Abstract
Background
Lymphocyte-to-monocyte ratio (LMR) is an independent predictive factor of clinical outcome of acute ischemic stroke and cancer, but the predictive effect of LMR in spontaneous intracerebral hemorrhage (ICH) is unknown. Thus, the aim of this study was to explore the impact of peripheral LMR on the neurological deterioration (ND) during the initial week after spontaneous ICH onset, as well as 90-day mortality.
Material/Method
The clinical data of 558 consecutive patients with ICH were retrospectively analyzed. LMR is calculated by absolute lymphocyte count divided by absolute monocyte count.
Results
Of these patients, 166 patients experienced ND during the first week after admission and 72 patients died within 90 days. Multivariate analysis indicated that white blood cells (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), neutrophil-to-lymphocyte ratio (NLR), LMR were significantly associated with ND during the initial week after ICH onset and also were associated with 90-day mortality. Moreover, NLR and LMR showed a higher predictive ability in ND during the initial week after ICH onset than 90-day mortality in receiver operating characteristic analysis. The best cut-off points of NLR and LMR in predicting ND and 90-day mortality were 10.24 and 2.21 and 16.81 and 2.19, respectively.
Conclusions
Our results suggest that LMR on admission is a predictive factor for ND during the initial week after ICH onset, as well as 90-day mortality.
MeSH Keywords: Intracranial Hemorrhage, Hypertensive; Lymphocyte Activation; Mortality
Background
Spontaneous intracerebral hemorrhage (ICH) is a common vascular disease with high rates of mortality and disability in the central nervous system (CNS) [1]. Despite the rapid development of medical technology, no treatment protocols with definitive effect have been confirmed [1]. Most patients with ICH will suffer from neurological deterioration (ND), which is a common complication in patients with CNS disease and is related to long l in-hospital stay and poor clinical outcomes [2]. Thus, it is important to identify risk factors for prognosis of patients with ICH and this may also improve the clinical outcome.
Some studies have indicated that 25% of patients with ICH experienced ND following admission, and ND was primarily evaluated by use of the National Institutes of Health Stroke Scale (NIHSS) or Glasgow Coma Scale (GCS) [3]. Hematoma expansion is a key to improving the development of ND and factors, including spot sign, larger hematomas, and hemorrhage into the ventricles of the brain, and these are associated with hematoma growth, which may also indirectly contribute to the development of ND [4]. However, there have been few studies on the association between ND and peripheral immune and inflammation biomarkers, such as neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR). Immune and inflammatory reactions play an important role in ICH-induced brain injury [5]. Systemic inflammatory response syndrome can result from many critical conditions, including ICH. The peripheral neutrophil count, lymphocytes, and monocytes were reported to be associated with secondary injury in ICH [6–15] and these biomarkers of immune and inflammation in peripheral blood have been demonstrated to be associated with long-term prognosis in patients with acute ICH. For example, inflammatory biomarkers including leukocyte count, absolute monocyte count (AMC), absolute lymphocyte count (ALC), and NLR were reported to be predictive factors for 30-day mortality in patients with ICH [6–15]. LMR was calculated by absolute lymphocyte count divided by absolute monocyte count and has been reported to be associated with peripheral immune and inflammation function [16,17]. Previous studies indicated that LMR is an independent predictor of overall survival in patients with cancers and acute ischemic stroke [16,17], but there are no studies on the relationship between LMR and ND and mortality in patients with ICH. Therefore, the primary aim of this study was to explore the association between LMR and ND during the initial week after ICH onset. We also evaluated the impact of LMR on 90-day mortality in these patients.
Material and Methods
Study design and patient enrolment
We retrospectively analyzed the clinical data of consecutive patients hospitalized at our hospital from January 2010 to January 2017 for stroke syndrome resulting from ICH who experienced admission routine blood sampling and brain computed tomographic (CT) scan within 24 h from symptom onset. All patients with supratentorial ICH were confirmed by brain CT scans within 24 h after symptom onset. We excluded patients who received surgery and immunomodulatory treatment before admission, including biological agents, methotrexate, corticosteroids, and azathioprine. In addition, we also excluded patients with aneurysm, hepatitis, arteriovenous malformation, heart failure, seizure, chronic obstructive pulmonary disease, hemorrhage following brain tumor, and isolated intraventricular hemorrhage.
Data collection
Data on demographics, lifestyle risk factors, medical history, total white blood cells (WBC), absolute neutrophil count (ANC), AMC, and absolute lymphocyte count (ALC) were collected. NIHSS and GCS at admission also were evaluated. NLR is calculated by absolute lymphocyte count divided by absolute neutrophil count, and LMR is calculated as ALC over AMC.
Blood pressure (BP) measurements and BP variability (BPV) definition
Noninvasive BP monitoring was performed at admission and subsequently at an interval of 4 h during the first 72 h after stroke symptoms. BP readings were using to calculate the minimum, mean, and maximum values of systolic and diastolic BP for each patient. BPV was defined as the maximum – minimum values, standard deviation (SD), and the coefficient of variation (CV; SD×100/mean).
ND evaluation and 90-day mortality
According to previous research [18], ND is determined by GCS or NIHSS score, which means that if the NIHSS score increased by 4 points or greater or if GCS decreased by 2 points or greater, or death occurred from the time of admission to 7 days post-hemorrhage, the patient may suffer from ND. Mortality at 90 days was documented by telephone interview or outpatient visit.
Infection assessment
Infection was microbiologically assessed using urine, sputum, and blood samples obtained during hospitalization. Only patients with infection symptoms such as hyperpyrexia, shivers, and expectoration received the diagnostic exam. The time of infection from stroke onset was also recorded.
Statistical analysis
Continuous variables are expressed as mean ±SD or median (interquartile range), while the categorical variables are presented as number or percent. For comparison analysis, the t test or Mann-Whitney test were performed for continuous variables, while the chi-squared test was used for categorical variables. Logistic regression models were used to evaluate the distribution of WBC, ALC, ANC, AMC, NLR, and LMR in patients with and without ND. Logistic regression modeling was also performed to assess the distribution of WBC, ALC, ANC, AMC, NLR, and LMR in patients with and without 90-day mortality. Variables with p value <0.05 indicated by comparison analysis were included in multivariate analysis and adjusted by sex, age, admission GCS, hematoma location, baseline volume, and intraventricular extension of ICH. In addition, receiver operating characteristic (ROC) analysis was performed to assess the ability of WBC, ALC, ANC, AMC, NLR, and LMR to predict ND and 90-day mortality. All p values were 2-sided, and p≤0.05 was considered as statistically significant. Statistical software used for analysis was SPSS version 17.0.
Results
There are 558 patients with ICH included in this study, with a mean age at admission of 57.6 years (range: 28–79). Of these patients, 166 patients experienced ND during the first week after admission. Table 1 summarizes the comparison data of patients with and without ND. Patients with ND tended to be older and smokers, and had higher systolic BP and systolic BP variability, higher diastolic BP and diastolic BP variability, higher frequency of hematoma growth, larger hematoma volume, lower GCS, higher NIHSS score, higher WBC, higher ANC, higher AMC, higher NLR, and lower ALC and LMR at admission compared to patients without ND (Table 1).
Table 1.
Baseline characteristics and neurological deterioration.
| Variable | ND (166) | Non-ND (392) | P value |
|---|---|---|---|
| Sex (Male/Female) | 102/64 | 266/126 | 0.454 |
| Age | 59.32±14.48 | 55.88±16.67 | 0.016 |
| Diabetes mellitus | 42/124 | 84/308 | 0.317 |
| Hyperlipidemia | 38/128 | 70/322 | 0.169 |
| Smoking | 51/115 | 81/311 | 0.011 |
| Mean SBP (mmHg) | 184.76±20.82 | 177.92±19.32 | <0.001 |
| Mean SBP (mmHg) | 109.82±14.41 | 104.31±11.87 | <0.001 |
| SBP CV | 11.5 (4.0) | 9.8 (3.8) | 0.012 |
| DBP CV | 11.3 (4.1) | 8.7 (4.3) | 0.001 |
| SBP SD | 16.75 (5.32) | 12.89 (4.38) | <0.001 |
| DBP SD | 9.36 (2.96) | 7.33 (1.89) | <0.001 |
| SBP mum-min | 56.23 (18.25) | 42.32 (17.22) | <0.001 |
| DBP mun-min | 32.15 (9.11) | 26.65 (6.54) | <0.001 |
| BP lowering strategy (intensive/conservative) | 85/81 | 173/219 | 0.126 |
| BP lowering agents | |||
| CCB (Yes/No) | 57/109 | 136/256 | 0.935 |
| ACEI (Yes/No) | 66/100 | 154/238 | 0.917 |
| Beta blocker (Yes/No) | 18/148 | 56/336 | 0.273 |
| ARB(Yes/No) | 31/135 | 61/331 | 0.881 |
| Diureticum (Yes/No) | 50/116 | 120/272 | 0.908 |
| NIHSS | 13.51±4.67 | 8.56±3.42 | <0.001 |
| GCS | 11.25±2.14 | 11.87±2.41 | 0.012 |
| Hematoma volume | 19.21±9.91 | 15.60±9.59 | <0.001 |
| Spot Sign (Yes/No) | 59/107 | 64/328 | <0.001 |
| Hematoma growth (Yes/No) | 75/91 | 63/329 | <0.001 |
| Hematoma location | |||
| Lobar (Yes/No) | 62/104 | 142/250 | 0.801 |
| Basal ganglia region (Yes/No) | 74/92 | 168/224 | 0.295 |
| Thalamus | 30/136 | 74/318 | 0.823 |
| Intraventricular extension (Yes/No) | 22/144 | 27/365 | <0.001 |
| Blood sampling time (h) | 13.5±3.3 | 14.3±4.1 | 0.806 |
| WBC | 13.39±5.24 | 10.67±4.15 | <0.001 |
| ALC | 0.93±0.53 | 1.44±0.75 | <0.001 |
| ANC | 11.82±4.85 | 8.77±4.01 | <0.001 |
| AMC | 0.69±1.07 | 0.62±0.39 | <0.001 |
| NLR | 15.98±8.83 | 8.03±6.44 | <0.001 |
| LMR | 2.06±1.95 | 3.49±2.37 | <0.001 |
SBP – systolic blood pressure; DBP – diastolic blood pressure; ND – neurological deterioration; NIHSS – National Institute of Health Stroke Scale; GCS – Glasgow Coma Scale; CV – coefficient of variation; SD – standard deviation; CCB – calcium channel blocker; ACEI – angiotensin-converting enzyme inhibitor; ARB – angiotensin II receptor inhibitor; WBC – white blood cells; ANC – absolute neutrophil count; AMC – absolute monocyte count; ALC – absolute lymphocyte count; NLR – neutrophil-to-lymphocyte ratio; LMR – lymphocyte-to-monocyte ratio.
In univariate logistic regression, WBC (OR: 1.132; 95%CI: 1.086–1.179, p≤0.001), ANC (OR: 1.166; 95%CI: 1.116–1.219, p≤0.001), ALC (OR: 0.216; 95%CI: 0.144–0.325, p≤0.001), NLR (OR: 1.143; 95%CI: 1.110–1.176, p≤0.001) and LMR (OR: 0.677; 95%CI: 0.599–0.765, p≤0.001) were significantly associated with ND (Table 2). These variables remained statistically significant when they were adjusted by age, sex, initial GCS, mean systolic and diastolic BP, systolic and diastolic BP variability, BP-lowering strategy, types of BP-lowering agents, unhealthy lifestyle including smoking and drinking, baseline ICH volume, time from stroke onset to blood sample, presence of intraventricular hemorrhage, hematoma location, hematoma expansion, and presence of spot sign and infection (all p values ≤0.001).
Table 2.
The relationship between NLR, LMR, and neurological deterioration.
| Variables | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | |
| WBC | 1.132 (1.086–1.179) | <0.001 | 1.126 (1.074–1.180) | <0.001 |
| ALC | 0.216 (0.144–0.325) | <0.001 | 0.210 (0.134–0.328) | <0.001 |
| ANC | 1.166 (1.116–1.219) | <0.001 | 1.162 (1.105–1.223) | <0.001 |
| AMC | 0.910 (0.730–1.135) | 0.403 | 0.994 (0.756–1.307) | 0.965 |
| NLR | 1.143 (1.110–1.176) | <0.001 | 1.142 (1.107–1.179) | <0.001 |
| LMR | 0.677 (0.599–0.765) | <0.001 | 0.664 (0.597–0.760) | <0.001 |
Adjusted by age, sex, initial GCS, systolic and diastolic BP, systolic and diastolic BP variability, BP-lowering strategy, types of BP-lowering agents, unhealthy lifestyle including smoking and drinking, baseline ICH volume, time from stroke onset to blood sample, presence of intraventricular hemorrhage, hematoma location, hematoma expansion, and presence of spot sign and infection.
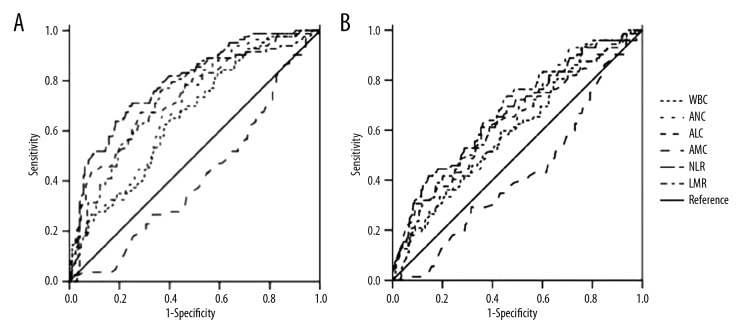
The results of ROC analysis showed that the area under the curves for WBC, ANC, ALC, AMC, NLR, and LMR in predicting ND were 0.659 (95%CI: 0.611–0.706, p≤0.001), 0.687 (95%CI: 0.641–0.732, p≤0.001), 0.738 (95%CI: 0.692–0.783, p≤0.001), 0.584 (95%: 0.534–0.634, p=0.002), 0.792(95%CI: 0.752–0.833, p≤0.001), and 0.726 (95%CI: 0.680–0.772, p≤0.001), respectively (Table 3). The best cut-offs of WBC, ANC, ALC, AMC, NLR, and LMR in predicting ND were 11.13, 8.45, 1.03, 0.53, 10.24, and 2.21, respectively (Figure 1).
Table 3.
The ability of NLR and LMR in predicting neurological deterioration.
| Variables | Area under the curve (95%CI) | p Value | Cut-off | Specificity | Sensitivity |
|---|---|---|---|---|---|
| WBC | 0.659 (0.611–0.706) | <0.001 | 11.13 | 62.70% | 61.27% |
| ALC | 0.738 (0.692–0.783) | <0.001 | 1.03 | 71.40% | 67.50% |
| ANC | 0.687 (0.641–0.732) | <0.001 | 8.45 | 78.30% | 54.10% |
| AMC | 0.584 (0.534–0.634) | 0.002 | 0.59 | 48.20% | 67.30% |
| NLR | 0.792 (0.752–0.833) | <0.001 | 10.24 | 71.10% | 75.00% |
| LMR | 0.726 (0.680–0.772) | <0.001 | 2.21 | 63.80% | 75.90% |
Figure 1.
The ability of NLR and LMR to predict neurological deterioration and 90-day mortality.
There are 72 patients who died during the first 90 days. Patients who died had higher systolic BP, higher diastolic BP, larger hematoma volume, lower GCS, higher NIHSS score, higher WBC, higher ANC, higher NLR, and lower ALC and LMR at admission compared to patients who survived the first 90 days (Table 4). In univariate logistic regression, WBC (OR: 1.083; 95%CI: 1.033–1.136, p=0.001), ANC (OR: 1.099; 95%CI: 1.045–1.155, p≤0.001), ALC (OR: 0.555; 95%CI: 0.359–0.858, p=0.008), NLR (OR: 1.143; 95%CI: 1.074–1.102, p≤0.001), and LMR (OR: 0.758; 95%CI: 0.648–0.886, p=0.001) were significantly associated with 90-day mortality (Table 5). Furthermore, all of these factors were associated with 90-day mortality after being adjusted by age, sex, initial GCS, mean systolic and diastolic BP, systolic and diastolic BP variability, BP-lowering strategy, types of BP-lowering agents, unhealthy lifestyle including smoking and drinking, baseline ICH volume, time from stroke onset to blood sample, presence of intraventricular hemorrhage, hematoma location and hematoma expansion, presence of spot sign, presence of infection, and ND (all p value ≤0.001, Table 5).
Table 4.
Baseline characteristics and 90-day mortality.
| Variable | 90-day mortality | P value | |
|---|---|---|---|
| Death (72) | Survival (486) | ||
| Sex (Male/Female) | 52/20 | 316/170 | 0.229 |
| Age | 57.46±15.11 | 58.42±14.89 | 0.611 |
| Diabetes mellitus (Yes/No) | 26/46 | 100/386 | 0.005 |
| Hyperlipidemia (Yes/No) | 19/53 | 89/397 | 0.105 |
| Smoking (Yes/No) | 25/47 | 107/379 | 0.018 |
| Mean SBP (mmHg) | 185.14±23.60 | 176.19±19.36 | 0.044 |
| Mean DBP (mmHg) | 90.52±15.91 | 86.12±12.21 | 0.002 |
| SBP CV | 11.8 (4.1) | 10.1 (3.7) | 0.028 |
| DBP CV | 10.3 (4.0) | 8.3 (4.2) | 0.001 |
| SBP SD | 15.97 (5.52) | 13.19 (4.08) | <0.001 |
| DBP SD | 9.06 (2.23) | 7.01 (1.22) | <0.001 |
| SBP mum-min | 50.12 (16.12) | 39.12 (18.11) | <0.001 |
| DBP mun-min | 31.22 (8.99) | 26.22 (5.34) | <0.001 |
| BP lowering strategy (intensive/conservative) | 29/43 | 229/257 | 0.277 |
| BP lowering agents | |||
| CCB | 24/48 | 169/317 | 0.810 |
| ACEI | 26/46 | 194/292 | 0.537 |
| Beta blocker | 11/61 | 63/423 | 0.589 |
| ARB | 12/60 | 74/412 | 0.752 |
| Diureticum | 21/51 | 149/337 | 0.797 |
| NIHSS | 12.37±4.31 | 7.41±4.11 | <0.001 |
| GCS | 11.94±2.31 | 12.83±2.33 | 0.003 |
| Hematoma volume | 20.91±10.21 | 16.32±8.89 | 0.003 |
| Spot Sign (Yes/No) | 26/46 | 97/389 | 0.002 |
| Hematoma growth (Yes/No) | 41/31 | 95/391 | <0.001 |
| Hematoma location involvement | |||
| Lobar (Yes/No) | 25/47 | 179/307 | 0.729 |
| Basal ganglia region (Yes/No) | 29/43 | 213/273 | 0.571 |
| Thalamus (Yes/No) | 18/54 | 86/400 | 0.137 |
| Intraventricular extension (Yes/No) | 18/54 | 40/446 | <0.001 |
| Blood sampling time(h) | 14.1±3.5 | 14.4±4.5 | 0.843 |
| WBC | 13.22±5.43 | 11.22±4.49 | 0.004 |
| ALC | 1.08±0.73 | 1.32±0.73 | 0.008 |
| ANC | 11.55±5.13 | 9.40±4.33 | <0.001 |
| AMC | 0.62±0.36 | 0.68±0.98 | 0.599 |
| NLR | 15.44±10.80 | 9.65±7.33 | <0.001 |
| LMR | 2.15±1.68 | 3.20±2.40 | <0.001 |
SBP – systolic blood pressure; DBP – diastolic blood pressure; ND – neurological deterioration; NIHSS – National Institute of Health Stroke Scale; GCS – Glasgow Coma Scale; CV – coefficient of variation; SD – standard deviation; CCB – calcium channel blocker; ACEI – angiotensin-converting enzyme inhibitor; ARB – angiotensin II receptor inhibitor; WBC – white blood cells; ANC – absolute neutrophil count; AMC – absolute monocyte count; ALC – absolute lymphocyte count; NLR – neutrophil-to-lymphocyte ratio; LMR – lymphocyte-to-monocyte ratio.
Table 5.
The relationship between NLR, LMR and 90-day mortality.
| Variables | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | |
| WBC | 1.083 (1.033–1.136) | 0.001 | 1.084 (1.026–1.146) | 0.004 |
| ALC | 0.555 (0.359–0.858) | 0.008 | 0.635 (0.411–0.981) | 0.041 |
| ANC | 1.099 (1.045–1.155) | <0.001 | 1.096 (1.033–1.162) | 0.002 |
| AMC | 0.919 (0.671–1.259) | 0.601 | 1.083 (0.746–1.572) | 0.676 |
| NLR | 1.074 (1.046–1.102) | <0.001 | 1.061 (1.030–1.094) | <0.001 |
| LMR | 0.758 (0.648–0.886) | 0.001 | 0.765 (0.641–0.912) | 0.003 |
Adjusted by age, sex, initial GCS, systolic and diastolic BP, systolic and diastolic BP variability, BP lowering strategy, types of BP-lowering agents, unhealthy lifestyle including smoking and drinking, baseline ICH volume, time from stroke onset to blood sample, presence of intraventricular hemorrhage, hematoma location and hematoma expansion, presence of spot sign, presence of infection, and ND.
The ROC results showed that the area under the curves for WBC, ANC, ALC, AMC, NLR, and LMR in predicting 90-day mortality were 0.605 (95%CI: 0.537–0.674, p=0.004), 0.623 (95%CI: 0.558–0.639, p=0.001), 0.631 (95%CI: 0.558–0.704, p≤0.001), 0.575 (95%: 0.508–0.642, p=0.041), 0.669 (95%CI: 0.600–0.738, p≤0.001), and 0.648 (95%CI: 0.580–0.716, p≤0.001), respectively. The best cut-offs of WBC, ANC, ALC, AMC, NLR, and LMR in predicting 90-day mortality were 7.83, 6.24, 1.03, 0.55, 16.81, and 2.19, respectively (Table 6, Figure 1).
Table 6.
The ability of NLR and LMR in predicting 90-day mortality.
| Variables | AUC (95%CI) | p Value | Cut-off | Specificity | Sensitivity |
|---|---|---|---|---|---|
| WBC | 0.605 (0.537–0.674) | 0.004 | 7.83 | 24.50% | 93.10% |
| ALC | 0.631 (0.558–0.704) | <0.001 | 1.03 | 63.90% | 63.40% |
| ANC | 0.623 (0.558–0.639) | 0.001 | 6.24 | 29.10% | 93.10% |
| AMC | 0.575 (0.508–0.642) | 0.041 | 0.55 | 61.50% | 56.90% |
| NLR | 0.669 (0.600–0.738) | <0.001 | 16.81 | 85.60% | 41.70% |
| LMR | 0.648(0.580–0.716) | <0.001 | 2.19 | 72.20% | 55.60% |
Furthermore, we also evaluated the association between above blood markers and infections in patients with ICH. There were 89 patients who had infections after admission. Of these patients, 43 were diagnosed with pneumonia, 26 patients had urinary tract infection, and 20 patients had sepsis. The mean time from infection to stroke onset was 14.45±3.56 days. Patients with infections had higher WBC, higher ANC, higher NLR, and lower ALC and LMR compared to patients without infections (all p value ≤0.001, Table 7).
Table 7.
The relationship of LMR, NLR associated with infections.
| Variable | Infection (89) | Non-infection (469) | P value |
|---|---|---|---|
| WBC | 13.74±5.32 | 10.83±4.28 | <0.001 |
| ALC | 0.97±0.68 | 1.38±0.72 | <0.001 |
| ANC | 11.13±4.81 | 8.96±4.14 | <0.001 |
| AMC | 0.65±0.38 | 0.67±1.03 | 0.859 |
| NLR | 16.10±8.42 | 8.76±7.22 | <0.001 |
| LMR | 2.03±2.04 | 3.37±2.34 | <0.001 |
Discussion
The aim of this study was to identify risk factors for ND and 90-day mortality in patients with ICH. Our main results showed that higher WBC, higher ANC, higher NLR, lower ALC, and LMR on admission were predictive factors for ND during the initial week after ICH onset and 90-day mortality. To the best of our knowledge, the present study is the first to evaluate the relationship between LMR and ND and 90-day mortality in patients with ICH.
Our results are in line with previous publications which indicated that elevated WBC, ANC, and NLR and lower ALC were associated with ND [19] and 90-day mortality [9–17], which suggests that immune and inflammatory response play an important role in the pathological process following ICH [5]. The pathophysiological process after ICH is involved with leukocyte infiltration and release of various inflammatory mediators, contributing to neuron death or apoptosis, which results in a poor outcome in patients with ICH [20–22]. The present study primarily explained the role of LMR in ICH from 2 aspects: lymphocytes and monocytes.
Lymphocytes, a subtype of leukocytes, are the main immune regulatory cells, which play a defensive role in the invasion of diseases. Some studies with animal models also indicated that lymphocytes were involved in the pathology following ICH [23], which suggested that lymphocytes have a role in the pathological changes after ICH. Patients with stroke may experience lymphopenia or decreasing absolute lymphocyte count, a phenomenon of immunodepression, which might result from activation of the hypothalamic-pituitary-adrenal system, sympathetic nervous system, and parasympathetic nervous system, via the secretion and release of acetylcholine, catecholamines, and cortisol, which may lead to lymphocyte apoptosis [24]. Some studies suggested that higher ALC can upregulate the anti-inflammatory cytokine interleukin (IL)-10 and suppress inflammatory cytokines including tumor necrosis factor (TNF)-α and IL-6, resulting in a neuroprotective effect [25,26]. In addition, there are studies demonstrating that decreased lymphocyte count was associated with infection in patients with ICH [27], which is consistent with our result. Therefore, not only the length of hospital stay was prolonged in patients with decreased lymphocytes, but also the clinical course was aggravated by infection owing to the suppression of lymphocyte in defensive effect [28]. NLR calculated from neutrophil and lymphocyte is a biomarker conveying important information about inflammatory and immune status in the vascular bed [29]. Increased neutrophils and reduced lymphocytes are involved in secondary injury after ICH, so NLR combined with the above 2 is an independent predictor of ND and 90-day mortality.
Monocyte was another important immunoregulator involved with secondary injury following ICH [30]. Migration of monocytes into the hemorrhagic brain via monocyte chemoattractant protein-1 and its receptor chemokine receptor 2 which were elevated after ICH [31]. TNF-α, IL-6, and IL-1b secreted from monocyte exerted a pro-inflammatory effect on brain lesions after stroke onset [32]. Furthermore, evidence suggests that monocytes migrate into the lesion following ICH and meditated by infiltrated neutrophil and Toll-like receptors, adhere to the vascular endothelial and destroy the blood–brain barrier and then aggravate the brain edema [33]. In addition, monocytes promote neuronal death and vascular injury and increase the expression of Toll-like receptor 2, 4 pro-inflammatory molecules, and inducible nitric oxide synthase, leading to secondary brain damage by combining with vascular endothelial chemotactic protein, all of which also promote and aggravate brain edema [34,35] and then the development of ND. Previous studies indicated that increased monocyte was associated with ND and long-term clinical outcome in patients with ICH [36,37], while monocytes were unrelated to ND and 90-day mortality. However, the peripheral LMR was a predictive factor for ND and 90-day mortality.
As far as we know, this is the first study to evaluate the relationship of LMR with short- and long-term clinical outcome in patients with ICH. Although previous studies assessed the correlation of NLR with ND [19] and long-term mortality [9–11,13,15,16], the importance of LMR in ICH remains unclear. In the present study, the association of LMR with ND and 90-day mortality may be explained by the following. On the one hand, just like NLR, LMR is a biomarker reflecting the inflammatory and immune status caused by ICH. The inflammatory response following ICH is meditated by lymphocytes and monocytes, leading to secondary brain injury including white matter damage, blood–brain barrier breakdown, and brain edema [24–28,31–35], which may have a critical role in the development of ND and 90-day mortality. On the other hand, immunosuppression caused by ICH may led to some post-stroke complications such as pneumonia, which promotes patient mortality [27]. As a result, LMR as well as NLR were combined in an index reflecting both the proinflammatory status and immunosuppression and predicted ND and poor clinical outcome in the present study.
We also found that BPV, including CV, SD, and maximum -minimum values, were significantly higher in patients with ND and 90-day mortality, which was consistent with previously published studies [38,39]. Lattanzi et al. [38]. performed a retrospective study with 138 patients of ICH and suggested that BPV during 72 hours after stroke onset was independently associated with poor clinical outcome at 3-month. The mechanism of poor clinical outcome mediated by BPV may include the following aspects. Recurrent sudden rises or falls in BP may contribute to the arterial bleeding and hematoma enlargement [39,40] which were the risk factors for patients with ICH. In addition, fluctuation of BP may have an affect brain perfusion and blood flow, therefore aggravating the secondary brain injury [41]. The evidence presented above shows that stabilization of BPV during the first 72 h after stroke onset can improve the clinical outcome of patients with ICH.
Besides LMR, BPV, and spot sign, many other factors are meditated secondary brain injury following spontaneous ICH, such as hyperglycemia, ficolin-1, and glycosylated hemoglobin [42–44], indicating that the pathophysiological processes involved in secondary-induced damage are associated with metabolic, hemodynamic, and pharmacological factors. Therefore, it is important to perform multidimensional modeling to predict treatment complications and prognosis in patients with ICH. A multidimensional model could involve immunological, imaging, serological, genetic, clinical, and proteomic information of patients [45]. This multidimensional model may be used to identify patients at high risk and customize treatment strategies, thus comprehensively improving the clinical outcome. However, it is difficult to predict clinical outcome based on multidimensional models due to the heterogeneity and complexity of all the potentially involved determinants [45]. In the near future, artificial intelligence with a large-scale stroke database based on multiple hospitals and centers may build this model [45].
The results of this study should be interpreted prudently. To begin with, this was a retrospective study and lacks prospective and multi-center studies using specific blood sampling analyses. Times of blood collection varied following admission, so it is difficult to assess the dynamic changes of NLR and LMR during the hospital stay. Thus, the relationships of NLR associated with ND and 90-day mortality are unclear. Furthermore, patients who underwent surgery were excluded in the present study, which may have contributed to selection bias. Finally, NLR and LMR can be affected by many factors, including chronic or acute systemic inflammation, unhealthy lifestyle, and concomitant conditions such as pneumonia, hypertension, diabetes, hepatitis, and heart failure. In the present study, we excluded patients with pneumonia, hepatitis, and heart failure, while unhealthy lifestyle including smoking and drinking and concomitant conditions such as hypertension and diabetes were identified as confounding factors adjusting for NLR and LMR. Therefore, further studies based on LMR and NLR should clearly define the exclusion and inclusion criteria.
Conclusions
Our results indicate that LMR upon admission is a simple, inexpensive, and immediately obtainable biomarker useful in predicting ND and 90-day mortality in patients with ICH. This reliable and easy-to-use predictor could contribute to clinical treatment strategy design in patients with ICH. Further studies should be performed to investigate the relevant inflammation and immune pathways to find new targets for ND and 90-day mortality and to improve the clinical outcome.
Acknowledgements
We are grateful to all patients and their relatives for their cooperation and support.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Feigin VL, Lawes CM, Bennett DA, et al. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 2.Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM. 2006;99:625–33. doi: 10.1093/qjmed/hcl082. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Safdar K, Weil J, et al. Predictors of early deterioration and mortality in Black Americans with spontaneous intracerebral hemorrhage. Stroke. 1995;26:1764–67. doi: 10.1161/01.str.26.10.1764. [DOI] [PubMed] [Google Scholar]
- 4.Ovesen C, Christensen AF, Havsteen I, et al. Prediction and prognostication of neurological deterioration in patients with acute ICH: A hospital-based cohort study. BMJ Open. 2015;5(7):e008563. doi: 10.1136/bmjopen-2015-008563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 6.Kazui S, Naritomi H, Yamamoto H, et al. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783–87. doi: 10.1161/01.str.27.10.1783. [DOI] [PubMed] [Google Scholar]
- 7.Leira R, Dávalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–67. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 8.Sansing LH, Harris TH, Kasner SE, et al. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:173–78. doi: 10.1007/978-3-7091-0693-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, Wang L, Jiang TT, et al. Neutrophil-to-lymphocyte ratio is an independent predictor of 30-day mortality of intracerebral hemorrhage patients: A validation cohort study. Neurotox Res. 2018 doi: 10.1007/s12640-018-9890-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Xu F, Quan Y, et al. Early increase of neutrophil-to-lymphocyte ratio predicts 30-day mortality in patients with spontaneous intracerebral hemorrhage. CNS Neurosci Ther. 2018 doi: 10.1111/cns.12977. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lattanzi S, Cagnetti C, Rinaldi C, et al. Neutrophil-to-lymphocyte ratio and outcome prediction in acute intracerebral hemorrhage. J Neurol Sci. 2018;387:98–102. doi: 10.1016/j.jns.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Walsh KB, Sekar P, Langefeld CD, et al. Monocyte count and 30-day case fatality in intracerebral hemorrhage. Stroke. 2015;46:2302–4. doi: 10.1161/STROKEAHA.115.009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Hu S, Ding Y, et al. Neutrophil-to-lymphocyte ratio and 30-day mortality in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2016;25:182–87. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Agnihotri S, Czap A, Staff I, et al. Peripheral leukocyte counts and outcomes after intracerebral hemorrhage. J Neuroinflammation. 2011;8:160. doi: 10.1186/1742-2094-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lattanzi S, Cagnetti C, Provinciali L, et al. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. 2016;47:1654–57. doi: 10.1161/STROKEAHA.116.013627. [DOI] [PubMed] [Google Scholar]
- 16.Ho CL, Lu CS, Chen JH, et al. Neutrophil/lymphocyte ratio, lymphocyte/monocyte ratio, and absolute lymphocyte count/absolute monocyte count prognostic score in diffuse large B-cell lymphoma: Useful prognostic tools in the rituximab era. Medicine (Baltimore) 2015;94(24):e993. doi: 10.1097/MD.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren H, Han L, Liu H, et al. Decreased lymphocyte-to-monocyte ratio predicts poor prognosis of acute ischemic stroke treated with thrombolysis. Med Sci Monit. 2017;23:5826–33. doi: 10.12659/MSM.907919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord AS, Gilmore E, Choi HA, Mayer SA VISTA-ICH Collaboration. Time course and predictors of neurological deterioration after intracerebral hemorrhage. Stroke. 2015;46:647–52. doi: 10.1161/STROKEAHA.114.007704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lattanzi S, Cagnetti C, Provinciali L, et al. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017;8(34):57489–94. doi: 10.18632/oncotarget.15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansing LH, Harris TH, Kasner SE, et al. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:173–78. doi: 10.1007/978-3-7091-0693-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tapia-Perez JH, Karagianis D, Zilke R, et al. Assessment of systemic cellular inflammatory response after spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg. 2016;150:72–79. doi: 10.1016/j.clineuro.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Mracsko E, Javidi E, Na SY, et al. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke. 2014;45:2107–14. doi: 10.1161/STROKEAHA.114.005801. [DOI] [PubMed] [Google Scholar]
- 23.Rolland WB, Lekic T, Krafft PR, et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp Neurol. 2013;241:45–55. doi: 10.1016/j.expneurol.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu L, Jian Z, Stary C, et al. T Cells and cerebral ischemic stroke. Neurochem Res. 2015;40:1786–91. doi: 10.1007/s11064-015-1676-0. [DOI] [PubMed] [Google Scholar]
- 25.Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–99. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Mao L, Zhou G, et al. Adoptive regulatory T-cell therapy preserves systemic immune homeostasis after cerebral ischemia. Stroke. 2013;44:3509–15. doi: 10.1161/STROKEAHA.113.002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood–brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol. 2011;70(3):218–35. doi: 10.1097/NEN.0b013e31820d94a5. [DOI] [PubMed] [Google Scholar]
- 28.Westendorp WF, Nederkoorn PJ, Vermeij JD, et al. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamhane UU, Aneja S, Montgomery D, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–57. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014;8:388. doi: 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Y, Tsirka SE. Chemokines and their receptors in intracerebral hemorrhage. Transl Stroke Res. 2012;3:70–79. doi: 10.1007/s12975-012-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyette LB, Macedo C, Hadi K, et al. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12:e0176460. doi: 10.1371/journal.pone.0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sansing LH, Harris TH, Kasner SE, et al. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochirurgica. 2011;111:173–78. doi: 10.1007/978-3-7091-0693-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Hallenbeck JM, Ruetzler C, et al. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23(6):748–55. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- 35.Urra X, Villamor N, Amaro S, et al. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009;29:994–1002. doi: 10.1038/jcbfm.2009.25. [DOI] [PubMed] [Google Scholar]
- 36.Adeoye O, Walsh K, Woo JG, et al. Peripheral monocyte count is associated with case fatality after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014;23(2):e107–11. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh KB, Sekar P, Langefeld CD, et al. Monocyte count and 30-day case fatality in intracerebral hemorrhage. Stroke. 2015;46(8):2302–4. doi: 10.1161/STROKEAHA.115.009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lattanzi S, Cagnetti C, Provinciali L, et al. Blood pressure variability and clinical outcome in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24(7):1493–99. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Menon RS, Burgess RE, Wing JJ, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71:199–205. doi: 10.1002/ana.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Luna D, Piñeiro S, Rubiera M, et al. Impact of blood pressure changes and course on hematoma growth in acute intracerebral hemorrhage. Eur J Neurol. 2013;20:1277–83. doi: 10.1111/ene.12180. [DOI] [PubMed] [Google Scholar]
- 41.Buratti L, Cagnetti C, Balucani C, et al. Blood pressure variability and stroke outcome in patients with internal carotid artery occlusion. J Neurol Sci. 2014;339(1–2):164–68. doi: 10.1016/j.jns.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Saxena A, Anderson CS, Wang X, et al. Prognostic significance of hyperglycemia in acute intracerebral hemorrhage: The INTERACT2 study. Stroke. 2016;47(3):682–88. doi: 10.1161/STROKEAHA.115.011627. [DOI] [PubMed] [Google Scholar]
- 43.Lattanzi S, Bartolini M, Provinciali L, et al. Glycosylated hemoglobin and functional outcome after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25(7):1786–91. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Zangari R, Zanier ER, Torgano G, et al. Early ficolin-1 is a sensitive prognostic marker for functional outcome in ischemic stroke. J Neuroinflammation. 2016;13:16. doi: 10.1186/s12974-016-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stroke Lattanzi S, Silvestrini M. Stroke outcome prediction: What do we know and where are we going? Eur J Neurol. 2018 doi: 10.1111/ene.13754. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]