Abstract
Background
Drug-resistant epilepsy is a common neurological disease in adults and children. This study aimed to undertake a systematic review of the literature with meta-analysis of the data from published studies to assess the effectiveness of magnetic resonance imaging (MRI)-guided laser interstitial thermal therapy (LITT) in treatment-resistant epilepsy.
Material/Methods
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed, MEDLINE, and EMBASE databases were systematically searched for indexed publications in the English language up to May 2018. Data on the prevalence, outcome using the Engel Epilepsy Surgery Outcome Scale (Class I to IV), and postoperative complications were analyzed with 95% confidence intervals (CIs). The Methodological Index for Non-Randomized Studies (MINORS) was used to assess the risk of bias in the included studies.
Results
Sixteen published studies that included a total of 269 patients with treatment resistant epilepsy were identified. The prevalence of Engel Class I, II, III and IV were 61% (95% CI, 0.54–0.68; I2=14.5%; P=0.302), 12% (95% CI, 0.07–0.16; I2=86.8%; P=0.000), 16% (95% CI, 0.10–0.22; I2=3.0%; P=0.397), and 15% (95% CI, 0.08–0.22; I2=13.2%; P=0.330), respectively. The prevalence of postoperative complications was 24% (95% CI, 0.16–0.32; I2=0%; P=0.629).
Conclusions
Meta-analysis of data from 16 studies that included 269 patients with treatment-resistant epilepsy showed that MRI-guided LITT significantly reduced the frequency of seizures and reduced postoperative complications, supporting the safety and effectiveness of MRI-guided LITT in the treatment of drug-resistant epilepsy.
MeSH Keywords: Epilepsies, Partial; Epilepsy, Absence; Laser Therapy; Magnetic Resonance Imaging; Meta-Analysis
Background
Epilepsy is a common neurological disorder occurring in approximately 3% of adults [1]. In 10% of patients with epilepsy, the first episode occurs in the first three years of life [2,3]. There are about one-third of newly diagnosed patients with epilepsy who do not become seizure-free with antiseizure medications alone [4]. Therefore, additional approaches are required to control seizures that are non-responsive to drug treatment.
Currently, there are three main treatment approaches to control drug-resistant seizures in adults and children. First, epilepsy surgery is considered to be a curative method with 80–90% of epileptic patients with identified lesions becoming free from seizures, but partial resection of the lesion or removal of epileptogenic foci at crucial sites can lead to serious complications [5,6]. Second, implantation of a neuromodulatory device is a traditional way to reduce seizure frequency and has been reported to be successful for between 68–76% of patients who undergo constant stimulation for at least five years [7]. Third, minimally invasive ablated approaches have recently been adopted, including stereo-electroencephalography (SEEG)-guided radiofrequency thermocoagulation (RFTC), focused magnetic resonance imaging (MRI)-guided ultrasound surgery, and focused MRI-guided laser interstitial thermal therapy (LITT). Although LITT was initially used in the 1980s for patients with intracranial tumors, with continued progress in technological development, including real-time MRI, LITT has delivered effective outcomes when compared with RFTC [8]. Also, LITT is a technique that is without the reported edema and radiation necrosis associated with RFTC [8], and without the risk of skull heating and cranial nerve damage that can be associated with ultrasound surgery [9].
LITT as a procedure does not require regulatory approval, but in 2008 the Visualase® Thermal Therapy System (Medtronic, MN, USA) used in MRI-guided laser technology was the first system to be approved by the US Food and Drug Administration (FDA) for use in neurosurgery. However, MRI-guided LITT remains a relatively new technology and the current status of its efficacy and safety in the treatment of patients with refractory epilepsy remains unknown. Therefore, this study aimed to undertake a systematic review of the literature with meta-analysis of the data from published studies to assess the effectiveness of MRI-guided LITT in treatment-resistant epilepsy, including postoperative seizure control, and the complications associated with the use of this procedure.
Material and Methods
Study design
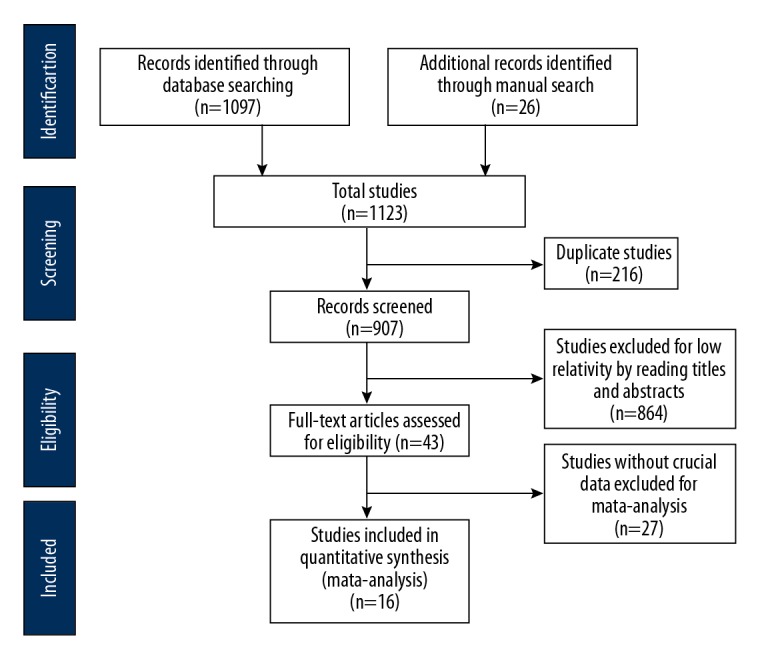
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (http://www.prisma-statement.org). Figure 1 is a flow diagram that illustrates the study design.
Figure 1.
Flowchart of the publication selection process used in the meta-analysis.
Data sources and search strategy
PubMed, MEDLINE, and EMBASE were systematically searched for all indexed publications in the English language up to May 2018. The search strategy combined the following search terms: “epilepsy” OR “drug-resistant epilepsy” OR “seizure” AND “laser interstitial thermal therapy” OR “laser-induced thermal therapy” OR “LITT”. Additional strategies included hand searches of journals that were not included in the electronic sources, internet searches of the literature, and screening of reference lists of retrieved studies.
Selection criteria
Studies were eligible for inclusion in the meta-analysis if they conformed to the following criteria: 1) patients with epilepsy who were medication-resistant with focal onset of seizures; 2) all patients were treated with magnetic resonance imaging (MRI)-guided laser interstitial thermal therapy (LITT), which was performed in a standard manner; 3) the studies contained comparable data that evaluated the efficacy of MRI-guided LITT. Studies without crucial and assessable data for statistical analysis or non-original studies such as reviews, letters, and commentaries were excluded. For relevant studies that did not provide necessary data for analysis, we contacted the corresponding author of the publication for information. If we did not receive a response from the author in a reasonable time, the study was excluded from the meta-analysis. During publication selection, any differences in opinion between the study members were resolved by discussion and consensus.
Data extraction
Study details of the selected publications were extracted using a data extraction form by a single reviewer and subsequently cross-checked for accuracy, consistency, and completeness by a second reviewer. Discrepancies were resolved by referring to the original publication. The following data elements were extracted to a standardized data collection sheet that included key patient characteristics, location of surgical foci, the length of follow-up, and the time taken for the surgery.
Quality assessment of the included studies
The Methodological Index for Non-Randomized Studies (MINORS) was used to assess the risk of bias in the included studies. Because this was a single rate meta-analysis, the following eight items were applied: a clearly stated aim; inclusion of consecutive patients; prospective collection of data; endpoints appropriate to the aim of the study; unbiased assessment of the study endpoint; a follow-up period appropriate to the aim of the study; loss to follow-up <5%; and prospective calculation of the study size. Each item was scored as, 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). A total score of 16 was considered to be an ideal score for non-comparative studies [10], and a total score of ≥13 was considered to represent a low risk of bias and was eligible for inclusion.
Outcome data from the Engel Epilepsy Surgery Outcome Scale (Class I to IV)
Currently, the Engel Epilepsy Surgery Outcome Scale (Class I to IV) is used to classify outcome after surgical treatment for epilepsy that is refractory to medical treatment. The Engel outcome classes included: Class I, free from disabling seizures; Class II, a rare occurrence of disabling seizures (almost seizure-free); Class III, worthwhile improvement with reduction in the frequency of seizures; Class IV, no worthwhile improvement or decrease in the frequency of seizures.
Statistical analysis
All statistical analysis was performed using STATA version 12.0 (STATA Corporation, College Station, TX). Prevalence rates were calculated from raw proportions and 95% confidence intervals (CIs) with the Wilson method [11]. Heterogeneity was tested by the I2 test and the chi-squared (χ2) test. Statistical heterogeneity occurred if the I2 statistic was >50% or the P-value was <0.05, and then a random effects model was selected. Otherwise, a fixed effects model was used. Forest plots were generated showing prevalence proportions with corresponding 95% CIs for each study. The overall pooled estimates used a random or fixed effects model. Sensitivity analysis was performed to confirm the robustness of the results by omitting one study at a time.
Results
Study selection process
As shown in Figure 1, according to the search criteria, 1,122 published studies were initially identified, with 1,097 publications from database searches, and 26 publications identified from manual searches. There were 907 publications that were excluded following review of the title and abstract, and a further 864 publications were excluded due to lack of relevance, resulting in 43 publications that were finally fully screened. Sixteen published studies that included a total of 269 patients with treatment-resistant epilepsy were identified and underwent meta-analysis (Tables 1, 2) [8,12–26].
Table 1.
Risk of bias of the included studies assessed by the Methodological Index for Non-Randomized Studies (MINORS) in 16 studies [8,12–26].
| Study | A | B | C | D | E | F | G | H | Total |
|---|---|---|---|---|---|---|---|---|---|
| Atsina et al., 2016 [26] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 15 |
| Curry et al., 2012 [12] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 15 |
| Grewal et al., 2018 [23] | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 13 |
| Ho et al., 2017 [13] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 16 |
| Jermakowicz et al., 2017 [14] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 16 |
| Kang et al., 2015 [8] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 15 |
| Lewis et al., 2015 [15] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 16 |
| Morris et al., 2017 [16] | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 13 |
| Perry et al., 2017 [17] | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 14 |
| Tao et al., 2018 [18] | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 13 |
| Vakharia et al., 2018 [24] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 15 |
| Hena et al., 2015 [19] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 15 |
| Gross and Willie, 2014 [20] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 15 |
| Yin et al., 2017 [25] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 15 |
| Youngerman et al., 2018 [21] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 15 |
| Drane et al., 2015 [22] | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 14 |
Study categories: A: a clearly stated aim; B: inclusion of consecutive patients; C: prospective collection of data; D: endpoints appropriate to the aim of the study; E: unbiased assessment of the study endpoint; F: follow-up period appropriate to the aim of the study; G: loss to follow-up less than 5%; H: prospective calculation of the study size.
Table 2.
| Study | Patient No. | Age (years) | Type of epilepsy | Follow-up (months) | Research period | Region |
|---|---|---|---|---|---|---|
| Atsina et al., 2016 [26] | 23 | 35±17 (7–66) | MTLE | 7days–51 | 2011–2015 | USA |
| Curry et al., 2012 [12] | 5 | NA | Lesional and localized epilepsy | 2–13 | NA | USA |
| Grewal et al., 2018 [23] | 20 | 43.8±13.9 (22–58) | MTLE | 12 | 2012–2015 | USA |
| Ho et al., 2017 [13] | 8 | 42.1 | TLE | >3 | 2014–2016 | USA |
| Jermakowicz et al., 2017 [14] | 23 | 40.9±11.9 | MTLE | 12–37 | NA | USA |
| Kang et al. 2015 [8] | 20 | 38.9±16.9 | MTLE | 6–24 | 2011–2014 | USA |
| Lewis et al., 2015 [15] | 17 | 15.3 (5.9–20.6) | Lesional and localized epilepsy | 3.5–35.9 | 2011–2014 | Canada |
| Morris et al., 2017 [16] | 13 | 35.5±12.7 | Lesional and localized epilepsy | 5–8 | NA | USA |
| Perry et al., 2017 [17] | 20 | 13.2±3.9 | Insular epilepsy | 7–39 | 2013–2016 | USA |
| Tao et al., 2018 [18] | 21 | 40±13 | MTLE | 7–43 | 2014–2017 | USA |
| Vakharia et al., 2018 [24] | 25 | 41.4±17.2 | MTS | 10.3–38.5 | 2012–2016 | USA |
| Hena et al., 2015 [19] | 7 | 60 (54–67) | MTLE | 12–15.6 | NA | USA |
| Gross and Willie, 2014 [20] | 13 | 24 (16–64) | MTLE | 5–26 | 2011–2013 | USA |
| Yin et al., 2017 [25] | 5 | 45 (28–69) | MTLE | 3–9 | 2015–2015 | USA |
| Youngerman et al., 2018 [21] | 30 | 13 (1–51) | TLE | 12–36 | 2013–2016 | USA |
| Drane et al., 2015 [22] | 19 | 38.2±17.1 | TLE | 6 | NA | USA |
MTLE – mesial temporal lobe epilepsy; TLE – temporal lobe epilepsy; MTS – temporal sclerosis; NA – not applicable.
Characteristics of the 16 included studies
The total scores of the risk of bias assessment by the Methodological Index for Non-Randomized Studies (MINORS) ranged from 13–16, which indicated a low risk of bias of the included studies, which are presented in Table 1 [8,12–26]. The studies included postoperative follow-up of between 7 days to 51 months. In 11 publications, the studies were undertaken between 2011 to 2018, 15 studies were performed in the United States, and one in Canada [8,12–26]. Eight publications focused on mesial temporal lobe epilepsy (MTLE), three on temporal lobe epilepsy (TLE), and another three publications on focal epilepsy. Four studies included both adult and juvenile patients with epilepsy. The characteristics of the 16 included studies in the meta-analysis are shown in Table 2 [8,12–26].
Postoperative seizure frequency using the Engel Epilepsy Surgery Outcome Scale (Class I to IV)
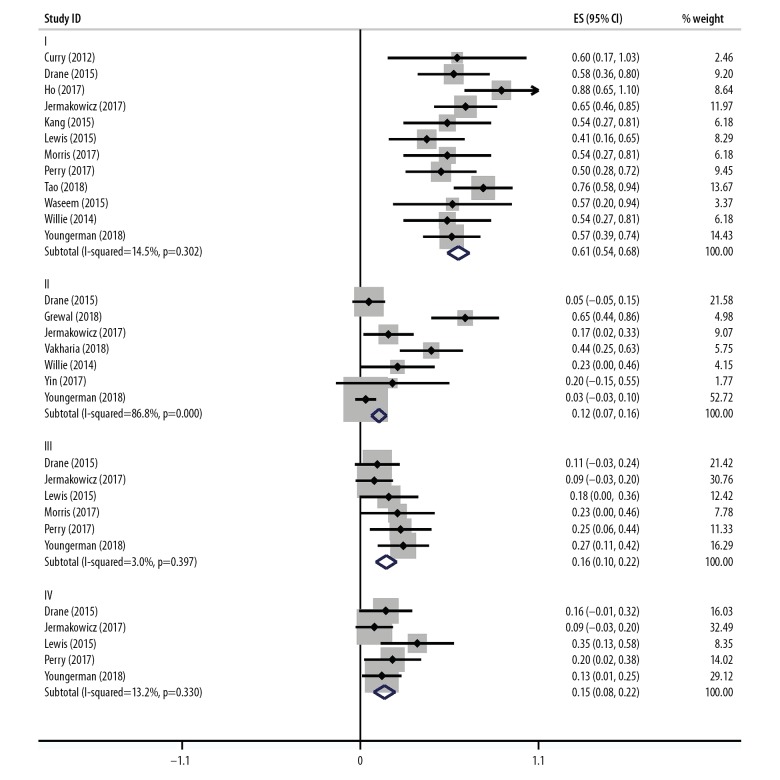
The prevalence of Engel Class I (free from disabling seizures) after ablation were reported in 12 studies [8,12–22] that included a total of 189 individuals. The pooled prevalence of patients who achieved postoperative freedom from epileptic seizures was 61% (95% CI, 0.54–0.68). Estimates ranged from 41–88% and low study heterogeneity were found (I2=14.5%; P=0.302).
Estimates for Engel Class II (a rare occurrence of disabling seizures or almost seizure-free), included 34 out of 135 patients who were evaluated, was reported in seven studies [14,20,22–25]. Estimates ranged from 3–65% with a pooled prevalence of 12% (95% CI, 0.07–0.16), but there was evidence to suggest significant study heterogeneity (I2=86.8%; P=0.000). Removal of the study by Grewal et al. [23] affected the overall pooled and heterogeneity, and the pooled prevalence changed to 6%, estimates ranged from 3–23% and heterogeneity was reduced to a low level (I2=26.9%; P=0.242).
Estimates for Engel Class III (worthwhile improvement with reduction in the frequency of seizures) were reported in six studies [14–17,21,22], with a pooled prevalence of 18% (95% CI, 0.10–0.22) including 23 out of 135 patients who were evaluated. Estimates ranged from 9–27% and low study heterogeneity was found (I2=3.0%; P=0.397).
Estimates for Engel Class IV (no worthwhile improvement or reduction in the frequency of seizures) were reported in five studies [14,15,17,21,22], including 19 out of 109 patients who were evaluated. The pooled prevalence was 15% (95% CI, 0.08–0.22), with estimates that ranged from 9–27%. Low study heterogeneity was found (I2=13.2%; P=0.330). The results are summarized in Figure 2.
Figure 2.
Prevalence of patients with Engel Class I, II, III and IV following magnetic resonance imaging (MRI)-guided laser interstitial thermal therapy (LITT) in treatment-resistant epilepsy. Engel Class I: 61% (95% CI, 0.54–0.68) of patients who were free from disabling seizures (I2=14.5%; P=0.302); Engel Class II: 12% (95% CI, 0.07–0.16) patients with a rare occurrence of disabling seizures who became almost free from seizures (I2=86.8%; P=0.000); Engel Class III: 16% (95% CI, 0.10–0.22) patients who had a worthwhile improvement with reduction in the frequency of seizures (I2=3.0%; P=0.397); Engel Class IV: 15% (95% CI, 0.08–0.22) patients with no worthwhile improvement or reduction in the frequency of seizures (I2=13.2%; P=0.330).
Postoperative complications
As shown in Figure 3, seven studies [15,17–20,26] reported postoperative complications, with a total of 26 complications in 101 patients with drug-resistant epilepsy. The pooled prevalence was 24% (95% CI, 0.16–0.32) and estimates ranged from 15–43%. Low study heterogeneity was detected (I2=0%; P=0.629).
Figure 3.
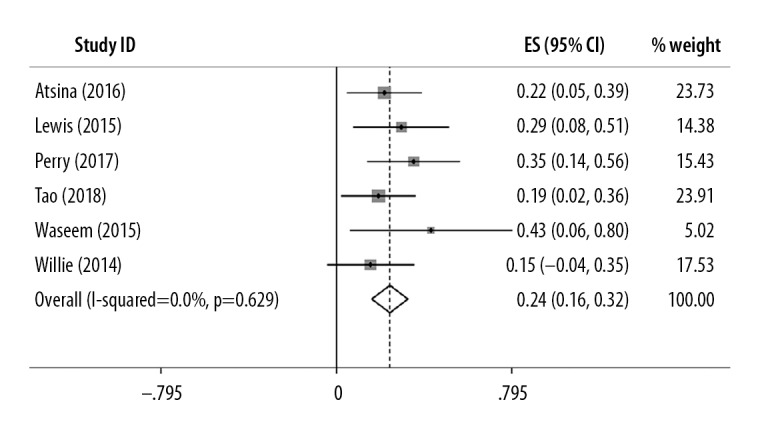
Prevalence of postoperative complications following magnetic resonance imaging (MRI)-guided laser interstitial thermal therapy (LITT) in treatment-resistant epilepsy. Only 24% of patients who received magnetic resonance imaging (MRI)-guided laser interstitial thermal therapy (LITT) had postoperative complications (I2=0%; P=0.629).
Discussion
To the best of our knowledge, this is the first meta-analysis of the safety and effectiveness of magnetic resonance imaging (MRI)-guided laser interstitial thermal therapy (LITT) in treatment-resistant epilepsy. There were 16 identified published clinical studies that included 269 participants that underwent a meta-analysis. Data on postoperative outcome included the Engel Epilepsy Surgery Outcome Scale (Class I to IV), and the Methodological Index for Non-Randomized Studies (MINORS) was used to assess the risk of bias in the included studies. The results of the meta-analysis showed that seizure reduction after LITT compared favorably with conventional open surgical techniques [8,12–26]. In the short-term follow-up, two-thirds (61%) of patients with refractory epilepsy were free from seizures or from disabling seizures after LITT and only 24% had postoperative complications. These findings indicated that MRI-guided LITT was an effective and well-tolerated approach to the treatment of drug-resistant epilepsy.
Although open surgery is an effective method for seizure control, it is also associated with postoperative neurologic and cognitive deficit [27]. Currently, LITT has become increasingly used by many epilepsy surgery centers as a minimally invasive alternative to open surgery for patients with medically refractory epilepsy who may or may not be candidates for open surgical intervention. Some of the potential advantages of LITT have been reported, including reduced morbidity, fewer complications, and a shorter length of postoperative hospital stay [28]. A study by Hena et al. [19] showed that there was no significant difference, in term of surgical outcome and neuropsychological outcomes measured at the last follow-up at 15.6 months, between open surgery and LITT, which supports the efficacy of LITT and its potential as an alternative to open resection. Also, in most studies reviewed for this meta-analysis, 50% of patients were free from seizures or from disabling seizures following MRI-guided LITT [8,12–26].
The Engel scale (Class I to IV) is an established method used to classify outcome after surgical treatment for refractory epilepsy and was used in the present study. In this meta-analysis, the pooled prevalence of Engel Class I (free from disabling seizures) was 61%, which is comparable with the median seizure-free rates of 64% reported for open surgical resection [29]. Also, due to some known reasons, which included inadequate ablation, low ablation power, and expanded epileptogenic zones, seizures may recur and further laser ablation is required for improved outcome [18]. These findings have been shown in a study by Tao et al., who showed that when recurrent seizures occurred within the first year following surgery, freedom from seizures was achieved following a second LITT procedure during follow-up [18]. Because significant heterogeneity was detected in Engel Class II (the rare occurrence of disabling seizures, or almost seizure-free), a sensitivity analysis was performed. The inclusion of the study by Grewal et al. [23] in the meta-analysis had a significant influence on the pooled results, accounting for a large number of patients who became free from seizures within one year after surgery.
Surgical complications of LITT have been reported in several clinical studies, and have been reported to include adverse functional effects (visual deficit, hemiparesis, and expressive language dysfunction), wound complications (edema, hemorrhage, infection, and pain), psychiatric symptoms (anxiety, insomnia, and depression) and complications induced by technical difficulties associated with operating the thermal therapy system. However, in studies reported by Perry et al. and Tao et al., some complications following LITT have been shown to resolve within six months on follow-up, including mild hemiparesis, language dysfunction, wound pain, and psychiatric symptoms [17,18].
Although MRI-guided LITT can achieve good outcomes, some factors may hinder its practical clinical use application. Firstly, the accurate and precise location of the epileptogenic network plays an important role in the use of LITT treatment, as most patients did not respond to LITT either underwent nonlocalized presurgical functional imaging and neurophysiology studies or had multiple lesion on MRI, suggesting a wider epileptogenic zone [19]. Therefore, some patients may not benefit from this technology due to inadequate resection of the epileptogenic focus, and so the use of electroencephalography (EEG) may be required to localize epileptogenic zones adequately, but this needs verification with further studies. Also, the use of MRI-guided LITT requires training, and there will be a learning curve associated with the use of this procedure clinically. It is important to note that Lewis et al. reported complications and treatment failure associated with LITT were induced by improper machine operation, which included inaccurate fiber placement and failure of the cooling mechanism around the catheter [15].
This study had several limitations. The study population was relatively small with a short follow-up, and the long-term outcome is an important endpoint to evaluate new technology. Because there are currently no standard preoperative protocols to guide candidate selection for MRI-guided LITT, it is possible that some patients did not receive benefits from the therapy due to inappropriate selection. Also, the use of MRI-guided LITT now includes the use of more than one type of machine, and it remains to be determined whether any one machine provides optimal results, although two clinical trials are in progress to compare outcomes from the use of two machines for LITT. Therefore, the findings of this meta-analysis support the need for further large-scale controlled clinical studies that take the above limitations into account, including the optimal choice of equipment.
Conclusions
A literature review and meta-analysis of data from 16 studies that included 269 patients with drug-resistant epilepsy treated by magnetic resonance imaging (MRI)-guided laser interstitial thermal therapy (LITT) demonstrated that this technique was effective and well tolerated, resulting in control of epileptic seizures with few complications.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Brown MG, Drees C, Nagae LM, et al. Curative and palliative MRI-guided laser ablation for drug-resistant epilepsy. J Neurol Neurosurg Psychiatry. 2018;89(4):425–33. doi: 10.1136/jnnp-2017-316003. [DOI] [PubMed] [Google Scholar]
- 2.Berg AT, Shinnar S, Levy SR, et al. Early development of intractable epilepsy in children: a prospective study. Neurology. 2001;56(11):1445–52. doi: 10.1212/wnl.56.11.1445. [DOI] [PubMed] [Google Scholar]
- 3.Olafsson E, Hauser WA. Prevalence of epilepsy in rural Iceland: A population-based study. Epilepsia. 1996;37(10):1529–34. doi: 10.1111/j.1528-1157.1999.tb02036.x. [DOI] [PubMed] [Google Scholar]
- 4.Buckley R, Estronza-Ojeda S, Ojemann JG. Laser ablation in pediatric epilepsy. Neurosurg Clin N Am. 2016;27(1):69–78. doi: 10.1016/j.nec.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Téllezzenteno JF, Hernández RL, Moienafshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: A systematic review and meta-analysis. Epilepsy Res. 2010;89(2–3):310–18. doi: 10.1016/j.eplepsyres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Englot DJ, Breshears JD, Sun PP, et al. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr. 2013;12(2):126–33. doi: 10.3171/2013.5.PEDS1336. [DOI] [PubMed] [Google Scholar]
- 7.Geller EB, Skarpaas TL, Gross RE, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58(6):994–1004. doi: 10.1111/epi.13740. [DOI] [PubMed] [Google Scholar]
- 8.Kang JY, Wu C, Tracy J, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2015;57(2):325–34. doi: 10.1111/epi.13284. [DOI] [PubMed] [Google Scholar]
- 9.Nowell M, Miserocchi A, Mcevoy AW, Duncan JS. Advances in epilepsy surgery. J Neurol Neurosurg. 2014;85(11):1273–79. doi: 10.1136/jnnp-2013-307069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. Anz J Surg. 2003;73(9):712–16. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 11.Newcombe RG. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med. 1998;17(8):857–72. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Curry DJ, Gowda A, Mcnichols RJ, Wilfong AA. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 2012;24(4):408–14. doi: 10.1016/j.yebeh.2012.04.135. [DOI] [PubMed] [Google Scholar]
- 13.Ho AL, Sussman ES, Pendharkar AV, et al. Improved operative efficiency using a real-time MRI-guided stereotactic platform for laser amygdalohippocampotomy. J Neurosurg. 2018;128(4):1165–72. doi: 10.3171/2017.1.JNS162046. [DOI] [PubMed] [Google Scholar]
- 14.Jermakowicz WJ, Kanner AM, Sur S, et al. Laser thermal ablation for mesiotemporal epilepsy: Analysis of ablation volumes and trajectories. Epilepsia. 2017;58(5):801–10. doi: 10.1111/epi.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis EC, Weil AG, Duchowny M, et al. MR-guided laser interstitial thermal therapy for pediatric drug-resistant lesional epilepsy. Epilepsia. 2015;56(10):1590–98. doi: 10.1111/epi.13106. [DOI] [PubMed] [Google Scholar]
- 16.Morris SA, Rollo M, Rollo P, et al. Prolonged blood–brain barrier disruption following laser interstitial ablation in epilepsy: A case series with a case report of post-ablation optic neuritis. World Neurosurg. 2017;104:467–75. doi: 10.1016/j.wneu.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Perry MS, Donahue DJ, Malik SI, et al. Magnetic resonance imaging-guided laser interstitial thermal therapy as treatment for intractable insular epilepsy in children. J Neurosurg Pediatr. 2017;20(6):1–8. doi: 10.3171/2017.6.PEDS17158. [DOI] [PubMed] [Google Scholar]
- 18.Tao JX, Wu S, Lacy M, et al. Stereotactic EEG-guided laser interstitial thermal therapy for mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2018;89(5):542–48. doi: 10.1136/jnnp-2017-316833. [DOI] [PubMed] [Google Scholar]
- 19.Hena W, Osborn KE, Schoenberg MR, et al. Laser ablation therapy: An alternative treatment for medically resistant mesial temporal lobe epilepsy after age 50. Epilepsy Behav. 2015;51:152–57. doi: 10.1016/j.yebeh.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Gross RE, Willie JT. Response to Journal Club: Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2015;77(3):502–4. doi: 10.1227/NEU.0000000000000876. [DOI] [PubMed] [Google Scholar]
- 21.Youngerman BE, Oh JY, Anbarasan D, et al. Laser ablation is effective for temporal lobe epilepsy with and without mesial temporal sclerosis if hippocampal seizure onsets are localized by stereoelectroencephalography. Epilepsia. 2018;59(3):595–606. doi: 10.1111/epi.14004. [DOI] [PubMed] [Google Scholar]
- 22.Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56(1):101–13. doi: 10.1111/epi.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grewal SS, Gupta V, Vibhute P, et al. Mammillary body changes and seizure outcome after laser interstitial thermal therapy of the mesial temporal lobe. Epilepsy Res. 2018;141:19–22. doi: 10.1016/j.eplepsyres.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Vakharia VN, Sparks R, Li K, et al. Automated trajectory planning for laser interstitial thermal therapy in mesial temporal lobe epilepsy. Epilepsia. 2018;59(4):814–24. doi: 10.1111/epi.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin D, Thompson JA, Drees C, et al. Optic radiation tractography and visual field deficits in laser interstitial thermal therapy for amygdalohippocampectomy in patients with mesial temporal lobe epilepsy. Stereotact Funct Neurosurg. 2017;95(2):107–13. doi: 10.1159/000454866. [DOI] [PubMed] [Google Scholar]
- 26.Atsina KB, Sharan AD, Wu C, et al. Journal Club. Longitudinal qualitative characterization of MRI features after laser interstitial thermal therapy in drug-resistant epilepsy. Am J Roentgenol. 2017;208(1):48–56. doi: 10.2214/AJR.16.16144. [DOI] [PubMed] [Google Scholar]
- 27.Shukla ND, Ho AL, Pendharkar AV, et al. Laser interstitial thermal therapy for the treatment of epilepsy: Evidence to date. Neuropsychiatr Dis Treat. 2017;13:2469–75. doi: 10.2147/NDT.S139544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du VX, Gandhi SV, Rekate HL, Mehta AD. Laser interstitial thermal therapy: A first-line treatment for seizures due to hypothalamic hamartoma? Epilepsia. 2017;58(S2):77–84. doi: 10.1111/epi.13751. [DOI] [PubMed] [Google Scholar]
- 29.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–18. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]