Abstract
A network of a few hundred neurons in the Drosophila central complex carries an estimate of the fly’s heading in the world, akin to the mammalian head-direction system. Here we describe how anatomically defined neuronal classes in this network are poised to implement specific sub-processes for building and updating this population-level heading signal. The computations we describe in the fly central complex strongly resemble those posited to exist in the mammalian brain, in computational models for building head-direction signals. By linking circuit anatomy to navigational physiology, the Drosophila central complex should provide the first detailed example of how a heading signal is built.
Introduction
If we wake up in the middle of the night, we can make our way to the bathroom, even in complete darkness. When we start off on this short journey, we walk in the correct direction using an internal sense of heading combined with a memory of the spatial layout of our home and our position inside it. How do brains build an internal sense of heading to guide navigation?
A major step forward in answering this question was the discovery, in rodents, of head-direction cells: neurons that are active when an animal’s head is oriented toward one specific direction in space [1–3]. Head direction cells are part of a larger network of interconnected neurons that carry navigational signals, which includes place cells (neurons that are active when an animal is in one or a few specific locations) [4,5] and grid cells (neurons that are active when an animal is located along a hexagonal grid of positions) [6,7], among others [8,9]. A fundamental next step is to describe the circuit-level interactions that give rise to the physiological activity patterns of head direction and other spatially-responsive cells and to understand how the activity of these cells influences navigational behavior.
Physiological signatures of a sense of heading have also been described in insects, in a set of neuropils called the central complex [10–12]. Because the central complex of small insects contains at least two orders of magnitude fewer neurons than navigation-related brain regions in rodents, the circuit mechanisms underlying these heading signals may be easier to dissect. In particular, behavioral neurophysiology in the fruit fly, Drosophila melanogaster, has recently revealed a system of heading-sensitive cells in the central complex [12] whose core physiological features echo those of head-direction cells in mammals [2]. Recent reviews have described the pioneering work on navigational behavior and central complex physiology in ants, bees, beetles, locusts, cockroaches, and other insects [13–16]. Here, we aim to illustrate how the physiological properties of neurons in the Drosophila heading system could be mediated by anatomically defined cell classes. The circuit architectures we highlight, many of which have been recently instantiated in formal models [17–21], resemble those proposed for rodent head-direction cells [22–25], hinting at the possibility that they will generalize beyond flies. Large-scale ongoing efforts to fully map out the connectivity of the fly central complex [26,27] should provide an understanding of these architectures at an unprecedented level of resolution in the coming years.
Central complex anatomy
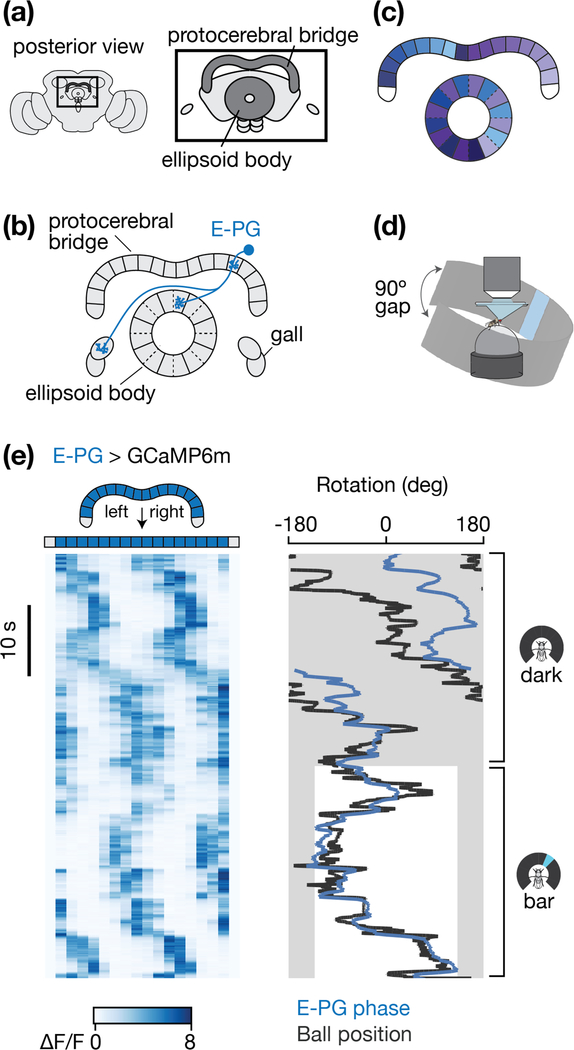
We focus on two neuropils in the Drosophila central complex (Figure 1a) [28–30]: the linear protocerebral bridge––which consists of 18 glomeruli that straddle the brain’s midline [29]––and the circular ellipsoid body––which consists of 8 pizza-slice-like tiles, with each tile split into two wedges [25] (Figure 1b). Both the ellipsoid body and bridge consist purely of axons and dendrites, like typical insect brain structures, with cell bodies in the periphery. Columnar neurons interconnect single glomeruli in the bridge with single tiles or wedges in the ellipsoid body (e.g. Figure 1b). Each half of the bridge contains arrays of columnar neurons that project to the entire circumference of the ellipsoid body (e.g. the E-PG projection pattern in Figure 1c). For a detailed description of the anatomy of neurons innervating the protocerebral bridge, see ref [29].
Figure 1 |. A heading signal in the fly central complex.
a, The protocerebral bridge and ellipsoid body represent two structures in the central complex, a set of interconnected neuropils located in the middle of the fly brain. b, The anatomy of one E-PG neuron is shown. Glomeruli in the protocerebral bridge and tiles in the ellipsoid body are delineated by solid lines. Wedges in the ellipsoid body are delineated by dashed lines. c, The full array of E-PGs innervate the entire ellipsoid body and most of the bridge. For E-PGs, each half of the bridge maps to every second wedge in the ellipsoid body. Colors represent the mapping of E-PG neurons between the ellipsoid body and bridge. d, Preparation for imaging a fly walking on an air-supported spherical treadmill with a panoramic LED display. The LED display covered 270˚ around the fly, with a 90˚ gap in the back (arrows) e, (Left) E-PG activity in the protocerebral bridge as the fly walks in complete darkness (top) and as it walks with a tall, bright, vertical bar presented on the LED display, which rotates in closed-loop with the fly’s turning to indicate its virtual heading. (Right) The position, or phase, of the E-PG activity in the bridge (blue) and the ball’s yaw orientation (black). We shifted the E-PG phase (blue curve) by a constant offset so as to best match the ball/bar position (black curve) when the bar is visible. Grey areas highlight the 90º gap in the arena behind the fly where the bar is not visible.
Neurons in the central complex track the fly’s heading
Heading signals in Drosophila were first described in E-PG (ellipsoid body-protocerebral bridge-gall) cells [12], a set of columnar neurons that project between the ellipsoid body and the protocerebral bridge (Table 1, Figure 1b-c). (The “E” is placed before the dash in the acronym to indicate putative inputs and the “PG” after the dash to indicate putative outputs, based on the spiny versus blebby morphology of processes; however, it is important to note that many neurites in Drosophila contain both input and output synapses.) When imaging [Ca2+] in E-PGs as a fly walks on an air-cushioned spherical treadmill, one observes a single activity peak in the ellipsoid body (not shown) and two to three periodically spaced peaks in the bridge (Figure 1de). The multiple peaks in the bridge are a simple consequence of the anatomical projection pattern of E-PGs (Figure 1b-c); they represent high [Ca2+] in the same neurons that are active within the one activity peak in the ellipsoid body [31]. When the fly stands still, the activity peaks are stationary [12,31]. When the fly turns left, the activity peaks rotate rightward in the bridge and clockwise in the ellipsoid body (when viewed from the posterior), and vice versa for right turns [12,31] (Figure 1d, note that here the black trace represents the ball/bar heading – the inverse of the fly’s heading – to better visually compare the fly’s heading and the E-PG signal). Whereas in darkness the positions of the peaks tend to drift relative to the fly’s virtual heading over time [12,31] (Figure 1e, dark), they track the fly’s heading without drift when the fly walks in a virtual environment with a prominent visual stimulus (e.g., a bright vertical bar) that rotates in closed-loop with the fly’s left/right turns on the ball, simulating a distant, static visual landmark [12,31] (Figure 1e, bar). Together, these properties are interpreted to mean that E-PGs carry a heading signal that is yoked to visual inputs when these are available, but which can also update based on the animal’s turns in complete darkness. These physiological properties of E-PG neurons in flies are very similar to those of head direction cells in rodents [2,3,32,33].
Table 1.
Cell types discussed in this review.
| E-PG | Ellipsoid body-protocerebral bridge-gall |
| P-EG | Protocerebral bridge-ellipsoid body-gall |
| P-EN | Protocerebral bridge-ellipsoid body-noduli |
| Δ7 | Protocerebral bridge local neurons |
| SPS-P | Superior posterior slope-protocerebral bridge |
| Ring neurons | Lateral triangle-ellipsoid body |
The E-PG heading peaks have at least four central properties whose underlying mechanisms one would wish to understand: (1) they persist when the fly is standing still in darkness, (2) they have a specific shape, (3) they rotate along the ellipsoid body and bridge when the fly turns and (4) their position along the ellipsoid body and bridge can be yoked to the angular position of visual landmarks. We next highlight putative circuit mechanisms for how these properties could be implemented. Because the synaptic connectivity and physiological activity profiles of most cells described herein are still being worked out, the proposed framework is highly speculative. Nevertheless, we hope it may serve as a useful guide for future experiments.
Persistent activity
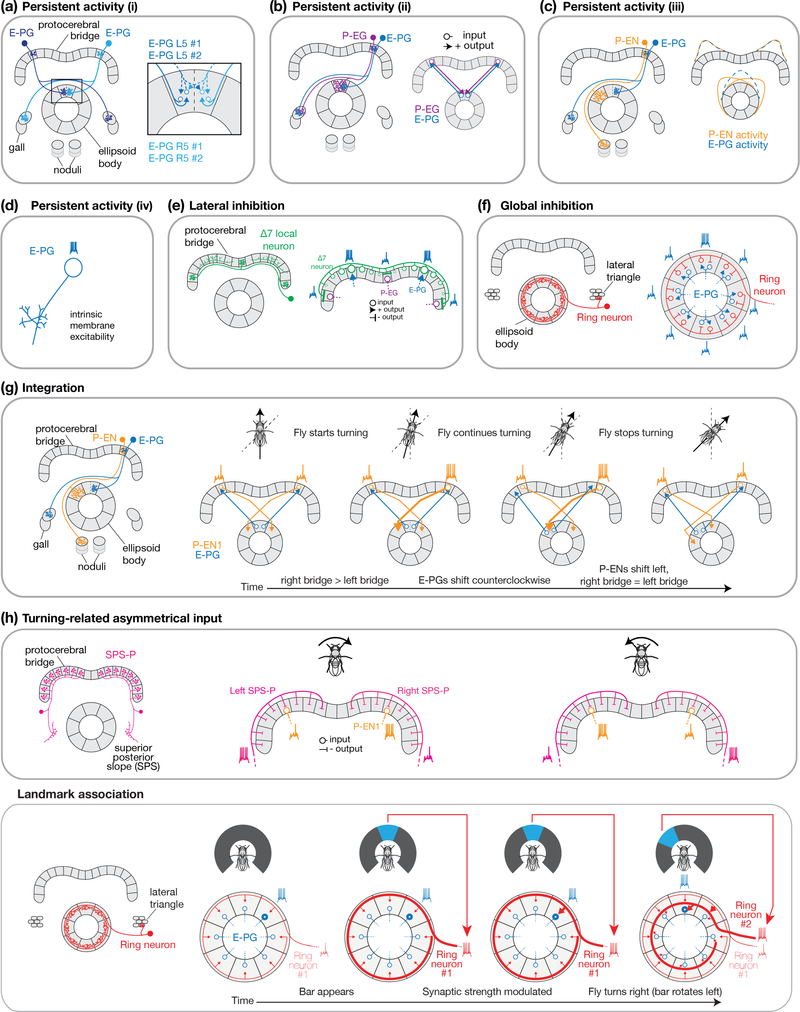
The E-PG activity peaks persist when a fly stands in complete darkness––when there are no dynamic vestibular, proprioceptive, visual or motor signals available to the system––raising the possibility that the E-PG signal is sustained by physiological processes confined to the central complex. Based on first-order assessments of neurite shape, E-PGs have dendrites in the ellipsoid body and axons in the bridge and gall [29]. However, as is typical in many invertebrate neurons, E-PG neurite fields are actually mixed and, most prominently, E-PGs appear to have both input and output synapses in the ellipsoid body ([29,30] and personal communication from Atsuko Adachi). Thus, E-PGs may strongly excite each other within each ellipsoid-body wedge as well as more weakly across neighboring wedges (Figure 2a) and thus generate persistent activity. E-PGs may also form recurrent excitatory connections with P-EGs (Figure 2b), which have dendrites in the bridge and axons in the ellipsoid body, thus projecting in the opposite direction as E-PGs. A third possibility, considered in recent papers [20,31], is for E-PGs to form recurrent excitatory connections with P-ENs, which, like P-EGs, project from the bridge to the ellipsoid body (though with a different projection pattern, the consequences of which we describe in more detail below) (Figure 2c). When synaptic output from P-ENs is strongly impaired, the E-PG activity peak becomes weaker [20,31], and although P-ENs and E-PGs project to non-overlapping tiles/wedges in the ellipsoid body [29], the measured P-EN activity peak in the ellipsoid body partially overlaps with the measured E-PG peak, allowing P-ENs to potentially serve a role in persistent E-PG activation [20,31]. A fourth possibility is that E-PGs have intrinsic membrane properties that support sustained spiking without synaptic inputs (Figure 2d). Beyond these possibilities, persistent activity in E-PGs could also be inherited from other cell classes in the fly brain (not depicted in Fig. 2), such as tonically-active proprioceptive signals from the legs [34]. Direct physiological evidence for all of these circuit mechanisms is missing, except for P-EN feedback. These possibilities are not mutually exclusive.
Figure 2 |. Building a heading signal with anatomically defined cell types.
a, Persistent activity in E-PG neurons through putative direct E-PG↔E-PG local excitation, within and across wedge boundaries. b, Persistent activity in E-PG neurons through putative E-PG➜P-EG excitation in the bridge, and return putative P-EG➜E-PG excitation in the ellipsoid body. c, Persistent activity through the partial overlap of the E-PG and P-EN activity peaks in the ellipsoid body. Activity profiles are schematized based on experimental measurements [20,31]. d, Persistent activity via intrinsic membrane excitability of neurons in the circuit, with E-PGs as one possible example. e, Lateral inhibition through Δ7 neurons, which have neurites wholly confined to the bridge. Single Δ7s have (blebby) axonal terminals anti-phase (or 4 glomeruli) relative to where they have the highest density of (spiny) dendrites. The specific Δ7 cell shown would receive inputs from active E-PGs (blue arrows) and provide inhibition to E-PGs (through inhibition of P-EGs that project to the ellipsoid body) anti-phase to the E-PG peaks. Other Δ7s, not shown, would provide additional lateral inhibition, at other glomeruli surrounding the E-PG peak. f, Global inhibition through the ring neurons in the ellipsoid body. Each ring neuron receives input from one microglomerulus in the lateral triangle, and sends outputs encircling the ellipsoid body. g, Angular velocity integration through a left-versus-right bridge asymmetry in P-EN activity driving a directional rotation of the E-PG peak. h, Asymmetric inputs via left- and right-bridge innervating SPS-Ps. Each SPS-P neuron receives inputs in the superior posterior slope and sends outputs to one half of the bridge. If SPS-Ps inhibit P-ENs, only P-ENs that were excited by E-PGs (directly or indirectly) and not inhibited by SPS-Ps fire action potentials. The SPS-P to P-EN synapse is inhibitory based on ref [26]. Other models where the asymmetric input is excitatory are also possible. i, Landmark association through ring neurons. Each ring neuron receives inputs from one microglomerulus in the lateral triangle and sends outputs that encircle the ellipsoid body. Each ring neuron responds to a small visual feature at a specific retinal position [39]. In this model, synapses between ring neurons and E-PGs are modulated over time to associate visual features at specific retinal positions with the activation of specific E-PG neurons, or internal heading estimates. The two ring neurons shown are separated spatially within the schematic ellipsoid body for clarity; they do not necessarily represent ring neurons that innervate different radii of the ellipsoid body. Solid lines delineate glomeruli in the protocerebral bridge and tiles in the ellipsoid body. Dashed lines delineate wedges in the ellipsoid body.
Shaping the heading signal
To first order, the E-PG activity peaks have a consistent shape, but their position along the ellipsoid body and bridge changes over time. The fact that E-PG activity takes the form of a single peak in the ellipsoid body, and periodically spaced peaks in the bridge, suggests that cells exist which inhibit E-PG activity elsewhere in these structures. Moreover, when one experimentally stimulates E-PGs at an inactive locus, the stimulated E-PGs activate and the previously active E-PGs inactivate [31,35]. A circuit mechanism that could explain the abovementioned results is lateral inhibition. That is, E-PG neurons participating in the currently active [Ca2+] peaks could inhibit those E-PGs located elsewhere in the ellipsoid body and/or protocerebral bridge. Another circuit mechanism that could explain the abovementioned results is global inhibition. That is, if E-PG neurons within the activity peaks locally excite each other (Figure 2a), then uniform (rather than lateral) inhibition throughout the ellipsoid body or bridge could effectively prevent multiple peaks.
Of the known anatomical cell types in the central complex, a good candidate to implement lateral inhibition is the protocerebral bridge Δ7 cell class (Table 1). Based on the anatomical evidence, Δ7 neurons send outputs four glomeruli (or 180°) offset from where they receive their strongest inputs (Figure 2e, Figure 18B in [29]). (The name “Δ7” refers to the 7-glomerulus spacing between axonal terminals in single cells of this anatomical class.) Although Δ7 neurons appear to not express GABA [30], they could inhibit E-PGs via an intermediate inhibitory cell class, or, if some or all Δ7 cells are glutamatergic, then they could inhibit E-PGs via a glutamate-gated chloride conductance [36], a possibility that has not yet been tested.
Global inhibition, on the other hand, could be achieved by neurons that project uniformly throughout the ellipsoid body or bridge. For example, ellipsoid-body ring neurons (Table 1) have neurites that encircle the ellipsoid body [28] and many of these cells stain positively for GABA [37] (Figure 2f). If ring neuron neurites were to function as bi-directional input/output devices in the ellipsoid body [38], receiving excitation from E-PGs and providing reciprocal inhibition back onto E-PGs in all wedges, then the active set of E-PGs in the activity peak could inhibit all E-PGs, including themselves, via ring neurons. With global inhibition, recurrent excitation of E-PGs within the active locus would obviously have to exceed feedback inhibition in that locus for an activity peak to persist. By means of stimulation experiments and modeling, a recent study argued that the feedback inhibition between E-PGs takes the form of global rather than lateral inhibition [35], suggesting that a mechanism like the one just described may be at play. Aside from ring neurons, several other neuron classes with arbors that span the entire ellipsoid body, protocerebral bridge, or layers of a third central complex structure called the fan shaped body [28–30] could, in principle, also contribute toward global inhibition.
Integration
When there are no visual cues available to reliably indicate the fly’s heading, like when walking in complete darkness, the E-PG activity peaks must update their position in the bridge and ellipsoid body based on how fast the fly measures itself to be turning (based on, for example, proprioceptive cues or copies of motor commands). Specifically, the system seems to perform mathematical integration, converting an estimate of the fly’s angular velocity into an updated estimate of the fly’s angular heading. P-ENs seem to serve a central role in this integration process (Figure 2g) [20,31]. P-ENs in the left bridge project to the ellipsoid body one tile clockwise relative to E-PGs and P-ENs in the right bridge project one tile counterclockwise (Figure 2g). Thus, P-ENs are poised to nudge the E-PG ellipsoid-body peak clockwise or counterclockwise depending on an asymmetry in their activity in the left versus right bridge. Recent studies have confirmed that P-ENs are asymmetrically active in the left versus right bridge when the fly turns, that this asymmetry is quantitatively tuned to the fly’s angular velocity, and that this asymmetry has the correct sign to rotate the E-PG activity peaks in the expected direction [20,31]. Clockwise- and counterclockwise-projecting P-EN neurons are expected to receive an asymmetric drive from cells that carry angular velocity signals and which project to the entire left or right bridge, like SPS-P neurons (Table 1, Figure 2h), or from neurons that project to the left or right noduli, where anatomical cell classes are only now starting to be defined.
There also exists a second class of P-ENs, P-EN2s, whose [Ca2+] physiology suggests that they may act to help brake a moving E-PG bolus during integration or perform some other, still unclear, function [31].
Landmark tracking
The position of the E-PG heading signal is updated not just via angular velocity integration, but also in reference to visual landmarks (Figure 1e). The mapping between angular positions of a visual landmark on the retina and E-PG-peak positions in the ellipsoid body or bridge differs across flies [12,31]; for example, when a landmark is directly in front of the fly, the E-PG peak might be at the top of the ellipsoid body in fly 1 and at the bottom (or anywhere else) in fly 2. In other words, the E-PG peak rotates with a different offset in each fly relative to visual reference points. Within a fly, the E-PG offset relative to a visual landmark is typically stable, but can also change over time [12,31]. These data speak against a model in which neurons responsive to visual features at specific retinal positions invariably excite the same E-PGs in the ellipsoid body or bridge.
Instead, visual neurons may provide synapses to the entire bridge or ellipsoid body and the strength of these synapses might be modified by short-term plasticity mechanisms to activate different E-PGs in different circumstances (Figure 2g) [18]. The idea could work as follows. Consider a fly standing in the dark, with its E-PG activity peak at some position in the ellipsoid body. When a visual landmark appears, neurons sensitive to small visual features (like oriented contours) at the landmark’s retinal position become active, simultaneously with the E-PGs constituting the current activity peak. The synapses that connect these two sets of neurons strengthen via a Hebbian-like plasticity rule. As the fly turns, the E-PG activity peak rotates in the ellipsoid body (via angular integration, described above) and the landmark rotates on its retina, activating different visual feature-detecting cells; the connections between this newly co-active set of visual neurons and E-PGs are in turn strengthened. Eventually, a mapping between the activity of feature-detecting neurons and E-PG peak positions in the ellipsoid body can be learned and used to update the E-PG peak’s position without drift as the fly turns. Richer panoramas, consisting of multiple landmarks [12], could be learned as well. Indeed, any visual feature that consistently rotates with the fly’s heading would associate with the E-PG peak over time whereas visual features that are poorly correlated with heading would not. This property may help the system ignore visual features that are not consistently informative of heading in the world, like moving animals or nearby objects that the fly walks past (assuming the system aims to track the fly’s world-centered heading and not heading relative to local objects).
Ellipsoid body ring neurons are the best known candidates to serve as the visual feature detectors in this model (Figure 2g). Each ring neuron sends an axon that encircles the entire ellipsoid body and responds to small visual features at specific retinal positions; as a population, rings neurons have receptive fields that tile azimuthal and vertical space [39,40]. Moreover, ring neurons are numerous, which could support the detection of many different visual (or other sensory) features at many retinal positions. Ring neurons have been implicated in place [41] and orientation [42] memories and could readily serve both feature detection and global inhibition (proposed earlier) through multiplexing functions in single neurons or through different ring neurons serving different roles. If ring neurons functionally inihibit rather than excite E-PGs, then the above model could work with anti-Hebbian plasticity (weakening the synapses between co-active cells) rather than Hebbian plasticity (strengthening the synapses between co-active cells). Recent work highlights that the feature-detecting signals in ring-neuron dendrites in the lateral triangle (outside the ellipsoid body) are far from a veridical reflection of the retinal input, but rather emphasize behaviorally relevant visual features over others [40,43], which could aid the above mentioned computation within the ellipsoid body.
Similarities between the fly and rodent heading system models
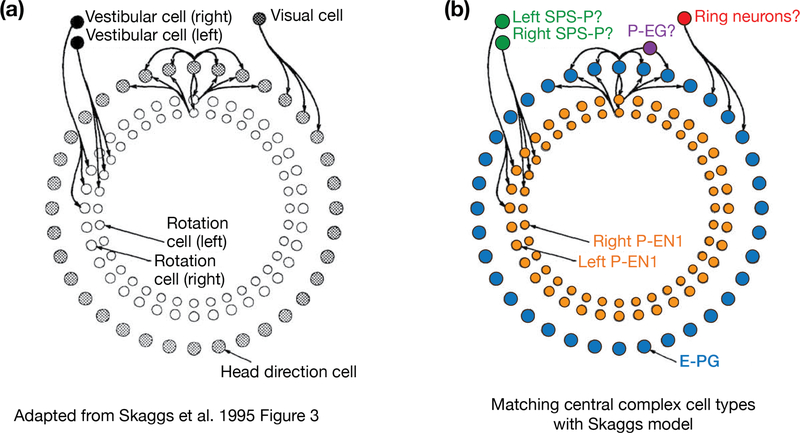
Intriguingly, the conceptual model of the Drosophila heading system just described maps, cell type for cell type, onto computational models for the rodent head-direction system [22–25], including the Skaggs et al. model on which we focus here [22] (Figure 3). For example, E-PGs conform to the role of head direction cells in the Skaggs et al. model [22], based on their ring-like organization within the topology of the circuit and their basic physiological properties, summarized above, which closely resemble those of rat head direction cells [2,12]. (Because all imaging experiments in Drosophila to date have been performed in flies with their heads glued in fixed alignment to their bodies, it is not yet clear whether the E-PG heading signal reflects a genuine head direction signal, which would update when the fly rotates its head relative to its body, or a “body direction” signal, which would only update when the fly rotates its body. For this reason, we operationally refer to the E-PG signal as a heading signal, which encompasses both such possibilities, rather than specifically as a head direction signal.) The excitatory connections among neighboring head direction cells in the Skaggs et al. model may be implemented by direct E-PG-to-E-PG connections, or indirect positive feedback via P-EGs or P-ENs. P-ENs map perfectly onto rotation cells in the Skaggs et al. model, given that both cell classes project either clockwise or counterclockwise along the ring-like circuit. P-ENs are most similar in physiology to rat head direction cells in the lateral mammillary nucleus, whose activity are also modulated by angular head velocity [44,45]. Based on their anatomy, SPS-P neurons map well onto vestibular cells in the Skaggs et al. model – or more generally cells that might signal angular velocity to the system also via optic flow, proprioceptive or motor-command-based cues. (Rats rely heavily on their vestibular system to update their head direction signal as evidenced by the severe impairments to the head-direction signal in animals with perturbed vestibular inputs [46]; since tethered flies receive no vestibular indication that they are turning but still effectively update their E-PG heading signal when turning on a ball, proprioceptive or motor-command copies appear to serve a more prominent role as indicators of angular velocity in flies.) Finally, ellipsoid body ring neurons map perfectly onto the visual cells in the Skaggs et al. model – which might be generalized as multimodal landmark-sensitive cells. Other cell type mappings are also possible (see above for other possible cell types within each functional category), given that much remains unknown in both the fly and rodent circuits.
Figure 3 |. Similarities between circuit models for the fly and rat heading systems.
a, Adapted from Skaggs et al. (1995) [22]. Schematic representing a formal computational model for how the rat head direction signal is built and rotates around a hypothesized ring of head-direction cells. b, Mapping the cell types in the Drosophila protocerebral-bridge/ellipsoid-body circuit onto the model by Skaggs et al. The physiology of E-PGs and P-ENs has been reported whereas the role of the other cell types in this schematic remains speculative (represented by question marks).
From heading signals to behavior
Very little is known about how Drosophila – or any species – use heading signals to guide navigational behavior [47,48]. Behavioral experiments have argued that flies can navigate to remembered 2D locations in space [41,49]. Flies can also effectively disperse far from a start location by traveling along a constant, but arbitrary, angular direction for seconds to hours [50–53]. To perform either such task, flies must, at each moment in time, ultimately choose an angle at which to walk or fly, which can be considered the instantaneous goal angle of the fly. When performing arbitrary-angle dispersal, this goal angle stays constant for seconds to hours. When navigating to remembered 2D locations, the goal angle continuously updates with respect to the fly’s estimate of its current position and heading in 2D space in relation to the 2D goal location. Thus, one hypothesis to explain such behaviors is that, flies, ultimately, internally compare an estimate of their current heading, as carried by E-PGs (or other heading neurons in the central complex) with a goal heading direction. A difference between these two signals would create an urgency to turn, inducing the fly to rotate its body so as to bring its internal heading signal in alignment with its goal angle, thus driving directed navigation [53]. If this framework is correct, future work should seek to discover angular goal signals, as well as representations of 2D goals, that interact with the heading signals described here, to guide navigational action.
Conclusions
Many neural correlates of spatial navigation have been described, but biologically-grounded circuit mechanisms for explaining how these signals are built are scarce. Precise anatomical and physiological measurements in Drosophila are yielding clear functional hypotheses for cell type specific implementations of spatial computations. A detailed understanding of how the fly brain computes heading, and potentially other stored spatial variables, should mark an important step forward for the study of spatial cognition.
Highlights.
The Drosophila central complex contains neurons that track the fly’s angular heading.
Anatomical projection patterns for many central complex neurons are known.
This anatomy suggests how specific neuron classes contribute to building a heading signal.
The heading computations proposed for flies resemble computational models in rats.
Acknowledgments
Funding.
This work was supported by the McKnight Foundation (G.M.) and the National Institutes of Health (DP2DA035148 and R01NS104934) (G.M.)
References
- 1.Ranck JB Jr: Head direction cells in the deep cell layer of dorsal presubiculum in freely moving rats. Soc Neurosci Abstr; 1984. [Google Scholar]
- 2.Taube JS, Muller RU, Ranck JB: Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci 1990, 10:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taube JS, Muller RU, Ranck JB: Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J. Neurosci 1990, 10:436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Keefe J: Place units in the hippocampus of the freely moving rat. Exp. Neurol 1976, 51:78–109. [DOI] [PubMed] [Google Scholar]
- 5.O’Keefe J, Nadel L: The hippocampus as a cognitive map. Clarendon Press Oxford; 1978. [Google Scholar]
- 6.Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI: Microstructure of a spatial map in the entorhinal cortex. Nature 2005, 436:801–806. [DOI] [PubMed] [Google Scholar]
- 7.Rowland DC, Roudi Y, Moser MB: Ten Years of Grid Cells. Annual review of … 2016, doi: 10.1146/annurev-neuro-070815-013824. [DOI] [PubMed] [Google Scholar]
- 8.Kropff E, Carmichael JE, Moser M-B, Moser EI: Speed cells in the medial entorhinal cortex. Nature 2015, 523:419. [DOI] [PubMed] [Google Scholar]
- 9.Solstad T, Boccara CN, Kropff E, Moser M-B, Moser EI: Representation of geometric borders in the entorhinal cortex. Science 2008, 322:1865–1868. [DOI] [PubMed] [Google Scholar]
- 10.Heinze S, Homberg U: Maplike Representation of Celestial E-Vector Orientations in the Brain of an Insect. Science 2007, 315:995–997. [DOI] [PubMed] [Google Scholar]
- 11.Homberg U, Heinze S, Pfeiffer K, Kinoshita M, Jundi el B: Central neural coding of sky polarization in insects. Philosophical Transactions of the Royal Society B: Biological Sciences 2011, 366:680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. **.Seelig JD, Jayaraman V: Neural dynamics for landmark orientation and angular path integration. Nature 2015, 521:186–191. This study provided physiological evidence for an abstract heading signal in Drosophila. The authors imaged [Ca2+] in E-PG neurons of the fly ellipsoid body while flies walked on a spherical treadmill. They found a peak of activity that rotated around the ellipsoid body to track the fly’s virtual heading both in complete darkness and by reference to visual landmarks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collett M, Chittka L, Collett TS: Spatial Memory in Insect Navigation. Current Biology 2013, 23:R789–R800. [DOI] [PubMed] [Google Scholar]
- 14.Heinze S: Unraveling the neural basis of insect navigation. Current Opinion in Insect Science 2017, 24:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeiffer K, Homberg U: Organization and Functional Roles of the Central Complex in the Insect Brain. Annual Review of Entomology 2014, 59:165–184. [DOI] [PubMed] [Google Scholar]
- 16.Homberg U: Evolution of the central complex in the arthropod brain with respect to the visual system. Arthropod Structure & Development 2008, 37:347–362. [DOI] [PubMed] [Google Scholar]
- 17. *.Kakaria KS, Bivort D, L B: Ring Attractor Dynamics Emerge from a Spiking Model of the Entire Protocerebral Bridge. Frontiers in Behavioral Neuroscience 2017, 11 This study represented the first comprehensive modeling effort of multiple cell types in the Drosophila central complex. The resultant dynamically modeled circuit recapitulated key features of the E-PG heading signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cope AJ, Sabo C, Vasilaki E, Barron AB, Marshall JAR: A computational model of the integration of landmarks and motion in the insect central complex. PLoS ONE 2017, 12:e0172325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Givon LE, Lazar AA, Yeh C-H: Generating Executable Models of the Drosophila Central Complex. Frontiers in Behavioral Neuroscience 2017, 11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. **.Turner-Evans D, Wegener S, Rouault H, Franconville R, Wolff T, Seelig JD, Druckmann S, Jayaraman V: Angular velocity integration in a fly heading circuit. eLife 2017, 6:e23496 This study described a neural circuit to explain how the E-PG heading peak rotates around the fly ellipsoid body as a function of the angular velocity with which the fly turns in complete darkness. This biological circuit for integration resembled theoretical models for angular integration developed in the rodent head-direction system. This paper had similar conclusions to reference [27], but included electrophysiological evidence and a formal computational model to support its conclusions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. *.Stone T, Webb B, Adden A, Ben Weddig N, Honkanen A, Templin R, Wcislo W, Scimeca L, Warrant E, Heinze S: An Anatomically Constrained Model for Path Integration in the Bee Brain. Current Biology 2017, 27:3069–3085.e11. This study provided evidence in bees for heading signals, optic-flow speed signals and neurons that combine the two in the central complex. The authors suggest a computational model for path integration based on these data and they implement the model in an autonomous robot, to show its plausibility in real world conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skaggs WE, Knierim JJ, Kudrimoti HS, McNaughton BL: A model of the neural basis of the rat’s sense of direction. Adv Neural Inf Process Syst 1995, 7:173–180. [PubMed] [Google Scholar]
- 23.Zhang K: Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. J. Neurosci 1996, 16:2112–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redish AD, Elga AN, Touretzky DS: A coupled attractor model of the rodent head direction system. Network: Computation in Neural Systems 1996, 7:671–685. [Google Scholar]
- 25.Sharp PE, Blair HT, Brown M: Neural network modeling of the hippocampal formation spatial signals and their possible role in navigation: a modular approach. Hippocampus 1996, 6:720–734. [DOI] [PubMed] [Google Scholar]
- 26.Franconville R, Beron C, Jayaraman V: Building a functional connectome of the Drosophila central complex. 2018, doi: 10.22541/au.151537454.41878908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Z, Lauritzen JS, Perlman E, Robinson CG, Nichols M, Milkie D, Torrens O, Price J, Fisher CB, Sharifi N, et al. : A Complete Electron Microscopy Volume Of The Brain Of Adult Drosophila melanogaster. bioRxiv 2017, doi: 10.1101/140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanesch U, Fischbach K-F, Heisenberg M: Neuronal architecture of the central complex in Drosophila melanogaster. Cell and Tissue Research 1989, [no volume]. [Google Scholar]
- 29. **.Wolff T, Iyer NA, Rubin GM: Neuroarchitecture and neuroanatomy of the Drosophila central complex: A GAL4-based dissection of protocerebral bridge neurons and circuits. J. Comp. Neurol 2015, 523:997–1037. This study provided a comprehensive light-microscopy analysis of most of the cell classes of the Drosophila protocerebral bridge. The authors showed that the bridge contains 18, not 16, glomeruli. They further characterized the projection patterns of approximately 18 anatomically defined cell types that innervate the bridge, whose anatomical arrangements have served to inspire many functional models in the central complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin C-Y, Chuang C-C, Hua T-E, Chen C-C, Dickson BJ, Greenspan RJ, Chiang A-S: A comprehensive wiring diagram of the protocerebral bridge for visual information processing in the Drosophila brain. Cell Reports 2013, 3:1739–1753. [DOI] [PubMed] [Google Scholar]
- 31. **.Green J, Adachi A, Shah KK, Hirokawa JD, Magani PS, Maimon G: A neural circuit architecture for angular integration in Drosophila. Nature 2017, 546:101–106. This study described a neural circuit to explain how the E-PG heading peak rotates around the fly ellipsoid body as a function of the angular velocity with which the fly turns in complete darkness. This biological circuit for integration resembled theoretical models for angular integration developed in the rodent head-direction system. This paper had similar conclusions to reference [18], but also provided evidence for two integrator neurons: P-EN1s, whose anatomy and physiology suggest they act like gas pedals to drive the rotation of the E-PG peak at the start of a turn, and P-EN2s whose anatomy and physiology suggest they serve a role later in the turn, perhaps to brake the E-PG signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNaughton BL, Chen LL, Markus EJ: “Dead reckoning,” landmark learning, and the sense of direction: a neurophysiological and computational hypothesis. J. Cog. Neurosci 1991, 3:190–202. [DOI] [PubMed] [Google Scholar]
- 33.Mizumori SJ, Williams JD: Directionally selective mnemonic properties of neurons in the lateral dorsal nucleus of the thalamus of rats. J. Neurosci 1993, 13:4015–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamiya A, Gurung P, Tuthill J: Neural coding of leg proprioception in Drosophila. bioRxiv 2018, doi: 10.1101/274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. *.Kim SS, Rouault H, Druckmann S, Jayaraman V: Ring attractor dynamics in the Drosophila central brain. Science 2017, 356:849–853. This study used stimulation experiments and modeling to argue that E-PG heading system functions like a ring attractor with global inhibition. [DOI] [PubMed] [Google Scholar]
- 36.Liu WW, Wilson RI: Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. PNAS 2013, 110:10294–10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Li X, Guo J, Li Y, Guo A: Two clusters of GABAergic ellipsoid body neurons modulate olfactory labile memory in Drosophila. J. Neurosci 2013, 33:5175–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martín-Peña A, Acebes A, Rodríguez J-R, Chevalier V, Casas-Tinto S, Triphan T, Strauss R, Ferrús A: Cell types and coincident synapses in the ellipsoid body of Drosophila. Eur. J. Neurosci 2014, 39:1586–1601. [DOI] [PubMed] [Google Scholar]
- 39.Seelig JD, Jayaraman V: Feature detection and orientation tuning in the Drosophila central complex. Nature 2013, 503:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y, Nern A, Franconville R, Dana H, Schreiter ER, Looger LL, Svoboda K, Kim DS, Hermundstad AM, Jayaraman V: Neural signatures of dynamic stimulus selection in Drosophila. Nat Neurosci. 2017, 503:262. [DOI] [PubMed] [Google Scholar]
- 41.Ofstad TA, Zuker CS, Reiser MB: Visual place learning in Drosophila melanogaster. Nature 2011, 474:204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuser K, Triphan T, Mronz M, Poeck B, Strauss R: Analysis of a spatial orientation memory in Drosophila. Nature 2008, 453:1244–1247. [DOI] [PubMed] [Google Scholar]
- 43.Shiozaki HM, Kazama H: Parallel encoding of recent visual experience and self-motion during navigation in Drosophila. Nat Neurosci. 2017, 20:1395. [DOI] [PubMed] [Google Scholar]
- 44.Stackman RW, Taube JS: Firing properties of rat lateral mammillary single units: head direction, head pitch, and angular head velocity. J. Neurosci 1998, 18:9020–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blair HT, Cho J, Sharp PE: Role of the Lateral Mammillary Nucleus in the Rat Head Direction Circuit. Neuron 1998, 21:1387–1397. [DOI] [PubMed] [Google Scholar]
- 46.Stackman RW, Taube JS: Firing Properties of Head Direction Cells in the Rat Anterior Thalamic Nucleus: Dependence on Vestibular Input. J. Neurosci 1997, 17:4349–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarel A, Finkelstein A, Las L, Ulanovsky N: Vectorial representation of spatial goals in the hippocampus of bats. Science 2017, 355:176–180. [DOI] [PubMed] [Google Scholar]
- 48.Butler WN, Smith KS, van der Meer MAA, Taube JS: The Head-Direction Signal Plays a Functional Role as a Neural Compass during Navigation. Current Biology 2017, 27:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim IS, Dickinson MH: Idiothetic Path Integration in the Fruit Fly Drosophila melanogaster. Current Biology 2017, 27:2227–2238.e3. [DOI] [PubMed] [Google Scholar]
- 50.Warren TL, Weir PT, Dickinson MH: Flying Drosophila maintain arbitrary but stable headings relative to the angle of polarized light. J. Exp. Biol 2018, doi: 10.1242/jeb.177550. [DOI] [PubMed] [Google Scholar]
- 51.Dickinson MH: Death Valley, Drosophila, and the Devonian Toolkit. Annual Review of Entomology 2014, 59:51–72. [DOI] [PubMed] [Google Scholar]
- 52.Giraldo YM, Leitch KJ, Ros IK, Warren TL, Weir PT, Dickinson M: Sun navigation requires compass neurons in Drosophila. bioRxiv 2018, doi: 10.1101/315176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green J, Vijayan V, Pires PM, Adachi A, Maimon G: Walking Drosophila aim to maintain a neural heading estimate at an internal goal angle. bioRxiv 2018, doi: 10.1101/315796. [DOI] [Google Scholar]