Abstract
This article primarily represents the contributions of two young investigators to the understanding of the neuropsychological consequences of epilepsy and its treatment. The authors have reviewed two key areas of importance: the complex relationship between cognitive dysfunction and epilepsy and the risks of cognitive dysfunction in children as a consequence of in utero exposure to antiepileptic drug treatment. The work of two young investigators is presented and future research needs are outlined.
Keywords: Epilepsy, Cognitive dysfunction, Pregnancy, Neurodevelopment, Antiepileptic drugs
1. Neurodevelopmental effects of fetal antiepileptic drug exposure
1.1. Gus A. Baker
1.1.1. Introduction
The majority of women with epilepsy (WWE) require antiepileptic drugs (AEDs) throughout pregnancy. Although the majority of pregnancies result in healthy babies [1], there is an increased risk of congenital malformations and a risk of poorer cognitive functioning as a consequence of exposure in utero to AEDs; which are known teratogens [2]. Consequently there is an established need to understand the teratogenic risks of all AEDs.
In an area typically dominated by physical outcomes, the effects of exposure in the womb to AEDs on neurodevelopment or cognition constitute an area of research that is gathering momentum [3]. Historically, pregnancy registers have been formed to monitor the incidence of congenital malformations [4] and have, over the years, provided reliable information regarding the incidence of major congenital malformations. Over the last 10 years there has been a welcomed twist toward investigation of the risk posed to cognitive development by in utero exposure to AEDs.
1.1.2. Published research
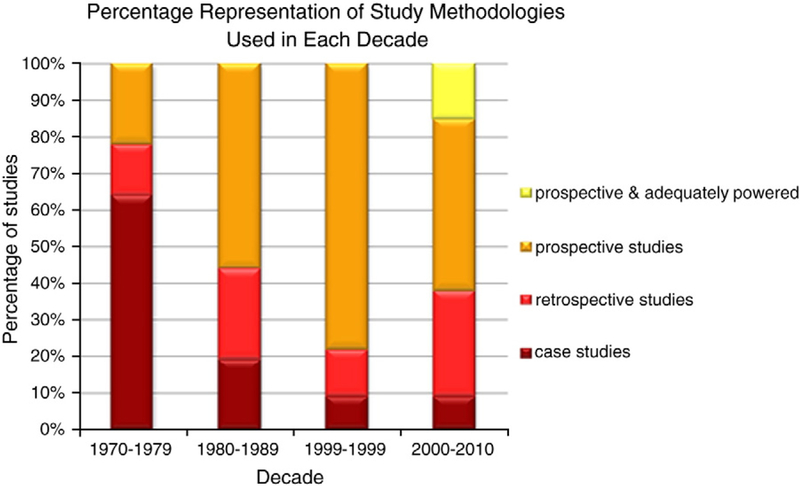
Early studies documented the potential teratogenic effects of AEDs in the 1960s and 1970s, with some documenting increased levels of “development delay” [5]. According to Bromley [3], there have been in excess of 80 observational studies and case reports conducted over the last 25 years commenting on, although not always formally assessing, the cognitive abilities of children born to WWE. Over time the typical methodology has increasingly moved from case series reports and retrospective studies to large well-designed prospective studies (Fig. 1).
Fig. 1.
Study methodologies employed to investigate in utero exposure to AEDs over the last three decades. Republished with permission [6].
Published information regarding the cognitive effects of in utero exposure to phenytoin and/or phenobarbital has been conflicting probably because of methodological differences and the combination of these two groups in some reports. More instances of global cognitive impairment (defined as an IQ ˂ 70) have been reported [5,7], whereas others have failed to find an effect [8]. A small number of cohorts of adolescents and adults with a history of prenatal exposure to phenytoin and/or phenobarbital suggest difficulties are present and persist [9].
Carbamazepine has been investigated by more prospective observational studies than any other AED. In a Finnish prospective study, a history of prenatal exposure to carbamazepine did not have a significant impact on IQ in children aged between 5 and 9 years [10]. A lack of significant influence of carbamazepine on cognitive development and functioning was replicated in the Liverpool and Manchester Neuro development Group’s prospective study [11] and by Wide and colleagues [12]. Recently, however, findings from the NEAD study have raised questions in relation to higher doses [13]. Further, a recent study reported significantly increased rates of below-average performance for the children exposed to carbamazepine [14], though no information was presented regarding the dose of carbamazepine and the age of the children in the group exposed to carbamazepine ranged from 8 to 90 months, which may have introduced bias.
A picture consistent with that of major congenital malformations is beginning to emerge that highlights exposure to sodium valproate as associated with the largest risk for later impairment of cognitive functioning. A relatively large retrospective study by the Liverpool and Manchester Neurodevelopment Group found a significant association between in utero exposure to sodium valproate and poorer Verbal IQ [15]. The Liverpool and Manchester Neurodevelopment Group’s prospective study found a significant impact of sodium valproate exposure that was not attributed to confounding factors in children under the age of 2 years [11]. The NEAD study group1 found that in utero exposure to sodium valproate significantly impacted the IQ of children aged 3 in comparison to children exposed to carbamazepine, lamotrigine, and phenytoin [16]. Prospective data from the Indian Pregnancy Register [17] and the observational study by Cummings and colleagues from the UK Pregnancy and Epilepsy Register [14] replicated findings of poorer early development following in utero exposure to sodium valproate.
With changes in prescribing practices, research investigating exposure to lamotrigine and levetiracetam is emerging. IQ in children exposed to lamotrigine is reported to be significantly higher than that in children exposed to sodium valproate [11,14,16], but more research is needed to draw conclusions. Collaboration between the UK Pregnancy and Epilepsy Register and the Liverpool and Manchester Neurodevelopment Group has shown that in utero exposure to levetiracetam is associated with better cognitive performance in children under the age of 2 years in comparison to children exposed to sodium valproate in utero [18].
1.1.3. Criticism of published research and research to be undertaken in the future
As the number of prospective studies increases, the results become more harmonious, particularly with respect to in utero exposure to sodium valproate. The previous lack of consistency over the risk AED exposure poses to the cognitive development of the child was probably due to methodological differences. According to Nicolai and colleagues [19], the majority of research conducted and published prior to 2008 could be criticized on the following grounds: selection bias, ill-defined terms, inadequate power, absence of a control group, failure to account for confounding variables, and inappropriate outcome measures. On this basis, a number of recommendations have been made (see Table 1).
Table 1.
Recommendations for future research proposed by Nicolai and colleagues [19].
| 1. Studies should be prospective and longitudinal. |
| 2. Women should be included in the study before conception. |
| 3. Compliance should be monitored throughout pregnancy and dose changes should be monitored. |
| 4. Serum levels should be monitored. |
| 5. Maternal use of tobacco, alcohol and other drugs should be recorded. |
| 6. Use of folic acid should be recorded. |
| 7. Each hospital visit and injury should be recorded. |
| 8. Seizure diaries should be kept. |
| 9. Mothers with seizures during pregnancy should not be excluded. |
| 10. Subjects should be recruited by random population-based selection through pregnancy registers. |
| 11. Monotherapy or specific polytherapy combinations should be included. |
| 12. Mothers with medical complications and children who develop epilepsy should be excluded. |
| 13. Comparisons across AED types should be made rather than in comparison to a general population control group. |
| 14. Information on parental IQ and educational level should be collected. |
| 15. Outcomes with sufficient psychometrics should be used. |
It is proposed here that future research should consider further points in addition to those listed in Table 1: different AEDs should not be combined, the assessments should consider more than simply IQ, and the follow-up should extend throughout childhood. Differential out-comes have been reported for different AEDs [10–14,16–18]; combining different AED types into a single group for analysis may result in unreliable outcomes. IQ is considered an aggregate score comprising of a number of cognitive functions (e.g., attention, language, processing speed, memory, reasoning, inhibition) [20]. It should not be assumed that AEDs will impact all aspects of cognitive functioning and that certain functions are not more affected than others. As noted below, in the section by Bromley, the documentation of specific cognitive abilities provides a more comprehensive and reliable understanding of the implications posed by AED exposure in utero. Further, the age of the children should be considered carefully as certain cognitive skills may not be assessed reliably until school age or even later, leading to premature conclusions when based on young cohorts [21].
The majority of the recommendations by Nicolai and colleagues are reasonable and should be implemented in future research. The recommendation, however, that WWE should be recruited prior to pregnancy will likely lead to an unrepresentative sample because of the number of pregnancies that are unplanned. It is acknowledged that data pertaining to paternal IQ and education as well as maternal IQ and education is important, but it poses practical difficulties. Parental IQ influences child development through both genetic and environmental pathways, but the levels of contact a father has with his children will vary from family to family and, in some cases, his identity may be unknown. Finally, the use of AED comparisons is useful but may lead to unreliable conclusions if there is no control group representative of the general population. For example, the NEAD study [13,16] provides useful information about sodium valproate, but is unable to provide information on other AEDs other than they are associated with better outcomes than sodium valproate. The utilization of a general population control group is necessary for investigation of milder deficits.
Significant methodological progress has been made with more recent studies that have produced reliable and valid information. Gaily et al. [10], Wide et al. [12], Meador et al. [13,16], the Liverpool and Manchester Neurodevelopment Group [3,11], the Kerala Registry [17], and the UK Epilepsy and Pregnancy Registry [14,18] have all published prospective data with adequate numbers to detect differences in neuropsychological functioning. Although Meador et al. [13,16] and Thomas et al. [17] did not include a control group, they provided comparisons between AED types and control for potential confounding variables, but were unable to comment on milder effects that may be associated with AED exposure. The NEAD group, in particular, has drawn useful conclusions pertaining to doses of different monotherapy AEDs [13]. The Liverpool and Manchester Neurodevelopment Group (outlined in detail below), Gaily et al. [10], and Wide et al. [12] report results in comparison to a control group representative of the general population and report no effect of exposure to carbamazepine in utero. The collection of neuropsycho-logical data from children enrolled into Pregnancy and Epilepsy Registries has shown that it is a useful method by which to ascertain prospective cohorts [14,17,18], although they should not be prioritized above prospective observational studies in which the main aim is to document cognitive development over the childhood years. The follow-up of children to assess cognitive development is expensive and practically challenging, but neither of these should be taken as impossible barriers.
1.1.4. Summary
The need for further high-quality research on currently prescribed AEDs is warranted if WWE are going to be provided with reliable and valid information about the risks for their unborn children. Current knowledge suggests that sodium valproate carries the largest risk to cognitive development and functioning. Future research should be prospective and longitudinal, include a control group, be adequately powered, consider individual AED groups, document AED dose changes and seizure frequency, use standardized neuropsychological methods with up-to-date normative samples, follow up until at least school age, be completed by qualified assessors blind to the AED exposure type, and aim to document abilities in individual domains as well as IQ. Finally, the analysis should seek to statistically control for the influence of confounding factors [3].
2. Promising Areas of Research and Young Investigators
2.1. Rebecca L. Bromley
Cognitive and neurodevelopmental effects of antiepileptic drugs: Cognitive outcomes in the child
2.1.1. Summary of research
The Liverpool and Manchester Neurodevelopment Group has centered on the consequences of in utero exposure to AEDs. The particular focus for myself surrounds the neurodevelopmental and cognitive consequences posed by in utero exposure. Historically, this area of research has focused on the physical development of the child, with a few exceptions. Only recently have the risks to cognitive development begun to become apparent in studies with improved methodology.
My doctoral research comprised a prospective study assessing the cognitive development of children born to WWE and women without epilepsy. Four main AED groups were recruited from antenatal clinics in the northwest of England and reflected prescribing practices at the time (mainly carbamazepine, lamotrigine, sodium valproate, and polytherapy). Details regarding maternal epilepsy, lifestyle, IQ, and socioeconomic status were collected and considered within the analysis [3,11].
A significant risk of major congenital malformations was found for children exposed to sodium valproate in utero (11%, OR= 5.94, 95% CI = 1.84–19.19) [1]. Results pertaining to the children’s early cognitive development revealed that children exposed to sodium valproate during pregnancy had a significantly poorer level of cognitive development, in comparison to both the control children and the children exposed to other AEDs. Exposure to sodium valproate was causally related to child assessment scores when confounding factors were controlled for [11]. An increased number of children exposed to sodium valproate (29%, RR= 3.6, 95% CI 1.7–7.5) fell below the average range, indicating that the differences are not merely statistical, but represent a clinically relevant decrease in cognitive development and functioning [11].
At 6 years of age, children in this cohort were reassessed with a comprehensive battery informing on IQ, language, memory, learning, and attentional abilities. Consistent with the early development results, school-aged children exposed to sodium valproate in utero differed significantly from control children across all cognitive assessments (the exception being visual attention), with an increased number falling below the average range (36–46% depending on the cognitive skill) [3]. Significantly more children exposed to sodium valproate in utero required educational support (34%) and speech and language therapy (46%). In addition to poorer cognitive abilities, our program of research has also documented an increased risk of autistic spectrum disorders (ASD) in the children exposed to sodium valproate [22], which is consistent with the findings of retrospective research [23,24] and animal data [25,26].
Carbamazepine or lamotrigine exposure in utero was not found to be associated with poorer cognitive development or ASD [3,11,22]. The exception was that in utero exposure to carbamazpine was associated with poorer general memory performance. These results were reported as an interim analysis [3], with the full cohort results expected in 2011.
Recently, the Liverpool and Manchester Neurodevelopment Group collaborated with the UK Epilepsy and Pregnancy Registry to investigate the cognitive development of children exposed to levetiracetam in utero. The cognitive abilities of the children exposed to levetiracetam under 2 years of age did not differ from those of the control children and were significantly higher in comparison to those of the children exposed to sodium valproate in utero [18].
2.1.2. Research Implications
Our research findings have a number of clinical and research implications.
2.1.2.1. Documentation of the cognitive abilities of children exposed to antiepileptic drugs.
Our research has highlighted that there is an increased risk of cognitive difficulties, particularly for children exposed to sodium valproate. The results provide evidence that the detrimental effects of certain AEDs on early neuronal development [27] likely translate into clinically measurable cognitive difficulties in the child. It is apparent from our program of research that as the cognitive demand increases, the discrepancy between the children exposed to sodium valproate and their peers increases [3]. However, questions in relation to the other AEDs remain more difficult to answer.
2.1.2.2. Increased incidence of cognitive difficulties.
The increased frequency of children with cognitive difficulties is larger than that reported for major congenital malformations following exposure in utero. In our prospective cohort, for example, 11% of children exposed to sodium valproate were found to have major congenital malformations, but under 2 years of age, 29% scored outside of the average range for their early development. At 6 years of age, this had increased to 36% for global cognitive ability and around 46% for attentional and memory abilities [3]. It is noted that the frequency of below-average cognitive ability is higher in the background population than major congenital malformations, but what is highlighted here is that more children are likely to present with cognitive difficulties than major congenital malformations following exposure to sodium valproate.
2.1.2.3. Are the group differences clinically meaningful?
An increased incidence of children scoring below the average range indicates that our results represent more than statistical differences between group means [11]. Although the incidence of formal intellectual disability (IQ ˂ 70) is lower than reported in the retrospective literature [15], a significantly increased number of children exposed to sodium valproate had a global cognitive ability score ˂ 84. Formal psychometric scores less than 1 SD lower than the mean are associated with poorer educational achievements that will impact occupational attainment [21] and, therefore, have lifelong consequences. Cognitive difficulties also translate into increased rates of educational support and speech therapy.
2.1.2.4. A more comprehensive understanding of the cognitive domains affected.
The assessment of specific cognitive domains rather than IQ in isolation provides a more reliable and comprehensive understanding of the child’s cognitive functioning. At 6 years of age, attention, language, and memory abilities appear most commonly affected in the children exposed to sodium valproate in utero [3], which likely translates into poor IQ scores reported in the literature [15,16]. Documenting specific cognitive deficits also provides avenues for intervention and support.
2.1.2.5. Influence of preconceptual counseling on women with epilepsy.
Health care professionals now have more reliable evidence to discuss with the potential mother to allow her to make an informed decision regarding her treatment. The provision of percentages of children falling below the average range provides treating physicians with a level of risk, but percentages will vary over neuropsychological measures, particularly those with older normative samples [28]. Adjusted coefficients may be a more reliable way to communicate information. For example, in our 6-year outcome data, a child’s attentional, memory, language, and global cognitive abilities were between 8 and 14 points lower following in utero exposure to sodium valproate when the effects of confounding variables are held constant. Considering that most cognitive abilities are measured with assessments with a mean 100 with a SD of 15, these represent significant implications.
Epidemiological reports document a decrease in the use of sodium valproate in women of childbearing age and an increase in the use of other AEDs, including lamotrigine and levetiracetam, which is likely due to increased concerns regarding risks to the fetus [29,30].
2.1.3. Directions for the future
Although recent research has increased our knowledge regarding the risks posed to cognitive development, questions remain to be answered.
The effects of sodium valproate are most often reported because of a higher incidence of negative outcomes, enabling risk and profile to be documented with ease [3]. Less clear are the risks posed by the other established and newer AEDs. A number of prospective studies have shown that carbamazepine is not associated with poorer cognitive abilities [10–12]; however, recent data from the NEAD study have shown that there may be a risk with higher doses [13]. Research is needed that uses large groups to enable investigation of subtle deficits that may be present in school-aged children exposed to any AED in utero and considering dose effects.
The increased incidence of ASD following in utero exposure to AEDs requires consideration. Few prospective studies have included ASD as an outcome but there is retrospective evidence [23,24], and our prospective evidence adds weight to concerns over sodium valproate [22]. Additional studies are required to outline the exact nature of the risk of ASD from in utero exposure to AEDs, particularly sodium valproate.
Research also needs to address the mediating factors between AED exposure and negative outcomes in the child. The principles of teratogenicity would assume that there is genetic variability [31]. Further, animal research proposes that certain AEDs disrupt normal brain development [26,27], but human studies are lacking. Investigations around potential neuroanatomical changes in the brain detected by imaging will provide insight into the mechanisms underpinning cognitive impairments and, in the future, may even help to identify children at risk of impaired cognitive development or neurodevelopmental disorders.
Research should also be directed toward understanding the developmental trajectory of children exposed to AEDs through adolescence and adulthood. Only then can it be said that the full implications of in utero exposure to AEDs are known.
Future research should aim to address the possible contributory nature of confounding variables such as transient seizure exposure, socioeconomic status, home environment, and parental education and consider their possible interaction with AED exposure. Children do not live in isolated environments, and therefore, multiple factors are likely to be influential over cognitive development.
Finally, AED use during pregnancy is likely to be unavoidable for the majority of WWE. Therefore, future research needs to consider intervention and support for WWE and their children. Children with a history of prenatal exposure to AEDs should be viewed as an “at risk population” for poor cognitive development and neuro developmental disorders. Research needs to investigate the best way to enhance variables in the child’s environment to ensure the best possible development, despite in utero exposure. Health and educational professionals who have contact with children in their early years need to be provided with information on the best way to assess and support children who are at an increased risk of cognitive and neurodevelopmental difficulties.
In summary, we are only a fraction of the way through and there is a lot more to be done! Future research should be designed considering the points above. Further, this type of research should also be applied to other chronic conditions in which medication use during pregnancy is essential.
3. Cognitive effects of antiepileptic drugs
3.1. Kimford J. Meador
3.1.1. Introduction
Although marked individual variability exists, people with epilepsy are at increased risk for impaired cognitive performance. Multiple factors can contribute to these deficits including the etiology of the epilepsy; cerebral abnormalities existing prior to onset of seizures; age at seizure onset; seizure type, frequency, duration, and severity; intraictal and interictal physiological effects of seizures; structural brain damage from repetitive or prolonged seizures; hereditary factors; psychosocial factors; and effects of treatments including AEDs and surgery [32,33].
3.1.2. Cognitive effects of antiepileptic drugs
The magnitude of AED-induced cognitive effects is usually smaller than that of the effects of epilepsy-related factors [34]. However, AEDs are the primary therapeutic modality for epilepsy, and the choice of AED is under the control of the physician. Thus, understanding the cognitive effects of AEDs is important in assessing the risk-to-benefit ratio of specific AED therapies. AEDs reduce neuronal irritability but also reduce neuronal excitability and, so, may impair cognition. The primary effects of AEDs on cognition include reductions in psycho-motor processing speed, sustained attention (i.e., vigilance), and learning. All AEDs may impair cognition, but the side effects are usually modest for monotherapy when anticonvulsant blood levels are within standard therapeutic ranges [34]. Even these “modest” cognitive side effects can be clinically pertinent as greater AED neurotoxicity symptoms are associated with lower perceived quality of life, even in the absence of overt toxicity on examination [35]. The risks of adverse cognitive side effects increase with greater AED dosages and blood levels and the use of polypharmacy [34]. Differential cognitive effects of AEDs exist. The most consistent adverse effects are observed with barbiturates, benzodiazepines, and topiramate. Intermediate effects are seen with carbamazepine, oxcarbazepine, phenytoin, and valproate. Fewer cognitive side effects occur with lamotrigine, levetiracetam, tiagabine, and vigabatrin [34]. However, many AEDs have not been tested, and the relative effects of many AEDs have not been fully determined.
3.1.3. Design issues
The cognitive effects of AEDs may not be detectable if conduct of the investigation is flawed. Many investigations examining the cognitive effects of AEDs are limited by deficits in experimental design, analysis, and interpretation [34]. Experimental design issues include subject selection bias, adequate power, appropriate cognitive tests, control for test–retest effects, nonequivalence of clinical variables, and nonequivalence of dependent variables. Selection bias may occur if there is no randomization of AED treatment. Problems with nonequivalence of clinical variables include lack of control for anticonvulsant blood levels or seizure frequency. Nonequivalence of dependent measures may occur when there is no assurance that treatment groups performed similarly on dependent measures prior to treatment. The assessment of cognitive effects may be difficult, if not impossible, in studies designed primarily to demonstrate efficacy of an AED. Such studies are designed to have patients fail seizure control on the placebo or comparison drug. Assessment of cognitive effects is difficult, if not impossible, if a sizable number of patients drop out early for seizures or side effects. The noncompleter patients cannot be compared directly to completers because the noncompleter patients will be tested off AED, or even if still on AED, the patient will have a shorter test interval, which could alter test performance by affecting test–retest practice effects or by reducing the time for brain habituation to AED effects. One possible alternative is to test all subjects earlier during treatment so that if patients drop out their early performance may be used to impute later cognitive performance based on the relationship of early to late performance in completers. In multicenter studies, reliability and consistency of assessments at each center need to be ensured by training and monitoring of testers. An alternative is the use of computerized assessments, but the computer test battery needs to demonstrate reliability, validity, and sensitivity to AED-induced cognitive effects. Analysis and interpretation problems include type I error, use of inappropriate statistics, nonorthogonal contrasts, and comparison of studies with nonequivalent designs/statistics. Because of individual variability in cognitive performance and test–retest effects on performance, reliable change indices may be of utility in assessing the clinical significance of changes. Finally, the magnitude and impact of the statistical findings need to be evaluated for clinical significance, taking into account the overall risk-to-benefit ratio of the AED and the severity of the seizure disorder.
Future studies need to learn from past mistakes and apply appropriate designs and analyses. Systematic evaluation of all AEDs with head-to-head comparisons should be conducted. Systematic testing should be a routine part of the drug development process. This is particularly a concern for populations at special risk (see below). The use of sensitive batteries that employ common elements across studies should be encouraged. The tests could include physiological measures (e.g., EEG-based), which give insight into direct cerebral effects of the AEDs.
3.1.4. Special populations
Cognitive impairments induced by AEDs may be of particular concern for adults with jobs requiring speed or sustained vigilance and for children in whom the additive effects during neuro development may have long-lasting consequences. In addition, the elderly have increased susceptibility to cognitive effects of drugs for both pharmacokinetic and pharmacodynamic reasons. The elderly are more likely to be on multiple drugs and have deficits from cognitive disorders, both of which can increase the risk of AED-induced impairments. Thus, additional investigations are needed to delineate the cognitive effects of all AEDs at age extremes, especially in the fetus, children, and the elderly.
3.1.5. Psychotropic effects
Antiepileptic drugs may also produce positive or negative behavioral alterations (e.g., mood stabilization, irritability/agitation, psychosis) [35]. Most AEDs can produce negative behavioral effects in some patients. Carbamazepine, lamotrigine, and valproate have established positive psychotropic effects. Several of the other AEDs may also have positive psychotropic effects. AED psychotropic effects are of special importance given the high comorbidity of psychiatric disorders in patients with epilepsy [35]. Future research is needed to better profile the positive and negative psychotropic effects of all AEDs and delineate the impact of these effects on psychiatric comorbidities [36].
3.1.6. Individual variability
Considerable variability in susceptibility to AED-induced cognitive and behavioral side effects exists across individuals. Investigations are needed to understand the factors contributing to this variability. Such studies could lead to methods and biomarkers that can predict individual adverse responses. These might include cognitive, electro-physiological, or pharmacogenetic measures. Techniques to monitor AED cognitive effects in a clinical setting need to be developed. At present, clinical monitoring is largely done by asking the patient about side effects. However, a patient’s perception of his or her cognitive performance is more related to mood than actual objective performance [37]. Techniques are required that are brief, are cost effective, and have defined reliable change indices (RCIs).
3.1.7. Neurobiological mechanisms
The actual neurobiological mechanisms of AED-induced adverse cognitive and psychotropic effects are poorly understood. AEDs largely affect neurotransmission (e.g., GABA, sodium channels, calcium channels, and glutamate receptors) [38]. Potential mechanisms might include reduction of sustained high-frequency repetitive firing, altered neuronal responses (firing rate or threshold effects), reduced speed of response, and disruption of coherent activity, long-term potentiation, or synaptogenesis. Animal models of neural processing, neurotransmitters, long-term potentiation, memory, and apoptosis may provide insights. In humans, direct measures of brain activity are needed such as alterations in EEG spectral patterns, event-related potential latencies, Granger causality, and MRI. For example, several older AEDs produce an EEG pattern similar to that of a mild diffuse encephalopathy, with generalized slowing and increased power in the lower-frequency ranges, but some newer AEDs produce a different pattern [39].
3.1.8. Cognitive enhancement
There is a need to develop treatments that enhance cognition in patients with epilepsy. Despite the high frequency of cognitive impairments in patients with epilepsy, there has been little research on how such deficits might be ameliorated. The approach may include not only drugs to enhance cognition, but also an improved understanding of how seizures and interictal discharges affect cognition and behavior and if AEDs can alter these effects across the short term and long term.
3.1.9. Translation into clinical practice
Research findings should be efficiently translated into clinical practice, but this is frequently not the case. Assessments are needed to determine the knowledge of patients and physicians with respect to AED-induced cognitive and behavioral effects, and how this knowledge translates into changes in care including prescription and monitoring practices.
3.1.10. Conclusions
Antiepileptic drugs can induce clinically meaningful adverse cognitive and behavioral side effects. Our understanding of these effects is incomplete. Additional studies are needed to improve the care of patients with epilepsy (Table 2).
Table 2.
Research needs related to the cognitive effects of antiepileptic drugs.
| 1. Testing should be systematic with appropriate design and head-to-head AED comparisons to assess the many untested and inadequately tested AEDs. |
| 2. Systematic testing should be performed during drug development. |
| 3. Effects in special populations (e.g., children and elderly) should be assessed. |
| 4. Psychotropic effects of AEDs and the impact of these effects on psychiatric comorbidities should be assessed. |
| 5. Understand, measure, and predict individual variability in a clinical setting. |
| 6. Understand the neurobiological mechanisms of AED-induced neuropsychological effects. |
| 7. Develop treatments to enhance cognition in patients with epilepsy. |
| 8. Understand how research findings can be efficiently translated into clinical practice. |
4. Promising Areas of Research and Young Investigators
4.1. Beth A. Leeman
Cognitive effects of epilepsy and its treatments
4.1.1. Introduction
Patients with epilepsy often demonstrate cognitive deficits, which may compromise quality of life [40–42]. A variety of factors may contribute to cognitive dysfunction in the setting of epilepsy, including seizure type, frequency, and severity; interictal discharges; underlying brain injuries; age at onset; side effects of treatment; and psychosocial and psychiatric comorbidities [43–60].
The types of deficits that may be present, and the types of epilepsy in which they occur, however, remain unclear. Furthermore, the mechanisms underlying cognitive dysfunction in epilepsy are unknown. Without a clear understanding of these issues, treatment options are limited. We are developing a research program that will help to better understand cognition in the context of epilepsy, with a focus on the characterization of deficits, prediction of cognitive outcomes after epilepsy surgery, identification of underlying mechanisms, and development of methods for treatment of cognitive dysfunction. Our goal is to translate knowledge gained from this research into the care of patients, to enhance cognitive performance and avoid detrimental effects of epilepsy and its treatments.
4.1.2. Cognitive effects of epilepsy surgery
Patients may experience cognitive decline following surgical resection for the treatment of refractory seizures [61,62]. The types of deficits sustained, long-term outcomes, and methods for outcome prediction, however, are not fully understood.
Although anterograde memory dysfunction is common in the setting of epilepsy, for example, the extent to which remote memory deficits occur is uncertain. We recently examined remote memory function in healthy controls and patients with temporal lobe epilepsy (TLE) after standard anterior temporal lobectomy (ATL) [63]. Data revealed poorer memory in subjects with epilepsy compared with controls for events dating up to 31–36 years prior to testing, without significant relationships to anterograde memory function. The data supported the hypothesis that memory deficits in the setting of epilepsy occur not only with anterograde tasks, but with tests of remote memory as well. It was not clear, however, whether the deficits were caused by resection and to what degree performance was dependent on hippocampal involvement. In our current study, subjects with localization-related epilepsy will undergo cognitive testing to evaluate remote, autobiographical, and prospective memory pre-and postresection. Results will be compared across patients with TLE, patients with extra-TLE, and healthy controls to assess the effects of localization.
It is also important to identify the time course of deficits sustained with epilepsy surgery. Standard ATL can worsen memory impairments immediately after surgery, and limited data suggest continued impairments over the several years following resection [64–66]. The long-term effects of surgery on memory ability in conjunction with aging, however, are unclear. Our preliminary results in patients up to 18 years post-ATL suggest poorer verbal memory performance compared with normative values obtained in healthy controls, without a significant effect of time since surgery. Predictive factors of poor outcome included left-sided resections and lower preoperative verbal memory test scores.
To identify those at greatest risk for cognitive decline after surgery, we also examined whether hippocampal asymmetries in fluorodeoxyglucose (FDG) uptake on preoperative PET scans would predict post temporal lobectomy verbal memory decline in patients with left TLE [67]. Preoperative asymmetries in hippocampal metabolism were not related to postoperative changes in verbal memory. Post hoc analyses revealed a trend for relatively greater preoperative metabolism in left superior and inferior temporal gyri to predict better verbal memory outcomes. This finding suggested reorganization of function and/or retained function of remaining tissue.
Findings of these investigations serve to underscore the need to fully characterize the types of, and risks for, deficits with epilepsy and epilepsy surgery. Results may ultimately affect testing strategies, surgical planning, counselling of patients, and methods for cognitive rehabilitation.
4.1.3. Cognitive effects of interictal epileptiform discharges
Prior studies found an association between disruptions of cognitive task performance and the presence of focal or generalized interictal epileptiform discharges (IEDs) in both animals and humans [43–57,68]. Memory processes may be particularly vulnerable to the effects of IEDs [69]. In humans, IEDs impair verbal and non-verbal short-term [57] and long-term [70] memory and correlate with accelerated rates of long-term forgetting [70]. In our pilot data from adults with focal-onset seizures, a subject with frequent left temporal discharges had the lowest baseline scores across a number of cognitive tasks, including measures of verbal memory, verbal fluency, and executive function, suggesting that focal IEDs may impair cognition more broadly [71].
These studies reflect the importance of understanding the role of IEDs in cognitive deficits, and our ongoing protocol examines the characteristics of hippocampal IEDs that lead to disruption of memory formation in humans with TLE. In a pilot study, patients with epilepsy undergoing intracranial monitoring completed verbal and nonverbal list learning tasks. The data demonstrated no significant relationship between the occurrence of hippocampal IEDs during encoding of stimuli and later recognition of the stimulus items [72]. Hence, it is possible that certain characteristics of IEDs are necessary for disruption to occur. These necessary features may relate to discharge duration, spatial extent, timing relative to stimulus onset, or presence of embedded high-frequency components. It is also possible that the effect of IEDs depends on the underlying functional and structural integrity of the hippocampus. Further study of these variables in a larger patient sample is necessary to determine in more detail the effects of IEDs on memory formation.
4.1.4. Mechanisms underlying cognitive dysfunction resulting from interictal epileptiform discharges
Although cognitive deficits may be associated with seizures, IEDs, anticonvulsant medications, and surgical interventions, the mechanisms underlying the cognitive impairments are unknown. We are currently conducting a study to investigate the mechanism by which IEDs impair memory, evaluating the hypothesis that pathological high-frequency oscillations (HFOs) embedded within focal IEDs disrupt memory formation by interference with the normal EEG HFOs that underlie encoding. Although pathological oscillatory activity may signal the region of epileptogenesis in patients with focal epilepsy, normal oscillations are believed to underlie long-term potentiation, a process of enhancing synaptic transmission that leads to synaptic plasticity and memory formation [73–83]. Our study examines the differences between normal oscillatory activity and pathological HFOs that occur in the setting of hippocampal IEDs.
4.1.5. Treatment options
Current treatment strategies for memory loss in epilepsy have limited efficacy and fail to target the likely pathophysiology. One mission of our research program is to develop new treatments for cognitive dysfunction in epilepsy based on our understanding of the underlying mechanisms.
Finding that IEDs disrupt memory formation, for example, would further highlight the need for investigations of IED suppression. Defining the properties of IEDs that lead to memory dysfunction may also serve to identify the groups of patients most in need of intervention. We are currently conducting a study to evaluate whether suppression of IEDs leads to improvements in cognitive task performance in adults with focal onset seizures who are placed on levetiracetam initial monotherapy. EEG recordings with concurrent tests of attention, language, and memory are conducted pre-and posttreatment. In a preliminary analysis, subjects with infrequent discharges showed no significant changes in discharge frequency or task performance across sessions. In contrast, a subject with frequent left temporal discharges at baseline demonstrated a large decrease in IED activity and marked improvement in performance across multiple domains with treatment [71]. Improvements were seen in verbal and nonverbal recognition memory, as well as quality of life. These preliminary data provide initial evidence for a beneficial effect of spike reduction on cognition. Data collection is ongoing and includes subjects with generalized discharges treated with lamotrigine.
Additional studies evaluate the possible cognition-enhancing effects of other classes of medications. As memory deficits in the setting of epilepsy are thought to result from excitotoxic injury involving NMDA receptor hyperactivity, we are conducting a randomized, placebo-controlled, double-blinded study to evaluate the efficacy of an NMDA receptor antagonist, memantine, for the treatment of memory deficits in subjects with localization-related seizures. Future studies will also include a trial of vinpocetine, a sodium and calcium channel inhibitor believed to improve cerebral metabolism, enhance long-term potentiation and memory, elevate seizure thresholds, and potentially control release of excitotoxic neurotransmitters. We propose to conduct pilot studies in healthy volunteers and patients with epilepsy to assess the potential efficacy and safety of different dosages of vinpocetine in improving cognition. Future research may also focus on nonpharmacological methods for improving memory, including stimulation techniques to enhance normal or reduce abnormal oscillatory activity. If beneficial, these treatments would provide much-needed therapeutic options.
4.1.6. Conclusions
Cognitive dysfunction may be disabling for many patients with epilepsy. A better understanding of the character and etiology of cognitive deficits in epilepsy is needed, to help develop appropriate treatments. Our research program aims to understand cognition in the setting of epilepsy, including studies to help characterize the types of memory deficits seen in epilepsy, predict memory outcomes after epilepsy surgery, identify mechanisms underlying memory dysfunction, and develop pharmacological and nonpharmacological methods for the treatment of cognitive deficits.
Footnotes
From a special issue of Epilepsy & Behavior: “The Future of Clinical Epilepsy Research” in which articles synthesize reviews from senior investigators with the contributions and research directions of promising young investigators.
It should be noted that 108 (39%) of the children of WWE from the Liverpool and Manchester study are also enrolled in the NEAD study [1].
References
- [1].Mawer G, Briggs M, Baker GA, et al. Pregnancy with epilepsy: obstetric and neonatal outcome of a controlled study. Seizure 2010;19:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hill RM, Tennyson LM. Significant anomalies in the antiepileptic drug syndrome: comment. In: Janz D, Dam M, Richens A, Bossi L, Helge H, Schmidt D, editors. Epilepsy, pregnancy and the child New York: Raven Press; 1982. p. 309–11. [Google Scholar]
- [3].Bromley RL. Foetal exposure to antiepileptic drugs: cognitive outcomes in the child Thesis, University of Liverpool; 2009. [Google Scholar]
- [4].Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2006;77:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Speidel BD, Meadow SR. Maternal epilepsy and abnormalities of the fetus and newborn. Lancet 1972;21:839–41. [DOI] [PubMed] [Google Scholar]
- [6].Shallcross R Unpublished Ph.D thesis, University of Liverpool; 2011. [Google Scholar]
- [7].Hill RM, Verniaud WM, Rettig GM, Tennyson LM, Craig JP. Relationship between antiepileptic drug exposure of the infant and developmental potential. In: Janz D, editor. Epilepsy, pregnancy and the child New York: Raven Press; 1982. p. 409–17. [Google Scholar]
- [8].Leavitt AM, Yerby MS, Robinson N, Sells CJ, Erickson DM. Epilepsy in pregnancy: developmental outcome of offspring at 12 months. Neurology 1992;42:141–3. [PubMed] [Google Scholar]
- [9].Reinisch JM, Sanders S, Mortensen EL, Rubin DB. In utero exposure to phenobarbital and intelligence deficits in adult men. JAMA 1995;274:1518–25. [PubMed] [Google Scholar]
- [10].Gaily E, Kantola-Sorsa E, Hiilesmaa V, et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology 2004;62:28–32. [DOI] [PubMed] [Google Scholar]
- [11].Bromley R, Mawer G, Love J, et al. Early cognitive development in children born to women with epilepsy: a prospective report. Epilepsia 2010;51:2058–65. [DOI] [PubMed] [Google Scholar]
- [12].Wide K, Henning E, Thomson T, Winbladh B. Psychomotor development in preschool children exposed to antiepileptic drugs in utero. Acta Paediatr 2002;91: 409–14. [DOI] [PubMed] [Google Scholar]
- [13].Meador KJ, Baker GA, Browning N, et al. , for the NEAD Study Group. Fetal antiepileptic drug exposure and verbal vs. non-verbal abilities at age 3. Brain 2011;134:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cummings C, Stewart M, Stevenson M, Morrow J, Nelson J. Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Arch Dis Childhood Published Online First: 17 March 2011. doi: 10.1136/adc.2009.176990. [DOI] [PubMed] [Google Scholar]
- [15].Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry 2004;75:1575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Meador K, Baker G, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009;360:1597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thomas SV, Ajaykumara B, Sindhua K, et al. Motor and mental development of infants exposed to antiepileptic drugs in utero. Epilepsy Behav 2008;13:229–36. [DOI] [PubMed] [Google Scholar]
- [18].Shallcross R, Bromley R, Irwin B, Bonnett L, Morrow J, Baker G. Child development following in utero exposure: levetiracetam in comparison to sodium valproate. Neurology 2011;76:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nicolai J, Vles JSH, Aldenkamp AP. Neurodevelopmental delay in children exposed to antiepileptic drugs in utero: a critical review directed at structural study-bias. J Neurol Sci 2008;271:1–14. [DOI] [PubMed] [Google Scholar]
- [20].Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment 4th ed. London/New York: Oxford Univ. Press; 2004. [Google Scholar]
- [21].Deary I, Strand S, Smith P, Fernandes C. Intelligence and educational achievement. Intelligence 2007;35:13–21. [Google Scholar]
- [22].Bromley R, Mawer G, Clayton-Smith J, Baker GA, on behalf of the Liverpool and Manchester Neurodevelopment Group. Autism spectrum disorders following in utero exposure to antiepileptic Drugs. Neurology 2008;71:1923–4. [DOI] [PubMed] [Google Scholar]
- [23].Moore SJ, Turnpenny PD, Quinn A, et al. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet 2000;37:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rasalam AD, Hailey H, Williams JH, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol 2005;47:551–5. [DOI] [PubMed] [Google Scholar]
- [25].Arndt T, Stodgell C, Rodier P. The teratology of autism. Int J Dev Neurosci 2005;23: 189–99. [DOI] [PubMed] [Google Scholar]
- [26].Rinaldi T, Silberberg G, Markram H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex 2008;18:763–70. [DOI] [PubMed] [Google Scholar]
- [27].LaJoie J, Moshe S. Effects of seizures and their treatment on fetal brain. Epilepsia 2004;45:48–52. [DOI] [PubMed] [Google Scholar]
- [28].Flynn J Searching for justice: the discovery of IQ gains over time. Am Psychol 1999;54:5–20. [Google Scholar]
- [29].Ackers R, Besag F, Wade A, Murray M, Wong I. Changing trends in antiepileptic drug prescription in girls of child-bearing potential. Arch Dis Childhood 2009;94:443–7. [DOI] [PubMed] [Google Scholar]
- [30].Meador KJ, Penovich P, Baker GA, et al. Antiepileptic drug use in women of childbearing age. Epilepsy Behav 2009;15:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Beckman D, Brent R. Mechanisms of teratogenesis. Annu Rev Pharmacol Toxicol 1984;24:483–500. [DOI] [PubMed] [Google Scholar]
- [32].Lennox WG. Brain injury, drugs and environment as causes of mental decay in epilepsy. Am J Psychiatry 1942;99:174–80. [Google Scholar]
- [33].Lesser RP, Lüders H, Wyllie E, et al. Mental deterioration in epilepsy. Epilepsia 1986;27(Suppl 2):S105–23. [DOI] [PubMed] [Google Scholar]
- [34].Meador KJ. Cognitive effects of epilepsy and of antiepileptic medications. In: Wyllie E, Cascino G, Gidal B, Goodkin H, editors. The treatment of epilepsy: principles & practice 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2010, pp. 1028–1036. [Google Scholar]
- [35].Gilliam F, Kuzniecky R, Faught E, et al. Impact of adverse antiepileptic drug effects on quality of life in refractory epilepsy. Epilepsia 1999;40(Suppl):64–5. [Google Scholar]
- [36].Ettinger AB, Kanner AM. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 2007. [Google Scholar]
- [37].Marino SE, Meador KJ, Loring DW, et al. Subjective perception of cognition is related to mood and not performance. Epilepsy Behav 2009;14:459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Levy RH, Mattson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs 5th ed. Philadelphia: Lippincott, Williams & Wilkins; 2002. [Google Scholar]
- [39].Salinsky M, Storzbach D, Oken B, Spencer D. Topiramate effects on the EEG and alertness in healthy volunteers: a different profile of antiepileptic drug neurotoxicity. Epilepsy Behav 2007;10:463–9. [DOI] [PubMed] [Google Scholar]
- [40].Perrine K, Hermann BP, Meador KJ, et al. The relationship of neuropsychological functioning to quality of life in epilepsy. Arch Neurol 1995;52:997–1003. [DOI] [PubMed] [Google Scholar]
- [41].Engelberts NHJ, Klein M, van der Ploeg HM, et al. Cognition and health-related quality of life in a well-defined subgroup of patients with partial epilepsy. J Neurol 2002;249:294–9. [DOI] [PubMed] [Google Scholar]
- [42].Giovagnoli AR, Avanzini G. Quality of life and memory performance in patients with temporal lobe epilepsy. Acta Neurol Scand 2000;101:295–300. [DOI] [PubMed] [Google Scholar]
- [43].Hovey HB, Kooi KA. Transient disturbances of thought processes and epilepsy. AMA Arch Neurol Psychiatry 1955;74:287–91. [DOI] [PubMed] [Google Scholar]
- [44].Dodrill CB, Wilkus RJ. Relationships between intelligence and electroencephalo-graphic epileptiform activity in adult epileptics. Neurology 1976;26(6, Pt 1):525–31. [DOI] [PubMed] [Google Scholar]
- [45].Kooi KA, Hovey HB. Alterations in mental function and paroxysmal cerebral activity. AMA Arch Neurol Psychiatry 1957;78:264–71. [PubMed] [Google Scholar]
- [46].Rausch R, Lieb JP, Crandall PH. Neuropsychologic correlates of depth spike activity in epileptic patients. Arch Neurol 1978;35:699–705. [DOI] [PubMed] [Google Scholar]
- [47].Browne TR, Penry JK, Porter RJ, Dreifuss FE. Responsiveness before, during, and after spike–wave paroxysms. Neurology 1974;24:659–65. [DOI] [PubMed] [Google Scholar]
- [48].Selldén U Psychotechnical performance related to paroxysmal discharges in EEG. Clin Electroencephalogr 1971;2:18–27. [Google Scholar]
- [49].Schwab RS. Reaction time in petit mal epilepsy. Assoc Res Nerv Ment Dis 1947;26: 339–41. [Google Scholar]
- [50].Tromp SC, Weber JW, Aldenkamp AP, Arends J, vander Linden I, Diepman L. Relative influence of epileptic seizures and of epilepsy syndrome on cognitive function. J Child Neurol 2003;18:407–12. [DOI] [PubMed] [Google Scholar]
- [51].Shewmon DA, Erwin RJ. The effect of focal interictal spikes on perception and reaction time: I. General considerations. Electroencephalogr Clin Neurophysiol 1988;69:319–37. [DOI] [PubMed] [Google Scholar]
- [52].Shewmon DA, Erwin RJ. The effect of focal interictal spikes on perception and reaction time: II. Neuroanatomic specificity. Electroencephalogr Clin Neurophysiol 1988;69:338–52. [DOI] [PubMed] [Google Scholar]
- [53].Tizard B, Margerison JH. The relationship between generalized paroxysmal E.E.G. discharges and various test situations in two epileptic patients. J Neurol Neurosurg Psychiatry 1963;26:308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Aldenkamp A, Arends J. The relative influence of epileptic EEG discharges, short nonconvulsive seizures, and type of epilepsy on cognitive function. Epilepsia 2004;45:54–63. [DOI] [PubMed] [Google Scholar]
- [55].Goode DJ, Penry JK, Dreifuss FE. Effects of paroxysmal spike–wave on continuous visual–motor performance. Epilepsia 1970;11:241–54. [DOI] [PubMed] [Google Scholar]
- [56].Kasteleijn-Nolst Trenité DG, Riemersma JB, Binnie CD, Smit AM, Meinardi H. The influence of subclinical epileptiform EEG discharges on driving behaviour. Electroencephalogr Clin Neurophysiol 1987;67:167–70. [DOI] [PubMed] [Google Scholar]
- [57].Aarts JH, Binnie CD, Smit AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain 1984;107(Pt 1):293–308. [DOI] [PubMed] [Google Scholar]
- [58].Pavone P, Bianchini R, Trifiletti RR, Incorpora G, Pavone A, Parano E. Neuropsycho-logical assessment in children with absence epilepsy. Neurology 2001;56:1047–51. [DOI] [PubMed] [Google Scholar]
- [59].Motamedi G, Meador K. Epilepsy and cognition. Epilepsy Behav 2003;4(Suppl 2): S25–38. [DOI] [PubMed] [Google Scholar]
- [60].Meador KJ. Cognitive outcomes and predictive factors in epilepsy. Neurology 2002;58(8, Suppl 5):S21–6. [DOI] [PubMed] [Google Scholar]
- [61].Ivnik RJ, Sharbrough FW, Laws ER Jr. Anterior temporal lobectomy for the control of partial complex seizures: information for counseling patients. Mayo Clin Proc 1988;63:783–93. [DOI] [PubMed] [Google Scholar]
- [62].Hermann BP, Seidenberg M, Haltiner A, Wyler AR. Relationship of age at onset, chronologic age, and adequacy of preoperative performance to verbal memory change after anterior temporal lobectomy. Epilepsia 1995;36:137–45. [DOI] [PubMed] [Google Scholar]
- [63].Leeman BA, Macklin EA, Schomer DL, O’Connor MG. Transient news events test: feasibility in assessment of post-temporal lobectomy remote memory deficits. Epilepsy Behav 2009;16:113–9. [DOI] [PubMed] [Google Scholar]
- [64].Alpherts WC, Vermeulen J, van Rijen PC, da Silva FH, van Veelen CW. Verbal memory decline after temporal epilepsy surgery? A 6-year multiple assessments follow-up study. Neurology 2006;67:626–31. [DOI] [PubMed] [Google Scholar]
- [65].Rausch R, Kraemer S, Pietras CJ, Le M, Vickrey BG, Passaro EA. Early and late cognitive changes following temporal lobe surgery for epilepsy. Neurology 2003;60:951–9. [DOI] [PubMed] [Google Scholar]
- [66].Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol 2003;54:425–32. [DOI] [PubMed] [Google Scholar]
- [67].Leeman BA, Leveroni CL, Johnson KA. Does hippocampal FDG-PET asymmetry predict verbal memory dysfunction after left temporal lobectomy? Epilepsy Behav 2009;16:274–80. [DOI] [PubMed] [Google Scholar]
- [68].Shatskikh TN, Raghavendra M, Zhao Q, Cui Z, Holmes GL. Electrical induction of spikes in the hippocampus impairs recognition capacity and spatial memory in rats. Epilepsy Behav 2006;9:549–56. [DOI] [PubMed] [Google Scholar]
- [69].Wilkus RJ, Dodrill CB. Neuropsychological correlates of the electroencephalogram in epileptics: I. Topographic distribution and average rate of epileptiform activity. Epilepsia 1976;17:89–100. [DOI] [PubMed] [Google Scholar]
- [70].Mameniskiene R, Jatuzis D, Kaubrys G, Budrys V. The decay of memory between delayed and long-term recall in patients with temporal lobe epilepsy. Epilepsy Behav 2006;8:278–88. [DOI] [PubMed] [Google Scholar]
- [71].Leeman BA, Moo LR, Leveroni CL, Cole AJ, Schachter SC, Hoch DB. Cognitive effects of treatment of focal interictal discharges with levetiracetam [abstract]. Epilepsia 2008;49(Suppl 7):136–7. [Google Scholar]
- [72].Leeman B, Schachter S, Macklin E, et al. Interictal discharges during encoding and effects on recognition. Poster presentation, San Antonio, TX: American Epilepsy Society; December 2010. [Google Scholar]
- [73].Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 1998;18:10464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gruber T, Tsivilis D, Giabbiconi CM, Muller MM. Induced electroencephalogram oscillations during source memory: familiarity is reflected in the gamma band, recollection in the theta band. J Cogn Neurosci 2008;20:1043–53. [DOI] [PubMed] [Google Scholar]
- [75].Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci 1998;18:4244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tallon-Baudry C, Kreiter A, Bertrand O. Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis Neurosci 1999;16:449–59. [DOI] [PubMed] [Google Scholar]
- [77].Miltner WH, Braun C, Arnold M, Witte H, Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature 1999;397:434–6. [DOI] [PubMed] [Google Scholar]
- [78].Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. Neural correlates of consolidation in working memory. Hum Brain Mapp 2007;28:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Axmacher N, Mormann F, Fernandez G, Cohen MX, Elger CE, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci 2007;27:7807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Meltzer JA, Zaveri HP, Goncharova II, et al. Effects of working memory load on oscillatory power in human intracranial EEG. Cereb Cortex 2008;18:1843–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Howard MW, Rizzuto DS, Caplan JB, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex 2003;13:1369–74. [DOI] [PubMed] [Google Scholar]
- [82].Osipova D, Takashima A, Oostenveld R, Fernandez G, Maris E, Jensen O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci 2006;26:7523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].King C, Henze DA, Leinekugel X, Buzsáki G. Hebbian modification of a hippocampal population pattern in the rat. J Physiol 1999;521(Pt 1):159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]