Significance
Studying weak interactions in transition metal complexes is of interest from a fundamental perspective to advance our knowledge of chemical bonding. It is also crucial to gain better understanding of factors influencing the structure and properties of transition metal complexes and provides useful insight into their chemical reactivity. Here, we report the synthesis of gold(I) complexes featuring Au∙∙∙H–N interactions. Their bonding situation was thoroughly analyzed by a combination of experimental and computational means, and the presence of attractive Au∙∙∙H–N hydrogen bonds was authenticated.
Keywords: gold, hydrogen bonding, noncovalent interactions, relativistic effects
Abstract
The ability of gold to act as proton acceptor and participate in hydrogen bonding remains an open question. Here, we report the synthesis and characterization of cationic gold(I) complexes featuring ditopic phosphine-ammonium (P,NH+) ligands. In addition to the presence of short Au∙∙∙H contacts in the solid state, the presence of Au∙∙∙H–N hydrogen bonds was inferred by NMR and IR spectroscopies. The bonding situation was extensively analyzed computationally. All features were consistent with the presence of three-center four-electron attractive interactions combining electrostatic and orbital components. The role of relativistic effects was examined, and the analysis is extended to other recently described gold(I) complexes.
Hydrogen bonds are ubiquitous and play a major role in chemistry. In addition to the classical hydrogen-bond acceptors (namely, N, O, the halogens, S, and P), transition metals have been recognized to also participate in hydrogen bonding toward protonic H‒X fragments. Since the 1990s, such M∙∙∙H–X interactions have garnered great interest (1, 2). Numerous studies have been carried out to better understand this unusual bonding situation, to delineate the influence of M∙∙∙H‒X interactions on the structure and properties of transition metal complexes. It is also of note that M∙∙∙H‒X interactions are relevant to the protonation of transition metals to form metal hydrides (1, 3, 4). M∙∙∙H‒X interactions have been unambiguously authenticated both intra- and intermolecularly with various hydrogen-bond donor moieties (e.g., ammoniums, amides, and water) and electron-rich transition metals (mainly Pt and Co).
The case of gold is very singular and deserves special attention. Due to strong relativistic effects (5, 6), gold displays very different properties compared to other transition metals. It is a very peculiar element among the d block of the periodic table that has attracted considerable interest over the last two to three decades due to its recently discovered and unique efficiency in catalysis (7–11), both heterogeneous and homogeneous. Considerable efforts have also been involved in better understanding the structure and reactivity of gold compounds. In this respect, it is striking to note that the ability of gold to participate in hydrogen bonding still remains an open question, despite intense experimental and theoretical research in the last 12 y. The most relevant contributions in the field will be briefly presented here. For a comprehensive and authoritative state of the art, the reader is invited to refer to the review published in 2014 by Schmidbaur et al. (12).
In 2006, Nuss and Jansen (13) prepared and characterized by X-ray diffraction (XRD) an ammonia complex of the auride ion Au−. The presence of Au∙∙∙H–N hydrogen bonding was inferred based on the relatively short Au∙∙∙H distance [2.58(1) Å] and wide Au∙∙∙H–N angle [158(1)°]. A number of Au(I) and Au(III) complexes were also reported to display relatively short Au∙∙∙H distances in the solid state (2.3 to 3.0 Å), but identifying the precise location of H atoms in close proximity of such a heavy element as gold by XRD is intrinsically challenging (14). As emphasized in the review by Schmidbaur et al. (12), the observed Au∙∙∙H contacts are likely to result from factors other than hydrogen bonding (cation–anion interactions, crystal packing, etc.) and their attractive nature has not been substantiated. Last year, the existence of Au∙∙∙H–C interactions akin to hydrogen bonds (15–17) within divalent hexagold clusters stabilized by diphosphine ligands was discussed by Konishi and coworkers (18) based on the short Au∙∙∙H–C contacts observed by XRD (2.62 Å) and on the substantial downfield shift of the corresponding 1H and 13C NMR signals. Kryachko et al. (19–21) performed early computational studies on Au∙∙∙H–X (X = N, O, or F) hydrogen bonds in gold complexes and clusters. In addition, recent theoretical studies (22–24) combining geometry optimizations and bonding analyses have examined and supported the possible existence of Au∙∙∙H–X hydrogen bonding in Au(I) molecular complexes, stimulating and providing guidelines for forthcoming experimental studies: Esterhuysen and coworkers (22, 23) investigated extensively the interaction between anionic divalent gold(I) complexes and water, while Berger et al. (24) performed a detailed study on a neutral gold(I) complex with a chelated NH-pyridinium moiety.
The absence of compelling evidence for hydrogen bonding involving gold is widely recognized and well summarized by the following statements excerpted from recent authoritative contributions:
A screening of the literature on gold compounds in which the gold atoms may be involved in intra- and intermolecular hydrogen bonding has not produced consistent evidence for any major influences of interactions of the type Au∙∙∙H–X on the molecular structure and dynamics of the systems concerned [Schmidbaur et al. (12)].
Au∙∙∙H interactions, particularly in complexes, cannot be discussed with confidence as yet [Esterhuysen and coworkers (22)].
While certain classes of H-bonds to Au(I) coordination centers have been proposed…on the basis of quantum chemical calculations…, evidence from the experimental side is sparse at most and even the principal possibility of effective Au∙∙∙H hydrogen bonds is set into question [Berger et al. (24)].
Even now there are no examples of spectroscopically identified “hydrogen-bond type” Au∙∙∙H interactions [Konishi and coworkers (18)].
Two main challenges must be overcome to establish and authenticate hydrogen bonding in gold complexes. On the one hand, the inherently weak Au∙∙∙H–X interaction must be favored over other competitive bonding situations such as intermolecular Au∙∙∙Au aurophilic interactions (25) and/or hydrogen bonding of the H–X moiety with electron-rich sites of the ligands surrounding the metal (26). On the other hand, conspicuous evidence for the existence of the Au∙∙∙H–X bond should be obtained, in addition to the observation of short Au∙∙∙H contacts in XRD analysis.
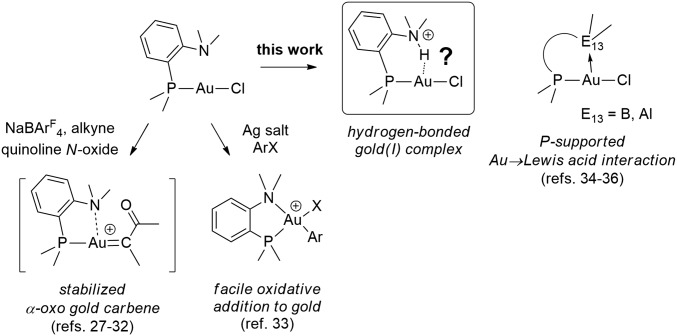
Based on recent developments in gold chemistry, we reasoned that (P,N) ligands such as ortho-amino phenylphosphines (DalPhos) may be good candidates and give access to hydrogen-bonded complexes (Fig. 1). The hemilabile character of the DalPhos ligands has been shown by Zhang and coworkers (27–32) to efficiently temper the reactivity of α-oxocarbenes. In addition, our group recently demonstrated the feasibility and easiness of oxidative addition to gold thanks to such (P,N) ligands [the hard N center stabilizes the Au(III) product and lowers the activation barrier] providing a new pathway for Au(I)/Au(III) catalysis (33). Due to its basic character, the nitrogen center also gives the opportunity to form an ammonium with a protonic N–H bond in close proximity to gold(I). As reported herein, this strategy proved fruitful and enabled us to form and authenticate hydrogen-bonded gold(I) complexes. It is worthwhile to note the structural analogy existing between the target compounds and the Au complexes deriving from (P,B) and (P,Al) ambiphilic ligands we reported previously, in which the Lewis acid moiety coordinates as a σ-acceptor Z-type ligand, with Au acting as a Lewis base (34–36).
Fig. 1.
Gold complexes deriving from chelating (P,N) and ambiphilic (P,E13) ligands.
Results and Discussion
Synthesis and Characterization of the Cationic Gold Complex 2.
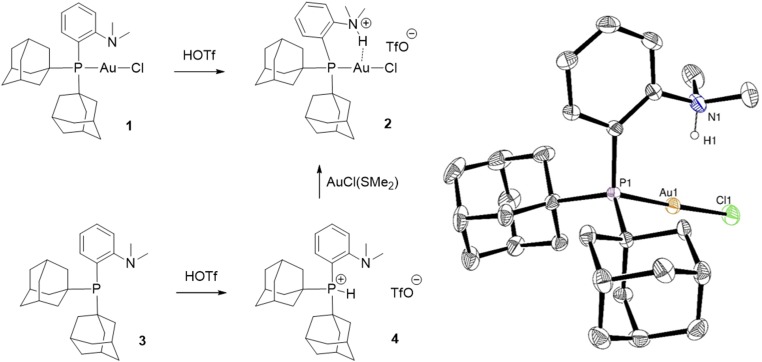
Addition of one equivalent of trifluoromethane sulfonic acid (HOTf) to the MeDalPhos gold complex 1 immediately leads to a new complex 2 according to 31P NMR spectroscopy (Scheme 1), and 1H NMR unambiguously indicates protonation of the nitrogen atom. A broad N–H signal appears at δ 10.9 ppm, while the N(CH3)2 signal is shifted to low field by about 1 ppm and resonates as a doublet with a 3JH–H coupling constant of 5.0 Hz. Of note, complex 2 can also be prepared by protonating the (P,N) ligand 3 before coordination to gold. Upon addition of HOTf, the phosphorus atom, not the nitrogen, is protonated, and the P–H phosphonium salt 4 is obtained. Diagnostic NMR features are the low-field shift of the 31P NMR signal (δ 17.5 ppm), the large 1JP–H coupling constant (485.3 Hz), and the absence of low-field shift of the N(CH3)2 1H NMR signal. As shown by XRD (SI Appendix), the same tautomer is present in the solid state. Despite the protonation of phosphorus, compound 4 reacts rapidly with AuCl(SMe2) to give complex 2. The proton shifts from P to N and the P atom coordinates to gold, displacing the SMe2 ligand. Density functional theory (DFT) calculations predict that protonation of the P atom of 3 is slightly more favored thermodynamically (ΔG = 1.6 kcal/mol) than that of the N atom, and that the activation barrier for the proton shift from P to N is fairly accessible at room temperature (ΔG≠ = 7.7 kcal/mol) (SI Appendix).
Scheme 1.
Synthesis (two routes) and X-ray structure of the (P,NH+) gold(I) complex 2 derived from MeDalPhos. Thermal ellipsoids are drawn at 50% probability; the TfO− counteranion and H atoms, except for that on nitrogen, are omitted for clarity. Selected bond lengths (Å) and bond angles (°): P–Au 2.270(1), Au–Cl 2.283(1), Au∙∙∙H 2.24(3), P–Au–Cl 176.98(2), N–H∙∙∙Au 165(2).
Crystals of 2 were grown from a dichloromethane/pentane solution at −30 °C and analyzed by XRD (SI Appendix). The H atom at N was located in the difference Fourier map and refined freely. There is no interaction between the acidic proton at N and the TfO− counteranion (shortest distance, >4.4 Å); compound 2 adopts a separated ion-pair structure. The N–H bond points toward Au, and the N–H∙∙∙Au skeleton is close to linear [165(2)°]. The Au∙∙∙H distance [2.24(3) Å] is well within the sum of van der Waals radii (2.86 Å) (37) and falls in the very low range of those previously reported (12). As pointed out previously, short N–H∙∙∙Au contacts are not indicative of attractive interactions, and the associated metrical parameters should be considered with caution (14).
Multinuclear NMR spectroscopy confirms the general connectivity, and according to 2D 1H–1H NOESY experiments, complex 2 adopts in solution the same conformation as in the solid state (the adamantyl groups at P and the Me groups at N show correlations with the adjacent H atoms of the ortho-phenylene spacer). The very high 1H NMR chemical shift of the N–H proton (10.9 ppm) is typical of hydrogen bonding (1, 2, 38). Spin–spin coupling has also been inferred as a characteristic of hydrogen-bonding M → H–X interactions, although such couplings have been measured only very rarely. The 1JN–H coupling in complex 2 was determined precisely at natural 15N abundance via a 1H–15N HSQC experiment using a homonuclear band selective detection scheme (SI Appendix). The obtained value (69.7 Hz) is very close to that reported by Pregosin et al. (39) and van Koten and coworkers (40) for a Pt(II) complex featuring an intramolecularly hydrogen-bonded NMe2H+ moiety.
IR spectroscopy can also be a useful analytical probe. For example, a significant decrease of the O–H stretching frequency was reported for the hydrogen bonding of perfluoroalcohols H‒ORf to Co, Rh, and Ir (3). Recent computational studies suggest that gold may follow a similar trend (24, 41). No characteristic band was detected for the N–H stretch of 2. Considering that it may be masked by the C–H stretches, we turned to the deuterium-labeled complex 2-D, which was readily prepared using DOTf (SI Appendix). A N–D band appeared at 2,124 cm−1 in the IR spectrum, corresponding to a N–H stretch at about 3,000 cm−1. This is 500 cm−1 lower than typical wavenumbers for free N–H (ammonium) oscillators. This shift confirms the presence of Au∙∙∙H–N bonding in solution and provides direct spectroscopic evidence for such hydrogen bonding.
To probe the chemical influence of the Au∙∙∙H–N interaction in complex 2, we then examined the possibility of proton transfer. Mixing the neutral gold(I) complex 1 with its protonated form 2 in solution give two well-resolved 31P NMR signals at room temperature, indicating slow, if any, proton transfer and chemical exchange between 1 and 2 at the NMR timescale. Cross-peaks in 31P/31P exchange spectroscopy experiments indicate that there is some chemical exchange, but it is slow at the NMR timescale, with no coalescence and even no broadening of the NMR signals being observed upon heating at 45 °C (SI Appendix). The gold complex 2 behaves very differently than the corresponding anilinium salt PhNMe2H,OTf for which proton exchange between the base and acid forms is fast by NMR at room temperature and even at low temperature (a unique set of 1H NMR signals is observed for the two species down to −70 °C).
Computational Studies of the Cationic Gold Complex 2: Geometry Optimization and Bonding Analysis.
A comprehensive theoretical study was performed to analyze the bonding situation in the gold complex 2 (SI Appendix). The main objective of these quantum chemical calculations was to establish unambiguously the presence of an attractive N–H∙∙∙Au interaction and to determine its precise nature.
Geometry optimization.
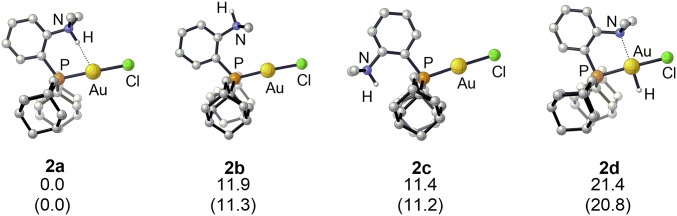
The B3PW91/SDD+f(Au),6-31G**(other atoms) level of theory was used, and solvent effects (CH2Cl2) were taken into account by means of the continuum standard solvation model based on density. Calculations were first carried out on the naked cations. Several minima were located on the potential energy surface (Fig. 2, Table 1, and SI Appendix, Table S2). The ground-state structure 2a parallels that observed by XRD, with the N–H bond pointing toward Au. Two other minima, 2b and 2c, correspond to conformers associated with rotations around the CPh‒N and CPh‒P bonds. Another local minimum is the square-planar gold(III) hydride 2d, in which the N center is coordinated to gold; 2d results from oxidative addition of the N–H bond to gold and is reminiscent of the gold(III) pincer hydride complex recently reported by Bezuidenhout and coworkers (42). In 2a, the H atom at N enters the coordination sphere of gold (Au∙∙∙H = 2.134 Å) with a quasi-linear N‒H∙∙∙Au arrangement (174.2°), in line with that expected for hydrogen bonding. Comparison of the metrical data shows that the N‒H bond is noticeably elongated upon interaction with gold (1.046 Å in 2a versus 1.024 to 1.025 Å in 2b,c). The conformers 2b and 2c are located 11 to 12 kcal/mol higher in energy than 2a, which suggests some stabilization of 2a thanks to N–H∙∙∙Au interaction. The gold(III) hydride 2d is even higher in energy, about 21 kcal/mol above 2a. Inclusion of TfO− in the calculations induces only small changes in the optimized geometries and relative energies of the gold(I) conformers, indicating that the counteranion has little influence (SI Appendix, Table S4). Relativistic effects are known to profoundly influence the properties of gold (5, 24, 43). To assess their impact on the gold–ammonium interaction in 2a, Amsterdam Density Functional calculations were performed at the COSMO(DCM)-BP86/TZ2P//B3PW91/SDD+f(Au),6-31G**(other atoms) level of theory. When relativistic effects were not taken into account for the geometry optimization (calculation carried out without zeroth-order regular approximation), the N–H bond departs from Au and strengthens (SI Appendix, Table S3), reflecting their importance in the structure of 2a. In turn, this shows that the N–H∙∙∙Au interaction is assisted by chelation but not imposed geometrically in 2a.
Fig. 2.
Energy minima located on the potential energy surface of complex 2. Relative energies in kcal/mol (ΔG values, with ΔE values in brackets).
Table 1.
Key geometric features and spectroscopic data for the three conformers, 2a–c
| Geometric and spectroscopic data | Conformer | ||
| 2a | 2b | 2c | |
| P‒Au, Å | 2.303 | 2.304 | 2.305 |
| Au‒Cl, Å | 2.338 | 2.344 | 2.339 |
| N‒H, Å | 1.046 | 1.024 | 1.025 |
| Au∙∙∙H, Å | 2.134 | —* | — |
| P‒Au‒Cl, ° | 178.53 | 176.51 | 178.14 |
| N‒H∙∙∙Au, ° | 174.18 | — | — |
| νNH, cm−1 | 3,032 | 3,457 | 3,445 |
| ΔνNH with PhNMe2H+ | −443 | −18 | −30 |
| δ 1H NMR, N–H ppm | 11.94 | 5.64 | 6.31 |
—, not relevant.
Spectroscopic data.
The 1H NMR chemical shifts and IR stretching frequencies computed for the three gold(I) conformers (2a–c) corroborate the experimental assignment and confirm the presence of a N–H∙∙∙Au hydrogen-bond interaction in 2a (Table 1). Upon interaction with gold, the 1H NMR resonance signal for the N–H moiety is predicted to shift from 5.6 to 6.3 ppm for 2b,c to 11.9 ppm for 2a, in good agreement with the signal observed at 10.9 ppm experimentally. The N–H stretches computed for 2b and 2c are very similar in energy to that of the anilinium PhNMe2H+ (∼3,500 cm−1), while that of 2a is about 500 cm−1 red shifted, indicating substantial weakening of the N–H bond upon interaction with gold (24, 41). For completeness, the N–D stretch for the deuterium-labeled 2-D was also computed (2,089 cm−1). The obtained value matches quite well with that determined experimentally (2,124 cm−1).
Bonding analysis.
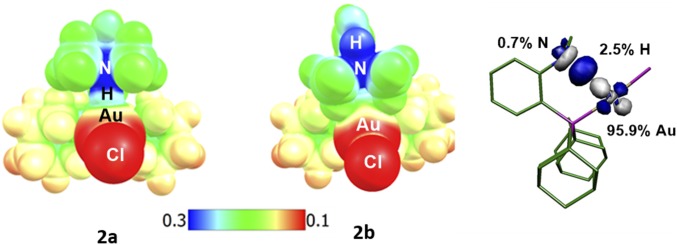
The bonding situation in 2a was then analyzed using various methods. Natural bond orbital (NBO) analyses (SI Appendix, Table S6) substantiate the weakening of the N–H bond upon interaction with gold. The Wiberg bond index (WBI) of the N–H bond decreases from 0.71 to 0.73 in 2b,c to 0.61 in 2a, while the WBI for the Au∙∙∙H interaction in 2a is small but significant (0.124). In the meantime, the computed natural population analysis (NPA) charges show some charge transfer from the metal fragment to the ammonium moiety: the charge for the PAuCl fragment increases from 0.65 to 0.71 in 2b,c to 0.76 in 2a, while the charge for the NMe2H fragment decreases from 0.66 to 0.68 in 2b,c to 0.61 in 2a. This charge transfer is also apparent from the electrostatic potential maps. Side views of 2a and 2b are displayed in Fig. 3, Left. The ammonium moiety is located in a positive (blue) area in both cases, but when hydrogen bonds to gold, its charge is reduced and the surface is lighter.
Fig. 3.
Electrostatic potential maps for 2a (Left, with the N–H bond pointing and hydrogen-bonded toward Au) and 2b (Center, with the N–H bond pointing opposite to Au) plotted over the range 0.1 a.u. (red) to 0.3 a.u. (blue). The isosurfaces are drawn at 0.002 e.a.u.−3. (Right) Superposition of the donor and acceptor NBO orbitals (cutoff = 0.08) involved in the N–H∙∙∙Au interaction; percent participation of each atom in the associated natural localized molecular orbital is noted.
Besides this electrostatic component, the NBO analysis revealed some orbital contribution to the N–H∙∙∙Au interaction. A weak donor–acceptor interaction [delocalization energy ΔE(2) = 12.8 kcal/mol] between an occupied d(Au) orbital and the σ*(N–H) orbital was found at the second-order perturbation theory (Fig. 3, Right). This picture is consistent with a 3c–4e interaction, as inferred for hydrogen-bonded complexes and as opposed to the 3c–2e interaction involved in agostic complexes (1, 44).
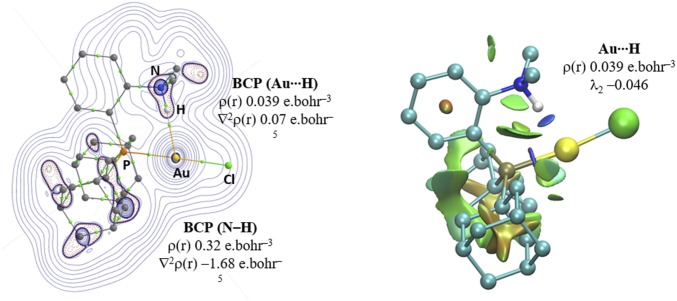
We then turned to atoms in molecules (AIM) analysis. This electron density-based theoretical method is very powerful to analyze chemical bonding, including weak interactions. It has been recently complemented with noncovalent interaction (NCI) plots to detect, visualize, and authenticate NCIs, in particular hydrogen bonds (45). These methods have been used to probe and actually support theoretically the possibility for water to form hydrogen bonds with anionic and neutral gold(I) complexes (22). The electron density at the N–H bond critical point (BCP) is smaller for 2a than for the two conformers 2b,c (ρ = 0.319 versus 0.343 e.bohr−3), in line with the bond weakening delineated by IR and NBO. In addition, the ground-state structure shows a BCP between the Au and H atoms, with ρ = 0.039 e.bohr−3 (see Fig. 4, Left for the Laplacian of the electron density map). The Laplacian is positive (0.068 e.bohr−3) and the second Hessian eigenvalue λ2 is negative (−0.046), in line with bonding noncovalent Au∙∙∙H interaction. This picture is corroborated by the NCI plot (Fig. 4, Right). The large and negative value of sign(λ2)ρ between Au and H is indicative of attractive NCI and is consistent with N–H∙∙∙Au hydrogen bonding (45).
Fig. 4.
(Left) Contour plot of the Laplacian distribution ∇2ρ(r) for 2a with relevant bond paths and BCPs (green spheres). Hydrogen atoms have been omitted for clarity except for that on the nitrogen atom. (Right) NCI plot for 2a. Gradient isosurface (s = 0.5 a.u.) colored according to a blue-green-red scheme over the range of −0.05 < sign (λ2)ρ < 0.05 a.u.). Blue indicates strong attraction, green indicates very weak interaction, and red indicates strong repulsion.
This detailed analysis thus provides compelling evidence for the presence of N–H∙∙∙Au hydrogen bonding in 2a. The computational methods and obtained results complete each other and are all consistent with a 3c–4e attractive interaction. Most diagnostic are the Au → NH+ transfer of electron density and the weakening of the N–H bond.
Generalization: Preparation and Bonding Analysis of Other Gold(I) Complexes.
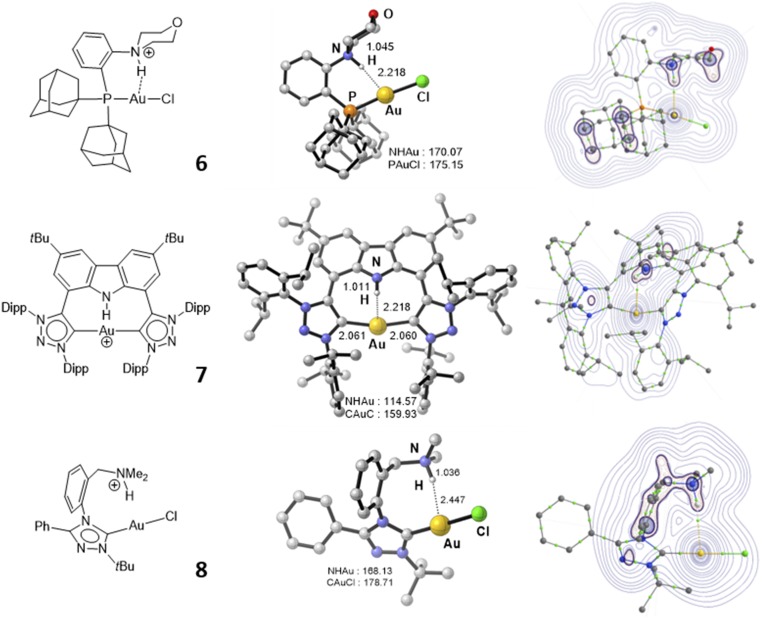
To complete the study, we then aimed to generalize the bonding situation of 2a to other gold complexes. First, we turned to the MorDalPhos ligand 5 with a morpholine instead of a dimethylamine group next to phosphorus. The corresponding cationic gold complex 6 (Fig. 5) was prepared and characterized by multinuclear NMR spectroscopy (SI Appendix). Exchange of the TfO counteranion for the tetraarylborate BArF4 [ArF = 3,5-(F3C)2C6H3] enables the growth of crystals suitable for XRD analysis. The proton at N resonates at low field in 1H NMR (11.4 ppm). As apparent from the X-ray structure (SI Appendix), complex 6 adopts the same conformation as 2. The N–H bond points toward Au [with a quasi-linear N–H∙∙∙Au arrangement, 174(8)°] and the H∙∙∙Au distance is quite short [2.27(2) Å]. DFT calculations (SI Appendix) support further the analogy between 2 and 6. The ground-state structure of the MorDalPhos complex 6 is that observed experimentally. Its geometric and spectroscopic features are very similar to those of 2 (SI Appendix, Table S5). Bonding analysis indicates the presence of Au∙∙∙H–N hydrogen bonding, whose magnitude is slightly weaker than for the MeDalPhos complex 2 according to NBO and AIM analyses (compare the numerical values of the WBI, NPA charges, second-order NBO delocalization energies, and electron density at BCPs; SI Appendix, Table S6).
Fig. 5.
Chemdraw structures, optimized geometries (distances in Å, angles in °), and calculated electron density maps (Laplacian plot) of the gold(I) complexes 6, 7, and 8.
In addition, two gold(I) complexes susceptible to display Au∙∙∙H hydrogen bonding were identified from recent literature (Fig. 5). Complex 7 was characterized by NMR and considered, but ruled out, as intermediate in the formation of a pincer Au(III) hydride complex (42). Complex 8 was envisioned as possible subunit in the assessment of aurophilic binding energies in the gas phase by mass spectrometry, without further identification of Au∙∙∙H–N hydrogen-bonding attractive interaction (41). According to the optimized geometries (SI Appendix), the H atom at N enters the coordination sphere of Au in both cases, with computed H∙∙∙Au distances of 2.22 and 2.45 Å, respectively. NBO and AIM analyses do support the presence of some hydrogen-type bonding (SI Appendix), albeit substantially weaker than those observed in the (P,NH+) gold complexes derived from the Me and MorDalPhos ligands.
Conclusion
By simple protonation of a ditopic (P,N) ligand, the cationic complex 2 featuring short contact between the gold(I) center and the proximal NH+ ammonium moiety was prepared. The presence of Au∙∙∙H–N hydrogen bonding was delineated experimentally by NMR, IR, and XRD. The bonding situation was confirmed and further assessed computationally. Geometry optimization, exploration of the potential energy surface, and thorough analysis of the Au∙∙∙H–N interaction provided compelling evidence for noncovalent attractive hydrogen-type bonding. Similar situations were authenticated in other gold(I) complexes, demonstrating that 2 is not a unique case.
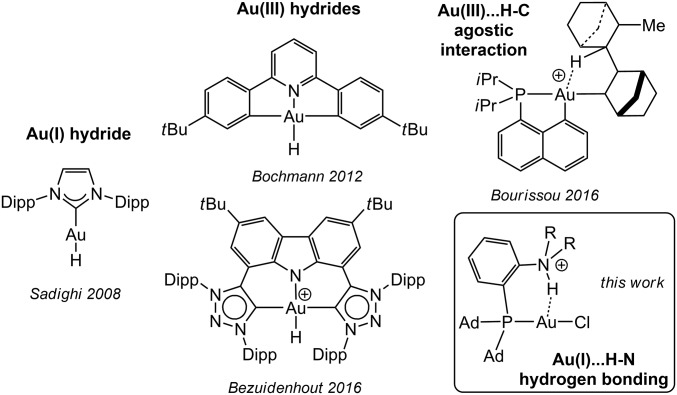
The hydrogen bonding evidenced here represents a type of gold–hydrogen interaction complementary to covalent Au–H bonds (gold hydrides) (42, 46–49) and Au∙∙∙H–C agostic interactions (50, 51) that have been recently authenticated and attract much interest (Fig. 6). This work contributes to further advance our knowledge of gold chemistry, which, despite recent advances (12, 52–57), remains far behind that of the other transition metals in terms of structures and reactivity.
Fig. 6.
Different types of molecular gold complexes featuring Au∙∙∙H interactions authenticated so far [selected examples for gold(III) hydrides].
Methods and Data
Experimental procedures, analytical data including NMR spectra, and crystallographic and computational details (including the Cartesian coordinates of the computed structures) are provided in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported financially by the Centre National de la Recherche Scientifique and the Université de Toulouse. Université de Pau et des Pays de l’Adour, Mésocentre de Calcul Intensif Aquitain, and Centre Informatique National de l’Enseignement Supérieur, under allocation A003080045 made by Grand Equipement National de Calcul Intensif, are acknowledged for computational facilities. E.D.S.C. thanks Communauté d’Agglomération Pau Pyrénées for funding part of his postdoctoral contract.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom, www.ccdc.cam.ac.uk (accession nos. CCDC-1868951–CCDC-1868953)
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817194116/-/DCSupplemental.
References
- 1.Brammer L. Metals and hydrogen bonds. Dalton Trans. 2003:3145–3157. [Google Scholar]
- 2.Martín A. Hydrogen bonds involving transition metal centers acting as proton acceptors. J Chem Educ. 1999;76:578–583. [Google Scholar]
- 3.Kazarian SG, Hamley PA, Poliakoff M. Is intermolecular hydrogen-bonding to uncharged metal centers of organometallic compounds widespread in solution? A spectroscopic investigation in hydrocarbon, noble gas, and supercritical fluid solutions of the interaction between fluoro alcohols and (η5-C5R5)ML2 (R = H, Me; M = Co, Rh, Ir; L = CO, C2H4, N2, PMe3) and its relevance to protonation. J Am Chem Soc. 1993;115:9069–9079. [Google Scholar]
- 4.Vedernikov AN, Caulton KG. N-PtIV-H/N-H...PtII intramolecular redox equilibrium in a product of H-C(sp2) cleavage and unusual alkane/arene C-H bond selectivity of ([2.1.1]pyridinophane)PtII(CH3)+ Chem Commun (Camb) 2003:358–359. doi: 10.1039/b207797n. [DOI] [PubMed] [Google Scholar]
- 5.Gorin DJ, Toste FD. Relativistic effects in homogeneous gold catalysis. Nature. 2007;446:395–403. doi: 10.1038/nature05592. [DOI] [PubMed] [Google Scholar]
- 6.Pyykkö P. Theoretical chemistry of gold. Angew Chem Int Ed Engl. 2004;43:4412–4456. doi: 10.1002/anie.200300624. [DOI] [PubMed] [Google Scholar]
- 7.Hashmi ASK, Hutchings GJ. Gold catalysis. Angew Chem Int Ed Engl. 2006;45:7896–7936. doi: 10.1002/anie.200602454. [DOI] [PubMed] [Google Scholar]
- 8.Hashmi ASK, Toste DF. Modern Gold Catalyzed Synthesis. Wiley-VCH; Weinheim, Germany: 2012. [Google Scholar]
- 9.Toste FD, Michelet V, editors. 2014. Gold Catalysis: An Homogeneous Approach, Catalytic Science Series (Imperial College Press, London), Vol 13.
- 10.Hutchings GJ. Heterogeneous gold catalysis. ACS Cent Sci. 2018;4:1095–1101. doi: 10.1021/acscentsci.8b00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciriminna R, Falletta E, Della Pina C, Teles JH, Pagliaro M. Industrial applications of gold catalysis. Angew Chem Int Ed Engl. 2016;55:14210–14217. doi: 10.1002/anie.201604656. [DOI] [PubMed] [Google Scholar]
- 12.Schmidbaur H, Raubenheimer HG, Dobrzańska L. The gold-hydrogen bond, Au-H, and the hydrogen bond to gold, Au∙∙∙H-X. Chem Soc Rev. 2014;43:345–380. doi: 10.1039/c3cs60251f. [DOI] [PubMed] [Google Scholar]
- 13.Nuss H, Jansen M. [Rb([18]crown-6)(NH3)3]Au·NH3: Gold as acceptor in N—H∙∙∙Au− hydrogen bonds. Angew Chem Int Ed Engl. 2006;45:4369–4371. doi: 10.1002/anie.200601093. [DOI] [PubMed] [Google Scholar]
- 14.Lusi M, Barbour LJ. Determining hydrogen atom positions for hydrogen bonded interactions: A distance-dependent neutron-normalized method. Cryst Growth Des. 2011;11:5515–5521. [Google Scholar]
- 15.Kraus F, Schmidbaur H, Al-juaid SS. Tracing hydrogen bonding Au···H-C at gold atoms: A case study. Inorg Chem. 2013;52:9669–9674. doi: 10.1021/ic4014762. [DOI] [PubMed] [Google Scholar]
- 16.Schaper L-A, et al. Gold(I) complexes with “normal” 1,2,3-triazolylidene ligands: Synthesis and catalytic properties. Organometallics. 2013;32:3376–3384. doi: 10.1021/ic400533u. [DOI] [PubMed] [Google Scholar]
- 17.Koskinen L, Jääskeläinen S, Kalenius E, Hirva P, Haukka M. Role of C–H···Au and aurophilic supramolecular interactions in gold–thione complexes. Cryst Growth Des. 2014;14:1989–1997. [Google Scholar]
- 18.Bakar MA, Sugiuchi M, Iwasaki M, Shichibu Y, Konishi K. Hydrogen bonds to Au atoms in coordinated gold clusters. Nat Commun. 2017;8:576. doi: 10.1038/s41467-017-00720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kryachko ES, Karpfen A, Remacle F. Nonconventional hydrogen bonding between clusters of gold and hydrogen fluoride. J Phys Chem A. 2005;109:7309–7318. doi: 10.1021/jp052460q. [DOI] [PubMed] [Google Scholar]
- 20.Kryachko ES, Remacle F. The gold-ammonia bonding patterns of neutral and charged complexes Aum0±1–(NH3)n. I. Bonding and charge alternation. J Chem Phys. 2007;127:194305. doi: 10.1063/1.2786996. [DOI] [PubMed] [Google Scholar]
- 21.Kryachko ES. Where gold meets a hydrogen bond? J Mol Struct. 2008;880:23–30. [Google Scholar]
- 22.Groenewald F, Dillen J, Raubenheimer HG, Esterhuysen C. Preparing gold(I) for interactions with proton donors: The elusive [Au]⋅⋅⋅HO hydrogen bond. Angew Chem Int Ed Engl. 2016;55:1694–1698. doi: 10.1002/anie.201508358. [DOI] [PubMed] [Google Scholar]
- 23.Groenewald F, Raubenheimer HG, Dillen J, Esterhuysen C. Gold setting the “gold standard” among transition metals as a hydrogen bond acceptor–A theoretical investigation. Dalton Trans. 2017;46:4960–4967. doi: 10.1039/c7dt00329c. [DOI] [PubMed] [Google Scholar]
- 24.Berger RJF, Schoiber J, Monkowius U. A relativity enhanced, medium-strong Au(I)···H-N hydrogen bond in a protonated phenylpyridine-gold(I) thiolate. Inorg Chem. 2017;56:956–961. doi: 10.1021/acs.inorgchem.6b02613. [DOI] [PubMed] [Google Scholar]
- 25.Schmidbaur H, Schier A. Aurophilic interactions as a subject of current research: An up-date. Chem Soc Rev. 2012;41:370–412. doi: 10.1039/c1cs15182g. [DOI] [PubMed] [Google Scholar]
- 26.Sen S, Gabbaï FP. An ambiphilic phosphine/H-bond donor ligand and its application to the gold mediated cyclization of propargylamides. Chem Commun (Camb) 2017;53:13356–13358. doi: 10.1039/c7cc06065c. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Z, Wang Z, Wang Y, Zhang L. Au-catalysed oxidative cyclisation. Chem Soc Rev. 2016;45:4448–4458. doi: 10.1039/c5cs00887e. [DOI] [PubMed] [Google Scholar]
- 28.Luo Y, Ji K, Li Y, Zhang L. Tempering the reactivities of postulated α-oxo gold carbenes using bidentate ligands: Implication of tricoordinated gold intermediates and the development of an expedient bimolecular assembly of 2,4-disubstituted oxazoles. J Am Chem Soc. 2012;134:17412–17415. doi: 10.1021/ja307948m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji K, Zhao Y, Zhang L. Optimizing P,N-bidentate ligands for oxidative gold catalysis: Efficient intermolecular trapping of α-oxo gold carbenes by carboxylic acids. Angew Chem Int Ed Engl. 2013;52:6508–6512. doi: 10.1002/anie.201301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji K, Zheng Z, Wang Z, Zhang L. Enantioselective oxidative gold catalysis enabled by a designed chiral P,N-bidentate ligand. Angew Chem Int Ed Engl. 2015;54:1245–1249. doi: 10.1002/anie.201409300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Zheng Z, Zhang L. Intramolecular insertions into unactivated C(sp3)-H bonds by oxidatively generated β-diketone-α-gold carbenes: Synthesis of cyclopentanones. J Am Chem Soc. 2015;137:5316–5319. doi: 10.1021/jacs.5b02280. [DOI] [PubMed] [Google Scholar]
- 32.Zeineddine A, et al. Isolation of a reactive tricoordinate α-oxo gold carbene complex. Angew Chem Int Ed Engl. 2018;57:1306–1310. doi: 10.1002/anie.201711647. [DOI] [PubMed] [Google Scholar]
- 33.Zeineddine A, et al. Rational development of catalytic Au(I)/Au(III) arylation involving mild oxidative addition of aryl halides. Nat Commun. 2017;8:565. doi: 10.1038/s41467-017-00672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bontemps S, Bouhadir G, Miqueu K, Bourissou D. On the versatile and unusual coordination behavior of ambiphilic ligands o-R2P(Ph)BR’2. J Am Chem Soc. 2006;128:12056–12057. doi: 10.1021/ja0637494. [DOI] [PubMed] [Google Scholar]
- 35.Amgoune A, Bourissou D. σ-Acceptor, Z-type ligands for transition metals. Chem Commun (Camb) 2011;47:859–871. doi: 10.1039/c0cc04109b. [DOI] [PubMed] [Google Scholar]
- 36.Devillard M, et al. Dative Au→Al interactions: Crystallographic characterization and computational analysis. Chemistry. 2015;21:74–79. doi: 10.1002/chem.201405610. [DOI] [PubMed] [Google Scholar]
- 37.Batsanov SS. Van der Waals radii of elements. Inorg Mater. 2001;37:871–885. [Google Scholar]
- 38.Zhang Y, Lewis JC, Bergman RG, Ellman JA, Oldfield E. NMR shifts, orbitals, and M···H−X bonding in d8 square planar metal complexes. Organometallics. 2006;25:3515–3519. [Google Scholar]
- 39.Pregosin PS, et al. New P⋯H–N bonds characterized by 15N-filtered and 2D NOESY 1H NMR spectroscopy. Magn Reson Chem. 1992;30:548–551. [Google Scholar]
- 40.Wehman-Ooyevaar ICM, et al. A hydrogen atom in an organoplatinum-amine system. 1. Synthesis and spectroscopic and crystallographic characterization of novel zwitterionic complexes with a Pt(II)-⋯H–N+ unit. J Am Chem Soc. 1992;114:9916–9924. [Google Scholar]
- 41.Andris E, et al. Aurophilic interactions in [(L)AuCl]...[(L’)AuCl] dimers: Calibration by experiment and theory. J Am Chem Soc. 2018;140:2316–2325. doi: 10.1021/jacs.7b12509. [DOI] [PubMed] [Google Scholar]
- 42.Kleinhans G, et al. Nucleophilic T-shaped (LXL)Au(I)-pincer complexes: Protonation and alkylation. J Am Chem Soc. 2016;138:15873–15876. doi: 10.1021/jacs.6b11359. [DOI] [PubMed] [Google Scholar]
- 43.Jerabek P, Vondung L, Schwerdtfeger P. Tipping the balance between ligand and metal protonation due to relativistic effects: Unusually high proton affinity in gold(I) pincer complexes. Chemistry. 2018;24:6047–6051. doi: 10.1002/chem.201800755. [DOI] [PubMed] [Google Scholar]
- 44.Yao W, Eisenstein O, Crabtree RH. Interactions between C–H and N–H bonds and d8 square planar metal complexes: Hydrogen bonded or agostic? Inorg Chim Acta. 1997;254:105–111. [Google Scholar]
- 45.Johnson ER, et al. Revealing noncovalent interactions. J Am Chem Soc. 2010;132:6498–6506. doi: 10.1021/ja100936w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsui EY, Müller P, Sadighi JP. Reactions of a stable monomeric gold(I) hydride complex. Angew Chem Int Ed Engl. 2008;47:8937–8940. doi: 10.1002/anie.200803842. [DOI] [PubMed] [Google Scholar]
- 47.Roşca D-A, Smith DA, Hughes DL, Bochmann M. A thermally stable gold(III) hydride: Synthesis, reactivity, and reductive condensation as a route to gold(II) complexes. Angew Chem Int Ed Engl. 2012;51:10643–10646. doi: 10.1002/anie.201206468. [DOI] [PubMed] [Google Scholar]
- 48.Pintus A, Rocchigiani L, Fernandez-Cestau J, Budzelaar PHM, Bochmann M. Stereo- and regioselective alkyne hydrometallation with gold(III) hydrides. Angew Chem Int Ed Engl. 2016;55:12321–12324. doi: 10.1002/anie.201607522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocchigiani L, Fernandez-Cestau J, Chambrier I, Hrobárik P, Bochmann M. Unlocking structural diversity in gold(III) hydrides: Unexpected interplay of cis/trans-influence on stability, insertion chemistry, and NMR chemical shifts. J Am Chem Soc. 2018;140:8287–8302. doi: 10.1021/jacs.8b04478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rekhroukh F, et al. Experimental and theoretical evidence for an agostic interaction in a gold(III) complex. Angew Chem Int Ed Engl. 2016;55:3414–3418. doi: 10.1002/anie.201511111. [DOI] [PubMed] [Google Scholar]
- 51.Rekhroukh F, et al. β-Hydride elimination at low-coordinate gold(III) centers. J Am Chem Soc. 2016;138:11920–11929. doi: 10.1021/jacs.6b07035. [DOI] [PubMed] [Google Scholar]
- 52.Heinze K. The quest for mononuclear gold(II) and its potential role in photocatalysis and drug action. Angew Chem Int Ed Engl. 2017;56:16126–16134. doi: 10.1002/anie.201708349. [DOI] [PubMed] [Google Scholar]
- 53.Hashmi ASK. Fire and ice: A gold(III) monohydride. Angew Chem Int Ed Engl. 2012;51:12935–12936. doi: 10.1002/anie.201208603. [DOI] [PubMed] [Google Scholar]
- 54.Teles JH. Oxidative addition to gold(I): A new avenue in homogeneous catalysis with Au. Angew Chem Int Ed Engl. 2015;54:5556–5558. doi: 10.1002/anie.201501966. [DOI] [PubMed] [Google Scholar]
- 55.Joost M, Amgoune A, Bourissou D. Reactivity of gold complexes towards elementary organometallic reactions. Angew Chem Int Ed Engl. 2015;54:15022–15045. doi: 10.1002/anie.201506271. [DOI] [PubMed] [Google Scholar]
- 56.Benitez D, et al. A bonding model for gold(I) carbene complexes. Nat Chem. 2009;1:482–486. doi: 10.1038/nchem.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Muratore ME, Echavarren AM. Gold carbene or carbenoid: Is there a difference? Chemistry. 2015;21:7332–7339. doi: 10.1002/chem.201406318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.