Significance
Pseudomonas aeruginosa uses quorum-sensing–mediated communication to coordinate group behaviors, including biofilm formation and virulence. Quorum sensing relies on extracellular signal molecules called autoinducers. In P. aeruginosa, the LasR receptor binds the cognate autoinducer 3OC12-homoserine lactone. Using genetic, biochemical, and structural approaches, we define a molecular basis underlying LasR ligand specificity. Mutations in key residues alter LasR binding preferences for cognate and noncognate autoinducers, altering P. aeruginosa quorum-sensing responses. We solve crystal structures of the LasR ligand-binding domain complexed with different autoinducers and show that LasR accommodates these ligands via a flexible loop near the ligand-binding region. These findings establish a mechanism a receptor can use to distinguish between related ligands, an issue fundamental to systems beyond quorum sensing.
Keywords: crystal structure, LasR, Pseudomonas aeruginosa, quorum sensing, homoserine lactone
Abstract
Quorum sensing is a cell–cell communication process that bacteria use to orchestrate group behaviors. Quorum sensing is mediated by signal molecules called autoinducers. Autoinducers are often structurally similar, raising questions concerning how bacteria distinguish among them. Here, we use the Pseudomonas aeruginosa LasR quorum-sensing receptor to explore signal discrimination. The cognate autoinducer, 3OC12 homoserine lactone (3OC12HSL), is a more potent activator of LasR than other homoserine lactones. However, other homoserine lactones can elicit LasR-dependent quorum-sensing responses, showing that LasR displays ligand promiscuity. We identify mutants that alter which homoserine lactones LasR detects. Substitution at residue S129 decreases the LasR response to 3OC12HSL, while enhancing discrimination against noncognate autoinducers. Conversely, the LasR L130F mutation increases the potency of 3OC12HSL and other homoserine lactones. We solve crystal structures of LasR ligand-binding domains complexed with noncognate autoinducers. Comparison with existing structures reveals that ligand selectivity/sensitivity is mediated by a flexible loop near the ligand-binding site. We show that LasR variants with modified ligand preferences exhibit altered quorum-sensing responses to autoinducers in vivo. We suggest that possessing some ligand promiscuity endows LasR with the ability to optimally regulate quorum-sensing traits.
Quorum sensing is a cell–cell communication process that enables bacteria to collectively control behavior (1). Quorum sensing relies on the production, release, and detection of extracellular signal molecules, called autoinducers (2–4). At low cell density, autoinducer concentration is low, and bacteria act as individuals. As cell density increases, autoinducer concentration also rises. Under this condition, autoinducers bind to cognate receptors, initiating the population-wide regulation of genes underlying collective behaviors.
Many species of Gram-negative bacteria use acylated homoserine lactones (HSLs) as autoinducers (5–9). HSL autoinducers possess identical lactone head groups, but they vary in acyl chain length and decoration. The chain modifications promote specificity between particular HSL autoinducers and partner receptors (10). There are two kinds of HSL receptors. First, there are LuxR-type receptors, which are cytoplasmic HSL-binding transcription factors that possess variable ligand-binding domains (LBD) and well-conserved helix-turn-helix DNA-binding domains (DBD) (11, 12). There are also LuxN-type receptors, which are membrane-spanning two-component signaling proteins that bind HSLs in their periplasmic regions and transduce information regarding ligand occupancy internally by phosphorylation/dephosphorylation cascades (1, 13, 14).
Ligand specificity has been examined in the founding member of the LuxN receptor family from Vibrio harveyi (15). LuxN is exquisitely selective for its cognate autoinducer 3OHC4HSL. Specific amino acids that confer selectivity for chain length and for chain decoration were identified in the predicted LuxN transmembrane-spanning region. Longer HSLs competitively inhibit LuxN, suggesting that, in mixed-species consortia, V. harveyi monitors the vicinity for competing species, and in response to their presence, exploits LuxN antagonism to delay the launch of its quorum-sensing behaviors, thus avoiding loss of expensive public goods to nonkin.
Some analyses of HSL preference in LuxR-type receptors have also been performed (16). TraR from Agrobacterium tumefaciens excludes noncognate HSLs (11, 17–19), LasR from Pseudomonas aeruginosa detects several long-chain HSLs (20), and SdiA from Escherichia coli is highly promiscuous and avidly responds to HSLs with variable chain lengths (21–23). Comparison of structures of the LasR and TraR LBDs suggests that increased hydrogen bonding to the ligand in TraR, compared with LasR, accounts for the selectivity difference (20). Here, we systematically explore the LasR response to 3OC12HSL and noncognate HSLs. We use mutagenesis to establish the amino acid determinants that enable LasR to discriminate between HSLs. We identify LasR S129 as an amino acid residue that, when altered, decreases the LasR response to 3OC12HSL, and consequently restricts the set of HSLs capable of activation. In contrast, we find that LasR L130F improves the LasR response to 3OC12HSL, and as a result, allows LasR to respond to a broader set of HSLs than wild-type LasR. We also investigate how LasR ligand sensitivity and promiscuity affect the timing and strength of quorum-sensing control of P. aeruginosa behaviors. Finally, we solve crystal structures of the LasR LBD L130F bound to noncognate autoinducers to establish the structural basis underlying ligand selectivity and sensitivity. We find that a flexible loop located near the ligand-binding pocket promotes ligand promiscuity in the wild-type protein. This loop exists in SdiA, which is promiscuous, but not in TraR, which is highly specific for its cognate ligand. We propose that evolution has established a balance between ligand discrimination and ligand sensitivity in LasR. Because LasR requires higher concentrations of noncognate autoinducers than its cognate autoinducer for activation, this trade-off could allow LasR to robustly respond to its own signal molecule, even in the presence of other bacteria that are producing HSLs. Nonetheless, because LasR can detect high concentrations of other HSLs, P. aeruginosa would be capable of reacting to the presence of these other bacteria when it is outnumbered.
Results
LasR Responds to Multiple HSL Autoinducers.
To investigate the preference LasR displays for different HSLs, we employed a plasmid reporter system in which transcription from a LasR-controlled promoter fused to luciferase (plasB-lux) was assessed in E. coli. Arabinose-inducible lasR was cloned on a second plasmid (24, 25). A set of HSLs differing in both carbon chain length and in functionality at the C-3 position was synthesized using a modification of a previously reported method (SI Appendix) (26). Fig. 1A shows reporter output following addition of 100 nM of the cognate autoinducer, 3OC12HSL, and four other HSLs (3OC14HSL, 3OC10HSL, 3OC8HSL, and 3OC6HSL). All five HSLs activated LasR, but to differing levels: 3OC12HSL, 3OC14HSL, and 3OC10HSL elicited maximal LasR activity, and 3OC8HSL and 3OC6HSL stimulated 7-fold and 18-fold less activity, respectively. Using dose–response analyses, we examined these five compounds and seven other HSLs harboring different functionalities on the C-3 carbon in combination with the various chain lengths (Table 1 and SI Appendix, Table S1). The most potent ligand was 3OC12HSL, with an EC50 of 2.8 nM; 3OC14HSL was half as potent, with an EC50 of 6.2 nM. From there, the EC50 values followed the order 3OC10HSL < 3OC8HSL < 3OC6HSL. SI Appendix, Table S1 shows data for the C-3 modifications, and that the ketone versions of the molecules are the most potent for every chain length. For this reason, we used the five HSLs shown in Table 1 for much of the remainder of this work. In all assays, appropriate concentrations of HSLs were chosen based on EC50 data from the dose–response assays (Table 1 and SI Appendix, Table S1).
Fig. 1.
LasR is activated by multiple homoserine lactone autoinducers. (A) LasR-dependent bioluminescence was measured in E. coli. Arabinose-inducible LasR was produced from one plasmid and the plasB-lux reporter construct was carried on a second plasmid; 0.1% arabinose was used for LasR induction. (B) Elastase activity was measured from ΔlasI P. aeruginosa using elastin-Congo red as the substrate. In A and B, 100 nM of the designated HSLs were tested. Two technical replicates were performed for each biological sample and three biological replicates were assessed. Error bars denote SDs of the mean. Paired two-tailed t tests were performed comparing each compound to the DMSO control. *P < 0.05, **P < 0.01, ***P < 0.0001. (C) Comparison of LasR LBD protein levels in whole-cell lysates (W) and in the soluble fractions (S) of E. coli cells harboring DNA encoding the LasR LBD on a plasmid; 1 mM IPTG was used for LasR LBD induction and either 1% DMSO or 10 μM of the indicated HSL was supplied. In all lanes, protein from 0.05 OD of cells was loaded. Results are representative of three trials. (D) Thermal-shift analyses of purified LasR LBD bound to 3OC10HSL (Left), 3OC12HSL (Center), and 3OC14HSL (Right) without (designated DMSO) and following supplementation with an additional 10 μM of the indicated HSLs. Normalized fluorescence represents the first derivative of the raw fluorescence data (59). Each line represents the average of three replicates.
Table 1.
EC50 values (nM) for LasR HSL compounds in the plasB-lux assay
| LasR allele | |||||||
| Homoserine lactone | Wild-type | S129C | S129W | S129F | S129T | S129M | L130F |
| 3OC12HSL | 2.8 | 5.9 | 77 | 177 | 870 | 6,600 | 2.0 |
| 3OC14HSL | 6.2 | 12 | 13 | 41 | 4,100 | NR | 5.3 |
| 3OC10HSL | 8.0 | 32 | 2,600 | 4,980 | 4,500 | NR | 5.0 |
| 3OC8HSL | 900 | 2,900 | 2,200 | NR | NR | NR | 81 |
| 3OC6HSL | 2,400 | 4,400 | NR | 55,000 | NR | NR | 1,400 |
NR, Nonresponsive; 95% Confidence intervals for EC50 values are provided in SI Appendix, Table S2.
We measured in vivo LasR activity in response to the test HSLs using an elastase assay (27). Elastase is encoded by lasB and, thus, is driven by the same promoter as we employed in the above recombinant E. coli lux reporter assay. For the elastase analyses, we used a ΔlasI P. aeruginosa strain that makes no endogenous 3OC12HSL. When supplied at 100 nM, all of the test compounds elicited some elastase activity, but 3OC12HSL stimulated the highest elastase production (Fig. 1B). To ensure that 100 nM was an appropriate ligand concentration for this assay, we measured elastase activity at 3OC12HSL concentrations ranging from 1 nM to 1 μM (SI Appendix, Fig. S1). We do note that in P. aeruginosa, 3OC14HSL and 3OC8HSL stimulated lower activity than expected based on their EC50 values in E. coli. Indeed, as shown below, these two molecules had reduced activity in all assays in all P. aeruginosa strains used here. While we do not know the underlying molecular mechanism, we suspect that efflux pumps present in P. aeruginosa but not in E. coli could be responsible. For example, the P. aeruginosa MexAB-OprM efflux pump can eliminate homoserine lactones as evidenced by reductions in EC50 values in the ∆mexAB-oprM mutant (28). Nonetheless, our results indicate that with respect to the HSLs we tested, LasR is most responsive to its cognate autoinducer 3OC12HSL. However, noncognate HSLs can induce production of the quorum-sensing product elastase, and presumably other quorum-sensing–regulated outputs.
LasR and most other LuxR-type receptors fold around their cognate HSL ligands. Thus, they are only soluble, capable of dimerizing, and capable of binding DNA when ligand is present (18, 29). The results in Fig. 1 A and B suggest that LasR can fold around HSLs in addition to 3OC12HSL. To verify this notion, we tested whether LasR could be solubilized by noncognate HSLs. To do this, we grew E. coli producing the LasR LBD in the presence of each of the HSL compounds in our collection (Fig. 1C shows the 5 test compounds and SI Appendix, Fig. S2A shows the 11 other compounds in the collection). Consistent with previous results, in the absence of any ligand (DMSO control), the LasR LBD is present in the whole-cell lysate, but not in the soluble fraction, indicating that the protein is insoluble (30, 31). With respect to the main HSL test compounds, all except for 3OC6HSL solubilized the LasR LBD (Fig. 1C). Nonetheless, we could only purify to homogeneity the LasR LBD bound to the ligands containing the longer acyl chains: 3OC12HSL, 3OC14HSL, and 3OC10HSL. Taken together, the results in Fig. 1 A–C suggest that although 3OC8HSL and 3OC6HSL can bind to and activate LasR, their interactions must be less stable than ligands with longer acyl chains. To test this hypothesis, we performed thermal-shift analyses on LasR LBD–ligand complexes without and with the addition of either the same or a different HSL. The LasR LBD bound to 3OC10HSL, 3OC12HSL, and 3OC14HSL had apparent melting temperatures of 42.3 °C, 49.1 °C, and 50.5 °C, respectively (Fig. 1D, black lines), showing that LasR stability increases with increasing ligand chain length. Notably, the LasR LBD is slightly more stable when bound to the noncognate ligand 3OC14HSL, than when bound to the cognate ligand 3OC12HSL. The discrepancy between the enhanced stability of purified LasR LBD:3OC14HSL relative to LasR LBD:3OC12HSL in the thermal-shift assay and the higher EC50 LasR displays for 3OC14HSL compared with 3OC12HSL could result from increased hydrophobic interactions with the C14 chain in the stably purified complex that do not drive LasR activation.
Exogenously supplied autoinducers can shift the apparent melting temperature of an existing receptor–ligand complex if the added ligand has the ability to stabilize the unfolding protein as it releases the prebound ligand. Importantly, exogenously supplied HSLs can only stabilize the LasR LBD if they have affinities that are equal to or higher than the originally bound ligand (25). For the LasR LBD:3OC10HSL complex, exogenously added 3OC10HSL, 3OC12HSL, and 3OC14HSL stabilize the LasR LBD, increasing the apparent melting temperature 3.4 °C, 6.4 °C, and 5.5 °C, respectively (Fig. 1D). In contrast, the LasR LBD:3OC12HSL and the LasR LBD:3OC14HSL complexes could be further stabilized by exogenously supplied 3OC12HSL and 3OC14HSL, but not by 3OC10HSL (Fig. 1D). While the short acyl chain HSLs could activate LasR with high micromolar EC50 values in the plasB-lux reporter assay, 3OC6HSL and 3OC8HSL did not stabilize the LasR LBD protein sufficiently in E. coli for purification, presumably due to their low affinities (Fig. 1C and Table 1). Thus, they could not be tested in the traditional thermal-shift assay. They also did not enhance the stabilization of the LasR LBD prebound with other ligands, analogous to the inability of 3OC10HSL to stabilize the LasR LBD:3OC12HSL complex as it melted (Fig. 1D). Taken together, our results indicate that in an environment containing a mixture of HSL autoinducers, LasR, while capable of detecting several HSLs, will preferentially detect long-chain HSLs, with superior preference for its own autoinducer 3OC12HSL, followed closely by 3OC14HSL.
LasR S129 Mutations Alter Ligand Sensitivity and Ligand Discrimination.
Our above results show that LasR detects multiple HSL ligands with different preferences. To alter those preferences, we mutagenized the LasR ligand-binding pocket using the existing LasR LBD crystal structure as a guide (29). Among the set of mutants that we made, we identified one residue of interest: LasR S129. Previous work demonstrated that alteration of serine to alanine at this position transformed some LasR activators into inhibitors, and vice versa (32, 33). Moreover, the LasR LBD structure suggests that S129 is part of the network that interacts with the ligand acyl chain (29). Finally, S129 is well conserved across LuxR-type receptors, so we predicted that it could play a crucial role in ligand binding (34). We constructed LasR S129C, S129W, S129F, S129T, and S129M and examined their activities in the E. coli plasB-lux reporter assay, using 1 μM of each compound (Fig. 2A). Wild-type LasR and LasR S129C showed some response to every test compound. Each of the other four mutants failed to respond to one or more of the HSL test compounds (Fig. 2A). We used Western blot analysis to confirm that all of the LasR S129 mutant proteins were produced at similar levels (SI Appendix, Fig. S2B), demonstrating that the phenotypes are due to alterations in responses to ligands. Dose–response analyses with the complete set of HSLs enabled us to obtain EC50 values for each mutant. These values reveal that each LasR S129 mutant displays reduced activity with 3OC12HSL compared with wild-type LasR (Table 1 and SI Appendix, Table S1). Moreover, there exists a consistent relationship between the activity LasR mutants display with 3OC12HSL and the range of HSLs to which they respond. Mutants with the highest activity with 3OC12HSL interact with a larger range of HSLs than mutants with reduced activity with 3OC12HSL. For example, LasR S129M had the highest EC50 for 3OC12HSL, and it showed no response to any other HSL in our set of test compounds (Table 1 and SI Appendix, Table S1). Conversely, LasR S129C responded to all of the HSLs we tested except 3OHC8HSL, C8HSL, and 3OHC6HSL, and it has the lowest EC50/highest activity with 3OC12HSL (Table 1 and SI Appendix, Table S1). With respect to EC50 for 3OC12HSL, our alleles followed the pattern: wild-type < LasR S129C < LasR S129W < LasR S129F < LasR S129T < LasR S129M. They followed the exact reverse order in terms of breadth of response to different HSLs. These findings, and the location of S129 in the LasR ligand-binding pocket, support the proposal that substitutions disrupt a crucial hydrogen bond between S129 and the ketone in the autoinducer acyl chain, thus negatively affecting ligand sensitivity (32).
Fig. 2.
LasR S129 mutants alter LasR responses to HSL autoinducers. (A) Wild-type LasR and LasR S129 mutant bioluminescence from the E. coli plasB-lux reporter; 1 μM of the indicated HSLs were provided (see Fig. 1A for detail). (B) Wild-type LasR and LasR S129F-driven elastase activity in ΔlasI P. aeruginosa; 10 μM of the indicated HSLs were provided (see Fig. 1B for detail). In A and B, two technical replicates were performed for each biological sample and three biological replicates were assessed. Error bars denote SDs of the mean. Unpaired t tests were performed to compare each mutant LasR-HSL combination to that of wild-type LasR with the same compound. Due to space constraints, statistics for A are provided in SI Appendix, Table S3. **P < 0.01, ***P < 0.0001. (C) Comparison of LasR S129F LBD protein levels in whole-cell lysates (W) and in the soluble fractions (S) of E. coli cells harboring DNA encoding the LasR S129F LBD on a plasmid; 1 mM IPTG was used for LasR S129F LBD induction and either 1% DMSO or 10 μM of the indicated HSL was supplied. In all lanes, protein from 0.05 OD of cells was loaded. Results are representative of three trials. (D) Thermal-shift analyses of purified LasR S129F LBD bound to 3OC12HSL (Left) and 3OC14HSL (Right) without (designated DMSO) and following supplementation with an additional 10 μM of the indicated HSLs. Normalized fluorescence represents the first derivative of the raw fluorescence data (59). Each line represents the average of three replicates.
To explore the hydrogen-bonding role of the amino acid at position 129 on ligand selection, we focused on LasR S129F. Our rationale was that a serine to phenylalanine substitution eliminates hydrogen bonding and, moreover, because the LasR S129F mutant has an intermediate phenotype in our assays (i.e., not the strongest and not the weakest), this allele would allow us to probe activity in vitro and in vivo. First, we examined the consequences of the LasR S129F alteration in vivo by incorporating the lasR S129F mutation onto the chromosome of the ΔlasI P. aeruginosa strain and assaying elastase activity (Fig. 2B). We used 10 μM of each test HSL compound because increased ligand concentration is required to activate LasR S129F relative to wild-type LasR (Table 1). LasR S129F was capable of promoting elastase production, but only in response to a subset of the HSLs that were capable of stimulating wild-type LasR and, as noted, in every case, at a higher ligand concentration than required for wild-type LasR.
To investigate the mechanism underlying the reduction in LasR S129F response to the HSL test compounds, we assessed whether it could be solubilized by them. Fig. 2C shows that, similar to wild-type LasR, LasR S129F is not solubilized by our shortest test compound 3OC6HSL, but unlike wild-type LasR, LasR S129F is also not solubilized by 3OC8HSL. These results support our above finding regarding the inability of 3OC8HSL to elicit a response from LasR S129F and they underpin the low sensitivity LasR S129F displays for 3OC6HSL (Fig. 2A and Table 1). To evaluate the stability of LasR S129F, we used thermal-shift measurements. The LasR S129F:3OC12HSL complex had an apparent melting temperature of 35 °C showing that it is markedly less stable than the wild-type LasR:3OC12HSL complex (Figs. 1D and 2D). Both 3OC12HSL and 3OC14HSL stabilized LasR S129F during melting and release of prebound 3OC12HSL. Unlike wild-type LasR (Fig. 1D), LasR S129F preferred 3OC14HSL over 3OC12HSL (Fig. 2D), consistent with its fourfold lower EC50 for 3OC14HSL than for 3OC12HSL (Table 1). These findings indicate that the loss of hydrogen bonding between the C-1 ketone and the S129 residue can be compensated by enhanced hydrophobic interactions with the C14 chain.
The LasR L130F Substitution Increases LasR Ligand Sensitivity.
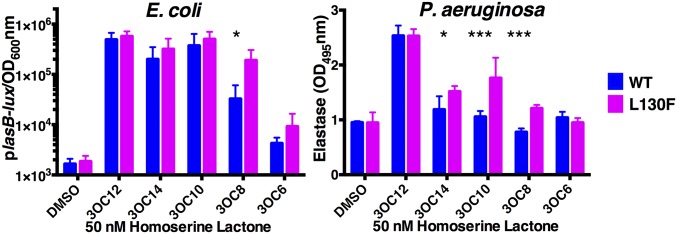
Based on our results with the LasR S129 mutants, we suspected that other LasR autoinducer binding-pocket residues located in the vicinity of the ligand acyl chain could participate in ligand selection. For this reason, we exchanged L130 and L128 for phenylalanine residues. As above, we tested their responses to the set of representative HSLs using the E. coli plasB-lux reporter assay (Fig. 3, Left, Table 1, and SI Appendix, Table S1). Table 1 shows that, surprisingly, LasR L130F exhibited a lower EC50 for every autoinducer tested. Shorter-chain HSLs showed the most dramatic increases in activity. LasR L128F also conferred improved sensitivity to some short-chain HSLs (SI Appendix, Table S1), but its phenotype was less pronounced than that of LasR L130F, so we further analyzed the LasR L130F mutant here. Fig. 3, Left, shows the wild-type LasR and the LasR L130F responses to a low concentration (50 nM) of the test HSLs. At this concentration, LasR L130F was roughly equivalent to wild-type LasR with respect to the response to 3OC12HSL, 3OC14HSL, and 3OC10HSL. However, LasR L130F was approximately fivefold more responsive to 3OC8HSL than wild-type LasR and it was modestly more responsive to 3OC6HSL. In vivo examination of LasR L130F ligand responses support these findings. When we assayed low concentrations (50 nM) of the test compounds, wild-type LasR stimulated elastase production only in response to 3OC12HSL, whereas LasR L130F responded to 3OC14HSL, 3OC10HSL, and 3OC8HSL in addition to 3OC12HSL (Fig. 3, Right).
Fig. 3.
LasR L130F displays enhanced responses to HSL autoinducers. (Left) Wild-type LasR and LasR L130F-driven bioluminescence from the E. coli plasB-lux reporter; 50 nM of the indicated HSLs were provided (see Fig. 1A for detail). (Right) Wild-type LasR and LasR L130F-driven elastase activity in ΔlasI P. aeruginosa; 50 nM of the indicated HSLs were provided (see Fig. 1B for detail). In both A and B, two technical replicates were performed for each biological sample and three biological replicates were assessed. Error bars denote SDs of the mean. Unpaired t tests were performed to compare each LasR L130F-HSL combination to that of wild-type LasR with the same compound. *P < 0.05, ***P < 0.0001.
We used thermal-shift analyses to explore the mechanism underlying the increased sensitivity of the LasR L130F mutant for HSLs. We used 3OC12HSL and 3OC14HSL as our test ligands. Compared with the wild-type LasR LBD, the LasR LBD L130F is more stable when bound to each ligand (Fig. 4A), suggesting that the L130F alteration increases the overall stability of the LasR protein. We exploited this feature to successfully purify the LasR LBD L130F bound to 3OC8HSL. As mentioned above, we could not purify the wild-type LasR LBD bound to 3OC8HSL. We performed thermal-shift analyses on the LasR LBD L130F bound to 3OC8HSL, 3OC10HSL, 3OC12HSL, and 3OC14HSL, to which we added different HSLs. Similar to the wild-type LasR LBD, exogenous addition of HSLs with long acyl chains further enhanced the stability of the LasR LBD L130F:HSL complexes compared with when HSLs with shorter acyl chains were added (Figs. 1C and 4B). For example, the LasR LBD L130F:3OC8HSL stability was enhanced by the addition of 3OC8HSL, 3OC10HSL, 3OC12HSL, and 3OC14HSL, but not 3OC6HSL (Fig. 4B). Protein solubility analyses track with these findings; long acyl chain ligands solubilize the LasR LBD L130F protein, whereas short-chain ligands do not (Fig. 4C and SI Appendix, Fig. S2C). Consistent with our EC50 values, chain length appears to be the most important factor driving protein solubility and stabilization for both the LasR LBD and the LasR LBD L130F (Fig. 4B and SI Appendix, Fig. S2C).
Fig. 4.
The LasR LBD L130F is more stable than the wild-type LasR LBD. (A) Thermal-shift analyses of purified LasR LBD (solid lines) and LasR LBD L130F (dotted lines) bound to 3OC12HSL (orange) or 3OC14HSL (green). Each line represents the average of three replicates. (B) Thermal-shift analyses of purified LasR LBD L130F bound to 3OC8HSL (Upper Left), 3OC10HSL (Lower Left), 3OC12HSL (Upper Right), and 3OC14HSL (Lower Right) without (designated DMSO) and following supplementation with an additional 10 μM of the indicated HSLs. In A and B, normalized fluorescence represents the first derivative of the raw fluorescence data (59). (C) Comparison of LasR L130F levels in the whole-cell lysates (W) and the soluble fractions (S) of E. coli cells harboring DNA encoding LasR LBD L130F on a plasmid (see Fig. 1C for details). Either 1% DMSO or 10 μM of the indicated HSL molecule was added.
LasR Ligand Selectivity and Sensitivity Influence the Timing and Strength of in Vivo Quorum-Sensing–Controlled Behaviors.
LasR L130F responds to HSLs at lower concentrations than does wild-type LasR, whereas LasR S129F requires higher concentrations (Table 1). Thus, we predicted that introducing these alleles into P. aeruginosa should influence its quorum-sensing responses in opposing manners. To test this prediction, we assayed pyocyanin production over time as the quorum-sensing readout in response to 3OC12HSL, in a ΔlasI P. aeruginosa strain containing wild-type lasR, lasR S129F, or lasR L130F. For comparison, we also evaluated pyocyanin production over time in wild-type P. aeruginosa under the same conditions (SI Appendix, Fig. S3). Guided by the EC50 value of LasR with 3OC12HSL, we tested an appropriate low (50 nM) and high concentrations (1 μM) of 3OC12HSL to enable us to observe responses from the wild-type and each mutant. Consistent with their relative EC50 values, when a low concentration of 3OC12HSL (50 nM) was added, the strain with LasR L130F made significantly more pyocyanin than the strain with wild-type LasR at every time point (Fig. 5, Left). At this HSL concentration, the strain carrying LasR S129F never activated pyocyanin production, presumably because the concentration of 3OC12HSL was far below the EC50 for LasR S129F (Fig. 5, Left). When the same assay was performed with 1 μM 3OC12HSL, rapid and maximal pyocyanin output occurred for P. aeruginosa carrying both wild-type LasR and LasR L130F. Furthermore, this high ligand concentration reveals that LasR S129F can drive pyocyanin production in response to 3OC12HSL, albeit weakly and only after 5 h (Fig. 5, Right). These results suggest that altering the sensitivity of LasR can change the production of quorum-sensing products such as pyocyanin.
Fig. 5.
Wild-type LasR, LasR S129F, and LasR L130F display distinct pyocyanin production phenotypes in response to high and low concentrations of 3OC12HSL. Pyocyanin production was measured in ΔlasI P. aeruginosa strains over the growth curve. The y axis “Pyocyanin” is the amount of pyocyanin pigment (OD695 nm) over cell density (OD600 nm). Designations are: red, DMSO control; blue, wild-type LasR; green, LasR S129F; pink, LasR L130F. Concentrations used are: (Left) 50 nM 3OC12HSL, (Right) 1 μM 3OC12HSL. Data show the mean of three biological replicates. Error bars denote SDs of the mean. Two-way ANOVAs were performed to compare LasR S129F and LasR L130F to wild-type LasR under each condition. A two-way ANOVA was also performed to compare wild-type LasR with 3OC12HSL and with DMSO alone. *P < 0.05, ***P < 0.0001.
Structural Basis Underlying LasR Ligand Preferences.
To define the molecular basis enabling LasR to accommodate noncognate autoinducers, we determined the structures of the LasR LBD L130F bound to 3OC10HSL and 3OC14HSL. As mentioned, the structure of the wild-type LasR LBD bound to 3OC12HSL was reported previously (29). We used LasR LBD L130F for our studies, reasoning that its enhanced stability might allow crystallization with noncognate ligands. Indeed, we were able to determine the structures of LasR LBD L130F:3OC10HSL and LasR LBD L130F:3OC14HSL to 2.1 and 1.9 Å, respectively (SI Appendix, Table S4). This resolution shows unambiguous ligand density enabling us to compare our structures with the LasR LBD:3OC12HSL structure (Fig. 6A and SI Appendix, Fig. S4 A and B). The side chain of F130 is buried in a hydrophobic pocket distal to the ligand-binding pocket (SI Appendix, Fig. S4 C and D). Compared with leucine, phenylalanine provides increased hydrophobic interactions with amino acid residues L23, L30, F32, I35, L114, L118, L128, L151, and L154. These interactions may stabilize LasR, in turn allowing it to accommodate an expanded set of HSLs compared with wild-type LasR.
Fig. 6.
Crystal structures of LasR LBD L130F bound to 3OC10HSL and 3OC14HSL. (A) Crystal structures of LasR LBD L130F:3OC10HSL (gold) and LasR LBD L130F:3OC14HSL (magenta) compared with the wild-type LasR LBD:3OC12HSL structure [blue, modified from PDB: 2UV0 (29)]. The Lower images show 90° rotations of the crystal structures relative to the images above. In the two Upper rightmost structures, the asterisks highlight the LasR loop region that includes residues 40–51 and that undergoes a conformational shift when 3OC14HSL is bound. (B) Structural comparison of LasR LBD:3OC12HSL (protein: blue, ligand: orange; modified from PDB ID code 2UVO) (29) and LasR LBD L130F:3OC10HSL crystal structures (protein: gold, ligand: magenta). Amino acids drawn in stick format show important residues for lactone head binding (Y56, W60, R61, D73, T75, W88, Y93, and S129) and acyl chain binding (G38, L40, A50, I52, A70, V76, L125, and A127). (C) Structural comparison of LasR LBD:3OC12HSL (protein: blue, ligand: orange; modified from PDB ID code 2UVO) (29) and LasR LBD L130F:3OC14HSL crystal structures (protein: magenta, ligand: green). Amino acids drawn in stick format are the same as in B. Note the shift in the position of the loop region corresponding to residues 40–51.
To understand how LasR can accommodate multiple long-chain HSL ligands in its binding site, we used the crystal structures to examine the binding pocket in detail. In all of the structures, the lactone head groups have the exact same placement, likely due to hydrogen bonding between the lactone ring ketone moiety and residue W60 (Fig. 6 B and C). The head group ketone and the two ketones on the acyl chain are further stabilized by an extensive hydrogen-bonding network consisting of residues Y56, R61, D73, T75, W88, Y93, and S129 (Fig. 6 B and C). In the three structures, the C10, C12, and C14 chains take similar paths until carbon 6, where C10 and C14 diverge from the path taken by the C12 chain. As a result, C14 and C12 fill the same volume (SI Appendix, Fig. S4A). However, the absence of two carbons in the C10 chain compared with the C12 chain could account for the increase in EC50 and lower stability of the LasR LBD L130F:3OC10HSL complex compared with the LasR LBD:3OC12HSL and LasR LBD L130F:3OC14HSL complexes (SI Appendix, Fig. S4B).
We next investigated whether there were any structural rearrangements in the crystals that could account for the expanded HSL binding capabilities of LasR L130F. An ∼2-Å shift occurs in the loop corresponding to residues 40–51 (highlighted by asterisks in Fig. 6A and displayed in SI Appendix, Fig. S4 D and E) in the LasR LBD L130F:3OC14HSL structure compared with the LasR LBD L130F:3OC10HSL and the LasR LBD:3OC12HSL structures (Fig. 6 B and C), and we propose the change in the placement of this loop underpins the altered binding capabilities of LasR L130F. Our structures with the different HSLs are consistent with recently reported data showing that the LasR loop conformation can be altered by synthetic agonists and, moreover, potency of the nonnative ligands correlates with the thermal stability of the complexes (35).
In each of our described structures, there are two LasR monomers in the asymmetric unit. In the LasR LBD L130F:3OC14HSL structure, the crystallographic contacts made by the loop regions in monomer A and monomer B differ slightly, while in the LasR LBD L130F:3OC10HSL structure, the monomer A and monomer B loop regions are similar, and each makes crystal contacts similar to those in monomer B in the LasR LBD L130F:3OC14HSL structure. It was possible that crystal contacts, rather than ligand binding, drove the different loop conformations in the LasR LBD L130F:3OC14HSL structure. Because we know that the conformations of the loop regions in the previously published wild-type LasR LBD:3OC12HSL structure are not influenced by crystallographic contacts, we can reliably compare the positions of the loop regions between LasR LBD L130F:3OC14HSL monomer A and monomer A from the LasR LBD:3OC12HSL structure. Both are free from crystallographic contacts, allowing us to conclude that the loop shifts in our crystal structures are ligand-induced (Fig. 6 A and C).
The crystal structures show that the majority of the LasR LBD exists as an interacting core that binds the lactone head and a portion of the acyl chain, whereas the loop consisting of residues 40–51 appears to act as a deformable region that enables LasR to accept ligands with different length acyl chains (Fig. 6 B and C). In the LasR LBD L130F:3OC14HSL structure, Y47 lies parallel to the ligand (Fig. 6C). We contrast this arrangement to the orientation of Y47 in the LasR LBD L130F:3OC10HSL and LasR LBD:3OC12HSL structures (Fig. 6C and SI Appendix, Fig. S4 E and F). In those cases, Y47 lies perpendicular to the ligands. We suggest that the ability of Y47 to adopt multiple conformations helps accommodate the different ligands. To test this possibility, we mutated LasR residue Y47. LasR Y47R and LasR Y47S displayed decreased sensitivity to both 3OC12HSL and 3OC14HSL in the E. coli reporter assay (SI Appendix, Fig. S5), suggesting that the loop provides interactions with the ligand chains that foster increased protein stability, allowing LasR to be activated. As previously noted, the packing of Y47 against the ligand acyl chain shields the ligand-binding pocket from the bulk solvent (36). We infer that alterations at this site, and perhaps in the loop region generally, lead to protein instability.
To understand the structural basis underlying specificity and promiscuity in this family of proteins, we compared all reported structures of the LBDs of LuxR-type receptors to our structure of LasR LBD L130F:3OC14HSL. The structures are SdiA LBD:3OC8HSL, CviR LBD:C6HSL, TraR LBD:3OC8HSL, and QscR:3OC12HSL (Fig. 7A and SI Appendix, Fig. S6). Similar to what we show here for LasR, SdiA is promiscuous with respect to ligand selectivity (21–23). A loop similar to the one we pinpoint in Fig. 6A as critical for LasR to accommodate different ligands exists in the SdiA LBD (Fig. 7A). Similarly, the orphan P. aeruginosa LuxR-type receptor QscR contains such a loop (SI Appendix, Fig. S6), and QscR displays ligand promiscuity (37, 38). Conversely, CviR and TraR display strict specificity for their cognate autoinducers (11, 18, 39, 40). No such large loop exists in the CviR and TraR LBD structures (Fig. 7A). Compared with the 12-residue loop in LasR and the 10-residue loop in SdiA, the corresponding CviR and TraR loops are eight and four residues long, respectively. These findings are consistent with the idea that this flexible loop confers ligand promiscuity.
Fig. 7.
A conserved flexible loop region confers promiscuity to LuxR-type receptors. (A) Upper images: structural comparison of the LuxR-type receptors LasR LBD L130F:3OC14HSL (magenta) and SdiA LBD:3OC8HSL [green, modified from PDB: 4Y17 (21) PDB ID code AY17] that exhibit promiscuity with respect to ligand binding. Lower images: structural comparison of the LuxR-type receptors CviR LBD:C6HSL [pink, modified from PDB: 3QP1 (39)] and TraR LBD:3OC8HSL [silver, modified from PDB: 1L3L (40)] that display strict ligand specificity. (B) Structural comparison of the protein:ligand interfaces for LasR LBD L130F:3OC14HSL (Upper Left, magenta), SdiA LBD:3OC8HSL (Upper Right, green), CviR LBD:C6HSL (Lower Left, pink), and TraR LBD:3OC8HSL (Lower Right, silver). Residues that make important hydrophobic side chain interactions in each protein:ligand complex are shown in stick format and named.
In addition to the differences in the overall structures of the receptors, we noted different conformations for the acyl chains in the LasR LBD structures compared with those in the other LuxR-type receptors. The autoinducer acyl chains in the CviR, TraR, and SdiA LBD structures have similar conformations within the ligand binding pockets terminating between residues Y88 and M89 for CviR, Y61 and F62 for TraR, and Y71 and Q72 for SdiA. In contrast, the acyl chains of the different ligands in the LasR LBD L130F structures orient their terminal carbons toward the opposing face of the ligand binding pocket (Fig. 7B). These different paths appear to be driven by the hydrophobic interactions we described above for the different LasR ligands (Fig. 6 B and C). We identified eight hydrophobic residues (G38, L40, A50, I52, A70, V76, L125, and A127) that dictate the shape of the ligand binding pocket in LasR. Indeed, these residues have generally hydrophobic characteristics in all LuxR-type proteins, but the size of each side chain varies (Fig. 7B). For example, A127 in LasR corresponds to F132 in SdiA. The smaller residue in LasR accommodates the altered path taken by its autoinducer, allowing the terminal carbon of the acyl chain to bind proximal to residue A127. A bulkier residue at this position, as in SdiA, sterically hinders this path for the ligand, forcing the acyl chain to bend in the opposite direction. Thus, in SdiA, F132 forces the terminal carbon of 3OC8HSL to bind distally. CviR I153 and TraR M127 appear to perform analogous roles.
To investigate whether a bulkier residue at position 127 would prevent LasR from binding to HSL ligands with long acyl chains, we constructed LasR A127W and assayed it in our E. coli plasB-lux reporter assay with 3OC12HSL, 3OC10HSL, 3OC8HSL, and 3OC6HSL. Fig. 8 shows that LasR A127W did not respond well to 3OC12HSL; therefore, we could not determine an EC50 value. However, the LasR A127W mutant did exhibit responses to shorter-chain HSLs. The EC50 value of LasR A127W for 3OC10HSL was 3,530 nM, ∼500 times higher than wild-type LasR for 3OC10HSL (8 nM). LasR A127W had EC50 values similar to wild-type LasR for 3OC8HSL and 3OC6HSL. Indeed, in the case of 3OC8HSL, the LasR A127W EC50 was somewhat lower than wild-type LasR (244 nM and 885 nM, respectively) (Fig. 8). We suggest that the large hydrophobic tryptophan side chain at position 127 cannot accommodate the long-chain 3OC12HSL ligand, but the protein is responsive to 3OC8HSL and 3OC6HSL because the enlarged amino acid side chain in the protein compensates for the missing carbons on the ligands. This finding suggests that larger amino acid residues at A127 improve the LasR response to shorter chain HSLs by enabling more stable protein–ligand complexes to form.
Fig. 8.
LasR A127W occludes long chain HSLs from the binding pocket. E. coli plasB-lux reporter assays were performed with wild-type LasR and LasR A127W in response to 3OC12HSL, 3OC10HSL, 3OC8HSL, and 3OC6HSL. EC50 values were obtained by performing dose–response assays for each HSL and analyzing the resulting data using nonlinear regression analysis. Two technical replicates were performed for each biological sample and at least three biological replicates were assessed. In the case of wild-type LasR, three biological replicates were performed for each experiment and then combined across all assays.
Discussion
P. aeruginosa often occupies niches containing other bacterial species that produce HSL autoinducers (41). Our results show that LasR can, with reduced sensitivity, detect noncognate HSLs and in response, activate transcription of quorum-sensing target genes. We suggest that determining the mechanisms that promote or restrict ligand access to LasR is important for understanding how the P. aeruginosa quorum-sensing response could be naturally or synthetically manipulated. Here, we identified mutations that alter how effectively LasR interacts with particular HSLs. Changing S129 increases LasR specificity for long-chain ligands over short-chain ligands but at a cost of reduced ligand potency, the consequence of which is delayed and dampened quorum-sensing output by P. aeruginosa (Fig. 5). In contrast, the LasR L130F alteration increases LasR response to 3OC12HSL, but again at a cost; this time, the penalty is a diminished ability to discriminate against other HSL ligands. We presume, based on the pyocyanin data in Fig. 5, such a change would lead to premature and increased quorum-sensing activity. Such mistimed and misregulated release of public goods may not benefit P. aeruginosa.
We propose that LasR has evolved to finely balance ligand sensitivity with ligand promiscuity. We investigated the published sequences of hundreds of clinical isolates of P. aeruginosa and S129 and L130 are strictly conserved (42), suggesting that they are crucial for P. aeruginosa fitness. Striking the ideal balance between LasR ligand-sensitivity and ligand-specificity may mean that detection of some noncognate HSLs must be tolerated, although importantly, only at higher concentrations relative to the cognate autoinducer.
We propose that LasR promiscuity could serve an important function in the environment (i.e., soil) and in eukaryotic hosts where P. aeruginosa encounters other species of bacteria. For example, in the soil, Pseudomonas putida produces 3OC12HSL, 3OC10HSL, 3OC8HSL, and 3OC6HSL (43). Klebsiella pneumoniae, which resides in human airways, produces C12HSL (44). LasR promiscuity may allow P. aeruginosa to “eavesdrop” on its competitors. Nonetheless, wild-type LasR detects 3OC12HSL more efficiently than other HSLs. Therefore, when P. aeruginosa is at high cell density, it is likely that 3OC12HSL outcompetes all other autoinducers. In contrast, when P. aeruginosa is at low cell density, provision of noncognate ligands made by other bacterial species that are at higher cell density could induce P. aeruginosa to activate its quorum-sensing behaviors prematurely. It could be beneficial for P. aeruginosa to engage in such behavior, for example, to enable the synthesis of toxic defensive products, such as pyocyanin and rhamnolipids that endow P. aeruginosa with an advantage over competing species (45, 46). Beyond defensive products, perhaps some of the quorum-sensing–controlled products produced under such conditions can be used exclusively by P. aeruginosa and not by competing species. If so, LasR promiscuity could grant P. aeruginosa a “last ditch” opportunity to survive in environments in which it is vastly outnumbered by competing species.
Unlike LasR, SdiA, and QscR, other quorum-sensing receptors are exquisitely specific (e.g., TraR and CviR). Thus, evolution can build receptors to possess or to lack ligand specificity. Presumably, the particular niche in which a quorum-sensing bacterium resides drives the optimal receptor ligand-detection preference strategy. Promiscuous quorum-sensing receptors could be superior when quorum sensing stimulates the production of defensive products that aid in competition with other bacterial species. Conversely, a receptor possessing strict ligand specificity could be optimal in cases in which quorum sensing activates production of vital public goods that require protection from cheaters.
Our combined genetic, biochemical, and structural work revealed the molecular basis for noncognate autoinducer recognition by LasR. A key flexible loop present in LasR, SdiA, and QscR appears to endow these receptors with the ability to bind to multiple HSL ligands. Conversely, the shorter and apparently less flexible loop present in TraR and CviR confers high specificity. Indeed, this structure–function analysis may help explain why a competitive inhibitor of CviR, chlorolactone, behaves as an agonist for LasR (31). These findings are particularly enlightening when considering attempts to design inhibitors that specifically target different LuxR-type receptors. The flexible loop and hydrophobic residues in LasR near the acyl chain binding site will need to be taken into account when developing small-molecule inhibitors that target LasR or other LuxR-type proteins that possess this feature. Designing molecules that target the flexibility of the loop region and that destabilize the protein could be explored for promiscuous receptors, such as LasR. In contrast, focused screening around molecules that resemble chlorolactone could yield inhibitors for LuxR-type proteins that display strict specificity for their cognate autoinducers, such as CviR.
Materials and Methods
Site-Directed Mutagenesis.
Mutations in lasR were constructed on the pBAD-A-lasR plasmid (25). Primers were designed using the Agilent Quikchange primer design tool and PCR with pFUltra polymerase (Agilent). PCR reactions were treated with DpnI to eliminate parental plasmid DNA and the plasmids with the mutant lasR genes were transformed into One Shot TOP10 chemically competent E. coli cells (Invitrogen). Reactions were plated on LB agar plates containing ampicillin (50 μg/mL) and individual mutants were verified via sequencing with primers for the lasR gene (ARM203 and ARM204). Primers and strains used in this work are listed in SI Appendix, Tables S5 and S6, respectively.
P. aeruginosa Strain Construction.
In-frame, marker-less lasR mutations were engineered onto the chromosome of P. aeruginosa PA14 using pEXG2-suicide constructs with gentamicin selection and sacB counter selection (47, 48). The lasR gene and 500 bp (base pairs) of flanking regions were cloned into pUCP18 (49). Site-directed mutagenesis was performed as described above to construct point mutations in plasmid-borne lasR. The DNA carrying the mutant lasR genes was obtained from pUCP18 by restriction enzyme digestion with BamHI and EcoRI (New England Biolabs), and subsequently the fragments were ligated into pEXG2. The recombinant pEXG2 plasmids were transformed into E. coli SM10λpir and, from there, the plasmids were mobilized into ΔlasI P. aeruginosa PA14 via biparental mating (50, 51). Exconjugants were selected on LB plates containing 30 μg/mL gentamicin and 100 μg/mL irgasan after overnight growth at 37° C. After recovery, 5% sucrose was used to select for loss of the plasmid. Candidate mutants were patched onto LB plates and LB plates containing 30 μg/mL gentamicin to select against the resistance marker. Colony PCR was performed on gentamicin sensitive patches with primers that annealed 500-bp upstream and downstream of lasR (ARM455 and ARM456). These PCR products were sequenced with lasR forward and reverse primers (ARM203 and ARM204).
E. coli plasB-lux Reporter Assay for LasR Activity.
The development of an assay that reports on LasR activity in response to exogenous ligands using luciferase as the readout has been described previously (25). In brief, 2 μL of overnight cultures containing plasB-luxCDABE and pBAD-A with either wild-type lasR or mutant lasR alleles were back-diluted into 200 μL LB medium and placed into clear-bottom 96-well plates (Corning). The plates were shaken at 30 °C for 4 h and 0.1% arabinose was added to each well along with a test HSL at the concentrations designated in the text and figures. To perform dose–response analyses, 1 mM of each HSL was serially diluted threefold 10 times, and 2 μL of each dilution was added to the wells. Higher or lower HSL concentrations were assayed when EC50 values did not fall into this range. Plates were shaken at 30 °C for 4 h. Bioluminescence and OD600 were measured using a Perkin-Elmer Envision Multimode plate reader. Relative light units were calculated by dividing the bioluminescence measurement by the OD600-nm measurement. Nonlinear regression was performed in Graphpad Prism6 to obtain EC50 values.
Elastase Assay.
The P. aeruginosa PA14 ΔlasI strains carrying either wild-type or mutant lasR genes were grown overnight with shaking at 37 °C in LB medium. Cultures were back diluted 1:50 in 3 mL of LB and grown for an additional 8 h with shaking at 37 °C. Strains were back-diluted 1:1,000 into 3 mL of LB medium and test HSLs or an equivalent volume of DMSO were added to each culture. These cultures were grown overnight at 37 °C with shaking. One milliliter of each culture was removed and the cells were pelleted by centrifugation at 16,100 × g. The supernatant was removed and filtered through a 0.22-μm filter (Millipore) and 100 μL of supernatant was added to 900 μL of 10 mM Na2HPO4 containing 10 mg of elastin-Congo red substrate (Sigma-Aldrich). These preparations were incubated at 37 °C for 2 h. The mixtures were subjected to centrifugation at 16,100 × g for 10 min. The resulting supernatants were removed and OD495 nm measured with a Beckman Coulter DU730 spectrophotometer against a blank of H2O.
Thermal-Shift Assay.
Thermal-shift analyses of 6xHis-LasR LBD and 6xHisLasR L130F LBD bound to HSLs were performed as previously described (25). In short, ligand-bound protein was diluted to 5 μM in reaction buffer (20 mM Tris⋅HCl pH 8, 200 mM NaCl, and 1 mM DTT) containing DMSO or 10 μM HSL test compound in 18-μL total volume. The mixtures were incubated at room temperature for 15 min. Next, 5000× SYPRO Orange (Thermo-Fisher) in DMSO was diluted to 200× in reaction buffer and used at 20× final concentration. Two microliters of 200× SYPRO Orange was added to the 18-μL sample immediately before analysis. Samples were subjected to a linear heat gradient of 0.05 °C/s, from 25 °C to 99 °C in a Quant Studio 6 Flex System (Applied Biosystems) using the melting-curve setting. Fluorescence was measured using the ROX reporter setting.
Pyocyanin Time Course.
Overnight cultures of the P. aeruginosa wild-type, ΔlasI, ΔlasI lasR S129F, and ΔlasI lasR L130F strains were grown in LB medium with shaking at 37 °C. 1.5 mL of each culture was diluted into 50 mL of fresh LB medium. Next, 3OC12HSL was added at the concentrations described in the text and figures and the cultures were shaken at 37 °C for an additional 3 h. From there forward, 1-mL aliquots were removed every 30 min for 300 min and cell density (OD600 nm) was measured immediately using a Beckman Coulter DU730 Spectrophotometer. The aliquots were subjected to centrifugation at 16,100 × g for 2 min and the clarified supernatants were removed. The OD695 nm of the supernatants were measured. Pyocyanin activity was determined by plotting the OD695 nm/OD600 nm over time for each strain.
Protein Production, Purification, and Crystallography.
Recombinant 6xHis-LasR LBD and 6xHis-LasR LBD L130F proteins bound to 3OC8HSL, 3OC10HSL, 3OC12HSL, or 3OC14HSL were purified as previously described for LasR LBD:3OC12HSL using Ni-NTA affinity columns followed by size-exclusion chromatography (25). The 6xHis-LasR LBD bound to 3OC10HSL and 6xHis-LasR LBD bound to 3OC14HSL were crystallized by the hanging-drop diffusion method in a solution of 200 mM Mg(NO3)2 and 20% PEG 3350. Diffraction data were processed using the HKL-3000 software package (52). The structures were solved using Phaser in Phenix (53, 54) by molecular replacement, with the structure of LasR LBD:3OC12HSL used as the search model (29). The space group was P1211. Model building was performed using Coot (55, 56) and further refinement was accomplished using Phenix (53). Images of the structures were generated with PyMOL (57). We note that the Rfree is higher in the higher-resolution structure of LasR LBD L130F:3OC14HSL than it is for the lower-resolution structure of LasR LBD L130F:3OC10HSL. The data from the LasR LBD L130F:3OC14HSL structure were of lower quality than that for the LasR LBD L130:3OC10HSL structure, resulting in a higher Rfree value in the highest-resolution bin for the LasR LBD L130F:3OC14HSL structure, and therefore an overall higher Rfree value. Lowering the resolution cut-off for LasR LBD L130F:3OC14HSL and rerefinement in Phenix did not lead to a demonstrable change in the Rfree value.
Protein Solubility Assay.
E. coli BL21 DE3 (Invitrogen) containing plasmid-borne 6xHis-LasR LBD or 6xHis-LasR LBD L130F were grown overnight and back-diluted 1:500 in 20 mL of LB medium containing ampicillin (100 μg/mL). Cultures were grown to OD600 of 0.5 and protein production was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). Upon addition of IPTG, the desired test HSL was also added at a final concentration of 10 μM, and the cultures were incubated at 25 °C with shaking for 4 h. Cells were harvested at 3,000 × g and resuspended in lysis buffer (500 mM NaCl, 20 mM Tris⋅HCl pH 8, 20 mM imidazole, 5% glycerol, 1 mM EDTA, and 1 mM DTT). The cells were lysed using sonication (1-s pulses for 15 s with a 50% duty cycle). The fraction we call the whole-cell lysate was harvested after sonication. The soluble fraction was isolated by centrifugation at 32,000 × g. Samples were subjected to electrophoresis on SDS/PAGE gels (Bio-Rad) and imaged with an Image Quant LAS4000 gel dock using the chemiluminescent setting (GE Healthcare).
Western Blotting to Assess LasR Protein Production Levels.
Whole-cell lysates and soluble fractions from E. coli carrying wild-type 6xHis-LasR and 6xHis-LasR S129C, S129F, S129M, S129T, S129W, and L130F were prepared as described in the preceding section, and then quantified using a Pierce BCA Protein Assay Kit (Thermo-Fisher). Samples were subjected to immunoblotting using a procedure adapted from Hurley and Bassler (58). Ten micrograms of each sample were loaded into each well, and samples were probed with a monoclonal antibody to the 6xHis tag (Thermo-Fisher) followed by goat anti-mouse IgG2b cross adsorbed secondary antibody HRP (Thermo-Fisher). Both antibodies were used at 3 μg/mL. The blot was imaged with an Image Quant LAS4000 gel dock using the transillumination setting (GE Healthcare). Samples were tested in triplicate.
Statistical Methods.
In all experiments, values are the average of three biological replicates, each of which was assessed in two or three technical replicates as noted. For EC50 analyses, wild-type LasR was used as the control. EC50 values that appear in multiple experiments represent the mean from all experiments (Fig. 8, Table 1, and SI Appendix, Fig. S5 and Table S1). In these cases, two technical replicates of three biological replicates were assayed for every protein/molecule in each experiment. The 95% confidence intervals were calculated for each EC50 value in Table 1, and are provided in SI Appendix, Table S2. Error bars represent SDs of the means. Two-tailed t tests were performed to compare experimental groups as noted in the figures. Two-way ANOVAs were performed to compare LasR variants to wild-type LasR as noted in Fig. 5. P values are noted with asterisks: *P < 0.05, **P < 0.01, ***P < 0.0001.
Supplementary Material
Acknowledgments
We thank Dr. Fred Hughson and Dr. Philip Jefferey for assistance with crystallography; and Dr. Chari Smith and the entire B.L.B. group for insightful ideas about this research. This work was supported by the Howard Hughes Medical Institute, National Institutes of Health Grant 5R37GM065859, and National Science Foundation Grant MCB-1713731 (to B.L.B.); National Institute of General Medical Sciences T32GM007388 (to A.R.M.); and a Jane Coffin Childs Memorial Fund for Biomedical Research Postdoctoral Fellowship (to J.E.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 6MVN (LasR LBD bound to 3OC10HSL) and 6MVM (LasR LBD bound to 3OC14HSL)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817239116/-/DCSupplemental.
References
- 1.Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albus AM, Pesci EC, Runyen-Janecky LJ, West SE, Iglewski BH. Vfr controls quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3928–3935. doi: 10.1128/jb.179.12.3928-3935.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latifi A, et al. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol. 1995;17:333–343. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x. [DOI] [PubMed] [Google Scholar]
- 4.Engebrecht J, Nealson K, Silverman M. Bacterial bioluminescence: Isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 5.Hanzelka BL, et al. Acylhomoserine lactone synthase activity of the Vibrio fischeri AinS protein. J Bacteriol. 1999;181:5766–5770. doi: 10.1128/jb.181.18.5766-5770.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson JP, et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brint JM, Ohman DE. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberhard A, et al. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 9.Cao JG, Meighen EA. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989;264:21670–21676. [PubMed] [Google Scholar]
- 10.Churchill MEA, Chen L. Structural basis of acyl-homoserine lactone-dependent signaling. Chem Rev. 2011;111:68–85. doi: 10.1021/cr1000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vannini A, et al. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 2002;21:4393–4401. doi: 10.1093/emboj/cdf459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasser W, Reverchon S. New insights into the regulatory mechanisms of the LuxR family of quorum sensing regulators. Anal Bioanal Chem. 2007;387:381–390. doi: 10.1007/s00216-006-0702-0. [DOI] [PubMed] [Google Scholar]
- 13.Freeman JA, Lilley BN, Bassler BL. A genetic analysis of the functions of LuxN: A two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol Microbiol. 2000;35:139–149. doi: 10.1046/j.1365-2958.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- 14.Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: Sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 15.Ke X, Miller LC, Bassler BL. Determinants governing ligand specificity of the Vibrio harveyi LuxN quorum-sensing receptor. Mol Microbiol. 2015;95:127–142. doi: 10.1111/mmi.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galloway WR, Hodgkinson JT, Bowden SD, Welch M, Spring DR. Quorum sensing in Gram-negative bacteria: Small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev. 2011;111:28–67. doi: 10.1021/cr100109t. [DOI] [PubMed] [Google Scholar]
- 17.You YS, et al. Use of bacterial quorum-sensing components to regulate gene expression in plants. Plant Physiol. 2006;140:1205–1212. doi: 10.1104/pp.105.074666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Winans SC. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc Natl Acad Sci USA. 2001;98:1507–1512. doi: 10.1073/pnas.98.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawver LA, Jung SA, Ng WL. Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol Rev. 2016;40:738–752. doi: 10.1093/femsre/fuw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerdt JP, et al. Chemical interrogation of LuxR-type quorum sensing receptors reveals new insights into receptor selectivity and the potential for interspecies bacterial signaling. ACS Chem Biol. 2017;12:2457–2464. doi: 10.1021/acschembio.7b00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen Y, et al. Structural and mechanistic roles of novel chemical ligands on the SdiA quorum-sensing transcription regulator. MBio. 2015;6:e02429-14. doi: 10.1128/mBio.02429-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael B, Smith JN, Swift S, Heffron F, Ahmer BM. SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J Bacteriol. 2001;183:5733–5742. doi: 10.1128/JB.183.19.5733-5742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sitnikov DM, Schineller JB, Baldwin TO. Control of cell division in Escherichia coli: Regulation of transcription of ftsQA involves both rpoS and SdiA-mediated autoinduction. Proc Natl Acad Sci USA. 1996;93:336–341. doi: 10.1073/pnas.93.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson JP, Pesci EC, Iglewski BH. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paczkowski JE, et al. Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum-sensing receptors. J Biol Chem. 2017;292:4064–4076. doi: 10.1074/jbc.M116.770552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chhabra SR, et al. Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-L-homoserine lactone as immune modulators. J Med Chem. 2003;46:97–104. doi: 10.1021/jm020909n. [DOI] [PubMed] [Google Scholar]
- 27.Gambello MJ, Iglewski BH. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore JD, Gerdt JP, Eibergen NR, Blackwell HE. Active efflux influences the potency of quorum sensing inhibitors in Pseudomonas aeruginosa. ChemBioChem. 2014;15:435–442. doi: 10.1002/cbic.201300701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bottomley MJ, Muraglia E, Bazzo R, Carfì A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J Biol Chem. 2007;282:13592–13600. doi: 10.1074/jbc.M700556200. [DOI] [PubMed] [Google Scholar]
- 30.Schuster M, Urbanowski ML, Greenberg EP. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc Natl Acad Sci USA. 2004;101:15833–15839. doi: 10.1073/pnas.0407229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Loughlin CT, et al. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci USA. 2013;110:17981–17986. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerdt JP, McInnis CE, Schell TL, Rossi FM, Blackwell HE. Mutational analysis of the quorum-sensing receptor LasR reveals interactions that govern activation and inhibition by nonlactone ligands. Chem Biol. 2014;21:1361–1369. doi: 10.1016/j.chembiol.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerdt JP, McInnis CE, Schell TL, Blackwell HE. Unraveling the contributions of hydrogen-bonding interactions to the activity of native and non-native ligands in the quorum-sensing receptor LasR. Org Biomol Chem. 2015;13:1453–1462. doi: 10.1039/c4ob02252a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuqua C, Winans SC, Greenberg EP. Census and consensus in bacterial ecosystems: The LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 35.O’Reilly MC, et al. Structural and biochemical studies of non-native agonists of the LasR quorum-sensing receptor reveal an L3 loop “out” conformation for LasR. Cell Chem Biol. 2018;25:1128–1139.e3. doi: 10.1016/j.chembiol.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou Y, Nair SK. Molecular basis for the recognition of structurally distinct autoinducer mimics by the Pseudomonas aeruginosa LasR quorum-sensing signaling receptor. Chem Biol. 2009;16:961–970. doi: 10.1016/j.chembiol.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wysoczynski-Horita CL, et al. Mechanism of agonism and antagonism of the Pseudomonas aeruginosa quorum sensing regulator QscR with non-native ligands. Mol Microbiol. 2018;108:240–257. doi: 10.1111/mmi.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lintz MJ, Oinuma K, Wysoczynski CL, Greenberg EP, Churchill MEA. Crystal structure of QscR, a Pseudomonas aeruginosa quorum sensing signal receptor. Proc Natl Acad Sci USA. 2011;108:15763–15768. doi: 10.1073/pnas.1112398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G, et al. A strategy for antagonizing quorum sensing. Mol Cell. 2011;42:199–209. doi: 10.1016/j.molcel.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang RG, et al. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature. 2002;417:971–974, and erratum (2011) 476:240. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
- 41.Tashiro Y, Yawata Y, Toyofuku M, Uchiyama H, Nomura N. Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes Environ. 2013;28:13–24. doi: 10.1264/jsme2.ME12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feltner JB, et al. LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa. MBio. 2016;7:9. doi: 10.1128/mBio.01513-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steidle A, et al. Identification and characterization of an N-acylhomoserine lactone-dependent quorum-sensing system in Pseudomonas putida strain IsoF. Appl Environ Microbiol. 2002;68:6371–6382. doi: 10.1128/AEM.68.12.6371-6382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin WF, et al. N-acyl homoserine lactone production by Klebsiella pneumoniae isolated from human tongue surface. Sensors (Basel) 2012;12:3472–3483. doi: 10.3390/s120303472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baron SS, Rowe JJ. Antibiotic action of pyocyanin. Antimicrob Agents Chemother. 1981;20:814–820. doi: 10.1128/aac.20.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smalley NE, An D, Parsek MR, Chandler JR, Dandekar AA. Quorum sensing protects Pseudomonas aeruginosa against cheating by other species in a laboratory coculture model. J Bacteriol. 2015;197:3154–3159. doi: 10.1128/JB.00482-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borlee BR, Geske GD, Blackwell HE, Handelsman J. Identification of synthetic inducers and inhibitors of the quorum-sensing regulator LasR in Pseudomonas aeruginosa by high-throughput screening. Appl Environ Microbiol. 2010;76:8255–8258. doi: 10.1128/AEM.00499-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kukavica-Ibrulj I, et al. In vivo growth of Pseudomonas aeruginosa strains PAO1 and PA14 and the hypervirulent strain LESB58 in a rat model of chronic lung infection. J Bacteriol. 2008;190:2804–2813. doi: 10.1128/JB.01572-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweizer HP. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene. 1991;97:109–121. doi: 10.1016/0378-1119(91)90016-5. [DOI] [PubMed] [Google Scholar]
- 50.Mukherjee S, Moustafa D, Smith CD, Goldberg JB, Bassler BL. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer. PLoS Pathog. 2017;13:e1006504. doi: 10.1371/journal.ppat.1006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic-engineering—Transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 52.Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: The integration of data reduction and structure solution—From diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- 53.Adams PD, et al. The Phenix software for automated determination of macromolecular structures. Methods. 2011;55:94–106. doi: 10.1016/j.ymeth.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Afonine PV, et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr. 2012;68:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 56.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeLano WL. PyMOL molecular viewer: Updates and refinements. Abstr Pap Am Chem S. 2009;238:1. [Google Scholar]
- 58.Hurley A, Bassler BL. Asymmetric regulation of quorum-sensing receptors drives autoinducer-specific gene expression programs in Vibrio cholerae. PLoS Genet. 2017;13:e1006826. doi: 10.1371/journal.pgen.1006826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grøftehauge MK, Hajizadeh NR, Swann MJ, Pohl E. Protein-ligand interactions investigated by thermal shift assays (TSA) and dual polarization interferometry (DPI) Acta Crystallogr D Biol Crystallogr. 2015;71:36–44. doi: 10.1107/S1399004714016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.