Abstract
Background
Osteoprotegerin (OPG) is a soluble glycoprotein that belongs to the tumor necrosis factor (TNF) receptor superfamily. OPG is mainly secreted by bone. The relationship between acute resistance training, serum OPG levels and metabolic syndrome, including insulin resistance, remains unclear. The purpose of this study was to determine the effect of resistance exercise on serum OPG levels and insulin resistance in middle-aged women with metabolic syndrome.
Material/Methods
Twenty-four middle-aged women were divided into those with metabolic syndrome (n=12) and a normal control group without metabolic syndrome or insulin resistance (n=12). Metabolic syndrome was diagnosed according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria. The quantitative insulin-sensitivity check index (QUICKI) and the homeostatic model assessment (HOMA) index for assessing beta-cell function and insulin resistance were used. The intensity of the resistance exercise was 60–70% of the repetition maximum, for 40 minutes with 10–12 repetitions, performed three times per week. Venous blood samples were tested using standard laboratory procedures.
Results
Before exercise, the metabolic syndrome group showed a significant increase in waist circumference (P=0.030) and serum triglyceride (TG) (P=0.014), and lower high-density lipoprotein-cholesterol (HDL-C) (P=0.010) compared with the control group. After the eight-week resistance exercise program, waist circumference, and the QUICKI decreased and OPG levels were significantly increased in the metabolic syndrome group compared with the normal control group.
Conclusions
A resistance exercise program was effective in reducing factors associated with metabolic syndrome including insulin resistance and increases serum levels of OPG in middle-aged women.
MeSH Keywords: Exercise, Insulin Resistance, Metabolic Syndrome X, Osteoprotegerin
Background
Metabolic syndrome includes insulin resistance, abdominal obesity, hypertension, and hyperlipidemia, and is recognized to be a risk factor for cardiovascular disease [1]. Osteoprotegerin (OPG) is a soluble member of the tumor necrosis factor (TNF) receptor superfamily [2]. OPG has a role in the activation of nuclear factor kappa B (NF-κB) ligand (RANKL)-mediated osteoclastic bone resorption [3].
In a large cohort study of postmenopausal women, Xue et al. did not show an association between increased serum levels of OPG and reduced bone density and an increased rate of bone fracture [4]. However, these authors found that serum levels of OPG were significantly associated with increased mortality due to cardiovascular disease [4]. Further studies have reported that increased serum levels of OPG were associated with coronary artery atherosclerosis, stroke, and increased mortality from cardiovascular disease [5,6].
Serum levels of OPG have been shown to be significantly increased in patients who have diabetes, poor blood glucose control, and in obese non-diabetic subjects [7,8]. However, there is limited published data on the relationship between serum OPG levels and metabolic syndrome. In a study that examined a cohort of patients with peripheral artery disease, serum concentrations of OPG were significantly increased in patients who were obese and who had metabolic syndrome [9]. Levels of OPG and other biomarkers of vascular inflammation have been shown to be raised in patients with type 2 diabetes [10]. Increased serum levels of OPG have been shown in obese postmenopausal Korean women who also had hypercholesterolemia [11]. However, another study showed an inverse relationship between serum OPG levels and fasting blood glucose levels, fasting insulin levels, and the homeostatic model assessment (HOMA) index for assessing beta-cell function and insulin resistance [12].
Adipocytokines are secreted by adipose tissue and regulate glucose homeostasis [13], but the relationship with OPG has not previously been studied in middle-aged women. However, for middle-aged men, there has been shown to be no statistically significant difference in OPG values between men with or without metabolic syndrome [7,8].
Chronic inflammation is associated with the metabolic changes that are related to excess caloric intake, obesity, metabolic syndrome, and decreased physical activity [14,15]. Metabolic syndrome increases with increasing age and is most common in postmenopausal women. However, few studies have investigated the changes in serum OPG levels in women with metabolic syndrome in premenopausal, menopausal, and postmenopausal women.
Physical activity has been shown to be effective in the management and prevention of osteoporosis [16]. Resistance exercise is reported to have a positive effect on bone density as it increases both muscle and bone mass [17,18]. Resistance training also activates the immune system, as shown by increased levels of circulating inflammatory cytokine [19,20]. Because women experience symptoms associated with the menopause and are at increased risk of cardiovascular disease and bone fracture, preventive measures in this group now include exercise.
The purpose of this study was to determine the effect of resistance exercise on serum OPG levels and insulin resistance in middle-aged women with metabolic syndrome.
Material and Methods
Ethical approval and determination of study size
This study was approved by the institutional review board (IRB) of Kangwon National University, Kangwon province, South Korea (KNUIRB-2017-03-005-002) and was conducted in accordance with the ethical standards of the declaration of Helsinki. All subjects were told about the purpose of the study and the study design, and all participants signed written informed consents.
To determine the sample size, the G*Power version 3.19 statistical power analysis software program was used. The alpha level was 0.05. According to a prior pilot test, at least 12 participants were required in each of the two study groups.
Study participants
The study participants were middle-aged women who visited the Athletics Department, Kangwon University, South Korea to participate in a resistance exercise program. A total of 24 study participants were chosen as subjects of study and were divided into those with metabolic syndrome (n=12), and a normal control group without metabolic syndrome or insulin resistance (n=12) (Figure 1). Metabolic syndrome was defined according to the criteria used by the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III). A diagnosis of metabolic syndrome requires any combination of three or more of the following: a fasting blood glucose of at least 5.56 mmol/l; a blood pressure of at least 130/85 mmHg; triglyceride (TG) level of at least 150 mg/dl; a high-density lipoprotein cholesterol (HDL-C) level <50 mg/dl [21], and a waist circumference of at least 85 cm [22]. The characteristics of the participants in the study are shown in Table 1.
Figure 1.
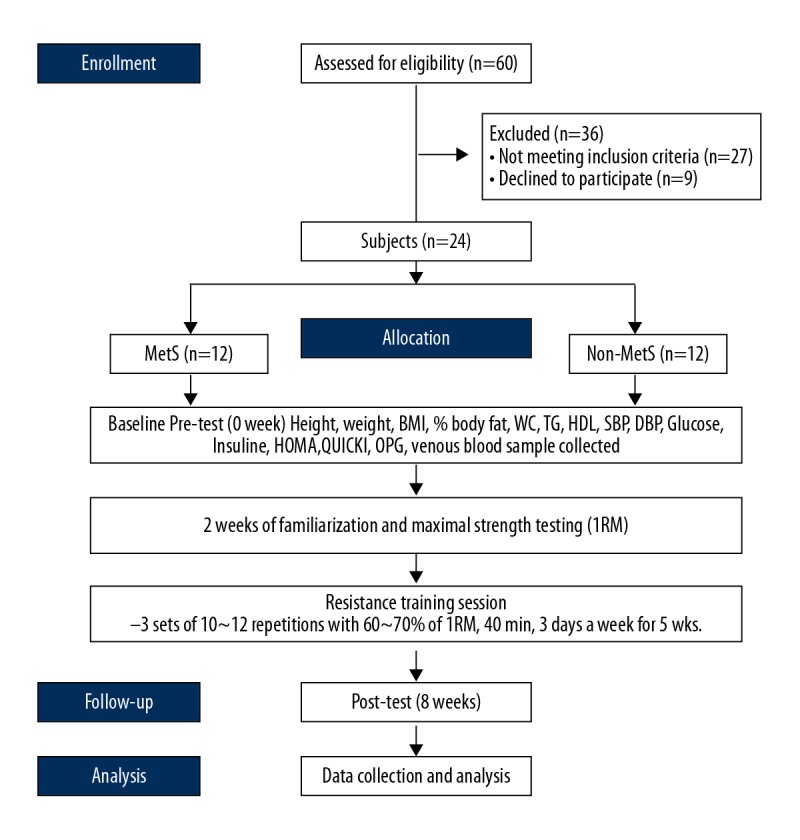
Flow diagram of the study design.
Table 1.
Characteristics of the participants (N=24).
| Groups | Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) | %Body fat (%) |
|---|---|---|---|---|---|
| MetS (n=12) | 47.24±2.20 | 155.43±4.17 | 60.05±6.73 | 24.44±1.31 | 35.66±2.38 |
| Non-MetS (n=12) | 48.36±2.60 | 155.87±4.25 | 57.43±3.76 | 24.30±3.04 | 33.55±3.41 |
| t (p) | −1.160 (.259) | −1.193 (.246) | 1.180 (.251) | .145 (.886) | 1.755 (.093) |
Mean ± standard deviation.
MetS – metabolic syndrome (n=12); Non-MetS – non-metabolic syndrome (n=12); BMI – body mass index.
Morphometric and clinical measurements
Morphometric measurements included were height, weight, body mass index (BMI), and percentage of body fat. Height, weight, and percentage of body fat were measured using Inbody 720 (Biospace, Seoul, Korea). The body mass index (BMI) was calculated by dividing the body weight (kg) by the square of the height (m2). Waist circumference was measured at the midpoint between the lower part of the ribs and the iliac crest. Systolic blood pressure and diastolic blood pressure were measured with a mercury thermometer. Blood pressure measurements were performed in triplicate, separated by 5 min intervals, and the mean values were recorded for systolic and diastolic blood pressure.
Laboratory tests
Venous blood samples were taken from all study participants following a 12-hour overnight fast. The following tests were performed under the same conditions at the beginning of the study before the exercise program began and after eight weeks of resistance exercise. The serum was then stored separately at −80°C for other measurements. Laboratory investigations were performed according to standard diagnostic laboratory procedures and included measurement of white blood cell count (WBC), plasma TG, HDL-C, systolic blood pressure, diastolic blood pressure, glucose levels, and insulin levels. Serum osteoprotegerin (OPG) levels were measured from venous blood samples from all study participants, using a commercial enzyme immunoassay (Biomedica Gruppe, Vienna, Austria) with inter-assay and intra-assay variation being <10%. Serum glucose levels were determined by the glucose oxidase color method (Diagnosticum Zrt, Hungary) while triglycerides and HDL cholesterol were measured by an enzymatic, colorimetric method (Diagnosticum Zrt, Budapest, Hungary). Insulin was measured using an enzyme-linked immunosorbent assay (ELISA) kit (DRG Instruments, GmbH, Marburg, Germany).
Insulin resistance was determined by the homeostasis model assessment of insulin resistance (HOMA) and defined as: fasting insulin (μlU/ml) × fasting glucose (mmol/l)/22.5 [23]. Insulin sensitivity was determined using the quantitative insulin sensitivity check index (QUICKI) defined as 1/log fasting insulin (μlU/ml) + log fasting glucose (mmol/l) [24]. A low QUICKI indicated low insulin sensitivity, and a high QUICKI indicated high insulin sensitivity.
Method of resistance exercises
All 24 study participants, including women with metabolic syndrome (n=12) and a normal control group without metabolic syndrome or insulin resistance (n=12), underwent an eight-week exercise program. An initial preparatory session was undertaken for each participant that included 15 repetitions of each exercise to determine 30% of the maximum intensity of that participant’s resistance intensity. Then, the intensity of the resistance exercise was 60–70% of the repetition maximum, for 40 minutes with 10–12 repetitions, performed three times per week. The participants conducted a full body resistance training program, including seven different exercises: leg press; leg extension; leg curl; chest press; front pull-down; shoulder press; and abdominal crunch. One minute of rest time was given to the participants between each set and exercise. All sessions were supervised by experienced strength education professionals. The progression of resistance movements was ‘personalized,’ and untrained patients with metabolic syndrome specifically targeted in this study [25]. After two weeks of adaptation, the one repetition maximum test was performed and there was a 10-minute rest interval between the exercises. Only study participant who wished to continue with the study were included.
Statistical analysis
Statistical analysis was performed using SPSS version 23.0 for Windows (IBM, Chicago, Ill, USA). Data were presented as the mean ± standard division (SD). The Shapiro-Wilk test for normality was used to confirm the normal distribution of all outcome variables. A t-test, paired t-test, and independent t-test were used to compare the physical characteristics and laboratory findings of the two study groups before and after exercising. Statistical significance was represented by P-value <0.05.
Results
Twenty-four middle-aged women were divided into those with metabolic syndrome (n=12) and a normal control group without metabolic syndrome or insulin resistance (n=12). Table 2 shows the changes in serum osteoprotegerin (OPG) levels and insulin resistance after eight weeks of resistance exercise in the two groups.
Table 2.
The changes in serum OPG levels and insulin resistance after 8 weeks of resistance exercise.
| Variables | MetS | Non-MetS | t-Value# | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | t-Value* | p-Value | Before | After | t-Value* | p-Value | |||
| Waist circumference (cm) | 87.63±6.01 | 85.70±5.51 | 5.796 | <.001 | 83.13±2.97 | 82.70±2.35 | 1.265 | .232 | −3.137 | .005 |
| Triglyceride (mg/dl) | 184.50±25.41 | 157.00±17.75 | 7.482 | <.001 | 157.50±23.90 | 142.42±17.61 | 3.946 | .002 | −1.915 | .069 |
| HDL cholesterol (mg/dl) | 46.42±6.30 | 48.58±4.85 | −2.377 | .037 | 52.67±4.31 | 53.58±3.26 | −1.733 | .111 | 1.186 | .248 |
| Systolic blood pressure (mmHg) | 123.33±21.85 | 121.25±14.79 | .861 | .408 | 122.08±10.33 | 120.83±6.69 | .821 | .429 | −.291 | .773 |
| Diastolic blood pressure (mmHg) | 73.33±12.85 | 73.75±10.47 | −.321 | .754 | 74.58±7.22 | 72.92±7.22 | 1.173 | .266 | 1.082 | .291 |
| Glucose (mmol/l) | 5.79±.59 | 5.35±.58 | 5.870 | <.001 | 5.48±.55 | 5.03±.50 | 6.231 | <.001 | .088 | .930 |
| Insulin (μlU/ml) | 15.48±2.83 | 12.73±2.44 | 5.001 | <.001 | 14.78±4.02 | 11.83±2.56 | 4.196 | .001 | .233 | .818 |
| HOMA | 3.98±.78 | 3.01±.59 | 6.798 | <.001 | 3.64±1.22 | 2.64±.63 | 5.050 | <.001 | .127 | .900 |
| QUICKI | .52±.03 | .55±.03 | −7.395 | <.001 | .53±.04 | .57±.03 | −7.055 | <.001 | .190 | <.001 |
| Serum OPG (pg/ml) | 72.17±15.66 | 81.01±20.36 | −3.084 | .010 | 76.29±14.65 | 83.92±17.43 | −2.312 | .041 | 14.249 | <.001 |
Mean ±SD. MetS – metabolic syndrome (n=12); Non-MetS – non-metabolic syndrome (n=12); HOMA – homeostasis model assessment of insulin resistance; QUICKI – quantitative insulin sensitivity check index; OPG – osteoprotegerin;
paired t-test;
independent t-test for change variation (Δ; after-before value).
Before the exercise program began, the metabolic syndrome group showed significant increases in waist circumference (t=2.324; P=0.030) and serum triglyceride (TG) (t=2.681; P=0.014) and a lower high-density lipoprotein-cholesterol (HDL-C) value (t=−2.835; P=0.010) compared with the control group. After the eight-week resistance exercise program, there was a difference between the change in waist circumference, the quantitative insulin-sensitivity check index (QUICKI), and serum levels of OPG between the two groups.
In the metabolic syndrome group, after eight weeks of resistance exercise, waist circumference was reduced, the QUICKI improved, TG levels, HDL-C, fasting blood glucose, insulin levels, and the homeostasis model assessment of insulin resistance (HOMA) index were significantly decreased. OPG levels were significantly increased in the metabolic syndrome group compared with the normal control group.
In the control group, after eight weeks of resistance exercise, TG levels, fasting blood glucose, insulin levels, and the HOMA index were significantly decreased, and the QUICKI and serum OPG levels were significantly increased.
Discussion
Osteoprotegerin (OPG) is a glycoprotein that has been recently identified as an inhibitor of nuclear factor kappa B (NF-κB) ligand (RANKL)-mediated osteoclastic bone resorption [3]. An increase in circulating levels of OPG has been shown to be associated with an increased risk of cardiovascular disease due to coronary artery atherosclerosis that leads to ischemic heart disease [26] and is associated with an increase in cardiovascular mortality [7]. Therefore, recent studies have shown that OPG is a metabolic biomarker for human disease.
There have been conflicting studies reporting both an increase and a decrease in serum levels of OPG following exercise in postmenopausal women. In 2012, Bergström et al. showed that serum OPG levels were found to increase in a group of postmenopausal women that had undergone physical training for a one-year period when compared with a sedentary group [27]. These authors hypothesized that an exercise-induced OPG increment compensates for the loss of OPG due to reduced estrogen levels in postmenopausal women [27]. However, in 2009, in a study by West et al., serum OPG levels were found to be higher in a group of premenopausal women with a sedentary lifestyle [28].
Premenopausal women who have high serum levels of OPG are likely to have improved metabolic indices and their incidence of cardiovascular disease may be reduced. Previous studies have shown that obesity, which is a component of metabolic syndrome, is associated with increased levels of circulating inflammatory mediators [10,29]. The findings of the present study have shown that the increase in serum OPG levels in response to an eight-week resistance exercise program in middle-aged women with metabolic syndrome showed significant protective effects, which are supported by the findings from previously published studies [30,31]. Resistance exercise in this study may have contributed to the increase in serum OPG levels as an index of reduced inflammation.
In this study, before undertaking an eight-week exercise program, middle-aged women with metabolic syndrome showed higher baseline levels of OPG. Therefore, as shown by previous studies, exercise can have a significant effect on serum levels of OPG, which may be explained by the fact that serum OPG levels increase under conditions of bone loss, including osteoporosis [32,33]. Osteoporosis and arteriosclerosis are major health issues, especially in postmenopausal women [34]. Postmenopausal women with osteoporosis report an increased mortality rate from cardiovascular disease that correlates with osteoporosis, independent of age and other risk factors for cardiovascular disease [35].
The resistance exercises used in this study are believed to have resulted in increased regional decomposition through increased energy consumption. It is assumed that the positive effects that were observed in the decrease in fasting blood glucose with increased serum OPG levels, and the improvement in insulin resistance were due to the effects on resistance exercise on bone. The clinical implications of these study findings should be qualified by the caveat that the progress of a resistance training program depends on the development of a proper and specific training goal.
The findings of the present study have shown that in middle-aged women with metabolic syndrome, circulating or serum levels of OPG were correlated with waist circumference and the quantitative insulin-sensitivity check index (QUICKI), with the number of risk factors for metabolic syndrome having decreased from four to three. The results are consistent with the hypothesis that OPG is associated with some of the components of metabolic syndrome. Resistance exercise may have the beneficial effect of increasing the serum OPG levels of middle-aged women with metabolic syndrome, as well as improving insulin resistance.
Conclusions
The aim of this study was to evaluate the effect of resistance exercise on serum osteoprotegerin (OPG) levels and insulin resistance in middle-aged women with metabolic syndrome compared with a control group without metabolic syndrome. Before the exercise program began, waist circumference and triglyceride (TG) levels were higher in the metabolic syndrome group compared with the normal control group, and HDL cholesterol was lower. After eight weeks of resistance exercise training, there was a difference between the waist circumference, the quantitative insulin-sensitivity check index (QUICKI), and OPG levels between the two groups. The use of resistance exercise training had the effect of increasing the serum OPG levels of the middle-aged woman with metabolic syndrome, as well as improving insulin resistance, and metabolic syndrome. In postmenopausal women, increased body fat is associated with reduced bone mineral density, and the prevalence of both osteoporosis and ischemic cardiovascular disease cardiovascular disease increases. The findings of the present study support the need for large-scale, multi-center, controlled studies to determine whether metabolic syndrome is a significant risk factor for postmenopausal osteoporosis and whether measurement of serum levels of OPG levels can be used as a clinical biomarker for metabolic syndrome or osteoporosis.
Footnotes
Source of support: This work was supported by the National Research Foundation of Korea Grant, funded by the Korean Government (NRF-2015S1A5135A07042779)
Conflict of interest
None.
References
- 1.Dallmeier D, Larson MG, Vasan RS, et al. Metabolic syndrome and inflammatory biomarkers: A community-based cross-sectional study at the Framingham Heart Study. Diabetol Metab Syndr. 2012;4(1):28. doi: 10.1186/1758-5996-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozer FF, Dagdelen S, Erbas T. Relation of RANKL and OPG levels with bone resorption in patients with acromegaly and prolactinoma. Horm Metab Res. 2018;50(7):562–67. doi: 10.1055/a-0630-1529. [DOI] [PubMed] [Google Scholar]
- 3.Blázquez-Medela AM, López-Novoa JM, Martínez-Salgado C. Osteoprotegerin and diabetes-associated pathologies. Curr Molecular Med. 2011;11:401–16. doi: 10.2174/156652411795976565. [DOI] [PubMed] [Google Scholar]
- 4.Xue Y, Jiang L, Cheng Q, et al. Adipokines in psoriatic arthritis patients: The correlations with osteoclast precursors and bone erosions. PLoS One. 2012;7:467–78. doi: 10.1371/journal.pone.0046740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayina CN, Sobngwi E, Essouma M, et al. Osteoprotegerin in relation to insulin resistance and blood lipids in sub-Saharan African women with and without abdominal obesity. Diabetol Metab Syndr. 2015;7:47. doi: 10.1186/s13098-015-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siasos G, Oikonomou E, Maniatis K, et al. Prognostic significance of arterial stiffness and osteoprotegerin in patients with stable coronary artery disease. Eur J Clin Invest. 2018;48(3) doi: 10.1111/eci.12890. [DOI] [PubMed] [Google Scholar]
- 7.Nabipour I, Kalantarhormozi M, Larijani B, et al. Osteoprotegerin in relation to type 2 diabets mellitus and the metabolic syndrome in postmenopausal women. Metabolism. 2010;59:742–47. doi: 10.1016/j.metabol.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Musialik K, Szulinska M, Hen K, et al. The relation between osteoprotegerin, inflammatory processes, and atherosclerosis in patients with metabolic syndrome. Eur Rev Med Phamacol Sci. 2017;21:4379–85. [PubMed] [Google Scholar]
- 9.Esteghamati A, Aflatoonian M, Rad MV, et al. Association of osteoprotegerin with peripheral artery disease in patients with type 2 diabetes. Arch Cardiovasc Dis. 2015;108:412–19. doi: 10.1016/j.acvd.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan EP, Ashley DT, Devlin N, et al. Osteoprotegerin and biomarkers of vascular inflammation in type 2 diabetes. Diabetes Metab Res Rev. 2010;26:496–502. doi: 10.1002/dmrr.1109. [DOI] [PubMed] [Google Scholar]
- 11.Oh ES, Rhee EJ, Oh KW, et al. Circulating osteoprotegerin levels are associated with age, waist to hip ratio, serum total cholesterol and low-density lipoprotein cholesterol levels in healthy Korean women. Metabolism. 2005;54:49–54. doi: 10.1016/j.metabol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Mashavi M, Menaged M, Shargorodsky M. Circulating osteoprotegerin in postmenopausal osteoporotic women: Marker of impaired glucose regulation or impaired bone metabolism. Menopause. 2017;24:1264–68. doi: 10.1097/GME.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 13.Akinci B, Celtik A, Yuksel F, et al. Increased osteoprotegerin levels in women with previous gestational diabetes developing metabolic syndrome. Diabetes Res Clin Pract. 2011;91:26–31. doi: 10.1016/j.diabres.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Cooke AA, Connaughton RM, Lyons CL, et al. Fatty acids and chronic low-grade inflammation associated with obesity and the metabolic syndrome. Eur J Pharmacol. 2016;785:207–14. doi: 10.1016/j.ejphar.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Riva N, Donadini MP, Ageno W. Epidemiology and pathophysiology of venous thromboembolism: Similarities with atherothrombosis and the role of inflammation. Thromb Haemost. 2015;113:1176–83. doi: 10.1160/TH14-06-0563. [DOI] [PubMed] [Google Scholar]
- 16.McMillan LB, Zengin A, Ebeling PR, et al. Prescribing physical activity for the prevention and treatment of osteoporosis in older adults. Healthcare (Basel) 2017;5(4) doi: 10.3390/healthcare5040085. pii: E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castrogiovanni P, Trovato FM, Szychlinska MA, et al. The importance of physical in osteoporosis. From the molecular pathways to the clinical evidence. Histol Histopathol. 2016;31(11):1183–94. doi: 10.14670/HH-11-793. [DOI] [PubMed] [Google Scholar]
- 18.Morreira LD, Oliveira ML, Lirani-Galvao AP, et al. Physical exercise and osteoporosis: Effects of different types of exercises on bone and physical function of postmenopausal women. Arg Bras Endocrinol Metabol. 2014;58:514–22. doi: 10.1590/0004-2730000003374. [DOI] [PubMed] [Google Scholar]
- 19.Pietrzak M. Adhesive capsulitis: An age-related symptom of syndrome and chronic low-grade inflammation? Med Hypotheses. 2016;88:12–17. doi: 10.1016/j.mehy.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Koelwyn GJ, Wennerberg E, Demaria S, et al. Exercise in regulation of inflammation-immune axis function in cancer initiation and progression. Oncology. 2015;29:908–20. [PMC free article] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Park HS, Kim SM. Cut-off points of waist circumference for defining abdominal obesity in the Korean population. Korean J Obes. 2006;15:1–9. [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–19. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 25.Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Chen WJ, Rijzewijk LJ, van der Meer RW, et al. Association of plasma osteoprotegerin and adiponectin with arterial function, cardiac function and metabolism in asymptomatic type 2 diabetic men. Cardiovasc Diabetol. 2011;10:67. doi: 10.1186/1475-2840-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergstöm I, Parini P, Gustafsson SA, et al. Physical training increases osteoprotegerin in postmenopausal women. J Bone Miner Metab. 2012;30:202–7. doi: 10.1007/s00774-011-0304-6. [DOI] [PubMed] [Google Scholar]
- 28.West SL, Scheid JL, De Souza MJ. The effect of exercise and estrogen on osteoprotegerin in premenopausal women. Bone. 2009;44:137–44. doi: 10.1016/j.bone.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Pereira GB, Tibana RA, Navalta J, et al. Acute effects of resistance training on cytokines and osteoprotegerin in women with metabolic syndrome. Clin Physiol Funct Imaging. 2013;33:122–30. doi: 10.1111/cpf.12004. [DOI] [PubMed] [Google Scholar]
- 30.Boutagy NE, McMillan RP, Frisard MI, et al. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie. 2016;124:11–20. doi: 10.1016/j.biochi.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tibana RA, Nascimento DC, de Sousa NM, et al. Enhancing of women functional status with metabolic syndrome by cardioprotective and anti-inflammatory effects of combined aerobic and resistance training. PLoS One. 2014;9:1–8. doi: 10.1371/journal.pone.0110160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott JP, Sale C, Greeves JP, et al. The effect of training status on the metabolic response of bone to an acute bout of exhaustive treadmill running. J Clin Endocrinol Metab. 2010;95:3918–25. doi: 10.1210/jc.2009-2516. [DOI] [PubMed] [Google Scholar]
- 33.An N, Li Y, Tang ZL, et al. Expression of osteoprotegerin and receptor activator of nuclear factor kappa-B ligand in mandibular ramus osteotomy healing with administration of different doses of parathyroid hormone. Zhonghua Kou Qiang Yi Xue Za Zhi. 2018;53:413–18. doi: 10.3760/cma.j.issn.1002-0098.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Samelson EJ, Miller PD, Christiansen C, et al. RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J Bone Miner Res. 2014;29:450–57. doi: 10.1002/jbmr.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SN, Cho JY, Eun YM, et al. Associations between osteoporosis and coronary artery disease in postmenopausal women. Climacteric. 2016;19:458–62. doi: 10.1080/13697137.2016.1200550. [DOI] [PubMed] [Google Scholar]


