Abstract
Human corneal endothelial cells are notorious for their restricted proliferative ability in vivo and in vitro. Hence, injury or dysfunction of these cells may easily result in blindness. Currently, the only treatment is to transplant a donor cornea that contains a healthy corneal endothelium. However there is a severe global shortage of donor corneas and there remains an unmet clinical need to engineer human corneal grafts with healthy corneal endothelium. In this review, we present current advances in the culture, expansion, and molecular understandings of corneal endothelial cells in vitro in order to help establish methods of engineering human corneal endothelial grafts.
Keywords: Cornea, Endothelium, Expansion, Mechanism
Background
The front part of the eye (i.e. cornea) is one of the most important human organs. In particular, the corneal endothelial cells are a vital component that regulate nutrition and hydration to maintain clear vision. Unfortunately, these cells are notorious for their limited proliferative ability. Consequently, loss in cell number or function caused by aging, injury or surgery (termed corneal endothelial dysfunction) can lead to corneal blindness. Treatment of this dysfunction traditionally requires a corneal transplant utilizing cadaver donor tissue however there is an increasing global shortage of donor corneal tissues. Therefore, it is paramount to find a replacement solution. In order to resolve this urgent problem, many scientists have researched alternatives instead of using cadaver tissue. One of the emerging methods is to use fetal or adult corneal stem cells/progenitors (which inherently have proliferative capabilities) to engineer appropriate corneal tissue grafts for surgical therapies. Another method is to reprogram adult human corneal cells to their progenitor status and then creating surgical grafts. Overall, these developments in human corneal stem cell therapies may lead to new discoveries in human corneal regenerative medicine and provide mechanistic understanding for other fields of medicine.
Substrates required for effective expansion of corneal endothelial cells (CEC)
Collagen IV is basement membrane-specific collagen which was found in the endothelial and epithelial layers [62], suggesting collagen IV is important for endothelial structure and functions. Since the first discovery of collagen IV by Kefalides in 1966, collagen IV has been investigated extensively by many research laboratories around the world. So far, six evolutionary related mammalian genes encoding six different polypeptide chains of collagen IV α-chain polypeptides (α1–α6) have been identified and characterized [22]. The NC1 domain (a long triple-helical collagenous domain and a globular non-collagenous C4 domain at the N and C terminus) is considered important for the assembly of the type IV collagen trimeric structure. The assembly of the trimer begins when three NC1 domains initiate a molecular interaction between three α-chains. Protomer trimerization proceeds like a zipper from the carboxy-terminal end, resulting in a fully assembled protomer and dimer formation of type IV collagen.
Collagen IV is very important to maintain the normal phenotype of CEC and prevent endothelial-mesenchymal transition (EMT). For example, bovine corneal endothelial cells lose their phenotype on glass or tissue culture polystyrene without Collagen IV coating. Additionally bovine corneal endothelial phenotype lose their phenotype when cultured on fibronectin and collagen I coating. In contrast, with collagen IV coating, the endothelial cells exhibit polygonal morphology, ZO-1 at the cell border, and F-actin located cortically [48], suggesting collagen IV plays a critical role in maintaining the phenotype of endothelial cells [37]. In addition, collagen IV may facilitate attachment and promote expansion of CEC [3, 80]. Consistent with this view, normal endothelial cells produce collagen IV as the major type of collagen while fibroblastic corneal endothelial cells produce collagen I as the major type of collagen [23]. Although collagen IV exerts unique functions for corneal endothelium as mentioned above, the mechanism remains unclear how collagen IV uniquely affects the behavior of human CEC aggregates (not single cells) [35, 36, 75–78].
Media and growth factors for expansion of human corneal endothelial cells (HCEC)
A number of cell culture media have been used for growth of HCECs, including DMEM, DMEM and F12, Ham’s F12 and M199, and Opti-MEM-I [44]. HCECs cultured in DMEM or DMEM and F12 could not be passaged more than once or twice [51]. On the contrary, HCECs expanded in Ham’s F12 and M199 or Opti-MEM-I could be passaged up to 3 times (reviewed in [44]) with HCEC markers, for example, Na + K+/ATPase and ZO-1 (reviewed in [44]). However, the cultured HCEC were not hexagonal in shape suggesting these medias are not optimal. In other reports, MEM, MESCM, and OptiMEM-I with 8% FBS, 20 ng/mL NGF, 5 ng/mL EGF have been found to be effective [19, 74, 79].
Growth Factors such as bFGF [7, 10–12, 57, 69], EGF [7, 33, 57, 58, 69, 75, 77], LIF [35, 79], NGF [7], may stimulate expansion of HCEC. We have reported that LIF delays contact-inhibition and is significantly more effective with bFGF without serum to promote HCEC growth without change of phenotype [35, 79].
Corneal differentiation from ESCs
Many cell types can be differentiated into HCEC [49]. CEC-like cells can be differentiated from hESCs [31]. The differentiation of hESCs to HCEC occurs via the periocular mesenchymal precursor (POMP) by a two-step inducement [7, 49]. The cells obtained from this method were characteristics of HCEC [71]. Cord blood-derived MSCs might also be differentiated into HCEC-like cells [20].
Traditional expansion of CEC
Traditionally, disruption of the contact inhibition has been adopted to unlock the mitotic block of CEC through the use of EDTA ± trypsin to break cell-cell junctions [50, 59]. In the presence of basic fibroblast growth factor (bFGF) [24, 26, 30, 32] or epithelial growth factor (EGF) [53], this conventional method has been used by several laboratories to promote human CEC proliferation as a strategy to engineer CEC grafts for tissue transplantation [6, 10, 13, 17, 18, 28, 35, 41, 43, 60, 68]. However, according to many studies including those conducted by the laboratory of Dr. Eundak Kay [26], this conventional strategy causes pathologic endothelial mesenchymal transition (EMT) [30, 32].
Mechanism of EMT
The key events of EMT in disruption of cell-cell junctions are: 1. loss of apical–basal polarity and acquisition of a front–rear polarity; 2. reorganization of the cytoskeletal architecture and changes in cell shape; 3. downregulation of an epithelial gene expression signature, activation of mesenchymal phenotype genes, increased cell protrusions and motility; and, 4. in many cases, an ability to degrade extracellular matrix proteins to enable invasive behavior [29, 39].
During the destabilization of adherent junctions, cell junctional proteins are cleaved at the plasma membrane and subsequently degraded or relocated [67]. Epithelial cadherin (E-cadherin) is cleaved at the plasma membrane and subsequently degraded. Consequently, β-catenin can no longer interact with E-cadherin and it is either degraded or protected from degradation (for example, in response to WNT signaling), so that it can act in transcription [45]. p120 catenin (also known as catenin δ1) also accumulates in the nucleus and participates in transcription following a decrease in E-cadherin levels [27]. In addition, the downregulation of E-cadherin is balanced by the increased expression of N-cadherin, which results in a ‘cadherin switch’ that alters cell adhesion [64, 67]. Through this switch, the transitioning cells lose their association with epithelial cells and acquire an affinity for mesenchymal cells through homotypic N-cadherin interactions, facilitating cell migration and invasion.
Cells that undergo EMT reorganize their cortical actin cytoskeleton into one that enables dynamic cell elongation and directional motility [61, 67]. New actin-rich membrane projections facilitate cell movement and act as sensory extension of the cytoskeleton. These projections include sheet-like membrane protrusions called lamellipodia and spike-like extensions called filopodia at the edge of lamellipodia [54]. EMT is characterized by increased cell contractility and actin stress fiber formation. These dynamic changes in actin organization are probably mediated by regulatory proteins such as moesin [14], but the molecular mechanisms controlling F-actin dynamics during EMT remain to be elucidated. Keratin and vimentin filaments regulate the trafficking of organelles and membrane-associated proteins, but they show differences in the proteins that they target to the membrane. Changes in intermediate filament composition also enable cell motility, possibly because of the interaction of vimentin with motor proteins [40]. However, how change of cytoskeleton is linked to change of cell-cell junctions and how the change of cytoskeleton and cell-cell junctions is linked to EMT remain unclear.
The change of cytoskeleton, actin dynamics and control of actin rearrangement during EMT are at least partially regulated by Rho GTPases. Among these, RhoA promotes actin stress fiber formation, whereas RAC1 and CDC42 mainly promote the formation of lamellipodia and filopodia. Rho GTPases also regulate the formation of cell–cell junction complexes and the stability of adherent junctions. After dissolution of cell-cell junctions, cytoplasmic p120 dissociates from the cell membrane and represses RhoA activity but activates RAC and CDC42 to induce the formation of membrane protrusions and cell motility [2]. In addition, RAC1 promotes microtubule polymerization and is activated by itself at the leading edge of cells, which sets up a positive feedback loop that participates in the reorganization of the microtubule cytoskeleton [66]. The reorganization of the cytoskeletal architecture and polarity complexes, which result in cell shape changes, cell elongation, membrane protrusions and front–rear polarity, are essential in EMT and enable directional migration. These results suggest balance of RhoA and Rac1 are major regulators of EMT. However, we do not know exactly how RhoA and Rac1 regulate EMT.
Novel approach for expansion of CEC without EMT
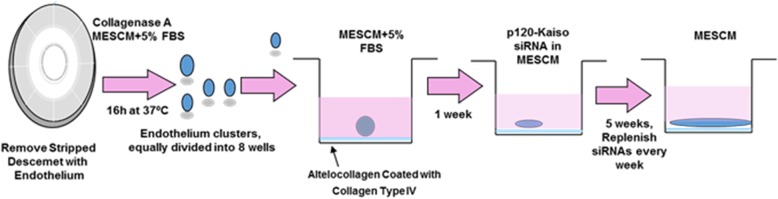
A novel strategy of effective expansion of human CEC monolayer with normal cell phenotype has been established by collagenase digestion (without disruption of cell-cell junctions) and p120-Kaiso knockdown (effective expansion). Because collagenase digestion selectively removes interstitial but not the basement membrane collagens, this method does not disrupt intercellular junctions nor interaction with the basement membrane. Indeed, collagenase digestion results in compact aggregates of human CEC, which retain intercellular junctions mediated by ZO-1 and connexin-43, and maintain their adhesion to such basement membrane components as collagen IV, laminin 5, and perlecan [33]. Using p120-Kaiso siRNA knockdown, human CEC monolayers have been successfully expanded in supplemental hormonal epithelial medium (SHEM) to an average size of 5.0 ± 0.4 mm in diameter from Descemet membrane stripped from 1/8 of the corneoscleral rim [75]. Knockdown by p120 siRNA selectively activates p120/Kaiso signaling as evidenced by nuclear translocation of p120 to release nuclear Kaiso (a repressor) and to induce colocalization of nuclear bromodeoxyuridine (BrdU) labeling [5, 75]. The knockdown regimen of p120 siRNA regarding dosing, frequency, and starting time has been further optimized and this optimized p120 siRNA knockdown is synergized by addition of Kaiso siRNA knockdown to further expand the size of human CEC monolayers to 11.0 ± 0.6 mm in diameter [76].
To confirm the phenotype of the generated HCEC, we have performed immunostaining for HCEC markers. The specific corneal endothelial markers include cytoplasmic γ-tubulin and p75NTR and junctional α-catenin, β-catenin, F-actin, N-cadherin, Na-K-ATPase, and p120 (all are markers of HCEC), without expression of LEF1 and S100A4 (both are markers of EMT) [75, 76, 78]. Such expression of HCEC markers was consistent with the in vivo expression pattern previously reported by us [75, 76, 78].
Mechanism of reprogramming CEC without EMT
Previously, it is reported that mitotic block of human CEC due to contact inhibition is established after three weeks of culturing in an EGF- and serum-containing medium termed SHEM [77]. However, a switch from SHEM to a serum-free LIF-containing medium termed MESCM delays contact inhibition by 2 weeks [35]. The delay in contact inhibition may permit human CEC to be responsive to reprogramming into NC progenitors by p120-Kaiso signaling, resulting in further expansion of human CEC monolayers to a transplantable size [78].
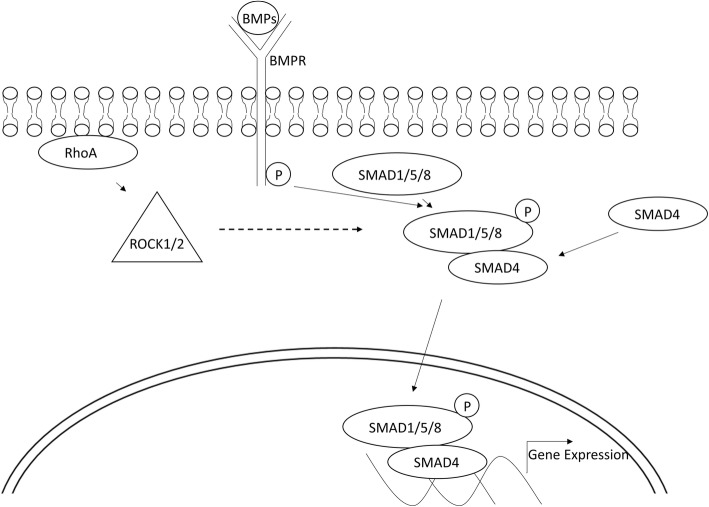
Subsequent investigation suggests that the expansion of human CEC in SHEM is closely correlated with the activation of RhoA signaling to promote BrdU labeling, which requires nuclear translocation of nuclear factor κB (pNFκB, p65, S276) [76]. Inhibition of RhoA by CT-04, ROCK by Y27632, bone morphological protein (BMP) by Noggin, TAK1 by 5Z-7-oxozeaenol, or NFκB by CAY10512 abolished nuclear translocation of pNFκB, which is required for nuclear translocation of p120 and BrdU labeling [76]. The nuclear translocation of pNFκB is mediated by BMPRI-TAK1-XIAP complex as a result of activation of the non-canonical BMP signaling [16, 18], evidenced by upregulation of BMP2, BMP4, BMPR1A, and BMPR1B transcripts but not nuclear location of pSmad1/5/8, and the lack of activation of inhibitor of differentiation 1–4 (ID1–4), which are known targets of nuclear Smads triggered by the canonical BMP signaling [76]. Interestingly, activation of canonical BMP signaling by p120-Kaiso siRNAs requires a switch of medium from EGF-containing serum-containing SHEM to leukemia Inhibitory Factor (LIF)-containing serum free MESCM [76]. As proposed, p120 acts, at least in part, through regulation of Rho GTPases and its downstream target ROCK1/2 [1].
RhoA-GTP can be activated by p120 siRNA and further by p120-Kaiso siRNAs [76]. This novel approach is summarized in Fig. 1. Activation of p120/Kaiso-RhoA-ROCK pathway may promote nuclear translocation of p120 to relieve the repressor activity of Kaiso, a member of BTB/POZ-ZF transcription factor family without disruption of cell-cell junctions, without activation of canonical Wnt signaling and thus without EMT [25, 55, 70, 75, 76], (also reviewed in [8]). Because activation of RhoA-ROCK signaling is clearly linked to inhibition of canonical Wnt signaling, we believe that inhibition of RhoA-ROCK signaling may relieve inhibition of canonical Wnt signaling and as a result, cause EMT.
Fig. 1.
Novel approach for expansion of HCEC without EMT
It remains unclear how p120-Kaiso siRNAs leads to activation of canonical BMP signaling. As reported [75, 76], weekly knockdown with p120-Kaiso siRNAs activates RhoA-ROCK signaling. RhoA may regulate the actin cytoskeleton and the expression of genes associated with cell proliferation. As suggested, RhoA may upregulate cJUN expression through ROCK (independently from the ability of ROCK to promote actin polymerization), which either indirectly via activation [38] of JNK or directly via phophorylation of cJUN and ATF2, which then bind with the AP1 site of cJUN promoter in NIH 3 T3 and HEK293T cells to promote cJUN expression [38]. DNA sequence analysis has revealed 4 AP-1 sites and 12 AP-1 sites in the BMP4 and BMP6 promoter, respectively (ENCODE data at UCSC). Because overexpression of cJUN promotes BMP4 transcription in Xenopus embryos, and because the AP-1 site serves as additional activatory component for the auto-regulation of BMP4 [9, 21, 56], we believe that JNK-cJUN signaling serves as a “missing” link for p120-RhoA-ROCK signaling to activate canonical BMP signaling that is required to promote the reprogramming of human CEC to progenitors. The RhoA-ROCK-canonical BMP signaling is summarized in Fig. 2.
Fig. 2.
RhoA-ROCK-canonical BMP signaling in reprogramming of HCEC
p16INK4a belongs to the family of cyclin-dependent kinase inhibitors (CKIs) [4]. Nuclear translocation of phosphorylated p16INK4a is one hallmark for CEC senescence, an irreversible arrest during the G1 transition of the cell cycle [15, 47, 52]. This a notion consistent with what has been previously reported [42] and senescence has been recognized as a barrier for reprogramming into iPSC [4, 15, 34]. Therefore, it is important to study whether nuclear exclusion of p16INK4a is critical for reprogramming adult human CEC into neural crest progenitors, and if so, how nuclear exclusion of p16INK4a is achieved. JAK2 signaling may serve as a “missing” link for p120-RhoA-ROCK signaling to activate STAT3 Tyr 705 signaling which is required to promote reprogramming of human CEC to progenitors via activation of Bmi1-mediated inhibition (Bmi1 is an inhibitor of p16) of p16-mediated senescence.
Lineage-tracing technology
Zhao et al. have reported corneal endothelial cells could be induced from human iPSCs [73], resulting in differentiation of iPSCs into corneal stem cells by expressing corneal lineage transcription factors, LHX2, PAX6, and VSX2 and subsequent expression of corneal neural crest stem cells and corneal endothelial cells (reviewed in [63]). Therefore, it is assumed that a subset of neural crest cells are corneal specific and can be corneal endothelial cells. Zhao et al. also reported generation of HCEC from iPSCs obtained from human foreskin fibroblasts [73]. Another study obtained iPSCs from human dermal fibroblasts [65], suggesting that we may obtain corneal endothelial cells from human iPSCs. We have reported that HCEC can be induced from human trabecular meshwork cells [72]. However, it remains unclear whether the stem cell-CEC resemble mature HCEC? To our knowledge, it is best to obtain HCEC from a subset of HCEC progenitors [76, 78], not from other origins [5], because those cells are more phenotypic than other HCEC induced from iPSCs or other resources.
Prospect of engineering of human CEC grafts for clinical applications
In the clinical application of cultured HCEC, many issues require consideration. For example, unwanted issues associated with application of cultured HCEC are usually linked to the microenvironment in which cultured HCEC engraft, causing unfavorable differentiation of transplanted cultured cells. This may be caused by many factors including unfavorable substrates, isolation method (substrates other than collagen IV, isolation method EDTA and trypsin), media for cell culture (culture media other than MESCM) as we have previously tested and reported (reviewed in [80]), or tumor growth (reviewed in [46]). In addition, applications of cultured HCEC may require immune-suppression for prevention of rejection of cultured HCEC grafts as we have experienced in a mini pig model. Furthermore, anatomical non-limbus HCEC, for example, human central CEC are extremely difficult to expand, while human peripheral CEC are expandable. However, it remains unclear whether HCEC expanded from peripheral areas are functional as those in the central area.
Pre-clinical animal models are required for the examination of the safety and efficacy of engineered corneal endothelial grafts. The current challenges for engineering human CEC are the difficulties for effective isolation and expansion of CEC without EMT. Still there are no cell-based therapies to cure human corneal endothelial dysfunction up to now. Therefore, it is important to strive for resolving the engineering issues of human CEC grafts in the near future.
Conclusions
This review has summarized the latest progress in substrate selection, in vitro expansion, and tissue engineering of human corneal endothelial grafts. Based on recent progress, the future is bright and this novel technique may generate a new treatment of CEC dysfunction and human blindness.
Acknowledgements
Not applicable.
Funding
This review has been supported by the National Eye Institute, National Institutes of Health (grant numbers R43 EY 022502–01 and R44 EY 022502–02, to Yingting Zhu), USA.
Availability of data and materials
Not applicable.
Abbreviations
- bFGF
Basic fibroblast growth factor
- BMP
Bone morphological protein
- BrdU
Bromodeoxyuridine
- C4
Domain at the N and C terminus
- CEC
Corneal endothelial cells
- CKI
Cyclin-dependent kinase inhibitor
- E-cadherin
Epithelial cadherin
- EGF
Epithelial grow factor
- EMT
Endothelial-mesenchymal transition
- GAP
GTPase-activating protein
- GEF
Guanine nucleotide exchange factor
- HCEC
Human corneal endothelial cells
- ID
Inhibitor of differentiation
- JAK
Janus kinase/signal transducer
- JNK
Jun N-terminal kinase
- LIF
Leukemia Inhibitory Factor
- MAPK
Mitogen-activated protein kinase
- NC1 domain
A long triple-helical collagenous domain and a globular non-collagenous
- N-cadherin
Neural cadherin
- NFκB
Nuclear factor κB
- p16INK4a
Cyclin-dependent kinase 4 inhibitor A
- SHEM
Supplemental hormonal epithelial medium
- STAT
Activators of transcription
- ZO-1
Tight junction protein 1
Authors’ contributions
SC, QZ and HS participated in collecting information and drafting the manuscript. YZ, ST and LX contributed to collecting information and editing the manuscript. YZ directed the work and finalized the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
No applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuangling Chen, Email: shuanglingchen@gmail.com.
Qin Zhu, Email: zhuqin619@163.com.
Hong Sun, Email: hollysh8@sina.com.
Yuan Zhang, Email: yZhang@tissuetechinc.com.
Sean Tighe, Email: stighe@tissuetechinc.com.
Li Xu, Email: xuli-113@163.com.
Yingting Zhu, Phone: (786) 456-7632, Email: yzhu@tissuetechinc.com.
References
- 1.Anastasiadis PZ. p120-ctn: A nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta. 2007;1773(1):34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Anastasiadis PZ, Reynolds AB. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol. 2001;13(5):604–610. doi: 10.1016/s0955-0674(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 3.Ando J, Albelda SM, Levine EM. Culture of human adult endothelial cells on liquid-liquid interfaces: a new approach to the study of cell-matrix interactions. In Vitro Cell Dev Biol. 1991;27A(7):525–532. doi: 10.1007/BF02631282. [DOI] [PubMed] [Google Scholar]
- 4.Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, Vallier L, Gil J. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23(18):2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HC, Zhu YT, Chen SY, Tseng SC. Selective Activation of p120(ctn)-Kaiso Signaling to Unlock Contact Inhibition of ARPE-19 Cells without Epithelial-Mesenchymal Transition. PLoS One. 2012;7(5):e36864. doi: 10.1371/journal.pone.0036864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen KH, Azar D, Joyce NC. Transplantation of adult human corneal endothelium ex vivo: a morphologic study. Cornea. 2001;20(7):731–737. doi: 10.1097/00003226-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Chen JZ, Shao CY, Li CY, Zhang YD, Lu WJ, Fu Y, Gu P, Fan X. Treatment with retinoic acid and lens epithelial cell-conditioned medium in vitro directed the differentiation of pluripotent stem cells towards corneal endothelial cell-like cells. Exp Ther Med. 2015;9(2):351–360. doi: 10.3892/etm.2014.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel JM. Dancing in and out of the nucleus: p120(ctn) and the transcription factor Kaiso. Biochim Biophys Acta. 2007;1773(1):59–68. doi: 10.1016/j.bbamcr.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 9.Dennler S, Prunier C, Ferrand N, Gauthier JM, Atfi A. c-Jun inhibits transforming growth factor beta-mediated transcription by repressing Smad3 transcriptional activity. J Biol Chem. 2000;275(37):28858–28865. doi: 10.1074/jbc.M910358199. [DOI] [PubMed] [Google Scholar]
- 10.Engelmann K, Bohnke M, Friedl P. Isolation and long-term cultivation of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 1988;29(11):1656–1662. [PubMed] [Google Scholar]
- 11.Engelmann K, Friedl P. Growth of human corneal endothelial cells in a serum-reduced medium. Cornea. 1995;14(1):62–70. [PubMed] [Google Scholar]
- 12.Engelmann K, Friedl P. Optimization of culture conditions for human corneal endothelial cells. In Vitro Cell Dev Biol. 1989;25(11):1065–1072. doi: 10.1007/BF02624143. [DOI] [PubMed] [Google Scholar]
- 13.Hatou S, Yoshida S, Higa K, Miyashita H, Inagaki E, Okano H, Tsubota K, Shimmura S. Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and Wnt/beta-catenin signaling. Stem Cells Dev. 2013;22(5):828–839. doi: 10.1089/scd.2012.0286. [DOI] [PubMed] [Google Scholar]
- 14.Haynes J, Srivastava J, Madson N, Wittmann T, Barber DL. Dynamic actin remodeling during epithelial-mesenchymal transition depends on increased moesin expression. Mol Biol Cell. 2011;22(24):4750–4764. doi: 10.1091/mbc.E11-02-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirosue A, Ishihara K, Tokunaga K, Watanabe T, Saitoh N, Nakamoto M, Chandra T, Narita M, Shinohara M, Nakao M. Quantitative assessment of higher-order chromatin structure of the INK4/ARF locus in human senescent cells. Aging Cell. 2012;11(3):553–556. doi: 10.1111/j.1474-9726.2012.00809.x. [DOI] [PubMed] [Google Scholar]
- 16.Hofer-Warbinek R, Schmid JA, Stehlik C, Binder BR, Lipp J, de MR. Activation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J Biol Chem. 2000;275(29):22064–22068. doi: 10.1074/jbc.M910346199. [DOI] [PubMed] [Google Scholar]
- 17.Hsiue GH, Lai JY, Chen KH, Hsu WM. A novel strategy for corneal endothelial reconstruction with a bioengineered cell sheet. Transplantation. 2006;81(3):473–476. doi: 10.1097/01.tp.0000194864.13539.2c. [DOI] [PubMed] [Google Scholar]
- 18.Ishino Y, Sano Y, Nakamura T, Connon CJ, Rigby H, Fullwood NJ, Kinoshita S. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci. 2004;45(3):800–806. doi: 10.1167/iovs.03-0016. [DOI] [PubMed] [Google Scholar]
- 19.Jackel T, Knels L, Valtink M, Funk RH, Engelmann K. Serum-free corneal organ culture medium (SFM) but not conventional minimal essential organ culture medium (MEM) protects human corneal endothelial cells from apoptotic and necrotic cell death. Br J Ophthalmol. 2011;95(1):123–130. doi: 10.1136/bjo.2010.183418. [DOI] [PubMed] [Google Scholar]
- 20.Joyce NC, Harris DL, Markov V, Zhang Z, Saitta B. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol Vis. 2012;18:547–564. [PMC free article] [PubMed] [Google Scholar]
- 21.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 22.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 23.Kay EP. Expression of types I and IV collagen genes in normal and in modulated corneal endothelial cells. Invest Ophthalmol Vis Sci. 1989;30(2):260–268. [PubMed] [Google Scholar]
- 24.Kay EP, Lee MS, Seong GJ, Lee YG. TGF-beta s stimulate cell proliferation via an autocrine production of FGF-2 in corneal stromal fibroblasts. Curr Eye Res. 1998;17(3):286–293. doi: 10.1076/ceyr.17.3.286.5212. [DOI] [PubMed] [Google Scholar]
- 25.Kelly KF, Spring CM, Otchere AA, Daniel JM. NLS-dependent nuclear localization of p120ctn is necessary to relieve Kaiso-mediated transcriptional repression. J Cell Sci. 2004;117(Pt 13):2675–2686. doi: 10.1242/jcs.01101. [DOI] [PubMed] [Google Scholar]
- 26.Ko MK, Kay EP. Regulatory role of FGF-2 on type I collagen expression during endothelial mesenchymal transformation. Invest Ophthalmol Vis Sci. 2005;46(12):4495–4503. doi: 10.1167/iovs.05-0818. [DOI] [PubMed] [Google Scholar]
- 27.Kourtidis A, Ngok SP, Anastasiadis PZ. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci. 2013;116:409–432. doi: 10.1016/B978-0-12-394311-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laing RA, Neubauer L, Oak SS, Kayne HL, Leibowitz HM. Evidence for mitosis in the adult corneal endothelium. Ophthalmology. 1984;91(10):1129–1134. doi: 10.1016/s0161-6420(84)34176-8. [DOI] [PubMed] [Google Scholar]
- 29.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HT, Lee JG, Na M, Kay EP. FGF-2 induced by interleukin-1 beta through the action of phosphatidylinositol 3-kinase mediates endothelial mesenchymal transformation in corneal endothelial cells. J Biol Chem. 2004;279(31):32325–32332. doi: 10.1074/jbc.M405208200. [DOI] [PubMed] [Google Scholar]
- 31.Lee JB, Song JM, Lee JE, Park JH, Kim SJ, Kang SM, Kwon JN, Kim MK, Roh SI, Yoon HS. Available human feeder cells for the maintenance of human embryonic stem cells. Reproduction. 2004;128(6):727–735. doi: 10.1530/rep.1.00415. [DOI] [PubMed] [Google Scholar]
- 32.Lee JG, Kay EP. FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Exp Eye Res. 2006;83(6):1309–1316. doi: 10.1016/j.exer.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Sabater AL, Chen YT, Hayashida Y, Chen SY, He H, Tseng SC. A novel method of isolation, preservation, and expansion of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2007;48(2):614–620. doi: 10.1167/iovs.06-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Zhou H, Abujarour R, Zhu S, Young JJ, Lin T, Hao E, Scholer HR, Hayek A, Ding S. Generation of Human-Induced Pluripotent Stem Cells in the Absence of Exogenous Sox2. Stem Cells. 2009;27(12):2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Tseng SC, Zhang MC, Chen SY, Tighe S, Lu WJ, Zhu YT. LIF-JAK1-STAT3 signaling delays contact inhibition of human corneal endothelial cells. Cell Cycle. 2015;14(8):1197–1206. doi: 10.1080/15384101.2015.1013667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu WJ, Tseng SC, Chen S, Tighe S, Zhang Y, Liu X, Chen SY, Su CW, Zhu YT. Senescence Mediated by p16INK4a Impedes Reprogramming of Human Corneal Endothelial Cells into Neural Crest Progenitors. Sci Rep. 2016;6:35166. doi: 10.1038/srep35166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madden PW, Lai JN, George KA, Giovenco T, Harkin DG, Chirila TV. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials. 2011;32(17):4076–4084. doi: 10.1016/j.biomaterials.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 38.Marinissen MJ, Chiariello M, Tanos T, Bernard O, Narumiya S, Gutkind JS. The small GTP-binding protein RhoA regulates c-jun by a ROCK-JNK signaling axis. Mol Cell. 2004;14(1):29–41. doi: 10.1016/s1097-2765(04)00153-4. [DOI] [PubMed] [Google Scholar]
- 39.Medici D, Kalluri R. Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin Cancer Biol. 2012;22(5-6):379–384. doi: 10.1016/j.semcancer.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mimura T, Amano S, Usui T, Araie M, Ono K, Akihiro H, Yokoo S, Yamagami S. Transplantation of corneas reconstructed with cultured adult human corneal endothelial cells in nude rats. Exp Eye Res. 2004;79(2):231–237. doi: 10.1016/j.exer.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Mimura T, Joyce NC. Replication competence and senescence in central and peripheral human corneal endothelium. Invest Ophthalmol Vis Sci. 2006;47(4):1387–1396. doi: 10.1167/iovs.05-1199. [DOI] [PubMed] [Google Scholar]
- 43.Mimura T, Yamagami S, Yokoo S, Usui T, Tanaka K, Hattori S, Irie S, Miyata K, Araie M, Amano S. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci. 2004;45(9):2992–2997. doi: 10.1167/iovs.03-1174. [DOI] [PubMed] [Google Scholar]
- 44.Mimura T, Yokoo S, Yamagami S. Tissue engineering of corneal endothelium. J Funct Biomater. 2012;3(4):726–744. doi: 10.3390/jfb3040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13(12):767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 46.Nuzzi R, Marolo P, Tridico F. From DMEK to Corneal Endothelial Cell Therapy: Technical and Biological Aspects. J Ophthalmol. 2018;2018:6482095. doi: 10.1155/2018/6482095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohlsson R, Lobanenkov V, Klenova E. Does CTCF mediate between nuclear organization and gene expression? Bioessays. 2010;32(1):37–50. doi: 10.1002/bies.200900118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palchesko RN, Lathrop KL, Funderburgh JL, Feinberg AW. In vitro expansion of corneal endothelial cells on biomimetic substrates. Sci Rep. 2015;5:7955. doi: 10.1038/srep07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parekh M, Ferrari S, Sheridan C, Kaye S, Ahmad S. Concise Review: An Update on the Culture of Human Corneal Endothelial Cells for Transplantation. Stem Cells Transl Med. 2016;5(2):258–264. doi: 10.5966/sctm.2015-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel SP, Bourne WM. Corneal endothelial cell proliferation: a function of cell density. Invest Ophthalmol Vis Sci. 2009;50(6):2742–2746. doi: 10.1167/iovs.08-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peh GS, Toh KP, Wu FY, Tan DT, Mehta JS. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PLoS One. 2011;6(12):e28310. doi: 10.1371/journal.pone.0028310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137(7):1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raymond GM, Jumblatt MM, Bartels SP, Neufeld AH. Rabbit corneal endothelial cells in vitro: effects of EGF. Invest Ophthalmol Vis Sci. 1986;27(4):474–479. [PubMed] [Google Scholar]
- 54.Ridley A, Heald R. Cell structure and dynamics. Curr Opin Cell Biol. 2011;23(1):1–3. doi: 10.1016/j.ceb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Rodova M, Kelly KF, VanSaun M, Daniel JM, Werle MJ. Regulation of the rapsyn promoter by kaiso and delta-catenin. Mol Cell Biol. 2004;24(16):7188–7196. doi: 10.1128/MCB.24.16.7188-7196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rzeczkowski K, Beuerlein K, Muller H, Dittrich-Breiholz O, Schneider H, Kettner-Buhrow D, Holtmann H, Kracht M. c-Jun N-terminal kinase phosphorylates DCP1a to control formation of P bodies. J Cell Biol. 2011;194(4):581–596. doi: 10.1083/jcb.201006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samples JR, Binder PS, Nayak SK. Propagation of human corneal endothelium in vitro effect of growth factors. Exp Eye Res. 1991;52(2):121–128. doi: 10.1016/0014-4835(91)90252-a. [DOI] [PubMed] [Google Scholar]
- 58.Schultz G, Cipolla L, Whitehouse A, Eiferman R, Woost P, Jumblatt M. Growth factors and corneal endothelial cells: III. Stimulation of adult human corneal endothelial cell mitosis in vitro by defined mitogenic agents. Cornea. 1992;11(1):20–27. doi: 10.1097/00003226-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Senoo T, Obara Y, Joyce NC. EDTA: a promoter of proliferation in human corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41(10):2930–2935. [PubMed] [Google Scholar]
- 60.Sumide T, Nishida K, Yamato M, Ide T, Hayashida Y, Watanabe K, Yang J, Kohno C, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB J. 2006;20(2):392–394. doi: 10.1096/fj.04-3035fje. [DOI] [PubMed] [Google Scholar]
- 61.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 62.Tseng SC, Smuckler D, Stern R. Comparison of collagen types in adult and fetal bovine corneas. J Biol Chem. 1982;257(5):2627–2633. [PubMed] [Google Scholar]
- 63.Wagoner MD, Bohrer LR, Aldrich BT, Greiner MA, Mullins RF, Worthington KS, Tucker BA, Wiley LA. Feeder-free differentiation of cells exhibiting characteristics of corneal endothelium from human induced pluripotent stem cells. Biol Open. 2018;7(5). [DOI] [PMC free article] [PubMed]
- 64.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121(Pt 6):727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 65.Wiley LA, Burnight ER, DeLuca AP, Anfinson KR, Cranston CM, Kaalberg EE, Penticoff JA, Affatigato LM, Mullins RF, Stone EM, Tucker BA. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci Rep. 2016;6:30742. doi: 10.1038/srep30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J Cell Biol. 2003;161(5):845–851. doi: 10.1083/jcb.200303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1-2):15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 68.Yokoo S, Yamagami S, Yanagi Y, Uchida S, Mimura T, Usui T, Amano S. Human corneal endothelial cell precursors isolated by sphere-forming assay. Invest Ophthalmol Vis Sci. 2005;46(5):1626–1631. doi: 10.1167/iovs.04-1263. [DOI] [PubMed] [Google Scholar]
- 69.Yue BY, Sugar J, Gilboy JE, Elvart JL. Growth of human corneal endothelial cells in culture. Invest Ophthalmol Vis Sci. 1989;30(2):248–253. [PubMed] [Google Scholar]
- 70.Zhang J, O'Donnell JJ, III, Holian O, Vincent PA, Kim KS, Lum H. P120 catenin represses transcriptional activity through Kaiso in endothelial cells. Microvasc Res. 2010;80(2):233–239. doi: 10.1016/j.mvr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang K, Pang K, Wu X. Isolation and transplantation of corneal endothelial cell-like cells derived from in-vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2014;23(12):1340–1354. doi: 10.1089/scd.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Cai S, Tseng SCG, Zhu YT. Isolation and Expansion of Multipotent Progenitors from Human Trabecular Meshwork. Sci Rep. 2018;8(1):2814. doi: 10.1038/s41598-018-21098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao JJ, Afshari NA. Generation of Human Corneal Endothelial Cells via In Vitro Ocular Lineage Restriction of Pluripotent Stem Cells. Invest Ophthalmol Vis Sci. 2016;57(15):6878–6884. doi: 10.1167/iovs.16-20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci. 2004;45(6):1743–1751. doi: 10.1167/iovs.03-0814. [DOI] [PubMed] [Google Scholar]
- 75.Zhu YT, Chen HC, Chen SY, Tseng SC. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. J Cell Sci. 2012;125(Pt 15):3636–3648. doi: 10.1242/jcs.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu YT, Han B, Li F, Chen SY, Tighe S, Zhang S, Tseng SC. Knockdown of both p120 catenin and Kaiso promotes expansion of human corneal endothelial monolayers via RhoA-ROCK-noncanonical BMP-NFkappaB pathway. Invest Ophthalmol Vis Sci. 2014;55(3):1509–1518. doi: 10.1167/iovs.13-13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu YT, Hayashida Y, Kheirkhah A, He H, Chen SY, Tseng SC. Characterization and comparison of intercellular adherent junctions expressed by human corneal endothelial cells in vivo and in vitro. Invest Ophthalmol Vis Sci. 2008;49(9):3879–3886. doi: 10.1167/iovs.08-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu YT, Li F, Han B, Tighe S, Zhang S, Chen SY, Liu X, Tseng SC. Activation of RhoA-ROCK-BMP signaling reprograms adult human corneal endothelial cells. J Cell Biol. 2014. [DOI] [PMC free article] [PubMed]
- 79.Zhu YT, Li F, Han B, Tighe S, Zhang S, Chen SY, Liu X, Tseng SC. Activation of RhoA-ROCK-BMP signaling reprograms adult human corneal endothelial cells. J Cell Biol. 2014;206(6):799–811. doi: 10.1083/jcb.201404032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu YT, Tighe S, Chen SL, John T, Kao WY, Tseng SC. Engineering of Human Corneal Endothelial Grafts. Curr Ophthalmol Rep. 2015;3(3):207–217. doi: 10.1007/s40135-015-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.