Summary
Animals react rapidly to external stimuli, such as an approaching predator, but in other circumstances they seem to act spontaneously, without any obvious external trigger. How do the neural processes mediating the execution of reflexive and spontaneous actions differ? We studied this question in tethered, flying Drosophila. We found that silencing a large, but genetically defined set of non-motor neurons virtually eliminates spontaneous flight turns while preserving the flies’ ability to perform two types of visually evoked turns, demonstrating that, at least in flies, these two modes of action are almost completely dissociable.
Keywords: Neuroscience, Drosophila, action initiation
eToc Blurb
Ferris et al. report that silencing neurons labeled by the NP0212 GAL4 line abolishes both graded and sharp spontaneous flight turns in Drosophila, but preserves the fly’s ability to perform two classes of visual responses. The neurons in NP0212 that mediate this effect are putatively cholinergic and are located in the thoracic ganglion.
Introduction
When scientists first tracked the trajectories of freely flying flies, they noted that their paths were composed of segments of straight flight interspersed with rapid rotations, which were dubbed saccades [1]. The purpose of a straight-run-and-saccade locomotor strategy is still not settled, although one influential idea is that insects navigate with rapid turns so as to restrict the amount of time that their retinal image is blurred during locomotion [1]. Drosophila that are glued in place will flap their wings in tethered flight. In this preparation flies perform frequent, sharp wing-steering movements, which likely represent saccadic attempts to turn left or right [2]. Like others before us [2, 3], we will refer to these attempted turns in tethered flight as saccades, although more work will be required to determine the exact relationship between free-flight maneuvers and any specific tethered-flight measurement made here [4, 5].
In both tethered and free flight, saccades often occur at unpredictable times relative to any obvious external sensory event [1, 3–6]. Though some of these seemingly random turns may ultimately be shown to have some relationship to the time history of sensory experience [7, 8], we will operationally refer to such turns as spontaneous saccades. In free flight, spontaneous saccades may help flies search efficiently for randomly distributed resources when no obvious sensory stimulus is available to guide them [6]. In tethered flight, it has also been suggested that spontaneous saccades reflect an internal action-initiation process that can serve as a substrate for operant learning [2, 9–12].
In addition to spontaneous saccades, tethered, flying flies perform a wide suite of visual flight behaviors. Here we study two types of visually evoked turns that can be operationally separated from spontaneous ones. One class is loom-evoked saccades, which are rapid steering maneuvers away from the center of a rapidly expanding disc, which simulates an object that is on a collision course with the fly [13, 14]. The kinematics of loom-evoked saccades are subtly different from those of spontaneous turns in free flight, but both involve a roll of the body followed by a counter roll and a rotation about the yaw axis [5, 13]. Another class of visually evoked turns are optomotor responses which are syn-directional steering responses to wide-field optic flow that act to stabilize the flight trajectory.
Spontaneous saccades, loom-evoked saccades, and optomotor responses represent three easily measurable and well controlled steering maneuvers on the tether, which allows us to ask a fundamental question regarding motor behavior. Namely, are spontaneous flight turns dissociable from these types of visually evoked flight turns, beyond the trivial fact that the latter require vision? Psychologists and neuroscientists have long studied spontaneous and reflexive behaviors and their neurophysiological correlates [15–25]. Here we ask whether a physiological perturbation can abolish a spontaneous behavior while preserving reflexive execution of the same (or similar) behavior. We find that impairing synaptic transmission in a large, but genetically defined population of neurons in Drosophila causes flies to markedly reduce the rate at which they generate spontaneous saccades while preserving their ability to perform both loom-evoked saccades and graded optomotor responses. These results argue that there exists a set of neurons in flies whose normal synaptic activity is essential for the regular execution of spontaneous turns, and whose same activity is dispensable for these two types of visually evoked turns.
Results
A set-up for studying spontaneous flight turning in Drosophila
To study spontaneous and visually evoked flight turns, we glued Drosophila to a custom platform [26] and tracked their wing movements during tethered flight with a camera from below (Figs 1A–B, see Methods). We computed the left-minus-right wingbeat angle (L–R WBA) with respect to the fly’s body axis, a signal correlated with yaw torque [27], as a measure of the fly’s intention to turn. Positive deflections in L–R WBA indicate that the fly is attempting to turn right, and negative deflections indicate that the fly is attempting to turn left. We note that L–R WBA is not a perfect measure of the fly’s yaw torque (let alone attempted maneuvers along other rotational axes) because it does not capture many important kinematic aspects of the wing’s motion, like the angle of attack and timing of the wing flip, but it serves as a sufficient measure for our first-order purposes here: determining if the fly is attempting any turns at all.
Figure 1. A behavioral setup for measuring both spontaneous and visually evoked flight turns in Drosophila.
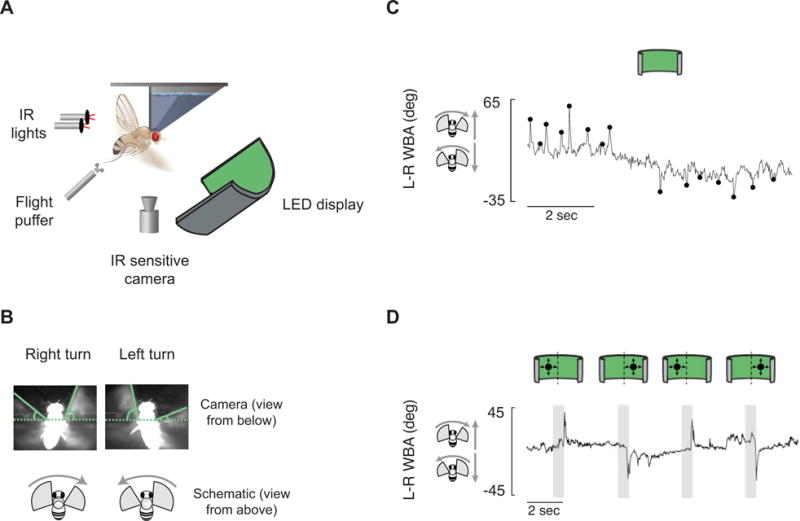
(A) Experimental apparatus. (B) Still images showing a fly turning right and left. (C) An example trace of a fly flying in blank screen conditions making spontaneous saccades. Saccades were picked algorithmically (Methods) and are marked at their peaks (black dots). (D) An example trace shows a fly responding to loom stimuli whose center of expansion is to the left or right of the fly. Gray boxes indicate stimulus expansion. See also Figure S1.
Flies flew in front of a panoramic LED display (see Methods). With the display uniformly lit, we observed frequent, sharp deflections of the L–R WBA signal, or saccades (Figure 1C) [2]. These saccades were not tied closely in time to any obvious external stimulus, which is the basis by which we and others [2, 28, 29] operationally define them as spontaneous. We computationally identified spontaneous saccades (black dots in Figure 1C) by subtracting the slow changes of L–R WBA from the raw signal and using a threshold-crossing algorithm on this baseline-subtracted signal (Methods). Rigidly tethered flies typically perform saccades in bursts of turns in one direction, followed by bursts of turns in the other direction, and so on (Figure 1C) [2]. In a more naturalistic behavioral paradigm, in which flies were attached to a thread so they were free to rotate about their yaw axis, others have observed this same propensity to execute bursts of turns in the same direction [30]. In a similar paradigm, in which tethered, flying flies are free to actually rotate about their yaw axis within a vertically oriented magnetic field [31], we observed the same pattern of syn-directional bursts of saccades (Figures S1A–D). This tendency to perform saccades in syn-directional bursts was independent of any individual fly’s overall tendency to perform more left turns or right turns overall in an experiment in both paradigms (Figs S1E–F).
In addition to performing spontaneous saccades, Drosophila also perform saccades in response to looming discs in the same tethered-flight paradigm. When a loom stimulus appears on the right, flies typically turn left, and vice versa (Figure 1D) [5, 13]. Thus, tethered flying flies perform rapid flight turns that can be operationally characterized as spontaneous or as visually evoked, allowing us to ask if the mechanisms for executing spontaneous saccades and this sub-class of visually evoked turns are dissociable.
Normal synaptic activity in NP0212-GAL4 cells is essential for spontaneous turns
While performing experiments for another purpose, we serendipitously discovered that when we silenced synaptic transmission in neurons targeted by the NP0212-GAL4 driver line (Figure 2A), flies performed very few spontaneous saccades. To silence these cells, we expressed shibirets, a temperature-sensitive dominant-negative allele of dynamin. Above the restrictive temperature of 29°C, shibirets blocks synaptic transmission by inhibiting the recycling of synaptic vesicles [32]. We controlled the flies’ temperature by passing temperature-regulated water over the flies’ head and front tip of their thorax while their wings remained dry and free to perform tethered flight. We monitored the flies’ saccade behavior at 19°C, when the flies should behave normally, and at 34°C, when shibirets is expected to block vesicle recycling.
Figure 2. Inhibiting synaptic transmission in NP0212 eliminates spontaneous saccades, but not expansion-evoked flight turns.
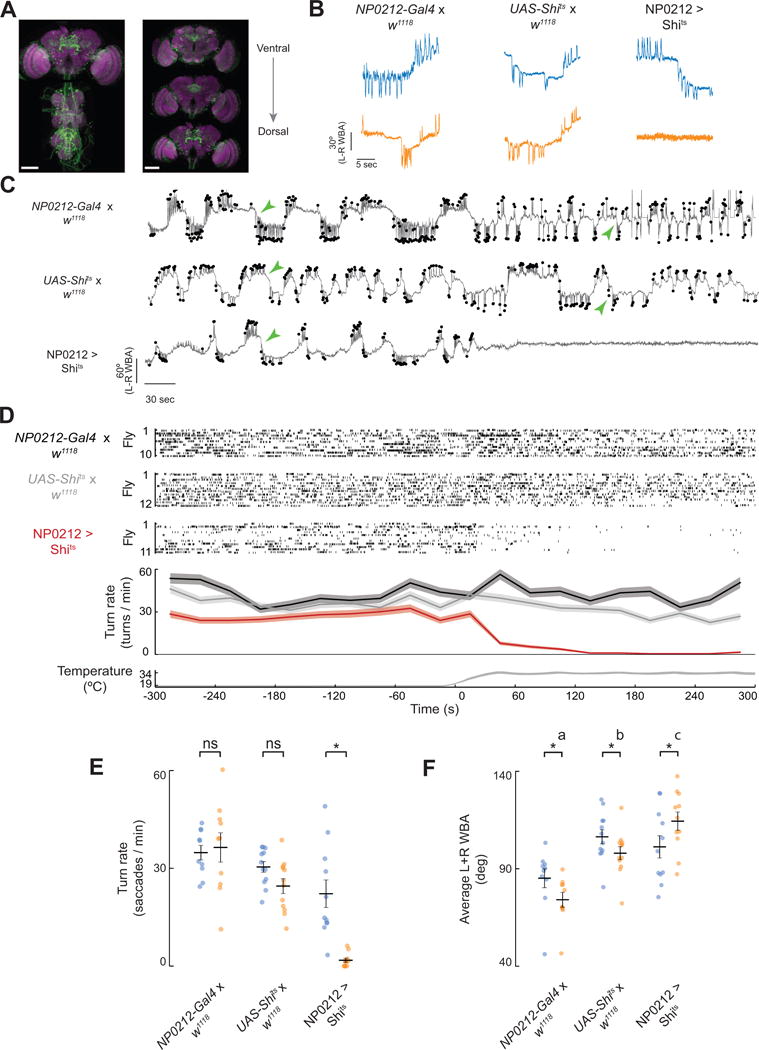
(A) Immunofluorescence of mCD8-GFP (green) and Brp (magenta) in the brain and thoracic ganglion of a NP0212 > GFP fly. Left: Max z-projection. Right: max z-projections of three subsets in z. Scale bars: 100 μm. (B) Example traces of spontaneous turning behavior in NP0212 > Shits and parental control flies at 19°C (blue) and 34°C (orange). (C) Ten-minute-long example traces. Saccades are indicated black dots. Traces are aligned to the x axis in D. Green arrowheads indicate examples of persistent turns. (D) Top three sections: raster plot of turns in NP0212 > Shits and parental controls. Every line represents an individual fly, and each saccade is indicated by a tick. Second from bottom: the average turning rate of each genotype, binned every 30s. Filled regions indicate 95% confidence intervals. Bottom: temperature traces from 5 flies from each genotype overlaid. Time zero was set to when the bath hit 26°C. (E) Spontaneous turning rate of NP0212 > Shits flies and parental controls at 19°C (blue) and 34°C (orange) (black bars: mean ± SEM, * p < 0.05, Wilcoxon Signed Rank Test with a Bonferroni Correction, n = 10–12). (F) Average L+R WBA of NP0212 > Shits flies and parental controls at 19°C (blue) and 34°C (orange), (n = 10–12, black bars: mean ± SEM, * p < 0.05, Wilcoxon Signed Rank Test with a Bonferroni Correction. Genotypes labeled with different letters have average L+R WBA values at 34°C that are significantly different, p < 0.05, Mann-Whitney U Test with Bonferroni correction). See also Figures S2, S3.
Upon heating, the mean saccade rate of NP0212-GAL4; UAS-Shits flies (NP0212 > Shits) decreased from 24.8 ± 4.9 to 2.9 ± 1.1 turns per minute (mean ± SEM). Three out of eleven flies, including the example fly shown (Figure 2C, bottom row), did not saccade for as long as we kept them at 34°C. Control flies did not show this effect upon heating (Figure 2B–E). While NP0212 > Shits flies exhibited a markedly reduced saccade rate compared to parental controls at 34°C, the average size of each saccade, when a saccade was rarely observed, was not significantly smaller than that of saccades at 19°C (Figure S2). In addition, NP0212 > Shits saccade magnitude at 34°C was not significantly smaller than those of the UAS-Shits parental controls, although the NP0212 > Shits average saccade magnitude was 28% smaller than that of the NP0212-GAL4 control (Figure S2). Flight persisted when synaptic transmission in NP0212 was inhibited. Indeed, NP0212 > Shits flies significantly increased their left + right wingbeat angle (L+R WBA) (Figure 2F), a proxy for flight power [33]. High wingbeat angles are unlikely to prevent turning on their own, since we observed no significant correlation between average L+R WBA and saccade rate in any of the above genotypes at either temperature (data not shown).
Saccades in tethered flight are relatively fast wing movements (~350 ms duration) that ride on top of slower fluctuations of the L–R WBA signal (Figure 2C, green arrows). In addition to the observed deficit in spontaneous saccades, NP0212 > Shits flies also stopped making these slow turns. To isolate the slower component of the flies’ wing movements, we plotted the distribution of observed wingbeat amplitudes in a low-pass filtered L–R WBA signal (see Methods), which we call L–R WBA(lpf). This low-pass-filtered signal is the floor from which we detect saccades, so by definition it excludes the saccades we defined computationally. Different flies had different median values of L–R WBA(lpf) – i.e. they sometimes had a bias for tonically turning left or right, which may be due to slight differences in the way the flies were tethered. We therefore subtracted the median from every fly’s L–R WBA(lpf) distribution so we could compare each distribution’s shape. When we did so, the distributions of L–R WBA(lpf) were relatively similar across all genotypes at 19°C (Figure 3A), but at 34°C, NP0212 > Shits flies’ showed L–R WBA(lpf) values that were much more tightly distributed around zero than those of parental controls (Figure 3B). At 19°C, we also observed a small variance drop in NP0212 > Shits flies compared to controls (Figure 3A), which may be due to a small amount of shibirets-mediated inhibition at 19°C.
Figure 3. Slow turns are eliminated by inhibiting synaptic transmission in NP0212.
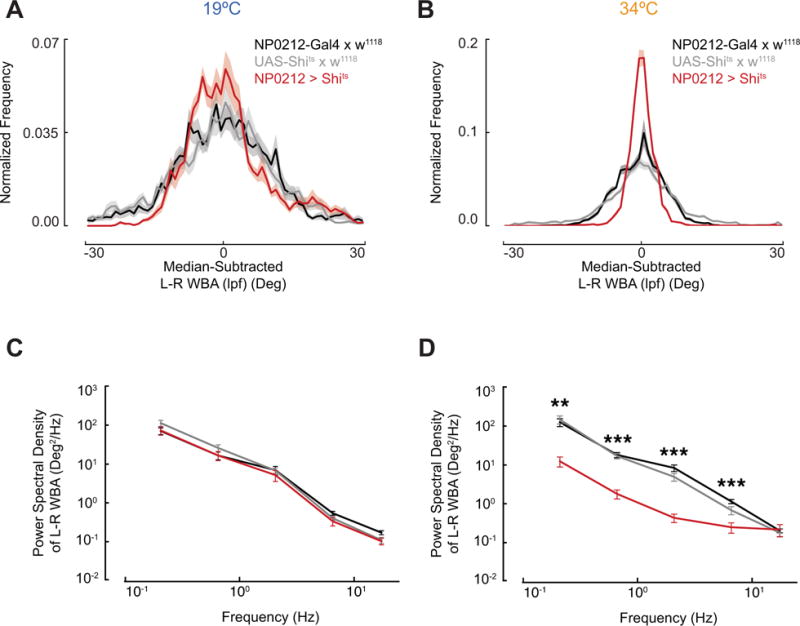
(A) Distribution of L–R WBA(lpf) during flight at 19°C for NP0212 > Shits (n = 11 flies, red) and parental controls (NP0212-GAL4 × w1118, n = 10, black, and UAS-Shits × w1118, n = 12, gray), mean ± 95% C.I. (B) Same as A, but at 34°C. NP0212 > Shits flies’ L–R WBA(lpf) distributions display lower variance than both parental controls at both temperatures, p <<< 0.001, Levene’s test of variance. (C) Average power spectra of the L–R WBA of NP0212 > Shits flies (n = 11) and parental controls (NP0212-GAL4 × w1118, n = 10, and UAS-Shits × w1118, n = 12, colors as above) at the 19°C, binned between half-powers of ten. Spectra are plotted on a log10-log10 scale. Mean ± SEM. (D) Same as C, but at 34°C. Significance asterisks indicate that NP0212 > Shits is significantly different from both parental controls, *** p < 0.001, ** p < 0.01, Mann-Whitney U Test. See also figure S3.
We also took a power spectrum of each fly’s L–R WBA, to determine whether inhibiting NP0212 neurons decreased the power spectral density at low frequencies. When we compared the power in each frequency band between genotypes, we found that NP0212 > Shits L–R WBA traces at 34°C displayed significantly less power at frequencies between 0.1 and 0.316 Hz, 0.316 and 1 Hz, 1 and 3.16 Hz, and 3.16 and 10 Hz, than those of control flies flying at the same temperature. At 19°C, this effect was not detectable (Figures 3C–D). By both measures described above, NP0212 > Shits flies exhibit less slow turning at 34°C than controls.
To determine whether the suppression of spontaneous turning in NP0212 could be observed at room temperature and with a different synaptic silencing reagent, we compared the spontaneous turning rate of flies in which NP0212-GAL4 drove expression of tetanus toxin light chain (TeTxLC-active), which blocks synaptic transmission by cleaving synaptobrevin [34], to the turning rate of flies that expressed an inactive form of the protein (TeTxLC-inactive). We made an effort to restrict tetanus toxin transcription to the 24 hours immediately prior to behavioral testing by using a temperature-sensitive variant of GAL80 (GAL80ts), an inhibitor of GAL4 [35], expressed in all cells under the control of the Tubulin promotor [36]. NP0212 > TeTxLC-active flies displayed a marked reduction in saccade rate when compared to NP0212 > TeTxLC-inactive controls (Figures S3A–B), corroborating the results seen with shibirets. Slow turning was also reduced in NP0212 > TeTxLC-active flies compared to controls (Figure S3C–D).
Normal synaptic activity in NP0212-GAL4 cells is dispensable for visually evoked turns
Did inhibiting synaptic transmission in NP0212 generally paralyze the flies’ ability to steer with their wings? In Drosophila, the power muscles that generate the mechanical force necessary for flight are distinct from the muscles that drive steering maneuvers [37], so one can easily imagine such a possibility. To test whether NP0212 > Shits flies were still capable of making both rapid and persistent wing movements, we tested NP0212 > Shits flies’ responses to visual stimuli that elicit such responses: loom and optomotor stimuli, respectively.
Flies in both free and tethered flight typically respond to looming visual objects by performing a saccade away from the expanding stimulus [7, 13, 14]. NP0212 > Shits flies and parental controls responded to our expanding disc stimuli at a fixed delay after the stimulus reached a critical size (Figures S4A–B) and many flies responded bi-modally to looms from the center (Figure S4C), phenomena which some consider to be characteristic of genuine loom responses (as opposed to, say, optomotor responses to the edge of the expanding disc) [38, 39]. Notably, NP0212 > Shits flies were able to execute these escape responses at the restrictive temperature of 34°C (Figure 4A). The overall shape of the loom response seemed quite normal, even though these responses were assessed directly after a long period of flight with a uniformly lit screen at 34°C, during which the same flies had performed virtually no spontaneous saccades (Figures S5A–B replicate Figures 2E–F in flies that performed loom-evoked turns immediately afterward). Moreover, the average size of the loom response in NP0212 > Shits flies did not significantly decrease compared to the same genotype at 19°C (Figure 4B, P > 0.05, Wilcoxon Signed Rank Test). NP0212 > Shits also did not display significantly smaller loom responses than those of one of the parental controls at 34°C (Figure 4B, p > 0.05, Mann-Whitney U Test with a Bonferroni Correction for NP0212 > Shits and NP0212-GAL4 / +), although their responses were significantly smaller than the other control (UAS-Shits / +), which may indicate that genetic background may have a small effect on the magnitude of this behavioral response. Finally, we note that, in some of our experiments (Figures S4 A–B), but not others (Figure 4A) loom response onset times of NP0212 > Shits flies at 34°C are delayed with respect to those of parental controls, an effect that could be caused by the expression of Shibire in one of the myriad visual or thoracic ganglion neuron classes labeled by NP0212.
Figure 4. The NP0212 circuitry required for spontaneous saccades is not required for two types of visually evoked turning.
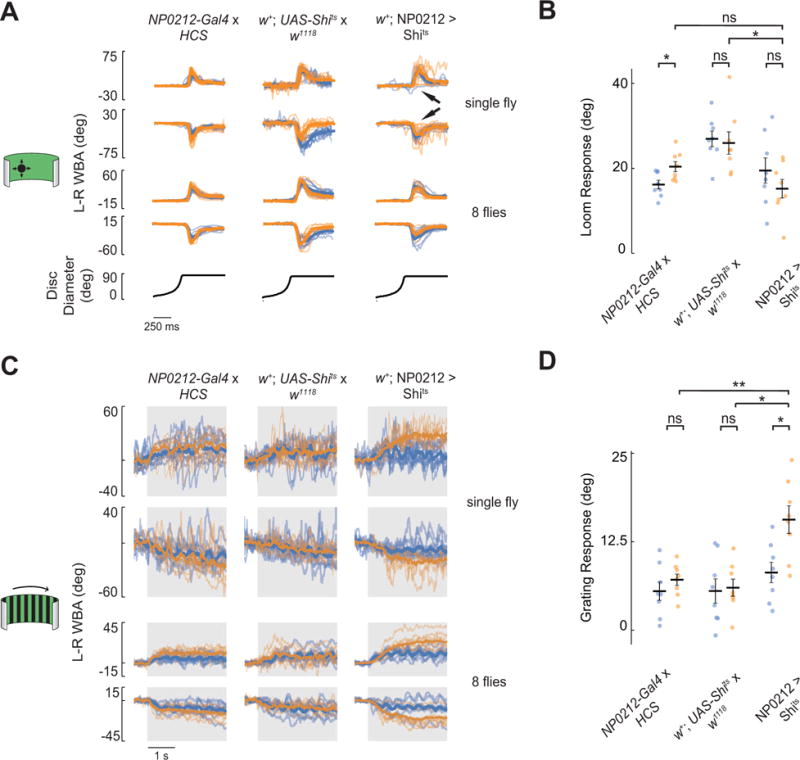
All panels: blue, 19°C; orange, 34°C. All traces: gray rectangles denote periods in which the visual stimulus was displayed. Top two rows: thin lines represent individual trials and thick lines denote the average response across trials for a single fly. Bottom two rows: thin lines represent the average responses of individual flies and thick lines denote the average response across flies. (A) Responses to looming disks expanding from the left (1st and 3rd rows) and right (2nd and 4th rows) of w+; NP0212 > Shits and parental controls, (n = 8 flies, 10 trials per fly). Arrowheads in 3rd column show trials with weak responses (see Main Text). (B) Average loom response in w+; NP0212 > Shits and parental controls (black bars: mean ± SEM) at 19°C and 34°C; each dot represents one fly. (C) Average responses to horizontally drifting grating to the right (1st and 3rd rows) and left (2nd and 4th rows) in w+; NP0212 > Shits and parental controls, (n = 8 flies, 10 trials per fly). (D) Average grating response in w+; NP0212 > Shits and parental controls (black bars: mean ± SEM) at 19°C and 34°C; each dot represents one fly. All panels: * p < 0.05, ** p < 0.01, Mann-Whitney U Test with a Bonferroni correction. See also Figures S4, S5.
While NP0212 > Shits flies showed grossly normal loom responses, we noticed that on some trials they did not seem to respond strongly to the loom (Figure 4A, arrows) – unlike control flies, which typically responded strongly on every trial. Analyzing this finding further, we found that, after ~10 loom trials at 34°C, loom responses of NP0212 > Shits flies began to weaken, until there was almost no behavioral response by the 30th trial. Control animals showed strong responses throughout 30 loom trials. However, when we minimized shibirets expression in certain visual and central complex neurons by driving GAL80 expression under the control of the R83H12 enhancer fragment (R83H12-GAL80) [40] (Fig S5C), we were able to rescue this depletion of the loom response while preserving the effect on spontaneous saccades (Figure S5D–E). That is, the spontaneous saccade rate of NP0212-GAL4, R83H12-GAL80 > Shits flies was still markedly reduced at 34°C, yet these flies responded reliably to looming stimuli for as long as we measured, even after 30 trials (Figure S5E). The spontaneous turn rate of NP0212-GAL4, R83H12-GAL80 > Shits flies was, barely, but significantly higher than that of NP0212 > Shits flies (Figure S5D), a difference that vanishes when a single outlier fly is excluded. However, even with the outlier included, the vast majority of the spontaneous turning deficit NP0212-GAL4, R83H12-GAL80 > Shits is still evident. We note that the elimination of loom responses was tied to the retinotopic location in which loom stimuli were repeatedly presented rather than to the direction in which the flies turned over and over again (data not shown). Taken together, we interpret these results to mean that the depletion of the loom response in NP0212 > Shits flies is due to the expression of shibirets in a retinotopic visual processing neuron class which is essential for sensing expansion, likely to be located in either the visual system or central complex.
In addition to rapid, spontaneous saccades, persistent turns were also affected in NP0212 > Shits flies. To test whether this effect could be explained by a general inability of NP0212 > Shits flies to tonically turn their wings, we tested classical, wing optomotor responses to coherent wide-field visual motion (in our experiment a drifting grating) presented for 3 seconds. Flies generally respond to horizontally drifting gratings by turning tonically in the direction of motion [38]. These responses were preserved in NP0212 > Shits flies; indeed, the response to this optomotor stimulus increased with respect to the same flies at 19°C (Figure 4C, D) and with respect to parental controls at 34°C. While the cause for the increase in optomotor-response magnitude in NP0212 > Shits is not clear, these observations demonstrate that the NP0212 > Shits flies can perform strong, tonic wing steering responses even while they barely perform any spontaneous tonic turns. Together, these experiments demonstrate the existence of neural processes that are required for the execution of spontaneous tonic turns and high rates of spontaneous saccades, but which are dispensable for generating loom-evoked and optomotor flight turns in Drosophila.
The NP0212 spontaneous turning phenotype is likely mediated by Cha+ neurons in the thoracic ganglion
NP0212-GAL4 drives expression in a large set of neurons spanning the visual lobes, the central complex, the mushroom bodies, and other regions of the brain and thoracic ganglion (Figure 2A). To gain a better handle on which cells in NP0212 might be required for the animal to execute spontaneous saccades, we co-expressed GAL80 transgenes driven by a variety of promotors, assessing the identity of the cells whose GAL4 expression was minimized or eliminated by crossing each GAL80 line to NP0212-Gal4; UAS-mCD8-GFP (NP0212 > GFP) (Figure 5A), and assessing the behavioral effect of minimizing the expression of GAL4 by measuring the spontaneous saccade rates of the NP0212, GAL80 > Shits flies.
Figure 5. The main cells that mediate the spontaneous saccade phenotype in NP0212 are Cha+ neurons in the thoracic ganglion.
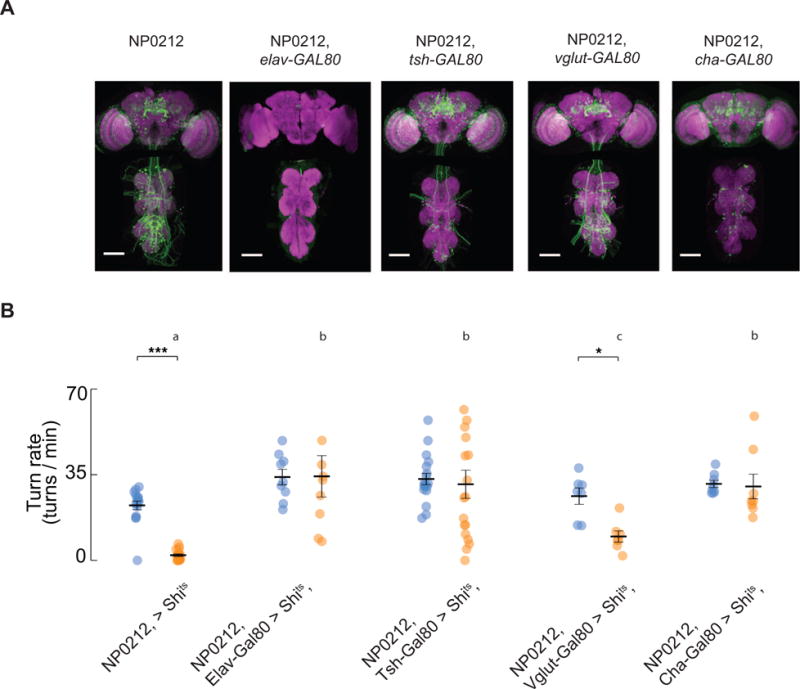
(A) Immunofluorescence of mCD8-GFP (green) and Brp (magenta) in the indicated driver lines in the brain and thoracic ganglion. (B) Mean spontaneous turning rate of flies of the indicated genotype at 19°C (blue) and 34°C (orange); each dot represents one fly. (Black bars: mean ± SEM, n = 7 - 18. Genotypes labeled with different letters have saccade rates that are significantly different at 34°C, Mann-Whitney U Test with Bonferroni correction. * p < 0.05, *** p < 0.001, Wilcoxon Signed Rank Test). An outlier NP0212 > Shits, elav-GAL80 fly that had a spontaneous saccade rate of 91.6 turns/min and an outlier NP0212 > Shits, tsh-GAL80 fly that had a spontaneous saccade rate of 89.4 turns/min at 34°C are not shown on the plot.
To test whether the spontaneous saccade defect in NP0212 was caused by neurons – rather than glia or muscles, for example – we crossed NP0212 > Shits flies to elav-GAL80. Elav is a gene expressed ubiquitously in neurons [41] and, as expected, elav-GAL80 eliminated UAS-driven transgene expression in the brain and thoracic ganglion while correspondingly rescuing the spontaneous saccade deficit (Figures 5A, B, 19°C vs 34°C, P > 0.05, Wilcoxon Signed Rank Test).
To ask whether the neurons in NP0212 required for spontaneous saccades reside in the brain or in the thoracic ganglion, we crossed NP0212 > Shits to tsh-GAL80. Tsh (teashirt) is widely expressed by neurons in the thoracic ganglion, with nearly no expression in the head [42]. As expected, in NP0212, tsh-GAL80 > GFP flies, GFP was expressed strongly in the brain, while it was virtually eliminated from the thoracic ganglion (Figure 5A). Tsh-GAL80 rescued the spontaneous saccade defect (Figs 5B, 19°C vs 34°C, P > 0.05, Wilcoxon Signed Rank Test), suggesting that the cells required for spontaneous turning in NP0212 reside in the thoracic ganglion. We note that the NP0212, tsh-GAL80 > Shits flies showed more fly-to-fly variability in saccade rates than wild type animals. This observation suggests that neurons whose expression of the teashirt gene is variable across individuals might contribute to this phenotype. Because the number of neurons targeted by NP0212 is so large, it was not feasible to examine the brains of NP0212, tsh-GAL80 > Shits flies and to determine (by co-expression of GFP, for example) whether different cells were labeled in flies that did or did not exhibit the behavioral rescue.
Motor neurons in Drosophila are glutamatergic and express the vesicular glutamate transport protein gene Vglut [43]. To test whether NP0212 > Shits saccade silencing was due to the inhibition of a steering motor neuron specific for spontaneous saccades, we crossed NP0212 > Shits to vglut-GAL80. As expected, NP0212, vglut-GAL80 > GFP flies showed reduced fluorescence and fewer large cell bodies in the lateral metathoracic ganglion, a region known to contain flight motor neurons [43] (Figure 5A). NP0212, vglut-GAL80 > Shits flies still showed significantly lower spontaneous turning rates at 34°C than at 19°C. However, the spontaneous turning rate at 34°C was significantly higher in NP0212, vglut-GAL80 > Shits than in NP0212 > Shits flies, although the effect size was only 32% smaller than that of NP0212 > Shits flies (61.2% vs 90.3% percent reduction in saccade rate). Such a mild rescue phenotype with GAL80 is difficult to interpret, as it may result from leaky expression of GAL80 in non-glutamatergic neurons or from partial contribution of glutamatergic neurons to the phenotype. However, these data suggest most of the NP0212 turning phenotype is not mediated by glutamatergic neurons.
In contrast, Cha-GAL80, which drives expression in cholinergic neurons in Drosophila [44], rescued the NP0212 spontaneous saccade defect in its entirety (Fig 5B, 19°C vs 34°C, P > 0.05, Wilcoxon Signed Rank Test). The precise identity of the Cha+ NP0212 neurons that likely mediate the phenotype is ambiguous, since the majority of neurons labeled by NP0212 > GFP were subtracted by Cha-GAL80, including all but the largest cells in the thoracic ganglion, which are putative glutamatergic motor neurons (Figure 5A).
We silenced neurons in 38 additional GAL4 lines that targeted subsets of the nervous system labeled in NP0212. While an additional 3 out of 38 GAL4 lines showed significant decreases in saccade rates (Figures 6A, B, lines 2–4, Student’s t-test, p < 0.05), none of these more specific driver lines fully recapitulated the nearly complete elimination of saccades associated with silencing cells in NP0212.
Figure 6. The NP0212 spontaneous saccade phenotype is not replicated in 38 additional GAL4 lines.
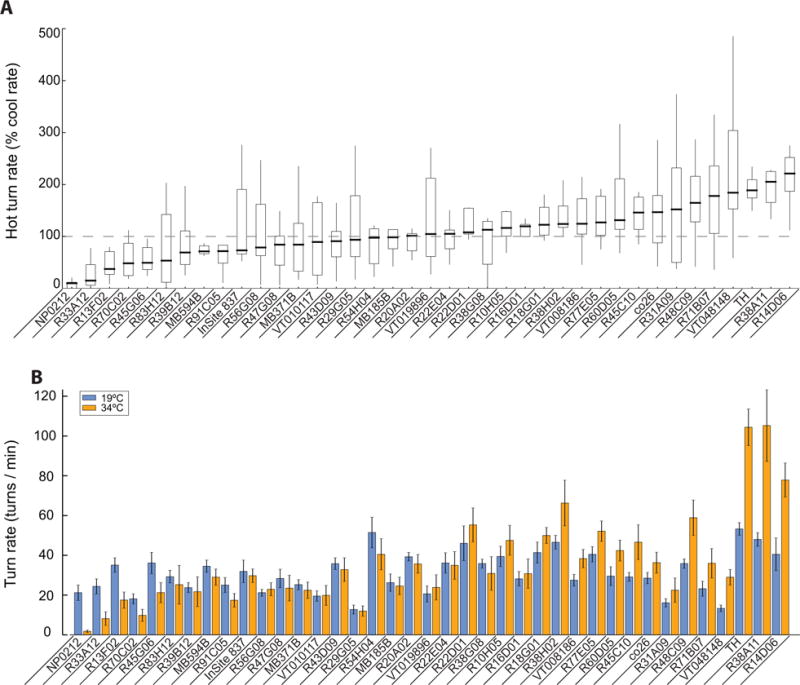
(A) Box plots showing the spontaneous turning rate at 34°C as a percentage of the turning rate at 19°C. The genotype median is indicated by the thick black horizontal line. Box boundaries show the first and third quartiles. Whiskers are 1.5 times the length of the interquartile range. (B). Raw turning rates of NP0212 > Shits and the other 38 GAL4 lines at 19°C (blue) and 34°C (orange). Genotypes are sorted by the median relative turning rate.
Discussion
In this study, we abolish almost all spontaneous saccades in tethered flying Drosophila by inactivating synaptic transmission in a large, genetically defined set of neurons. The observed suppression of spontaneous saccades spares loom-evoked saccades and optomotor responses. We thus demonstrate that the mechanisms underlying the execution of spontaneous turns and these two types of visually evoked turns are, at some level, dissociable. Conversely, we previously found that flies blinded with a prolonged depolarization afterpotential continue to perform spontaneous saccades [29]. Together, these results argue that spontaneous turning is doubly dissociable from both loom responses and optomotor responses in Drosophila; whereas certain neural processes that contribute to both classes of behaviors likely exist, it is possible to inhibit each one without abolishing the other.
We define spontaneous saccades as ones whose precise time of initiation cannot be easily linked to any abrupt external event. However, environmental stimuli are known to affect the rate of saccades that would still fall within this definition of spontaneous. For example, the first paper describing spontaneous saccades in tethered flight noted that flies perform saccades of unpredictable timing (i.e., spontaneous) much less frequently in the dark [2]. Also, it has recently been shown that magnetically tethered flies (who are free to rotate about their yaw axis) perform saccades in the direction of yaw optic flow or toward a visual object [8]. These saccades can be considered stimulus driven in that their average onset times can be predicted by a model that integrates optic-flow velocity, or visual-object position, over time. However, unlike saccades driven by loom stimuli, these stimulus-driven turns show considerable onset-time variability relative to the mean, suggesting that there exists a stochastic component to their initiation times, akin to spontaneous turns. It will be interesting to determine whether NP0212 > Shits flies can perform optic-flow and visual-object associated saccades, which are stimulus-driven in one sense and yet spontaneous (or stochastic) in another. The answer to this question may help to further refine the definition of a spontaneous action.
We note that NP0212 did not eliminate absolutely all measurable, non-stimulus-locked flight maneuvers. NP0212 > Shits flies at 34°C continued to make some sharp turns during optomotor stimuli (see NP0212 > Shits example trace in Figure 4C). Such turns, whose rate seems to be increased by optomotor stimuli and which have been noted previously [3, 29, 45], represent a potentially different class of flight maneuver from those blocked in NP0212 > Shits flies.
While we did not present any overt stimuli to elicit spontaneous saccades, the flies could almost certainly sense stimuli that we did not control, such as the air currents generated by their own flapping wings [46]. We therefore cannot fully exclude the possibility that every seemingly spontaneous turn in our tethered preparation represents an immediate or integrated response to an environmental stimulus that we do not measure. However, flies that have been blinded [29] or that have had their antennae glued (data not shown) – blocking smell and mechanosensation in these organs – still saccade. Furthermore, if spontaneous saccades represent responses to subtle environmental stimuli, these stimuli would have to change on a timescale commensurate with the structure of the turning behavior. Namely, spontaneous saccades in tethered flight typically occur in bursts [2, 30], all in the same direction, lasting tens of seconds. These bursts switch from a set of turns to the left, then a set of turns to the right, and back to the left, and so on, (See Figures 1C, 2B–C, S3A, S5A) and this pattern is recapitulated in magnetically tethered flies that rotate about their yaw axis (S1A–D). It seems to us unlikely that uncontrolled environmental stimuli would always show this structure across different preparations and experimental days.
It has been reported that the rate and direction of 93% of apparently spontaneous turns could be explained by the amount of visual expansion a freely flying fly experiences at any given moment in a 2-meter-diameter cylindrical arena [7]. In our experiments, flies that did not perform spontaneous turns still performed loom-evoked turns. These results might mean that neurons in NP0212 are required to execute the ~7% of saccades that could not be explained by visual expansion in the free flight experiments. It is also possible that, in free-flight conditions where flies do not encounter an arena wall as often, more than 7% of saccades would be internally initiated. Intriguingly, in the free flight study, flies performed spontaneous saccades at a rate of ~0.4 Saccades / second (~24 saccades / min), which is almost exactly the same as the rates detected in rigidly tethered flies [7].
It is possible that the causal neurons in NP0212 are necessary for both spontaneous and loom evoked turns, but chemical synaptic transmission is essential for executing spontaneous saccades whereas electrical transmission – which is not expected to be affected by shibirets – governs the loom-triggered behavior. Drosophila’s giant fiber neuron is an example of a cell in which chemical and electrical transmission serve different output functions [47]. In this scenario, the two behaviors can still be considered dissociable. However, the dissociation would take place at a sub-cellular locus.
It may be that the NP0212 enhancer drives gene expression in neurons where bursts of action potentials normally occur as an early step in driving a spontaneous turn to initiate and that these initiator neurons are cholinergic cells in the thoracic ganglion. However, it is important to note that this is not the only option, or even the most parsimonious one. Work by Schnell et al. [45] has identified a descending neuron, AX, whose membrane potential and [Ca2+] activity and membrane voltage are tightly correlated to fast wing steering in tethered flight, and whose exogenous activation induces directional steering responses. AX, or neurons like it in the brain, might initiate a spontaneous saccade or relay such an initiation signal originating in neurons upstream, and neurons in NP0212 may act as a downstream gate of such initiator neurons. In this scenario, silencing NP0212 cells would yield a phenotype of markedly reduced spontaneous saccades even though this driver line would not include neurons that participate in the process of initiation per se. Indeed, AX could further drive loom-evoked turns, and perhaps even optomotor turns, via coupling to a different thoracic-ganglion interneuron system than that targeted by NP0212.
We note that motor-related signals that precede wing steering maneuvers exist in brain cells beyond AX. For example, Horizontal-system (HS) neurons in the fly visual lobe show modulations of their membrane voltage that correlate with rapid flight steering maneuvers and these membrane voltage changes in HS cells precede spontaneous wing-steering changes [29]. Furthermore, exogenous activation of HS cells is sufficient to drive flies to turn during flight and walking [48, 49]. Because HS cells are located in the visual system and because these neurons receive antagonistic visual and motor-related inputs, their wing-steering-related modulations have been interpreted as efference copies rather than as motor commands [29]. AX cells, on the other hand, respond strongly to wing steering behavior, and the variation in their responses to visual stimuli covaries with the strength of the fly’s behavioral response. In addition, AX neurons have a prominent projection to the wing-steering neuropil in the thoracic ganglion. Thus, AX’s wing-steering-related signal has been interpreted as a motor command [45]. As such, one might imagine that silencing synaptic transmission in AX cells might impair wing steering behavior. We expressed shibirets in two different driver lines that target AX neurons, but were not able to detect a defect in saccade rate at 34°C (R91C05-GAL4 and R56G08-GAL4 in Figure 6). We note that it is possible that AX neurons drive rapid turns through gap junctions, which are not impaired by shibirets, or that our shibirets transgene was not expressed strongly enough in these GAL4 driver lines to inhibit the labeled neurons. That said, our inability to abolish spontaneous saccades with shibirets in AX cells or over 30 other Gal4 lines is generally consistent with the possibility that a relatively widespread network of neurons in the fly nervous system is involved in initiating or otherwise processing saccadic commands and that silencing any one component may not impair the fly’s ability to initiate action. NP0212-GAL4 may target a large swath of the initiating network or a downstream gate of such a network. Future work will be needed to tease apart these varying hypotheses.
Recent work by Lindsay et al. [3] has argued that the direct flight steering muscles in Drosophila fall into two main categories: tonic muscles, whose [Ca2+] activity scales linearly with the strength of flight turns, and which are preferentially recruited during graded changes in wing steering; and phasic muscles, which are recruited preferentially during very strong, sharp saccades. In our experiments, both sharp and graded spontaneous turns were depleted in NP0212 > Shits flies (Figures 2, 3), whereas sharp and graded turns induced by looming (Figures 4A–B) and optic flow stimuli, respectively (Figures 4C–D), were preserved. Thus, our results add another dimension to the categorization of flight turns in Drosophila; spontaneous turns and both loom and optomotor visual responses should be considered to have a neural distinction outside of the steering motor-neuron system, much like graded and sharp turns do within it.
The precise identity of the neurons labeled by NP0212 that mediate spontaneous turning remains unclear. We hypothesize that the locus of NP0212 inhibition of spontaneous flight turns is upstream of steering motor neurons, since NP0212 > Shits flies that could not execute spontaneous saccades were still capable of responding to visual stimuli (Figure 4), because average saccade size did not measurably change in NP0212 > Shits compared to the UAS-Shits parental control (Figure S2), and because vglut-GAL80 did not fully rescue the NP0212 spontaneous saccade deficit (Figure 5B.). Since tsh-GAL80 and cha-GAL80 rescued the deficit, we hypothesize that the causal neurons in NP0212 are cholinergic and in the thoracic ganglion. This result is intriguing in light of results from Berni et al. [50], who show that, in Drosophila larvae, synaptic transmission in neurons in the thoracic ganglion are required for periodic turning during an exploratory routine, but that synaptic transmission in the brain lobes is dispensable for this behavior.
One class of cholinergic neurons in the thoracic ganglion that express GAL4 in the NP0212 driver line are the afferent fibers from the halteres (Figure 2A, white arrows) [51], which are mechanosensory balance organs involved in dipteran flight control [52]. The halteres regulate wing steering [52] and are monosynaptically coupled to the b1 wing steering motor neuron in Drosophilids [51]. To test the hypothesis that the NP0212 > Shits saccade phenotype arises from silencing synaptic transmission in haltere afferent neurons, we inhibited neurons in seven GAL4 lines that target subsets of haltere afferents. However, inhibiting synaptic transmission in these cells with shibirets did not lead to a consistent drop in the rate of spontaneous saccades (R33A12, R70C02, R39B12, R29G05, R22E04, R31A09, and R71B07 in Fig S6), suggesting that these are not the causal neurons for the NP0212 saccade phenotype (Though this conclusion is tentative because without further anatomical experiments one cannot be 100% certain that the seven GAL4 lines we tested targeted the identical haltere afferents in NP0212 with a similar level of GAL4 expression).
We impaired synaptic transmission in smaller subsets of neurons to those that are targeted by NP0212-GAL4 driver line, by using other, more specific, GAL4 lines (Figure 6). While the majority of lines we tested showed no significant depletion in spontaneous saccade rate when crossed to UAS-Shits, this does not mean that these driver lines do not target cells necessary for the generation of spontaneous saccades, since Shibire inhibits only chemical synapses, not electrical ones [32]. In addition, Shibire may not get expressed at sufficient levels to elicit a complete phenotype in all GAL4 lines that target a cell class of interest. Still, because none of these other GAL4 drivers yielded a full spontaneous-saccade-silencing phenotype, it remains possible that more than one anatomically-defined neuronal class must be silenced to fully eliminate spontaneous turning, though further work will be needed to definitively prove this idea.
Specific neuronal perturbations that alter spontaneous locomotion can also be found in natural contexts. The jewel wasp Ampulex compressa, which hunts the cockroach Periplanta americana as live food for their larvae, captures its quarry by injecting venom into precise locations in the cockroach’s central nervous system [53]. The wasp’s venom induces a hypokinetic state, blocking spontaneous walking without paralyzing the cockroach [54, 55]. The venom-induced hypokinetic state in Periplanta and the NP0212 flight turning phenotype in Drosophila are not perfectly analogous. Notably, injecting wasp venom into the cockroach’s brain is sufficient to induce the hypokinetic state [56], whereas restricting inhibition of NP0212 neurons to the brain is not sufficient to rescues the block of spontaneous saccades (Figures 5A–B). Still, the existence of both of these phenomena suggest that circuitry that is functionally tuned toward spontaneous behavior exist in many species of insects, and that these circuits may be studied to teach us how animals implement spontaneous behavior more generally.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to the Lead Contact, Gaby Maimon (maimon@rockefeller.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fly Stocks
We studied female, Drosophila melanogaster, 1–4 days post-eclosion. Flies were reared with standard corn-meal agar, in 18°C (tubP-GAL80ts, NP0212 > TeTxLC-active and tubP-GAL80ts, NP0212 > TeTxLC-inactive flies) or 25°C incubators (all others) with a 12 hour light/dark cycle. In spontaneous turning experiments, unless otherwise indicated, w− experimental flies and parental controls were used (i.e. w−; +; GAL4 and w−; +; UAS-Shits flies were crossed each other or to w1118 flies to generate parental controls). In visual response experiments (Figures 4, S4, S5), the experimental fly and parental controls each carried a single wild type copy of the white gene (w+) derived from the Heisenberg Canton S (HCS) wild type strain. In addition, the second chromosome of each fly contained a single “NP+” chromosome, which was derived from back-crossing the NP background fly to UAS-Shibirets nine times.
In magnetic tether experiments and the accompanying rigid tether experiments in Figure S1 HCS flies were used.
Transgenic Flies
We amplified the R83H12 fragment from genomic DNA (y; cn bw sp) using the same primers as in [57] (forward: gaaaggacctctgcccctagttaaa, reverse: gatatgaagacaacaagggggcgtg). This R83H12 fragment was first cloned into pCR8/GW/TOPO (Invitrogen), and then recombined into pBPGal80Uw (Nilay Yapici) using Gateway cloning as in [58]. pBPGal80Uw contains attR1 and attR2 sequences upstream of the Drosophila Synthetic Core Promoter (DSCP), the Gal80 sequence, the Woodchuck Hepatitis Virus (WHP) Posttranscriptional Regulatory Element (WPRE), and the SV40 terminator sequence. The plasmid also contains mini-white. This R83H12-Gal80 vector was then integrated into VK00027 using PhiC31 recombinase (Genetic Services, Inc), and transformants selected as in [58].
METHOD DETAILS
Immunohistochemistry
We dissected fly brains in 19°C S2 medium, and fixed them in 1% paraformaldehyde at 4°C overnight. Fixed brains were washed 3 times for 30–60 min with PAT3 (0.5% Triton X-100 and 0.5% Bovine Serum Albumin (BSA) in PBS), then blocked with 3% NGS in PAT3 for 1.5 hours at room temperature. We incubated brains with primary and secondary antibodies as previously described 59], and mounted them in VectaShield (Vector Labs). We stained with anti-GFP (Rockland) at a dilution of 1:1000 along with nc82 antibody (DSHB) at 1:50 to label neuropil. We imaged brains using a 20× 1.0 NA objective on an Inverted Leica DMI 6000 confocal microscope with 1 or 1.5 μm separating each optical slice.
Behavioral measurements
We estimated wingbeat amplitudes of the left and right wings in real time, using the strokelitude plugin, as previously described [26]. We illuminated the fly with infrared light (880 nm) directly from behind such that the wings generated a high luminance smear of reflected light. Video data were collected at 50 Hz, triggered externally, with an AVT-GE680 camera with an Infinistix lens (94 mm working distance). Data were digitized at 10 kHz using a Digidata 1440 (Axon Instruments).
In magnetic tether experiments we took videos of flies with an infrared-sensitive camera (AVT-GE680) triggered externally, recording frames (320×240 pixels) at 350 Hz. The yaw body angle was estimated as previously described [59].
Visual Stimuli
In rigid tether experiments we used a cylindrical visual display [60] covering 216° and 90° in azimuth and elevation, respectively (IORodeo) with each pixel (570 nm LEDs) subtending ~2.25° on the fly’s retina. For behavioral genetic experiments we tilted the arena ~45° downward from upright so as to roughly match the fly’s pitch-down head angle. Expanding disk (loom) stimuli, whose ratio of radius to expansion velocity (l/v) were held constant, were projected on the visual display as dark spots against a bright background. The stimuli were centered either directly in front of the fly or 45° to the left or right and expanded with a pixel update rate of 56 frames/sec. The angular size of the spot in degrees varied according to the function θ(t) = 2*arctan((l/v) / t), where t represents time in ms prior to collision (i.e. θ(0) = 180°), such that θ(t) varied between 5° when the stimulus appeared to 90° when expansion ended. A loom stimulus with l/v = 22 ms was used in Figures 1, 4A–B, and S5E. In Figure S4, stimuli with l/v of 22, 44, and 88 ms were used. Grating stimuli were square waves with an 18° spatial wavelength. Smooth gratings rotated about the fly with a temporal frequency of 2.5 cycles/s. Both the looming disk and the grating had a nominal contrast of 100%, though reflections likely reduced this value. In magnetic tether experiments we used a cylindrical LED display covering 360 in azimuth and 94 in elevation with each pixel subtending ~3.75°.
Behavioral experiments
In rigid tether experiments, females were collected 1–4 days after eclosion. Flies were anesthetized on a Peltier stage at ~4° C, and attached by the dorsal part of their prothorax either to a custom holder (rigid tether experiments) or to a steel pin (magnetically tether experiments) using blue-light activated glue (Bondic, Canada).
For Shits experiments, tethered flies were placed in front of a blank, bright visual display (see above). Water was perfused onto the chamber above the fly’s head, and the water’s temperature was controlled by an in-line peltier device (Harvard Apparatus) connected to a CL-100 bipolar temperature controller (Harvard Apparatus). We took images with an A655sc thermal camera (FLIR) calibrated to a BB701 blackbody radiator (Omega) to confirm that the fly’s thorax and head equilibrated above the restrictive temperature of shibire (30–34°C) less than 20 seconds after the bath temperature reached 34°C. In Shits spontaneous saccade experiments, flies flew in the context of a blank screen for 5 minutes (Figures 2, 3, 5, 6) or 3.5 minutes (Figures 4, S5) with the temperature held at 19°. The temperature was then increased above 26°C, equilibrating at 34°C, and the process was repeated. If experiments included visual stimuli, these were presented after blank screen flight above 26°C for 3.5 minutes (Figures 4A–B, S5) or 1 minute (Figures 4C–D, Figure S4).
For TeTxLC; GAL80ts experiments, flies were collected 1–3 days after eclosing and incubated at 31°C for 24 hours prior to tethering. Flies’ spontaneous flight behavior was assayed less than 2 hours after removal from 31°C.
In magnetic-tether experiments, each fly flew before a blank screen for 3 separate bouts of 100s each. These blank screen periods were one of six different types of stimuli were presented pseudorandomly to the fly.
QUANTIFICATION AND STATISTICAL ANALYSIS
Saccade detection
In rigid tether experiments, spontaneous saccades were detected using an algorithm which worked by comparing the raw L–R WBA signal to a low-pass filtered “baseline” signal (cutoff frequency = 0.5 Hz). The baseline signal, L–R WBA(lpf), was computed by linearly interpolating regions of the filtered L–R WBA signal that were both relatively flat (absolute slope < 4 °/s) and had a low magnitude second derivative (absolute acceleration < 200 deg/s2). If the raw signal crossed at least 8° above or below this calculated baseline and remained beyond this threshold for at least 60 ms, then the algorithm classified this event as a saccade. Saccade amplitude was measured as the largest difference between L–R WBA and L–R WBA(lpf). In Figures S2A–B, the average saccade was plotted so that saccades were aligned to their onset time, which we computed as the last inflection of the derivative of L–R WBA preceding the point when the L–R WBA had reached 40% of the saccade’s peak height, or the end of the previous saccade, whichever came last.
In magnetic tether experiments, we unwrapped the time course of the fly’s body rotation, took the derivative, and low-pass filtered it at 10 Hz. If this low-pass filtered derivative signal crossed positive or negative 140 deg/s for more than 50 ms, we classified this event as a saccade.
Behavioral response quantification
In rigid tether experiments, saccade rate was determined by dividing the number of turns by the amount of time the fly spent flying. Flies flew 3.5 minutes (Figures 4, S5) or 5 minutes (Figures 2, 3, 5, 6) at 19°C, then the same amount of time at 34°C. Time zero was set to when the bath hit 26°C on its way from 19°C to 34°C. The period one minute before and one minute after this heating transition was not taken into consideration when calculating the spontaneous saccade rate or the rate of slow turning.
To quantify the power spectrum of the L–R WBA signal, we slid a 20s window in 50 ms steps. If the fly flew for the entire window, we then took a power spectrum of the L–R WBA within the window and, within each fly, averaged these spectra together. We then averaged the power within five frequency bands (boundaries of 0.1 Hz, 0.316 Hz, 1 Hz, 3.16 Hz, 10 Hz, and 25 Hz).
Loom response amplitude of average fly traces was calculated by subtracting the average L–R WBA in a window 560–800 ms after expansion began from the average during a 200 ms window prior to expansion. In Figure S4, loom response onset times were taken by determining the time of the peak response, and finding the last time before the peak that the L–R WBA was below three standard deviations of the mean L–R WBA during inter-trial periods. We estimated the collision time of each stimulus by using the curve_fit function from the SciPy library to fit an arctangent to each stimulus’ real diameter in degrees and extrapolated it to estimate the time when the stimulus diameter would equal 180°. We then plotted onset times with respect to the estimated collision time against the stimulus l/v and took a linear regression. The threshold stimulus size may be calculated from the slope of this line, as described previously [38, 39]. In [38], the authors reported that the peak firing time of a visual neuron (tpeak) occurred with a fixed delay (δ) after the stimulus had reached a threshold stimulus size (θcrit). They found that these parameters fit the following linear relationship: tpeak = α* (l/v) – δ. Where α, the slope of the linear regression, is related to the θcrit such that θcrit = 2*arctan(1/α). In our case we used tonset instead of tpeak, but used the same type of analysis. Only trials in which the fly was flying 100% of the time were analyzed. In w+; NP0212 > Shits flies at 34°C we only analyzed the first 6 trials of a given speed to ensure we were not analyzing trials on which the loom response had adapted.
Grating response amplitude was calculated by subtracting the average L–R WBA in a window 1–1.5 s after the visual stimulus began to move from the average during a 500 ms period beforehand. Single fly average traces were composed of the average of the first 10 trials for loom, and all trials for gratings (up to a maximum of 10) in which the fly was flying for at least 95% of the time in both the baseline period and the duration of the stimulus.
To calculate the likelihood that flies on the magnetic tether and rigid tether would saccade so many times in the same direction, we performed 50,000 simulations where we randomly sampled 9 (magnetic tether) or 7 (rigid tether) flies from the appropriate population of flies (with replacement). In each simulated fly, we randomly shuffled the turn sequence. Then, in the real and simulated turn sequences we counted turns that were in the same direction of the turn that preceded it, and divided by the number of total turns minus 1. Flies’ turn direction bias was calculated by taking the absolute value of the difference between the number of left turns and the number of right turns and dividing this by the number of total turns. A turn bias value of 1 indicates that a fly executed all of its turns in the same direction, whereas a bias value of 0 indicates that a fly made 50% left and 50% right turns.
Statistics
Details about statistical tests are provided in the figure legends. In Figure S1, P-values were derived by calculating the probability of observing an experimental fraction of syn-directional saccades as large as the one actually observed in the permuted data. For statistical tests, we treated each fly as an independent sample unless. All statistical analysis was conducted using the scipy.stats module of Python.
Data and Software Availability
Raw data and analysis/stimulus code will be provided upon request by the Lead Contact Gaby Maimon (maimon@rockefeller.edu).
Supplementary Material
Highlights.
We silence spontaneous flight turns in Drosophila
Spontaneous sharp turns and slow turns are both silenced
Visually driven flight turns are preserved
Neurons required for spontaneous flight turns are located in the thoracic ganglion
Acknowledgments
We thank the laboratories of Vanessa Ruta, Leslie Vosshall, and Anne von Philipsborn for fly stocks; Anne von Philipsborn and Angela O’Sullivan for identifying thoracic ganglion motor neurons labeled by NP0212; Atsuko Adachi for generating fly stocks and assistance with immunofluorescence; Cheng Lyu for collecting magnetically tether data and for extracting body angle trajectories; and Jonathan Hirokawa for technical support. Research reported in this publication was supported by the New York Stem Cell Foundation (NYSCF-R-NI13), Searle Scholars Foundation (12-SSP-153), McKnight Foundation, and the National Institute on Drug Abuse of the NIH (DP2DA035148). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
BF, JG, and GM designed the experiments. JG made the initial discovery and characterization of the phenotypes in Figs 2, 4A–B, and Supplemental Figure 2. These results were confirmed and further characterized by BF. BF and JG collected and analyzed the data with input from GM. BF and GM wrote the paper.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Collett TS, Land MF. Visual control of flight behavior in the hoverfly Syritta Pipiens L. J Comp Physiol. 1975;99:1–66. [Google Scholar]
- 2.Kraus BJ, Brandon MP, Robinson RJ, 2nd, Connerney MA, Hasselmo ME, Eichenbaum H. During Running in Place, Grid Cells Integrate Elapsed Time and Distance Run. Neuron. 2015;88:578–589. doi: 10.1016/j.neuron.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavelis GS, Hayakawa S, White RA, 3rd, Gojobori T, Suttle CA, Keeling PJ, Leander BS. Eye-like ocelloids are built from different endosymbiotically acquired components. Nature. 2015;523:204–207. doi: 10.1038/nature14593. [DOI] [PubMed] [Google Scholar]
- 4.Tammero LF, D MH. The influence of the visual landscape on the free flight behavior of the fruit fly Drosophila melanogaster. J Exp Biol. 2002;205:327–343. doi: 10.1242/jeb.205.3.327. [DOI] [PubMed] [Google Scholar]
- 5.Muijres FT, Elzinga MJ, Iwasaki NA, Dickinson MH. Body saccades of Drosophila consist of stereotyped banked turns. J Exp Biol. 2015;218:864–875. doi: 10.1242/jeb.114280. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds AM, F MA. Free-flight odor tracking in Drosophila is consistent with an optimal intermittent scale-free search. PLoS ONE. 2007;2:e354. doi: 10.1371/journal.pone.0000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Censi A, Straw AD, Sayaman RW, Murray RM, Dickinson MH. Discriminating external and internal causes for heading changes in freely flying Drosophila. PLoS Comput Biol. 2013;9:e1002891. doi: 10.1371/journal.pcbi.1002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mongeau JM, Frye MA. Drosophila Spatiotemporally Integrates Visual Signals to Control Saccades. Curr Biol. 2017;27:2901–2914 e2902. doi: 10.1016/j.cub.2017.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf R, Heisenberg M. Visual orientation in motion-blind flies is an operant behavior. Nature. 1986;323:154–156. [Google Scholar]
- 10.Wolf R, Heisenberg M. Basic organization of operant behavior as revealed in Drosophila flight orientation. J Compar Physiol A Sense Neur Behav Physiol. 1991;169:699–705. doi: 10.1007/BF00194898. [DOI] [PubMed] [Google Scholar]
- 11.Wolf R, Voss A, Hein S, Heisenberg M. Can a fly ride a bicycle? Discussion on natural and artificial low-level seeing systems. Philos transact Royal Soc London Series B Biol Sci. 1992;337:261–269. [Google Scholar]
- 12.Brembs B. The importance of being active. J Neurogenet. 2009;23:120–126. doi: 10.1080/01677060802471643. [DOI] [PubMed] [Google Scholar]
- 13.Muijres FT, Elzinga MJ, Melis JM, Dickinson MH. Flies evade looming targets by executing rapid visually directed banked turns. Science. 2014;344:172–177. doi: 10.1126/science.1248955. [DOI] [PubMed] [Google Scholar]
- 14.Tammero LF, D MH. Collision avoidance and the landing response are mediated by separate pathways in the fruit fly, Drosophila melanogaster. J Exp Biol. 2002;285:2785–2798. doi: 10.1242/jeb.205.18.2785. [DOI] [PubMed] [Google Scholar]
- 15.Skinner BF. The behavior of organisms: An experimental analysis. New York: Appleton-Century-Crofts; 1938. [Google Scholar]
- 16.Tinbergen N. The Study of Instinct. New York: Oxford University Press; 1951. [Google Scholar]
- 17.Von Holst E, Mittelstaedt H. The principle of reafference. Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- 18.Murakami M, Vicente MI, Costa GM, Mainen ZF. Neural antecedents of self-initiated actions in secondary motor cortex. Nat Neurosci. 2014;17:1574–1582. doi: 10.1038/nn.3826. [DOI] [PubMed] [Google Scholar]
- 19.Dunn TW, Mu Y, Narayan S, Randlett O, Naumann EA, Yang C, Schier AF, Freeman J, Engert F, Ahrens MB. Brain-wide mapping of neural activity controlling zebrafish exploratory locomotion. eLife. 2016;5 doi: 10.7554/eLife.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn TW, Gebhardt C, Naumann EA, Riegler C, Ahrens MB, Engert F, Del Bene F. Neural Circuits Underlying Visually Evoked Escapes in Larval Zebrafish. Neuron. 2016;89:613–628. doi: 10.1016/j.neuron.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;18:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- 22.Maimon G, Assad JA. A cognitive signal for the proactive timing of action in macaque LIP. Nat Neurosci. 2006;9:948–955. doi: 10.1038/nn1716. [DOI] [PubMed] [Google Scholar]
- 23.Lee IH, Assad JH. Putaminal activity for simple reactions or self-timed movements. J Neurophysiol. 2003;89:2528–2537. doi: 10.1152/jn.01055.2002. [DOI] [PubMed] [Google Scholar]
- 24.Libet B. Unconscious cerebral initiative and the role of conscious wil in voluntary action. Behav Brain Sci. 1985;8:529–539. [Google Scholar]
- 25.Fried I, Mukamel R, Kreiman G. Internally generated preactivation of single neurons in human medial frontal cortex predicts volition. Neuron. 2011;69:548–562. doi: 10.1016/j.neuron.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci. 2010;13:393–399. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- 27.Tammero LF. Spatial organization of visuomotor reflexes in Drosophila. Journal of Experimental Biology. 2004;207:113–122. doi: 10.1242/jeb.00724. [DOI] [PubMed] [Google Scholar]
- 28.Maye A, Hsieh CH, Sugihara G, Brembs B. Order in spontaneous behavior. PLoS One. 2007;2:e443. doi: 10.1371/journal.pone.0000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim AJ, Fitzgerald JK, Maimon G. Cellular evidence for efference copy in Drosophila visuomotor processing. Nat Neurosci. 2015;18:1247–1255. doi: 10.1038/nn.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer M, Vogtmann K, Bausenwein B, Wolf R, Heisenberg M. Flight control during ‘free yaw turns’ in Drosophila melanogaster. Journal of Comparative Physiology A. 1988;163:389–399. [Google Scholar]
- 31.Bender JA, Dickinson MH. A comparison of visual and haltere-mediated feedback in the control of body saccades in Drosophila melanogaster. J Exp Biol. 2006;209:4597–4606. doi: 10.1242/jeb.02583. [DOI] [PubMed] [Google Scholar]
- 32.Konig JH, Saito K, Ikeda K. Reversible control of synaptic transmission in a single-gene mutant Drosophila melanogaster. J Cell Biol. 1993;96:1517–1522. doi: 10.1083/jcb.96.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickinson MH, Lehmann FO, Chan WP. The control of mechanical power in insect flight. Am Zool. 1998;38:718–728. [Google Scholar]
- 34.Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 35.Suster ML, Seugnet L, Bate M, Sokolowski MB. Refining GAL4-driven transgene expression in Drosophila with a GAL80 enhancer trap. Genesis. 2004;39:240–245. doi: 10.1002/gene.20051. [DOI] [PubMed] [Google Scholar]
- 36.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 37.Dickinson MH. The initiation and control of rapid flight maneuvers in Fruit Flies. Integr Comp Biol. 1999;45:274–281. doi: 10.1093/icb/45.2.274. [DOI] [PubMed] [Google Scholar]
- 38.Gabbiani F, Krapp HG, Laurent G. Computation of object approach by a wide-field, motion-sensitive neuron. J Neurosci. 1999;19:1122–1141. doi: 10.1523/JNEUROSCI.19-03-01122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bender JA, Dickinson MH. Visual stimulation of saccades in magnetically tethered Drosophila. J Exp Biol. 2006;209:3170–3182. doi: 10.1242/jeb.02369. [DOI] [PubMed] [Google Scholar]
- 40.Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart-Guerin G, Placais PY, Robie AA, Yamagata N, Schnaitmann C, et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife. 2014;3:e04580. doi: 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang C, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan Y. Control of the post-mating behavioral switch in Drosophila by internal sensory neurons. Neuron. 2009;61:519–526. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fasano L, Röder L, Coré N, Alexandre E, Vola C, Jac B, Kerridge S. The role of the teashirt gene in trunk segmental identity in Drosophila. Development. 1992;117:1017–1033. doi: 10.1242/dev.115.4.1017. [DOI] [PubMed] [Google Scholar]
- 43.Mahr A, Aberle H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr Patterns. 2006;6:299–309. doi: 10.1016/j.modgep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 45.Schnell B, Ros IG, Dickinson MH. A Descending Neuron Correlated with the Rapid Steering Maneuvers of Flying Drosophila. Curr Biol. 2017;27:1200–1205. doi: 10.1016/j.cub.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mamiya A, Dickinson MH. Antennal mechanosensory neurons mediate wing motor reflexes in flying Drosophila. J Neurosci. 2015;35:7977–7991. doi: 10.1523/JNEUROSCI.0034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Reyn CR, Breads P, Peek MY, Zheng GZ, Williamson WR, Yee AL, Leonardo A, Card GM. A spike-timing mechanism for action selection. Nat Neurosci. 2014;17:962–970. doi: 10.1038/nn.3741. [DOI] [PubMed] [Google Scholar]
- 48.Haikala V, Joesch M, Borst A, Mauss AS. Optogenetic control of fly optomotor responses. J Neurosci. 2013;33:13927–13934. doi: 10.1523/JNEUROSCI.0340-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujiwara T, Cruz TL, Bohnslav JP, Chiappe ME. A faithful internal representation of walking movements in the Drosophila visual system. Nat Neurosci. 2017;20:72–81. doi: 10.1038/nn.4435. [DOI] [PubMed] [Google Scholar]
- 50.Berni J, Pulver SR, Griffith LC, Bate M. Autonomous circuitry for substrate exploration in freely moving Drosophila larvae. Curr Biol. 2012;22:1861–1870. doi: 10.1016/j.cub.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trimarchi JR, Murphey RK. The shaking-B2 mutation disrupts electrical synapses in a flight circuit in adult Drosophila. J Neurosci. 1997;17:4700–4710. doi: 10.1523/JNEUROSCI.17-12-04700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickinson MH. Haltere-mediated equilibrium reflexes of the fruit fly, Drosophila melanogaster. Phil Trans R Soc Lond B. 1999;354:903–916. doi: 10.1098/rstb.1999.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams FX. Ampulex compressa (Fabr.), a cockroach-hunting wasp introduced from New Caledonia into Hawaii. Proc Haw Ent Soc. 1942;11:221–233. [Google Scholar]
- 54.Gal R, Libersat F. A parasitoid wasp manipulates the drive for walking of its cockroach prey. Curr Biol. 2008;18:877–882. doi: 10.1016/j.cub.2008.04.076. [DOI] [PubMed] [Google Scholar]
- 55.Fouad K, Rathmayer W, Libersat F. Neuromodulation of the escape behavior of the cockroach Periplaneta americana by the venom of the parasitic wasp Ampulex compressa. J Comp Physiol A. 1996;178:91–100. [Google Scholar]
- 56.Fouad K, Libersat F, Rathmayer W. The venom of the cockroach-hunting wasp Ampulex compressa changes motor thresholds – a novel tool for studying the neural control of arousal. Zoology. 1994;98:23–34. [Google Scholar]
- 57.Jenett A, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfeiffer BD, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim AJ, Fenk LM, Lyu C, Maimon G. Quantitative Predictions Orchestrate Visual Signaling in Drosophila. Cell. 2017;168:280–294 e212. doi: 10.1016/j.cell.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reiser MB, Dickinson MH. A modular display system for insect behavioral neuroscience. Journal of Neuroscience Methods. 2008;167:127–139. doi: 10.1016/j.jneumeth.2007.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


