization 50 (Neut50) = 1,588] and cell culture-derived (mean Neut50 = 10,306) DENV1 virions (Fig. 4A). On average, the homotypic immune sera neutralized the cell culture-derived virions about sixfold better than the plasma viruses (Fig. 4C). When heterotypic primary immune sera from DENV2 and DENV3 infected individuals were tested against DENV1, we observed strong neutralization of cell culture-grown P1 virus (mean Neut50 = 1,025) and very low to undetectable neutralization of plasma virus (mean Neut50 = 81) (Fig. 4B). On average, heterotypic immune sera neutralized cell culture virus 13-fold better than plasma virus (Fig. 4C). These results establish that a single passage in cell culture generates virions that are hypersensitive to neutralization by heterotypic primary immune sera. In contrast, the homotypic immune sera efficiently neutralized DENV1 virions irrespective of their human plasma or cell culture origin. When we controlled for maturation state by producing DENV1 in furin-overexpressing Vero cells, plasma and Vero (furin)-derived virions were equally sensitive to neutralization by human immune sera (Fig. 4 A, B, and D).
Fig. 4.
Neutralization ability of homotypic and heterotypic human immune sera against plasma and cell culture DENV1. Various dilutions of homotypic (SI Appendix, Fig. S7) and heterotypic (SI Appendix, Fig. S8) human DENV immune sera were used to neutralize DENV1 from SJT008 plasma and P1 isolate from Vero and Vero Furin cells. Neut50 values for four homotypic (A) and four heterotypic (B) sera were plotted, followed by fold differences in Neut50 between Vero and plasma DENV1 (C), and fold difference in Neut50 between Vero and Vero Furin derived DENV1 (D). Colors represent a single serum. Lines indicates the mean. Two-way ANOVA was performed to compare the neutralization variations for plasma DENV1 versus Vero P1 isolate among homotypic and heterotypic dengue immune sera. Vero Furin, furin-overexpressing Vero.
DENV1 Produced in Humans and in Cell Culture Have Nearly Identical Genomes.
The structure of DENVs released from infected cells will depend on the genome of the virus as well as posttranslational processing events dictated by the infected cells. To determine if laboratory cell lines selectively amplified plasma virions that were genetically different from the dominant population circulating in people, we sequenced the entire viral genome present in two plasma samples and the corresponding P1 viruses amplified on Vero and C6/36 cells from two different sources. The consensus sequences of the plasma and P1 viruses were nearly identical, except for a few minority variants in cell culture P1 viruses that did not map to DENV1 structural proteins (SI Appendix, Table S4). Based on this analysis, we conclude that the structural differences between DENV1 virions circulating in people and after a single passage on cell lines are caused by the actual infected target cells producing virions, and not viral genetic differences.
Discussion
We have demonstrated that a single passage in cell culture has a major impact on the structural and functional properties of DENVs circulating in people. DENVs in human plasma were fully mature, whereas most virions released by laboratory cell lines were immature and contained unprocessed prM protein on the surface. Vogt et al. (19) have reported that West Nile virus (WNV) circulating in mice was more mature than virions produced by C6/36 insect cells. In the same study, the investigators noted that WNV generated in Vero cells had a mature phenotype that was similar to virions circulating in mice. Therefore, WNV appears to be different from DENV1 with respect to the efficiency of prM processing in Vero cells. It is well documented that mature virions are more infectious than partially or fully immature virions because unprocessed prM bound to E protein inhibits viral fusion required to infect cells (20). In addition to maturation state, other factors such as the lipid composition, posttranslational modifications (including addition and processing of N-linked glycans), and binding of human plasma factors to virions may contribute to the observed differences in specific infectivity between human plasma and cell culture DENVs.
Virions in human plasma and cell culture had nearly identical genome sequences, excluding strong selective pressure as an explanation for phenotypic differences. Laboratory cell lines are likely to release immature virions because the robust replication of DENVs will overwhelm the host furin protease in the Golgi that cleaves prM to generate mature virions. In support of this model, we observed that Vero cells engineered to overexpress furin released DENVs that were more mature than DENVs released from regular Vero cells. While the precise cells that produce DENVs in people have not been defined well, the level of viral replication and furin protease expression in human target cells may lead to efficient prM cleavage and only the release of mature virions. In people, DENVs have been observed in various organs such as the spleen, lymph nodes, liver, and lungs and found to infect phagocytes (including blood monocytes, tissue macrophages, and dendritic cells) and hepatocytes (reviewed in refs. 21, 22). In a recent comprehensive histological study of tissues from 13 fatal cases of DHF/dengue shock syndrome (DSS) in children, DENV infection was confirmed in hepatocytes and Kupffer cells in the liver and in macrophage-like cells in spleens and lymph nodes (23). Within germinal centers, the investigators noted a reduction in lymphocytes and an increase in eosinophilic deposits containing dengue antigens, immunoglobulins, and complement components. Recently, investigators have also reported on the presence of atypical DENVs present in infectious vesicles derived from CD61+ cells of the megakaryocyte lineage in human plasma (24, 25). Further studies are needed to fully define the human target cells.
We observed that human plasma and cell culture-derived DENV1 virions were differentially neutralized by human antibodies. DENV cross-reactive, fusion loop-directed mAbs and heterotypic immune sera (from primary DENV2 and DENV3 patients) efficiently neutralized cell culture viruses but not the human plasma viruses. Indeed, several groups have demonstrated that the maturation state of virions produced on laboratory cell lines has a strong impact on the ability of some cross-reactive mAbs to neutralize DENVs (11–13). Moreover, Mukherjee et al. (11) demonstrated that the DENV maturation state has a strong impact on the ability of some DENV vaccine-immune sera to neutralize DENVs as well. The fusion loop on EDII, a major target of cross-reactive antibodies, is well exposed on immature virions (26). In contrast, the fusion loop is hidden in the head-to-tail–oriented E protein homodimers that cover the surface of mature virions circulating in human plasma (6). We conclude that poor exposure of the fusion loop on mature plasma virions renders them less sensitive to neutralization by cross-reactive mAbs and heterotypic DENV immune sera.
DENV1 type-specific mAbs and DENV1 homotypic immune sera (from primary DENV1 cases) strongly neutralized both human plasma and cell culture-derived virions. However, even with DENV1 type-specific antibodies, we observed that cell culture-grown immature virions were more sensitive to neutralization than mature human plasma virions. This result was anticipated because immature virions contain fewer fusion-competent E protein homodimers than fully mature virions, leading to a lower threshold of neutralization by type-specific mAbs. Compared with mature DENV1 particles, immature particles would be more sensitive to neutralization by homotypic DENV1 immune sera because of the exposure of the fusion loop recognized by cross-reactive antibodies and the reduced number of fusion-competent E molecules on immature virions. The most important finding relevant to protective immunity in people was that DENV1 particles in human plasma were efficiently neutralized by type-specific human mAbs and homotypic DENV1 immune sera and poorly neutralized by fusion loop mAbs and heterotypic human immune sera.
The highly successful yellow fever and Japanese encephalitis vaccines establish the importance of vaccination for protecting people from flavivirus infections. For both of these attenuated live-virus vaccines, neutralizing antibodies are strongly correlated with vaccine efficacy. In addition to antibody responses, a single dose of yellow fever and Japanese encephalitis vaccines augments effective T cell immunity (27–29). DENV vaccines faced a major setback recently in clinical trials when people developed vaccine breakthrough infections despite developing relatively high levels of neutralizing antibodies (14). Our results here provide an explanation for the failure of cell culture virus neutralizing antibodies to predict DENV vaccine efficacy. Laboratory propagated, partially immature DENV virions will overestimate levels of protective antibodies because of their sensitivity to heterotypic neutralizing antibodies. Indeed, we recently demonstrated that a leading tetravalent live-attenuated DENV vaccine with poor efficacy against DENV2 mainly induced heterotypic neutralizing antibodies against this serotype (30). Our results provide a strong rationale for using fully mature virions that better represent the structure of virions circulating in people when evaluating vaccine immunogenicity and efficacy.
In this study, we establish the strong impact of cell culture passage on the structure of DENVs and a rationale for developing more relevant assays for studying pathogenesis and assessing vaccine performance. Our study was performed using multiple patients and isolates from a single DENV1 epidemic in Sri Lanka. By focusing on a single epidemic and also sampling primary cases within the first 4 d after onset of symptoms, we were able to control for other variables such as viral serotype and genotype, immune status of the host, and the effect of preexisting antibodies on circulating virions. Further studies are needed to compare people experiencing primary or secondary DENV infections with different DENV serotypes and genotypes to more broadly assess the impact of virion structure on human disease and vaccine performance.
Methods
Patient Blood Plasma.
Blood samples were collected from patients suspected of having DF in a clinical setting during the 2014 Sri Lankan DF epidemic. More information is provided in SI Appendix, SI Methods. Ethical approval for this research was obtained from the Ethical Review Committee of the Faculty of Medicine, University of Colombo, Sri Lanka (serving as the institutional review board for Genetech Research Institute). The University of North Carolina (UNC) Institutional Review Board determined that its approval was not required because participating UNC investigators were not involved in human subject research. Only children whose parents or legal guardians provided written informed consent were enrolled in the study.
Cells Lines and Viruses.
Detailed information is provided in SI Appendix, SI Methods.
Isolation of Virus from Clinical Samples.
DENV1 clinical isolates were made in C6/36, Vero, and furin-overexpressing Vero cells by inoculating virus from plasma samples. More information is provided in SI Appendix, SI Methods.
Digital Drop PCR.
RNA copies of DENV1 were detected in blood plasma samples and cell culture isolates using digital drop PCR. More information is provided in SI Appendix, SI Methods.
Vero Infectious Titer Assay.
DENV1 from plasma and cell culture isolates were serially diluted and infected on Vero cells. Cells were stained after foci formation and counted manually. More information is provided in SI Appendix, SI Methods.
Antibody-Dependent Enhancement Assay.
Human anti-prM antibody was incubated with DENV1 from Vero cells, furin-overexpressing Vero cells, and plasma. The virus–antibody immune complex was infected on K562 cells and stained after 24 h to check infection. More information is provided in SI Appendix, SI Methods.
Depletion Assay.
We used 50 μL of Dynabeads Protein G magnetic beads (Novex) to bind 10 μg of 2H2, 1M7, and 8A1 mAbs according to the manufacturer’s protocol. Briefly, magnetic beads and mAbs were kept at room temperature for 1 h in a rotator for binding. Unbound mAbs were removed by applying the magnet and removing the supernatant. PBS was used to wash remaining unbound mAbs. About 50 infectious units of virus was captured in triplicate with mAb bound to magnetic beads by keeping them at room temperature for 1 h in a rotator. Unbound virus was used to infect a Vero cell monolayer grown for 48 h in a 24-well plate. Plates were incubated and stained as explained above. Undepleted control was kept for processing during the assay. An equal amount of normal human plasma was spiked in cell culture virus while performing the assay.
Neutralization Assay.
Various dilutions of mAbs or sera were incubated with DENV1 from plasma and cell culture isolates and infected on Vero cells. Cells were stained after foci formation and counted manually. More information is provided in SI Appendix, SI Methods.
Library Preparation and Sequencing Plasma and Cell Culture DENV1.
Viral RNA was isolated from human plasma samples and their cell culture isolates. Sequencing libraries were prepared, and sequencing was performed on Illumina systems. More information is provided in SI Appendix, SI Methods.
Supplementary Material
Acknowledgments
We thank all the patients who provided samples for the study. We also thank Dr. Ted Pierson (NIH) for providing the Vero cells overexpressing furin protease. We thank Caroline Pellegry, Bhumi Patel, and Alice Liou for their suggestions and/or technical help in optimizing the digital drop PCR, initial antibody depletion optimizations, and ELISA, respectively. We also thank Mathew Collins for his input in the initial stages of manuscript preparation. Training support (to K.S.C.) was provided by the NIH [University of North Carolina (UNC) Virology Training Grant T32AI007419 and Initiative for Minority Student Development Grant 5R25GM055336] and a UNC Graduate School’s off-campus dissertation fellowship. These studies were supported by National Institute of Allergy and Infectious Diseases Grants 1-R01-AI107731-01 (primary investigator A.M.d.S., UNC) and P01 AI106695 (primary investigator E. Harris, University of California at Berkeley; A.M.d.S. is the leader of project #2 of this NIH program project).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 17.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812055115/-/DCSupplemental.
References
- 1.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flipse J, Smit JM. The complexity of a dengue vaccine: A review of the human antibody response. PLoS Negl Trop Dis. 2015;9:e0003749. doi: 10.1371/journal.pntd.0003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 4.Halstead SB, et al. Dengue hemorrhagic fever in infants: Research opportunities ignored. Emerg Infect Dis. 2002;8:1474–1479. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slon Campos JL, Mongkolsapaya J, Screaton GR. The immune response against flaviviruses. Nat Immunol. 2018;19:1189–1198. doi: 10.1038/s41590-018-0210-3. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn RJ, et al. Structure of dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodenhuis-Zybert IA, Wilschut J, Smit JM. Partial maturation: An immune-evasion strategy of dengue virus? Trends Microbiol. 2011;19:248–254. doi: 10.1016/j.tim.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Pierson TC, Diamond MS. Degrees of maturity: The complex structure and biology of flaviviruses. Curr Opin Virol. 2012;2:168–175. doi: 10.1016/j.coviro.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowd KA, DeMaso CR, Pierson TC. Genotypic differences in dengue virus neutralization are explained by a single amino acid mutation that modulates virus breathing. MBio. 2015;6:e01559-15. doi: 10.1128/mBio.01559-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goo L, VanBlargan LA, Dowd KA, Diamond MS, Pierson TC. A single mutation in the envelope protein modulates flavivirus antigenicity, stability, and pathogenesis. PLoS Pathog. 2017;13:e1006178. doi: 10.1371/journal.ppat.1006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee S, et al. Enhancing dengue virus maturation using a stable furin over-expressing cell line. Virology. 2016;497:33–40. doi: 10.1016/j.virol.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowd KA, Mukherjee S, Kuhn RJ, Pierson TC. Combined effects of the structural heterogeneity and dynamics of flaviviruses on antibody recognition. J Virol. 2014;88:11726–11737. doi: 10.1128/JVI.01140-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouvinski A, et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature. 2015;520:109–113. doi: 10.1038/nature14130. [DOI] [PubMed] [Google Scholar]
- 14.Hadinegoro SR, et al. CYD-TDV Dengue Vaccine Working Group Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 15.de Alwis R, et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci USA. 2012;109:7439–7444. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dejnirattisai W, et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol. 2015;16:170–177. doi: 10.1038/ni.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SA, et al. The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. MBio. 2013;4:e00873-13. doi: 10.1128/mBio.00873-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teoh EP, et al. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med. 2012;4:139ra83. doi: 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 19.Vogt MR, et al. Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fcgamma receptor and complement-dependent effector mechanisms. J Virol. 2011;85:11567–11580. doi: 10.1128/JVI.05859-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu IM, et al. Association of the pr peptides with dengue virus at acidic pH blocks membrane fusion. J Virol. 2009;83:12101–12107. doi: 10.1128/JVI.01637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmid MA, Diamond MS, Harris E. Dendritic cells in dengue virus infection: Targets of virus replication and mediators of immunity. Front Immunol. 2014;5:647. doi: 10.3389/fimmu.2014.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun P, Kochel TJ. The battle between infection and host immune responses of dengue virus and its implication in dengue disease pathogenesis. Sci World J. 2013;2013:843469. doi: 10.1155/2013/843469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aye KS, et al. Pathologic highlights of dengue hemorrhagic fever in 13 autopsy cases from Myanmar. Hum Pathol. 2014;45:1221–1233. doi: 10.1016/j.humpath.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Hsu AY, et al. Infectious dengue vesicles derived from CD61+ cells in acute patient plasma exhibited a diaphanous appearance. Sci Rep. 2015;5:17990. doi: 10.1038/srep17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noisakran S, et al. Role of CD61+ cells in thrombocytopenia of dengue patients. Int J Hematol. 2012;96:600–610. doi: 10.1007/s12185-012-1175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beltramello M, et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monath TP. Yellow fever vaccine. Expert Rev Vaccines. 2005;4:553–574. doi: 10.1586/14760584.4.4.553. [DOI] [PubMed] [Google Scholar]
- 28.Hepburn MJ, et al. Neutralizing antibody response to booster vaccination with the 17d yellow fever vaccine. Vaccine. 2006;24:2843–2849. doi: 10.1016/j.vaccine.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 29.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2-3 September, 2004. Vaccine. 2005;23:5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Henein S, et al. Dissecting antibodies induced by a chimeric yellow fever-dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in naive and dengue-exposed individuals. J Infect Dis. 2017;215:351–358. doi: 10.1093/infdis/jiw576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.