Abstract
Sphingolipids are bioactive lipids that participate in a wide variety of biological mechanisms, including cell death and proliferation. The myriad of pro-death and pro-survival cellular pathways involving sphingolipids provide a plethora of opportunities for dysregulation in cancers. In recent years, modulation of these sphingolipid metabolic pathways has been in the forefront of drug discovery for cancer therapeutics. About two decades ago, researchers first showed that standard of care treatments, e.g., chemotherapeutics and radiation, modulate sphingolipid metabolism to increase endogenous ceramides, which kill cancer cells. Strikingly, resistance to these treatments has also been linked to altered sphingolipid metabolism, favoring lipid species that ultimately lead to cell survival. To this end, many inhibitors of sphingolipid metabolism have been developed to further define not only our understanding of these pathways but also to potentially serve as therapeutic interventions. Therefore, understanding how to better use these new drugs that target sphingolipid metabolism, either alone or in combination with current cancer treatments, holds great potential for cancer control. While sphingolipids in cancer have been reviewed previously (Hannun & Obeid, 2018; Lee & Kolesnick, 2017; Morad & Cabot, 2013; Newton, Lima, Maceyka, & Spiegel, 2015; Ogretmen, 2018; Ryland, Fox, Liu, Loughran, & Kester, 2011) in this chapter, we present a comprehensive review on how standard of care therapeutics affects sphingolipid metabolism, the current landscape of sphingolipid inhibitors, and the clinical utility of sphingolipid-based cancer therapeutics.
1. EFFECTS OF CHEMO- AND RADIATION THERAPIESON SPHINGOLIPIDS
1.1. Chemotherapy
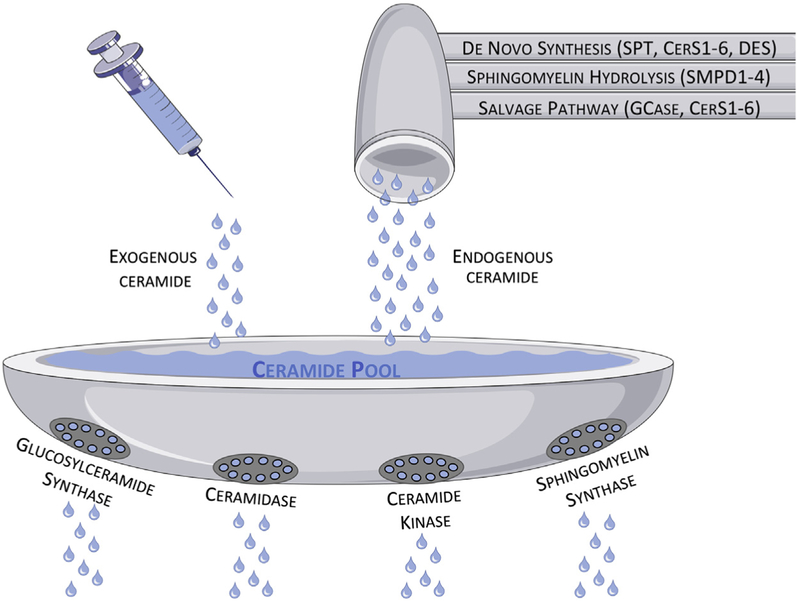
Chemotherapeutic or cytotoxic agents are drugs commonly used as standard of care for multiple types of cancers due to their ability to kill or damage highly proliferative cells. These agents are usually divided into classes based on their mechanisms of action, and, strikingly, a wide range of these classes have been described as leading to accumulation of sphingolipids (particularly ceramide) in cells. As shown in Fig. 1, we depict the steady state accumulation of endogenous ceramide via chemo- and radiation therapies as a combination of “faucets” and “drains.” Enzymes underlying sphingomyelin hydrolysis (sphingomyelinased—SMase), de novo sphingolipid synthesis (serine palmitoyltransferased—SPT, dihydroceramide synthased—CerS, dihydroceramide desaturased—DES), and the sphingolipid salvage pathway (glucosylceramidased—GCase, CerS) are the “faucets” that generate ceramide, whereas enzymes that metabolize ceramide (ceramidased—CDase, glucosylceramide synthased—GCS, sphingomyelin synthased—SMS, ceramide kinased—CERK) are the “drains.” In essence, when too much ceramide accumulates and the metaphorical sink overflows the cell dies. Complicating this system, some of these sphingolipids have separate “faucets” or “drains” due to different isoenzymes of sphingolipid enzymes that generate distinct ceramide species with unique biological and biophysical properties. This diversity in ceramide species is due to distinct ceramide synthases with specific fatty acyl-CoA specificities. As a generalization for the major ceramide species observed in cancer, CerS1 preferentially generates C18-ceramide, CerS2 and CerS4 generate long chain C24 species, and CerS5 and CerS6 generate C16 species. The “fluid dynamics” of these enzyme activities determines both the efficacy of, and resistance to, chemo- and radiation therapies. In fact, this more interconnected and compensatory metabolic system is just a new version of the “sphingolipid rheostat,” by which the pro-death actions of ceramide are counterbalanced by the pro-survival functions of sphingosine1-phosphate (S1P) (Cuvillier et al., 1996). A detailed metabolic pathway for sphingolipid regulation in cancer is depicted in Fig. 2 (top panel).
Figure 1.
The accumulation of ceramide can be generated from three main pathways (sphingomyelin hydrolysis, de novo synthesis, or salvage pathway) or exogenously added (in the form of short chain ceramides), whereas the degradation of ceramide is facilitated by four main enzymes (SMS, GCS, CDase, and CerK). At a steady state, the level of ceramide is held stable, as flux through the system remains fairly constant. However, similarly to increasing flow through the faucet or clogging the drain, increasing the generation of ceramide or preventing its degradation can lead to an accumulation of ceramide, which can be selectively toxic to cancer cells.
Figure 2.
This figure depicts a ceramide-centric view of sphingolipid metabolism. The upper panel outlines the major sphingolipid species (blue boxes) and the enzymes that metabolize these lipids (multicolored boxes). Inhibitors and activators of specific enzymes in the pathway are listed in the lower panel using enzyme color-coded “sticky notes.”
Studies performed in yeast showed that under stress conditions, cells responded by generating ceramide, demonstrating that sphingolipid metabolism is an evolutionary response to stress (Cowart & Obeid, 2007). Chemotherapeutics induce stress in cancer cells, and numerous investigations have shown that in response to this stress, ceramide levels are increased, both by sphingomyelin hydrolysis as well as through de novo synthesis of ceramide (Beckham, Cheng, Marrison, Norris, & Liu, 2013). This increase in ceramide levels has been validated in many different classes of chemotherapies including daunorubicin (anthracycline), etoposide (topoisomerase II inhibitor), and gemcitabine (nucleotide analog DNA replication inhibitor). These drugs have all been described as inducers of de novo generation of ceramide, either by activating ceramide synthases or serine palmitoyl transferase (Bose et al., 1995; Chalfant et al., 2002; Jaffrezou et al., 1996; Perry et al., 2000). Inhibition of enzymes important for de novo generation of ceramide decreases the cytotoxicity of these chemotherapeutics, and therefore, decreases their overall efficacy. However, in glioma cells treated with etoposide, the increase in ceramide levels was caused by increased activation of neutral SMase, which is responsible for converting sphingomyelin to ceramide (Sawada et al., 2001). In one clinical trial for head and neck squamous cell carcinoma, administration of a combination of gemcitabine plus doxorubicin increased serum levels of C18-ceramide that correlated with better drug responses (Saddoughi et al., 2011).
Interestingly, altered ceramide levels are not the only biological connection between sphingolipids and chemotherapy; the Cabot laboratory demonstrated that levels of glucosylceramide are increased in breast cancer cell lines (Lavie et al., 1997) and in patients who were resistant to chemotherapy (Lucci et al., 1998). The enzyme that generates glucosylceramide, GCS, is upregulated in several different tumor types such as lung cancer (Zhang et al., 2014), breast cancer (Liu, Patwardhan, Xie, et al., 2011), bladder cancer (Sun et al., 2010), and colorectal cancer (Wang, Liu, Xu, Mu, & Sun, 2014). Given that increased conversion of ceramide to glucosylceramide decreases killing of cancer cells exposed to chemotherapeutic agents and the increased expression/activity of glucosylceramide synthase in several tumor models, focus has shifted to developing inhibitors for this enzyme that could synergize with standard of care drugs.
Cancer cells may also decrease ceramide levels by increasing activity of the CDase enzymes that cleave ceramide to sphingosine, which can then be converted to S1P. Due to this conversion from ceramide, a pro-apoptotic lipid, to S1P, a pro-survival lipid, CDase can modulate cell fate under stress conditions. CDase enzymes are divided in three different types according to their optimum pH: neutral, acid, or alkaline. In cancer research, a vast majority of the studies have been devoted to acid ceramidase (ACDase). This enzyme was reported as overexpressed in a myriad of tumor types including prostate (Saad et al., 2007), head and neck squamous cell (Elojeimy et al., 2007), and acute myeloid leukemia (AML) (Tan, Pearson, Feith, & Loughran, 2017). In vitro and in vivo studies have shown that by overexpressing ACDase, tumors are more aggressive and resistant to chemotherapies (Beckham et al., 2012; Saad et al., 2007). In prostate cancer cells, ACDase overexpression promoted resistance to etoposide, cisplatin (platinum-based agent), and gemcitabine by stopping accumulation of ceramide (Beckham, Cheng, et al., 2013). Interestingly, ACDase has been shown in hepatocellular cells to be upregulated after exposure to daunorubicin, a phenomenon that was not observed in normal hepatocytes, suggesting a specific mechanism for cancer cells to resist cytotoxicity (Morales et al., 2007).
Recently, the use of medical marijuana was legalized to mitigate side effects of chemotherapies in 30 states (http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html). In 1998, the Sanchez group first reported the relationship between sphingolipids and cannabinoids, the active ingredients of medical marijuana (Sanchez, Galve-Roperh, Canova, Brachet, & Guzman, 1998; Sanchez, Galve-Roperh, Rueda, & Guzman, 1998). They demonstrated that THC (tetrahydrocannabinol) caused an increase in ceramide levels through de novo synthesis, which led to apoptosis in C6 glioma cells. This increase of ceramide levels through THC or other cannabinoids was also shown in prostate and colon cancer cells, as well as mantle cell lymphoma (MCL) and multiple myeloma (Barbado et al., 2017; Cianchi et al., 2008; Gustafsson, Christensson, Sander, & Flygare, 2006; Mimeault, Pommery, Wattez, Bailly, & Henichart, 2003). Further work in MCL showed that cannabinoids induced CerS3 and CerS6 expression. Notably, blocking ceramide metabolism into S1P or glucosylceramide also increased the cytotoxic effect of cannabinoids (Gustafsson, Sander, Bielawski, Hannun, & Flygare, 2009).
Increases in S1P levels at the expense of sphingosine and ceramide in cancer are often manifested by an increase in sphingosine kinase (SphK) expression or activity. The conversion of sphingosine to S1P is executed by two SphK isoenzymes that phosphorylate sphingosine. SphK1 is upregulated in paclitaxel-resistant prostate cancer cell lines (Aoyama et al., 2017) as well as in several tumor xenograft models such as stomach, breast, lung, brain, colon, and kidney (Pyne & Pyne, 2010). Moreover, SphK1 expression was shown to be upregulated in AML patient samples (Dick et al., 2015). Although the specific role of SphK2 in cancer is not as well described, specific SphK2 inhibitors are showing efficacy in the clinic (Orr Gandy & Obeid, 2013). In vitro and in vivo studies in prostate cancer and none small cell lung carcinoma showed that overexpression of SphK1 was responsible for resistance to docetaxel (taxane), camptothecin (topoisomerase inhibitor), and doxorubicin (Akao et al., 2006; Pchejetski et al., 2008; Sauer et al., 2009; Song et al., 2011). However, other studies have also shown that in leukemia cells, treatment with actinomycin D (peptide antibiotic), doxorubicin, or etoposide resulted in reduction of SphK1 due to accumulation of p53 (Taha et al., 2004). SphK1 was also found to be responsible for resistance to cetuximab (a monoclonal antibody chemotherapeutic against Epidermal Growth Factor Receptor (EGFR)) in colorectal cancer both in vitro as well as in patients (Rosa et al., 2013). An interesting mechanism for cell death is to alter the sphingolipid rheostat, not by increasing ceramide levels, but by decreasing S1P levels (French et al., 2003). For example, Oskouian et al. (2006) showed that treating several cancer cell lines with etoposide led to increased levels of S1P lyase and concomitant increases in apoptosis. Taken together, inhibitors of SphK or activators of sphingosine lyase or phosphatases may sensitize patients to conventional therapeutics and prevent drug resistance mechanisms. Although manipulating sphingolipid metabolism as new therapeutic avenue might be enticing, understanding each patient’s sphingolipid and enzymatic profile is vital for interfering with this very intricate, complex, and highly regulated process.
1.2. Radiation
Radiotherapy, as currently used in the clinic, was first developed and tested as a treatment for cancer in 1934 by Henri Coutard. As described by Halperin et al., Coutard developed a fractionized protocol and observed that by administrating lower doses of X-rays over a course of days, he could kill cancer cells while allowing normal cells to recover from the effects of radiation (Halperin, Perez, & Brady, 2008). Contrary to chemotherapeutics, radiotherapy is frequently limited to tumor locations and not systemic. By using high-energy liberation of electrons, it is possible to induce cell death specifically in malignant cells (Aureli et al., 2014). This process occurs by induction of DNA damage, as radiation causes double-strand breaks in DNAs that are lethal to cells (Beckham, Cheng, et al., 2013; Carroll, Donaldson, & Obeid, 2015). Cancer cells can attempt to repair these double-strand breaks, but as repair mechanisms are usually dysregulated in cancer, it leads to errors and subsequent mitosis-associated cell death or p53-induced apoptosis (Aureli et al., 2014; Beckham, Cheng, et al., 2013). Strikingly, the work performed by the Kolesnick lab has shown that even p53-independent cell death mechanisms after radiation exposure are regulated by sphingolipid metabolism (Haimovitz-Friedman et al., 1994; Kolesnick & Fuks, 2003). Among sphingolipid species, ceramide was shown to be the major mediator of cellular stress after radiation exposure (Modrak, Gold, & Goldenberg, 2006).
One of the earliest evidences for the role of ceramide in cell death upon radiation led to the discovery of the rapid hydrolysis of sphingomyelin to ceramide by SMase after exposure to ionizing radiation (Haimovitz-Friedman et al., 1994). The identification of different isoforms of SMase that work under different optimal pH conditions, led to the discovery that sphingomyelin metabolism is triggered by different SMase enzymes in a cell-type specific manner (Beckham, Cheng, et al., 2013; Li & Zhang, 2015). In a very elegant set of experiments, the Kolesnick lab showed that by ablating acid SMase in human type I Niemanne Pick disease lymphoblastoid cells, or in p53 knockout mice, ceramide induced apoptosis after radiation was ablated (Lozano et al., 2001; Santana et al., 1996). Ectopic expression of acid SMase as well as by exogenous treatment with ceramide restored sensitivity to irradiation. These results indicated a critical role of ceramide in cell death induced by radiation. Moreover, SMases can be present extracellularly, and in a small group of cancer patients, fractionated high dose radiation was correlated with an increase of SMase extracellular content and ceramide levels (Oskouian & Saba, 2010).
Sphingomyelin hydrolysis is not the only metabolic pathway to generate ceramide in response to radiation. An in vitro study in radiation-resistant DU145 prostate cancer cells showed that pretreatment with resveratrol (an antioxidant agent) resulted in sensitization to radiation. This shift had occurred by induction of de novo synthesis of ceramide, a finding that was validated when sphingolipid synthesis inhibitors blocked sensitization and reverted DU145 cells to radiation-resistant status (Scarlatti et al., 2007).
Different ceramide chain length species are now known to have different roles in cells. Therefore it is not surprising that different responses to radiation treatments have been correlated with changes in various ceramide species or ceramide synthases. CerS5 and CerS6 are activated following radiation and induce cell death. However, C24 ceramide generated by CerS2, conferred resistance to radiation in the same human cervical cancer (HeLa) cells (Galadari, Rahman, Pallichankandy, & Thayyullathil, 2015). This paradigm of differential activities and cellular roles between C16/C18 versus C24 species is actively being investigated in various other cancer therapeutic models. In addition, the role of saturated versus unsaturated ceramide species of similar chain length is also an area deserving further investigation.
In addition to sphingomyelin hydrolysis and de novo synthesis, ceramide synthase activity has long been known to increase in cells after exposure to radiation (Vit & Rosselli, 2003). The current rationale in the field is that ceramide levels are initially increased by activation of sphingomyelin hydrolysis as described above, and then, between 8 and 24 h after irradiation, the increase in ceramide levels shifts to become driven by increased CerS activity (Carroll et al., 2015). Strikingly, the second wave of ceramide increase seems to be dependent on the first one. In the aforementioned type I Niemanne—Pick disease cells that lack acid SMase, after radiation treatment, there is an increase in CerS activity but not an increase in ceramide levels (Carroll et al., 2015). This dual wave system of cell death was also validated in Jurkat leukemia cells (Ardail et al., 2009).
Another important sphingolipid pathway is the generation of sphingosine by ceramide cleavage via CDases. Hara et al. (2004) reported that in vitro irradiated glioblastoma cells showed increased expression of ACDase. The authors further showed that by inhibiting this enzyme, glioblastoma cells accumulated ceramide on irradiation and increased levels of apoptosis (Hara et al., 2004). A different laboratory working with prostate cancer cells showed that this upregulation of ACDase after radiation exposure was mediated by c-Jun (Cheng et al., 2013). Similarly to glioblastoma cells, reducing ACDase expression in prostate cancer cells resulted in ceramide accumulation and cell death after radiation treatments (Cheng et al., 2013). Considering the evidence above, conversion of ceramide to sphingosine can be a mechanism to counteract the proapoptotic role of ceramide and lead to cell survival and insensitivity to radiation.
2. CURRENT LANDSCAPE OF INHIBITORS OFSPHINGOLIPID METABOLISM
Sphingolipids are vital to normal cell function and thus the dysregulation of the enzymes responsible for their production and maintenance can lead to a number of diseases, including cancer. As such, the ability to regulate the activity of these enzymes using inhibitors or activators is important for developing novel treatments for diseases in which sphingolipid imbalance occurs. Thus, the search for effective inhibitors for the enzymes that decrease ceramide levels are worth exploring. The following section will discuss in vitro and in vivo anti-cancer effects of some of the best-characterized inhibitors and activators of the main sphingolipid enzymes. The lower panel of Fig. 2 provides a comprehensive list of these drugs, their targets, and their impact in sphingolipid metabolism.
2.1. Targeting Sphingomyelin Synthesis
SMSs generate sphingomyelin from two substrates, ceramide and phosphatidylcholine. Tricyclodecan-9-yl-xanthogenate (D609) is a cytotoxic drug that causes selective tumor cell death by inhibiting SMS leading to increased levels of the pro-apoptotic lipid ceramide (Bai et al., 2004; Meng et al., 2004). However, due to the presence of a xanthate group on D609, cells may metabolize the drug and render it useless. Thus, a series of D609 prodrugs have been developed, which are more stable and effective at inhibiting SMS at lower LD50 values (56.6 mM prodrug vs. 117 mM D609) (Bai et al., 2004). Another inhibitor of SMS, MS-209, has been used in numerous studies for its ability to reverse multidrug resistance in tumors (Delgado, Casas, Llebaria, Abad, & Fabrias, 2006; Robert, 2004). Finally, the SMS inhibitor, Dy105, was initially developed as an atherosclerosis treatment but may be useful for future cancer studies (Lou et al., 2014).
2.2. Targeting Ceramide Formation
The first step in the de novo synthesis of ceramide is the condensation of serine and palmitoyl CoA by the enzyme SPT (Hanada, 2003). Myriocin, also known as ISP-1 or thermozymocidin, is the most studied inhibitor of SPT and can inhibit the enzyme at picomolar concentrations (Miyake, Kozutsumi, Nakamura, Fujita, & Kawasaki, 1995; Sauane et al., 2010). More natural products such as sphingofungins, lipoxamycin, and, to a lesser extent, viridiofungins have been shown to inhibit SPT as well (Mandala et al., 1994; Mandala, Thornton, Frommer, Dreikorn, & Kurtz, 1997; Zweerink, Edison, Wells, Pinto, & Lester, 1992). Other previously used less-specific compounds, L-cycloserine and β-chloro-L-alanine, have received some attention as SPT inhibitors but both are known to have off-target effects. Specifically, L-cycloserine also inhibits 3-ketodihydrosphingosine reductase, which is downstream of SPT in the de novo synthesis pathway, whereas β-chloro-L-alanine has off-target effects inhibiting other pyridoxal-5’phosphate—dependent enzymes (Medlock & Merrill, 1988; Sundaram & Lev, 1984).
The CerS family of six isoenzymes is involved in both the de novo synthesis and the recycling of sphingosine into ceramide in the salvage pathway. One of the most well-known general inhibitors of CerS is Fumonisin B1, a mycotoxin produced by Fusarium moniliforme (Bose et al., 1995; Wang, Norred, Bacon, Riley, & Merrill, 1991; Yoo, Norred, Wang, Merrill, & Riley, 1992). A structurally similar toxin, AAL toxin produced by Alternaria alternata, has also been shown to inhibit CerS (Delgado et al., 2006; Winter, Gilchrist, Dickman, & Jones, 1996). Australifungin, a mycotoxin from Sporormiella australis, potently inhibits CerS but has limited use because of its high chemical reactivity (Delgado et al., 2006; Mandala et al., 1995). Interestingly, despite showing efficacy in preclinical development, N-(4-hydroxyphenyl)retinamide (4-HPR or fenretinide) activates CerS but also inhibits DES, the next enzyme in the de novo pathway (Schulz et al., 2006). Fenretinide is now being tested in clinical trials for its potential as a broad anti-tumor inhibitor in several tumor models.
The sphingolipid-based compound, GT11, is a competitive inhibitor of DES that has been modified to generate more effective versions of the compound (Bedia, Triola, Casas, Llebaria, & Fabrias, 2005; Triola, Fabrias, Casas, & Llebaria, 2003; Triola, Fabrias, & Llebaria, 2001). The utility of these compounds that effect multiple sphingolipid metabolites in cancer are undefined.
Desipramine, imipramine, and amitriptyline, which are all tricyclic antidepressants, are also functional aSMase inhibitors that increase aSMase degradation by lysosomal proteases (Albouz et al., 1981; Arimochi & Morita, 2008; Bhabak & Arenz, 2013; Delgado et al., 2006; Huang et al., 2007; Lu et al., 2009). L-alpha-phosphatidyl-D-myo-inositol-3,5-bisphosphate (PtdIns3,5P2) and the more potent phosphatidyl-myo-inositol 3,4,5triphosphate (PtdIns3,4,5P3) are both physiological inhibitors of aSMase that are found in humans (Kolzer et al., 2003; Testai, Landek, Goswami, Ahmed, & Dawson, 2004). A calcium channel blocker that sensitized multidrug resistant cells to doxorubicin, SR33557, also functions to inhibit aSMase (Jaffrezou et al., 1991). NB6, a structurally similar molecule to the tricyclic antidepressants, decreases aSMase activity in a different manner, by inhibiting its transcription (Deigner et al., 2001). α-Mangostin, cowanin, and cowanol, compounds isolated from the bark of Garcinia speciosa, each inhibit aSMase with varying degrees of potency (14.1, 19.2, and 10.9 μM IC50 values, respectively) (Delgado et al., 2006; Okudaira et al., 2000). SMA-7 is another aSMase inhibitor that was shown to inhibit Dextran Sulfate Sodium (DSS)-induced colitis in mice, though other studies have suggested that it may also inhibit nSMase (Canals, Perry, Jenkins, & Hannun, 2011; Sakata et al., 2007; Yokomatsu et al., 2001). A sphingomyelin analogue that was developed to inhibit aSMase, AD2765, increased cellular ceramide levels and cell death in cancer cells in vitro (Darroch et al., 2005). Treatment with fluphenazine, an anti-psychotic drug that induces lysosomal stress and inhibits aSMase activity, led to accumulation of sphingomyelin and subsequent cell death in hypoxic tumors (Klutzny et al., 2017). In contrast to the inhibitors listed above, bismonoacylgycerophopsohate was found to be an activator of aSMase that increased the generation of ceramide (Canals et al., 2011; Linke et al., 2001). Other functional aSMase inhibitors (FIASMAs) that cause aSMase to dissociate from the inner lysosomal membrane and become inactivated include Dextromethorphan, Fluoxetine, Maprotilin, Nortriptyline, Orphenadrine, Sertralin, and Triflupromazine (Kornhuber et al., 2008, 2010).
Numerous studies have explored the effects of inhibition of the SMase family member, neutral SMase (nSMase), utilizing GW4869, a noncompetitive nSMase inhibitor (Luberto et al., 2002; Marchesini, Luberto, & Hannun, 2003; Takahashi, Inanami, Asanuma, & Kuwabara, 2006). The antioxidant glutathione, which is present in mammalian cells at relatively high concentrations (1—20 mM), was reported to almost completely inhibit nSMase at a concentration of 5 mM, suggesting that nSMase is relatively inactive in vivo under normal conditions (Liu & Hannun, 1997). Another nSMase inhibitor, C11AG, which was originally used to inhibit herpes simplex virus-1 replication, was later shown to enhance apoptosis of Jurkat T cell lymphoma cells (Amtmann & Zoller, 2005; Amtmann, Zoller, & Schilling, 2000; Delgado et al., 2006). Macquarimicin A and scyphostatin, two natural products that are primarily nSMase inhibitors, are also weak ACDase inhibitors (Nara, Tanaka, Hosoya, Suzuki-Konagai, & Ogita, 1999; Nara, Tanaka, Masuda-Inoue, et al., 1999; Tanaka et al., 1999). Alutenusin and chlorogentisylquinone are both nSMase inhibitors that were originally purified from culture media of different fungal species (Uchida, Tomoda, Arai, & Omura, 2001; Uchida, Tomoda, Dong, & Omura, 1999). Manumycin A is an antibiotic produced by the bacterium Streptomyces parvulus, which selectively inhibits nSMase, not aSMase, and was also found to be a useful inhibitor of Ras farnesyltransferase (Arenz et al., 2001; Kouchi et al., 1999). Cambinol is another molecule with dual uses, as it was originally discovered as a regulator of SIRT1/2 and was later found to be dramatically more effective for its ability to inhibit nSMase (Figuera-Losada et al., 2015).
2.3. Targeting Glucosylceramide Generation and Degradation
Glucosylceramidase (GCase) hydrolyzes glucosylceramide back to ceramide and glucose. In very early studies, α-gluconolactone was found to inhibit glucosylceramidase, but more potent inhibitors have since been developed (Overkleeft et al., 1998; van Weely, Brandsma, Strijland, Tager, & Aerts, 1993). Deoxynojirimycin is an a-glucosidase inhibitor isolated from mulberry leaves that has been extensively used as a GCase inhibitor, although interestingly, its derivatives, N-nonyl-1-deoxynojirimycin (NNDNJ) and N-Butyldeoxynojirimycin (NBDNJ), have been shown to function as chaperones of GCase that increase its activity (Alfonso et al., 2005; Delgado et al., 2006; Sawkar et al., 2002). Other natural product inhibitors, such as castanospermine, isofagomine, valienamine, and validamine, have also been used to inhibit GCase activity (Delgado et al., 2006; Sasak, Ordovas, Elbein, & Berninger, 1985; Saul, Chambers, Molyneux, & Elbein, 1983).
GCS is the enzyme responsible for adding a glucose molecule to ceramide. A pair of the most widely used GCS inhibitors, D-threo-1-phenyl-2palmitoylamino-3-morpholino-l-propanol (PPMP) and D-threo-1-phenyl2-decanoylamino-3-morpholino-propanol (PDMP), are ceramide analogues (Kovacs, Pinter, & Csaba, 2000; Vunnam & Radin, 1980). PPMP has previously been shown to resensitize multidrug-resistant cancer cells to chemotherapies in vitro and in vivo, whereas PDMP has shown cytotoxicity in AML in vitro (Cakir, Saydam, Sahin, & Baran, 2011; Gouaze et al., 2005). A derivative of PDMP, ethylenedioxy-P4, showed specific inhibition of human GCS but did not inhibit other animal, plant, fungal, or bacterial versions of the enzyme (Hillig, Warnecke, & Heinz, 2005). Miglustat, or NBDNJ, has been shown to reduce the effects of Gaucher disease by inhibiting GCS to prevent glucosylceramide buildup (Cox, 2005; Platt, Neises, Dwek, & Butters, 1994). A structurally similar molecule, N-(n-Butyl)deoxygalactonojirimycin (NBDGJ), also functions to inhibit GCS (Platt, Neises, Karlsson, Dwek, & Butters, 1994).
2.4. Targeting Ceramide-1-Phosphate Formation
CERK, a highly homologous relative of SphK, catalyzes the ATPdependent specific phosphorylation of ceramide to ceramide-1-phosphate. Two main CERK inhibitors have been developed that block its activity: K1 and NVP-231 (Graf et al., 2008; Kim, Inagaki, et al., 2005; Pastukhov et al., 2014). K1 is an isomer of F-12509A, a SphK inhibitor discussed below, that non-competitively inhibits CERK (Kim, Inagaki, et al., 2005). NVP-231 is a competitive CERK inhibitor (Graf et al., 2008) and inhibits breast and lung-cancer cell growth (Pastukhov et al., 2014).
2.5. Targeting Sphingosine Formation
Similar to the three types of SMase enzymes, CDaseare classified according to their optimal pH and thus are named acid ceramidase (ACDase), neutral ceramidase (nCDase), and alkaline ceramidase (alkCDase). B13 (also referred to as D-NMAPPD) is a ceramide analogue that inhibits ACDase and has been used extensively in vitro and in in vivo models of melanoma, pancreatic cancer, and metastatic colon cancer (Raisova et al., 2002; Samsel et al., 2004; Selzner et al., 2001). This has led to the generation of a number of analogs (LCL204, LCL385, LCL521) that have been designed to target ACDase in the lysosomal compartment that B13 struggles to reach (Bai et al., 2017; Holman et al., 2008; Mahdy et al., 2009). LCL 204 has been shown to inhibit in vitro and in vivo models of AML (Tan et al., 2016). N-oleoylethanolamine (NOE) is a synthetic analogue of ceramide that inhibits ACDase that has been used to sensitize cancer cells to chemotherapies and reduce pro-survival signals (Monick et al., 2004; Morales et al., 2007; Strelow et al., 2000; Sugita, Willians, Dulaney, & Moser, 1975). Like NOE, AD2646, SABRAC, and RBMI-12 are other ceramide analogues that were designed to inhibit ACDase (Camacho et al., 2013; Dagan, Wang, Fibach, & Gatt, 2003). In contrast, D-e-MAPP (or D-MAPP) is a ceramide analogue that inhibits alkCDase (Bielawska et al., 1996). DM102 meanwhile is a specific ACDase inhibitor as it shows no activity toward other CDases and causes cell death in vitro (Gouaze-Andersson et al., 2011). Carmofur, a chemotherapeutic drug used to treat colorectal cancer, was discovered to be an ACDase inhibitor suggesting that its mechanism of action is promoting ceramide buildup in cancer cells (Realini et al., 2013). Cystatin SA, a member of the cystatin family, was identified as one of the first physiological inhibitors of ACDase (Eliyahu et al., 2011). Finally, a very potent ACDase inhibitor, SABRAC (IC50 52 nM) induces ceramide accumulation and reduces cell growth of PC-3 prostate cancer cells in vitro (Camacho et al., 2013).
C16-urea-ceramide, as well as shorter-chain C6-urea-ceramide, are potent inhibitors of nCDase (Garcia-Barros et al., 2016; Saied & Arenz, 2014; Usta et al., 2001). C18-Ceramine (ceramine), a synthetic ceramide derivative, is also able to inhibit nCDase (Garcia-Barros et al., 2016; Saied & Arenz, 2014). Two additional inhibitors, Ceranib-1 and Ceranib-2, have been identified that broadly inhibit ACDase, nCDase, and alkCDase (Draper et al., 2011).
2.6. Targeting Sphingosine-1-Phosphate Formation and Degradation
Dimethylsphingosine (DMS), a methylated version of sphingosine, is the first SphK inhibitor that was shown to promote apoptosis in cancer cells (Edsall, Van Brocklyn, Cuvillier, Kleuser, & Spiegel, 1998) and to inhibit growth of xenografted breast cancer tumors in mice (Nava et al., 2000).
Based on the lead compound dihydroxyaurone, which was reported to be a SphK inhibitor that has anti-tumor properties in a mouse model of breast cancer, a group of SphK inhibitors (termed SKI I—V) were developed (French et al., 2003). Of these, SKI-II (also called SKi) has found the most use although it inhibits both SphK1 and SphK2 and possibly other targets, complicating understanding of the mechanism by which it promotes cell death in a variety of cancer types (French et al., 2003; Truman, GarciaBarros, Obeid, & Hannun, 2014). L-threo-dihydrosphingosine, also known as Safingol, was initially reported to be a protein kinase C inhibitor but was later proven to also inhibit SphKs (Buehrer & Bell, 1992; Tsukamoto et al., 2015). Additionally, a number of SphK inhibitors from various bacterial and fungal sources have been discovered. S-15183a and S-15183b were produced by the fungus Zopfiella inermis, B-5354a, B-5354b, and B-5454c were all derived from a marine bacteria from the genus Ruegeria, and F-12509A was isolated from the fungus Trichopezizella barbata (Kono, Sugiura, & Kohama, 2002; Kono, Tanaka, Mizuno, et al., 2000; Kono, Tanaka, Ogita, Hosoya, & Kohama, 2000; Kono et al., 2001).
SK1-I (BML-258), a sphingosine analogue that is an isozyme-specific SphK1 inhibitor, promoted cell death in a number of in vitro cancer models (Paugh et al., 2008). Two other SphK1 inhibitors, SKI-178 and PF-543, also occupy the substrate-binding site of SphK and thus prevent the phosphorylation of sphingosine (Hengst et al., 2017; Schnute et al., 2012; Wang, Knapp, Pyne, Pyne, & Elkins, 2014).
SG-12 and SG-14 were the first two SphK2 inhibitors discovered, but ABC294640 has been used more frequently. ABC294640 displays activity in vitro and in vivo to prevent cancer growth and is currently in phase II clinical trials (Antoon et al., 2010; Beljanski, Knaak, & Smith, 2010; Kim, Kim, et al., 2005). K145 was recently developed as a more specific and potent SphK2 inhibitor and has shown promise in vivo in murine xenografts for suppressing breast tumor growth (K. Liu et al., 2013).
S1P is not only a bioactive mediator; it is also the ultimate sphingolipid metabolite and the only exit point for metabolism of all sphingolipids. S1P lyase is responsible for the irreversible cleavage of S1P into hexadecenal and ethanolamine phosphate, which are substrates for metabolism in various metabolic pathways. 1-deoxysphinganine-1-phosphate, an S1P analogue, is a naturally occurring phosphorylated sphingoid base that inhibits S1P lyase (Stoffel & Grol, 1974). S1P lyase activity can also be inhibited by 2-vinylsphinganine-1-phosphate (Boumendjel & Miller, 1994). S1P lyase is a pyridoxal phosphate (B6)-dependent enzyme but the often used S1P lyase inhibitor 4-deoxypyridoxine, which interferes with many B6dependent enzymes is generally not used anymore because this lack of specificity results in high levels of toxicity (Serra & Saba, 2010).
In addition, therapeutics can regulate S1P activity by altering S1P receptor expression or activity. As a prime example, FTY720, also known as fingolimod/gilenya, is a myriocin derivative and sphingosine analogue that is phosphorylated in vivo to its active form FTY720-P mainly by SphK2; inhibiting S1P activity through S1P receptor 1, 3, 4, and 5 binding (Brinkmann et al., 2002). In vivo studies have shown that FTY720-P is also able to inhibit S1P lyase activity directly, as S1P lyase mRNA and protein expression were not altered (Bandhuvula, Tam, Oskouian, & Saba, 2005). Thus FTY720, already FDA approved for multiple sclerosis, is now being actively investigated as a cancer therapeutic.
3. DELIVERABLE AND CLINICALLY RELEVANTSPHINGOLIPID-BASED DRUGS FOR CANCER
3.1. Methods for Delivery of Sphingolipids and Mimetics to Cells
Instead of relying on increasing “flow through the faucets or clogging the drains,” we can also simply add water to the sink externally to overflow the system (Fig. 1). Over the past 10 to 15 years, a number of approaches have been developed to enhance delivery of sphingolipids and analogues directly to cells in an effort to alter cell physiology, usually to disrupt the growth of tumor cells. Of these delivered sphingolipids, the most common is ceramide, with numerous chemical modifications and synthetic derivatives created to increase cell permeability, efficacy, and chemotoxicity. One method of increasing specificity of ceramide derivatives for negatively charged cellular compartments (such as the mitochondria) is the introduction of a positive charge on the fatty acid residue by the addition of a pyridine structure. Studies with pyridine-ceramides showed that they localized more readily to the mitochondria and induced the release of cytochrome c, a marker of apoptosis (Novgorodov et al., 2005; Senkal et al., 2006). These positively charged ceramides selectively induced pancreatic cancer cell death (Beckham, Lu, et al., 2013).
Another approach is to shorten the ceramide fatty-acid chain length to C2, C6, or C8 (Liu, Beckman, & Foroozesh, 2013). These synthetic shortchain ceramides have increased cell permeability and have been shown to have potent anti-tumor effects (Obeid, Linardic, Karolak, & Hannun, 1993; Wolff, Dobrowsky, Bielawska, Obeid, & Hannun, 1994). C6 ceramide has been utilized for the development of a novel nanoliposome formulation termed ceramide nanoliposomes (CNLs). These CNLs can deliver C6 ceramides effectively as well as synergizing with current chemotherapeuitcs by encapsulating them within the same nanoliposomes (Adiseshaiah et al., 2013; Kester et al., 2015; Ma, Mou, Ding, Zou, & Huang, 2016; Sun, Fox, Adhikary, Kester, & Pearlman, 2008). The efficacy of these CNLs is currently being investigated in a phase 1 clinical trial in patients with solid tumors.
Finally, several sphingosine analogues have been researched as promising sphingolipid drugs, such as safingol (Schwartz et al., 1995, 1997), N,N-DMS (Edsall et al., 1998; Sweeney et al., 1996), fingolimod (Chun & Hartung, 2010; White, Alshaker, Cooper, Winkler, & Pchejetski, 2016), and (R)-FTY720 methyl ether (ROME) (Watson et al., 2013). These sphingosine analogues display anti-tumor effects as inhibitors of SphK enzyme activity and/or S1P receptors signaling.
3.2. Sphingolipid-Based Therapeutics Clinical Pipeline
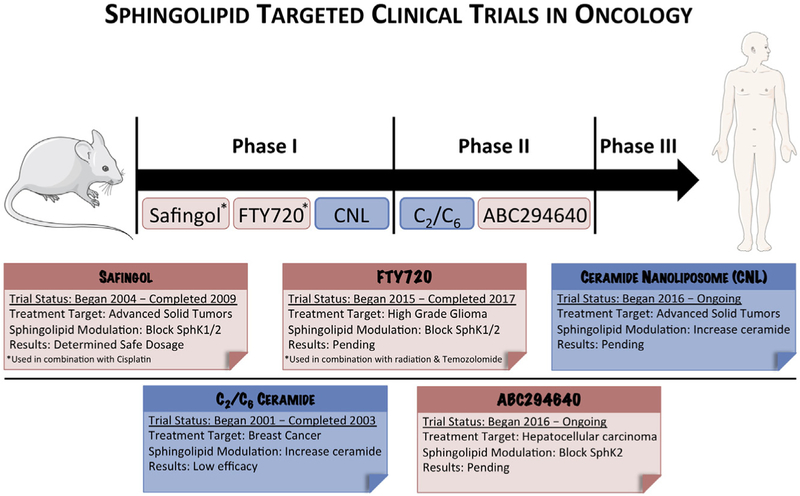
A search through the clinicaltrials.gov database for various sphingolipid keywords such as S1P, sphingosine, ceramide, and sphingomyelin returned an impressive number of completed, ongoing, or recruiting clinical trials. For the sake of brevity and clarity, this review is focused on the three largest classes of sphingolipid-related drugs currently in clinical trials: synthetic ceramides, S1P antagonists, and SphK inhibitors. While clinical trials for other sphingolipid pathway inhibitors exist (notably amitriptyline, an acid SMase inhibitor), they are not as far along in the clinical pipeline and thus will not be discussed here (ClinicalTrials.gov) (Beckmann, Sharma, Gulbins, Becker, & Edelmann, 2014). Fig. 3 depicts various sphingolipid-based therapeutics that have entered clinical oncology trials.
Figure 3.
An outline of the sphingolipid-based therapeutics currently undergoing clinical trials for cancer treatment. Although there are few sphingolipid therapies currently in the clinic, many other sphingolipid-based therapeutics are showing promise in preclinical models.
Synthetic ceramides are ceramide analogues that have been modified to increase their tumor-specific toxicity, membrane permeability, or efficacy. As mentioned earlier, numerous synthetic ceramides exist, but the most common involve modifications to the hydrocarbon chain length, rendering it shorter. In 2003, topical administration of C2 and C6 ceramides were employed in a phase II study against cutaneous breast cancer, with mixed results (Clinical trial #: NCT00008320) (ClinicalTrials.gov). The trial found that while the C2 and C6 ceramides had little to no toxicity, patients yielded only a 4% response rate, shelving topical administration of short chain ceramides as a potential chemotherapeutic (Jatoi et al., 2003). However, by encapsulating a C6 ceramide into stable nontoxic nanoliposomes, researchers found that they could increase chemotoxicity as compared to free ceramide alone in in vivo models (Kester et al., 2015; Sun et al., 2008). These CNLs entered a phase I study beginning in 2017 for patients with advanced solid tumors at the University of Maryland, the University of Virginia, and the Medical University of South Carolina (Clinical trial #: NCT02834611) (ClinicalTrials.gov).
For the past decade, S1P has been the focus of intense study due to its tumorigenic properties. As a result, numerous inhibitors against the enzyme responsible for its generation, SphK, have been advanced as candidates for clinical trials. Of these, the furthest down the clinical pipeline is ABC294640. ABC294640 acts as a specific SphK2 inhibitor, with numerous studies demonstrating its efficacy and utility as a potential chemotherapeutic across a broad range of cancer types (Schrecengost, Keller, Schiewer, Knudsen, & Smith, 2015; Yang et al., 2015). According to clinicaltrials. gov, six separate trials used ABC294640 against a variety of cancers including pancreatic cancer, cholangiocarcinoma, multiple myeloma, and hepatocellular carcinoma. Trademarked as YELIVA, ABC294640 is currently being employed in a single-arm phase IIA clinical study in the treatment of cholangiocarcinoma at the Mayo Clinic and the MD Anderson Cancer Center (Clinical trial #: NCT03377179).
By far the most common sphingosine analogue in clinical trials today, FTY720 (also known as fingolimod and trademarked as gilenya) has been referenced 94 times on clinicaltrials.gov and is an FDA-approved therapy for multiple sclerosis (Ingwersen et al., 2012). Though reports differ, the most universal mechanism for its potential anti-cancer properties is outcompeting sphingosine for the binding pocket of SphK, thus limiting the conversion of sphingosine to S1P (Billich et al., 2003; Lim et al., 2011; Paugh, Payne, Barbour, Milstien, & Spiegel, 2003). The first trial using FTY720 as a potential treatment for cancer began in 2015, when a phase I safety study of FTY720 with radiation and temozolomide against glioblastoma or anaplastic astrocytoma was conducted at Johns Hopkins University (Clinical trial #: NCT02490930) (ClinicalTrials.gov). For more information regarding FTY720’s role in cancer treatment, see the 2016 review by White et al. (2016). FTY720 has shown promise as a cancer therapy partly because of its ability to prevent pro-tumorigenic T cell transport out of the thymus by blocking the S1P receptor (S1PR) on T cells (Matloubian et al., 2004; Segui, Andrieu-Abadie, Jaffrezou, Benoist, & Levade, 2006). In addition, safingol is currently in the clinical setting for a phase 1 clinical trial for metastatic solid tumors in combination with fenretinide. Another clinical trial already completed used safingol combined with cisplatin for metastatic solid tumors. This trial showed that administering safingol with cisplatin was safe for cancer patients and ascertained as a recommended dose for safingol in a future phase 2 trial (Dickson et al., 2011).
Other, glyco- and sphingolipid analogues have also been shown to regulate immune components, which are dysregulated in cancer. α-Galactosylceramide, a sphingolipid produced by marine sponges, activates invariant natural killer T cells, which recruits anti-tumor immune cells (Berkers & Ovaa, 2005) (McEwen-Smith, Salio, & Cerundolo, 2015). A chemical modified α-galactosylceramide, KRN7000, has been used in a phase 1/2 clinical trial for treatment of patients with NCT00698776 myeloma (Berkers & Ovaa, 2005; Ishikawa et al., 2005). Other sphingolipid-targeting drugs are being developed that have immune-regulating properties. The ACDase inhibitor LCL521 decreased viability of the tumor-promoting myeloidderived suppressor cells (MDSCs) in mice (F. Liu et al., 2016; Umansky & Sevko, 2013). In addition, SphK2-dependent dihydrosphingosine-1phosphate also ameliorates MDSCs in mice models of solid tumors (Barth et al., 2013). Finally, GM3 ganglioside, overexpressed in human melanomas, was recently targeted with a preventative vaccine in mice models that resulted in rejection of B16 melanoma tumors in a CD8+ dependent manner (Mazorra, Mesa, Fernandez, & Fernandez, 2008).
4. SPHINGOLIPIDS SYNERGIZE WITH CHEMOTHERAPEUTICS
Recent studies have shown that elevating endogenous levels of ceramide by opening metabolic “faucets” or closing some of the enzymatic “drains” can augment the efficacy of conventional treatments in cancer. In addition, exogenous delivery platforms for sphingolipid mimetics can also elevate endogenous ceramide levels (Fig. 1). This section will review underlying combinatorial/synergistic cytotoxic mechanisms between elevated ceramide levels/decreased S1P levels and chemotherapeutic regulation of signal transduction cascades.
4.1. Synergistic Therapeutic Strategies Against Glucosylceramide Synthase
Perhaps the most prevalent area of research regarding synergy of sphingolipid therapies involves inhibiting GCS to limit conversion of pro-apoptotic ceramide into the less toxic glucosylceramide in cancer cells. Additionally, inhibitors of GCS downregulate the multidrug resistance gene 1 (MDR1) and its protein product P-glycoprotein (P-gp) reducing drug resistance (Gouaze et al., 2005; Morad & Cabot, 2013).
In breast cancer cell lines, an antisense-driven GCS knockdown led to higher ceramide levels and increased caspase-3 activity, which resensitized these cells to doxorubicin (Liu, Han, Giuliano, Hansen, & Cabot, 2000). The same lab later showed that using a pharmacological approach to inhibit GCS could also synergize with doxorubicin (an anthracycline chemotherapeutic). However, the novel, mixed backbone oligonucleotide antisense to GCS (MBO-asGCS), showed greater than 10-fold sensitivity to doxorubicin in doxorubicin-resistant lines of breast and ovarian cancer, through increased drug uptake and C18-ceramide—induced apoptosis (Liu et al., 2004; Patwardhan et al., 2009). The MBO-asGCS was also found to decrease levels of MDR-1, cSrc kinase, nuclear β-catenin, and P-gp that sensitized breast, ovarian, cervical, and colon cancer cells to doxorubicin (Liu et al., 2010). Finally, the sensitization of ovarian cancer cells by PPMP or MBO-asGCS to doxorubicin was shown to only be effective in p53 mutants and could be mitigated by CerS inhibition using FB1 (Liu, Patwardhan, Bhinge, et al., 2011).
Using GCS antisense RNA and paclitaxel together enhanced ceramide formation, but, interestingly, also showed 10-fold higher levels of intracellular drug and 80% less P-gp beginning to suggest the relationship between P-gp and GCS. PPMP also restored sensitivity to vinblastine by enhancing its uptake by threefold, and diminished MDR1 expression by 84% in a vinblastine-resistant cell line (Gouaze et al., 2005). Additional studies using shRNAs against GCS confirmed increased apoptosis following treatment with doxorubicin and vinblastine while also displaying synergy with daunorubicin (Sun et al., 2006) and paclitaxel (Sun et al., 2010).
A study in 2001 that showed the GCS inhibitor DL-threo-phenyl-2hexadecanoylamino-3-pyrrolidino-1-propanol (PPPP) synergizes with vincristine in leukemic cell lines (Olshefski & Ladisch, 2001) paved the way for a more in-depth analysis of 65 human leukemic samples. This study established not only that GCS expression levels correlated with increased multidrug resistance in patients and cell lines but also that PPMP could sensitize leukemic cell lines to doxorubicin (Xie et al., 2008). Counterintuitively, one study in leukemia cell lines showed that by blocking GCS using both PDMP and PPMP, cells were no longer sensitive to daunorubicin due to an anti-apoptotic effect of accumulated galactosylceramides (Grazide et al., 2004).
GCS inhibition using PDMP has also been shown to synergize with new treatment options, including BCL2-inhibitor ABT-737 (Casson et al., 2013) and Imatinib (Baran, Bielawski, Gunduz, & Ogretmen, 2011) in multiple leukemia cell lines, with temozolomide in glioblastoma multiforme (Giussani, Tringali, Riboni, Viani, & Venerando, 2014), with curcumin in melanoma (Yu, Li, Qiu, & Sun, 2012), and with sorafenib in hepatocellular carcinoma (Stefanovic et al., 2016). Efficacy of glucosylceramide synthase inhibition by PPMP and tamoxifen (an anti-estrogen with P-gp antagonistic properties) was also shown to be augmented in combination with C6 ceramide in neuroepithelioma and ovarian cancer cells, respectively (Chapman, Gouaze-Andersson, Karimi, Messner, & Cabot, 2011; Spinedi, Bartolomeo, & Piacentini, 1998).
4.2. Synergistic Therapeutic Strategies Against Sphingosine Kinase
As mentioned previously, S1P is the most pro-mitogenic sphingolipid. SphK inhibitors decrease S1P levels in cells and increase levels of the more-toxic sphingosine leading frequently to increased ceramides as well.
The first of many synergy studies investigating SphK inhibition in blood cancers was performed in 2006 when a combination of F-12509A, a microbially derived weak inhibitor of SphK and CerK, and doxorubicin or etoposide effectively resensitized AML cell lines, which had developed chemoresistance (Bonhoure et al., 2006). Additionally, decreased SphK1 activity resulting from treatment with etoposide in combination with Perifosine (an Akt inhibitor) was able to increase Fas-receptore—mediated cell death in human T-leukemia—mimicking Jurkat cells (Nyakern, Cappellini, Mantovani, & Martelli, 2006). Another SphK1 inhibitor (Ski), but not a SphK2 inhibitor (ROME), was able to synergistically induce autophagic cell death in T-ALL cells in combination with vincristine (Evangelisti et al., 2014). A different SphK2 inhibitor (Ski-II) was able to synergize with ABT-263 (a BCL2-like protein inhibitor) by causing accumulation of sphingosine and ceramide, leading to in vitro death of multiple human leukemia cell lines (Casson et al., 2013). In myeloma lines, K145 (a specific SphK2 inhibitor) in combination with Bortezomib, a receptor tyrosine kinase (RTK) inhibitor, synergistically induced ER stress and led to apoptosis (Wallington-Beddoe et al., 2017).
This combination of SphK and RTK inhibitors was also successful in solid tumor models. A combination of ABC294640 (a SphK2 inhibitor) and another RTK inhibitor, sorafenib, synergistically induced cytotoxicity in hepatocellular carcinoma (Beljanski, Lewis, & Smith, 2011), pancreatic adenocarcinoma, kidney carcinoma (Beljanski, Knaak, Zhuang, & Smith, 2011), and cholangiocarcinoma (Ding et al., 2016). Finally, there appears to be promise in combining the previously mentioned Ski-II with temozolomide (a monofunctional alkylator) to synergistically increase caspasedependent cell death in glioblastoma multiforme (Noack, Choi, Richter, Kopp-Schneider, & Regnier-Vigouroux, 2014).
4.3. Synergistic Therapeutic Strategies Against Acid Ceramidase
In multiple prostate cancer cell lines, the ACDase inhibitor NOE synergized with the EGFR inhibitor PD135035 to show drastic increases in both the estimated levels of ceramide as well as the percentage of cells undergoing apoptosis (Mimeault, Pommery, & Henichart, 2003). Interestingly, despite the success of NOE in the previous study, another group studying prostate cancer found that NOE did not synergize when combined with another compound, 4-HPR (fenretinide). However, using a different ACDase inhibitor, DM102, with fenretinide synergistically decreased cell viability, enhanced caspase activity, led to a 30-fold increase in ROS levels, and increased dihydroceramide levels. Although these results suggested activation of de novo ceramide synthesis, myriocin was unable to rescue from this cytotoxic effect, whereas surprisingly, antioxidant vitamin E did convey a partial rescue (Gouaze-Andersson et al., 2011).
Further work in head and neck squamous cell carcinoma showed that while overexpression of ACDase promoted resistance to Fas ligand-induced cell death, Fas-induced death was also potentiated by siRNAs against ACDase. Additionally, inhibition of ACDase using LCL 204 in the presence of Fas ligand led to cellular effects including: decreased survivin protein, increased cleaved caspase-8, and increased caspase-3 levels in vitro. The combination of these same two compounds also decreased tumor volume and promoted survival in in vivo xenograft models (Elojeimy et al., 2007). ACDase inhibition additionally showed synergy with daunorubicin in hepatocarcinoma cells. While NOE or siRNAs against ACDase in combination with daunorubicin increased the ceramide:S1P ratio resulting in increased cell death in vitro, the siRNA and daunorubicin combination was also efficacious in decreasing tumor volume in in vivo xenograft models. This synergistic cell death, which did not occur in healthy mouse hepatocytes, was preceded by markers of mitochondrial-induced apoptosis including ultrastructural changes, generation of ROS, release of cytochrome C, and caspase-3 activation (Morales et al., 2007).
4.4. Synergistic Therapeutic Strategies Using Exogenous Sphingolipids
Short-chain ceramides including C2 and C6 species have increased solubility and cell permeability compared with longer-chain, more physiological, ceramides. Short-chain ceramides have proven to be synergistic with docetaxel, doxorubicin, methotrexate and pemetrexed (antifolates) in murine melanoma cell lines, and osteosarcoma (Chapman et al., 2011; Feng, Li, Liu, Yang, & Zhang, 2014; Zhai, Sun, Han, Jin, & Zhang, 2015). Combining C6-ceramide with ACDase inhibitor (DM102), AZD-8055 (mTORC1/2 dual inhibitor), or AT406 (a small molecule IAP antagonist) was synergistically efficacious in breast cancer cell lines, hepatocellular carcinoma lines, or pancreatic cell lines and primary samples, respectively (Flowers et al., 2012; M. Liu, Gu, Guo, & Fan, 2016; Zhao, Sun, Zhang, Zhang, & Zhang, 2016).
Nanotechnology platforms to improve ceramide delivery including CNL have shown synergy with sorafenib, PPMP, FTY720, gemcitabine, vinblastine, and tamoxifen/verapamil/cyclosporine A in in vitro and in vivo models of melanoma, breast, natural killer cell leukemia, pancreatic, hepatocellular carcinoma, and colorectal cancer, respectively (Adiseshaiah et al., 2013; Chapman et al., 2010; Jiang et al., 2011; Tran, Smith, Kester, & Robertson, 2008; Watters et al., 2013). It has been hypothesized that vinblastine and other microtubule destabilizers such as chloroquine and vincristine synergize with C6 ceramide by reducing autophagosome formation and switching ceramide-induced autophagy into ceramide-induced cell death (Adiseshaiah et al., 2013; Lima, Milstien, & Spiegel, 2017; Young et al., 2016). A recent study showed that the use of CNL combined with adoptive T cell transfer immunotherapy in mice bearing liver tumors was able to reduce the number of tumor-associated macrophages and decreased the ability of these immune cells to suppress the anti-tumor properties (Li et al., 2018).
5. CONCLUSION
In the ever-growing quest to better understand the basis of cancer, numerous tools and collaborative efforts have emerged as powerful initiatives for research and medicine. The Cancer Genome Atlas represents one of the largest such endeavors, chronicling patient samples and organizing them by cancer type, gene expression, RNA profile, and mutation profile, among others. With the increasing sensitivity and selectivity as well as availability of mass spectrometry devices capable of creating full sphingolipid profiles for patients, we would argue that these vital class of lipids should be included when discussing patient diagnosis and treatment plans, as every year new research is published establishing links between sphingolipids and cancer.
As researchers continue to define the essential cellular functions of sphingolipids, their pivotal role in cancer progression is beginning to come into focus. Discoveries that established links between sphingolipid metabolism and carcinogenesis have cemented the need for a more complete understanding of these bioactive lipids. The medical advances in systems biology, big data, genomics, and epigenetics will synergize with advances in lipidomics to further the precision medicine revolution. All of these tools will further advance the utility of sphingolipid-based chemotherapeutics in oncology. Sphingolipid metabolism inhibitors, sphingolipidmodulated immunotherapies, cannabinoids, and sphingolipid analogues hold the promise for entirely new approach to cancer treatments.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants 5 P01 CA171983 and R01s CA208396, CA167535 to MK; R01 GM043880 to SS.
Footnotes
Conflict of Interest
Penn State Research Foundation has licensed ceramide nanoliposomes and other sphingolipid-based nanoscale therapeutics to Keystone Nano, Inc (State College, PA). MK is Chief Medical Officer and cofounder of Keystone Nano, Inc.
REFERENCES
- Adiseshaiah PP, Clogston JD, McLeland CB, Rodriguez J, Potter TM, Neun BW, et al. (2013). Synergistic combination therapy with nanoliposomal C6-ceramide and vinblastine is associated with autophagy dysfunction in hepatocarcinoma and colorectal cancer models. Cancer Letters, 337(2), 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akao Y, Banno Y, Nakagawa Y, Hasegawa N, Kim TJ, & Murate T (2006). High expression of sphingosine kinase 1 and S1P receptors in chemotherapy-resistant prostate cancer PC3 cells and their camptothecin-induced up-regulation. Biochemical and Biophysical Research Communications, 342(4), 1284–1290. [DOI] [PubMed] [Google Scholar]
- Albouz S, Hauw JJ, Berwald-Netter Y, Boutry JM, Bourdon R, & Baumann N (1981). Tricyclic antidepressants induce sphingomyelinase deficiency in fibroblast and neuroblastoma cell cultures. Biomedicine, 35(7e8), 218–220. [PubMed] [Google Scholar]
- Alfonso P, Pampin S, Estrada J, Rodriguez-Rey JC, Giraldo P, Sancho J, et al. (2005). Miglustat (NB-DNJ) works as a chaperone for mutated acid beta-glucosidase in cells transfected with several Gaucher disease mutations. Blood Cells, Molecules & Diseases, 35(2), 268–276. [DOI] [PubMed] [Google Scholar]
- Amtmann E, & Zoller M (2005). Stimulation of CD95-induced apoptosis in T-cells by a subtype specific neutral sphingomyelinase inhibitor. Biochemical Pharmacology, 69(8), 1141–1148. [DOI] [PubMed] [Google Scholar]
- Amtmann E, Zoller M, & Schilling G (2000). Neutral sphingomyelinase-inhibiting guanidines prevent herpes simplex virus-1 replication. Drugs Under Experimental and Clinical Research, 26(2), 57–65. [PubMed] [Google Scholar]
- Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, Elliott S, et al. (2010). Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology, 151(11), 5124–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama Y, Sobue S, Mizutani N, Inoue C, Kawamoto Y, Nishizawa Y, et al. (2017). Modulation of the sphingolipid rheostat is involved in paclitaxel resistance of the human prostate cancer cell line PC3-PR. Biochemical and Biophysical Research Communications, 486(2), 551–557. [DOI] [PubMed] [Google Scholar]
- Ardail D, Maalouf M, Boivin A, Chapet O, Bodennec J, Rousson R, et al. (2009). Diversity and complexity of ceramide generation after exposure of jurkat leukemia cells to irradiation. International Journal of Radiation Oncology, Biology, Physics, 73(4), 1211–1218. [DOI] [PubMed] [Google Scholar]
- Arenz C, Thutewohl M, Block O, Waldmann H, Altenbach HJ, & Giannis A (2001). Manumycin A and its analogues are irreversible inhibitors of neutral sphingomyelinase. Chembiochem, 2(2), 141–143. [DOI] [PubMed] [Google Scholar]
- Arimochi H, & Morita K (2008). Desipramine induces apoptotic cell death through nonmitochondrial and mitochondrial pathways in different types of human colon carcinoma cells. Pharmacology, 81(2), 164–172. [DOI] [PubMed] [Google Scholar]
- Aureli M, Murdica V, Loberto N, Samarani M, Prinetti A, Bassi R, et al. (2014). Exploring the link between ceramide and ionizing radiation. Glycoconjugate Journal, 31(6e7), 449–459. [DOI] [PubMed] [Google Scholar]
- Bai A, Mao C, Jenkins RW, Szulc ZM, Bielawska A, & Hannun YA (2017). Anticancer actions of lysosomally targeted inhibitor, LCL521, of acid ceramidase. PLoS One, 12(6), e0177805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai A, Meier GP, Wang Y, Luberto C, Hannun YA, & Zhou D (2004). Prodrug modification increases potassium tricyclo[5.2.1.0(2,6)]-decan-8-yl dithiocarbonate (D609) chemical stability and cytotoxicity against U937 leukemia cells. The Journal of Pharmacology and Experimental Therapeutics, 309(3), 1051–1059. [DOI] [PubMed] [Google Scholar]
- Bandhuvula P, Tam YY, Oskouian B, & Saba JD (2005). The immune modulator FTY720 inhibits sphingosine-1-phosphate lyase activity. Journal of Biological Chemistry, 280(40), 33697–33700. [DOI] [PubMed] [Google Scholar]
- Baran Y, Bielawski J, Gunduz U, & Ogretmen B (2011). Targeting glucosylceramide synthase sensitizes imatinib-resistant chronic myeloid leukemia cells via endogenous ceramide accumulation. Journal of Cancer Research and Clinical Oncology, 137(10), 1535–1544. [DOI] [PubMed] [Google Scholar]
- Barbado MV, Medrano M, Caballero-Velazquez T, Alvarez-Laderas I, SanchezAbarca LI, Garcia-Guerrero E, et al. (2017). Cannabinoid derivatives exert a potent anti-myeloma activity both in vitro and in vivo. International Journal of Cancer, 140(3), 674–685. [DOI] [PubMed] [Google Scholar]
- Barth BM, Shanmugavelandy SS, Kaiser JM, McGovern C, Altinoglu EI, Haakenson JK, et al. (2013). PhotoImmunoNanoTherapy reveals an anticancer role for sphingosine kinase 2 and dihydrosphingosine-1-phosphate. ACS Nano, 7(3), 2132–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham TH, Cheng JC, Marrison ST, Norris JS, & Liu X (2013). Interdiction of sphingolipid metabolism to improve standard cancer therapies. Advances in Cancer Research, 117, 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham TH, Lu P, Cheng JC, Zhao D, Turner LS, Zhang X, et al. (2012). Acid ceramidase-mediated production of sphingosine 1-phosphate promotes prostate cancer invasion through upregulation of cathepsin B. International Journal of Cancer, 131(9), 2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham TH, Lu P, Jones EE, Marrison T, Lewis CS, Cheng JC, et al. (2013). LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria. The Journal of Pharmacology and Experimental Therapeutics, 344(1), 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann N, Sharma D, Gulbins E, Becker KA, & Edelmann B (2014). Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons. Frontiers in Physiology, 5, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedia C, Triola G, Casas J, Llebaria A, & Fabrias G (2005). Analogs of the dihydroceramide desaturase inhibitor GT11 modified at the amide function: Synthesis and biological activities. Organic & Biomolecular Chemistry, 3(20), 3707–3712. [DOI] [PubMed] [Google Scholar]
- Beljanski V, Knaak C, & Smith CD (2010). A novel sphingosine kinase inhibitor induces autophagy in tumor cells. The Journal of Pharmacology and Experimental Therapeutics, 333(2), 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljanski V, Knaak C, Zhuang Y, & Smith CD (2011). Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Investigational New Drugs, 29(6), 1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljanski V, Lewis CS, & Smith CD (2011). Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biology & Therapy, 11(5), 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkers CR, & Ovaa H (2005). Immunotherapeutic potential for ceramide-based activators of iNKT cells. Trends in Pharmacological Sciences, 26(5), 252–257. [DOI] [PubMed] [Google Scholar]
- Bhabak KP, & Arenz C (2013). Novel drugs targeting sphingolipid metabolism. Handbook of Experimental Pharmacology, 215, 187–196. [DOI] [PubMed] [Google Scholar]
- Bielawska A, Greenberg MS, Perry D, Jayadev S, Shayman JA, McKay C, et al. (1996). (1S,2R)-D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol as an inhibitor of ceramidase. Journal of Biological Chemistry, 271(21), 12646–12654. [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, & Baumruker T (2003). Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. Journal of Biological Chemistry, 278(48), 47408–47415. [DOI] [PubMed] [Google Scholar]
- Bonhoure E, Pchejetski D, Aouali N, Morjani H, Levade T, Kohama T, et al. (2006). Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia, 20(1), 95–102. [DOI] [PubMed] [Google Scholar]
- Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, & Kolesnick R (1995). Ceramide synthase mediates daunorubicin-induced apoptosis: An alternative mechanism for generating death signals. Cell, 82(3), 405–414. [DOI] [PubMed] [Google Scholar]
- Boumendjel A, & Miller SP (1994). Synthesis of sphingosine-1-phosphate and dihydrosphingosine-1-phosphate. The Journal of Lipid Research, 35(12), 2305–2311. [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. (2002). The immune modulator FTY720 targets sphingosine 1-phosphate receptors. Journal of Biological Chemistry, 277(24), 21453–21457. [DOI] [PubMed] [Google Scholar]
- Buehrer BM, & Bell RM (1992). Inhibition of sphingosine kinase in vitro and in platelets. Implications for signal transduction pathways. Journal of Biological Chemistry, 267(5), 3154–3159. [PubMed] [Google Scholar]
- Cakir Z, Saydam G, Sahin F, & Baran Y (2011). The roles of bioactive sphingolipids in resveratrol-induced apoptosis in HL60: Acute myeloid leukemia cells. J Cancer Res Clin Oncol, 137(2), 279–286. [DOI] [PubMed] [Google Scholar]
- Camacho L, Meca-Cortes O, Abad JL, Garcia S, Rubio N, Diaz A, et al. (2013). Acid ceramidase as a therapeutic target in metastatic prostate cancer. The Journal of Lipid Research, 54(5), 1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals D, Perry DM, Jenkins RW, & Hannun YA (2011). Drug targeting of sphingolipid metabolism: Sphingomyelinases and ceramidases. British Journal of Pharmacology, 163(4), 694–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B, Donaldson JC, & Obeid L (2015). Sphingolipids in the DNA damage response. Advances in Biological Regulation, 58, 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson L, Howell L, Mathews LA, Ferrer M, Southall N, Guha R, et al. (2013). Inhibition of ceramide metabolism sensitizes human leukemia cells to inhibition of BCL2-like proteins. PLoS One, 8(1), e54525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, et al. (2002). De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. Journal of Biological Chemistry, 277(15), 12587–12595. [DOI] [PubMed] [Google Scholar]
- Chapman JV, Gouaze-Andersson V, Karimi R, Messner MC, & Cabot MC (2011). P-glycoprotein antagonists confer synergistic sensitivity to short-chain ceramide in human multidrug-resistant cancer cells. Experimental Cell Research, 317(12), 1736–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JV, Gouaze-Andersson V, Messner MC, Flowers M, Karimi R, Kester M, et al. (2010). Metabolism of short-chain ceramide by human cancer cellse implications for therapeutic approaches. Biochemical Pharmacology, 80(3), 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JC, Bai A, Beckham TH, Marrison ST, Yount CL, Young K, et al. (2013). Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. Journal of Clinical Investigation, 123(10), 4344–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, & Hartung HP (2010). Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clinical Neuropharmacology, 33(2), 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianchi F, Papucci L, Schiavone N, Lulli M, Magnelli L, Vinci MC, et al. (2008). Cannabinoid receptor activation induces apoptosis through tumor necrosis factor alphamediated ceramide de novo synthesis in colon cancer cells. Clinical Cancer Research, 14(23), 7691–7700. [DOI] [PubMed] [Google Scholar]
- Cowart LA, & Obeid LM (2007). Yeast sphingolipids: Recent developments in understanding biosynthesis, regulation, and function. Biochimica et Biophysica Acta, 1771(3), 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TM (2005). Substrate reduction therapy for lysosomal storage diseases. Acta Paediatrica Supplement, 94(447), 69–75. discussion 57. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. (1996). Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature, 381(6585), 800–803. [DOI] [PubMed] [Google Scholar]
- Dagan A, Wang C, Fibach E, & Gatt S (2003). Synthetic, non-natural sphingolipid analogs inhibit the biosynthesis of cellular sphingolipids, elevate ceramide and induce apoptotic cell death. Biochimica et Biophysica Acta, 1633(3), 161–169. [DOI] [PubMed] [Google Scholar]
- Darroch PI, Dagan A, Granot T, He X, Gatt S, & Schuchman EH (2005). A lipid analogue that inhibits sphingomyelin hydrolysis and synthesis, increases ceramide, and leads to cell death. The Journal of Lipid Research, 46(11), 2315–2324. [DOI] [PubMed] [Google Scholar]
- Deigner HP, Claus R, Bonaterra GA, Gehrke C, Bibak N, Blaess M, et al. (2001). Ceramide induces aSMase expression: Implications for oxLDL-induced apoptosis. The FASEB Journal, 15(3), 807–814. [DOI] [PubMed] [Google Scholar]
- Delgado A, Casas J, Llebaria A, Abad JL, & Fabrias G (2006). Inhibitors of sphingolipid metabolism enzymes. Biochimica et Biophysica Acta, 1758(12), 1957–1977. [DOI] [PubMed] [Google Scholar]
- Dick TE, Hengst JA, Fox TE, Colledge AL, Kale VP, Sung SS, et al. (2015). The apoptotic mechanism of action of the sphingosine kinase 1 selective inhibitor SKI-178 in human acute myeloid leukemia cell lines. The Journal of Pharmacology and Experimental Therapeutics, 352(3), 494–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MA, Carvajal RD, Merrill AH Jr., Gonen M, Cane LM, & Schwartz GK (2011). A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clinical Cancer Research, 17(8), 2484–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Chaiteerakij R, Moser CD, Shaleh H, Boakye J, Chen G, et al. (2016). Antitumor effect of the novel sphingosine kinase 2 inhibitor ABC294640 is enhanced by inhibition of autophagy and by sorafenib in human cholangiocarcinoma cells. Oncotarget, 7(15), 20080–20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper JM, Xia Z, Smith RA, Zhuang Y, Wang W, & Smith CD (2011). Discovery and evaluation of inhibitors of human ceramidase. Molecular Cancer Therapeutics, 10(11), 2052–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, & Spiegel S (1998). N,Ndimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: Modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry, 37(37), 12892–12898. [DOI] [PubMed] [Google Scholar]
- Eliyahu E, Shtraizent N, He X, Chen D, Shalgi R, & Schuchman EH (2011). Identification of cystatin SA as a novel inhibitor of acid ceramidase. Journal of Biological Chemistry, 286(41), 35624–35633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elojeimy S, Liu X, McKillop JC, El-Zawahry AM, Holman DH, Cheng JY, et al. (2007). Role of acid ceramidase in resistance to FasL: Therapeutic approaches based on acid ceramidase inhibitors and FasL gene therapy. Molecular Therapy, 15(7), 1259–1263. [DOI] [PubMed] [Google Scholar]
- Evangelisti C, Evangelisti C, Teti G, Chiarini F, Falconi M, Melchionda F, et al. (2014). Assessment of the effect of sphingosine kinase inhibitors on apoptosis, unfolded protein response and autophagy of T-cell acute lymphoblastic leukemia cells; indications for novel therapeutics. Oncotarget, 5(17), 7886–7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng LX, Li M, Liu YJ, Yang SM, & Zhang N (2014). Synergistic enhancement of cancer therapy using a combination of ceramide and docetaxel. International Journal of Molecular Sciences, 15(3), 4201–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figuera-Losada M, Stathis M, Dorskind JM, Thomas AG, Bandaru VV, Yoo SW, et al. (2015). Cambinol, a novel inhibitor of neutral sphingomyelinase 2 shows neuroprotective properties. PLoS One, 10(5), e0124481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers M, Fabrias G, Delgado A, Casas J, Abad JL, & Cabot MC (2012). C6-ceramide and targeted inhibition of acid ceramidase induce synergistic decreases in breast cancer cell growth. Breast Cancer Research and Treatment, 133(2), 447–458. [DOI] [PubMed] [Google Scholar]
- French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, et al. (2003). Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Research, 63(18), 5962–5969. [PubMed] [Google Scholar]
- Galadari S, Rahman A, Pallichankandy S, & Thayyullathil F (2015). Tumor suppressive functions of ceramide: Evidence and mechanisms. Apoptosis, 20(5), 689–711. [DOI] [PubMed] [Google Scholar]
- Garcia-Barros M, Coant N, Kawamori T, Wada M, Snider AJ, Truman JP, et al. (2016). Role of neutral ceramidase in colon cancer. The FASEB Journal, 30(12), 4159–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani P, Tringali C, Riboni L, Viani P, & Venerando B (2014). Sphingolipids: Key regulators of apoptosis and pivotal players in cancer drug resistance. International Journal of Molecular Sciences, 15(3), 4356–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaze-Andersson V, Flowers M, Karimi R, Fabrias G, Delgado A, Casas J, et al. (2011). Inhibition of acid ceramidase by a 2-substituted aminoethanol amide synergistically sensitizes prostate cancer cells to N-(4-hydroxyphenyl) retinamide. The Prostate, 71(10), 1064–1073. [DOI] [PubMed] [Google Scholar]
- Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, & Cabot MC (2005). Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Research, 65(9), 3861–3867. [DOI] [PubMed] [Google Scholar]
- Graf C, Klumpp M, Habig M, Rovina P, Billich A, Baumruker T, et al. (2008). Targeting ceramide metabolism with a potent and specific ceramide kinase inhibitor. Molecular Pharmacology, 74(4), 925–932. [DOI] [PubMed] [Google Scholar]
- Grazide S, Terrisse AD, Lerouge S, Laurent G, & Jaffrezou JP (2004). Cytoprotective effect of glucosylceramide synthase inhibition against daunorubicin-induced apoptosis in human leukemic cell lines. Journal of Biological Chemistry, 279(18), 18256–18261. [DOI] [PubMed] [Google Scholar]
- Gustafsson K, Christensson B, Sander B, & Flygare J (2006). Cannabinoid receptormediated apoptosis induced by R(þ)-methanandamide and Win55,212–2 is associated with ceramide accumulation and p38 activation in mantle cell lymphoma. Molecular Pharmacology, 70(5), 1612–1620. [DOI] [PubMed] [Google Scholar]
- Gustafsson K, Sander B, Bielawski J, Hannun YA, & Flygare J (2009). Potentiation of cannabinoid-induced cytotoxicity in mantle cell lymphoma through modulation of ceramide metabolism. Molecular Cancer Research, 7(7), 1086–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, et al. (1994). Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. The Journal of Experimental Medicine, 180(2), 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin EC, Perez CA, & Brady LW (2008). Perez and Brady’s principles and practice of radiation oncology (5th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. [Google Scholar]
- Hanada K (2003). Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochimica et Biophysica Acta, 1632(1–3), 16–30. [DOI] [PubMed] [Google Scholar]
- Hannun YA, & Obeid LM (2018). Sphingolipids and their metabolism in physiology and disease. Nature Reviews. Molecular Cell Biology, 19(3), 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S, Nakashima S, Kiyono T, Sawada M, Yoshimura S, Iwama T, et al. (2004). p53-Independent ceramide formation in human glioma cells during gamma-radiationinduced apoptosis. Cell Death and Differentiation, 11(8), 853–861. [DOI] [PubMed] [Google Scholar]
- Hengst JA, Dick TE, Sharma A, Doi K, Hegde S, Tan SF, et al. (2017). SKI-178: A multitargeted inhibitor of sphingosine kinase and microtubule dynamics demonstrating therapeutic efficacy in acute myeloid leukemia models. Cancer Translational Medicine, 3(4), 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillig I, Warnecke D, & Heinz E (2005). An inhibitor of glucosylceramide synthase inhibits the human enzyme, but not enzymes from other organisms. Bioscience, Biotechnology, and Biochemistry, 69(9), 1782–1785. [DOI] [PubMed] [Google Scholar]
- Holman DH, Turner LS, El-Zawahry A, Elojeimy S, Liu X, Bielawski J, et al. (2008). Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemotherapy and Pharmacology, 61(2), 231–242. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Cheng HH, Chou CT, Kuo CC, Lu YC, Tseng LL, et al. (2007). Desipramine-induced Ca2þ movement and cytotoxicity in PC3 human prostate cancer cells. Toxicology in Vitro, 21(3), 449–456. [DOI] [PubMed] [Google Scholar]
- Ingwersen J, Aktas O, Kuery P, Kieseier B, Boyko A, & Hartung HP (2012). Fingolimod in multiple sclerosis: Mechanisms of action and clinical efficacy. Clinical Immunology, 142(1), 15–24. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, et al. (2005). A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clinical Cancer Research, 11(5), 1910–1917. [DOI] [PubMed] [Google Scholar]
- Jaffrezou JP, Herbert JM, Levade T, Gau MN, Chatelain P, & Laurent G (1991). Reversal of multidrug resistance by calcium channel blocker SR33557 without photoaffinity labeling of P-glycoprotein. Journal of Biological Chemistry, 266(29), 19858–19864. [PubMed] [Google Scholar]
- Jaffrezou JP, Levade T, Bettaieb A, Andrieu N, Bezombes C, Maestre N, et al. (1996). Daunorubicin-induced apoptosis: Triggering of ceramide generation through sphingomyelin hydrolysis. The EMBO Journal, 15(10), 2417–2424. [PMC free article] [PubMed] [Google Scholar]
- Jatoi A, Suman VJ, Schaefer P, Block M, Loprinzi C, Roche P, et al. (2003). A phase II study of topical ceramides for cutaneous breast cancer. Breast Cancer Research and Treatment, 80(1), 99–104. [DOI] [PubMed] [Google Scholar]
- Jiang Y, DiVittore NA, Kaiser JM, Shanmugavelandy SS, Fritz JL, Heakal Y, et al. (2011). Combinatorial therapies improve the therapeutic efficacy of nanoliposomal ceramide for pancreatic cancer. Cancer Biology & Therapy, 12(7), 574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester M, Bassler J, Fox TE, Carter CJ, Davidson JA, & Parette MR (2015). Preclinical development of a C6-ceramide NanoLiposome, a novel sphingolipid therapeutic. Biological Chemistry, 396(6e7), 737–747. [DOI] [PubMed] [Google Scholar]
- Kim JW, Inagaki Y, Mitsutake S, Maezawa N, Katsumura S, Ryu YW, et al. (2005). Suppression of mast cell degranulation by a novel ceramide kinase inhibitor, the F-12509A olefin isomer K1. Biochimica et Biophysica Acta, 1738(1e3), 82–90. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kim YW, Inagaki Y, Hwang YA, Mitsutake S, Ryu YW, et al. (2005). Synthesis and evaluation of sphingoid analogs as inhibitors of sphingosine kinases. Bioorganic & Medicinal Chemistry, 13(10), 3475–3485. [DOI] [PubMed] [Google Scholar]
- Klutzny S, Lesche R, Keck M, Kaulfuss S, Schlicker A, Christian S, et al. (2017). Functional inhibition of acid sphingomyelinase by Fluphenazine triggers hypoxiaspecific tumor cell death. Cell Death & Disease, 8(3), e2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick R, & Fuks Z (2003). Radiation and ceramide-induced apoptosis. Oncogene, 22(37), 5897–5906. [DOI] [PubMed] [Google Scholar]
- Kolzer M, Arenz C, Ferlinz K, Werth N, Schulze H, Klingenstein R, et al. (2003). Phosphatidylinositol-3,5-bisphosphate is a potent and selective inhibitor of acid sphingomyelinase. Biological Chemistry, 384(9), 1293–1298. [DOI] [PubMed] [Google Scholar]
- Kono K, Sugiura M, & Kohama T (2002). Inhibition of recombinant sphingosine kinases by novel inhibitors of microbial origin, F-12509A and B-5354c. Journal of Antibiotics, 55(1), 99–103. [DOI] [PubMed] [Google Scholar]
- Kono K, Tanaka M, Mizuno T, Kodama K, Ogita T, & Kohama T (2000). B-535a, b and c, new sphingosine kinase inhibitors, produced by a marine bacterium; taxonomy, fermentation, isolation, physico-chemical properties and structure determination. Journal of Antibiotics, 53(8), 753–758. [DOI] [PubMed] [Google Scholar]
- Kono K, Tanaka M, Ogita T, Hosoya T, & Kohama T (2000). F-12509A, a new sphingosine kinase inhibitor, produced by a discomycete. Journal of Antibiotics, 53(5), 459–466. [DOI] [PubMed] [Google Scholar]
- Kono K, Tanaka M, Ono Y, Hosoya T, Ogita T, & Kohama T (2001). S-15183a and b, new sphingosine kinase inhibitors, produced by a fungus. Journal of Antibiotics, 54(5), 415–420. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Tripal P, Reichel M, Muhle C, Rhein C, Muehlbacher M, et al. (2010). Functional inhibitors of acid sphingomyelinase (FIASMAs): A novel pharmacological group of drugs with broad clinical applications. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 26(1), 9–20. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Tripal P, Reichel M, Terfloth L, Bleich S, Wiltfang J, et al. (2008). Identification of new functional inhibitors of acid sphingomyelinase using a structureproperty-activity relation model. Journal of Medicinal Chemistry, 51(2), 219–237. [DOI] [PubMed] [Google Scholar]
- Kouchi H, Nakamura K, Fushimi K, Sakaguchi M, Miyazaki M, Ohe T, et al. (1999). Manumycin A, inhibitor of ras farnesyltransferase, inhibits proliferation and migration of rat vascular smooth muscle cells. Biochemical and Biophysical Research Communications, 264(3), 915–920. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Pinter M, & Csaba G (2000). Effect of glucosphingolipid synthesis inhibitor (PPMP and PDMP) treatment on Tetrahymena pyriformis: Data on the evolution of the signaling system. Cell Biochemistry and Function, 18(4), 269–280. [DOI] [PubMed] [Google Scholar]
- Lavie Y, Cao H, Volner A, Lucci A, Han TY, Geffen V, et al. (1997). Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. Journal of Biological Chemistry, 272(3), 1682–1687. [DOI] [PubMed] [Google Scholar]
- Lee WK, & Kolesnick RN (2017). Sphingolipid abnormalities in cancer multidrug resistance: Chicken or egg? Cellular Signalling, 38, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, & Zhang N (2015). Ceramide: Therapeutic potential in combination therapy for cancer treatment. Current Drug Metabolism, 17(1), 37–51. [DOI] [PubMed] [Google Scholar]
- Li G, Liu D, Kimchi ET, Kaifi JT, Qi X, Manjunath Y, et al. (2018). Nanoliposome C6-ceramide increases the anti-tumor immune response and slows growth of liver tumors in mice. Gastroenterology, 154(4), 1024–1036. e1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KG, Tonelli F, Li Z, Lu X, Bittman R, Pyne S, et al. (2011). FTY720 analogues as sphingosine kinase 1 inhibitors: Enzyme inhibition kinetics, allosterism, proteasomal degradation, and actin rearrangement in MCF-7 breast cancer cells. Journal of Biological Chemistry, 286(21), 18633–18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S, Milstien S, & Spiegel S (2017). Sphingosine and sphingosine kinase 1 involvement in endocytic membrane trafficking. Journal of Biological Chemistry, 292(8), 3074–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke T, Wilkening G, Lansmann S, Moczall H, Bartelsen O, Weisgerber J, et al. (2001). Stimulation of acid sphingomyelinase activity by lysosomal lipids and sphingolipid activator proteins. Biological Chemistry, 382(2), 283–290. [DOI] [PubMed] [Google Scholar]
- Liu B, & Hannun YA (1997). Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. Journal of Biological Chemistry, 272(26), 16281–16287. [DOI] [PubMed] [Google Scholar]
- Liu F, Li X, Lu C, Bai A, Bielawski J, Bielawska A, et al. (2016). Ceramide activates lysosomal cathepsin B and cathepsin D to attenuate autophagy and induces ER stress to suppress myeloid-derived suppressor cells. Oncotarget, 7(51), 83907–83925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Beckman BS, & Foroozesh M (2013). A review of ceramide analogs as potential anticancer agents. Future Medicinal Chemistry, 5(12), 1405–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Guo TL, Hait NC, Allegood J, Parikh HI, Xu W, et al. (2013). Biological characterization of 3-(2-amino-ethyl)-5-[3-(4-butoxyl-phenyl)-propylidene]-thiazolidine2,4-dione (K145) as a selective sphingosine kinase-2 inhibitor and anticancer agent. PLoS One, 8(2), e56471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Gu P, Guo W, & Fan X (2016). C6 ceramide sensitizes the anti-hepatocellular carcinoma (HCC) activity by AZD-8055, a novel mTORC1/2 dual inhibitor. Tumour Biology, 37(8), 11039–11048. [DOI] [PubMed] [Google Scholar]
- Liu YY, Gupta V, Patwardhan GA, Bhinge K, Zhao Y, Bao J, et al. (2010). Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Molecular Cancer, 9, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Han TY, Giuliano AE, Hansen N, & Cabot MC (2000). Uncoupling ceramide glycosylation by transfection of glucosylceramide synthase antisense reverses adriamycin resistance. Journal of Biological Chemistry, 275(10), 7138–7143. [DOI] [PubMed] [Google Scholar]
- Liu YY, Han TY, Yu JY, Bitterman A, Le A, Giuliano AE, et al. (2004). Oligonucleotides blocking glucosylceramide synthase expression selectively reverse drug resistance in cancer cells. The Journal of Lipid Research, 45(5), 933–940. [DOI] [PubMed] [Google Scholar]
- Liu YY, Patwardhan GA, Bhinge K, Gupta V, Gu X, & Jazwinski SM (2011). Suppression of glucosylceramide synthase restores p53-dependent apoptosis in mutant p53 cancer cells. Cancer Research, 71(6), 2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Patwardhan GA, Xie P, Gu X, Giuliano AE, & Cabot MC (2011). Glucosylceramide synthase, a factor in modulating drug resistance, is overexpressed in metastatic breast carcinoma. International Journal of Oncology, 39(2), 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou B, Dong J, Li Y, Ding T, Bi T, Li Y, et al. (2014). Pharmacologic inhibition of sphingomyelin synthase (SMS) activity reduces apolipoprotein-B secretion from hepatocytes and attenuates endotoxin-mediated macrophage inflammation. PLoS One, 9(7), e102641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano J, Menendez S, Morales A, Ehleiter D, Liao WC, Wagman R, et al. (2001). Cell autonomous apoptosis defects in acid sphingomyelinase knockout fibroblasts. Journal of Biological Chemistry, 276(1), 442–448. [DOI] [PubMed] [Google Scholar]
- Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, et al. (2002). Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. Journal of Biological Chemistry, 277(43), 41128–41139. [DOI] [PubMed] [Google Scholar]
- Lucci A, Cho WI, Han TY, Giuliano AE, Morton DL, & Cabot MC (1998). Glucosylceramide: A marker for multiple-drug resistant cancers. Anticancer Research, 18(1B), 475–480. [PubMed] [Google Scholar]
- Lu T, Huang CC, Lu YC, Lin KL, Liu SI, Wang BW, et al. (2009). Desipramine-induced Ca-independent apoptosis in Mg63 human osteosarcoma cells: Dependence on P38 mitogen-activated protein kinase-regulated activation of caspase 3. Clinical and Experimental Pharmacology & Physiology, 36(3), 297–303. [DOI] [PubMed] [Google Scholar]
- Ma YY, Mou XZ, Ding YH, Zou H, & Huang DS (2016). Delivery systems of ceramide in targeted cancer therapy: Ceramide alone or in combination with other antitumor agents. Expert Opinion on Drug Delivery, 13(10), 1397–1406. [DOI] [PubMed] [Google Scholar]
- Mahdy AE, Cheng JC, Li J, Elojeimy S, Meacham WD, Turner LS, et al. (2009). Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer. Molecular Therapy, 17(3), 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala SM, Frommer BR, Thornton RA, Kurtz MB, Young NM, Cabello MA, et al. (1994). Inhibition of serine palmitoyl-transferase activity by lipoxamycin. Journal of Antibiotics, 47(3), 376–379. [DOI] [PubMed] [Google Scholar]
- Mandala SM, Thornton RA, Frommer BR, Curotto JE, Rozdilsky W, Kurtz MB, et al. (1995). The discovery of australifungin, a novel inhibitor of sphinganine N-acyltransferase from Sporormiella australis. Producing organism, fermentation, isolation, and biological activity. Journal of Antibiotics, 48(5), 349–356. [DOI] [PubMed] [Google Scholar]
- Mandala SM, Thornton RA, Frommer BR, Dreikorn S, & Kurtz MB (1997). Viridiofungins, novel inhibitors of sphingolipid synthesis. Journal of Antibiotics, 50(4), 339–343. [DOI] [PubMed] [Google Scholar]
- Marchesini N, Luberto C, & Hannun YA (2003). Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. Journal of Biological Chemistry, 278(16), 13775–13783. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. (2004). Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature, 427(6972), 355–360. [DOI] [PubMed] [Google Scholar]
- Mazorra Z, Mesa C, Fernandez A, & Fernandez LE (2008). Immunization with a GM3 ganglioside nanoparticulated vaccine confers an effector CD8(þ) T cells-mediated protection against melanoma B16 challenge. Cancer Immunology, Immunotherapy, 57(12), 1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen-Smith RM, Salio M, & Cerundolo V (2015). The regulatory role of invariant NKT cells in tumor immunity. Cancer Immunology Research, 3(5), 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock KA, & Merrill AH Jr. (1988). Inhibition of serine palmitoyltransferase in vitro and long-chain base biosynthesis in intact Chinese hamster ovary cells by betachloroalanine. Biochemistry, 27(18), 7079–7084. [DOI] [PubMed] [Google Scholar]
- Meng A, Luberto C, Meier P, Bai A, Yang X, Hannun YA, et al. (2004). Sphingomyelin synthase as a potential target for D609-induced apoptosis in U937 human monocytic leukemia cells. Experimental Cell Research, 292(2), 385–392. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Pommery N, & Henichart JP (2003). Synergistic antiproliferative and apoptotic effects induced by epidermal growth factor receptor and protein kinase a inhibitors in human prostatic cancer cell lines. International Journal of Cancer, 106(1), 116–124. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Pommery N, Wattez N, Bailly C, & Henichart JP (2003). Antiproliferative and apoptotic effects of anandamide in human prostatic cancer cell lines: Implication of epidermal growth factor receptor down-regulation and ceramide production. The Prostate, 56(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, & Kawasaki T (1995). Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochemical and Biophysical Research Communications, 211(2), 396–403. [DOI] [PubMed] [Google Scholar]
- Modrak DE, Gold DV, & Goldenberg DM (2006). Sphingolipid targets in cancer therapy. Molecular Cancer Therapeutics, 5(2), 200–208. [DOI] [PubMed] [Google Scholar]
- Monick MM, Mallampalli RK, Bradford M, McCoy D, Gross TJ, Flaherty DM, et al. (2004). Cooperative prosurvival activity by ERK and Akt in human alveolar macrophages is dependent on high levels of acid ceramidase activity. The Journal of Immunology, 173(1), 123–135. [DOI] [PubMed] [Google Scholar]
- Morad SA, & Cabot MC (2013). Ceramide-orchestrated signalling in cancer cells. Nature Reviews Cancer, 13(1), 51–65. [DOI] [PubMed] [Google Scholar]
- Morales A, Paris R, Villanueva A, Llacuna L, Garcia-Ruiz C, & FernandezCheca JC (2007). Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene, 26(6), 905–916. [DOI] [PubMed] [Google Scholar]
- Nara F, Tanaka M, Hosoya T, Suzuki-Konagai K, & Ogita T (1999). Scyphostatin, a neutral sphingomyelinase inhibitor from a discomycete, Trichopeziza mollissima: Taxonomy of the producing organism, fermentation, isolation, and physico-chemical properties. Journal of Antibiotics, 52(6), 525–530. [DOI] [PubMed] [Google Scholar]
- Nara F, Tanaka M, Masuda-Inoue S, Yamasato Y, Doi-Yoshioka H, SuzukiKonagai K, et al. (1999). Biological activities of scyphostatin, a neutral sphingomyelinase inhibitor from a discomycete, Trichopeziza mollissima. Journal of Antibiotics, 52(6), 531–535. [DOI] [PubMed] [Google Scholar]
- Nava VE, Cuvillier O, Edsall LC, Kimura K, Milstien S, Gelmann EP, et al. (2000). Sphingosine enhances apoptosis of radiation-resistant prostate cancer cells. Cancer Research, 60(16), 4468–4474. [PubMed] [Google Scholar]
- Newton J, Lima S, Maceyka M, & Spiegel S (2015). Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Experimental Cell Research, 333(2), 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack J, Choi J, Richter K, Kopp-Schneider A, & Regnier-Vigouroux A (2014). A sphingosine kinase inhibitor combined with temozolomide induces glioblastoma cell death through accumulation of dihydrosphingosine and dihydroceramide, endoplasmic reticulum stress and autophagy. Cell Death & Disease, 5, e1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novgorodov SA, Szulc ZM, Luberto C, Jones JA, Bielawski J, Bielawska A, et al. (2005). Positively charged ceramide is a potent inducer of mitochondrial permeabilization. Journal of Biological Chemistry, 280(16), 16096–16105. [DOI] [PubMed] [Google Scholar]
- Nyakern M, Cappellini A, Mantovani I, & Martelli AM (2006). Synergistic induction of apoptosis in human leukemia T cells by the Akt inhibitor perifosine and etoposide through activation of intrinsic and Fas-mediated extrinsic cell death pathways. Molecular Cancer Therapeutics, 5(6), 1559–1570. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, & Hannun YA (1993). Programmed cell death induced by ceramide. Science, 259(5102), 1769–1771. [DOI] [PubMed] [Google Scholar]
- Ogretmen B (2018). Sphingolipid metabolism in cancer signalling and therapy. Nature Reviews Cancer, 18(1), 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okudaira C, Ikeda Y, Kondo S, Furuya S, Hirabayashi Y, Koyano T, et al. (2000). Inhibition of acidic sphingomyelinase by xanthone compounds isolated from Garcinia speciosa. Journal of Enzyme Inhibition, 15(2), 129–138. [DOI] [PubMed] [Google Scholar]
- Olshefski RS, & Ladisch S (2001). Glucosylceramide synthase inhibition enhances vincristine-induced cytotoxicity. International Journal of Cancer, 93(1), 131–138. [DOI] [PubMed] [Google Scholar]
- Orr Gandy KA, & Obeid LM (2013). Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: Review of sphingosine kinase inhibitors. Biochimica et Biophysica Acta, 1831(1), 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouian B, & Saba JD (2010). Cancer treatment strategies targeting sphingolipid metabolism. Advances in Experimental Medicine and Biology, 688, 185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouian B, Sooriyakumaran P, Borowsky AD, Crans A, Dillard-Telm L, Tam YY, et al. (2006). Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proceedings of the National Academy of Sciences of the United States of America, 103(46), 17384–17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overkleeft HS, Renkema GH, Neele J, Vianello P, Hung IO, Strijland A, et al. (1998). Generation of specific deoxynojirimycin-type inhibitors of the non-lysosomal glucosylceramidase. Journal of Biological Chemistry, 273(41), 26522–26527. [DOI] [PubMed] [Google Scholar]
- Pastukhov O, Schwalm S, Zangemeister-Wittke U, Fabbro D, Bornancin F, Japtok L, et al. (2014). The ceramide kinase inhibitor NVP-231 inhibits breast and lung cancer cell proliferation by inducing M phase arrest and subsequent cell death. British Journal of Pharmacology, 171(24), 5829–5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan GA, Zhang QJ, Yin D, Gupta V, Bao J, Senkal CE, et al. (2009). A new mixed-backbone oligonucleotide against glucosylceramide synthase sensitizes multidrug-resistant tumors to apoptosis. PLoS One, 4(9), e6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, et al. (2008). A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood, 112(4), 1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh SW, Payne SG, Barbour SE, Milstien S, & Spiegel S (2003). The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Letters, 554(1e2), 189–193. [DOI] [PubMed] [Google Scholar]
- Pchejetski D, Doumerc N, Golzio M, Naymark M, Teissie J, Kohama T, et al. (2008). Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Molecular Cancer Therapeutics, 7(7), 1836–1845. [DOI] [PubMed] [Google Scholar]
- Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, & Hannun YA (2000). Serine palmitoyltransferase regulates de novo ceramide generation during etoposideinduced apoptosis. Journal of Biological Chemistry, 275(12), 9078–9084. [DOI] [PubMed] [Google Scholar]
- Platt FM, Neises GR, Dwek RA, & Butters TD (1994). N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. Journal of Biological Chemistry, 269(11), 8362–8365. [PubMed] [Google Scholar]
- Platt FM, Neises GR, Karlsson GB, Dwek RA, & Butters TD (1994). N-butyldeoxygalactonojirimycin inhibits glycolipid biosynthesis but does not affect N-linked oligosaccharide processing. Journal of Biological Chemistry, 269(43), 27108–27114. [PubMed] [Google Scholar]
- Pyne NJ, & Pyne S (2010). Sphingosine 1-phosphate and cancer. Nature Reviews Cancer, 10(7), 489–503. [DOI] [PubMed] [Google Scholar]
- Raisova M, Goltz G, Bektas M, Bielawska A, Riebeling C, Hossini AM, et al. (2002). Bcl-2 overexpression prevents apoptosis induced by ceramidase inhibitors in malignant melanoma and HaCaT keratinocytes. FEBS Letters, 516(1e3), 47–52. [DOI] [PubMed] [Google Scholar]
- Realini N, Solorzano C, Pagliuca C, Pizzirani D, Armirotti A, Luciani R, et al. (2013). Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity. Scientific Reports, 3, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J (2004). MS-209 schering. Current Opinion in Investigational Drugs, 5(12), 1340e1347. [PubMed] [Google Scholar]
- Rosa R, Marciano R, Malapelle U, Formisano L, Nappi L, D’Amato C, et al. (2013). Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clinical Cancer Research, 19(1), 138–147. [DOI] [PubMed] [Google Scholar]
- Ryland LK, Fox TE, Liu X, Loughran TP, & Kester M (2011). Dysregulation of sphingolipid metabolism in cancer. Cancer Biology & Therapy, 11(2), 138–149. [DOI] [PubMed] [Google Scholar]
- Saad AF, Meacham WD, Bai A, Anelli V, Elojeimy S, Mahdy AE, et al. (2007). The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biology & Therapy, 6(9), 1455–1460. [DOI] [PubMed] [Google Scholar]
- Saddoughi SA, Garrett-Mayer E, Chaudhary U, O’Brien PE, Afrin LB, Day TA, et al. (2011). Results of a phase II trial of gemcitabine plus doxorubicin in patients with recurrent head and neck cancers: Serum C(1)(8)-ceramide as a novel biomarker for monitoring response. Clinical Cancer Research, 17(18), 6097–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saied EM, & Arenz C (2014). Small molecule inhibitors of ceramidases. Cellular Physiology and Biochemistry, 34(1), 197–212. [DOI] [PubMed] [Google Scholar]
- Sakata A, Ochiai T, Shimeno H, Hikishima S, Yokomatsu T, Shibuya S, et al. (2007). Acid sphingomyelinase inhibition suppresses lipopolysaccharide-mediated release of inflammatory cytokines from macrophages and protects against disease pathology in dextran sulphate sodium-induced colitis in mice. Immunology, 122(1), 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsel L, Zaidel G, Drumgoole HM, Jelovac D, Drachenberg C, Rhee JG, et al. (2004). The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. The Prostate, 58(4), 382–393. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Galve-Roperh I, Canova C, Brachet P, & Guzman M (1998). Delta9tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Letters, 436(1), 6–10. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Galve-Roperh I, Rueda D, & Guzman M (1998). Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the Delta9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Molecular Pharmacology, 54(5), 834–843. [DOI] [PubMed] [Google Scholar]
- Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, et al. (1996). Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell, 86(2), 189–199. [DOI] [PubMed] [Google Scholar]
- Sasak VW, Ordovas JM, Elbein AD, & Berninger RW (1985). Castanospermine inhibits glucosidase I and glycoprotein secretion in human hepatoma cells. The Biochemical Journal, 232(3), 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Dash R, Liu X, Norris JS, Sarkar D, et al. (2010). Ceramide plays a prominent role in MDA-7/IL-24-induced cancer-specific apoptosis. Journal of Cellular Physiology, 222(3), 546–555. [DOI] [PubMed] [Google Scholar]
- Sauer L, Nunes J, Salunkhe V, Skalska L, Kohama T, Cuvillier O, et al. (2009). Sphingosine kinase 1 inhibition sensitizes hormone-resistant prostate cancer to docetaxel. International Journal of Cancer, 125(11), 2728–2736. [DOI] [PubMed] [Google Scholar]
- Saul R, Chambers JP, Molyneux RJ, & Elbein AD (1983). Castanospermine, a tetrahydroxylated alkaloid that inhibits beta-glucosidase and beta-glucocerebrosidase. Archives of Biochemistry and Biophysics, 221(2), 593–597. [DOI] [PubMed] [Google Scholar]
- Sawada M, Nakashima S, Kiyono T, Nakagawa M, Yamada J, Yamakawa H, et al. (2001). p53 regulates ceramide formation by neutral sphingomyelinase through reactive oxygen species in human glioma cells. Oncogene, 20(11), 1368–1378. [DOI] [PubMed] [Google Scholar]
- Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, & Kelly JW (2002). Chemical chaperones increase the cellular activity of N370S beta-glucosidase: A therapeutic strategy for Gaucher disease. Proceedings of the National Academy of Sciences of the United States of America, 99(24), 15428–15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlatti F, Sala G, Ricci C, Maioli C, Milani F, Minella M, et al. (2007). Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Letters, 253(1), 124–130. [DOI] [PubMed] [Google Scholar]
- Schnute ME, McReynolds MD, Kasten T, Yates M, Jerome G, Rains JW, et al. (2012). Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. The Biochemical Journal, 444(1), 79–88. [DOI] [PubMed] [Google Scholar]
- Schrecengost RS, Keller SN, Schiewer MJ, Knudsen KE, & Smith CD (2015). Downregulation of critical oncogenes by the selective SK2 inhibitor ABC294640 hinders prostate cancer progression. Molecular Cancer Research, 13(12), 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz A, Mousallem T, Venkataramani M, Persaud-Sawin DA, Zucker A, Luberto C, et al. (2006). The CLN9 protein, a regulator of dihydroceramide synthase. Journal of Biological Chemistry, 281(5), 2784–2794. [DOI] [PubMed] [Google Scholar]
- Schwartz GK, Haimovitz-Friedman A, Dhupar SK, Ehleiter D, Maslak P, Lai L, et al. (1995). Potentiation of apoptosis by treatment with the protein kinase C-specific inhibitor safingol in mitomycin C-treated gastric cancer cells. Journal of the National Cancer Institute, 87(18), 1394–1399. [DOI] [PubMed] [Google Scholar]
- Schwartz GK, Ward D, Saltz L, Casper ES, Spiess T, Mullen E, et al. (1997). A pilot clinical/pharmacological study of the protein kinase C-specific inhibitor safingol alone and in combination with doxorubicin. Clinical Cancer Research, 3(4), 537–543. [PubMed] [Google Scholar]
- Segui B, Andrieu-Abadie N, Jaffrezou JP, Benoist H, & Levade T (2006). Sphingolipids as modulators of cancer cell death: Potential therapeutic targets. Biochimica et Biophysica Acta, 1758(12), 2104–2120. [DOI] [PubMed] [Google Scholar]
- Selzner M, Bielawska A, Morse MA, Rudiger HA, Sindram D, Hannun YA, et al. (2001). Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Research, 61(3), 1233–1240. [PubMed] [Google Scholar]
- Senkal CE, Ponnusamy S, Rossi MJ, Sundararaj K, Szulc Z, Bielawski J, et al. (2006). Potent antitumor activity of a novel cationic pyridinium-ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. The Journal of Pharmacology and Experimental Therapeutics, 317(3), 1188–1199. [DOI] [PubMed] [Google Scholar]
- Serra M, & Saba JD (2010). Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Advances in Enzyme Regulation, 50(1), 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Xiong H, Li J, Liao W, Wang L, Wu J, et al. (2011). Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-kappaB pathway in human non-small cell lung cancer. Clinical Cancer Research, 17(7), 1839–1849. [DOI] [PubMed] [Google Scholar]
- Spinedi A, Bartolomeo SD, & Piacentini M (1998). Apoptosis induced by N-hexanoylsphingosine in CHP-100 cells associates with accumulation of endogenous ceramide and is potentiated by inhibition of glucocerebroside synthesis. Cell Death and Differentiation, 5(9), 785–791. [DOI] [PubMed] [Google Scholar]
- Stefanovic M, Tutusaus A, Martinez-Nieto GA, Barcena C, de Gregorio E, Moutinho C, et al. (2016). Targeting glucosylceramide synthase upregulation reverts sorafenib resistance in experimental hepatocellular carcinoma. Oncotarget, 7(7), 8253–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W, & Grol M (1974). Chemistry and biochemistry of 1-desoxysphinganine 1-phosphonate (dihydrosphingosine-1-phosphonate). Chemistry and Physics of Lipids, 13(4), 372–388. [DOI] [PubMed] [Google Scholar]
- Strelow A, Bernardo K, Adam-Klages S, Linke T, Sandhoff K, Kronke M, et al. (2000). Overexpression of acid ceramidase protects from tumor necrosis factor-induced cell death. The Journal of Experimental Medicine, 192(5), 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M, Willians M, Dulaney JT, & Moser HW (1975). Ceramidase and ceramide synthesis in human kidney and cerebellum. Description of a new alkaline ceramidase. Biochimica et Biophysica Acta, 398(1), 125–131. [DOI] [PubMed] [Google Scholar]
- Sun Y, Fox T, Adhikary G, Kester M, & Pearlman E (2008). Inhibition of corneal inflammation by liposomal delivery of short-chain, C-6 ceramide. Journal of Leukocyte Biology, 83(6), 1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang T, Gao P, Meng B, Gao Y, Wang X, et al. (2010). Targeting glucosylceramide synthase downregulates expression of the multidrug resistance gene MDR1 and sensitizes breast carcinoma cells to anticancer drugs. Breast Cancer Research and Treatment, 121(3), 591–599. [DOI] [PubMed] [Google Scholar]
- Sun YL, Zhou GY, Li KN, Gao P, Zhang QH, Zhen JH, et al. (2006). Suppression of glucosylceramide synthase by RNA interference reverses multidrug resistance in human breast cancer cells. Neoplasma, 53(1), 1–8. [PubMed] [Google Scholar]
- Sundaram KS, & Lev M (1984). Inhibition of sphingolipid synthesis by cycloserine in vitro and in vivo. Journal of Neurochemistry, 42(2), 577–581. [DOI] [PubMed] [Google Scholar]
- Sweeney EA, Sakakura C, Shirahama T, Masamune A, Ohta H, Hakomori S, et al. (1996). Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. International Journal of Cancer, 66(3), 358–366. [DOI] [PubMed] [Google Scholar]
- Taha TA, Osta W, Kozhaya L, Bielawski J, Johnson KR, Gillanders WE, et al. (2004). Down-regulation of sphingosine kinase-1 by DNA damage: Dependence on proteases and p53. Journal of Biological Chemistry, 279(19), 20546–20554. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Inanami O, Asanuma T, & Kuwabara M (2006). Effects of ceramide inhibition on radiation-induced apoptosis in human leukemia MOLT-4 cells. Journal of Radiation Research, 47(1), 19–25. [DOI] [PubMed] [Google Scholar]
- Tan SF, Liu X, Fox TE, Barth BM, Sharma A, Turner SD, et al. (2016). Acid ceramidase is upregulated in AML and represents a novel therapeutic target. Oncotarget, 7(50), 83208–83222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SF, Pearson JM, Feith DJ, & Loughran TP Jr. (2017). The emergence of acid ceramidase as a therapeutic target for acute myeloid leukemia. Expert Opinion on Therapeutic Targets, 21(6), 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Nara F, Yamasato Y, Masuda-Inoue S, Doi-Yoshioka H, Kumakura S, et al. (1999). Macquarimicin A inhibits membrane-bound neutral sphingomyelinase from rat brain. Journal of Antibiotics, 52(7), 670–673. [DOI] [PubMed] [Google Scholar]
- Testai FD, Landek MA, Goswami R, Ahmed M, & Dawson G (2004). Acid sphingomyelinase and inhibition by phosphate ion: Role of inhibition by phosphatidyl-myoinositol 3,4,5-triphosphate in oligodendrocyte cell signaling. Journal of Neurochemistry, 89(3), 636–644. [DOI] [PubMed] [Google Scholar]
- Tran MA, Smith CD, Kester M, & Robertson GP (2008). Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clinical Cancer Research, 14(11), 3571–3581. [DOI] [PubMed] [Google Scholar]
- Triola G, Fabrias G, Casas J, & Llebaria A (2003). Synthesis of cyclopropene analogues of ceramide and their effect on dihydroceramide desaturase. The Journal of Organic Chemistry, 68(26), 9924–9932. [DOI] [PubMed] [Google Scholar]
- Triola G, Fabrias G, & Llebaria A (2001). Synthesis of a cyclopropene analogue of ceramide, a potent inhibitor of dihydroceramide desaturase. This work was supported by the Direccion General de Ensenanza Superior e Investigacion Cientifica (grant PB97–1171) and the Departament d’Universitats, Recerca i Societat de la Informacio, Generalitat de Catalunya (grant 1999-SGR 00187 and a Predoctoral fellowship to G.T.). We thank Dr. J. Casas, Dr. A. Delgado, and Dr. J. Joglar for their help in different aspects of this work Angewandte Chemie, 40(10), 1960–1962. [PubMed] [Google Scholar]
- Truman JP, Garcia-Barros M, Obeid LM, & Hannun YA (2014). Evolving concepts in cancer therapy through targeting sphingolipid metabolism. Biochimica et Biophysica Acta, 1841(8), 1174–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto S, Huang Y, Kumazoe M, Lesnick C, Yamada S, Ueda N, et al. (2015). Sphingosine kinase-1 protects multiple myeloma from apoptosis driven by cancerspecific inhibition of RTKs. Molecular Cancer Therapeutics, 14(10), 2303–2312. [DOI] [PubMed] [Google Scholar]
- Uchida R, Tomoda H, Arai M, & Omura S (2001). Chlorogentisylquinone, a new neutral sphingomyelinase inhibitor, produced by a marine fungus. Journal of Antibiotics, 54(11), 882–889. [DOI] [PubMed] [Google Scholar]
- Uchida R, Tomoda H, Dong Y, & Omura S (1999). Alutenusin, a specific neutral sphingomyelinase inhibitor, produced by Penicillium sp. FO-7436. Journal of Antibiotics, 52(6), 572–574. [DOI] [PubMed] [Google Scholar]
- Umansky V, & Sevko A (2013). Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenvironment, 6(2), 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usta J, El Bawab S, Roddy P, Szulc ZM, Hannun YA, & Bielawska A (2001). Structural requirements of ceramide and sphingosine based inhibitors of mitochondrial ceramidase. Biochemistry, 40(32), 9657–9668. [DOI] [PubMed] [Google Scholar]
- Vit JP, & Rosselli F (2003). Role of the ceramide-signaling pathways in ionizing radiation-induced apoptosis. Oncogene, 22(54), 8645–8652. [DOI] [PubMed] [Google Scholar]
- Vunnam RR, & Radin NS (1980). Analogs of ceramide that inhibit glucocerebroside synthetase in mouse brain. Chemistry and Physics of Lipids, 26(3), 265–278. [DOI] [PubMed] [Google Scholar]
- Wallington-Beddoe CT, Bennett MK, Vandyke K, Davies L, Zebol JR, Moretti PAB, et al. (2017). Sphingosine kinase 2 inhibition synergises with bortezomib to target myeloma by enhancing endoplasmic reticulum stress. Oncotarget, 8(27), 43602–43616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu JN, Xu L, Mu YL, & Sun P (2014). Expression and significance of glucosylceramide synthase in colorectal carcinoma tissues. European Review for Medical and Pharmacological Sciences, 18(23), 3632–3637. [PubMed] [Google Scholar]
- Wang E, Norred WP, Bacon CW, Riley RT, & Merrill AH Jr. (1991). Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. Journal of Biological Chemistry, 266(22), 14486–14490. [PubMed] [Google Scholar]
- Wang J, Knapp S, Pyne NJ, Pyne S, & Elkins JM (2014). Crystal structure of sphingosine kinase 1 with PF-543. ACS Medicinal Chemistry Letters, 5(12), 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DG, Tonelli F, Alossaimi M, Williamson L, Chan E, Gorshkova I, et al. (2013). The roles of sphingosine kinases 1 and 2 in regulating the Warburg effect in prostate cancer cells. Cellular Signalling, 25(4), 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters RJ, Fox TE, Tan SF, Shanmugavelandy S, Choby JE, Broeg K, et al. (2013). Targeting glucosylceramide synthase synergizes with C6-ceramide nanoliposomes to induce apoptosis in natural killer cell leukemia. Leukemia and Lymphoma, 54(6), 1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weely S, Brandsma M, Strijland A, Tager JM, & Aerts JM (1993). Demonstration of the existence of a second, non-lysosomal glucocerebrosidase that is not deficient in Gaucher disease. Biochimica et Biophysica Acta, 1181(1), 55–62. [DOI] [PubMed] [Google Scholar]
- White C, Alshaker H, Cooper C, Winkler M, & Pchejetski D (2016). The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget, 7(17), 23106–23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter CK, Gilchrist DG, Dickman MB, & Jones C (1996). Chemistry and biological activity of AAL toxins. Advances in Experimental Medicine and Biology, 392, 307–316. [DOI] [PubMed] [Google Scholar]
- Wolff RA, Dobrowsky RT, Bielawska A, Obeid LM, & Hannun YA (1994). Role of ceramide-activated protein phosphatase in ceramide-mediated signal transduction. Journal of Biological Chemistry, 269(30), 19605–19609. [PubMed] [Google Scholar]
- Xie P, Shen YF, Shi YP, Ge SM, Gu ZH, Wang J, et al. (2008). Overexpression of glucosylceramide synthase in associated with multidrug resistance of leukemia cells. Leukemia Research, 32(3), 475–480. [DOI] [PubMed] [Google Scholar]
- Yang J, Yang C, Zhang S, Mei Z, Shi M, Sun S, et al. (2015). ABC294640, a sphingosine kinase 2 inhibitor, enhances the antitumor effects of TRAIL in non-small cell lung cancer. Cancer Biology & Therapy, 16(8), 1194–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomatsu T, Takechi H, Akiyama T, Shibuya S, Kominato T, Soeda S, et al. (2001). Synthesis and evaluation of a difluoromethylene analogue of sphingomyelin as an inhibitor of sphingomyelinase. Bioorganic & Medicinal Chemistry Letters, 11(10), 1277–1280. [DOI] [PubMed] [Google Scholar]
- Yoo HS, Norred WP, Wang E, Merrill AH Jr., & Riley RT (1992). Fumonisin inhibition of de novo sphingolipid biosynthesis and cytotoxicity are correlated in LLC-PK1 cells. Toxicology and Applied Pharmacology, 114(1), 9–15. [DOI] [PubMed] [Google Scholar]
- Young MM, Takahashi Y, Fox TE, Yun JK, Kester M, & Wang HG (2016). Sphingosine kinase 1 cooperates with autophagy to maintain endocytic membrane trafficking. Cell Reports, 17(6), 1532–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Li J, Qiu Y, & Sun H (2012). 1-phenyl-2-decanoylamino-3-morpholino-1propanol (PDMP) facilitates curcumin-induced melanoma cell apoptosis by enhancing ceramide accumulation, JNK activation, and inhibiting PI3K/AKT activation. Molecular and Cellular Biochemistry, 361(1e2), 47–54. [DOI] [PubMed] [Google Scholar]
- Zhai L, Sun N, Han Z, Jin HC, & Zhang B (2015). Liposomal short-chain C6 ceramide induces potent anti-osteosarcoma activity in vitro and in vivo. Biochemical and Biophysical Research Communications, 468(1e2), 274–280. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lin X, Song Y, Zhang X, Li H, & Wang Q (2014). Overexpression of glucosylceramide synthase and its significance in the clinical outcome of non-small cell lung cancer. Chinese Medical Journal, 127(17), 3071–3076. [PubMed] [Google Scholar]
- Zhao X, Sun B, Zhang J, Zhang R, & Zhang Q (2016). Short-chain C6 ceramide sensitizes AT406-induced anti-pancreatic cancer cell activity. Biochemical and Biophysical Research Communications, 479(2), 166–172. [DOI] [PubMed] [Google Scholar]
- Zweerink MM, Edison AM, Wells GB, Pinto W, & Lester RL (1992). Characterization of a novel, potent, and specific inhibitor of serine palmitoyltransferase. Journal of Biological Chemistry, 267(35), 25032–25038. [PubMed] [Google Scholar]