Significance
A metabolite of ketamine, (2R,6R)-hydroxynorketamine [(2R,6R)-HNK], produces rapid and sustained antidepressant effects in animal models but without the side effects of ketamine. Notably, (2R,6R)-HNK does not block the NMDA receptor, the primary target of ketamine. Here, we demonstrate that the antidepressant effects of (2R,6R)-HNK require activity dependent release of BDNF that is mediated by stimulation of voltage-dependent Ca2+ channels. Importantly, increased BDNF release leads to activation of downstream TrkB and mechanistic target of rapamycin complex 1 signaling that increases synaptic function of pyramidal neurons in the medial prefrontal cortex. Stimulation of BDNF release and increased synaptic function block or reverse the detrimental effects of stress and depression and thereby underlie the antidepressant actions of (2R,6R)-HNK.
Keywords: BDNF; depression; mTORC1; ketamine; (2R,6R)-hydroxynorketamine
Abstract
Ketamine, a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, produces rapid and long-lasting antidepressant effects in major depressive disorder (MDD) patients. (2R,6R)-Hydroxynorketamine [(2R,6R)-HNK], a metabolite of ketamine, is reported to produce rapid antidepressant effects in rodent models without the side effects of ketamine. Importantly, (2R,6R)-HNK does not block NMDA receptors like ketamine, and the molecular signaling mechanisms for (2R,6R)-HNK remain unknown. Here, we examined the involvement of BDNF/TrkB/mechanistic target of rapamycin complex 1 (mTORC1) signaling in the antidepressant actions of (2R,6R)-HNK. Intramedial prefrontal cortex (intra-mPFC) infusion or systemic (2R,6R)-HNK administration induces rapid and long-lasting antidepressant effects in behavioral tests, identifying the mPFC as a key region for the actions of (2R,6R)-HNK. The antidepressant actions of (2R,6R)-HNK are blocked in mice with a knockin of the BDNF Val66Met allele (which blocks the processing and activity-dependent release of BDNF) or by intra-mPFC microinjection of an anti-BDNF neutralizing antibody. Blockade of L-type voltage-dependent Ca2+ channels (VDCCs), required for activity-dependent BDNF release, also blocks the actions of (2R,6R)-HNK. Intra-mPFC infusion of pharmacological inhibitors of TrkB or mTORC1 signaling, which are downstream of BDNF, also block the actions of (2R,6R)-HNK. Moreover, (2R,6R)-HNK increases synaptic function in the mPFC. These findings indicate that activity-dependent BDNF release and downstream TrkB and mTORC1 signaling, which increase synaptic function in the mPFC, are required for the rapid and long-lasting antidepressant effects of (2R,6R)-HNK, supporting the potential use of this metabolite for the treatment of MDD.
Major depressive disorder (MDD) is a widespread illness with an estimated lifetime prevalence in the United States of ∼17% and has high rates of relapse that have major social and economic consequences (1, 2). Currently available antidepressants take weeks to months to produce a therapeutic response, and more than 30% of patients fail to respond to these agents and are considered treatment resistant (3). These findings underscore a significant unmet need for more efficacious and rapid-acting antidepressants with mechanisms distinct from the currently available monoamine reuptake inhibitor medications.
Recent studies demonstrate that ketamine, a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, causes efficacious and rapid (within hours) antidepressant effects in MDD patients, including those considered treatment resistant (4–6). In addition, ketamine has been reported to rapidly reduce suicidal ideation in depressed patients (7). However, ketamine also has undesirable side effects, including psychotomimetic and dissociative symptoms, as well as abuse potential (8). A recent study demonstrates that (2R,6R)-hydroxynorketamine [(2R,6R)-HNK], a major metabolite of ketamine, produces rapid antidepressant effects in rodent models and may contribute significantly to the therapeutic actions of ketamine (9). Importantly, (2R,6R)-HNK does not have the side effects produced by ketamine in rodent models (9). Studies are currently underway to determine the clinical efficacy of (2R,6R)-HNK in depressed patients. However, the antidepressant actions of this interesting metabolite are reported to be independent of NMDA receptor blockade (9), and, to date, the molecular signaling mechanisms have not been identified.
Previous work has demonstrated that the antidepressant effects of ketamine and other rapid-acting agents, including the muscarinic receptor antagonist scopolamine and the NMDA-modulating agent rapastinel, are activity dependent, requiring activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and L-type voltage-dependent Ca2+ channels (VDCCs) (10–14). These studies also demonstrate a requirement for activity-dependent release of brain-derived neurotrophic factor (BDNF) and activation of the mechanistic target of rapamycin complex 1 (mTORC1) in the medial prefrontal cortex (mPFC) (10–13). Stimulation of BDNF-mTORC1 signaling by these rapid-acting antidepressants leads to increased synthesis of synaptic proteins and increased number and function of synapses in layer V pyramidal neurons in the mPFC (10–13, 15–18). Here, we tested the hypothesis that (2R,6R)-HNK produces antidepressant actions via activity-dependent release of BDNF and mTORC1 signaling.
Results
(2R,6R)-HNK in the mPFC Induces Antidepressant Effects.
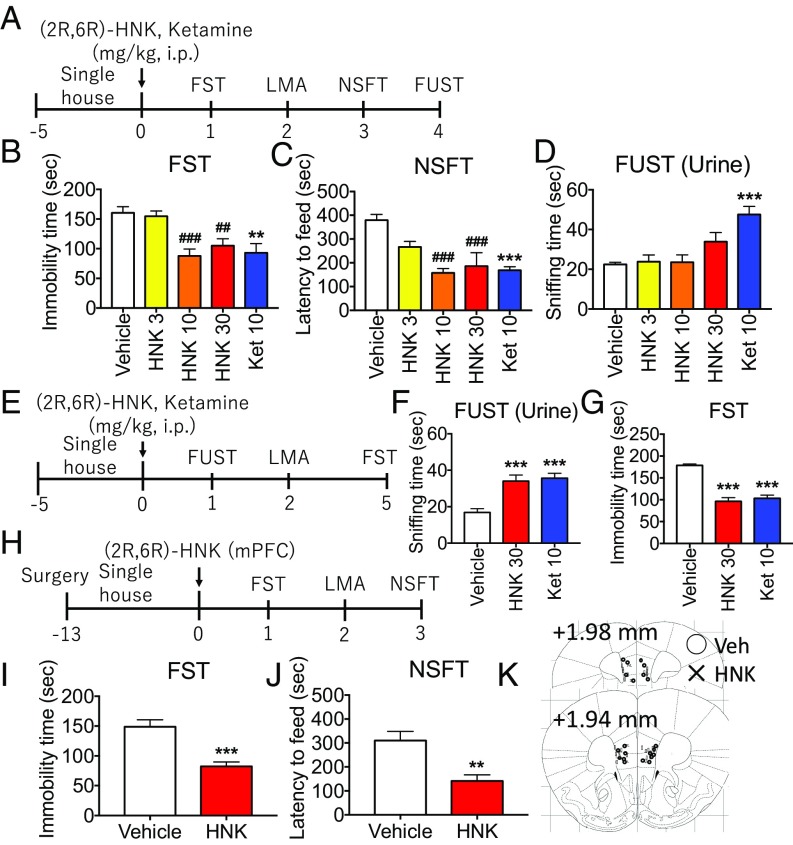
To examine the effects of (2R,6R)-HNK, we used several standard models, including the forced swim test (FST), a measure of behavioral despair; the novelty-suppressed feeding test (NSFT), a measure of anxiety and antidepressant response; and the female urine sniffing test (FUST), a measure of reward/anhedonia (Fig. 1A). Doses of 3, 10, or 30 mg/kg (i.p.) (2R,6R)-HNK were tested and compared with ketamine (10 mg/kg). Both (2R,6R)-HNK and ketamine significantly decreased immobility time in the FST 24 h after dosing (Fig. 1B) and reduced latency to feed in the NSFT 3 d after dosing (Fig. 1C); there was no effect on locomotor activity or food consumption (SI Appendix, Fig. S1 A and B). Testing in the FUST 4 d after dosing revealed no effects of (2R,6R)-HNK administration, even though ketamine produced a significant effect at this time point (Fig. 1D). However, when tested 1 d after dosing, (2R,6R)-HNK (30 mg/kg), like ketamine (10 mg/kg), induced antidepressant effects in the FUST (Fig. 1 E and F); we also observed significant effects 5 d after dosing in FST (Fig. 1G). There were no effects on water-sniffing time or locomotor activity (SI Appendix, Fig. S1 C–E). These findings indicate that (2R,6R)-HNK produces an antidepressant effect in the FUST, albeit at a shorter time point compared with ketamine.
Fig. 1.
Systemic or mPFC infusion of (2R,6R)-HNK induces antidepressant effects. (A) Mice were administered vehicle, (2R,6R)-HNK (3 to 30 mg/kg, i.p.) or ketamine (10 mg/kg, i.p.), followed by behavioral tests. (B and C) (2R,6R)-HNK and ketamine significantly decreased both immobility time 24 h after dosing in the FST and latency to feed 3 d after treatment in the NSFT. (D) Four days after treatment, (2R,6R)-HNK had no effect in the FUST, even though ketamine significantly increased female urine sniffing at this time point. (E–G) Studies of FUST 1 d after dosing show that (2R,6R)-HNK (30 mg/kg) or ketamine (10 mg/kg) administration induced antidepressant effects; there were also significant effects 5 d after treatment in the FST. (H) Mice received bilateral infusion of (2R,6R)-HNK (10 ng per side) in the mPFC, followed by behavioral tests. (I and J) Infusions of (2R,6R)-HNK into the mPFC induced significant antidepressant responses in the FST and NSFT. (K) Location of cannula placements in the mPFC. Bars represent mean ± SEM, n = 4 to 12 per group. ###P < 0.001, ##P < 0.01 compared with vehicle group; Dunnett’s multiple comparison test after significant results of one-way ANOVA. ***P < 0.001, **P < 0.01 compared with vehicle group; Student’s t test.
To examine the involvement of the mPFC, we infused (2R,6R)-HNK into the mPFC. Microinjection of very low doses of (2R,6R)-HNK (10 ng per side) induced significant antidepressant responses in the FST and NSFT (Fig. 1 H–J), with no effect on locomotor activity or food consumption (SI Appendix, Fig. S1 F and G). These findings indicate that the mPFC plays a critical role in the antidepressant actions of (2R,6R)-HNK.
Antidepressant Effects of (2R,6R)-HNK Require BDNF Release.
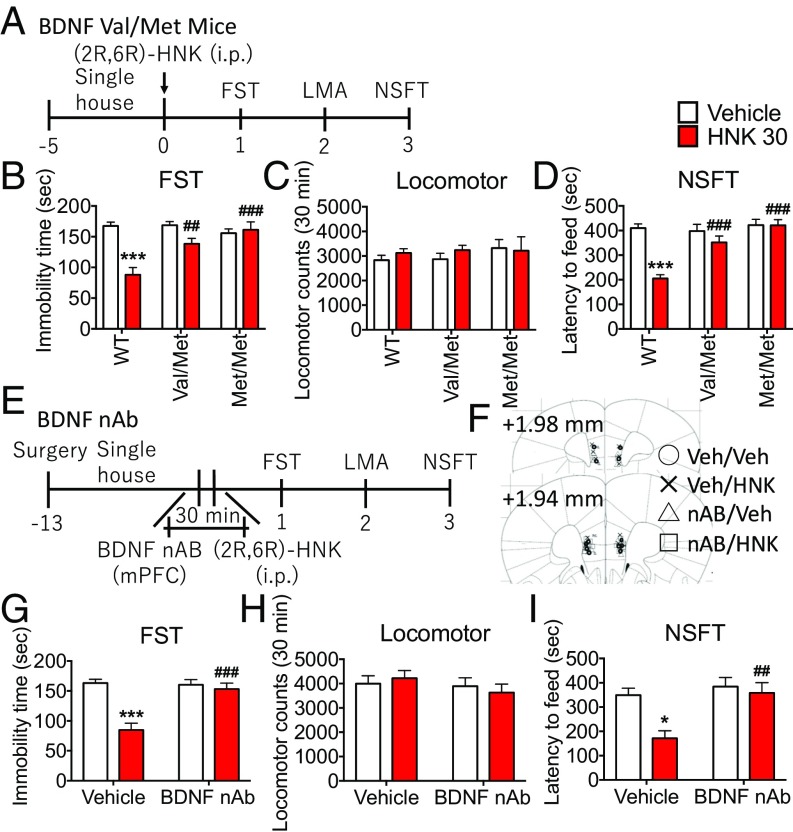
Here, we tested the role of BDNF in the actions of (2R,6R)-HNK using genetic and BDNF neutralizing antibody (nAb) approaches. First, we examined the actions of (2R,6R)-HNK in mice with a knockin of the BDNF Val66Met allele, which blocks the processing and activity-dependent release of mature BDNF (Fig. 2A) (19). (2R,6R)-HNK produced significant antidepressant effects in the FST and NSFT in Val/Val WT mice, but these effects were blocked in Val/Met and Met/Met mice (Fig. 2 B and D); there were no effects of (2R,6R)-HNK or genotype on locomotor activity or food consumption and no effects of genotype on baseline levels of immobility or latency to feed (Fig. 2 B–D and SI Appendix, Fig. S2A).
Fig. 2.
Antidepressant effects of (2R,6R)-HNK require BDNF release. (A) Val/Val (WT), Val/Met, and Met/Met mice were administered vehicle or (2R,6R)-HNK (30 mg/kg, i.p.), followed by behavioral testing. (B–D) (2R,6R)-HNK produced significant antidepressant effects in the FST and NSFT in Val/Val mice, but these effects were blocked in Val/Met and Met/Met mice. There were no effects on locomotor activity and no effects of genotype on immobility time or latency to feed. (E) Mice received bilateral infusions of BDNF nAb (0.2 μg per side) into the mPFC before administration of (2R,6R)-HNK, followed by behavioral tests. (F) Location of cannula placements in the mPFC. (G and I) Infusions of BDNF nAb blocked the antidepressant actions of (2R,6R)-HNK, including the reduction of immobility time in the FST and latency in the NSFT. (H) There were no effects of (2R,6R)-HNK or BDNF nAb infusion on locomotor activity and no effects of BDNF nAb alone on immobility time or latency to feed. Bars represent mean ± SEM, n = 5 to 8 per group. ***P < 0.001, *P < 0.05 compared with vehicle-treated WT group or vehicle-treated vehicle group; ###P < 0.001, ##P < 0.01 compared with (2R,6R)-HNK–treated WT group or (2R,6R)-HNK–treated vehicle group; Tukey’s multiple comparison test after significant results of two-way ANOVA.
A BDNF nAb was microinjected into the mPFC (0.2 μg per side; Fig. 2E) as previously described (14) to test the requirement for mPFC and to further test the requirement for BDNF release. The results demonstrate that BDNF nAb infusion completely blocked the antidepressant actions of (2R,6R)-HNK, including the reduction of immobility time in the FST and latency in the NSFT (Fig. 2 G and I); there were no effects of (2R,6R)-HNK or BDNF nAb infusion on locomotor activity or food consumption (Fig. 2H and SI Appendix, Fig. S2B) and no effects of BDNF nAb alone on immobility time or latency to feed (Fig. 2 G and I).
To test the involvement of activation of L-type VDCCs, we tested the influence of the L-type VDCC blocker verapamil on the antidepressant actions of (2R,6R)-HNK. Pretreatment with verapamil (10 mg/kg) significantly blocked the reduction of immobility time and latency to feed induced by (2R,6R)-HNK in the FST and NSFT (SI Appendix, Figs. S3 A, B, and D); there were no effects on locomotor activity or food consumption, and verapamil alone had no effect on immobility or latency to feed (SI Appendix, Fig. S3 B–E).
To further test the role of BDNF signaling, the role of TrkB, a high-affinity tyrosine kinase receptor, was examined by microinjection of the selective inhibitor ANA 12 (0.02 nmol per side) into the mPFC. Infusions of ANA 12 significantly blocked the reduction of immobility time and latency to feed induced by (2R,6R)-HNK in the FST and NSFT (SI Appendix, Fig. S4 A, C, and E); there were no effects on locomotor activity or food consumption, and ANA 12 alone had no effect on immobility or latency to feed (SI Appendix, Fig. S4 C–F).
(2R,6R)-HNK Stimulates BDNF Release in Primary Neuronal Cultures.
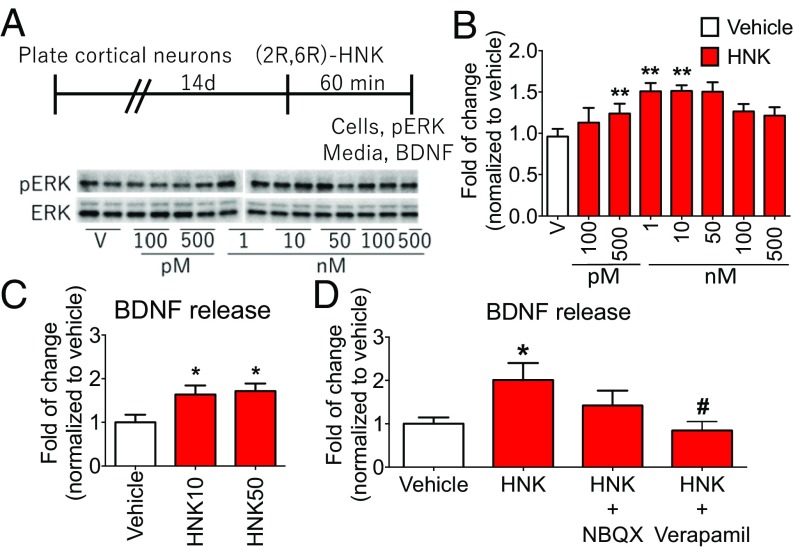
To directly test whether (2R,6R)-HNK induces BDNF release, we used a primary cortical cell culture system as previously described (13, 14). First, to characterize the concentration- and time-dependent effects of (2R,6R)-HNK, we measured levels of ERK phosphorylation, a reliable marker of in vitro drug effects (13). We found that (2R,6R)-HNK produced a concentration-dependent increase of phospho-ERK (with optimal effects at 1 to 50 nM) and reduced levels at higher doses (100 to 500 nM), demonstrating an inverted U-shaped dose response (Fig. 3 A and B). We also found that (2R,6R)-HNK incubation for 60 min, but not 15 or 30 min, stimulated phospho-ERK levels (SI Appendix, Fig. S5 A and B). (2R,6R)-HNK stimulation of phospho-ERK was blocked by preincubation with the selective TrkB inhibitor K252a or the AMPA receptor antagonist 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) (SI Appendix, Fig. S6 B and D); K252a or NBQX alone had no effect on phospho-ERK levels (SI Appendix, Fig. S6 B and D).
Fig. 3.
(2R,6R)-HNK stimulates BDNF release in primary neuronal cultures. (A) Schematic showing timeline for primary cortical culture experiments. After drug incubations, cells were collected, and the phosphorylation levels of ERK and total ERK were determined by Western blot analysis (B) and media was collected and BDNF release was determined by ELISA (C and D). (B) Primary cortical cultures were treated with (2R,6R)-HNK (100 pM to 500 nM) for 60 min. (Left) Representative Western blot images are shown. (Right) Levels of phospho-ERK are presented as a ratio by dividing phopho-ERK by total ERK. Treatment with (2R,6R)-HNK (1, 10, and 50 nM; 60 min) significantly increased phospho-ERK levels. (C) (2R,6R)-HNK at both 10 and 50 nM (60 min) significantly increased BDNF release into the media. (D) Influence of preincubation (20 min) with NBQX (50 μM) or verapamil (10 µM) on (2R,6R)-HNK (10 nM; 60 min) stimulation of BDNF release in primary cultured neurons. (2R,6R)-HNK–stimulated BDNF release was completely blocked by pretreatment with verapamil. BDNF levels were normalized to levels of protein and are presented as a ratio compared with vehicle to allow for values to be compared across experiments. The mean absolute value for BDNF level in the vehicle group is 15.2 ± 4.7 pg/mL. Bars represent mean ± SEM, n = 4 to 8 per group. **P < 0.01, *P < 0.05 compared with vehicle group or vehicle-treated vehicle group; #P < 0.05 compared with vehicle-treated (2R,6R)-HNK group; Fisher’s least significant difference multiple comparison test after significant results of one-way ANOVA.
Using the optimal dosing determined in the phospho-ERK studies, we tested the influence of (2R,6R)-HNK incubation on BDNF release in primary cortical neurons (Fig. 3C). (2R,6R)-HNK (60 min) at both 10 and 50 nM significantly increased BDNF release into the media (Fig. 3C). We also found that (2R,6R)-HNK (10 nM) stimulation of BDNF release was attenuated by addition of NBQX and was completely blocked by incubation with the VDCC blocker verapamil (Fig. 3D); previous studies have demonstrated that NBQX or verapamil alone had no effect on BDNF release in primary cortical cultures (13, 14, 20).
Inhibition of mTORC1 Signaling Blocks the Antidepressant Actions of (2R,6R)-HNK.
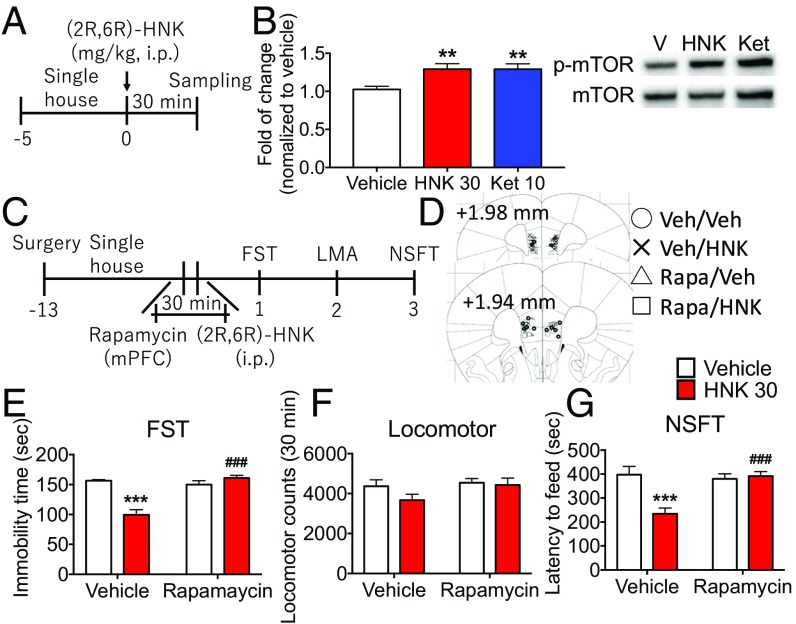
To test whether (2R,6R)-HNK activates mTORC1 signaling, we examined the effect of (2R,6R)-HNK on the phosphorylation of mTOR. In our preliminary studies conducted at 60 min, we found no effect of (2R,6R)-HNK administration on phospho-mTOR levels in the mPFC of mice (SI Appendix, Fig. S7 A and B). However, examination of additional time points revealed that (2R,6R)-HNK and ketamine increased phospho-mTOR levels at 30 min after administration, indicating a rapid but transient induction of mTORC1 signaling in mice (Fig. 4 A and B). We also found that (2R,6R)-HNK increased mTORC1 signaling in primary cortical cultures, determined by increased levels of phospho-p70S6K, a more stable marker of mTORC1 signaling in cultured cells, and this effect was blocked by the TrkB inhibitor K252a (SI Appendix, Figs. S5C and S6C). These results indicate that (2R,6R)-HNK activates mTORC1 signaling and that this effect is mediated via activation of TrkB receptors.
Fig. 4.
Inhibition of mTORC1 signaling blocks the antidepressant actions of (2R,6R)-HNK. (A) Mice received administration of (2R,6R)-HNK (30 mg/kg, i.p.) or ketamine (10 mg/kg, i.p.), and PFC dissections were collected 30 min later. (B) (2R,6R)-HNK and ketamine significantly increased phospho-mTOR. Levels of phospho-mTOR were determined by Western blot analysis (Right) and are presented as a ratio divided by total mTOR (Left). Bars represent mean ± SEM, n = 5 to 6 per group. **P < 0.01 compared with vehicle group, Fisher’s least significant difference multiple comparison test after significant results of one-way ANOVA. (C) Mice received bilateral infusion of rapamycin (0.02 nmol per side) in the mPFC before administration of (2R,6R)-HNK (30 mg/kg, i.p.), followed by behavioral tests starting 24 h later. (D) Location of cannula placements in the mPFC. (E–G) Infusions of rapamycin blocked the reduction of immobility time and latency to feed induced by (2R,6R)-HNK in the FST and NSFT; there were no effects on locomotor activity, and rapamycin alone had no effect on immobility time or latency to feed. Bars represent mean ± SEM, n = 6 to 8 per group. ***P < 0.001 compared with vehicle-treated vehicle group; ###P < 0.001 compared with vehicle-treated (2R,6R)-HNK group; Tukey’s multiple comparison test after significant results of two-way ANOVA.
To examine the involvement of mTORC1 signaling in the antidepressant actions of (2R,6R)-HNK, we used the selective mTORC1 inhibitor rapamycin (0.02 nmol per side). Microinjection of rapamycin into the mPFC blocked the reduction of immobility time and latency to feed induced by (2R,6R)-HNK in the FST and NSFT, respectively (Fig. 4 C, E, and G); there were no effects on locomotor activity or food consumption, and rapamycin alone had no effect on immobility time or latency to feed (Fig. 4 E–G and SI Appendix, Fig. S7C).
(2R,6R)-HNK Increases Synaptic Function in mPFC.
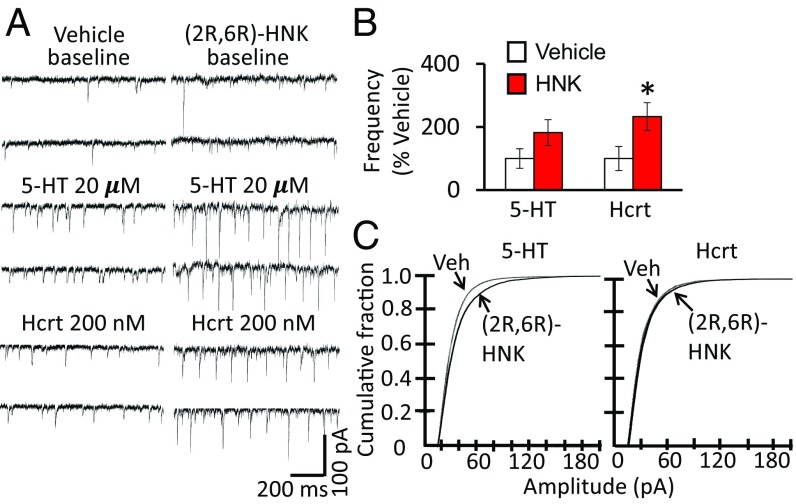
Here, we tested the effects of (2R,6R)-HNK on synaptic function by assessing excitatory postsynaptic currents (EPSCs). In the present study, we not only found that (2R,6R)-HNK administration significantly increased the frequency of hypocretin-induced EPSCs in layer V pyramidal neurons (determined 24 h after dosing), but also found a tendency for increased serotonin (5-HT)-induced EPSC frequency (Fig. 5 A and B). (2R,6R)-HNK significantly increased the amplitude of 5-HT–induced EPSCs and produced a small but significant increase in hypocretin-induced EPSC amplitude (Fig. 5C), similar to ketamine (11). These results demonstrate that (2R,6R)-HNK increases synaptic function of layer V pyramidal neurons in the mPFC.
Fig. 5.
(2R,6R)-HNK increases synaptic function in the mPFC. Mice were administered vehicle or (2R,6R)-HNK (30 mg/kg, i.p.) 24 h before slice preparation and electrophysiological recordings. Layer V pyramidal neurons were patched and 5-HT–induced or hypocretin (Hcrt)-induced EPSCs were determined. (A) Representative traces of EPSC recordings from saline vehicle and (2R,6R)-HNK–treated mice. (B) (2R,6R)-HNK administration significantly increased the frequency of hypocretin-induced EPSCs in layer V pyramidal neurons; there was also a tendency for increased 5-HT–induced EPSC frequency. EPSC frequencies are mean ± SEM, percent of control, n = 20 cells from six mice for vehicle, n = 23 cells from six mice for (2R,6R)-HNK; *P < 0.05 compared with vehicle group; Student’s t test. (C) Cumulative probability distributions showing that (2R,6R)-HNK significantly increased the amplitude of 5-HT–induced EPSCs as well as hypocretin-induced EPSCs. Cumulative probability distributions showing significantly increased amplitudes (Kolmogorov–Smirnov two-sample test). Veh, vehicle.
To examine spine number, layer V neurons were filled with neurobiotin while recording and then subjected to confocal imaging. The results demonstrate that (2R,6R)-HNK had no effects on total spine density or spine subtypes, including stubby, thin, or mushroom spines (SI Appendix, Fig. S8); similar effects were observed at three different levels from the neuronal cell body (SI Appendix, Fig. S8B). This was unexpected, as previous studies have found a correlation between synaptic function (i.e., 5-HT–induced and hypocretin-induced EPSCs) and spine density and diameter (11, 12, 16, 21, 22).
To extend our studies of BDNF signaling and synaptic function, we also examined the role of Rac1, a member of the Rho family of GTPase that is required for the synaptic alterations induced by BDNF/TrkB signaling in cultured neurons (23). Microinjection of the selective Rac1 inhibitor NSC 23766 (3.76 nmol per side) into the mPFC significantly attenuated the reduction of immobility time and latency to feed induced by (2R,6R)-HNK, as well as by ketamine, in the FST and NSFT (SI Appendix, Fig. S9 A, C, and E); there were no effects on locomotor activity or food consumption, and NSC 23766 alone had no effect on immobility time or latency to feed (SI Appendix, Fig. S9 C–F). These findings indicate that activation of Rac1 in the mPFC is required for the antidepressant effects of (2R,6R)-HNK and ketamine.
Discussion
Previous studies demonstrate that a single dose of (2R,6R)-HNK produces rapid and sustained antidepressant actions in rodent models without the side-effect profile of ketamine (9), prompting clinical studies of the therapeutic efficacy of this metabolite. However, (2R,6R)-HNK does not have high-affinity blocking activity of NMDA receptors as reported for ketamine, and the signaling pathways have not been identified. Here, we demonstrate that the antidepressant effects of (2R,6R)-HNK are mediated by activity-dependent release of BDNF and mTORC1 signaling, resulting in increased synaptic function in the mPFC.
We first conducted dose–response studies to establish optimal conditions for our mechanistic studies of (2R,6R)-HNK. The results demonstrate significant antidepressant actions of (2R,6R)-HNK in three different behavioral paradigms (the FST, NSFT, and FUST), consistent with initial reports (9). We did find that a slightly higher dose of (2R,6R)-HNK (30 mg/kg) was required to produce effects equivalent to ketamine (10 mg/kg) and that the effects of (2R,6R)-HNK in the FUST were not as long lasting as ketamine. There have been some discrepancies in reports of the antidepressant actions of (2R,6R)-HNK, particularly in models of stress (learned helplessness) and social defeat (24, 25). The exact reasons for these discrepancies are unclear but could be due to variations in strain, species, or testing conditions (26). One of these studies (25) examined only a single dose of (2R,6R)-HNK, and it is possible that a higher dose could have produced an antidepressant response.
While the present findings confirm that systemic (2R,6R)-HNK produces antidepressant actions, the key brain regions underlying this effect have not been tested. Here, we show that infusion of (2R,6R)-HNK into the mPFC is sufficient to produce antidepressant actions similar to systemic (2R,6R)-HNK administration. This is consistent with previous reports that the mPFC is sufficient and necessary for the antidepressant effects of ketamine (27) as well as the effects of other rapid-acting agents, including rapastinel (28) and scopolamine (29). A recent correspondence has also reported that intra-mPFC infusion as well as systemic administration of (2R,6R)-HNK produces antidepressant effects in the FST (30). Moreover, brain imaging and postmortem studies implicate the mPFC in both the pathophysiology and treatment of depression (31–33).Together, these studies provide strong evidence that the mPFC is a key region for the actions of (2R,6R)-HNK, although studies of additional areas, including the hippocampus and amygdala are needed to further characterize the regional effects of (2R,6R)-HNK.
BDNF has been established as an important mediator of activity-dependent synaptic plasticity (23, 34–37). Moreover, activity-dependent release of BDNF and TrkB signaling play a key role in the antidepressant effects of ketamine (12, 38). Notably, the synaptic and antidepressant behavioral responses to ketamine are blocked both in BDNF Val66Met knockin mice (12), in which the processing and activity-dependent release of BDNF is blocked (19), and by infusion of a BDNF nAb into the mPFC (14). In addition, BDNF infusion produces sustained antidepressant effects in rodents (39–41). Here, we found that the antidepressant effects of (2R,6R)-HNK are blocked in BDNF Val/Met and Met/Met mice and by infusion of a BDNF nAb into the mPFC, indicating a requirement for BDNF release. This is supported by studies of primary cortical neurons, which provide direct evidence that (2R,6R)-HNK increases BDNF release. These effects were observed at lower concentrations of (2R,6R)-HNK (10 to 50 nM) than observed in hippocampal tissues slices (9), possibly due to the better access of drug in the monolayer primary cell culture system used in the current study. Moreover, we found that BDNF release is blocked by addition of the VDCC antagonist verapamil, consistent with evidence that activity-dependent BDNF release requires VDCC activation (42). This is confirmed by evidence demonstrating that the antidepressant behavioral responses to (2R,6R)-HNK are blocked by verapamil. Finally, evidence for BDNF-TrkB signaling is provided by studies demonstrating that microinjection of the selective TrkB receptor inhibitor ANA 12 into the mPFC blocks the antidepressant actions of (2R,6R)-HNK. Together, the results indicate that BDNF release and TrkB receptor activation in the mPFC are necessary for the antidepressant effects of (2R,6R)-HNK.
Ketamine stimulates mTORC1 signaling in the mPFC via activity-dependent release of BDNF and TrkB receptor signaling (11, 13, 14, 25, 43–46). Here, we found that (2R,6R)-HNK increases the phosphorylated and activated form of mTOR in the mPFC. Zanos et al. (9) did not observe stimulation of mTORC1 signaling, but this could be due to the region analyzed (hippocampus vs. mPFC in the current study) and the time point (60 min vs. 30 min in the current study). We also found that (2R,6R)-HNK increases levels of phosphorylated p70S6K, a downstream target of mTORC1, through the activation of TrkB receptors. In addition, the current results provide direct causal evidence for a role of mTORC1 by showing that the antidepressant behavioral actions of (2R,6R)-HNK are blocked by mPFC microinjection of the selective mTORC1 inhibitor rapamycin.
(2R,6R)-HNK is rapidly eliminated from the brain within hours (9, 47), even though it produces long-lasting antidepressant actions for up to 3 to 5 d as shown in the current study. These findings indicate that the long-lasting antidepressant effects of (2R,6R)-HNK are initiated by the stimulation of signaling pathways that result in rapid and sustained synaptic and behavioral actions. Our results demonstrating that (2R,6R)-HNK stimulates the release of BDNF, which contributes to long-lasting synaptic effects in cellular models of learning and memory, are consistent with this possibility. Here, we show that (2R,6R)-HNK increases the function of spine synapses in layer V pyramidal neurons in the mPFC. This includes increased frequency and/or amplitude of 5-HT–induced and hypocretin-induced EPSCs, which act via apically targeted corticocortical and thalamocortical inputs, respectively (48, 49). Further evidence that synaptic function is involved in the actions of (2R,6R)-HNK is provided by studies of the Rac1 Rho GTPase, a critical downstream mediator of BDNF/TrkB-induced synaptic plasticity (23, 50), which we have found is required for the antidepressant effects of BDNF infused into the mPFC (51). Here, we show that Rac1 activation is necessary for the antidepressant effects of (2R,6R)-HNK as well as ketamine, providing further evidence for enhanced synaptic function in the actions of (2R,6R)-HNK. However, the lack of effect on spine morphology was surprising, and the reason for this difference with ketamine is unclear. One possibility is that the effects of (2R,6R)-HNK are more subtle, and the changes in synapse number and morphology are present but below our level of detection. Another possibility is that (2R,6R)-HNK does not produce the same level of synaptogenic response, which is supported by evidence that (2R,6R)-HNK did not influence levels of synaptic proteins in the mPFC.
In summary, the results demonstrate that a single dose of (2R,6R)-HNK induces long-lasting antidepressant behavioral responses via activity-dependent BDNF release and stimulation of signaling pathways that enhance synaptic function in the mPFC. These effects of (2R,6R)-HNK occur in the absence of NMDA receptor blockade (9) but converge with the downstream actions of ketamine, which produces activity-dependent effects on BDNF release via blockade of NMDA receptors on GABA interneurons and disinhibition of glutamate transmission (11, 52, 53). Zanos et al. (9) reported that (2R,6R)-HNK had no activity at NMDA receptors, but a recent study reported inhibition of NMDA receptor currents in hippocampal slices, albeit at a very high concentration of (2R,6R)-HNK (50 µM) (54). To directly address this question and identify the initial cellular target, further studies are needed to determine whether region- and cell-specific deletion of NMDA receptor subunits blocks the antidepressant actions of (2R,6R)-HNK.
Materials and Methods
Animals and Drug Administration.
(2R,6R)-HNK (National Institute of Mental Health) and ketamine (Sigma-Aldrich) were dissolved in saline and administered i.p. Verapamil (Sigma-Aldrich) was injected i.p. 30 min before injection of (2R,6R)-HNK. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committees.
Surgical and Infusion Procedures.
Mice were anesthetized with ketamine (100 mg/kg)/xylazine (10 mg/kg, i.p.) and bilateral 26-gauge guide cannulae (Plastics One) were implanted into 0.3 mm above the infusion sites in the mPFC (anteroposterior, 1.8 mm from bregma; lateral, ±0.4 mm; ventral, −2.5 mm). After at least 11 d of recovery, mice were bilaterally infused with (2R,6R)-HNK (10 ng per side), saline [vehicle for (2R,6R)-HNK], a function-blocking anti-BDNF antibody (0.2 μg per side, EMD Millipore), sheep IgG (vehicle for function-blocking anti-BDNF antibody), ANA 12 (0.02 nmol per side; Tocris Bioscience), 1% DMSO (vehicle for ANA 12), NSC 23766 (3.76 nmol per side; Tocris Bioscience), rapamycin (0.02 nmol per side; Cell Signaling) or 10% DMSO (vehicle for rapamycin) for 2 min at a rate of 0.1 μL/min (total volume, 0.2 μL). The dose and timing of each compound administration are based on previous reports (27, 51, 55, 56).
Behavior Studies.
The FST, locomotor activity test, NSFT, and FUST were carried out as previously described (51).
Primary Cortical Culture.
Primary cortical culture was performed as previously described (13). For phospho-ERK and phospho-p70S6K Western blot analysis, neurons were incubated with (2R,6R)-HNK (100 pM to 500 nM) for 60 min or with (2R,6R)-HNK (10 nM) for 15, 30, or 60 min, and cells were collected. For blockade studies, neurons were incubated with K252a (500 nM) or NBQX (50 μM) 20 min before incubation with (2R,6R)-HNK (10 nM) for 60 min, and cells were collected. For BDNF analysis, neurons were incubated with (2R,6R)-HNK (10 or 50 nM) for 60 min, and cells were collected. For blockade studies, neurons were incubated with NBQX (50 μM) or verapamil (10 μM) 20 min before incubation with (2R,6R)-HNK (10 nM) for 60 min, and cells were collected. Measurement of BDNF was performed as previously described (13).
Western Blot.
The phosphorylation levels of mTOR, ERK, and p70S6K and the expression of postsynaptic proteins were evaluated on crude synaptoneurosome or crude cell homogenates of mouse PFC and were determined by Western blot as previously reported (11, 13). Primary antibodies included phospho-mTOR (Ser2448), total mTOR, phospho-p70S6K (Thr389), total p70S6K, phospho-ERK (Thr202/Tyr204), and total ERK. Densitometric analysis of phospho- and total immunoreactivity for each protein was conducted using Image Lab (Bio-Rad). Immunoreactivity was normalized to vehicle-treated control group values for each protein.
Electrophysiology and Spine Analysis.
Brain slices were prepared as previously described (16). Pyramidal neurons in layer V of coronal slices of the mPFC (400 μm) were visualized by videomicroscopy, and whole-cell recordings were performed with an Axoclamp-2B amplifier (Axon Instruments). Neurobiotin (0.3%) was added to the pipette solution to mark cells for later imaging. Postsynaptic currents were studied in the continuous single-electrode voltage-clamp mode (3000 Hz low-pass filter) clamped at −65 mV to remove inhibitory postsynaptic currents from EPSCs. After completion of recording, slices were processed with streptavidin conjugated to Alexa 594 (1:1,000) for visualization of labeled cells. Neurobiotin-filled mPFC layer V neurons were imaged, and spine density was determined as previously described (16). Images were collected on an Olympus confocal laser scanning microscope (FV3000) equipped with a 60× 1.42 N.A. objective at a zoom of 4.65× (XY pixel dimensions, 0.095 μm × 0.095 μm). Dendrites were sampled within the apical dendritic tuft at sites distal, midway, and proximal to distal bifurcation (sampled 60, 90, and 130 μm from the midline, respectively). Computerized analysis of z-stack images was performed in deconvolved confocal image stacks (AutoquantX Version 3.0.1; Media Cybernetics), and spines were and quantified by an experimenter blinded to treatment, using NeuronStudio software (57).
The experimental procedures are described in detail in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health Grants MH093897 and MH105910 (to R.S.D.), the Connecticut Mental Health Center (R.S.D.), Yale University (R.S.D.), and Taisho Pharmaceutical Co., Ltd. (K.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814709116/-/DCSupplemental.
References
- 1.Kessler RC, et al. National Comorbidity Survey Replication The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Mueller TI, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156:1000–1006. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Aan Het Rot M, Zarate CA, Jr, Charney DS, Mathew SJ. Ketamine for depression: Where do we go from here? Biol Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 6.Zarate CA, Jr, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 7.Ionescu DF, et al. Rapid and sustained reductions in current suicidal ideation following repeated doses of intravenous ketamine: Secondary analysis of an open-label study. J Clin Psychiatry. 2016;77:e719–e725. doi: 10.4088/JCP.15m10056. [DOI] [PubMed] [Google Scholar]
- 8.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanos P, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeng S, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu RJ, et al. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242–252. doi: 10.1016/j.neuropharm.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18:pyu033. doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voleti B, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu RJ, et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology. 2017;42:1231–1242. doi: 10.1038/npp.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: Potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ZY, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosal S, et al. Activity-dependent brain-derived neurotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biol Psychiatry. 2018;83:29–37. doi: 10.1016/j.biopsych.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu RJ, et al. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedrick NG, et al. Rho GTPase complementation underlies BDNF-dependent homo- and heterosynaptic plasticity. Nature. 2016;538:104–108. doi: 10.1038/nature19784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirayama Y, Hashimoto K. Lack of antidepressant effects of (2R,6R)-hydroxynorketamine in a rat learned helplessness model: Comparison with (R)-ketamine. Int J Neuropsychopharmacol. 2018;21:84–88. doi: 10.1093/ijnp/pyx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C, et al. (R)-ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry. 2017;82:e43–e44. doi: 10.1016/j.biopsych.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto K, Shirayama Y. What are the causes for discrepancies of antidepressant actions of (2R,6R)-hydroxynorketamine? Biol Psychiatry. 2018;84:e7–e8. doi: 10.1016/j.biopsych.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Fuchikami M, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci USA. 2015;112:8106–8111. doi: 10.1073/pnas.1414728112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgdorf J, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarria A, et al. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis. 2015;82:254–261. doi: 10.1016/j.nbd.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham TH, et al. Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry. 2018;84:e3–e6. doi: 10.1016/j.biopsych.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murrough JW. Ketamine as a novel antidepressant: From synapse to behavior. Clin Pharmacol Ther. 2012;91:303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosch M, et al. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron. 2014;82:444–459. doi: 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leal G, Afonso PM, Salazar IL, Duarte CB. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015;1621:82–101. doi: 10.1016/j.brainres.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Song M, Martinowich K, Lee FS. BDNF at the synapse: Why location matters. Mol Psychiatry. 2017;22:1370–1375. doi: 10.1038/mp.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Naumenko VS, et al. Effect of brain-derived neurotrophic factor on behavior and key members of the brain serotonin system in genetically predisposed to behavioral disorders mouse strains. Neuroscience. 2012;214:59–67. doi: 10.1016/j.neuroscience.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 42.Jourdi H, et al. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29:8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koike H, Fukumoto K, Iijima M, Chaki S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav Brain Res. 2013;238:48–52. doi: 10.1016/j.bbr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Harraz MM, Tyagi R, Cortés P, Snyder SH. Antidepressant action of ketamine via mTOR is mediated by inhibition of nitrergic Rheb degradation. Mol Psychiatry. 2016;21:313–319. doi: 10.1038/mp.2015.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller OH, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. eLife. 2014;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang C, et al. VGF function in depression and antidepressant efficacy. Mol Psychiatry. 2018;23:1632–1642. doi: 10.1038/mp.2017.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maxwell CR, et al. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- 48.Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 49.Lambe EK, Aghajanian GK. Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron. 2003;40:139–150. doi: 10.1016/s0896-6273(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 50.Lai KO, et al. TrkB phosphorylation by Cdk5 is required for activity-dependent structural plasticity and spatial memory. Nat Neurosci. 2012;15:1506–1515. doi: 10.1038/nn.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato T, et al. BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry. December 5, 2017 doi: 10.1038/mp.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wohleb ES, Gerhard D, Thomas A, Duman RS. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15:11–20. doi: 10.2174/1570159X14666160309114549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–E3. doi: 10.1038/nature22084. [DOI] [PubMed] [Google Scholar]
- 55.Cazorla M, et al. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang JC, et al. Depression-like phenotype by deletion of α7 nicotinic acetylcholine receptor: Role of BDNF-TrkB in nucleus accumbens. Sci Rep. 2016;6:36705. doi: 10.1038/srep36705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.