Abstract
Background
The Hammersmith Infant Neurological Examination (HINE) is one of several useful tools for early identification of Cerebral Palsy (CP), however, cut-off scores for CP do not consistently distinguish infants with hemiplegia from those typically developing. We hypothesized that use of an asymmetry score, in addition to the assessment’s standard total cut-off score could remedy this problem in a clinical setting.
Methods
This retrospective study of a neonatal intensive care follow-up program with consistent clinical use of the HINE matched infants with a diagnosis of CP to infants without motor delays or evidence of neurodevelopmental impairments. Groups had same corrected and gestational ages at HINE assessment. Asymmetry presence was recorded.
Results
Of 74 infants with CP, 28 had quadriplegia, 11 had diplegia and 35 had hemiplegia. Median total HINE and asymmetry scores for hemiplegia were 57.5 and 10 vs. 76 and 0 for those without CP. Sensitivity and specificity to distinguish hemiplegia from typical development by combining a total HINE score <63 and an asymmetry score >5 were 91.8 and 100% respectively.
Conclusion
In a clinical setting, combining total HINE and asymmetry scores can help providers differentiate infants with hemiplegia from those typically developing.
Keywords: Neurological examination, asymmetry, cerebral palsy, hemiplegia
Introduction
The Hammersmith Infant Neurological Examination (HINE) [1] is a standardized neurological exam for infants adjusted age 2 to 24 months. The HINE evaluates nerve function, movements, reflexes and reactions, posture, and tone and can help clinicians identify movement disorders including cerebral palsy (CP). The HINE has good interobserver reliability [1] and published cut-off scores for CP [2], helpful in establishing an early diagnosis. Infants with hemiplegic CP, however, often have HINE scores above published thresholds for the disorder, making differentiation from infants with typical development or mild delays difficult at early ages [3]. The HINE incorporates an asymmetry score to quantify the number of items on the neurological exam that are different on right and left sides. When the high-risk infant follow-up program at our institution previously implemented the HINE as part of a larger set of guidelines for detection of CP [4], the documentation was set up to calculate an asymmetry score [5]. We hypothesized that use of an asymmetry score in addition to a total HINE score threshold would differentiate those infants with hemiplegia from those without CP in a NICU follow-up clinic where HINE use is standard care.
Methods
Clinical Characterization of Subjects
This retrospective study included infants cared for in the Neonatal Intensive Care Unit (NICU) Follow-Up clinic 07/2016–06/2017. The HINE training and implementation was previously described [5]. Reliability of clinical examination was previously established on observation and scoring of at least 3 exams per provider with a minimum concordance of 97% (no more than 2.5 points difference in total HINE score) compared to the standard scorer. Guided observation of providers is repeated annually for ongoing reliability. In the clinic, the diagnosis of CP [6] is based on a combination of clinical history, neuroimaging evidence, neurological exams and functional evaluations such as the General Movements Assessment, the Test of Infant Motor Performance, and the motor domains of the Bayley Scales of Infant and Toddler Development (3rd edition), as recommended in the international guidelines [7]. Infants with severe generalized hypotonia were excluded in this study due to difficulties in early identification of CP versus other neuromotor disorders such as those arising from genetic or metabolic etiologies. All included infants with CP had a documented cranial ultrasound or magnetic resonance imaging. For the current clinical observation, infants who received a diagnosis of CP before 24 months corrected age were matched by gestational age at birth and corrected age at HINE assessment to controls also seen in clinic, as close to the visit date of the children with CP as possible. Control infants had no neuroimaging evidence of severe injury or no imaging. When available, the Gross Motor Function Classification System (GMFCS) [8] and Mini-Manual Ability Classification System (MiniMACS) ratings were recorded [9]. Topology of CP classification for diagnostic purposes was consistent with Kuban et al, 2008 [8].
Scoring Process
As the study hypothesis was observational rather than predictive, the included HINE scores documented closest to the time of confirmatory diagnosis were recorded. If a child was diagnosed prior to the 9–12-month visit, later HINE scores and visit documentation were reviewed to ensure no change in neurologic status or diagnosis. Total HINE scores were calculated as the sum of all item scores. If an item contained an asymmetry between left and right sides, then that item’s score was calculated as an average. Asymmetry score was calculated by assigning a 1 or 0 to an item, regardless of actual score difference, based on whether clinical findings were different on each side of the body. The total asymmetry score was then calculated by summing the number of items with clinically detectable asymmetry. In theory, the score could range from 0–26 (see Supplementary HINE scoring form for visualization of scoring); in practice however, some items such as suck/swallow, are unlikely to be scored as asymmetric. Based on distribution and the primary hypothesis, we set HINE asymmetry score cutoff to 5 for calculations of sensitivity and specificity along with confidence intervals. The electronic health record programming team (Epic, Copyright 2016 © Epic Systems Corporation) included both scores. Institutional review board waiver of consent was obtained for access to medical records.
Statistical Analysis
Group comparisons were assessed using chi-square or Fisher’s exact tests for categorical variables and Kruskal-Wallis and Wilcoxon rank sum tests for continuous variables; where a significant overall group difference was found, Holm-Sidak multiplicity adjusted p-values are reported for pairwise comparisons. Values <63 on the HINE and/or >5 on HINE asymmetry were considered high-risk for CP. ROC curve analysis was used to estimate and compare the discriminatory ability of having a total HINE score below a cutoff alone (high-risk) vs. the discriminatory ability of having either a high-risk score on the HINE (<63) or asymmetry (>5). All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) with two-sided p-values <0.05 considered statistically significant.
Results
A total of 148 patient records were included, 74 with a diagnosis of CP. Thirty-five had hemiplegia, 11 had diplegia, and 28 had quadriplegia. Gestational age at birth and corrected age at HINE assessment were not different between groups. Of the 74 children with CP, 9 had localized infarct/thrombosis, 48 had severe encephalopathy of prematurity (intraventricular hemorrhage Grade III, IV, hydrocephalus or periventricular leukomalacia), 15 had neonatal encephalopathy, and 2 had other lesion types. All term infants with isolated infarct/thrombosis had hemiplegia while 11/15 (73%) of infants with neonatal encephalopathy had quadriplegia.
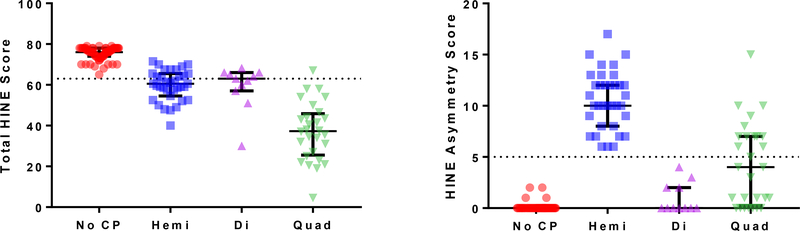
Of 74 infants with confirmed clinical diagnosis of CP, 18 had total HINE scores above the cut-off of 63, and 13/18 were hemiplegic. Asymmetry scores ranged from 6–17 in children with hemiplegia, 0–3 in children with diplegia, and 0–15 in children with quadriplegia. Children in the control group all had total HINE scores > 62 and asymmetry scores < 3. (Table 1). HINE total and asymmetry scores distribution and ranges are visualized according to CP subtype in Figure 1.
Table 1:
| Cohort characteristics | CP (n=74) | Controls(n=74) | ||
|---|---|---|---|---|
| GA at birth in weeks (median, IQR) | Hemi n = 35 | Di n = 11 | Quad n = 28 | |
| 30 (25,37) | 29 (26, 30) | 29 (24.5, 37.5) | 31 (28,35) | |
| Corrected age at HINE in months (median, IQR) Neuroimaging findings | 15 (9, 21) | 17 (15, 20) | 10 (8,19.5) | 14 (9, 22) |
| Infarct/thrombosis in term infant (n) | 9 | 0 | 0 | N/a |
| Severe encephalopathy of prematurity (n) | 22 | 10 | 16 | N/a |
| Neonatal encephalopathy (n) | 4 | 0 | 11 | N/a |
| Other (n) | 0 | 1 | 1 | N/a |
| 60.5 | 37.25 | |||
| HINE score (median, IQR) | (57.5,65.5) | 63 (57,66) | (26,45.25) | 76 (74,78) |
| HINE asymmetry score (median, IQR) | 10 (8,12) | 0 (0,2) | 4 (0.5,7) | 0 (0,0) |
| Functional Scaled Scores* | ||||
| GMFCS | 2 (1,2) | 2 (1,2) | 4 (3.5,4) | N/a |
| MINI-MACS | 2 (1,2) | 0.5 (0,1) | 3 (2.5,4) | N/a |
Scores not available for infants under 12 months CA; IQR: interquartile range (25th,75th); GMFCS: Gross Motor Function Classification System; MINI-MACS: Mini Manual Abilities Classification Scale; GA: gestational age at birth, CA: corrected age
Figure 1: Distribution of HINE and Asymmetry Scores:
Total Hammersmith Infant Neurological Exam Scores (A) and asymmetry scores (B) distribution and ranges are visualized according to cerebral palsy (CP) subtype: No CP; hemi: hemiplegic CP; Di: Diplegic CP; Quad: Quadriplegic CP. Dotted line demonstrates threshold for CP at <63 points (A) and threshold for asymmetry score cutoff as >5 points. The median and interquartile range are visualized.
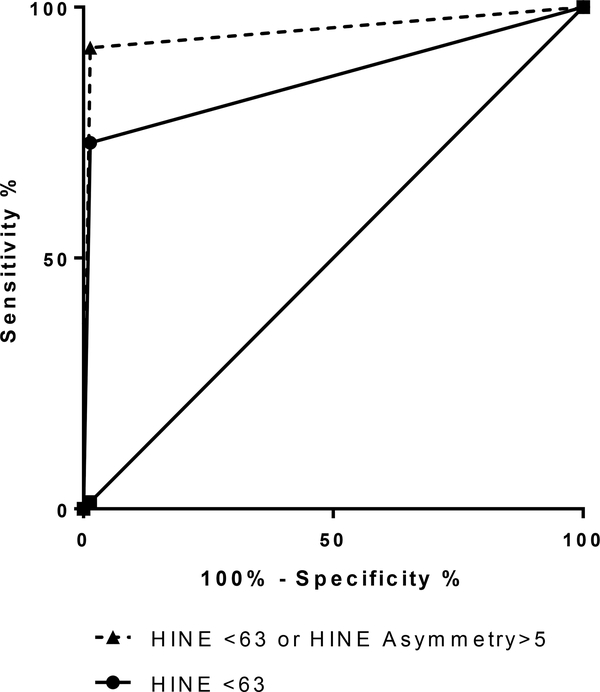
Table 2 summarizes the sensitivity, specificity, and overall area under the curve (AUC) for each of HINE scores. Specificity of total HINE score for any CP with a cut off of 63 was 73.2% and when combined with the asymmetry score increased to 92.9%. Discrimination between typical controls and patients with CP was greater using the HINE total /HINE asymmetry combination compared to using only the HINE total score (p<0.0001). The estimated difference in AUC between the two forms of screening was 0.0946 (0.05, 0.14), p<0.0001. ROC curves (Figure 2) for the HINE total score alone vs. total HINE score or HINE asymmetry score illustrate the increase in precision of adding asymmetry scores to the model.
Table 2:
Performance of HINE vs. HINE or Asymmetry score
| HINE<63 | HINE <63 OR Asymmetry >5 | |||
|---|---|---|---|---|
| Parameter: | Value | 95% CI | Value | 95% CI |
| Sensitivity | 0.7297 | (0.62, 0.82) | 0.9189 | (0.83, 0.96) |
| Specificity | 1.0 | (0.95, 1.0) | 1.0 | (0.95, 1.0) |
| PPV | 1.0 | (0.93, 1.0) | 1.0 | (0.95, 1.0) |
| NPV | 0.7872 | (0.69, 0.86) | 0.925 | (0.84, 0.97) |
| AUC | 0.8649 | (0.81, 0.92) | 0.9595 | (0.93, 0.99) |
HINE: Hammersmith Infant Neurological Exam; PPV: positive predictive value for CP; NPV negative predictive value for CP; AUC area under the curve; CI: confidence interval
Figure 2: ROC Curves for HINE alone vs. HINE with HINE Asymmetry.
Receiver operating characteristic (ROC) curves for the Hammersmith Infant Neurological Examination (HINE) total score alone vs. total score or HINE asymmetry score illustrate the increase in precision
Discussion
An asymmetry score derived from clinical assessment using the HINE appears, in combination with the total score, to be a sensitive method to differentiate typically developing infants from those with hemiplegic CP. Because the HINE is now a recommended tool for early detection of CP [7], the use of thresholds and optimality scores is essential to its clinical application. Infants with hemiplegic CP sometimes had total scores above optimality cut-offs [3] leading to possible misinterpretation of the validity of HINE scoring. The current observation demonstrates that asymmetry scores add to the utility of the HINE in differentiating milder forms of CP from typical development. The asymmetry score may also provide a useful categorization of topographic characteristics of CP in infancy. While the HINE has been recommended to help predict the topography of impairments in CP [7], the asymmetry score had not been studied for this purpose [7]. The asymmetry score may provide a useful measure of differential impairment in all children with CP, especially if it is studied prospectively in conjunction with standardized neuroimaging protocols.
Of note, infants diagnosed with quadriplegic CP in our setting often appeared to have asymmetric distribution of tone, sometimes with a discrepancy asymmetry between arm and leg, consistent with other observations in high-risk infant settings [10,11]. The current study suggests that infants with hemiplegic and quadriplegic distributions are most likely to demonstrate upper limb asymmetry, highlighting the value of evaluating the degree to which one extremity functions better than the other, as is possible with the Hand Assessment in Infants [12]. Infants with diplegic CP in our setting did not display asymmetry scores exceeding our cut off of 5, consistent with the adopted definitions and algorithm by Kuban et al. When one lower extremity was more affected than the other but no upper extremity was involved, the typology was described as diplegia. Limitations of this clinical observation study include the retrospective design and single clinic cohort. Future studies may evaluate the utility of the asymmetry score over a longer period of time, include a larger data set, and combine the HINE asymmetry score, total HINE score and the General Movements Assessment results for early diagnosis of CP. The combination of the HINE and GMA is reported to be more useful in early prediction of neurodevelopmental outcome of preterm infants than either of the two assessments alone [2]. Evaluations of the associations between neuroimaging characteristics and HINE asymmetry score can also examine its potential as a quantitative outcome metric after neonatal interventions.
Conclusion:
In a clinical setting for the follow-up of NICU graduates, the total HINE score combined with the asymmetry score can help providers in differentiating young infants with hemiplegia from those without CP, thus leading to recommendations for specific upper limb asymmetry rehabilitation services.
Supplementary Material
Acknowledgments
This work was supported by 1R01HD081120–01A1 from the NICHD to NL Maitre. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Haataja L, Mercuri E, Regev R, et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr. 1999;135(2):153–161. doi: 10.1016/s0022-3476(99)70016-8. [DOI] [PubMed] [Google Scholar]
- [2].Romeo DMM, Ricci D, Brogna C, Mercuri E. Use of Hammersmith Infant Neurological Examination in infants with cerebral palsy: a critical review of the literature. Developmental Medicine and Child Neurology. 2015; 58(3): 240–245 doi: 10.1111/dmcn.12876 [DOI] [PubMed] [Google Scholar]
- [3].Romeo DMM, Guzzetta A, Scoto M, et al. Early neurologic assessment in preterm-infants: Integration of traditional neurologic examination and observation of general movements. Eur J Paediatr Neurol. 2008;12(3):183–189. doi: 10.1016/j.ejpn.2007.07.008. [DOI] [PubMed] [Google Scholar]
- [4].Byrne R, Noritz G, Maitre NL. Implementation of early diagnosis and intervention guidelines for cerebral palsy in a high-risk infant follow-up clinic. Pediatr Neurol. 2017;76:66–71. doi: 10.1016/j.pediatrneurol.2017.08.002. [DOI] [PubMed] [Google Scholar]
- [5].Maitre NL, Chorna O, Romeo DM, Guzzetta A. Implementation of the Hammersmith Infant Neurological Examination in a high-risk infant follow-up program. Pediatr Neurol. 2016;65:31–38. doi: 10.1016/j.pediatrneurol.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol. 2007;49:8–14. doi: 10.1111/j.1469-8749.2007.tb12610.x [DOI] [PubMed] [Google Scholar]
- [7].Novak I, Morgan C, Adde L, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 2017;171(9):879–907. doi: 10.1001/jamapediatrics.2017.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- [9].Eliasson AC, Ullenhag A, Wahlström U, Sundholm-Krumlinde L. Mini-MACS: development of the Manual Ability Classification System for children younger than 4 years of age with signs of cerebral palsy. Dev Med Child Neurol. 2017;59(1):72–78. doi: 10.1111/dmcn.13162 [DOI] [PubMed] [Google Scholar]
- [10].Kuban KCK, Allred EN, O’Shea TM, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24(1):63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maitre NL, Slaughter JK, Aschner JL. Early prediction of cerebral palsy after neonatal intensive care using motor development trajectories in infancy. Early Hum Dev. 2013;89(10):781–786. doi: 10.1016/j.earlhumdev.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Krumlinde-Sundholm L, Ek L, Sicola E, et al. Development of the Hand Assessment for Infants: evidence of internal scale validity. Dev Med Child Neurol. 2017;59(12):1276–1283. doi: 10.1111/dmcn.13585. [DOI] [PubMed] [Google Scholar]
- [13].Kuban KCK, Allred EN, O’Shea M, Paneth N, Pagano M, Leviton A. An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr. 2008;153(4):466–472. doi: 10.1016/j.jpeds.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.