Regenerative medicine holds promise for the treatment of degenerative and genetic diseases. One goal is to rejuvenate damaged tissue by establishing processes for the transplantation of pluripotent or multipotent stem cells, which are able to differentiate into a wide range of cell types. Challenges to cell delivery include the need for invasive procedures to collect autologous cells, the tissue-specific uptake of cells, and the rejection of heterologous cells. In disorders such as osteo-arthritis, chondrocytes buried in large amounts of extracellular matrix complicate the problem of vascular access. New data from Johnson and colleagues1 show that endogenous progenitor cells can be stimulated by a small heterocyclic compound called kartogenin to differentiate and proliferate as matrix-producing chondrocytes that can spur the repair of osteoarthritis, thus bypassing the challenges of stem-cell delivery.
Osteoarthritis, a degenerative condition of articular cartilage, is caused by mechanical stress, ligamentous injuries, and genetic abnormalities affecting cartilage and bone. The protective cartilage cap is eroded, resulting in pain, inflammation, and loss of joint function; at the same time, cartilage matrix proteins, such as type II collagen peptides and metalloproteinases, are released into the serum.2 Johnson and colleagues have adapted an approach to stem-cell stimulation and differentiation that involves screening a library of more than 20,000 heterocyclic small molecules, compounds that mimic natural ligands involved in cell signaling and differentiation. One molecule, kartogenin, was able to promote the differentiation of human mesenchymal stem cells into cartilage nodules in vitro. The nodules had markers that were specific for cartilage, such as type II collagen and aggrecan; just as important, they did not have markers for hyper-trophic chondrocytes or bone cells. Long-term culture of differentiated cells with kartogenin did not trigger the production of enzymes known to degrade matrix, such as matrix metalloproteinases, but instead increased the level of protective tissue inhibitors of metalloproteinase. The authors also observed that kartogenin partially suppressed the release of both proinflammatory nitric oxide and the glycosaminoglycan by-products of tissue destruction in articular chondrocyte cultures and cartilage explants.
Johnson and colleagues went on to test the intra-articular injection of kartogenin in two different mouse models of osteoarthritis. In one model, osteoarthritis was induced with a collagenase injection to mimic chronic joint injury, and in the other it was induced by severing the knee ligament to model acute injury. Both mouse models showed regeneration of articular cartilage over the course of several weeks, a reduction in serum levels of the products of cartilage breakdown, and improved weight bearing. Kartogenin thus would seem to have both a regenerative and a protective effect. The amount of kartogenin absorbed from the intraarticular space into serum was estimated to be 0.1% of the intraarticular dose, a level that is consistent with negligible systemic toxic effects.
To get a handle on the intracellular events triggered by kartogenin in the mesenchymal stem cells and chondrocytes, the authors synthesized an analog of the compound, with an extension on one end that facilitated the cross-linking of kartogenin with its binding partners and a biotin extension on the other end that would allow the cross-linked complex (and thus the binding partner) to be detected on gels. Through this procedure, the researchers identified the cytosolic protein filamin A, known to bind actin and connect the intracellular filamentous network to cell-membrane proteins, as a ligand of kartogenin. They proceeded to determine the precise part of filamin A to which kartogenin binds.
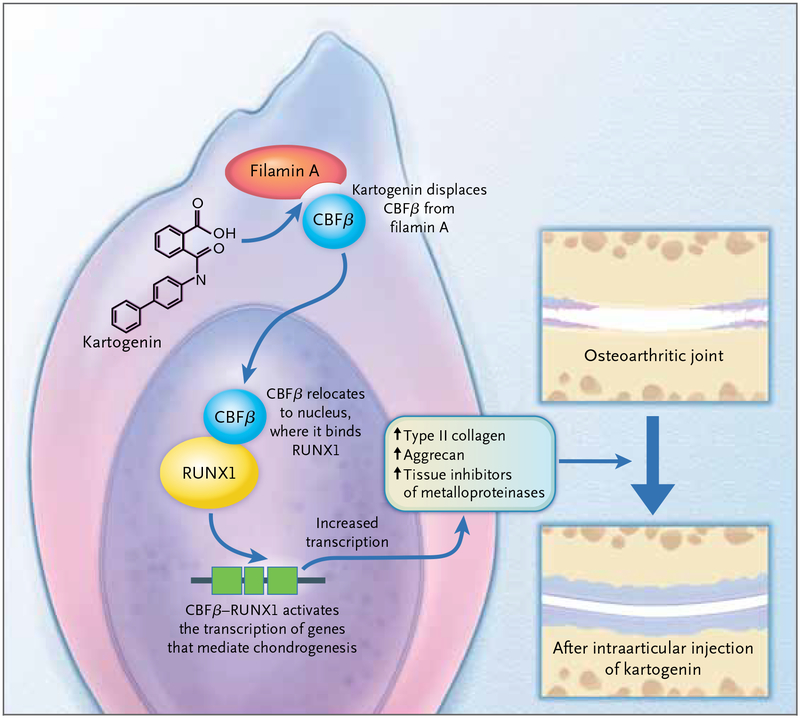
However, a key binding partner of human filamin A, a transcription factor called core-binding factor β (CBFβ), normally binds to the site on filamin A that also binds kartogenin.3 The extent of binding between filamin A and CBFβ regulates an established differentiation pathway; Johnson and colleagues proceeded to show in vitro, in human mesenchymal stem cells, that this pathway is activated by kartogenin. Filamin A retains CBFβ in the cytoplasm during rest. When CBFβ is activated, it dissociates from filamin A and translocates into the nucleus; the competitive binding of kartogenin to filamin A displaces CBFβ, increasing the amount of CBFβ available to enter the nucleus and dimerize with the DNA-binding transcription factor RUNX1 (Fig. 1). Together, the CBFβ–RUNX1 complex activates genes that are important for the differentiation of mesenchymal stem cells into cartilage. The researchers went on to show that decreasing levels of CBFβ or RUNX1 eliminates the differentiation effect of kartogenin, which indicates that both factors are essential to the pathway activated by this heterocyclic compound.
Figure 1. Cartilage Regeneration.
Johnson and colleagues1 recently described data from mouse models supporting the theory that the intraarticular injection of a small heterocyclic molecule, kartogenin, differentiates endogenous mesenchymal stem cells into cartilage-producing chondrocytes. According to in vitro experiments with human mesenchymal stem cells, kartogenin binds to the carboxyl end of filamin A, displacing CBFβ from its cytoplasmic binding site. CBFβ is thus freed Draft 6 to enter the nucleus, where it binds to the DNA-binding transcription factor RUNX1. The CBFβ–RUNX1 complex activates the transcription of proteins involved in cartilage differentiation, increases the synthesis of components 3 6/11/12 of the cartilage matrix (such as collagen type II, aggrecan, and tissue inhibitors of metalloproteinases), and protects cartilage against stress factors.
Cell-based regenerative therapies that use en dogenous stem cells have attractive features — a perfect genetic match, optimal localization, and nonsystemic application. Stimulating the differentiation of one’s own stem cells by means of an easily deliverable chemical compound would be more advantageous than using conventional drilling and microfracture techniques to stimulate endogenous stem cells for cartilaginous legions or grafting autologous cells onto a scaffold. It is easy to imagine the preventive articular application of kartogenin after an acute ligament tear or its restorative application for chronic osteoarthritis.
Kartogenin is not the first chemically synthesized small molecule to influence adult stem-cell differentiation. Another small molecule has been shown to stimulate the differentiation of hippocampal neural progenitor cells by inhibiting the progression of the cell cycle.4 Other types of small molecules, such as certain small RNA molecules (called RNA aptamers), which interfere directly with transcription factors, may also be used to regulate transcription and affect differentiation. These chemical tools would seem particularly useful for targeting tissues in which implantation is difficult, such as bone and neural tissue. These compounds might also prove helpful in the treatment of genetic conditions, which would require the transplantation of corrected autologous stem cells. The use of stem-cell transplantation in mouse models of osteogenesis imperfecta has improved bone mechanics despite very low level of cell engraftment in the bone compartment.5 Perhaps supplementing this approach with a small molecule, such as kartogenin, would enhance the success of transplantation by stimulating the differentiation and proliferation of the engrafted cells.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Johnson K, Zhu S, Tremblay MS, et al. A stem cell-based approach to cartilage repair. Science 2012. April 5 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 2.Hunter DJ. Pharmacologic therapy for osteoarthritis — the era of disease modification. Nat Rev Rheumatol 2011;7:13–22. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida N, Ogata T, Tanabe K, et al. Filamin A-bound PEBP2β/CBFβ is retained in the cytoplasm and prevented from functioning as a partner of the Runx1 transcription factor. Mol Cell Biol 2005;25:1003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wurdak H, Zhu S, Min KH, et al. A small molecule accelerates neuronal differentiation in the adult rat. Proc Natl Acad Sci U S A 2010;107:16542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panaroni C, Gioia R, Lupi A, et al. In utero transplantation of adult bone marrow decreases perinatal lethality and rescues the bone phenotype in the knockin murine model for classical, dominant osteogenesis imperfecta. Blood 2009;114:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]