Abstract
Huntington’s disease (HD) is a fatal genetic neurological disorder caused by a mutation in the human Huntingtin (HTT) gene. This mutation confers a toxic gain of function of the encoded mutant huntingtin (mHTT) protein, leading to widespread neuropathology including the formation of mHTT-positive inclusion bodies, gene dysregulation, reduced levels of adult dentate gyrus neurogenesis and ultimate neuron loss throughout many regions of the brain. Additionally, because HTT is ubiquitously expressed, several peripheral tissues are also affected. HD patients suffer from progressive motor, cognitive, psychiatric, and metabolic symptoms, including weight loss and skeletal muscle wasting. HD patients also show neuroendocrine changes including a robust, significant elevation in circulating levels of the glucocorticoid, cortisol. Previously, we confirmed that the R6/2 mouse model of HD exhibits elevated corticosterone (the rodent homolog of cortisol) levels and demonstrated that experimentally elevated corticosterone exacerbates R6/2 HD symptomology, resulting in severe and rapid weight loss and a shorter latency to death. Given that efficacious therapeutics are lacking for HD, here we investigated whether normalizing glucocorticoid levels could conversely serve as a viable therapeutic approach for this disease. We tested the hypothesis that normalizing glucocorticoids to wild-type levels would ameliorate HD symptomology. Wild-type (WT) and transgenic R6/2 mice were allocated to three treatment groups: 1) adrenalectomy with normalized, WT-level corticosterone replacement (10μg/ml), 2) adrenalectomy with high HD-level corticosterone replacement (35μg/ml), or 3) sham surgery with no corticosterone replacement. Normalizing corticosterone to WT levels led to an improvement in metabolic rate in male R6/2 mice, as indicated by indirect calorimetry, including a reduction in oxygen consumption and normalization of respiratory exchange ratio values (p<.05 for both). Normalizing corticosterone also ameliorated brain atrophy in female R6/2 mice and skeletal muscle wasting in both male and female R6/2 mice (p<.05 for all). Female R6/2 mice given WT-level corticosterone replacement also showed a reduction in HD neuropathological markers, including a reduction in mHTT inclusion burden in the striatum, cortex, and hippocampus (p<.05 for all). This data illustrates that ameliorating glucocorticoid dysregulation leads to a significant improvement in HD symptomology in the R6/2 mouse model and suggests that cortisol-reducing therapeutics may be of value in improving HD patient quality of life.
Keywords: glucocorticoids, corticosterone, Huntington’s disease, metabolism, indirect calorimetry, cachexia, neuropathology, neurogenesis, inclusion bodies, R6/2 mouse
INTRODUCTION
Huntington’s disease (HD) is a fatal genetic neurodegenerative disorder caused by a CAG triplet repeat expansion in the human huntingtin (HTT) gene (Huntington Disease Collaborative Research Group, 1993). If sufficiently long (40+ repeats), this mutation confers a toxic gain of function on the encoded mutant huntingtin protein (mHTT), leading to widespread neuropathology, including robust transcriptional abnormalities, the formation of mHTT+ inclusion bodies, regional reductions in brain volume, altered neurogenesis and neuroinflammation (astrogliosis and microgliosis) (Hodges et al., 2006; Huntington Disease Collaborative Research Group, 1993; Ross et al., 2014; Rub et al., 2015; Sapp et al., 2001; Vonsattel et al., 1985). The hallmark symptom of the disease is chorea, whereby patients show uncontrollable hyperkinetic movements that become progressively more severe (Huntington Study Group, 1996; Ross et al., 2014). However, although HD is typically conceptualized as a movement disorder, patients also suffer from severe cognitive, psychiatric, and metabolic symptoms (Anderson and Marder, 2001; Bamford et al., 1995; Kirkwood et al., 2001; Ross et al., 2014; Ross and Tabrizi, 2011; van der Burg et al., 2009). Metabolic symptoms in HD include weight loss, muscle wasting and insulin resistance (Aziz et al., 2008; van der Burg et al., 2009; van der Burg et al., 2011). Calorimetry data demonstrates that patients are in a chronic catabolic state, and thus in negative energy balance (Goodman et al., 2008). Accordingly, at end stage of disease, patients are often severely cachexic (Djousse et al., 2002; Kosinski et al., 2007). The disease typically onsets in the 3rd to 4th decade of life, progresses over 15–20 years, and always leads to death (Ross et al., 2014).
Glucocorticoids, which are chronically elevated in Huntington’s disease (HD), have long been suspected as a contributor to the metabolic phenotype shown by patients and in HD rodent models (Aziz et al., 2009; Bjorkqvist et al., 2006a; Goodman et al., 2008). This suspicion is logical, given that chronically elevated glucocorticoids in otherwise healthy individuals leads to widespread metabolic dysfunction, including muscle wasting (Braun et al., 2013; Grossberg et al., 2010; Lee et al., 2005), altered fat and protein metabolism (Lofberg et al., 2002), insulin resistance (Black et al., 1982), and osteoporosis (Moutsatsou et al., 2012; Toth and Grossman, 2013), all of which are symptoms of HD. There is also evidence that elevated glucocorticoids may potentially be exacerbating HD neuropathology, as chronically elevated cortisol in otherwise healthy individuals (i.e. Cushing’s syndrome) is associated with reductions in regional and whole brain volume (Andela et al., 2013; Bourdeau et al., 2002) and reduced adult neurogenesis in the dentate gyrus (Brummelte and Galea, 2010; Cameron and Gould, 1994). Cell culture studies have demonstrated that glucocorticoids are also a potent modulator of mHTT inclusion formation (Diamond et al., 2000). Furthermore, elevated glucocorticoids are also known to exacerbate neuropathology in other neurodegenerative disease models. For example, in Alzheimer’s disease rodent models, elevated glucocorticoids cause an increase in the accumulation of both amyloid beta plaques and hyperphosphorylated tau (Green et al., 2006), and blocking glucocorticoid receptors results in a reduction in both (Baglietto-Vargas et al., 2013).
However, while these findings provide abundant circumstantial evidence for a role for glucocorticoids in HD metabolic symptoms and neuropathology, there has been little direct evidence to support this speculation. Of the existing HD mouse models, a spontaneous elevation in corticosterone, the rodent homolog of cortisol, has only been described in R6/2 mice (Bjorkqvist et al., 2006a; Dufour and McBride, 2016). R6/2 transgenic mice show robust metabolic symptomology including severe and progressive weight-loss, muscle wasting, and insulin resistance (Bjorkqvist et al., 2005; Luthi-Carter et al., 2002; van der Burg et al., 2009). R6/2 mice are also in negative energy balance (Goodman et al., 2008), with indirect calorimetry measures showing that this may be driven by a chronic increase in metabolic rate, indicated by an increase in oxygen consumption (Bjorkqvist et al., 2006b; Goodman et al., 2008). While elevated corticosterone levels and metabolic symptomology co-occur in R6/2 mice, it remains unclear whether these metabolic symptoms are directly affected or even mediated by this spontaneous elevation in glucocorticoid levels, or whether it is driven by the widespread deleterious consequences of mHTT toxicity alone. Thus, this speculative relationship between corticosterone and metabolic symptoms in R6/2 mice has remained largely correlational. The role of glucocorticoid dysregulation has been more directly investigated in R6/1 mice, another transgenic HD line. Although R6/1 mice have not been shown to have a spontaneous elevation in corticosterone, experimental elevations in corticosterone, as well as experimenter induced stress, worsen HD cognitive symptoms and reduce hippocampal neurogenesis (Du et al., 2012; Grote et al., 2005; Lazic et al., 2004; Mo et al., 2014a; Mo et al., 2014b; Mo et al., 2014c). Surprisingly, experimentally elevated corticosterone only caused a mild and transient weight-loss in male R6/1 mice (Mo et al., 2014a).
We recently demonstrated that experimental elevation of corticosterone in adrenalectomized R6/2 mice dramatically exacerbates weight loss and shortens the latency to death (Dufour and McBride, 2016). While this provides direct evidence that an elevation in glucocorticoids can exacerbate HD symptomology, it remains unclear whether normalizing glucocorticoids to wild-type (WT) levels in HD mice would conversely attenuate HD symptomology. Given that there are no current FDA-approved medications that can slow the progression of HD symptoms, we investigated whether glucocorticoid dysregulation in HD could serve as a novel point of therapeutic intervention to alleviate HD symptomology. Thus, here, we tested the hypothesis that normalizing corticosterone to wild-type levels in R6/2 mice would attenuate the metabolic and neuropathological symptomology germane to this model as well as seen in patients suffering from HD.
MATERIALS AND METHODS
Animals.
All animals were group housed with littermates (3–5 mice per cage) under controlled conditions of temperature and light (12 hour light/dark cycle). Food (Laboratory Rodent Diet 5001, LabDiet, St. Louis, MO) and water were provided ad libitum. R6/2 mice (stock # 002810, carrying 160±5 cag repeats) were obtained from Jackson Laboratories (Bar Harbor, ME) and bred in the vivarium at the Oregon National Primate Research Center (ONPRC). Wild-type males were mated with ovary transplanted wild-type females. Transgenic mice were genotyped using primers specific for the mutant human HTT transgene (forward primer 5’ TCATCAGCTTTTCCAGGGTCGCCAT and reverse primer 5’ CGCAGGCTAGGGCTGTCAATCATGCT), and age-matched wild-type littermates were used for the indicated experiments. Average CAG repeat length for the colony was assessed from a subset of animals by sequencing, with mice from the colony showing an average of 165 ± 5 (SD) CAG repeats (Laragen Sequencing and Genotyping). Body weights of all animals were recorded weekly. All experimental procedures were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals, and were approved by the Oregon Health and Science University (OHSU) and ONPRC Institutional Animal Care and Use Committee.
Adrenalectomy Surgery.
Mice were anesthetized with 3% isoflurane. A 2cm × 2cm square was shaved on their lower dorsal surface and an incision was made in the skin at the animal’s dorsal midline (this opening was used for the removal of both adrenal glands). Next, a small (3–5mm) incision in the muscular wall was made, directly above the kidney. Small tweezers were used to grasp and gently remove the adrenal gland. This procedure was repeated for the animal’s contralateral side (bilateral adrenalectomy). The muscle incisions were closed using absorbable suture and the dorsal skin was closed with wound clips.
Corticosterone Replacement.
Corticosterone replacement was provided in the animals’ drinking water, which was available ad-libitum. Corticosterone (Sigma, cat. #27840) was dissolved in a small volume of ethanol (0.6%), and then added to dH20. Since the aldosterone producing cells of the adrenal gland are lost to adrenalectomy, NaCl (0.9%) was also added to replace salt loss. Sucrose (2.0%) was also added to increase palatability of the solution. A dose of 10μg/ml corticosterone was used for the physiological/WT level replacement group (therapeutic group) and 35μg/ml of corticosterone was used for the high/HD level replacement group. Vehicle alone (2% sucrose, 0.9% NaCl, and 0.6% ethanol in dH20) was given to the sham surgery animals.
Plasma Corticosterone Analysis.
The tail clip method was utilized to collect blood for assessing peak blood corticosterone levels. The tip of the tail was clipped (0.5mm), and approximately 20ul of whole blood was collected from the tip and collected into heparinized capillary tubes, processed for plasma, and stored at −80°C until analysis. Handling of mice during tail-clip method blood collection was minimized, and typically lasted approximately 30 seconds. Blood was collected from all adrenalectomized mice on corticosterone replacement (10μg/ml and 35μg/ml) at 9.5 weeks of age at midnight, to better assess peak levels of corticosterone due to mainly dark-cycle intake of replacement solution. All plasma was assayed for corticosterone by the ONPRC Endocrine Technology CORE using an in-house radio-immuno assay. Since R6/2 HD mice show circadian dysregulation in peak plasma corticosterone levels, with individuals peaking at variable times that is idiosyncratic to individual mice, we did not analyze blood samples from sham mice as it would have necessitated a circadian profile that would have been stressful for mice and possibly altered some of the metabolic measures of interest. Instead, we refer readers to our circadian profile of plasma corticosterone published previously in mice form the same colony (Dufour and McBride, 2016).
Indirect Calorimetry.
The comprehensive lab animal monitoring system (CLAMS Oxymax system, Columbus Instruments) was used to assess alterations in metabolic rate (indirect calorimetry) and food intake in subjects. Mice were measured at 5 and 10 weeks of age, and 24-hours of data were analyzed (6pm – 6pm). Mice were acclimated to the sealed chambers (3.5”) for 4 hours before the dark cycle (2pm-6pm), and these acclimation data were omitted from analysis. As there are diurnal variations in indirect calorimetry measures, dark cycle and light cycle data were analyzed separately. Mice had ad libidum access to food and water (water at 5 weeks of age, before surgery, and corticosterone replacement solution at 10 weeks) throughout their time in the chambers. Standard rodent chow was finely ground and placed in a specialized feeder at the base of the chambers, and food consumption was automatically measured by the CLAMS apparatus. Fluid intake was manually measured over the 24 hour period. Measures of oxygen intake (VO2) and carbon dioxide production (VCO2) were automatically collected by the software for each chamber every 18 minutes throughout the 24hour period, and the respiratory exchange ratio (RER, Ratio VCO2:VO2) was automatically calculated by the software for each interval. There was a malfunction of the oxygen sensors during some of the indirect calorimetry runs (up to 8 mice are run at a time, 3 runs were affected, leading to exclusion of 21 mice), and thus a portion of the animals from this experiment were specifically excluded from the calorimetry analyses. Although the malfunction only affected a single timepoint for these animals, both timepoints were omitted to ensure that repeated measures were complete and reliable.
Necropsy.
At 11 weeks of age, mice were deeply anesthetized with ketamine/xylazine and perfused with 15mLs of 0.9% saline. Brains and gastrocnemius muscles were carefully dissected and weighed. Brains were post-fixed in 4% paraformaldehyde for 24 hours, then stored in a 30% sucrose solution until they were sectioned.
Immunohistochemistry.
Brains were cut frozen into 40μm sections on a sliding microtome and stored in cryoprotectant solution (30% sucrose, 30% ethylene glycol, in PBS) until stained. Serial sections from 48 mice (balanced for sex, genotype, and treatment) were stained using standard DAB immunohistochemistry using the following primary antibodies: 1) NeuN (1:750, Millipore Catalog # ABN78) - a pan neuronal marker used here for volumetric analysis, 2) Ki67 (1:150, ThermoFisher Catalog # MA5–14520) – a mitosis marker that is used to identify proliferating cells in the adult brain as a marker of neurogenesis, and 3) EM48 (1:250, Millipore Catalog # MAB5374) – an antibody that preferentially binds to aggregated mutant huntingtin protein. Sections were blocked (for endogenous peroxidases) in sodium-meta-periodate for 20 minutes, then blocked (for non-specific antibody binding) in 5% goat serum for 1 hour, incubated in primary antibodies overnight (in 3% goat serum), washed and incubated in secondary antibody for 1 hour, amplified with VectaStain ABC kit, and developed with DAB. Nickel intensification was used for the Ki67 stain.
Neuropathology Quantification.
NeuN stained sections were used to analyze regional volume of striatum, cortex, and hippocampus using the Cavalieri method in Microbrightfield Stereo Investigator software (MBF Bioscience). The following parameters were used for the Cavalieri estimations from each region: 1) Striatum – 6 matched serial sections (every 12th section, from AP +1.70 to −1.94) were assessed with a grid size of 75μm, 2) Cortex – 9 matched serial sections (every 12th section, from AP +2.10 to −2.92) were assessed with a grid size of 150μm, and 3) Hippocampus – 5 matched serial sections (every 12th section, from AP −1.46 to −3.88) were assessed with a grid size of 75μm. Parameters were optimized with pilot analyses, aimed at minimizing Gunderson Coefficients of Error (m=0) to ≤0.100 (Average values: Striatum = 0.049, Cortex = 0.054, Hippocampus = 0.096). Ki67+ cells in the dentate gyrus were counted manually at 40x magnification in 5–6 serial sections (every 6th section) from AP −1.58 to −2.80. Counts were taken for both hemispheres, for a total of 10–14 counts per mouse and averaged. EM48 stained sections were assessed for inclusion number and size using imageJ software (National Institutes of Health), following the method outlined in Gharami et al. (2008). Images were acquired at 100x in three serial sections, with an image taken from both the left and right hemispheres, for a total of 6 images per mouse for each region of interest: hippocampus (CA1 and dentate gyrus: AP −1.70 to −3.16), hypothalamus (lateral hypothalamus: AP −0.82 to −2.18), striatum (dorsomedial: AP +0.86 to −0.46), and cortex (layer 5 motor cortex: AP +1.42 to +0.02). The acquired images were converted to 8-bit greyscale and the threshold was adjusted to a set level in each region of interest (ROI) to remove background staining. The number and size of EM48+ inclusion bodies were quantified using the particle analysis tool and averaged for each mouse across all 6 acquired images per ROI.
Statistical analysis.
All statistical analyses were performed by using JMP Version 11 statistical software (SAS Institute Inc.). A repeated measures mixed-model analysis was used for all repeated measures (Dependent variables: bodyweight, VO2, VCO2, RER, food intake, and fluid intake; Independent variables: genotype, treatment, time-point). A two-way ANOVA was used to assess the effects of genotype and treatment group on: organ mass (brain and gastrocnemius), regional brain volume (striatum, cortex, hippocampus), and dentate gyrus neurogenesis. A one-way ANOVA was used to assess the effects of treatment on inclusion burden (inclusion size and number) individually for each of the 6 regions of interest (striatum, motor cortex, cingulate cortex, CA1 hippocampus, dentate gyrus, and lateral hypothalamus) in R6/2 mice. All analyses were carried out separately for males and females, as there were sexually dimorphic patterns of treatment effects. Pre-planned post-hoc contrasts were used to assess within genotype and within treatment pairwise comparisons in bodyweight at each time-point assessed. The alpha level for contrasts were Bonferroni adjusted (total of 48 comparisons - .05/48 = .0011) to avoid Type I error. Contrasts were utilized for the bodyweight analysis instead of Tukey’s post-hoc comparisons to avoid Type II error (excessive alpha suppression) due to a high number of possible pairwise comparisons (435), most of which were not relevant to the hypotheses being tested. For all other analyses, Tukey’s post-hoc comparisons were used if there was a significant fixed effect for the independent variables of interest. For fixed effects in all models, as well as for Tukey’s posthoc comparisons, p<0.05 was considered statistically significant.
RESULTS
Assessment of plasma corticosterone levels
In order to assess the role of glucocorticoids in HD metabolic symptomology and neuropathology, WT and R6/2 HD mice were adrenalectomized at 6 weeks of age and treated with either low dose (10μg/ml, WT level, n=20 HD and n=25 WT) or high dose (35μg/ml, HD level, n=17 per genotype) corticosterone replacement, provided in their drinking water (See Fig. 1A for experimental timeline). Another cohort of WT and HD mice were given sham surgeries, and provided with vehicle only (no corticosterone replacement) for their drinking water (n=17 HD and n=18 WT). Corticosterone dosing was adjusted from our previously published study (Dufour and McBride, 2016) to reduce the HD-level replacement to a dose that is closer to the levels of hypercortisolinemia normally seen in transgenic R6/2 mice (35μg/ml instead of 60μg/ml). This new 35μg/ml HD-level replacement dose was determined in a pilot (Supplemental Fig. 1) with a target plasma corticosterone level of 300 ng/ml, similar to peak corticosterone levels shown by R6/2 HD mice (Dufour and McBride, 2016). The target plasma corticosterone levels for WT-level corticosterone replacement (10μg/ml) was 100 ng/ml, as demonstrated previously (Dufour and McBride, 2016).
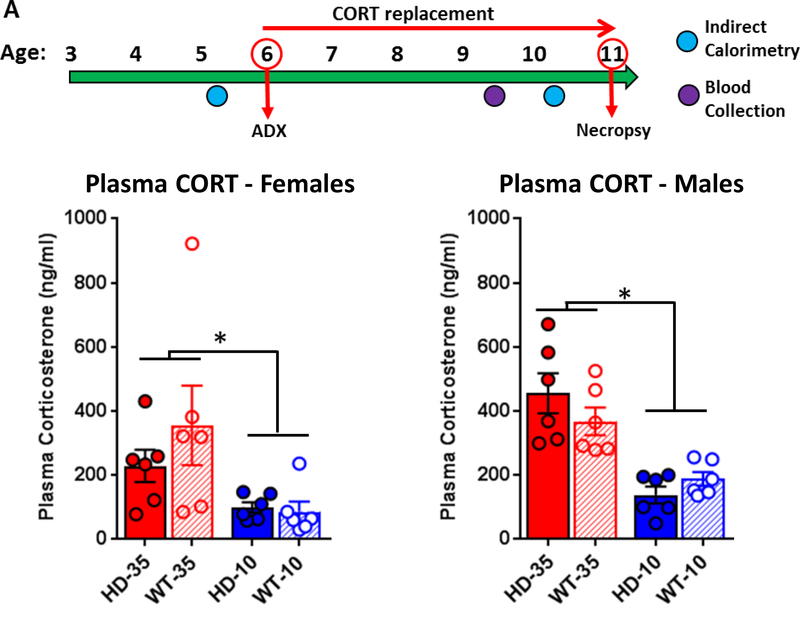
Figure 1. Experimental timeline and plasma corticosterone levels.
(A) Experimental timeline - WT and R6/2 HD mice underwent adrenalectomy surgery at 6 weeks of age and were given either low-dose corticosterone replacement (WT-level, 10ug/ml, blue) or high dose corticosterone replacement (HD-level, 35ug/ml, red) in their drinking water from 6 to 11 weeks of age, at which time mice were sacrificed. All subjects were measured for indirect calorimetry at 5 and 10 weeks of age, body weight weekly, and blood was collected at 9.5 weeks of age for peak plasma corticosterone levels. (B) Plasma corticosterone analysis (n=24 mice per sex, 6 per genotype/treatment group) indicates that high dose treatment (35μg/ml) led to increased plasma levels in both males and females relative to those on low dose treatment (10μg/ml). Plasma corticosterone values were significantly higher in males, overall (p<.05), but did not differ between genotypes in either sex (p>.05). Glucocorticoid replacement doses resulted in plasma corticosterone levels at the planned target levels near 300ng/ml for HD-level replacement and 100ng/ml for WT-level replacement. Values represent Mean ± SE. *Indicate significant differences as indicated by Tukey’s post-hoc comparisons.
Blood was collected from subjects at 9.5 weeks of age to identify peak plasma corticosterone levels due to corticosterone replacement (Fig. 1B). Blood collection took place at midnight in the middle of dark cycle when water intake is highest. There was a significant sex effect (F1,40 = 5.45, p<.05), and thus the two sexes were analyzed independently. As expected, treatment affected plasma corticosterone levels in both males (F1,20 = 35.86, p<.001) and females (F1,20 = 8.28, p<.01). In males, Tukey’s post-hoc comparisons indicate that high dose corticosterone (HD-level, 35μg/ml) replacement resulted in significantly elevated plasma corticosterone levels (WT: 367.5 ± 43.0 ng/ml, HD: 454.9 ± 62.4 ng/ml) relative to those on low dose (WT-level, 10μg/ml) replacement (WT: 187.6 ± 21.7 ng/ml, HD: 137.7 ± 25.9 ng/ml). For females, Tukey’s post-hoc comparisons indicate that high dose corticosterone (HD-level, 35μg/ml) replacement resulted in significantly elevated plasma corticosterone (WT: 355.5 ± 135.9 ng/ml, HD: 228.9 ± 50.3 ng/ml) relative to those on low dose (WT-level, 10μg/ml) replacement (WT: 86.3 ± 31.0 ng/ml, HD: 100.1 ± 16.0 ng/ml). Plasma corticosterone levels were not affected by genotype (Males - F1,20 = 0.20, p=.66, Females - F1,20 = 0.67, p=.42) nor was there a treatment*genotype interaction for either sex (Males – F2,20 = 2.73, p=.11, Females – F2,20 = 1.03, p=.32).
Normalizing glucocorticoid levels delays weight loss in R6/2 mice
We first tested the hypothesis that normalizing glucocorticoid levels (10μg/ml, low dose WT-level treatment) would slow the progression of weight loss in R6/2 mice relative to those on high dose (35μg/ml – HD level) treatment and sham surgery controls. In previous work, we’ve shown that high dose corticosterone was associated with precipitous weight loss in transgenic R6/2 mice (Dufour and McBride, 2016). Change in bodyweight from 6 weeks of age, at which point mice underwent adrenalectomy or sham surgery, was calculated weekly until 11 weeks of age and analyzed using a 2-way repeated measures ANOVA (Fig. 2). While percent change in bodyweight is presented here, as it best illustrates disease- and treatment- associated weight loss, actual (raw) bodyweight data is also presented in Supplemental Fig. 2. Pre-planned post-hoc orthogonal contrasts were used to make pairwise comparisons (both within treatment, and within genotype) and accordingly the alpha-level was Bonferoni adjusted to avoid type I error (α = .0011 = .05/45 comparisons). As there were numerous sex effects across metabolic and neuropathological markers throughout these experiments, males and females were assessed separately for all analyses. We found a significant divergence in bodyweight change between genotypes (Females: Genotype*timepoint - F4,192=70.70 p<.0001; Males: Genotype*timepoint - F4,220=156.27 p<.0001), with wild-type mice showing normal weight gain following surgery at 6 weeks, and transgenic mice showing a progressive and significant decline in bodyweight over time (Fig. 2 A, B). Bodyweight was further influenced by an interaction of both treatment and genotype across the span of the experiment (Treatment*Genotype*Timepoint interaction) for both males (F8,220=7.54 p<.0001) and females (F8,192=3.25 p<.005). Given that treatment affected bodyweight for the R6/2 and wildtype mice differently, both within-treatment post-hoc comparisons and within-genotype post-hoc comparisons were utilized to better assess how treatment affected the R6/2 weight loss phenotype.
Figure 2. Effect of glucocorticoid normalization on percent change in bodyweight.
All WT mice gained weight following surgery/treatment at week 6, while HD mice lost weight, although the degree of weight loss varied according to treatment and genotype (Significant Treatment*Genotype*Timepoint Fixed Effects. Males: p<.0001; Females: p<.005). Within-genotype post-hoc comparisons (indicated by symbols in green: at top for wildtype, below for HD) indicate that HD males on high dose CORT (35μg/ml) show significantly more weight loss than HD sham males from weeks 8–11 and low dose (10μg/ml) HD males from weeks 10–11 (B). High dose HD females show significantly more weight loss at week 11 only, relative to the other treatments. Sham male WT mice showed increased weight gain relative to WT males on high dose (weeks 8–11) and low dose corticosterone treatment (week 10 only). Within treatment comparisons (e.g. low dose. WT vs HD) indicate that low dose CORT delayed weight loss in male R6/2 mice, which only becomes significant at 11 weeks of age (B, blue arrow), while sham (B, black arrow) and high dose (B, red arrow) treated males show significant weight loss from weeks 9–11. Low dose treatment also delays weight loss in HD females (A, blue arrow) to week 11, while weight loss begins at week 9 for high dose (A, red arrow) and week 10 for sham controls (A, black arrow). Alpha level was Bonferoni adjusted for 45 post-hoc comparisons (.05/45 = .0011 critical alpha). Overall N=52 for females (n= 7–11 per group) and N=60 for males (n=9–14 per group). Values represent Mean ± SE.
Within-treatment comparisons (Fig. 2, indicated by blue, red, and black arrows below the x-axis) illustrate the timepoint at which transgenic R6/2 mice show significant weight loss relative to WT control mice on the same treatment. This within-treatment comparison is important statistically, since treatment had subtle but significant effects on WT controls and thus was accounted for when assessing the effect of treatment on the onset of the R6/2 weight-loss phenotype. Low dose corticosterone delayed the onset of weight loss in transgenic male and female R6/2 mice, which did not become significantly different from low-dose WT controls until 11 weeks (Fig. 2 A, B, blue arrows, p<.001). In contrast, male and female transgenic mice on high dose corticosterone showed weight loss significantly earlier, starting at 9 weeks and continuing through week 11, relative to wild-type controls on the same treatment (high dose corticosterone, red arrows, p<.001 for each of the 3 timepoints for both sexes). As expected, male R6/2 sham controls also showed significant weight loss relative to wild-type male sham controls from weeks 9 through 11 (black arrows, p<.0011 for all). There was a slight delay in female sham control, with transgenics showing significant weight loss in weeks 10 and 11 (black arrow, p<.001), one week earlier than low dose transgenics and one week later than high dose transgenics.
Within-genotype post-hoc comparisons (Fig. 2, indicated by $,#,* in green, below data lines for transgenics, above data lines for wild-type mice) revealed that R6/2 mice on high dose corticosterone show significantly more weight loss, particularly in males, relative to low-dose treated and sham control R6/2 mice. Male transgenics on high dose corticosterone showed significantly more weight loss than sham controls from weeks 8–11 (p<.001) and low dose treated mice from weeks 10–11 (p<.001). High dose corticosterone also exacerbated weight loss in female transgenics, relative to those on low dose treatment (p<.001) and sham controls (p<.0011), although this didn’t become significant until the last time point at 11 weeks of age. Withingenotype comparisons indicated that bodyweights were not significantly different between low-dose and sham control R6/2 mice, for either sex (p>.001 for all timepoints). Together, this data confirms that elevated corticosterone hastens weight loss in R6/2 mice and shows that normalizing corticosterone to wildtype levels delayed the weight loss phenotype in these mice.
Reduced glucocorticoid levels partially prevents the catabolic phenotype in male R6/2 mice
To assess the effect of glucocorticoid treatment on metabolism in R6/2 HD mice, 24-hour indirect calorimetry measures were taken from the subjects at 5 weeks of age (prior to surgery/treatment) and again at 10 weeks of age following 4 weeks of chronic treatment with corticosterone replacement (vehicle for sham controls). Gas exchange is measured with indirect calorimetry, including oxygen consumption (VO2) and carbon dioxide production (VCO2). From these values, the respiratory exchange ratio (RER) is calculated (ratio of VCO2:VO2) for each individual, which indicates on a cellular level what nutrient source is preferentially used for fuel (Frayn, 1983). Mice were provided with ad-libitum food and water (water at baseline/5 weeks, corticosterone replacement solution or vehicle at 10 weeks of age), which was measured for the 24 hours of treatment.
Previous studies with R6/2 mice have shown an increased in oxygen consumption relative to WT mice, an indicator of elevated metabolic rate (Goodman et al., 2008; van der Burg et al., 2008). Accordingly, here we tested the hypothesis that normalizing glucocorticoid levels would ameliorate the metabolic deficit in R6/2 mice, as measured by indirect calorimetry. As indirect calorimetry measures shift between the light and dark cycles, these two 12-hour periods were analyzed separately. In spite of differences in light and dark cycle values, the effect of sex, genotype, and treatment are largely consistent between the two data sets. For brevity, the darkcycle data is presented here in Fig. 3 and the light-cycle data is presented in Supplemental Fig. 3. As predicted, genotype had a strong and consistent impact on all indirect calorimetry measures (Fig. 3). R6/2 HD mice showed a significant and expected increase in VO2 levels across timepoints (Males – 14.6% increase: Genotype F1,41=43.92 p<.001, Fig. 3D; Females – 15.2% increase: Genotype F1,38=38.94 p<.0001, Fig. 3A), indicative of increased metabolic rate. We observed significantly increased VCO2 levels in R6/2 mice compared to controls (8.0% increase in Males: Genotype F1,41=16.47 p<.001, Fig. 3E; 10.7% increase in Females: Genotype F1,38=22.47 p<.001, Fig. 3B). Although both VO2 and VCO2 levels are elevated, VO2 is elevated to a larger degree than VCO2 in R6/2 mice, which drives a significant reduction in the RER that becomes particularly pronounced at the 10 weeks timepoint (Males: Genotype F1,41=15.21 p<.001; Females: Genotype F1,38=22.47 p<.001, Fig. 3C).
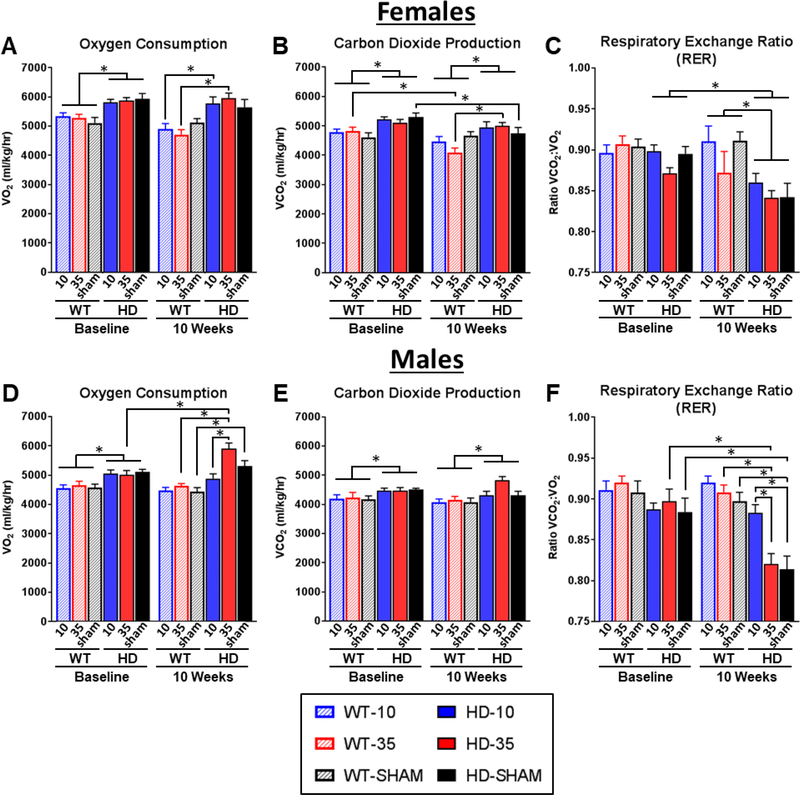
Figure 3. Effect of glucocorticoid replacement on 12-hour indirect calorimetry (CLAMS) during the dark cycle.
Mice were measured for indirect calorimetry at 5 weeks (baseline) and 10 weeks of age, following adrenalectomy at 6 weeks and subsequent corticosterone replacement. During the dark cycle (6pm-6am), R6/2 mice showed significant elevations in VO2 (A,D) and VCO2 (B,E) at baseline and at 10 weeks (Genotype Fixed Effect for VO2 and VCO2 in both sexes, all p<.05), indicative of increased metabolic rate. R6/2 mice also showed a significant reduction in respiratory exchange rate (RER) values at 10 weeks of age (Genotype*Timepoint Fixed effects for both sexes, p<.05), indicative of abnormal macronutrient utilization (C,F). Normalizing glucocorticoid levels (10μg/ml CORT replacement, solid blue) in male R6/2 mice led to normalization of VO2 (D) and a rescue of RER values to WT levels (F), as indicated by Tukey’s post-hoc comparisons (Significant Treatment*Genoptye*Timepoint Fixed Effects for VO2 and RER, p<.05 for both). Treatment effects in wildtype mice and in female R6/2 mice were inconsistent, and normalized glucocorticoid levels in female R6/2 mice did not improve metabolic deficits, as measured by indirect calorimetry. Overall N=44 (n= 7–8 per group) for females and N=47 for males (n=7–9 per group). Values represent Mean ± SE. *Indicate significant differences as indicated by Tukey’s post-hoc comparisons.
Glucocorticoid treatment also had strong impact on calorimetry measures in transgenic R6/2 male mice, reflected by significant Treatment*Genoptype*Timepoint fixed effects VO2 and RER (p<.05 for all). Tukey’s post-hoc tests indicate that in male R6/2s, elevated (high dose, HD-level) corticosterone drives a significant 15.6% elevation in VO2 relative to baseline (Fig. 3D). Normalizing glucocorticoids partially prevent these elevations in VO2 shown in high dose males, as male R6/2 mice treated with low dose corticosterone do not show any significant changes from baseline in VO2 (3.4% decrease, Fig. 3D) VCO2 (3.1% decrease, Fig. 3E). Normalizing glucocorticoids also prevents the reduction in the respiratory exchange ratio (RER) in males that is characteristic of R6/2 mice, described above. R6/2 males on low dose treatment showed a nonsignificant 0.1% reduction in RER (Fig. 3F) relative to baseline.
Additionally, low dose treated R6/2 mice were not significantly different from low dose treated WT controls for RER (3.9% decrease, Fig. 3F). In contrast, male R6/2 mice on high dose corticosterone showed a significant 8.0% reduction in RER (Fig. 3F) reflecting an increase in the utilization of fatty acids for energy relative to carbohydrates. Sham control male R6/2 mice showed a significant 9.3% reduction in RER showing a similar preferential utilization of fatty acids. Additionally, male R6/2 mice on high dose treatment and sham controls were both significantly lower than wildtype controls on the same treatment (7.4% decrease for high dose and 10.4% decrease for sham) for RER.
Although there were clear genotype effects in females, with transgenics showing an expected significantly increased VO2 and VCO2 and decreased RER, there were only a few effects of treatment, reflected in VO2 (Fig. 3A, Treatment*Genotype*Timepoint: F2,38=4.64 p<.05) and VCO2 (Fig. 3B, Treatment*Genotype*Timepoint: F2,39= 8.68 p<.05) measurements. Tukey’s post-hoc comparisons indicate that for dark cycle VO2, low dose and high dose corticosterone treated R6/2 females show a significant 17.7% and 26.5% elevation in VO2 at 10 weeks, respectively, relative to WT controls on the same treatments (Fig. 3A, p<.05 for all). R6/2 sham controls show a significant 4.8% reduction from baseline for VCO2 (Fig. 3B, p<.05). R6/2 females on high dose corticosterone show 26.5% higher VCO2 levels relative to high dose WT at 10 weeks (Fig. 3B, p<.05). In spite of these small treatment induced differences in VO2 and VCO2 levels, the RER values for female transgenics were unaffected by treatment (Fig. 3C), suggesting similar patterns of micronutrient utilization for cellular metabolism. Overall, these data indicate that normalizing glucocorticoid levels in male R6/2 mice was effective in partially preventing the disease-associated catabolic state in this model.
Normalizing glucocorticoids partially prevents disease-associated hypophagia in male R6/2 mice
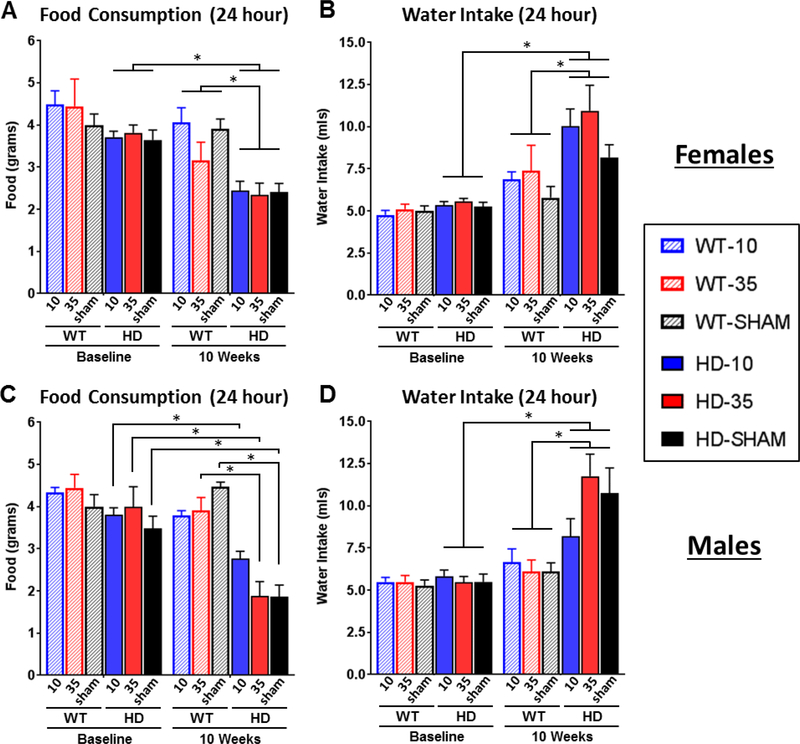
Food consumption and fluid intake (treatment or vehicle solution) were measured for all mice while in the CLAMS metabolic chambers. Measures reflect intake over a full 24 hour period, after acclimation to the chambers. Although small reductions in food consumption in late stage R6/2 mice have been previously documented (van der Burg et al., 2008), there was a much larger reduction in food intake detected here for both female (Fig. 4A - Genotype*Timepoint: F1,39=34.85 p<.05) and male (Fig. 4C - Genotype*Timepoint: F1,38=34.85 p<.0001) transgenic R6/2 mice at 10 weeks of age. There were no detectable differences in food intake between genotypes at baseline (Tukey’s post-hoc, p>.05 at 5 weeks), suggesting that late stage hypophagia may be a significant contributor to precipitous weight loss that occurs at end stages of disease in R6/2 mice. While treatment did not significantly affect food consumption in females (p>.05 for treatment and all of its interactions), it did affect food intake in male HD mice (Treatment*Genotype*Timepoint F1,38=34.85 p<.05). Tukey’s post-hoc comparisons indicate that all groups of male HD mice showed a significant reduction in food intake relative to baseline (Fig. 4C): 27.5% for low dose (3.80 ± 0.17g baseline vs 2.76 ± 0.18g post), 58.5% for high dose (3.99 ± 0.48g vs 1.65 ± 0.37g), and 46.5% for sham controls (3.48 ± 0.29g vs 1.86 ± 0.28). However, Tukey’s comparisons also indicate that the normalization of glucocorticoids led to slight attenuation of hypophagia in R6/2 males, as food intake levels at 10 weeks were not significantly different between male R6/2 and WT mice on low dose corticosterone, while sham and high dose treated R6/2 males were significantly different from treatment-matched WT controls. As expected, there was a significant increase in fluid intake in both female (Fig. 4B - Genotype*Timepoint: F1,39=8.38 p<.01) and male (Fig. 4D - Genotype*Timepoint: F1,41=17.53 p<.001) R6/2 HD mice at the 10 week time point – a phenotype that has been previously characterized (Wood et al., 2008). Treatment did not affect intake levels in any way (p>.05 for all), with all groups of HD mice showing a similar increase in consumption.
Figure 4. Food and water measurements in CLAMS metabolic chambers.
24-hour food and water intake were measured in CLAMS metabolic chambers at 5 (baseline) and at 10 weeks of age. Mice were adrenalectomized at 6 weeks of age and provided with continuous corticosterone replacement. Female (A) and male (C) R6/2 mice showed a significant reduction in food intake at 10 weeks (Genotype*Timepoint: p<.05 for both sexes); intake levels were not different at baseline (Tukey’s p>.05). Although food intake was reduced for males on all treatments, low dose CORT (10μg/ml) attenuated the severity of hypophagia in R6/2 males (not significantly different from WT mice on same treatment). Female and male R6/2 mice also showed a significant increase in fluid intake (water at baseline, corticosterone or vehicle at 10 weeks) at 10 weeks (Genotype*Timepoint: p<.05 for both males and females, B, D), which is an expected R6/2 phenotype. There was not an effect of treatment on fluid intake (Treatment and treatment*genotype: p>05 for both sexes). Overall N=44 (n= 7–8 per group) for females and N=47 for males (n=7–9 per group). Values represent Mean ± SE. *Indicate significant differences as indicated by Tukey’s post-hoc comparisons.
Normalizing glucocorticoid levels partially prevents skeletal muscle and brain atrophy
Both brain and skeletal muscle are known to be particularly sensitive to chronic alterations in glucocorticoid levels; it has been shown that chronically elevated cortisol is associated with Cushing’s like reductions in both brain (Bourdeau et al., 2002) and muscle mass (Braun et al., 2013). To assess whether glucocorticoids contribute to overall reductions in brain and skeletal muscle mass that is characteristic of R6/2 HD mice, wet weight of both tissues were assessed during necropsy at 11 weeks of age. We tested the hypotheses that normalizing glucocorticoid levels in R6/2 HD mice would prevent the reduction of gastrocnemius skeletal muscle mass and whole brain mass.
Glucocorticoid levels significantly affected gastrocnemius mass (Fig. 5), although it did so differently in wild-type and transgenic R6/2 HD mice (Males- Treatment*Genoptype: F2,54=3.83, p<.05, Females - Treatment*Genoptype: F2,46=7.25, p<.01). R6/2 mice with normalized glucocorticoid levels had less gastrocnemius muscle atrophy compared to controls, as indicated by Tukey’s post-hoc comparisons. Female R6/2 mice (Fig. 5A) on low dose corticosterone treatment showed a significant 27.0% increase in muscle mass (72.2 ± 1.6mg) relative to those on high dose treatment (55.9 ± 2.4mg), and a non-significant 16.6% increase relative to sham controls (62.0 ± 2.5mg). In males, there was a significantly less gastrocnemius muscle atrophy in those on low dose corticosterone (76.7 ± 2.3mg) relative to those on high dose treatment (59.8 ± 3.5mg). Sham control R6/2 mice show an intermediate mass (66.4mg for males, 62.0mg for females), which were not significantly different from either treatment group. In contrast, high dose glucocorticoids resulted in a significant Cushing’s disease-like reduction in gastrocnemius muscle mass in both female (Fig. 5A) and male (Fig. 5C) wild-type mice, with those on high dose corticosterone showing significantly reduced mass relative to those on low dose corticosterone and sham controls. For males, this represents a 12.5% decrease in muscle mass for mice on high dose treatment (107.9 ± 4.3mg) relative to those on low dose treatment (123.3 ± 2.6mg) and a 16.7% decrease relative to sham controls (129.6 ± 3.4mg). For females, this represents a 27.0% decrease for mice on high dose treatment (81.5 ± 2.9mg) relative to those on low dose treatment (111.3 ± 2.8mg) and a 23.1% decrease relative to sham controls (107.2 ± 3.0mg).
Figure 5. Effect of glucocorticoid normalization on brain and muscle mass.
Normalizing glucocorticoid levels (10μg/ml) in R6/2 mice resulted in a significant increase in gastrocnemius mass in females (A) and males (C), and brain mass in females (B) relative to those on high dose replacement – [Significant Genotype*Treatment effect for female (p<.01) and male gastrocnemius muscle (p<.05), and female brain (p<.05) but not male brain mass (p=.40)]. Tukey’s posthoc comparisons indicate that high dose glucocorticoid treatment (35μg/ml) also resulted in a significant Cushing’s like reduction in gastrocnemius muscle mass in WT females (A) and males (C), and in brains for WT females (B). Overall N=52 for females (n= 7–11 per group) and N=60 for males (n=9–14 per group). Values represent Mean ± SE. *Indicate significant differences as indicated by Tukey’s post-hoc comparisons.
As with muscle mass, treatment also affected brain mass in female WT and R6/2 mice differently (Treatment*Genoptype: F2,46=4.65, p<.05). Normalization of glucocorticoids partially prevented brain atrophy in R6/2 female mice (Fig. 5B), with those on low dose corticosterone showing a small but significant 7.0% larger brain mass (412.4 ± 3.9mg), relative to those on high dose corticosterone (385.6 ± 5.7mg). Tukey’s post-hoc comparisons also indicated that there was a Cushing’s like reduction in brain mass in wild-type female mice on high dose corticosterone (438.9 ± 9.6mg), which were 9.7% smaller than those on low-dose corticosterone (485.9 ± 3.1mg) and 8.8% lower than sham controls (481.3 ± 5.6mg). Interestingly, there was not a similar interaction of genotype and treatment in male R6/2 mice (Fig. 5D, Treatment*Genoptype: F2,54=0.93, p=.40), although the transgenics did show a significantly reduced brain mass relative to wild-type mice (Genotype: F1,54=3.32, p<.01), and a significant Cushing’s like reduction in brain mass for both R6/2 and wild- type males on high dose corticosterone (Treatment: F2,54=4.65, p<.05). Although glucocorticoid normalization did not fully prevent a loss in brain and muscle mass to wild-type levels, these data provide evidence that elevated glucocorticoids certainly exacerbate the HD disease phenotype, and that the normalization of glucocorticoids attenuates the reduction in skeletal muscle and brain mass that are key characteristic symptoms of the disease.
To potentially identify specific brain regions that contributed to the reduction of brain atrophy following corticosterone normalization, we measured volumes of the cortex, striatum and hippocampus, brain regions known to be sensitive to elevated glucocorticoid levels and also affected in HD (Andela et al., 2013; Bourdeau et al., 2002; Lupien et al., 1998). Both male and female R6/2 mice showed significantly reduced regional volume across all regions assessed (p<.001 for all) relative to WT mice (Supplemental Fig. 4). Although there was no effect of treatment on striatal or hippocampal volume (p>.05 for treatment and treatment*genotype for both regions, for males and females), there was an effect of treatment (F2,18=8.07, p<.005) on cortical volume in females, with those on high dose corticosterone showing significantly reduced cortical volume relative to those on low dose treatment, as assessed with Tukey’s post-hoc comparisons. However, the effect on both genotypes were similar (No genotype*treatment interaction: F2,18=1.58, p=.23) and thus elevated corticosterone induces a reduction in cortical volume regardless of genotype. As there was no difference between HD sham and low dose mice, normalizing glucocorticoids does not improve this phenotype beyond normal reduction in regional volume. For males, there was a trend for the effect of treatment (F2,18=2.97, p=.077) on cortical volume, likely reflecting a reduction for wild-type and transgenic mice on high dose corticosterone treatment. As with females, there was not a differential effect of treatment on cortical volume between genotypes for males (Treatment*genotype: F2,18=0.20, p=.82).
High levels of glucocorticoids exacerbate neurogenesis defects in R6/2 mice
Adult neurogenesis in the dentate gyrus is potently modulated by glucocorticoids, with high levels associated with reduced neurogenesis and low levels associated with increased neurogenesis (Wong and Herbert, 2004). R6/2 mice, which have elevations on glucocorticoids, also show reduced dentate gyrus neurogenesis (Gil et al., 2005) and experimentally elevated levels of glucocorticoids reduce cell proliferation in the related transgenic R6/1 HD mouse (Mo et al., 2014a). Thus, in another experiment, we tested the hypothesis that normalizing glucocorticoid levels would prevent neurogenesis defects in R6/2 mice, reflected by an increased number of ki67+ cells, a marker for cell proliferation, in the dentate gyrus. While ki67 is a marker of cell proliferation and expressed before neural progenitors have differentiated into their ultimate cell fate, it is often used as a marker for neurogenesis (Kee et al., 2002). Specific to HD, it has also been demonstrated that a deficit in ki67+ cells in R6/2 mice directly reflects impaired neurogenesis, as it is also associated with a concomitant loss in the number of doublecortin+ cells (Gil et al., 2005). Furthermore, the vast majority of proliferating cells in R6/2 mice, although reduced in total number, are neurons (BRDU+/NeuN) (Gil et al., 2005). Thus, here, serial coronal tissue sections were stained for ki67 (Fig. 6, A-L) and total counts of ki67+ cells in the hippocampus of each section were quantified and analyzed statistically, and used as a proxy for indicating alterations in dentate gyrus neurogenesis.
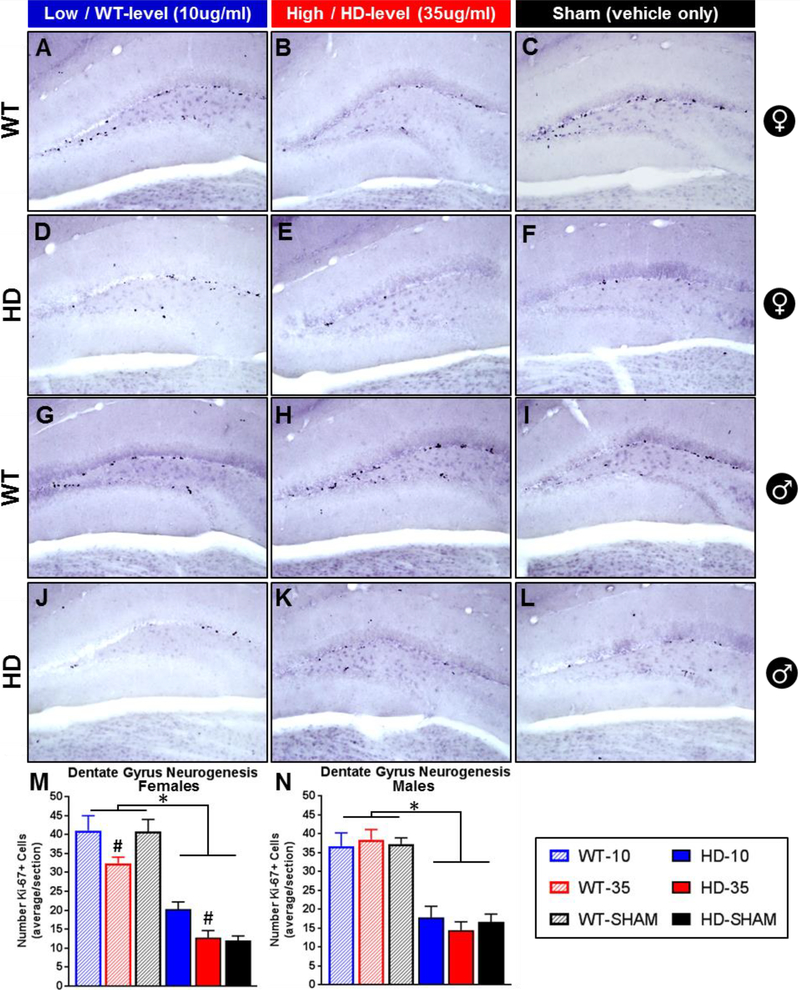
Figure 6. Effect of glucocorticoid replacement on neurogenesis in the dentate gyrus.
Representative images of Ki-67+ mitotic cells, a marker for neurogenesis, in the dentate gyrus of wild-type (females A-C, males G-I) and transgenic R6/2 HD mice (females D-F, males J-L). R6/2 mice show fewer ki67+ cells than WT (M,N - p<.05) and treatment with low dose CORT (WT-level, 10μg/ml) increases ki67+ cells in females relative to those on high dose (HD-level, 35μg/ml) (M, Treatment: p<.05, # Tukey’s post-hoc indicates difference between 10μg/ml and 35μg/ml treated female mice, regardless of genotype). Treatment had no effect on males (N, p>.05). N=24 for each sex, n=4 per group. Values represent Mean ± SE.
R6/2 mice show a significant reduction in the number of ki67+ cells in the dentate gyrus relative to WT mice (Fig. 6M and Fig. 6N, Genotype fixed effect, p<.0001 for males and females), replicating a disrupted neurogenesis phenotype shown previously (Gil et al., 2005; Mo et al., 2014a). Glucocorticoid treatment significantly affected the number of ki67+ cells in females (Treatment: F2,18=5.62, p<.05), with those on high dose corticosterone showing significantly fewer ki67+ cells than those on low dose, regardless of genotype (Tukey’s post-hoc, indicated by # in Fig. 6M). For male mice, although there was a clear effect of genotype on the number of ki67+ cells, there was no effect of treatment on either genotype (Treatment: F2,18=0.05 p>.05, Treatment*Genotype: F2,18=0.48 p>.05, Fig. 6N). Although there wasn’t a significant treatment*genotype interaction for females (Treatment*Genotype: F2,18=2.05, p=.16), there appears to be a divergent pattern in how corticosterone treatment affected neurogenesis between genotypes. R6/2 females on low dose corticosterone (20.3 ± 1.9 ki67+ cells/section) showed a 64.7% increase in the number of ki67+ cells relative to those on high dose (12.7 ± 1.9) and a 73.8% increase relative to sham controls (12.0 ± 1.2). The difference in the mean number of ki67+ cells in female R6/2 mice on high dose and sham controls is minimal, a differences of means of only 0.7 cells/section (a 5.9% increase for those on high dose corticosterone). This suggests that the difference in ki67+ cells between low dose and high dose reflects an increase in neurogenesis induced by the normalization of glucocorticoids. For wild-type females, those on high dose corticosterone (32.4 ± 1.6 ki67+ cells/section) showed a 22.7% reduction in ki67+ cells relative to those on low dose corticosterone (41.1 ± 3.9) and a 20.6% reduction relative to sham controls (40.8 ± 3.2). Average number of ki67+ cells were similar between the low dose group and sham controls in WT females, a difference of means of only 0.3 cells/section (a 0.7% increase in the low dose group relative to sham controls), suggesting that high dose corticosterone was sufficient to induce a Cushing’s like reduction in neurogenesis in WT females.
Normalizing glucocorticoids in female R6/2 mice reduces mHTT-positive inclusion burden
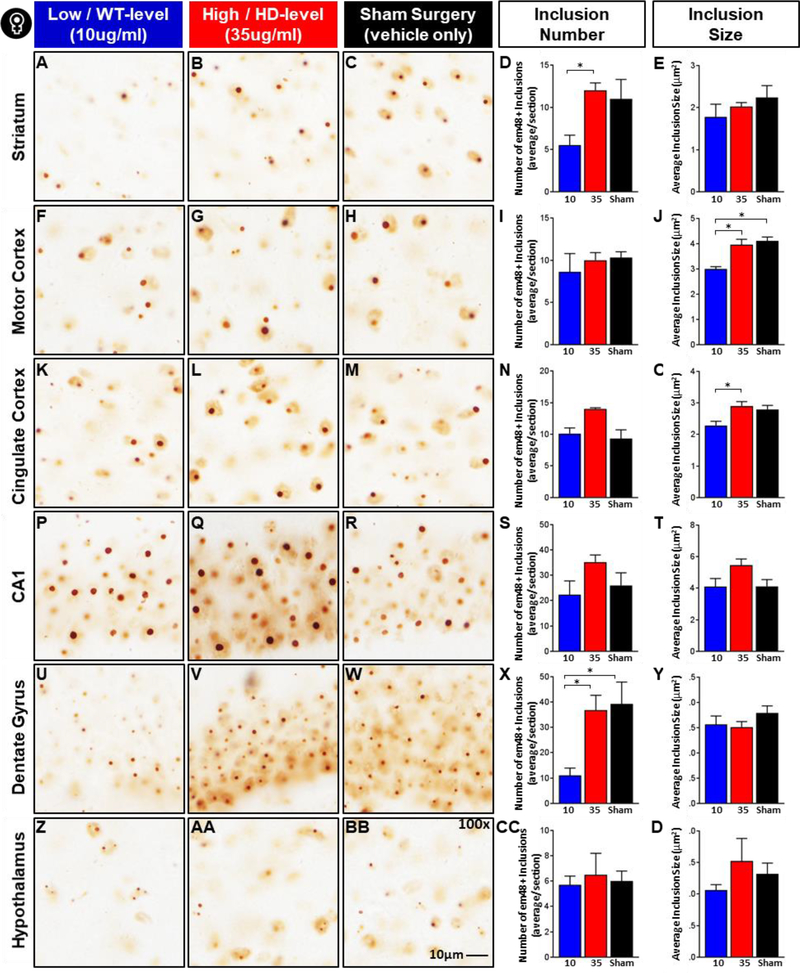
Since there is evidence that acute glucocorticoid receptor signaling has a potent effect on whether mutant huntingtin exists in aggregate or soluble form in vitro (Diamond et al., 2000), and worsens inclusion formation in other neurodegenerative disorders in vivo (Baglietto-Vargas et al., 2013; Green et al., 2006), we assessed the effect of glucocorticoid treatment here on inclusion burden in R6/2 mice. Sections from male and female R6/2 mice were stained with DAB immunohistochemistry using an anti-EM48 antibody, a specific marker for aggregated mutant huntingtin protein, and inclusion number and size were quantified systematically in ImageJ in a total of six brain regions known to be affected in HD, including the striatum, motor and cingulate cortices, the CA1 region of the hippocampus, the dentate gyrus and the hypothalamus. Interestingly, the dentate gyrus and CA1 region of the hippocampus both have a very high expression of glucocorticoid receptors and we were interested in assessing if they were particularly affected by variations in glucocorticoid levels. The lateral hypothalamus was assessed to provide a preliminary analysis of whether the effects of glucocorticoids on metabolism and body weight could be explained, in part, by decreased neuropathology in this key metabolic regulatory region.
As with neurogenesis and brain weight, there was a sexually dimorphic response to glucocorticoid treatment on EM48+ inclusions in R6/2 mice. In females, normalization of glucocorticoid levels significantly reduced the number of inclusions in the striatum (Fig. 7D, Treatment: F2,9=4.68, p<.05) and the dentate gyrus (Fig. 7X, Treatment: F2,9=6.25, p<.05), and resulted in a significant reduction in inclusion size in motor cortex (Fig. 7J, Treatment: F2,9=14.34, p<.01) and cingulate cortex (Fig. 7O, Treatment: F2,9=5.68, p<.05). For females, there was no effect of treatment in either the CA1 region of the hippocampus, or in the lateral hypothalamus (p>.05 for both). Tukey’s post-hoc tests indicated that in the striatum (Fig. 7, A-E), normalized glucocorticoid levels was associated with a significant 54% reduction in inclusion number (5.5 ± 1.2) in female R6/2 mice relative to those on high dose replacement (12.0 ± 0.9), and a non-significant 50% reduction relative to sham controls (11.0 ± 2.3). In the dentate gyrus (Fig. 7, U-Y), normalized glucocorticoid levels led to a large and significant 70% reduction in inclusion number (11.0 ± 2.9) relative to those on high-dose treatment (36.8 ± 5.9), and a significant 72% reduction relative to sham controls (39.3 ± 8.6). In the motor cortex (Fig. 7, F-J), females on low dose corticosterone showed a significant 24% reduction in inclusion size (3.00μm2 ± 0.09) relative to those on high dose corticosterone (3.96μm2 ± 0.21), and a significant 27% reduction relative to sham controls (4.11μm2 ± 0.15). In the cingulate cortex (Fig. 7, K-O), females on low dose corticosterone showed a significant 21% reduction in inclusion size (2.28μm2 ± 0.14) relative to high dose corticosterone (2.90μm2 ± 0.14), and a non-significant 18% reduction relative to sham controls (2.79μm2 ± 0.13). Interestingly, there was no effect of treatment on inclusion burden (number or size) in male R6/2 mice in any region examined (Supplemental Fig. 5, p>.05 for all). Taken together, these data show that normalizing glucocorticoids has the ability to reduce inclusion burden in R6/2 mice, although the effect of treatment is strongly affected by sex and varies by brain region.
Figure 7. Effect of glucocorticoid reduction on em48+ inclusion bodies in female R6/2 mice.
Representative images from Striatum (A-C), Motor cortex (F-H), Cingulate cortex (K-M), Hippocampus (CA1: P-R and Dentate Gyrus: U-W), and Lateral hypothalamus (Z-BB). Female R6/2 mice on low dose (WT-level) CORT replacement (10μg/ml) showed significantly fewer EM48+ inclusions in the striatum (D) and dentate gyrus (X), and reduced inclusion size in the motor (J) and cingulate cortices (O) (Treatment Fixed Effect: p<.05). Overall N=12, n=4 per treatment group. Scale bar reflects 10μm. Values represent Mean ± SE. *Indicate significant differences as indicated by Tukey’s post-hoc comparisons.
DISCUSSION
It has long been speculated that neuroendocrine changes may contribute the metabolic phenotype shown by HD patients, in particular that chronically elevated glucocorticoids contribute to increased metabolism, weight loss, and muscle wasting (Aziz et al., 2009; Bjorkqvist et al., 2006a; Goodman et al., 2008; Petersen and Bjorkqvist, 2006; Saleh et al., 2009). There is also indirect evidence that suggests chronically elevated glucocorticoids may possibly exacerbate HD neuropathology, including regional changes in brain volume (Bourdeau et al., 2002), reductions in neurogenesis (Brummelte and Galea, 2010; Cameron and Gould, 1994; McEwen, 1999), as well as mutant huntingtin protein aggregation (Diamond et al., 2000; Labbadia et al., 2011; Maheshwari et al., 2014). In previous work, we demonstrated that significant elevations in corticosterone do, indeed, impact the HD metabolic phenotype by exacerbating weight-loss in the transgenic R6/2 HD mouse model. Here, we extend these findings and demonstrate that normalizing corticosterone to WT levels in R6/2 mice attenuates disease-related changes to weight loss, the respiratory exchange ratio, muscle wasting, reductions in brain mass, deficits in neurogenesis and inclusion body burden. Although treatment did not fully rescue these metabolic or neuropathological symptoms, these data provide evidence that normalizing glucocorticoid levels or blocking the glucocorticoid receptor could possibly attenuate symptomology in clinical HD cases. These data also demonstrate that when glucocorticoids become elevated in HD mice, and likely HD patients, it profoundly further exacerbates the disease phenotype.
Progressive weight loss is a well-documented symptom of HD (Aziz et al., 2008; Trejo et al., 2004), which occurs simultaneously with a progressive increase in glucocorticoid levels (Bjorkqvist et al., 2006a; Saleh et al., 2009) and a chronic elevation of metabolic rate (Gaba et al., 2005; Pratley et al., 2000). All of these co-occurring symptoms are recapitulated in the R6/2 mouse model (Bjorkqvist et al., 2006a; She et al., 2011; van der Burg et al., 2008), and the relationship between glucocorticoids and specific metabolic abnormalities in HD are poorly characterized. In this study, the normalization of glucocorticoids resulted in a delay in weight loss in both males and female R6/2 mice. This compliments our previous data whereby experimentally increased glucocorticoid levels were associated with precipitous weight loss (Dufour and McBride, 2016), and illustrates that while glucocorticoids are not solely responsible for the weight loss HD phenotype, they do contribute significanty.
It is postulated that weight loss in HD is driven by a chronic elevation in whole organism metabolic rate (Gaba et al., 2005; Pratley et al., 2000), leading to a state of negative energy balance (Goodman et al., 2008). R6/2 mice are in a state of elevated metabolism, as indicated by indirect calorimetry data here and elsewhere, with mice showing increased oxygen consumption (VO2) (She et al., 2011; van der Burg et al., 2008). This increase in metabolic rate precedes the onset of weight loss, which is progressive and severe in R6/2 mice (She et al., 2011; van der Burg et al., 2008). We detected an elevation in VO2 in both male and female R6/2 mice here as early as 5 weeks of age. These data demonstrate, for the first time, that R6/2 mice also have an elevation in VCO2 production, which is present early on at 5 weeks of age. These patterns persist and worsen with time, with R6/2 mice showing even more significant elevations in VO2 and VCO2 at 10 weeks of age. While both were elevated in R6/2 mice here, VO2 was more highly elevated than VCO2, leading to a significan reduction in the respiratory exchange ratio (RER, ratio VCO2:VO2). RER is an indirect indicator of nutrient utilization, with higher values (near 1.00) reflecting preferential utilization of carbohydrates for cellular metabolism and lower values (near 0.70) reflecting preferential utilization of lipids (Frayn, 1983). Thus, this reduction in RER in R6/2 mice likely reflects a shift to increased utilization of fatty acids as a metabolic fuel source, rather than carbohydrates.
Treatment had a strong and consistent impact on indirect calorimetry measures in male R6/2 mice. Here, normalization of glucocorticoids (low-dose WT-level replacement) leads to a nearly normalized metabolic rate, as indicated by improved measures of VO2, VCO2, and RER. In fact, R6/2 males on wild-type level corticosterone replacement showed a full rescue in their RER values to wild-type levels, demonstrating that the normalization of glucocorticoids has the ability to improve whole organism metabolic rate and nutrient utilization. High dose corticosterone did not exacerbate metabolic rate in R6/2 females, and the normalization of glucocorticoids also failed to normalize any metabolic readout (VO2, VCO2, or RER) to WT levels. This was surprising, especially given that normalized glucocorticoid levels resulted in an attenuation in the weight loss phenotype in female R6/2 mice. This suggests that there might be another mechanism that is driving reduction in weight loss seen here, other than simply a normalization in metabolic rate. Wildtype mice of both sexes were also unresponsive to different glucocorticoid levels, with respect to indirect calorimetry. This is an intriguing finding, which illustrates that R6/2 male mice are particularly sensitive to alterations in glucocorticoids at the doses and time-points investigated here.
While chronically elevated glucocorticoids are known to be the key mediator of muscle wasting in a variety of conditions, including Cushing’s syndrome and chemotherapy associated cachexia (Braun et al., 2013; Grossberg et al., 2010), it was previously unclear if this elevation in HD contributes to the muscle wasting phenotype. The data here show that it does indeed contribute, as the normalization of glucocorticoids in R6/2 mice significantly reduced muscle wasting in both sexes. However, while there was significant attenuation of atrophy, the reduction in muscle mass was only partially rescued in these mice, again suggesting that chronically elevated glucocorticoids are only partial contributors to the HD cachexia phenotype. The lack of sexual dimorphism here is interesting, especially given the robust sexual dimorphism in the calorimetry measures. This emphasizes that muscle wasting in HD may not only be due to chronic elevations in metabolic rate, leading to muscle breakdown for nutrient utilization, as it still occurs in male R6/2 mice when their calorimetry measures are fully normalized. Conversely, there was significant partial prevention of muscle atrophy in female R6/2 mice without any treatment induced improvement in calorimetry measures. Instead of elevated metabolism being the driver of cachexia, an alternative possibility is that mutant huntingtin toxicity within skeletal muscle itself could be a main driver of cachexia in HD, which is further exacerbated by chronically elevated glucocorticoid levels. Mutant huntingtin protein is expressed in skeletal muscle, which shows significant pathology itself, including the presence of inclusion bodies and increased markers of inflammation and apoptosis (Magnusson-Lind et al., 2014; Moffitt et al., 2009). Glucocorticoids are also known to be modulators of apoptosis pathways, with chronically high levels being associated with increased apoptosis in a variety of tissues including muscle, brain, and bone (Gruver-Yates and Cidlowski, 2013; Lee et al., 2005; Moutsatsou et al., 2012; Sapolsky et al., 1985). Similarly, high doses of glucocorticoids are associated with an increase in protein breakdown and catabolism in skeletal muscle (Lofberg et al., 2002). Thus, while not directly tested here, reduction in muscle atrophy here may reflect the normalization of this hormonal factor, which worsens cachexia at high levels by further activating inflammatory and apoptotic pathways in muscle itself, and which are already activated by mutant huntingtin toxicity. The resulting partial prevention of skeletal muscle atrophy observed here could alone explain the attenuation in weight loss shown here in male and female R6/2 mice, rather than a treatment-induced improvement in metabolic rate.
One surprising finding from the calorimetry data is that R6/2 mice of both sexes here showed a dramatic decrease in food intake at 10 weeks of age. In previous studies, the R6/2 weight loss phenotype has been demonstrated to occur in spite of maintaining normal levels of food intake throughout disease progression (She et al., 2011), or with a very slight hypophagia only at end stage (van der Burg et al., 2008). It is not clear what is responsible for this discrepancy; it is possible that the mice had trouble obtaining and consuming food in the specialized feeders at the bottom of the calorimetry chambers due to motor impairment. Indeed, R6/2 mice have previously been shown to have an increase in time spent feeding (van der Burg et al., 2008), which suggests that they might be compensating for motor impairments in food consumption by spending more time consuming food, and thereby normalize the amount of calories consumed to wild-type levels. They have been shown to have a slight attenuation in caloric intake at very late stages (van der Burg et al., 2008), which appears to be insufficient to mediate weight loss. If the hypophagia detected here represents particular difficulty eating in the chambers, rather than a generalized reduction in food intake, then hypophagia would be limited to only the 24 hours spent in the chambers, and would likely have little impact on overall bodyweight or metabolic readouts. However, if the R6/2 mice here did show profound and chronic hypophagia, in contrast to previous studies, this could be a significant contributor to the overall metabolic findings.
In addition to improving metabolic phenotypes in R6/2 mice, the normalization of glucocorticoids significantly improved neuropathological readouts, leading to a partial prevention of global brain atrophy, altered neurogenesis, and a decrease in mutant huntingtin inclusion burden in female R6/2 mice. However, as with the metabolic findings, none of these neuropathological symptoms were fully rescued, which again demonstrates that while elevated glucocorticoids exacerbate many HD symptoms they do not exclusively mediate them. Unexpectedly, neuropathological markers in male R6/2 mice were not affected by different levels of glucocorticoid replacement, although they were more sensitive to the effects of glucocorticoids on metabolism.
Reduced brain mass is a hallmark R6/2 phenotype, and this reduction in brain mass was attenuated in female R6/2 mice with normalized glucocorticoid levels. Additionally, wild-type females on high dose corticosterone also showed an expected Cushing’s-like reduction in brain mass. Males of both genotypes were resistant to these effects on brain mass. Surprisingly, treatment had no effect on striatal and hippocampal regional brain volume in either sex in females, in spite of significant differences in brain mass. There was a slight, but significant effect of treatment on cortical volume in females of both genotypes, with those on high/HD-level corticosterone showing reduced cortical volume relative to those on low/WT dose treatment. R6/2 mice, and human HD patients, show alterations in neuronal volume and cell loss in the brain (Stack et al., 2005), which are both likely contributors to reductions in brain mass and regional brain volume. Glucocorticoids are known to be modulators of apoptosis pathways, with abnormally high levels being associated with increased apoptosis in brain (Gruver-Yates and Cidlowski, 2013; Sapolsky et al., 1985; Sapolsky et al., 1990).Although not directly measured here, it is plausable that partial prevention in whole brain atrophy in female R6/2 mice on low/WT-level corticosterone reflects an attenuation in glucocorticoid-induced apoptosis. However, this should be interpreted with caution; while there is abundant evidence that glucocorticoid-induced cell loss is mediated by apoptosis, there is debate on whether neuronal cell loss is mediated by apoptosis in HD (Sawa et al., 2003), as degenerating neurons in human HD and R6/2 mice are lacking some of the hallmarks of apoptotic cell death (e.g. blebbing, apoptotic bodies, DNA fragmentation) (Turmaine et al., 2000). However, while there is a lack of clear apoptotic morphology in degenerating neurons in R6/2 mice, there is abundant evidence that apoptotic machinery is activated in R6/2 brain and peripheral tissues, including upregulation in cytokines and caspases, as well as increased levels of caspase activation (Ona et al., 1999; She et al., 2011). Accordingly, while the exact mechanisms of cell death in HD and R6/2 mice remains debated, overlap between mHTT and glucocorticoid induced activation of apoptotic pathways suggest a possible common mechanism by which these two factors interact and possibly result in increased neuronal and peripheral tissue cell loss. As a separate issue, it remains unclear why female R6/2 mice would be more sensitive than males to the therapeutic effects of normalized glucocorticoids on apoptosis and brain mass. Interestingly, wild-type females were also more sensitive to the effects of experimentally elevated glucocorticoids here, showing a Cushing’s like reduction in brain mass and cortical volume, while wild-type males did not demonstrate such changes.
The normalization of glucocorticoids also impacted levels of neurogenesis in the dentate gyrus of R6/2 females. Here, there was a significant effect of treatment for both wild-type and HD females, with those on low/WT dose showing increased neurogenesis relative to those on high dose corticosterone. Although the data did not indicate a treatment by genotype interaction, neurogenesis levels in sham controls suggest that the finding in R6/2 females represents a low/WT-dose induced improvement in neurogenesis (the mean number of ki67+ cells are increased relative to both high/HD dose treated mice and sham controls). Conversely, it appears that the difference between high and low dose treatment in wild-type females represents a Cushing’s like reduction in neurogenesis (the mean number of ki67+ cells in the high/HD dose treated mice are lower than both the low/wt dose group and sham controls). The sample size is small here (n=4 per group), and insufficient power may account for the lack of significance for a more complex treatment*genoptype interaction. Thus, while this finding is preliminary and should be followed up in a larger cohort to confirm the finding statistically, it isn’t surprising that a chronic elevation in glucocorticoids would further impair neurogenesis in HD mice. The dentate gyrus has long been known to be particularly sensitive to chronic elevations in glucocorticoids, which suppresses differentiation of dentate gyrus progenitor cells (Brummelte and Galea, 2010; Cameron and Gould, 1994; Gould et al., 1992) and also activates apoptosis in mature dentate gyrus neurons (Crochemore et al., 2005; Lu et al., 2003). However, it is surprising that neurogenesis was unaffected by glucocorticoids in both wild-type and R6/2 males here, as it was for brain mass and regional volume. However, when contrasted with the female findings of a treatment induced increase in neurogenesis, brain mass, and cortical volume, it emphasizes that brains from female subjects here were uniquely sensitive to manipulations in glucocorticoid levels and suggests that improvements in brain mass and cortical volume in female R6/2s may, in part, be mediated by improved neurogenesis and the migration and differentiation of progenitor cells to regions affected by disease.
Normalizing glucocorticoid levels in female R6/2 mice here resulted in a marked reduction in inclusion number in the striatum and dentate gyrus, as well as reduction in inclusion size the motor and cingulate cortices. It is unclear why in certain brain regions there was a reduction in aggregate number, in others a reduction in aggregate size, and in others no effect of treatment whatsoever. While this may simply reflect region specific differences in glucocorticoid receptor expression, or in other molecular cofactors and targets of the activated receptor, further assessment of why these differences emerged are warranted. It also remains unclear whether or not the reduction in mHTT+ inclusion bodies results from a reduction in inclusion formation, or possibly an increase in protein clearance. The key finding here of a treatment induced reduction in inclusion burden is consistent with the Alzheimer’s disease (AD) literature, where chronically elevated glucocorticoids are associated with increased amyloid-beta and hyperphosphorylated tau accumulation (Green et al., 2006) and blocking glucocorticoid receptors attenuates inclusion burden in rodent models (Baglietto-Vargas et al., 2013). There is also recent evidence in that mHTT aggregation is particularly sensitive to glucocorticoids. Acute administration of dexamethasone (a potent glucocorticoid receptor agonist) on cultured cells expressing mutant huntingtin and glucocorticoid receptors (GRs) potently modulates inclusion burden; mHTT protein is predominately in soluble form when dexamethasone is present, and predominately in insoluble aggregate form when absent (Diamond et al., 2000). While this in vitro study provides a strong link between glucocorticoid signaling and mHTT aggregation, their findings represent the inverse of what was found here and in the AD literature. This discrepancy is probably best explained by the differences in administration here, as this study assessed the effects of chronic elevations of corticosterone (and its normalization) on inclusion burden. Chronic elevations in glucocorticoids lead to a compensatory downregulation in GR mRNA and protein levels and a reduction in glucocorticoid binding (Burnstein et al., 1991). It’s possible that normalizing glucocorticoid levels here to a more typical physiological range could help to maintain GR signaling mediated reduction in inclusion bodies (at peak levels), and that chronically high levels paradoxically impairs this process by attenuating GR signal transduction. Thus, although plasma levels of corticosterone are elevated, perhaps this elevation can no longer activate downstream processes that attenuate inclusion burden.
The data presented here further demonstrate that the elevated glucocorticoid phenotype seen in HD does indeed exacerbate other HD symptomology, including metabolic and neuropathological abnormalities. However, there was an unexpected and robust sexually dimorphic response to treatment, with normalized corticosterone improving in-vivo metabolic readouts predominately in male R6/2 mice and post-mortem neuropathology exclusively in female R6/2 mice. However, some metabolic effects of treatment were shared by both sexes, including a therapeutically induced attenuation in weight loss and improvement in skeletal muscle mass. It is not immediately clear why this sexual dimorphic response exists. One possibility is that male R6/2 mice show a slightly faster disease progression, losing weight and dying about a week earlier than females on average (Cummings et al., 2012; Wood et al., 2010). Since the majority of the post-treatment measures were taken at a single timepoint (muscle mass, brain mass, and neuropathology were assessed at week 11 and calorimetry at 10 weeks of age) the findings represent the cumulative changes induced by treatment at only that point in time. It is possible that the male R6/2 mice were already entering late stage phenotype with profound metabolic symptoms at 10 weeks of age, while female R6/2s had only more subtle alterations in metabolism. Instead of affecting calorimetry measures directly, perhaps normalized glucocorticoid treatment had a direct effect on end-stage symptom progression broadly. Similarly, neuropathological readouts are perhaps attenuated by low dose corticosterone earlier in disease progression, and female specific improvements in neuropathology simply reflect the stage of progression at 11 weeks of age. It is possible that normalized glucocorticoid treatment induced similar reductions in mHTT aggregation in males earlier in the experiment, but that they were no longer effective by 11 weeks of age. Another possible explanation for the sexual dimorphic response is simply differences in HD pathology that are specific to each sex. For example, male R6/2 mice (and human patients) show dramatic atrophy of the testes during end stages of disease and a concomitant reduction in circulating testosterone levels (Papalexi et al., 2005; Saleh et al., 2009; Van Raamsdonk et al., 2007), which would likely impact metabolism and muscle homeostasis (Herbst and Bhasin, 2004). Female specific abnormalities in Hypothalamic-Gonadal-Pituitary (HPG) axis have been demonstrated in R6/1 mice, including a reduction in Gonadotropin Releasing Hormone (GnRH) (Du et al., 2015). However, this does not seem to impact ovary derived sex steroids in females - in both female R6/1 mice and human HD patients, circulating estrogen levels are not reduced by mHTT toxicity (Du et al., 2015; Saleh et al., 2009), and may possibly have neuroprotective effects (Tunez et al., 2006).
While HD symptomology is clearly driven by mHTT toxicity across a variety of brain regions and tissues, it has remained unclear whether glucocorticoid dysregulation, itself a symptom of HD, further exacerbates HD symptomology at physiologically relevant levels. The experimental design presented here, adrenalectomy with glucocorticoid replacement, was purposefully and carefully chosen to best assess this question and to optimize both construct and predictive validity, as it enabled us to block HD-induced hypercoritsolinemia and to set circulating glucocorticoid replacement to physiologically relevant HD and WT levels based on both human clinical data as well as our previous findings in R6/2 mice. HD-level glucocorticoid replacement in this study resulted in roughly a 2.8-fold increase in circulating glucocorticoid levels relative to those on WT-level replacement (see Figure 1). While there are some inconsistencies in the human clinical literature regarding the magnitude and timecourse of glucocorticoid dysregulation in HD, the HD-level replacement utilized here is consistent with human clinical data from Bjorkqvist et al. (2006a) which showed a 3-fold elevation in urinary cortisol levels in human HD patients at late stages of disease. Bjorkqvist et al also showed that this elevation is progressive in nature, starting at mid-stages of disease with a two-fold elevation before ultimately reaching the higher end-stage level. This is consistent with Heuser et al. (1991), who was the first to identify an elevation in basal plasma glucocorticoid levels in clinical HD, with patients across disease stages showing a 2-fold increase. Saleh et al. (2009) performed the largest study to date, across HD patients of varying degrees of disease progression, and showed an approximate 1.5-fold increase in plasma cortisol levels, but failed to find any differences according to disease stage. In contrast with these three clinical studies, there are also a handful of studies that did not find an elevation in manifest clinical HD cases, but rather only found small elevations in salivary cortisol levels in pre-manifest cases, before the onset of motor symptomology (Shirbin et al., 2013a; Shirbin et al., 2013b; van Duijn et al., 2010). While it has been previously shown that R6/2 mice show an even more dramatic 6-fold elevation in glucocorticoid levels, subsequent and more comprehensive studies in our lab have demonstrated that this elevation is lower (roughly a 2-fold elevation) and closer in magnitude to both the clinical HD cases, as well as our HD-level CORT replacement dose here. Accordingly, our chosen HD-level corticosterone replacement dose is a conservative estimate when based on the R6/2 mouse literature, and is also consistent with the human clinical HD literature, albeit at the higher end of the spectrum. Another important aspect of our experimental design here, which we believe strengthens the translational nature of this study, was the inclusion of sham operated mice as a control. By doing so, we were able to clearly demonstrate that WT-level corticosterone replacement not only attenuates symptomology relative to those on experimentally elevated (HD-level) corticosterone replacement, but that it also attenuates HD metabolic and neuropathological symptomology relative to sham-operated R6/2 HD mice spontaneously producing their own high HD-levels of glucocorticoids.
Accordingly, the data resulting from this proof of concept study confirm our previous findings that chronically elevated glucocorticoids, itself a symptom of HD, further exacerbates HD symptomology. More importantly, though, these data also demonstrate that normalizing glucocorticoid homeostasis in a rodent model of HD has the ability to ameliorate metabolic and neuropathological symptomology. Given the poor prognosis for HD patients, and the lack of any FDA approved medications that can slow the progression of disease-associated symptomology, these data identify glucocorticoid dysregulation as a novel point of therapeutic intervention for the disease. Although adrenalectomy with cortisol replacement is not a practical therapeutic approach for human disease, reducing the deleterious consequences of chronic hypercortisolinemia by pharmacologically blocking glucocorticoid receptors is a clinically plausible strategy. Glucocorticoid receptor antagonists, such as Mifepristone, are FDA approved pharmacotherapeutic drugs used clinically to treat Cushing’s disease symptoms (Fleseriu et al., 2012; Morgan & Laufgraben, 2013), and may also show efficacy in clinical populations for treating HD symptoms that are associated with chronically elevated cortisol. Although Mifepristone has shown to be safe and effective in a non-HD population (Cushing’s patients) (Fleseriu et al., 2012; Morgan and Laufgraben, 2013), it will be critically important to carefully assess whether HD patients may be more negatively impacted by the side-effects of this medication, as the drug not only has potent glucocorticoid receptor antagonist properties, it is also a potent progesterone receptor antagonist and also acts to a lesser degree on androgen receptors (Morgan and Laufgraben, 2013). Given the severity of HD symptomology and the desperate need for efficacious therapeutics, we believe this study provides strong rationale for further assessment of both safety and efficacy of GR-antagonists to attenuate metabolic and neuropathological symptomology in rodent models and HD patients. Additionally, while not investigated in the current study, elevated glucocorticoids have also been associated with HD cognitive and mood symptoms in R6/1 mice (Mo et al., 2014a; Mo et al., 2013) and in human patients (Shirbin et al., 2013a). Accordingly, it is also possible that GR-antagonists could offer robust improvement across many HD symptom domains, including metabolism, cognition, mood, and neuropathology.
Supplementary Material
Acknowledgements.
We would like to thank Dr. Paul Kievet and Rene Lindsley at the ONPRC for assistance and use of the rodent metabolic chambers. We also thank Dana Button for assistance with organ weight measurements at necropsy. A special thank you to the Small Laboratory Animal Unit staff at the ONPRC for their exceptional animal care and for accommodating the experiments detailed here. This research was supported by an NIH/NINDS Ruth L. Kirschstein National Research Service Award F31NS092281 (BDD), ONPRC Core Grant P51OD011092 (JLM) and a generous donation from Quentin and Bee Neufeld (JLM).
Footnotes
COMPETING FINANCIAL INTEREST STATEMENT
The authors have no competing financial interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andela CD, et al. , 2013. Smaller grey matter volumes in the anterior cingulate cortex and greater cerebellar volumes in patients with long-term remission of Cushing’s disease: a case-control study. Eur J Endocrinol. 169, 811–9. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Marder KS, 2001. An overview of psychiatric symptoms in Huntington’s disease. Curr.Psychiatry Rep 3, 379–388. [DOI] [PubMed] [Google Scholar]
- Aziz NA, et al. , 2009. Increased hypothalamic-pituitary-adrenal axis activity in Huntington’s disease. J.Clin.Endocrinol.Metab 94, 1223–1228. [DOI] [PubMed] [Google Scholar]
- Aziz NA, et al. , 2008. Weight loss in Huntington disease increases with higher CAG repeat number. Neurology. 71, 1506–1513. [DOI] [PubMed] [Google Scholar]
- Baglietto-Vargas D, et al. , 2013. Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol Psychiatry. 74, 357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford KA, et al. , 1995. A prospective evaluation of cognitive decline in early Huntington’s disease: functional and radiographic correlates. Neurology. 45, 1867–73. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist M, et al. , 2005. The R6/2 transgenic mouse model of Huntington’s disease develops diabetes due to deficient beta-cell mass and exocytosis. Hum.Mol.Genet 14, 565–574. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist M, et al. , 2006a. Progressive alterations in the hypothalamic-pituitary-adrenal axis in the R6/2 transgenic mouse model of Huntington’s disease. Hum.Mol.Genet 15, 1713–1721. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist M, et al. , 2006b. Progressive alterations in the hypothalamic-pituitary-adrenal axis in the R6/2 transgenic mouse model of Huntington’s disease. Hum Mol Genet 15, 1713–21. [DOI] [PubMed] [Google Scholar]
- Black PR, et al. , 1982. Mechanisms of insulin resistance following injury. Ann Surg. 196, 420–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau I, et al. , 2002. Loss of brain volume in endogenous Cushing’s syndrome and its reversibility after correction of hypercortisolism. J.Clin.Endocrinol.Metab 87, 1949–1954. [DOI] [PubMed] [Google Scholar]
- Braun TP, et al. , 2013. Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle. FASEB J. 27, 3572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Galea LA, 2010. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience. 168, 680–90. [DOI] [PubMed] [Google Scholar]
- Burnstein KL, et al. , 1991. Autoregulation of glucocorticoid receptor gene expression. Steroids. 56, 52–8. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E, 1994. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 61, 203–9. [DOI] [PubMed] [Google Scholar]
- Crochemore C, et al. , 2005. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry. 10, 790–8. [DOI] [PubMed] [Google Scholar]
- Cummings DM, et al. , 2012. A critical window of CAG repeat-length correlates with phenotype severity in the R6/2 mouse model of Huntington’s disease. J Neurophysiol 107, 677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MI, et al. , 2000. Regulation of expanded polyglutamine protein aggregation and nuclear localization by the glucocorticoid receptor. Proc Natl Acad Sci U S A. 97, 657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djousse L, et al. , 2002. Weight loss in early stage of Huntington’s disease. Neurology. 59, 1325–1330. [DOI] [PubMed] [Google Scholar]
- Du X, et al. , 2012. Environmental enrichment rescues female-specific hyperactivity of the hypothalamicpituitary-adrenal axis in a model of Huntington’s disease. Transl.Psychiatry 2, e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, et al. , 2015. The influence of the HPG axis on stress response and depressive-like behaviour in a transgenic mouse model of Huntington’s disease. Exp Neurol. 263, 63–71. [DOI] [PubMed] [Google Scholar]
- Dufour BD, McBride JL, 2016. Corticosterone dysregulation exacerbates disease progression in the R6/2 transgenic mouse model of Huntington’s disease. Exp Neurol. 283, 308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleseriu M, et al. , 2012. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 97, 2039–49. [DOI] [PubMed] [Google Scholar]
- Frayn KN, 1983. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 55, 628–34. [DOI] [PubMed] [Google Scholar]
- Gaba AM, et al. , 2005. Energy balance in early-stage Huntington disease. Am J Clin Nutr. 81, 1335–41. [DOI] [PubMed] [Google Scholar]
- Gharami K, et al. , 2008. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington’s disease phenotypes in mice. J Neurochem. 105, 369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil JM, et al. , 2005. Reduced hippocampal neurogenesis in R6/2 transgenic Huntington’s disease mice. Neurobiol.Dis 20, 744–751. [DOI] [PubMed] [Google Scholar]
- Goodman AO, et al. , 2008. The metabolic profile of early Huntington’s disease--a combined human and transgenic mouse study. Exp Neurol. 210, 691–8. [DOI] [PubMed] [Google Scholar]
- Gould E, et al. , 1992. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 12, 3642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, et al. , 2006. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 26, 9047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg AJ, et al. , 2010. Hypothalamic mechanisms in cachexia. Physiol Behav. 100, 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote HE, et al. , 2005. Cognitive disorders and neurogenesis deficits in Huntington’s disease mice are rescued by fluoxetine. Eur J Neurosci. 22, 2081–8. [DOI] [PubMed] [Google Scholar]
- Gruver-Yates AL, Cidlowski JA, 2013. Tissue-specific actions of glucocorticoids on apoptosis: a doubleedged sword. Cells. 2, 202–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst KL, Bhasin S, 2004. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 7, 271–7. [DOI] [PubMed] [Google Scholar]
- Heuser IJ, et al. , 1991. The limbic-hypothalamic-pituitary-adrenal axis in Huntington’s disease. Biol.Psychiatry 30, 943–952. [DOI] [PubMed] [Google Scholar]
- Hodges A, et al. , 2006. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet. 15, 965–77. [DOI] [PubMed] [Google Scholar]
- Huntington Disease Collaborative Research Group, 1993. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 72, 971–983. [DOI] [PubMed] [Google Scholar]
- Huntington Study Group, 1996. Unified Huntington’s Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord. 11, 136–42. [DOI] [PubMed] [Google Scholar]
- Kee N, et al. , 2002. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 115, 97–105. [DOI] [PubMed] [Google Scholar]
- Kirkwood SC, et al. , 2001. Progression of symptoms in the early and middle stages of Huntington disease. Arch Neurol. 58, 273–8. [DOI] [PubMed] [Google Scholar]
- Kosinski CM, et al. , 2007. Myopathy as a first symptom of Huntington’s disease in a Marathon runner. Mov Disord. 22, 1637–1640. [DOI] [PubMed] [Google Scholar]
- Labbadia J, et al. , 2011. Altered chromatin architecture underlies progressive impairment of the heat shock response in mouse models of Huntington disease. J Clin Invest. 121, 3306–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic SE, et al. , 2004. Decreased hippocampal cell proliferation in R6/1 Huntington’s mice. Neuroreport. 15, 811–3. [DOI] [PubMed] [Google Scholar]
- Lee MC, et al. , 2005. Apoptosis of skeletal muscle on steroid-induced myopathy in rats. J Nutr. 135, 1806S–1808S. [DOI] [PubMed] [Google Scholar]
- Lofberg E, et al. , 2002. Effects of high doses of glucocorticoids on free amino acids, ribosomes and protein turnover in human muscle. Eur J Clin Invest. 32, 345–53. [DOI] [PubMed] [Google Scholar]
- Lu J, et al. , 2003. Ionotropic and metabotropic glutamate receptor mediation of glucocorticoid-induced apoptosis in hippocampal cells and the neuroprotective role of synaptic N-methyl-D-aspartate receptors. Neuroscience. 121, 123–31. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, et al. , 1998. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1, 69–73. [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R, et al. , 2002. Dysregulation of gene expression in the R6/2 model of polyglutamine disease: parallel changes in muscle and brain. Hum.Mol.Genet 11, 1911–1926. [DOI] [PubMed] [Google Scholar]
- Magnusson-Lind A, et al. , 2014. Skeletal muscle atrophy in R6/2 mice - altered circulating skeletal muscle markers and gene expression profile changes. J Huntingtons Dis. 3, 13–24. [DOI] [PubMed] [Google Scholar]
- Maheshwari M, et al. , 2014. Dexamethasone induces heat shock response and slows down disease progression in mouse and fly models of Huntington’s disease. Hum Mol Genet. 23, 2737–51. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 1999. Stress and hippocampal plasticity. Annu.Rev.Neurosci 22, 105–122. [DOI] [PubMed] [Google Scholar]
- Mo C, et al. , 2014a. High stress hormone levels accelerate the onset of memory deficits in male Huntington’s disease mice. Neurobiol.Dis 69, 248–262. [DOI] [PubMed] [Google Scholar]
- Mo C, et al. , 2014b. Effects of chronic stress on the onset and progression of Huntington’s disease in transgenic mice. Neurobiol.Dis 71, 81–94. [DOI] [PubMed] [Google Scholar]
- Mo C, et al. , 2014c. Ethological endophenotypes are altered by elevated stress hormone levels in both Huntington’s disease and wildtype mice. Behav.Brain Res 274, 118–127. [DOI] [PubMed] [Google Scholar]
- Mo C, et al. , 2013. Short-term memory acquisition in female Huntington’s disease mice is vulnerable to acute stress. Behav.Brain Res 253, 318–322. [DOI] [PubMed] [Google Scholar]
- Moffitt H, et al. , 2009. Formation of polyglutamine inclusions in a wide range of non-CNS tissues in the HdhQ150 knock-in mouse model of Huntington’s disease. PLoS One. 4, e8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan FH, Laufgraben MJ, 2013. Mifepristone for management of Cushing’s syndrome. Pharmacotherapy. 33, 319–29. [DOI] [PubMed] [Google Scholar]
- Moutsatsou P, et al. , 2012. Glucocorticoid receptor signaling in bone cells. Trends Mol Med. 18, 348–59. [DOI] [PubMed] [Google Scholar]
- Ona VO, et al. , 1999. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature. 399, 263–7. [DOI] [PubMed] [Google Scholar]
- Papalexi E, et al. , 2005. Reduction of GnRH and infertility in the R6/2 mouse model of Huntington’s disease. Eur.J.Neurosci 22, 1541–1546. [DOI] [PubMed] [Google Scholar]
- Petersen A, Bjorkqvist M, 2006. Hypothalamic-endocrine aspects in Huntington’s disease. Eur J Neurosci. 24, 961–7. [DOI] [PubMed] [Google Scholar]
- Pratley RE, et al. , 2000. Higher sedentary energy expenditure in patients with Huntington’s disease. Ann Neurol. 47, 64–70. [PubMed] [Google Scholar]
- Ross CA, et al. , 2014. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 10, 204–16. [DOI] [PubMed] [Google Scholar]
- Ross CA, Tabrizi SJ, 2011. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 10, 83–98. [DOI] [PubMed] [Google Scholar]
- Rub U, et al. , 2015. The Neuropathology of Huntington s disease: classical findings, recent developments and correlation to functional neuroanatomy. Adv Anat Embryol Cell Biol. 217, 1–146. [PubMed] [Google Scholar]
- Saleh N, et al. , 2009. Neuroendocrine disturbances in Huntington’s disease. PLoS One. 4, e4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, et al. , 1985. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 5, 1222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, et al. , 1990. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 10, 2897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp E, et al. , 2001. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J Neuropathol.Exp.Neurol 60, 161–172. [DOI] [PubMed] [Google Scholar]
- Sawa A, et al. , 2003. Mechanisms of neuronal cell death in Huntington’s disease. Cytogenet Genome Res. 100, 287–95. [DOI] [PubMed] [Google Scholar]
- She P, et al. , 2011. Molecular characterization of skeletal muscle atrophy in the R6/2 mouse model of Huntington’s disease. Am.J.Physiol Endocrinol.Metab 301, E49–E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirbin CA, et al. , 2013a. The relationship between cortisol and verbal memory in the early stages of Huntington’s disease. J Neurol. 260, 891–902. [DOI] [PubMed] [Google Scholar]
- Shirbin CA, et al. , 2013b. Cortisol and depression in pre-diagnosed and early stage Huntington’s disease. Psychoneuroendocrinology. 38, 2439–2447. [DOI] [PubMed] [Google Scholar]
- Stack EC, et al. , 2005. Chronology of behavioral symptoms and neuropathological sequela in R6/2 Huntington’s disease transgenic mice. J Comp Neurol. 490, 354–70. [DOI] [PubMed] [Google Scholar]
- Toth M, Grossman A, 2013. Glucocorticoid-induced osteoporosis: lessons from Cushing’s syndrome. Clin.Endocrinol.(Oxf) 79, 1–11. [DOI] [PubMed] [Google Scholar]
- Trejo A, et al. , 2004. Assessment of the nutrition status of patients with Huntington’s disease. Nutrition. 20, 192–196. [DOI] [PubMed] [Google Scholar]
- Tunez I, et al. , 2006. 17 beta-Estradiol may affect vulnerability of striatum in a 3-nitropropionic acid-induced experimental model of Huntington’s disease in ovariectomized rats. Neurochem Int. 48, 367–73. [DOI] [PubMed] [Google Scholar]
- Turmaine M, et al. , 2000. Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 97, 8093–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burg JM, et al. , 2008. Increased metabolism in the R6/2 mouse model of Huntington’s disease. Neurobiol.Dis. 29, 41–51. [DOI] [PubMed] [Google Scholar]; van der Burg JM, et al. , 2009. Beyond the brain: widespread pathology in Huntington’s disease. Lancet Neurol. 8, 765–774. [DOI] [PubMed] [Google Scholar]; van der Burg JM, et al. , 2011. Gastrointestinal dysfunction contributes to weight loss in Huntington’s disease mice. Neurobiol.Dis [DOI] [PubMed] [Google Scholar]
- van Duijn E, et al. , 2010. Hypothalamic-pituitary-adrenal axis functioning in Huntington’s disease mutation carriers compared with mutation-negative first-degree controls. Brain Res.Bull 83, 232–237. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, et al. , 2007. Testicular degeneration in Huntington disease. Neurobiol.Dis 26, 512520. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, et al. , 1985. Neuropathological classification of Huntington’s disease. J Neuropathol.Exp.Neurol 44, 559–577. [DOI] [PubMed] [Google Scholar]
- Wong EY, Herbert J, 2004. The corticoid environment: a determining factor for neural progenitors’ survival in the adult hippocampus. Eur J Neurosci. 20, 2491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood NI, et al. , 2010. Responses to environmental enrichment differ with sex and genotype in a transgenic mouse model of Huntington’s disease. PLoS One. 5, e9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood NI, et al. , 2008. Increased thirst and drinking in Huntington’s disease and the R6/2 mouse. Brain Res.Bull 76, 70–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.