Abstract
Background
This study investigated the changes in blood pressure and inflammatory cytokines in patients with chronic intractable insomnia, and explored the effects of chronic intractable insomnia on antihypertensive efficacy.
Material/Methods
A total of 248 patients with hypertension admitted to our hospital from 2008 to 2017 were enrolled. We enrolled 124 patients without chronic insomnia in the control group, while 124 patients with chronic insomnia were included in the treatment group. The treatment group received estazolam and was further subdivided into the effective group (n=96) and the ineffective group (n=28) according to Sleep Dysfunction Rating Scale (SDRS) scores. Sleep quality before and after treatment was determined.
Results
Antihypertensive treatment with eplerenone (50 mg) significantly reduced SDRS scores, C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), matrix metalloproteinase-9 (MMP-9), serum intercellular adhesion molecules (sICAM), and IL-1β levels, as well as systolic blood pressures (SBP) and diastolic blood pressures (DBP), with elevation of non-dipper blood pressure rhythm (P<0.05). The inhibition of intractable insomnia significantly downregulated SBP and DBP, as well as serum inflammatory cytokines such as CRP and TNF-α, showing a favorable effect on antihypertensive function.
Conclusions
Alleviation of chronic intractable insomnia facilitates hypertension therapy through decreasing levels of inflammatory cytokines and the proportion of non-dipper blood pressure rhythm, which offers insights for the treatment of hypertension.
MeSH Keywords: Circadian Rhythm, Cytokines, Hypertension
Background
Changes in societies and lifestyles have increased the proportion of people with sub-optimal health [1] and lead to sleep disorders and mental anxiety; middle-aged and young people are particularly affected, due to pressures at work. The lack of high-quality sleep over a long period of time causes anxiety, fatigue, pessimism, and other mental problems. Studies have revealed that lack of sleep can result in abnormal hormone secretion, weakened immunity, neuroendocrine disorders, hypersecretion of angiotensin II, and in high blood pressure [2–4]. In addition, obstructive apnea syndrome has been identified to trigger hypertension and affect the corresponding treatment [5]. In this study we investigated changes in blood pressures and levels of inflammatory cytokines in patients with intractable insomnia and hypertension, as well as the role of chronic intractable insomnia in hypertension.
Material and Methods
Study objects
We enrolled a total of 248 hypertensive patients, including 124 in the control group and 124 in the treatment group, according to the symptom of insomnia, in this study. Patients in the control group had no chronic insomnia and received antihypertensive treatment with eplerenone (50 mg), while patients in the treatment group suffered from chronic intractable insomnia and were administered with estazolam to improve their sleep quality based on the same antihypertensive treatment as the control group. Gender, age, and blood pressure of the 2 groups of patients were comparable (P>0.05). This study was pre-approved by the Ethics Committee of our hospital. All subjects signed the informed consent forms before recruitment in this study.
Research methods
The diagnosis of hypertension was based on the Guidelines for the Prevention and Treatment of Hypertension in China [6]. The Chinese Classification of Mental Disorders and Diagnostic Criteria [7] was used for the diagnosis of chronic intractable insomnia. The Sleep Dysfunction Rating Scale (SDRS) was used to score intractable insomnia in patients. SDRS scoring was performed for patients in the control and treatment groups at the beginning of admission. Blood pressure was measured using a 24-h ambulatory blood pressure monitor and the levels of serum inflammatory cytokines were detected. At 0, 7, 14, 21, and 28 d after drug treatment, SDRS scores, mean systolic blood pressure (SBP), mean diastolic blood pressure (DBP), and levels of serum inflammatory cytokines in patients were recorded, and the effects of drug treatment on blood pressure treatment and sleep improvement statuses were observed.
Index monitoring
Blood pressures and serum inflammatory cytokines, SDRS scores, and blood pressure rhythm before and after treatment were observed, and 24-h SBP and SDP were monitored. Mean nighttime blood pressure/mean daytime blood pressure >10% represents dipper blood pressure rhythm; otherwise, it is recognized as non-dipper blood pressure rhythm. SDRS scoring was conducted for sleep quality assessment. Score reduction ratio=1 minus total score after treatment/total score for baseline: ≥0.5 is effective whereas <0.5 is ineffective.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 17.0 was used for statistical analysis. The chi-square test was used for enumeration data. One-way analysis of variance with Tukey’s post hoc test was conducted for measurement data, and the t test was used for count data. P<0.05 represented a statistically significant difference.
Results
Comparisons of the basic data between the 2 groups of patients
There were no significant differences in gender, age, or course of hypertension parameters between the 2 groups, indicating that the groups were comparable (Table 1).
Table 1.
Basic data of patients from 2 groups.
| Item | Control group (n=124) | Treatment group (n=124) | P value |
|---|---|---|---|
| Age | 57.8±13.1 | 60.2±10.7 | 0.1154 |
| Male/Female | 83/41 | 80/44 | 0.6881 |
| Course of hypertension | 1.0–3.5 | 1.1–4.2 | 0.1150 |
| Chronic intractable insomnia | / | Difficulty in falling asleep and maintaining sleep, psychological conflict, emotional instability, and excessive anxiety | / |
Comparisons of SDRS scores between the 2 groups of patients
No significant change in SDRS scores was presented in the control group before and after treatment. However, we found the SDRS scores were significantly reduced at day 7, 14, 21 and 28 after the treatment, compared with that at day 0 (P<0.05). Moreover, the scores of the patients in the treatment group were similar to those of the control group at day 21 after treatment (Table 2).
Table 2.
Comparisons of SDRS scores between the 2 groups of patients (χ̄±s).
| Group | 0 d | 7th d | 14th d | 21st d | 28th d |
|---|---|---|---|---|---|
| Control group (n=124) | 16.9±9.6 | 15.2±9.2 | 16.5±10.1 | 16.8±9.1 | 15.9±8.8 |
| Treatment group (n=124) | 26.9±11.2 | 23.6±10.2* | 21.1±8.9* | 15.6±9.7* | 11.2±8.5* |
P<0.05, compared with treatment at day 0.
Comparisons of the levels of serum inflammatory cytokines between the 2 groups of patients
Antihypertensive treatment significantly reduced levels of C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and matrix metalloproteinase-9 (MMP-9), as well as serum intercellular adhesion molecules (sICAM) and IL-1β, in both groups (P<0.05). Additionally, after the treatment, serum inflammatory cytokines in the treatment group were significantly higher than those in the control group (P<0.05) (Table 3).
Table 3.
Comparisons of the levels of serum inflammatory cytokines between the 2 groups of patients (χ̄±s).
| Item | Control group | Treatment group | ||
|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | |
| CRP (mg/L) | 5.21±1.07 | 2.66±1.14* | 6.91±1.76 | 2.87±1.34*# |
| TNF-α (μg/L) | 1.41±0.26 | 0.91±0.15* | 1.83±0.31 | 0.99±0.21*# |
| IL-6 (ng/L) | 186.7±17.21 | 103.21±19.84* | 209.58±29.34 | 108.4±19.47*# |
| MMP-9 (pg/mL) | 82.5±9.16 | 40.7±7.07* | 116.3±10.28 | 53.2±6.29*# |
| Serum intercellular adhesion molecules (sICAM) (ng/mL) | 381.5±105.2 | 329.4±121.3* | 437.3±142.9 | 342.2±131.6*# |
| IL-1β (μg/L) | 0.41±0.10 | 0.19±0.05* | 0.41±0.13 | 0.27±0.04*# |
P<0.05, after treatment vs. before treatment;
P<0.05 control group vs. treatment group after treatment.
Comparison of blood pressure between the 2 groups
We then measured systolic blood pressures and diastolic blood pressures from both groups before and after the treatment (P>0.05). The treatment significantly decreased SBP and SDP in both groups (P<0.05). In addition, SBP and SDP were even further decreased in the control group after treatment compared with those in the treatment group (P<0.05) (Table 4). The blood pressure rhythm before treatment was significantly different between the 2 groups (P<0.05), while the non-dipper blood pressure rhythm of patients in the treatment group was obviously higher than that of patients in the control group, indicating that chronic intractable insomnia affects the blood pressure rhythm and antihypertensive efficacy in patients. However, there were no significant differences between the 2 groups after the end of treatment (Table 5).
Table 4.
Comparison of blood pressure between the 2 groups of patients (χ̄±s, mmHg).
| Item | Control group | Treatment group | |||
|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | ||
| 24-h | SBP | 156.2±15.7 | 140.1±10.8* | 163.6±11.5 | 144.6±12.5*# |
| DBP | 108.8±9.3 | 92.5±9.3* | 112.1±10.2 | 104.7±7.9*# | |
| Day | SBP | 157.2±9.1 | 142.6±8.3* | 159.2±10.4 | 145.7±9.5*# |
| DBP | 103.1±8.5 | 88.2±6.3* | 108.3±10.9 | 98.7±10.2*# | |
| Night | SBP | 149.8±8.5 | 139.5±8.8* | 154.7±9.7 | 143.5±7.7*# |
| DBP | 98.1±6.8 | 84.9±10.2* | 100.8±8.9 | 91.2±7.98*# | |
SBP – systolic blood pressure; DBP – diastolic blood pressure;
P<0.05, after treatment vs. before treatment;
P<0.05 control group vs. treatment group after treatment.
Table 5.
Comparison of the blood pressure rhythm between the 2 groups of patients.
| Group (n=124) | Before treatment | After treatment | ||
|---|---|---|---|---|
| Non-dipper | Dipper | Non-dipper | Dipper | |
| Control group | 68 | 56 | 42 | 82 |
| Treatment group | 85 | 39 | 30 | 94 |
| P value | 0.0264 | 0.0932 | ||
Effects of intractable insomnia on blood pressure
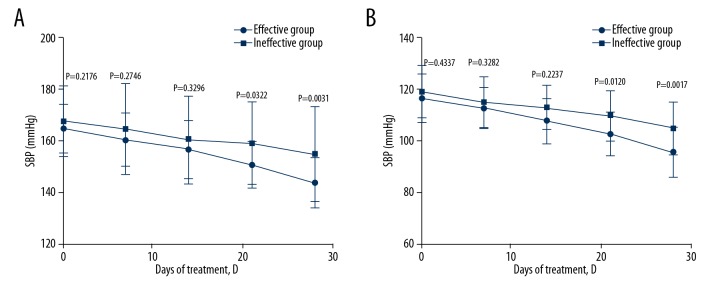
To further assess the effects of insomnia on blood pressure and inflammatory cytokines, the treatment group was divided into an effective group and an ineffective group, according to the SDRS scores. Then, the blood pressure levels in the 2 groups were observed. The results showed that, after treatment for 21 days, SBP and DBP in the effective group were significantly lower than those in the ineffective group (P<0.05), suggesting that treatment of intractable insomnia increases the efficacy of antihypertensive drugs (Figure 1).
Figure 1.
Effect of estazolam (anti-intractable insomnia) on SBP (A) and DBP (B) in patients. The blood pressure was detected at 0, 7, 14, 21, and 28 days after treatment in the effective treatment group and ineffective treatment group.
Effects of intractable insomnia on inflammatory cytokines in patients
We then assessed effects of treatment of intractable insomnia on the regulation of serum inflammatory cytokines CRP and TNF-α. Of note, treatment of intractable insomnia significantly reduced the levels in the effective group compared to those in the ineffective group (P<0.05) (Figures 2, 3). Similarly, the levels of other serum inflammatory cytokines (MMP-9, IL-1, IL-6, and sICAM) in patients in the effective insomnia treatment group were markedly lower than those in the ineffective insomnia treatment group (results not shown), suggesting that intractable insomnia affects the efficacy of antihypertensive drugs for serum inflammatory cytokines.
Figure 2.
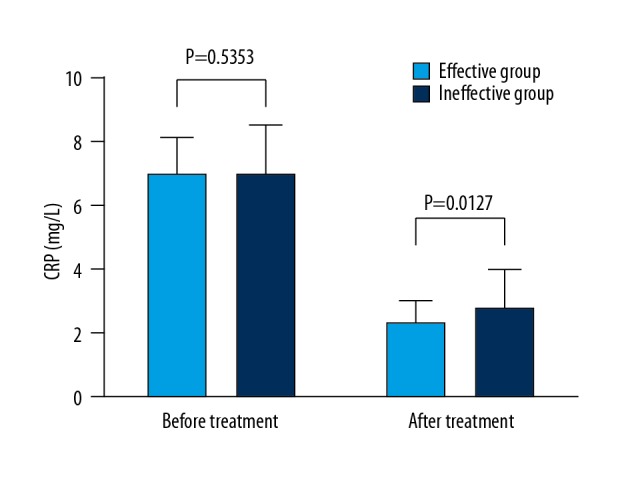
Effects of estazolam (anti-intractable insomnia) on CRP in patients. The differences in levels between the 2 groups before and after the treatment were determined.
Figure 3.
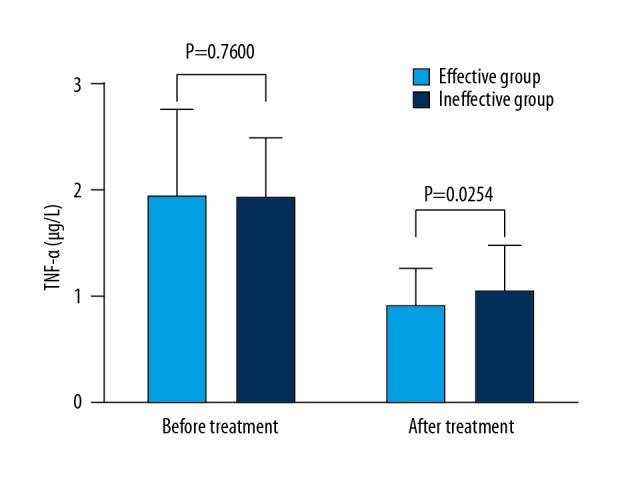
Effects of estazolam (anti-intractable insomnia) on TNF-α in patients. Differences in levels between the 2 groups before and after the treatment were determined.
Discussion
Insomnia primarily refers to a mental illness with common symptoms such as poor quality of sleep, short sleep duration, and frequent nocturnal awakenings [8]. Frequent insomnia, also called chronic insomnia, can cause mental and physical problems, reduce work efficiency and quality of life, and affect the progression of other diseases and treatments [9]. As the competition in modern society intensifies and the pressure of work increases, people’s mental burdens are also increasing. The incidence of insomnia has been increasing year by year, and the average age of people affected is decreasing. A study has reported that about 10% of people worldwide have chronic insomnia [10]. At present, there are 3 theories on the pathogenesis of insomnia: the psychogenic theory, the humor maladjustment theory, and the neurological theory [11,12]. These 3 theories are associated with the occurrence of hypertension, and many people with chronic insomnia also have hypertension. Patients with chronic insomnia tend to be anxious and manic, and to have continuous sympathetic nervous excitement. The sympathetic hyper-excitability can contract blood vessels, increase blood flow resistance, and accelerate kidney, cerebral vessel, and other organ injuries. The humor maladjustment theory of insomnia involves the parasecretion of hypothalamic-pituitary-adrenocortical hormones, which results in dysregulation of water sodium and blood pressure, disorder of blood pressure rhythm, narrowing of day-to-night difference, and increased non-dipper proportion [13,14]. Normally, the vagal and sympathetic excitements contain circadian rhythms, and the corresponding regulation of blood pressure also exhibits circadian rhythm. However, the disrupted sympathetic nerve rhythm of patients with chronic insomnia leads to disordered blood pressure in circadian rhythm (i.e., the formation of non-dipper blood pressure) [15], and chronic intractable insomnia impairs the immune system. Patients with chronic intractable often have high levels of inflammatory cytokines. Meanwhile, the occurrence of hypertension is associated with various influencing factors. Increased vascular pressure in the brain, triggered by hypertension and insufficient blood supply to the heart and lungs, can also cause changes in nervousness, irritability, headaches, and other emotional changes, thus eventually leading to insomnia [16]. Therefore, chronic intractable insomnia and hypertension interact with each other and co-occur [17,18]. Previous finding revealed the effects of chronic intractable insomnia on hypertension. In the studies of Vgontzas et al., chronic insomnia was found to be an important risk factor for the development of hypertension [19,20]. Previous evidence indicated the relationship between preoperative circadian blood pressure pattern and early postoperative course in patients undergoing coronary artery bypass graft (CABG) surgery [21]. Furthermore, chemerin, which plays a role in inflammation and atherosclerosis, shows positive correlations with blood pressure [22]. In the processes of the development and treatment of hypertensive disease, the crucial role of chronic intractable insomnia should be considered and provides clues to the appropriate treatment measures. However, the limitation of this study is that the exact mechanisms underlying the effect of chronic intractable insomnia on hypertension, as well as the potential signaling pathways, need to be further identified.
Conclusions
Our data demonstrate that chronic intractable insomnia affects blood pressure and the therapeutic effect against hypertension, which provides a scientific basis for the therapy of hypertension.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Zhao J, Liao X, Zhao H, et al. Evaluation on effectiveness and safety of Chinese herbs in treatment of sub-health: A systematic review and meta-analysis of randomized controlled trials. Chin J Integr Med. :2018. doi: 10.1007/s11655-018-2982-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Lorton D, Lubahn CL, Estus C, et al. Bidirectional communication between the brain and the immune system: implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation. 2006;13:357–74. doi: 10.1159/000104864. [DOI] [PubMed] [Google Scholar]
- 3.Bangash MF, Xie A, Skatrud JB, et al. Cerebrovascular response to arousal from NREM and REM sleep. Sleep. 2008;31:321–27. doi: 10.1093/sleep/31.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YK, Na KS, Shin KH, et al. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1044–53. doi: 10.1016/j.pnpbp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Roberts J, Gaduzo S. Finding the ‘missing millions’: Do we need incentives to optimise COPD outcomes? Prim Care Respir J. 2013;22:12–13. doi: 10.4104/pcrj.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Maan V, Agarwal P. Diagnostic criteria in pediatric intracranial hypertension. J AAPOS. 2018;22(4):333. doi: 10.1016/j.jaapos.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Dai Y, Yu X, Xiao Z, et al. Comparison of Chinese and international psychiatrists’ views on classification of mental disorders. Asia Pac Psychiatry. 2014;6:267–73. doi: 10.1111/appy.12146. [DOI] [PubMed] [Google Scholar]
- 8.Holst SC, Muller T, Valomon A, et al. Functional polymorphisms in dopaminergic genes modulate neurobehavioral and neurophysiological consequences of sleep deprivation. Sci Rep. 2017;7:45982. doi: 10.1038/srep45982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–19. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cirelli C. Sleep and synaptic changes. Curr Opin Neurobiol. 2013;23:841–46. doi: 10.1016/j.conb.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker MP. Cognitive consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S29–34. doi: 10.1016/S1389-9457(08)70014-5. [DOI] [PubMed] [Google Scholar]
- 12.Goel N, Rao H, Durmer JS, et al. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vernon G. The chronotherapy of hypertension: Or the benefit of taking blood pressure tablets at bedtime. Br J Gen Pract. 2017;67:171. doi: 10.3399/bjgp17X690269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orias M, Correa-Rotter R. Chronotherapy in hypertension: A pill at night makes things right? J Am Soc Nephrol. 2011;22:2152–55. doi: 10.1681/ASN.2011101012. [DOI] [PubMed] [Google Scholar]
- 15.Tang H, Zheng Q, Wang J. Pathogenic role of ion channels in pulmonary arterial hypertension. Exp Physiol. 2017;102:1075–77. doi: 10.1113/EP086426. [DOI] [PubMed] [Google Scholar]
- 16.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Lewis PE, Emasealu OV, Rohrbeck P, et al. Risk of type II diabetes and hypertension associated with chronic insomnia among active component, U.S. Armed Forces, 1998–2013. MSMR. 2014;21:6–13. [PubMed] [Google Scholar]
- 18.Bertisch SM, Pollock BD, Mittleman MA, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep. 2018;41(6) doi: 10.1093/sleep/zsy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Mendoza J, Baker JH, Vgontzas AN, et al. Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain Behav Immun. 2017;61:110–16. doi: 10.1016/j.bbi.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vgontzas AN, Liao D, Bixler EO, et al. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–97. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahcivan M, Gulel O, Kolbakir F. The effect of preoperative circadian blood pressure pattern on early postoperative outcomes in patients with coronary artery bypass graft surgery. Anadolu Kardiyol Derg. 2008;8:354–59. [PubMed] [Google Scholar]
- 22.Meric M, Soylu K, Avci B, et al. Evaluation of plasma chemerin levels in patients with non-dipper blood pressure patterns. Med Sci Monit. 2014;20:698–705. doi: 10.12659/MSM.890784. [DOI] [PMC free article] [PubMed] [Google Scholar]