Abstract
Osteogenesis imperfecta (OI) is a heritable disorder characterized by osteoporosis and increased susceptibility to fracture. All children with severe OI have extreme short stature and some have “popcorn” calcifications, areas of disorganized hyperdense lines in the metaphysis and epiphysis around the growth plate on lower limb radiographs. Popcorn calcifications were noted on radiographs of two children with non-lethal type VIII OI, a recessive form caused by P3H1 deficiency. To determine the incidence, progression, and molecular correlations of popcorn calcifications, we retrospectively examined serial lower limb radiographs of 45 children with type III or IV OI and known dominant mutations in type I collagen. Popcorn calcifications were present in 13 of 25 type III (52%), but only 2 of 20 type IV (10%), OI children. The mean age of onset was 7.0 years, with a range of 4–14 years. All children with popcorn calcifications had this finding in their distal femora, and most also had calcifications in proximal tibiae. While unilateral popcorn calcification contributes to femoral growth deficiency and leg length discrepancy, severe linear growth deficiency, and metaphyseal flare do not differ significantly between type III OI patients with and without popcorn calcifications. The type I collagen mutations associated with popcorn calcifications occur equally in both COL1A1 and COL1A2, and have no preferential location along the chains. These data demonstrate that popcorn calcifications are a frequent feature of severe OI, but do not distinguish cases with defects in collagen structure (primarily dominant type III OI) or modification (recessive type VIII OI).
Keywords: autosomal dominant osteogenesis imperfect, osteogenesis imperfect, popcorn calcification, calcification, collagen mutations
INTRODUCTION
Osteogenesis imperfecta (OI) is a heritable disorder of the extracellular matrix of bone, skin, and tendon characterized by osteoporosis, brittle bones, and susceptibility to fracture from minimal trauma [Byers and Cole, 2002; Marini, 2007]. The four types of OI described by the Sillence classification have auto-somal dominant inheritance and encompass about 85–90% of patients with clinical OI. Sillence types I–IV have a broad range of severity and are caused by mutations in COL1A1 or COL1A2, which encode the chains of type I collagen, the major protein of the extracellular matrix of bone and skin [Prockop and Kivirikko, 1995; Byers and Cole, 2002; Marini, 2007]. Mild type I OI is generally caused by mutations that decrease the quantity of collagen deposited in matrix. Perinatal lethal type II, severe progressive deforming type III, and moderately severe type IV OI are caused by structural changes, usually glycine substitutions or exon splicing defects, in the helical region of either collagen alpha chain [Marini et al., 2007b]. More recently, severe and lethal recessive forms of OI (types VII and VIII) have been described and are estimated to cause 5% of OI in North America. The recessive forms are caused by deficiency in one of the collagen modification systems. Null mutations have been identified in the genes coding for two proteins, cartilage-associated protein (CRTAP) and prolyl 3-hydroxylase 1 (P3H1) that form a complex with cyclophylin B in the endoplasmic reticulum which is responsible for 3-hydroxylation of the α1(I)Pro986 residue [Ward et al., 2002; Barnes et al., 2006; Morello et al., 2006; Cabral et al., 2007; Marini et al., 2007a].
In children with severe OI, radiographic studies of lower limb long bones have shown areas of disorganization around the growth plate, with sclerotic lines in the metaphysis and epiphysis. In the 1979 report establishing his classification for OI, Sillence noted that many type III OI patients had a progressive abnormality in the metaphyses of distal femora and proximal tibiae consisting of “whorls of radiodensity” [Sillence et al., 1979]. In 1980, Bullough [Goldman et al., 1980] coined the term “popcorn” to describe a collection of “scalloped radiolucencies, each with a sclerotic margin,” which were noted in a radiological survey of 46 American children with OI. Both Sillence et al. and Goldman et al. noted that the lesions were not present at birth, occurred in severe cases of OI primarily in the lower extremity, obscured the normal lucent line of the growth plate, progressed in abundance with age, and resolved with physeal closure. In the autopsy of an 11-year-old girl with severe OI and radiographic popcorn calcifications, Bullough et al. [1981] described irregular disordered nests of chondrocytes in the growth plate surrounded by soft marrow and minimal spongy bone, and proposed that popcorn calcifications represented growth plate fragmentation, from combined trauma and poor support by surrounding bone, as well as intrinsic disordered maturation of the physis. Both Bullough et al. and Sillence et al. speculated that the metaphyseal/epiphyseal dysplasia resulting in popcorn calcifications might contribute to the severe growth retardation of OI. A decade later, a longitudinal clinical study of over 200 children with OI in Germany [Vetter et al., 1992; Brenner et al., 1993] reported popcorn calcifications in both type III (47% in ages 6–10 years; 75% between 11–20 years) and type IV OI (15% in age 6–10 years; 23% between 11–20 years). A recent survey of 141 Polish children [Sulko, 2005] with OI described popcorn calcifications in 15 of 34 (44%) children with type III OI. Neither European study reported molecular correlations with popcorn calcifications.
We have recently described recessive types VII and VIII OI, caused by deficiency of the components of the collagen prolyl 3-hydroxylation modification complex [Marini et al., 2007a]. Popcorn calcifications have been demonstrated in children with both types VII and VIII OI [Baldridge et al., 2008]. These authors have speculated that popcorn epiphyses may be a distinguishing feature of recessive versus dominant OI. Since causative collagen structural mutations have been identified in many cases of dominant OI [Marini et al., 2007b], it is now feasible to focus on the occurrence of popcorn calcifications specifically in children with documented collagen mutations. The purpose of this study was to determine the incidence of popcorn calcifications in cases of severe and moderate OI with known collagen mutations. In the NICHD pediatric OI cohort, we examined the association of popcorn calcifications with OI type, the extent of growth retardation and the collagen chain in which the mutation is located.
MATERIALS AND METHODS
For this retrospective, non-blinded study of popcorn calcification in OI, children were selected from among participants in the NICHD longitudinal research program in osteogenesis impefecta whose collagen mutations had been identified. All children had multiple admissions spanning 4–20 years per patient under one or more of three NICHD IRB-approved protocols for natural history of OI, or treatment with growth hormone [Marini et al., 2003] or pamidronate [Letocha et al., 2005]. Forty-five children met our criteria of multiple NIH Clinical Center admissions, ≥5 sets of serial radiographs of lower limbs spanning multiple years with open physes, and known mutations in type I collagen. Height age (HA) for OI children was calculated as the age (years) for which their current length would be 50th centile for height on normal growth curves for their gender. Information on administration of pamidronate and growth hormone was derived from patient charts.
Serial lower limb AP radiographs taken at approximately 1-year intervals were examined retrospectively for the occurrence of popcorn calcifications, its location and pattern of onset and progression by two of us (AAO and JCM).Sillence type of OI (types III and IV) was assigned by JCM based on direct clinical examination and radiographs. The NICHD pediatric OI population does not include children with type I OI. For determination of age of onset, we included only the 11 of 15 popcorn calcification positive children for whom a prior unaffected radiograph was available. To examine the association of popcorn calcification progression with changes in bone morphology, metaphyseal flare was measured on serial AP films from six children, as the ratio of metaphyseal width to the narrowest diaphyseal width. Simple comparisons between two samples were by t-test if continuous and by Fisher’s exact test if categorical. To compare the growth deficiency of popcorn positive and negative type III OI children, regression plots were fit to the data for age (CA, chronological age) versus growth deficiency (CA–HA).
The type I collagen mutations of the patients were determined using cDNA sequencing and verification by restriction digestion of PCR products. All mutations were determined in the CLIA-certified laboratory of the BEMB, NICHD, and have been contributed to the OI Mutation Consortium [Marini et al., 2007b].
RESULTS
Among 45 children with autosomal dominant OI caused by structural defects in type I collagen, 15 children were found to have popcorn calcifications at the growth plates of lower limb long bones (Table I). The incidence of popcorn calcification was higher among children with more severe OI, P = 0.004 (Table I). Popcorn calcifications were identified in the serial radiographs of 13 of 25 (52%) children with severe progressive deforming type III OI, but only 2 of 20 (10%) children with type IV OI, the moderately severe form. Furthermore, the two positive type IV children both have phenotypes at the more severe end of the type IV spectrum (OI III/IV) based on subjective criteria of more severe growth deficiency (HA = 3.0–3.5 years at CA = 11–12 years), early scoliosis in patient 14, and more frequent lower limb fractures than expected for type IV OI.
TABLE I.
Incidence of Popcorn Calcification in Lower Limbs of Types III and IV Osteogenesis Imperfecta Children
| (+) Popcorn calcification | (−) Popcorn calcification | Total | CA (year) | HA (year) | CA–HA (year) | |
|---|---|---|---|---|---|---|
| Type III | 13 (52%) | 12 | 25 | 13.99 ± 5.12 | 3.08 ± 1.88 | 10.91 ± 4.90 |
| Type IV | 2 (10%) | 18 | 20 | 13.82 ± 5.50 | 6.51 ± 2.60 | 7.32 ± 5.09 |
| Total | 15 (53%) | 30 | 45 |
HA, height age, that is, age at which patient is currently 50th percentile; CA, chronological age.
There was no relationship between drug treatments received by some of the children and popcorn formation. Eleven of the 15 popcorn positive OI children had received treatment with growth hormone and/or the bisphosphonate pamidronate and had a prior unaffected radiograph available (Table II). These pharmacological interventions began before (four children) or after (seven children) popcorn calcification was first noted and did not appear to stimulate or inhibit the occurrence or progression of popcorn calcifications. Furthermore, 8 of 12 popcorn negative type III OI children also received these drugs.
TABLE II.
Comparison of All Popcorn-Positive Patients With Popcorn-Negative OI Type III Patients
| Stature | Mutation | Age of onset | Tibial popcorn calcification | Growth plate closure | Treatments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OI type | CA (years) | HA (years) | CA–HA (years) | Right femur | Left femur | Drug | Ageb (years) | ||||
| Positive popcorn calcification | |||||||||||
| 1 | III | 19.50 | 4.00 | 15.50 | α1 (I) gly76glu | a | a | + | + | GH/pamidronate | 8.5/9.67 |
| 2 | III | 15.50 | 2.25 | 13.25 | α1 (I) gly193ser | 9 years | 9 years | + | − | GH/pamidronate | 8.5/11.67 |
| 3 | III | 15.83 | 3.75 | 12.08 | α1 (I) gly217ser | 4 years | 5 years | + | + | GH/pamidronate | 8.17/9.33 |
| 4 | III | 17.00 | 5.50 | 11.50 | α1 (I) gly232ser | a | a | + | + | GH/pamidronate | 7.58/12.67 |
| 5 | III | 16.08 | 1.92 | 14.16 | α1 (I) gly286arg | 14 years | 13 years | − | − | Pamidronate | 12.17 |
| 6 | III | 20.92 | 1.42 | 19.50 | α1 (I) Δ E33–36 | a | a | + | + | GH | 8 |
| 7 | III | 10.67 | 3.50 | 7.17 | α1 (I) Δ E41 | 4 years | 4 years | − | − | Pamidronate | 7.67 |
| 8 | III | 8.83 | 3.25 | 5.58 | α1 (I) gly898ser | 7 years | — | − | − | Pamidronate | 6.83 |
| 9 | III | 18.00 | 4.50 | 13.50 | α1 (I) thr1120ile | a | a | + | + | n/a | n/a |
| 10 | III | 17.92 | 1.00 | 16.92 | α2 (I) gly250ser | 5 years | 4 years | + | − | n/a | n/a |
| 11 | III | 18.92 | 3.50 | 15.42 | α2 (I) gly337ser | 10 years | 11 years | − | + | GH/pamidronate | 8.42/15 |
| 12 | III | 14.25 | 2.33 | 11.92 | α2 (I) gly703arg | 6 years | 6 years | − | + | n/a | n/a |
| 13 | III | 21.00 | 3.00 | 18.00 | α2 (I) gly898val | 6 years | 6 years | + | + | GH | 7.75 |
| 14 | IV | 11.08 | 3.25 | 7.83 | α1 (I) gly187ala | 9 years | 5 years | + | − | GH/pamidronate | 5.42/7.08 |
| 15 | IV | 12.17 | 3.50 | 8.67 | α1 (I) gly448ser | — | 5 years | − | + | Pamidronate | 7.5 |
| Negative popcorn calcification | |||||||||||
| 16 | III | 6.25 | 3.25 | 3.00 | α1 (I) gly25val | − | n/a | n/a | |||
| 17 | III | 17.17 | 6.25 | 10.92 | α1 (I) gly154arg | + | Pamidronate | 11.25 | |||
| 18 | III | 4.83 | 0.42 | 4.41 | α1 (I) gly415ser | − | Pamidronate | 3.5 | |||
| 19 | III | 13.25 | 5.00 | 8.25 | α1 (I) gly589ser | − | GH/pamidronate | 7.67/8.67 | |||
| 20 | III | 15.25 | 8.50 | 6.75 | α1 (I) gly844ala | − | Pamidronate | 9.67 | |||
| 21 | III | 8.75 | 2.25 | 6.50 | α1 (I) gly862ser | − | GH/pamidronate | 6.92/6.92 | |||
| 22 | III | 10.50 | 3.50 | 7.00 | α1 (I) gly898ser | − | Pamidronate | 7.17 | |||
| 23 | III | 12.42 | 2.50 | 9.92 | α1 (I) gly997ser | − | GH/pamidronate | 6.42/7.92 | |||
| 24 | III | 6.83 | 1.67 | 5.16 | α2 (I) gly190val | − | Pamidronate | 4.33 | |||
| 25 | III | 8.58 | 0.92 | 7.66 | α2 (I) gly247cys | − | n/a | n/a | |||
| 26 | III | 8.58 | 0.33 | 8.25 | α2 (I) gly247cys | − | n/a | n/a | |||
| 27 | III | 23.00 | 2.50 | 20.50 | α2 (I) gly247cys | + | n/a | n/a | |||
HA, height age, that is, age at which patient is currently 50th percentile; CA, chronological age; GH, growth hormone. n/a, not applicable.
Popcorn calcification already present on earliest available films (Pt 1 at 8.2 years; Pt 4 at 6.3 years; Pt 6 at 11 years; Pt 9 at 13.7 years).
Age at initiation of drug treatment.
Similarly, most children with types III and IV OI have received femoral instrumentation with Bailey–Dubow, Rush or Fassier–Duval intramedullary rods. Among the type III OI children, 16 of 24 popcorn positive femora and 20 of 24 popcorn negative femora received instrumentation (Table III). All except three rod placements occurred prior to the appearance of popcorn because rodding is customarily begun during toddler years. However, Patient 6 developed popcorn but never had a rodding procedure. Patients 9 and 12 developed popcorn in one femur before rodding and in the second femur without ever being rodded. In addition, 23 of 34 type IV OI femora received instrumentation, but did not develop popcorn. Although these data suggest that rodding does not cause popcorn formation, we cannot exclude the possibility that instrumentation might increase the proportion of children with severe OI who develop popcorn because of local damage in susceptible fragile bones.
TABLE III.
Comparisons Within OI Type III Patients
| (+) Popcorn calcification (n = 13) | (−) Popcorn calcification (n = 12) | |
|---|---|---|
| CA (years) | 16.49 ± 3.64 | 11.28 ± 5.23 (P=0.008) |
| HA (years) | 3.07 ± 1.27 | 3.09 ± 2.45 (P=0.98) |
| CA–HA (years) | 13.42 ± 3.95 | 8.19 ± 4.46 (P=0.005) |
| Growth plate closure | 9 | 2 |
| Growth hormone Rx | 7 | 3 |
| Parnidronale Rx | 10 | 8 |
| Femora rodding | 16 | 20 |
| Tibal PCN | 10 (8 bilat, 2 unilat) | — |
| PCN resolution | 9 | — |
HA, height age, that is, age at which patient is currently 50th percentile; CA, chronological age; PCN, popcorn calcification.
Popcorn calcification occurred in the distal femora of all 15 children, and in the proximal tibiae of 10 patients (two cases unilateral, eight bilateral) (Table II). For 11 of the 15 patients with popcorn calcifications, we were able to determine the age and pattern of onset, because of the availability of a negative radiograph within the year prior to first popcorn detection. The mean age of onset for these children was 7.0 years (range of 4–14 years), with progression in quantity and exuberance with time (Fig. 1). Popcorn calcification onset in the femurs was approximately concurrent in many patients, and was first noted bilaterally in 58% of cases. When popcorn calcifications in one femur preceded the other, the onset in the second femur was noted about 1 year later, except for a single patient in whom the interval was 4 years. Ten of the 15 positive children also developed popcorn calcifications in their proximal tibiae. Popcorn calcification onset in tibiae always followed distal femoral onset; popcorn calcifications were never found only in the tibiae. Nine of the 15 patients with femoral popcorn calcifications progressed to physeal fusion (Tables II and III). Consistent with occurrence of popcorn changes at growth plates, the calcifications resolved with fusion and did not recur in subsequent radiographs. Fracture rate also decreases with puberty in severe OI, as it does in both our popcorn-positive and -negative type III OI children. There is also no apparent association of higher fracture incidence with popcorn positive than negative children during the years prior to puberty.
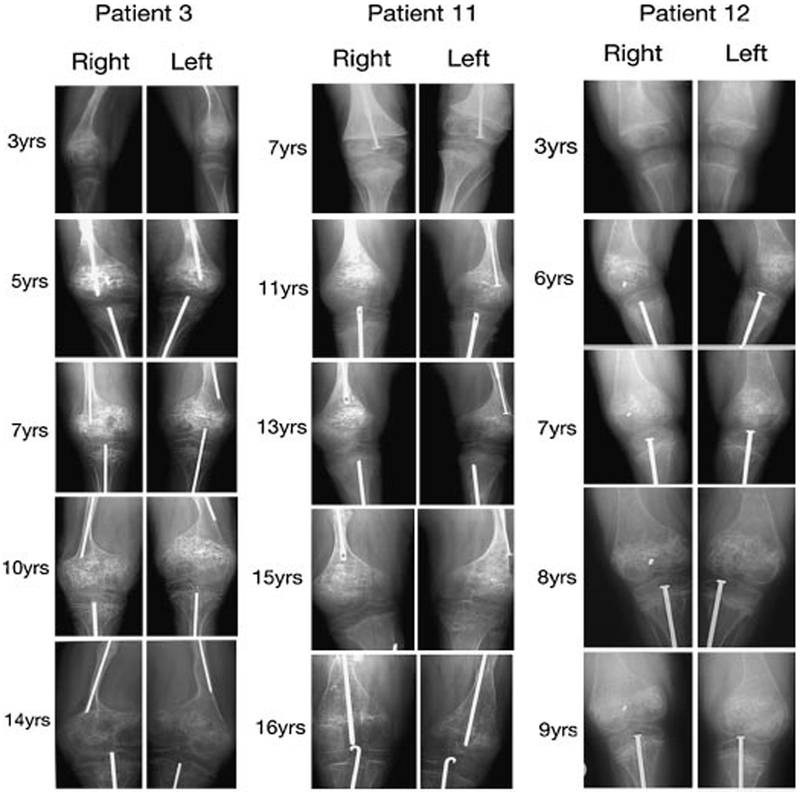
FIG. 1.
Examples of progression of popcorn calcifications over 6–11 years in lower limb radiographs of three patients with osteogenesis imperfecta. Note popcorn development in bilateral distal femora and proximal tibia in patient 3, while popcorn calcification is limited to bilateral distal femora in patients 11 and 12.
All 25 children with type III OI have severe growth deficiency (Table II). We examined whether growth deficiency was greater among the children with popcorn calcifications than those who were radiographically negative. Popcorn positive type III OI children had the same average HA as those without popcorn calcifications (3.1 ± 1.3 years vs. 3.1 ± 2.45 years, respectively, P = 0.98) (Table III). However, the children with popcorn calcifications were significantly older than children whose radiographs were negative (16.5 ± 3.6 years vs. 11.3 ± 5.2 years, respectively, P = 0.008). As a consequence, popcorn positive type III children had a greater discrepancy between CA and HA (CA–HA) than did popcorn negative children (13.4 ± 4 years vs. 8.2 ± 4.5 years, respectively, P = 0.005), However, we were unable to demonstrate a significant difference in the rate of increase of CA–HA between the popcorn positive and negative type III OI children in our study. Regression plots fitted to CA–HA versus age data using the current age of the patients show that growth discrepancy is increasing with age in the popcorn positive group (P < 0.0001), but that the rate of growth discrepancy increase did not differ from the popcorn negative group (P = 0.13).
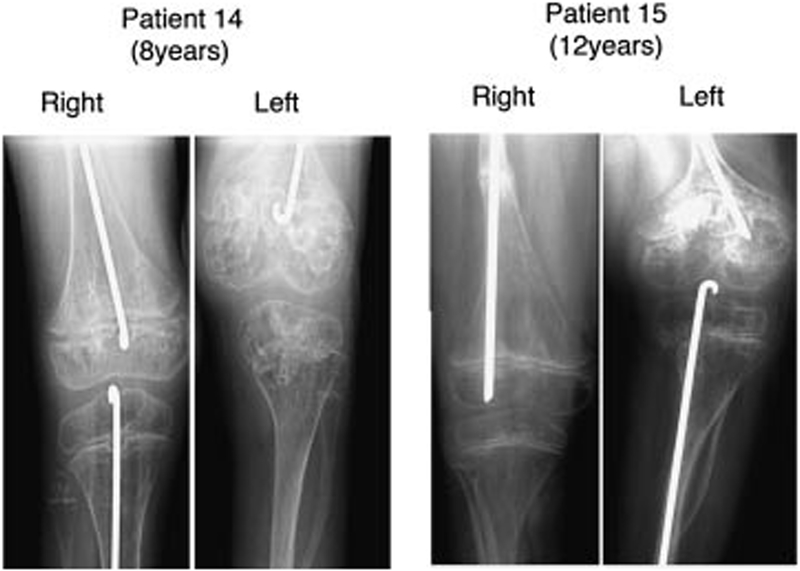
Although popcorn formation is apparently not the major factor associated with severe growth deficiency in type III OI, data from two patients with unilateral femoral popcorn calcifications and one patient with a 4-year interval in popcorn calcification onset between right and left femora demonstrated an effect on femoral growth (Fig. 2). Patient 8 has popcorn only in her right distal femur, with onset almost 2 years ago; she currently has a 3 cm leg length discrepancy (L > R). Patient 14 had a 4-year delay between popcorn calcification onset in left and right distal femora and has a 5 cm leg length discrepancy (R > L) at age 8 years. Patient 15, who has popcorn in his left femur, has a 5 cm leg length discrepancy (R > L) after epiphyseal closure.
FIG. 2.
Leg length discrepancy associated with popcorn calcification in osteogenesis imperfecta. Patient 14 had a 4-year time lag between onset of popcorn calcifications in left and right femur resulting in R > L femur length. Note beginning of popcorn calcification in proximal left tibia. Patient 15 had unilateral onset of popcorn in left femur resulting in R > L femur length.
We sought to determine whether the disruption of the orderly columns in the growth plate by popcorn calcifications was associated with a lateral increase in metaphyseal diameter, as compared to popcorn negative patients. We examined serial changes in femoral metaphyseal flare, the ratio of the largest metaphyseal diameter to the narrowest diaphyseal width on an AP radiograph, in type III OI children (three each, popcorn positive and negative). The change in metaphyseal flare did not differ between the groups; both groups had increasing flare over 4–6 years, by 30–50% above the baseline ratio.
In type III OI, popcorn calcification occurred in patients with mutations in either COL1A1 or COL1A2. There was no preferential association of popcorn with mutations located in one of the collagen chains (Table IV). Popcorn calcifications occurred in 53% of patients with α1(I) and 47% of patients with α2(I) mutations. The two positive type IV children both have α1(I) mutations. Mutations associated with popcorn calcifications are distributed evenly along the collagen chains (Table II). Genotype–phenotype correlations are generally, but not totally consistent. Three children with α2(I) gly247cys are all popcorn negative. In contrast, one of two children with α1(I) gly898ser, who is now almost 9 years old, developed popcorn by age 7 years, while a second child with the same mutation remains popcorn negative at age 10.5 years. It is possible that the second child might still develop popcorn, since we noted onset in other children as late as 14 years of age.
TABLE IV.
Comparison of COL1A1 versus COL1A2 Mutations in OI Type III Patients
| (+) Popcorn calcification | (−) Popcorn calcification | All patients | |
|---|---|---|---|
| OI type III patients | |||
| COL1A1 | 9 (53%) | 8 | 17 |
| COL1A2 | 4 (50%) | 4 | 8 |
| Total | 13 (52%) | 12 | 25 |
| OI Type IV patients | |||
| COL1A1 | 2 (29%) | 5 | 7 |
| COL1A2 | 0 | 13 | 13 |
| Total | 2 (10%) | 18 | 20 |
DISCUSSION
The original Sillence classification for OI described popcorn calcifications in the lower limb long bones of children with progressive deforming type III OI [Sillence et al., 1979]. Subsequently, the incidence of popcorn calcifications in severe OI and its location in distal femoral and proximal tibial long bones were delineated [Goldman et al., 1980; Sillence et al., 1986]. German and Polish pediatric OI studies corroborated the occurrence of popcorn calcifications in about half of children with type III OI [Vetter et al., 1992; Brenner et al., 1993; Sulko, 2005]. However, in previous studies, the mutations causing OI in the patients were unknown.
Our study revisits popcorn calcifications in the new context of causative mutations for OI, both dominant OI in type I collagen and recessive OI in CRTAP and LEPRE1/P3H1. Recessive OI has recently been molecularly delineated from dominant OI, and shown to be caused by defects in collagen prolyl 3-hydroxylation [Barnes et al., 2006; Morello et al., 2006; Cabral et al., 2007; Marini et al., 2007a]. Popcorn calcifications have been noted in both types VII and VIII OI [Baldridge etal., 2008],leading the authors to propose that the occurrence of popcorn calcifications might delineate the subset of severe OI cases with recessive inheritance. To add molecular genetic correlations to popcorn calcifications in OI, we selected 45 children from the NICHD Pediatric OI Research Cohort, who met the criteria of having type III or IV OI, serial lower limb radiographs and structural mutations identified in COL1A1 or COL1A2. Among these children with known collagen mutations, we found essentially the same prevalence and progression of popcorn calcifications as described two decades ago. About half of the children in our cohort with type III OI and a documented collagen mutation have popcorn calcifications. This is a conservative estimate of the incidence of popcorn in type III OI, since six children in the popcorn-negative group are less than 10 years old and might develop popcorn in the future. Among type III children in our cohort who are greater than 10 years old, 14 of 20 (70%) had developed popcorn. Popcorn calcification was infrequent in type IV OI but does occur at the more severe end of the clinical spectrum.
Popcorn formation occurs in distal femora, and subsequently in most proximal tibiae. The mean age of onset in our population was 7 years old, beginning in both right and left femora at about the same time, or within a year of each other, with one exception. Popcorn calcification progresses in exuberance during the growing years, then resolves with physeal closure. Although most children in our cohort were treated with rGH or bisphosphonates [Marini et al., 2003; Letocha et al., 2005], these drugs do not appear to have altered the incidence, age of onset or progression of popcorn calcifications. However, our data do not allow us to comment on whether treatment with pamidronate prior to age 4 years would have any effect on the natural history of popcorn calcifications.
The occurrence of disorganized calcifications around the growth plate in severe OI led to the suggestion of a causal relationship with OI growth deficiency. The histological appearance of a disrupted growth plate from a child with popcorn calcifications, showing irregular, disorganized nests of chondrocytes, supported this suggestion [Bullough et al., 1981]. Among our 25 children with type III OI, severe growth deficiency occurred in all children regardless of the presence of popcorn calcifications. We were not able to demonstrate a significant difference in rate of growth deficiency or metaphyseal flare in children with and without popcorn calcifications. Thus, popcorn calcifications do not appear to be the major mechanism for the extreme growth deficiency of type III OI, nor to be responsible for the lateral expansion of the metaphyses. However, the higher proportion of younger children in the popcorn-negative group may have limited our ability to detect significant differences in growth deficiency between the popcorn-positive and -negative groups. Two findings suggest that popcorn is likely to be a contributory factor to OI growth deficiency. First, the older popcorn-positive group has the same average height as the younger popcorn-negative children. Second, three children with unilateral femoral popcorn calcifications or delay in onset in the contralateral femur have 3–5 cm leg length discrepancies, with popcorn calcifications in the shorter leg, supporting a contributing role in growth deficiency.
We found popcorn calcifications in about the same percentage of our type III OI patients with collagen mutations, as reported in prior cohorts without identified mutations. Popcorn formation showed no preference for the chain in which the mutation was located, with about equal incidence in COL1A1 and COL1A2. There was also no preference for the position of glycine substitution along the chain associated with popcorn, and there was general, although not complete, consistency of genotype and popcorn calcifications. In conclusion, popcorn calcifications, which are radiological changes in the metaphyses and epiphyses of limb long bones representing disruption of the growth plate, occur in severe cases of OI with both collagen structural (primarily type III OI) and modification defects (type VIII OI) and cannot be used to distinguish dominant and recessive OI forms.
ACKNOWLEDGMENTS
We would like to thank Mona K. Abukhaled, MSN, CRNP for assistance with data collection for this project. We would also like to thank Ms Janet Stevens and Aileen Barnes, MS for their assistance in assembling radiographic images into figures. This research was supported by NICHD Intramural Funds to JCM. AAOwas supported by the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer, Inc. via a grant to the Foundation for NIH from Pfizer, Inc.
Grant sponsor: NICHD Intramural Funds; Grant sponsor: NIH; Grant sponsor: Pfizer, Inc.
REFERENCES
- Baldridge D, Schwarze U, Morello R, Lennington J, Bertin TK, Pace JM, Pepin MG, Weis M, Eyre DR, Walsh J, Lambert D, Green A, Robinson H, Michelson M, Houge G, Lindman C, Martin J, Ward J, Lemyre E, Mitchell JJ, Krakow D, Rimoin DL, Cohn DH, Byers PH, Cole WG, Lee B. 2008. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta Hum Mutat (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AM, Chang W, Morello R, Cabral WA, Weis M, Eyre DR, Leikin S, Makareeva E, Kuznetsova N, Uveges TE, Ashok A, Flor AW, Mulvihill JJ, Wilson PL, Sundaram UT, Lee B, Marini JC. 2006. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med 355: 2757–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner RE, Schiller B, Pontz BF, Lehmann H, Teller WM, Spranger J, Vetter U. 1993. Osteogenesis imperfecta in childhood and adolescence. Monatsschr Kinderheilkd 141: 940–945. [PubMed] [Google Scholar]
- Bullough PG, Davidson DD, Lorenzo JC. 1981. The morbid anatomy of the skeleton in osteogenesis imperfecta. Clin Orthop Relat Res 159:42–57. [PubMed] [Google Scholar]
- Byers PH, Cole WG. 2002. Osteogenesis imperfecta In: Royce PM, Steinman B, editors. Connective tissue and its heritable disorders. 2nd edition New York: Wiley-Liss, Inc. p 385–430. [Google Scholar]
- Cabral WA, Chang W, Barnes AM, Weis M, Scott MA, Leikin S, Makareeva E, Kuznetsova NV, Rosenbaum KN, Tifft CJ, Bulas DI, Kozma C, Smith PA, Eyre DR, Marini JC. 2007. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet 39:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AB, Davidson D, Pavlov H, Bullough PG. 1980. “Popcorn” calcifications: A prognostic sign in osteogenesis imperfecta. Radiology 136:351–358. [DOI] [PubMed] [Google Scholar]
- Letocha AD, Cintas HL, Troendle JF, Reynolds JC, Cann CE, Chernoff EJ, Hill SC, Gerber LH, Marini JC. 2005. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res 20:977–986. [DOI] [PubMed] [Google Scholar]
- Marini JC. 2007. Osteogenesis imperfecta In: Kliegman RM, Behrman RE, Jenson HB, Staton BF, editors. Nelson textbook of pediatrics. 18th ed Philadelphia: Saunders; p 2887–2890. [Google Scholar]
- Marini JC, Hopkins E, Glorieux FH, Chrousos GP, Reynolds JC, Gundberg CM, Reing CM. 2003. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: High predictive value of the carboxyterminal propeptide of type I procollagen. J Bone Miner Res 18:237–243. [DOI] [PubMed] [Google Scholar]
- Marini JC, Cabral WA, Barnes AM, Chang W. 2007a. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle 6:1675–1681. [DOI] [PubMed] [Google Scholar]
- Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, Hyland JC, Korkko J, Prockop DJ, De Paepe A, Coucke P, Symoens S, Glorieux FH, Roughley PJ, Lund AM, Kuurila-Svahn K, Hartikka H, Cohn DH, Krakow D, Mottes M, Schwarze U, Chen D, Yang K, Kuslich C, Troendle J, Dalgleish R, Byers PH, Cole WG. 2007b. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: Regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat 28:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello R, Bertin TK, Chen Y, Hicks J, Tonachini L, Monticone M, Castagnola P, Rauch F, Glorieux FH, Vranka J, Bachinger HP, Pace JM, Schwarze U, Byers PH, Cole WG, Weis M, Fernandes RJ, Eyre DR, Yao Z, Boyce BF, Lee B. 2006. CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell 127:291–304. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI. 1995. Collagens: Molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64:403–434. [DOI] [PubMed] [Google Scholar]
- Sillence DO, Senn A, Danks DM. 1979. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 16:101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence DO, Barlow KK, Cole WG, Dietrich S, Garber AP, Rimoin DL. 1986. Osteogenesis imperfecta type III. Delineation of the phenotype with reference to genetic heterogeneity. Am J Med Genet 23:821–832. [DOI] [PubMed] [Google Scholar]
- Sulko J 2005. Osteogenesis imperfecta–lower limb in osteogenesis imperfecta. Chir Narzadow Ruchu Ortop Pol 70:185–187. [PubMed] [Google Scholar]
- Vetter U, Pontz B, Zauner E, Brenner RE, Spranger J. 1992. Osteogenesis imperfecta: A clinical study of the first ten years of life. Calcif Tissue Int 50:36–41. [DOI] [PubMed] [Google Scholar]
- Ward LM, Rauch F, Travers R, Chabot G, Azouz EM, Lalic L, Roughley PJ, Glorieux FH. 2002. Osteogenesis imperfecta type VII: An autosomal recessive form of brittle bone disease. Bone 31:12–18. [DOI] [PubMed] [Google Scholar]