Abstract
Background
Elderly patients with Ewing sarcoma have a very poor prognosis, and treatment remains a challenge. However, the outcomes and potential prognostic factors of elderly Ewing sarcoma patients are rarely documented. Therefore, we investigated the prognosis of this special cohort and determine independent prognostic factors.
Material/Methods
A cohort of Ewing sarcoma patients aged over 40 years from 1973 to 2015 was identified from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database. The Kaplan-Meier method and a Cox proportional hazard regression model were used for the prognostic analysis.
Results
A total of 162 patients were included with a mean age of 53 years. The 5-year overall survival (OS) and cancer-specific survival (CSS) rates of the entire group were 43.7% and 47.9%, respectively. The sex, location, tumor size, and radiation treatment had no effect on survival outcomes on univariate analysis. Tumor stage, surgery, and chemotherapy were significant indicators of both OS and CSS on multivariable analysis.
Conclusions
Surgery in combination with chemotherapy had a significant survival benefit in elderly Ewing sarcoma patients and should be recommended.
MeSH Keywords: Adult; Prognosis; Sarcoma, Ewing; Treatment Outcome
Background
Ewing sarcoma (ES) is the second most frequent bone sarcomas and usually occurs in children and adolescents [1,2]. The demographic, prognostic, and outcome data of ES in children and young adults, or patients of all ages, are well documented. Current treatment strategies of ES include systemic chemotherapy, and local control such as surgical resection and radiation treatment [3]. The 10-year cancer-specific survival rate for patients with non-metastatic ES is about 70%, while it is less than 30% for patients with metastasis [4]. Age, tumor size, tumor site, metastasis at presentation, surgery, and systemic chemotherapy are all associated with the prognosis of ES [4–7]. ES is radiosensitive, and radiotherapy is an effect adjuvant treatment for local control. However, Wan et al. [5] recently reported that patients with ES showed poor survival when receiving radiotherapy alone. Thus, the prognostic role of radiotherapy in elderly EW patients should be assessed.
Unlike osteosarcoma, ES does not have the second incidence peak in the elderly; therefore, ES patients aged over 40 years are extremely rare. Elderly ES patients often have a poorer prognosis than younger patients. Recently, Cesari et al. [8] reported poor outcomes in 31 elderly ES patients, with a 5-year overall survival rate of 54%, but they did not explore the risk factors for survival of elderly ES patients. To obtain deeper insight into elderly ES patients, we analyzed ES patients aged over 40 years from 1973 to 2015 based on the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database. This is the largest investigation of elderly ES patients that aimed to confirm the predictors of survival.
Material and Methods
Patient population
From 1973 to 2015, a total of 2436 patients diagnosed with ES of bone were identified from the SEER database. The database is publicly available and does not include unique patient identifiers. This study was carried out in accordance with standard guidelines and was approved by the local Ethics Committee.
First, data on ES of bone was retrieved based on the International Classification of Diseases for Oncology, third edition (ICD-O-3; histologic type: 9260 and site code: C40.0–40.3, C40.8–41.4, C41.8–41.9), using the case-listing procedure. Only patients aged over 40 years were enrolled, by reference to the age at diagnosis. All patient diagnoses were confirmed histologically, based either on biopsy results or the surgical specimen. Twenty-three patients diagnosed only on the basis of the clinical presentation, or according to the radiography, or unknown, were excluded. Seven patients with unknown therapy were excluded. Fourteen patients with unknown stage were also excluded. The inclusion criteria are shown in Figure 1. Data extracted from the SEER database included age, sex, location, tumor stage, tumor type, tumor size, surgical treatment, radiation treatment, chemotherapy, cause of death, and survival time. Surgery or radiation treatment for tumors in our study refers to treatment for local primary tumors. We divided the location into 3 categories: (1) appendicular (long and short bones of the upper and lower extremities), (2) axial (pelvis and spine), and (3) other locations (mandible, skull, rib, sternum, clavicle, and other atypical locations).
Figure 1.
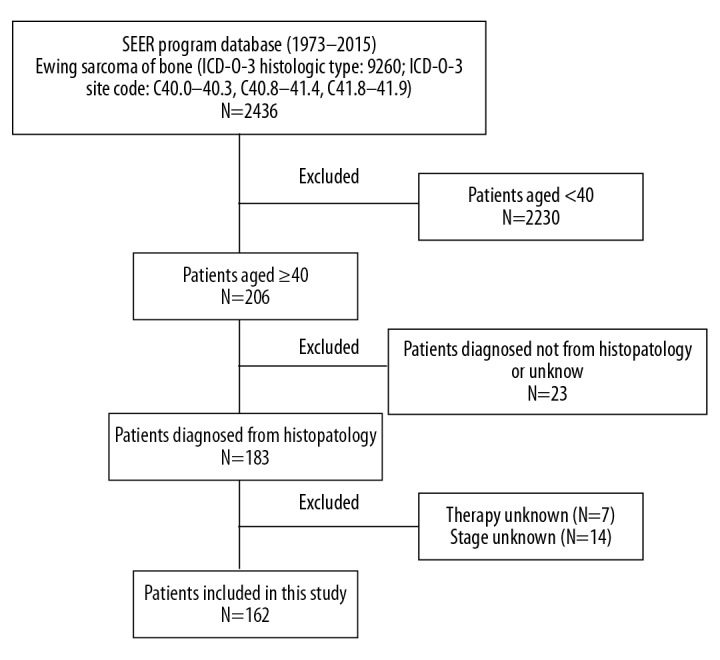
Flow chart for selection of the study population. SEER – surveillance, epidemiology, and end results; ICD-O-3 – international classification of diseases for oncology, 3rd edition; ES – Ewing sarcoma.
Statistical methods
The Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, USA) and SPSS software (ver. 22.0; SPSS Inc., Chicago, IL, USA) were used for statistical analyses. Overall survival (OS) was defined as the time from diagnosis to death from any cause, and cancer-specific survival (CSS) was regarded as the time from diagnosis to death due specifically to cancer. Univariate analysis was performed to select possible risk factors associated with survival using the Kaplan-Meier method. The independent predictors of OS and CSS were determined using multivariate analysis with a Cox proportional hazard regression. Statistical significance was set at P≤0.05.
Results
Demographic and clinical characteristics of ES patients aged over 40 years
From 1973 to 2015, data for a total of 162 patients with ES who met the inclusion criteria were collected from the SEER database. Demographic and clinical characteristics of patients are listed in Table 1. The median patient age at diagnosis was 51 years (ranging from 41 to 87 years). In terms of location, 34.0% of tumors were located in the extremities, 38.3% in the axial skeleton, and 27.8% in other sites. Information on tumor size was available in 65.4% cases, and was categorized into 3 groups. Half of the patients (50.6%) received local surgery, half of the patients (48.8%) received radiation treatment, and most of the patients (82.7%) had chemotherapy. Ultimately, 92 patients (56.8%) died, of whom 67 died of cancer. The 5-year OS and CSS rates of the study population were 43.7% and 47.9%, respectively. The 10-year OS and CSS rates of the study population were 31.8% and 39.9%, respectively (Table 1).
Table 1.
Demographic and clinical characteristics of 162 elderly patients with Ewing sarcoma identified in the SEER database from 1973 to 2015.
| Category | Value |
|---|---|
| Median age (range) | 51 (41–87) |
| Sex | |
| Female | 71 (43.8%) |
| Male | 91 (56.2%) |
| Location | |
| Appendicular | 55 (34.0%) |
| Axial | 62 (38.3%) |
| Other locations | 45 (27.8%) |
| Tumor stage | |
| Localized | 37 (22.8%) |
| Regional | 58 (35.8%) |
| Distant | 67 (41.4%) |
| Tumor size | |
| Mean (cm) | 8 |
| <8 cm | 67 (41.4%) |
| ≥8 cm | 39 (24.1%) |
| Unknown | 56 (34.6%) |
| Surgery | |
| Yes | 82 (50.6%) |
| No | 80 (49.4%) |
| Radiation treatment | |
| Yes | 79 (48.8%) |
| No | 83 (51.2%) |
| Chemotherapy | |
| Yes | 134 (82.7%) |
| No | 28 (17.3%) |
| Dead | |
| Yes | 92 (56.8%) |
| No | 70 (43.2%) |
| 5-year OS rate | 43.7% |
| 5-year CSS rate | 47.9% |
| 10-year OS rate | 31.8% |
| 10-year CSS rate | 39.9% |
OS – overall survival; CSS – cancer-specific survival.
Univariate analyses of variables associated with OS or CSS in ES patients aged over 40 years
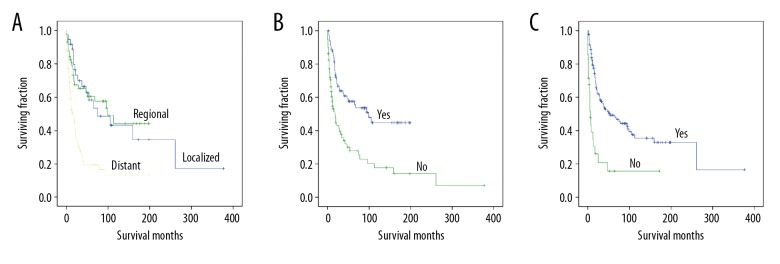
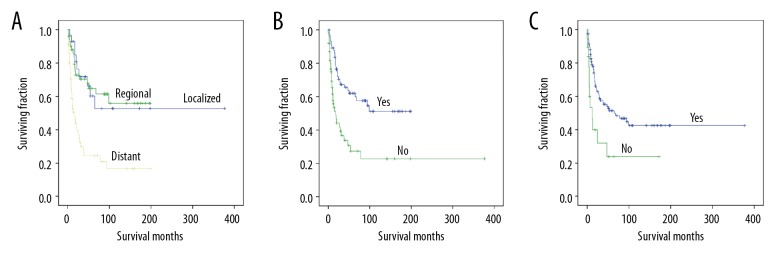
Univariate analyses revealed that sex, tumor location, tumor size, and radiation treatment were not associated with OS or CSS (Table 2). Tumor stage was associated with significant differences in OS and CSS (Table 2), with metastasis predicting a worse prognosis (Figures 2A, 3A). Both OS and CSS showed a significant difference based on surgery or chemotherapy (Table 2). Patients who underwent surgical treatment (Figures 2B, 3B) or received chemotherapy (Figures 2C, 3C) had better OS and CSS than those who did not.
Table 2.
Univariate analysis of variables in elderly patients with Ewing sarcoma using Kaplan-Meier method.
| Category | OS (log-rank p value) | CSS (log-rank p value) |
|---|---|---|
| Sex | 0.378 | 0.145 |
| Location | 0.428 | 0.977 |
| Tumor stage | 0.000 | 0.000 |
| Distant vs. localized | 0.000 | 0.000 |
| Distant vs. regional | 0.000 | 0.000 |
| Regional vs. localized | 0.985 | 0.960 |
| Tumor size (<8 cm vs. ≥8 cm) | 0.098 | 0.071 |
| Surgery | 0.000 | 0.000 |
| Radiation treatment | 0.183 | 0.296 |
| Chemotherapy | 0.000 | 0.005 |
OS – overall survival; CSS – cancer-specific survival.
Figure 2.
Kaplan-Meier method estimated OS in elderly patients with Ewing sarcoma of bone stratified by: (A) tumor stage, (B) surgery, and (C) chemotherapy.
Figure 3.
Kaplan-Meier method estimated CSS in elderly patients with Ewing sarcoma of bone stratified by: (A) tumor stage, (B) surgery, and (C) chemotherapy.
Multivariate analysis of independent predictors of OS or CSS in ES patients aged over 40 years
On multivariate analysis of all patients, tumor stage, surgery for primary tumors, and chemotherapy were determined to be independent risk factors of OS and CSS (Table 3).
Table 3.
Multivariate analysis for OS and CSS for elderly patients with Ewing sarcoma.
| Variable | OS | CSS | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Tumor stage | ||||
| Localized | 1 | 1 | ||
| Regional | 1.389 (0.734–2.628) | 0.313 | 1.373 (0.614–3.067) | 0.440 |
| Distant | 2.629 (1.494–4.626) | 0.001 | 2.976 (1.470–6.023) | 0.002 |
| Surgery | ||||
| Yes | 1 | 1 | ||
| No | 2.269 (1.429–3.601) | 0.001 | 2.099 (1.209–3.646) | 0.008 |
| Chemotherapy | ||||
| Yes | 1 | 1 | ||
| No | 3.785 (2.252–6.360) | 0.000 | 2.481 (1.292–4.762) | 0.006 |
OS – overall survival; CSS – cancer-specific survival.
Discussion
Ewing sarcoma is a highly aggressive sarcoma of bone, and occurs predominantly in children and young adults [9]. It is the second most frequent primary bone sarcoma, and accounts for about 34% of all primary bone tumors [10]. However, outcomes for ES patients aged over 40 remain poor. Because cases of ES patients aged over 40 are very rare, no previous study has determined the prognostic factors of this special cohort. Our study is the largest to describe the clinical features of such patients and to explore possible predictors of survival using the SEER program database.
Patients with ES over age 40 exhibit different clinical characteristics compared with children and young adults. This study found that 41.4% of the patients in our study presented metastatic disease at diagnosis, which was more frequent than in younger patients (21.0%, 217/1031) [11]. However, Cesari et al. [8] reported that 15.8% (3/19) of skeletal ES patients aged over 40 years presented metastatic disease. The small sample size of their study may explain this difference. Previous studies reported that axial osteosarcoma occurred more frequently in the elder than younger patients [12–14], but our study revealed that the incidence of axial ES in elderly persons was similar to that of young persons [4,7,11,15]. Bivas et al. [6] reported that ES patients with metastasis presented a larger mean tumor size (11.6 cm versus 9.4 cm; p=0.02), but our study showed that the mean tumor size was 8 cm. It is possible that one-third of patients in our study had unknown tumor size. Previous studies indicated that older ES patients experienced a survival disadvantage [5,11,16]. The 5-year OS rate of elderly ES patients in this study was 43.7%, and was similar to those with metastasis [9,11], suggesting a very poor prognosis. Thus, it is necessary to explore prognostic factors to better guide the management of such patients.
Many studies found that sex was not a prognostic factor for ES [5,7,17,18], consistent with our findings. However, Miller et al. [11] identified male sex (HR=1.33, 95%CI 1.04–1.70) as an independent negative prognostic factor at 5 years. Duchman et al. [4] found that male sex was associated with decreased CSS at 10 years, but it was not an independent prognostic factor. Further studies will need to clarify the reasons for sex differences in survival. Duchman et al. [4] and Wan et al. [5] analyzed ES patients identified in the SEER program database and found that axial tumor location was associated with a poorer outcome compared with an appendicular location. However, by multivariate analyses, Miller et al. [11] reported that tumor site was not associated with survival. In our study, tumor location was not associated with the prognosis of elderly ES patients. Tumor stage is generally recognized as a very important predictor of ES [6,7,11,18]. Uyeturk et al. [18] reported that tumor stage was an independent prognostic factor of ES in adults, which was similar to our study. Many studies have reported that larger tumor size is associated with poorer prognosis and decreased survival rate of ES patients [5,7,11]. However, in our cohort, tumor size was not associated with either OS or CSS. The fact that one-third of the patients had unknown tumor size may explain this difference in our results.
Surgery, radiotherapy, and chemotherapy are the current standard treatments of ES patients [19]. However, the appropriate treatment for elderly ES patients remains unknown. Chemotherapy-related toxicity was generally higher than in younger patients [20,21]. Therefore, treatment for elderly sarcoma patients remains a challenge. The majority of patients (134, 82.7%) received chemotherapy, and only 28 (17.3%) did not. Our study also suggests that chemotherapy can offer a favorable outcome for elderly ES patients. Cesari et al. [8] found that the chemotherapy toxicity of elderly ES patients was similar to that of younger patients and recommended aggressive chemotherapy in this age group. Moreover, surgical resection significantly prolonged the survival of our elderly ES patients, similar to other studies [5,6,11]. Thus, surgical excision in combination with chemotherapy should be recommended to this age group.
As ES is a radiosensitive tumor, radiotherapy can offer effective local control and reduce local recurrence [22]. However, there have been few studies on the effects of radiotherapy on survival of elderly ES patients. Elderly ES patients may receive radiotherapy due to its lower toxicity compared with systemic chemotherapy. Miller et al. [11] revealed that ES patients receiving radiotherapy alone were associated with shorter 5-year survival, but Ning et al. [15] reported that radiotherapy did not appear to be inferior to surgery alone for most ES patients in regard to survival. Arshi et al. [17] found that radiation treatment increased survival of spinal ES patients but did not reach statistical significance for both OS and CSS. Similarly, our study showed that about half of patients (48.8%) received radiotherapy, but this treatment was not associated with survival. The role of radiation treatment for elderly ES patients needs to be clarified in the future.
There are several limitations of this investigation. First, data on local recurrence or metastatic spread during the follow-up period were not recorded in the SEER database. Second, this database does not provide data on other prognostic factors in cancer survival, such as chemotherapy procedure and surgical method. Despite these shortcomings, the SEER database is of great value in the study of elderly patients with ES.
Conclusions
This is the largest investigation to determine the optimal management of elderly ES patients. OS and CSS rates of the study population at 5 years were 43.7% and 47.9%, respectively. Tumor stage, surgery, and chemotherapy were determined to be independent predictors of both OS and CSS. Elderly ES patients should receive chemotherapy and surgery if at all possible to achieve a good survival rate. Our study explored OS and CSS and their risk factors in this special cohort and lays a solid foundation for future research on standard therapy.
Acknowledgements
We appreciate the contribution of the SEER database.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Cotterill SJ, Parker L, Malcolm AJ, et al. Incidence and survival for cancer in children and young adults in the North of England, 1968–1995: A report from the Northern Region Young Persons’ Malignant Disease Registry. Br J Cancer. 2000;83:397–403. doi: 10.1054/bjoc.2000.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maheshwari AV, Cheng EY. Ewing sarcoma family of tumors. J Am Acad Orthop Surg. 2010;18:94–107. doi: 10.5435/00124635-201002000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Dunst J, Schuck A. Role of radiotherapy in Ewing tumors. Pediatric Blood Cancer. 2004;42:465–70. doi: 10.1002/pbc.10446. [DOI] [PubMed] [Google Scholar]
- 4.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with Ewing’s sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015;39:189–95. doi: 10.1016/j.canep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Wan ZH, Huang ZH, Chen LB. Survival outcome among patients with Ewing’s sarcoma of bones and joints: A population-based cohort study. Sao Paulo Med J. 2017;136(2):116–22. doi: 10.1590/1516-3180.2017.0236230917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas B, Rastogi S, Khan SA, et al. Outcomes and prognostic factors for Ewing-family tumors of the extremities. J Bone Joint Surg Am. 2014;96:841–49. doi: 10.2106/JBJS.M.00411. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Hoang BH, Ziogas A, Zell JA. Analysis of prognostic factors in Ewing sarcoma using a population-based cancer registry. Cancer. 2010;116:1964–73. doi: 10.1002/cncr.24937. [DOI] [PubMed] [Google Scholar]
- 8.Cesari M, Righi A, Cevolani L, et al. Ewing sarcoma in patients over 40 years of age: A prospective analysis of 31 patients treated at a single institution. Tumori. 2016;102:481–87. doi: 10.5301/tj.5000534. [DOI] [PubMed] [Google Scholar]
- 9.Gaspar N, Hawkins DS, Dirksen U, et al. Ewing sarcoma: Current management and future approaches through collaboration. J Clin Oncol. 2015;33:3036–46. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 10.Li YJ, Yang X, Zhang WB, et al. Clinical implications of six inflammatory biomarkers as prognostic indicators in Ewing sarcoma. Cancer Manag Res. 2017;9:443–51. doi: 10.2147/CMAR.S146827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller BJ, Gao Y, Duchman KR. Does surgery or radiation provide the best overall survival in Ewing’s sarcoma? A review of the National Cancer Data Base. J Surg Oncol. 2017;116:384–90. doi: 10.1002/jso.24652. [DOI] [PubMed] [Google Scholar]
- 12.Nishida Y, Isu K, Ueda T, et al. Osteosarcoma in the elderly over 60 years: A multicenter study by the Japanese Musculoskeletal Oncology Group. J Surg Oncol. 2009;100:48–54. doi: 10.1002/jso.21287. [DOI] [PubMed] [Google Scholar]
- 13.Manoso MW, Healey JH, Boland PJ, et al. De novo osteogenic sarcoma in patients older than forty: Benefit of multimodality therapy. Clin Orthop Relat Res. 2005;438:110–15. doi: 10.1097/01.blo.0000179587.42350.4d. [DOI] [PubMed] [Google Scholar]
- 14.Carsi B, Rock MG. Primary osteosarcoma in adults older than 40 years. Clin Orthop Relat Res. 2002;397:53–61. doi: 10.1097/00003086-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Ning MS, Perkins SM, Borinstein SC, et al. Role of radiation in the treatment of non-metastatic osseous Ewing sarcoma. J Med Imaging Radiat Oncol. 2016;60:119–28. doi: 10.1111/1754-9485.12389. [DOI] [PubMed] [Google Scholar]
- 16.Gupta AA, Pappo A, Saunders N, et al. Clinical outcome of children and adults with localized Ewing sarcoma: Impact of chemotherapy dose and timing of local therapy. Cancer. 2010;116:3189–94. doi: 10.1002/cncr.25144. [DOI] [PubMed] [Google Scholar]
- 17.Arshi A, Sharim J, Park DY, et al. Prognostic determinants and treatment outcomes analysis of osteosarcoma and Ewing sarcoma of the spine. Spine J. 2017;17:645–55. doi: 10.1016/j.spinee.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uyeturk U, Helvaci K, Demirci A, et al. Clinical outcomes and prognostic factors of adult’s Ewing sarcoma family of tumors: Single center experience. Contemp Oncol (Pozn) 2016;20:141–46. doi: 10.5114/wo.2016.58487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subbiah V, Anderson P, Lazar AJ, et al. Ewing’s sarcoma: Standard and experimental treatment options. Curr Treat Options Oncol. 2009;10:126–40. doi: 10.1007/s11864-009-0104-6. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari S, Bielack SS, Smeland S, et al. EURO-B.O.S.S.: A European study on chemotherapy in bone-sarcoma patients aged over 40: Outcome in primary high-grade osteosarcoma. Tumori. 2018;104:30–36. doi: 10.5301/tj.5000696. [DOI] [PubMed] [Google Scholar]
- 21.Grimer RJ, Cannon SR, Taminiau AM, et al. Osteosarcoma over the age of forty. Eur J Cancer. 2003;39:157–63. doi: 10.1016/s0959-8049(02)00478-1. [DOI] [PubMed] [Google Scholar]
- 22.Albergo JI, Gaston CLL, Parry MC, et al. Risk analysis factors for local recurrence in Ewing’s sarcoma: When should adjuvant radiotherapy be administered? Bone Joint J. 2018;100-b:247–55. doi: 10.1302/0301-620X.100B2.BJJ-2017-0222.R1. [DOI] [PubMed] [Google Scholar]