Abstract
Motor development and psychological development are fundamentally related, but researchers typically consider them separately. In this review, we present four key features of infant motor development and show that motor skill acquisition both requires and reflects basic psychological functions. (a) Motor development is embodied: Opportunities for action depend on the current status of the body. (b) Motor development is embedded: Variations in the environment create and constrain possibilities for action. (c) Motor development is enculturated: Social and cultural influences shape motor behaviors. (d) Motor development is enabling: New motor skills create new opportunities for exploration and learning that instigate cascades of development across diverse psychological domains. For each of these key features, we show that changes in infants’ bodies, environments, and experiences entail behavioral flexibility and are thus essential to psychology. Moreover, we suggest that motor development is an ideal model system for the study of psychological development.
Keywords: infant, locomotion, reaching, exploration, developmental cascade, culture
MOTOR DEVELOPMENT: WHAT DOES PSYCHOLOGY HAVE TO DO WITH IT?
Infant motor development includes some of the most beautiful, emblematic, surprising, and richly documented phenomena in developmental psychology. It also boasts one of the longest histories in developmental research. For more than a century, reviews of motor development have been included in a variety of prestigious venues (e.g., Adolph & Berger 2006, 2015; Adolph & Robinson 2015; Bertenthal & Clifton 1998; Gesell 1929, 1946; Smitsman & Corbetta 2010; Thelen 1995, 2000; Trettien 1900; von Hofsten 2004, 2007).
However, a review exclusively focused on motor development has never appeared in the Annual Review of Psychology. Why not? After all, the Annual Review of Psychology includes dozens of articles about other aspects of infant development—theory of mind, statistical learning, neural correlates of cognition, and so on. Most readers likely never noticed the omission. Indeed, many psychological researchers assume that motor behavior is not inherently psychological. As Rosenbaum (2005) put it, motor behavior is the “Cinderella of psychology”—important, but largely ignored. Although a few Annual Review of Psychology authors have discussed infant motor skills, they did so in the context of other topics—exploration (Gibson 1988), problem solving (Keen 2011), consciousness (Kopp 2011), and representation (Bertenthal 1996). However, motor development need not be dressed up by association with traditionally psychological topics. Motor development is fundamentally psychological in its own right.
A primary aim of this review is to dispel any notion that motor development is superfluous to psychology. Psychology is the study of mind and behavior, and all behavior is motor behavior. Thus, the study of motor development is really the study of behavioral development. As such, motor behavior both requires and reveals the workings of the mind (Thelen 2000). Infant motor development includes the acquisition of basic skills such as moving the head and eyes to look around, moving the arms and hands to grasp objects, and moving the body to sit up or go somewhere. It includes higher-order skills such as wielding a hammer to pound a peg and stacking boxes to reach an overhead lure (Kahrs et al. 2014, McGraw 1935). It includes skills that support human interaction such as moving the face to express emotions, the arms to point and gesture, and the mouth to eat and talk (Adolph & Berger 2015). And it includes spontaneous waking movements such as leg kicks and arm flails (Piek & Carman 1994) and the inadvertent twitches and sighs expressed during sleep (Blumberg & Dooley 2017).
In this review, we do not attempt to cover 100 years of research on infant motor development. Instead, we aim to show that motor skill acquisition is essential to psychology. Learning to move requires basic psychological functions, reflects fundamental cultural values, and facilitates learning and development across a wide range of psychological domains. As stated in our subtitle, movements are embodied, embedded, enculturated, and enabling (Figure 1). In less alliterative terms, movements are constrained by the current status of the body, situated in an environment that offers myriad possibilities for action, and shaped by social influences and culturally specific child-rearing practices. Moreover, new motor skills create new opportunities for learning and can instigate cascades of developments far afield from the original accomplishment.
Figure 1.
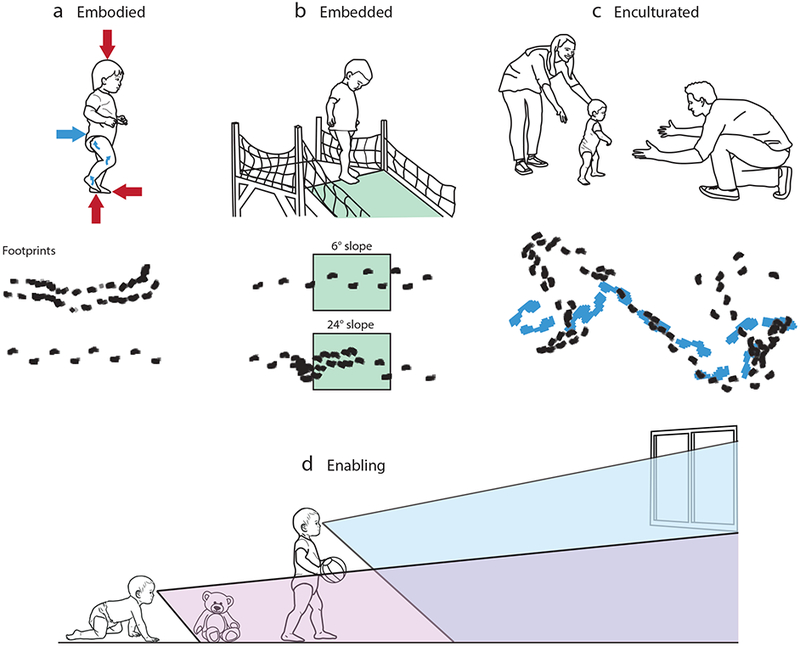
Motor development is embodied, embedded, enculturated, and enabling. (a) Embodied movement. Infant walker with red arrows denoting forces acting on the body (gravity, friction forces, ground reaction force) and blue arrows representing forces generated by the body (propulsive force and torques). Footprints show characteristic gait patterns of (top) a novice infant walker with short, irregularly spaced steps and the feet wide apart and (bottom) an experienced infant walker with longer, narrower, more regularly spaced steps (data taken from Lee et al. 2018). (b) Embedded movement. An infant is shown deciding whether and how to descend a steep slope. Footprints show characteristic gait modifications in an experienced infant walker while approaching and descending a shallow 6° slope and a steep 24° slope (data taken from Gill et al. 2009). Green boxes denote the middle sloping section of the walkway. (c) Enculturated movement. An infant is shown taking first steps from the arms of one caregiver into the open arms of another. The illustration is based on Van Gogh’s 1890 painting, “First Steps.” Footprints show the characteristic walking patterns of an infant (black) and caregiver (blue) during free play (data taken from O. Ossmy, J. Hoch, S. Hasan, W.G. Cole, and K. Adolph, unpublished data). (d) Movement enables new developments. The schematic drawing shows the average field of view for crawling and walking infants (data taken from Kretch et al. 2014).
Motor behavior must be adaptive and functional. Thus, a second and related aim of this review is to highlight the importance of behavioral flexibility—the ability to select and modify actions to suit changes in the body, environment, and task (Adolph & Robinson 2015, Bernstein 1996). Behavioral flexibility intimately links motor development with central issues in psychology because adaptive action involves planning, exploration, learning, and problem solving. Adaptive action is also generative and creative. Flexibility allows infants to achieve the same functional outcome using a variety of actions, for example, by pushing a button with the fingers, palm, forearm, or even the head (Meltzoff 1988). Thus, flexibility can entail transfer of existing means to a new situation or the invention of a new solution on the fly.
In the following sections, we provide examples to illustrate the embodied, embedded, enculturated, and enabling nature of infant motor development. In each case, we show that changes in infants’ bodies, environments, and experiences require and promote behavioral flexibility and are thus essential to psychology. Although we partition our examples into four sections, we do not intend to imply that any infant behavior reflects only one aspect of motor development. Rather, with the recognition that behavior involves all four aspects, the organization of our review reflects the typical historical and disciplinary divisions among researchers.
MOTOR DEVELOPMENT IS EMBODIED
The concept of embodiment has been embraced by many cognitive, perceptual, and social psychologists (Shapiro 2011). But researchers in motor behavior have always recognized movement as embodied. How could it be otherwise? Movements fundamentally depend on generating, controlling, and exploiting physical forces, and these forces in turn depend on the body (see Figure 1a). Developmental and temporary changes in infants’ bodies change the biomechanical constraints on action, so flexibility is imperative. Although the body constrains actions, it does not dictate them (Bernstein 1996, Bertenthal & Clifton 1998). There is no one-to-one correspondence between muscle activations and movement outcome. The same functional outcome can result from a variety of muscle activation patterns (e.g., the elbow can bend by contracting the biceps while relaxing the triceps or by cocontracting both pairs of muscles simultaneously), and the same muscle activation patterns can result in different movement outcomes (e.g., while moving an arm from a static position or while the arm is already in motion).
Walking is a prime example of an embodied, flexible skill that emerges in infancy. Over weeks and months of walking experience, infants become better at managing physical forces—generating disequilibrium to initiate a bout of walking, controlling dynamic equilibrium from step to step, and recapturing stationary equilibrium to end the bout (Adolph & Berger 2006, Adolph & Robinson 2015). The two footprint sequences in Figure 1a illustrate characteristic improvements in infant walking skill in a standard gait assessment where infants take continuous, forward steps along a straight, uniform path. When infants first begin walking, the side-to-side distance between steps often exceeds the distance from front to back. Gait is slow, halting, and inconsistent. Infants spend excessive amounts of time with two feet on the floor because they have trouble keeping balance with one foot in the air. With walking experience, infants’ steps become faster, longer, closer together from side to side, and less variable (Chang et al. 2006, Lee et al. 2018). These developments can be seen with the naked eye (compare the footprints of the novice and experienced walkers). Less obvious are the remarkable improvements in joint angles and intralimb coordination, increased efficiency of muscle activations, control over balance and propulsive forces, and acquisition of pendular mechanisms (Bisi & Stagni 2015; Bril et al. 2015; Chang et al. 2006; Hallemans et al. 2006; Ivanenko et al. 2004, 2005).
Changing Bodies, Changing Constraints, Changing Solutions
Infants acquire new motor skills in a body that is continually changing. Developmental changes due to growth, redistribution of muscle and fat, changes in muscle strength and elasticity, and so on alter the constellation of forces at work (Van Dam et al. 2011). Likewise, temporary changes to the body (due to clothing, footwear, backpacks, objects in hand, etc.) alter the relevant forces (Garciaguirre et al. 2007, Rochat et al. 1999). The same is true for moment-to-moment changes in head, limb, and body position (Bertenthal & Clifton 1998, Thelen 1995). The constraints of the moment can be detected through perceptual feedback from just-prior movements. Thus, learning to move entails learning to adapt behavior to the current status of the body at every moment.
Motor behavior begins during fetal development. Even before birth, infants must use perceptual information to adapt movements to their changing bodies. Over the course of several months, an infant transforms from a pea-sized embryo to a full-sized fetus—all the while moving every moveable body part. At 7 weeks gestation, fetuses bring hand to face by raising their arm at the shoulder because their arm buds are so short (Moore & Persaud 1993). Several weeks later, when their arms are much longer, fetuses must lower their shoulder and bend their elbow to produce the same hand-to-face action.
From fetus to adolescent, the body undergoes dramatic change. The mathematically smoothed curves in traditional growth charts give the impression that growth is continuous. But daily measurements show that growth is episodic (Lampl & Thompson 2007). Children grow in short (less than 24-h) bursts separated by long periods (days to weeks) of no growth. Variations in the frequency and amplitude of growth spurts lead to individual differences in trajectories and outcomes (Lampl & Thompson 2007). In a single day, infants’ height can increase by nearly 2 cm (Lampl 1993). Moreover, standing height temporarily decreases across the day because gravity compresses the trunk and legs while the child is upright. Children shrink by nearly a centimeter in height between the time they wake up and the time they go to sleep (Lampl 1992).
The particular characteristics of each infant’s changing body determine which movements are possible and how they are performed. For example, Johnny Eck, the famous sideshow actor born without legs, learned to “walk” upright by using his hands as “feet” beneath his torso (Blumberg & Dooley 2017). Infants with achondroplastic dwarfism crawl on hands and feet instead of hands and knees because it keeps their trunk level across their short limbs (Fowler et al. 1997). To accommodate their short arms and large heads, many “snowplow” by pushing against the floor with their feet and sliding on their head. However extreme, all bodies differ, and all infants must solve the problem of moving in the particular body that they have (Adolph & Robinson 2015, Blumberg & Dooley 2017).
Although motor development is traditionally portrayed as a series of universal milestones (Gesell 1929, 1946; McGraw 1935), infants’ initial solutions are idiosyncratic and reflect the changing facts of embodiment. More active infants achieve their first reaches by dampening the ongoing forces of spontaneous arm flaps; less active infants must power up their stationary arms (Thelen et al. 1993). Infants with weak arms and poor balance first achieve independent mobility with a variant of belly crawling, dragging or bouncing their abdomen along the floor. Stronger, more coordinated infants’ first crawling steps are in the characteristic hands-and-knees posture. Still others logroll, bum-shuffle, crawl-sit, or hitch with one leg (Adolph et al. 1998, Patrick et al. 2012). Likewise, infants’ first steps while learning to walk reflect individual, idiosyncratic solutions: “Steppers” take small, conservative steps to minimize disruptions to upright posture; “fallers” lurch forward while their feet hurry to catch up; “twisters” swing each leg around like a compass by twisting their trunk to generate angular momentum (Bisi & Stagni 2015, Snapp-Childs & Corbetta 2009). Within a few months, infants learn to exploit the pendular motions of their legs (Bisi & Stagni 2015, Ivanenko et al. 2004).
Coping with Change
Temporary changes to the body also have consequences for behavior. For example, merely wearing a diaper causes infants to adopt less mature gait patterns (shorter steps with legs splayed farther apart) compared to walking naked (Cole et al. 2012). Indeed, the cost of wearing a cloth diaper is equivalent to losing 2 months of walking experience compared to going naked; the cost of wearing a thin disposable diaper is 5 weeks. Similarly, toddlers take slower, shorter steps while walking in a diaper and trousers compared to walking in only a diaper (Theveniau et al. 2014).
New walkers respond to a sudden reduction in body weight (experimenters hold infants around the torso to reduce vertical force) by lifting their feet higher and overshooting foot placement as their leg swings forward (Dominici et al. 2007). In contrast, older children and adults maintain their normal foot trajectories even when body weight is partially supported. Adding weight to infants’ bodies also alters walking patterns. Experienced walkers can carry heavy loads—up to 25% of their body weight (Adolph & Avolio 2000). However, loads impair walking. With only 15% of their body weight strapped to their shoulders, waist, or ankles, infants take slower, smaller steps (Vereijken et al. 2009). With asymmetrical loads (all of the weight strapped to the front, back, or side of their torso), infants shorten and widen their steps and spend more time with both feet on the ground and less time with one foot in the air (Garciaguirre et al. 2007). Although asymmetrical loads pull the body off balance, infants never modify their posture to offset the load. Instead, infants always lean into the load and struggle onward as best they can, whereas older children and adults lean away from the direction of a load (e.g., leaning forward to offset a heavy backpack).
In some cases, infants flexibly compensate for body changes—they modify their actions to correct a problem. For example, slightly aroused neonates kick their legs in alternation while lying on their backs. With a weight on one leg, supine 6-week-olds decrease kicking in the weighted leg and increase kicking in the unweighted leg, thereby maintaining their overall unweighted kick rate (Thelen et al. 1987). With their legs tethered by an elastic band, supine 3-month-olds switch from alternating to simultaneous kicks (Thelen 1994). The band does not prohibit alternation, but it makes alternation more effortful. With the band in place, the typically more effortful simultaneous kicks become more attractive. In each case (weights and band), infants can choose to move their legs or not, but when they do move, their leg movements reflect the pattern that requires the least energy or that delivers the largest payoff given their current state of embodiment.
Similarly, infants compensate for uneven leg lengths induced by a platform shoe on one foot (Cole et al. 2014). At first, the difference between legs causes babies to limp, with the longer leg taking longer steps. After a few practice trials, infants take shorter steps with their longer leg and longer steps with their normal leg to reestablish the symmetry of their walking patterns. Adults, in contrast, do not compensate and continue to limp, even after 15 min of additional practice. Apparently, compensation requires the wherewithal to respond to a disruptive change in the body (perhaps impossible for precariously balanced infants strapped with a heavy, asymmetrical load) and an appreciation for the cost of failing to compensate (in the case of an elongated leg, adults can willingly limp, but limping causes infants to fall).
The most impressive evidence for behavioral flexibility is that infants take their altered bodies into account while planning future actions. With small weights attached to their wrists, sitting infants reduce the frequency of reaching to far-off targets but not to nearby ones, indicating that they perceive the increased risk of losing balance while leaning forward with a heavy hand (Rochat et al. 1999). With lead-weighted shoulder packs or Teflon-soled shoes, infants’ ability to walk down slopes is reduced compared to their abilities while wearing featherweight packs or rubber-soled shoes (Adolph & Avolio 2000, Adolph et al. 2010c). Infants correctly perceive the decreased stability of their altered bodies and restrict attempts to walk to shallower slopes while wearing the heavy packs or the slippery shoes.
MOTOR DEVELOPMENT IS EMBEDDED
Movement always occurs in a physical environment. For earth-bound infants, the constraints of gravity are constant. In contrast, the objects that fill the environment, ground surfaces that cover the environment, and larger layouts that structure the environment are continually in flux. Thus, motor behavior is situated—embedded—in the changing realities of the world. Variations in the environment make flexibility imperative. Infants must perceive what is out there and what to do about it. Should they visit or avoid, explore or ignore the shimmering pool, the flight of stairs, the bin of toys, and so on? Perception and cognition are required to plan and guide movements adaptively so as to achieve the goals of everyday life. As illustrated in Figure 1b, for an infant walker, a steep slope can present an impassable obstacle, a challenge for gait modifications, or an opportunity to use alternative descent methods such as scooting in a sitting position, backing feet first, or sliding head first with arms outstretched like Superman (Adolph 1997); note that an experimenter (not shown in the figure) follows alongside infants to ensure their safety.
Moreover, the functional environment develops because new motor skills make new aspects of the environment available (Gibson 1988). Objects take on new meaning after infants can grasp and manipulate them (Needham 2016). The landscape of surfaces (grass, sand, mattress, linoleum, carpet, etc.) and elevations (slopes, stairs, coffee table, crib rail, etc.) offers new possibilities for action after infants acquire independent mobility (Adolph et al. 2012). The environment that was available yesterday may offer new opportunities today. The continual flux of a variable and developing environment prevents infants from learning fixed movement patterns and promotes behavioral flexibility (Adolph & Robinson 2015).
Learning to Perceive Affordances
Acquiring behavioral flexibility is in fact a perceptual undertaking. In an ever-changing world, infants must learn to perceive which actions are possible and which are not (Gibson 1982, 1988). The term affordance describes the fit between the body and the environment that makes a particular action possible (Franchak & Adolph 2014). The same sized drop-off, for example, is functionally a step for an infant with long legs and good balance control but is a cliff for an infant with short legs or poor balance (Kretch & Adolph 2013a). The same degree of slope affords walking or slipping depending on the friction between feet and surface (Adolph et al. 2010a,c). Infants’ manual dexterity determines whether a spoon can function as an object to bang, a utensil to bring food to mouth, or a tool that can be grasped by the bowl to poke the handle into a narrow opening (Barrett et al. 2007, Connolly & Dalgleish 1989). Most affordances (e.g., a tool for poking) go unnoticed until the need arises. However, affordances exist regardless of whether they are perceived or used (Gibson 1982). Actions are possible or not, depending on the body–environment fit.
The ability to distinguish possible from impossible actions is largely absent at skill onset. For example, new sitters foolishly try to lean forward to retrieve objects beyond their reach or fail to lean forward for objects within their reach (Yonas & Hartman 1993). Only after weeks of sitting experience can infants precisely distinguish between distances that are within and those that are beyond a lean and reach (Adolph 2000). Experienced infants immediately adjust their sitting posture to cope with changes in the slant of the support surface (Rachwani et al. 2017). They lean backward to keep balance on forward slopes and lean forward to keep balance on backward slopes, and postural adjustments are closely aligned to each 2° change in slant.
In their first weeks of crawling and walking, infants plunge straight over the brink of impossibly high drop-offs, steep slopes, and wide gaps (Adolph 1997, 2000; Adolph et al. 2008; Karasik et al. 2016; Kretch & Adolph 2013a). Over weeks of crawling and walking, their judgments become increasingly accurate. After several months of experience, infants perceive affordances within 1 cm of their abilities on drop-offs and 2° of their abilities on slopes (Adolph 1997, Adolph et al. 2008, Karasik et al. 2016, Kretch & Adolph 2013a, Tamis-LeMonda et al. 2008). Similarly, experienced crawling infants refuse to venture over a water cliff—a deep water-filled pool (Burnay & Cordovil 2016); experienced crawlers also avoid an apparent drop-off on a visual cliff—a large drop-off covered with safety glass to give the visual illusion of a cliff (Dahl et al. 2013, Witherington et al. 2005). Experienced walking infants refuse to walk over impossibly narrow bridges, narrow ledges, and high barriers (Franchak & Adolph 2012; Kingsnorth & Schmuckler 2000; Kretch & Adolph 2013b, 2017; Schmuckler 1996).
The footprints in Figure 1b illustrate exquisite attunement to affordances in an experienced infant walker faced with shallow and steep slopes (Gill et al. 2009). The infant correctly perceived that the 6° slope was barely an impediment, evidenced by long, narrow, evenly spaced steps during approach and descent. The infant also correctly perceived that the 24° slope required gait modifications, shown by the decreasing step length during approach; cluster of footsteps at the brink; and small, overlapping, irregularly spaced steps during descent. Indeed, experienced walkers consistently modify their gait while approaching and navigating obstacles such as slopes, drop-offs, ledges, and bridges (Adolph 1997; Franchak & Adolph 2012; Gill et al. 2009; Kretch & Adolph 2013a, 2013b, 2017; McGraw 1935). When descending steep slopes, infants decrease step length and velocity to brake forward momentum, sometimes taking such tiny, slow steps that they barely lift their feet. When navigating large drop-offs, infants carefully lower themselves downward while balancing on one leg. When crossing narrow ledges and bridges, infants turn sideways and take tiny leading–following steps. For the most skilled infants, such gait modifications allow them to traverse 40° slopes, 28-cm drop-offs, 6-cm ledges, and 12-cm bridges (Franchak & Adolph 2012; Karasik et al. 2016; Kretch & Adolph 2013a, 2013b; Tamis-LeMonda et al. 2008). Moreover, in all cases, infants adjust their gait prospectively, before stepping over the brink, and gait modifications are precisely matched to incremental changes in the slant or size of the obstacle.
Improvements in affordance perception do not depend on experience with particular tasks or obstacles in the laboratory. For example, infants in control groups, matched for days of crawling or walking experience, respond similarly to infants tested repeatedly on slopes in the lab (Adolph 1997, Gill et al. 2009). These findings indicate that everyday experiences coping with the varied ground surfaces and elevations in the natural environment support learning to perceive affordances.
Perhaps most striking, learning does not transfer from an earlier-developing skill to a later-developing one. Experienced crawlers who detect affordances for crawling with centimeter precision attempt to walk over impossibly steep slopes and high drop-offs when they first begin walking (Adolph 1997, Adolph et al. 2008, Karasik et al. 2016). Infants who precisely perceive affordances for spanning gaps in an experienced sitting posture attempt to cross impossibly large gaps when tested moments later in a novice crawling posture (Adolph 2000). The same experienced cruisers who accurately gauge affordances for cruising across gaps in a handrail cruise blithely into gaps in the floor beneath their feet; new walkers do not perceive affordances for traversing a gap in a handrail or the floor (Adolph et al. 2011). Indeed, infants display distinct learning curves for sitting, crawling, cruising, and walking and do not show any evidence that they learn faster the next time around.
Why does learning not transfer across action systems? Why should it? The body–environment relations that support sitting, crawling, cruising, and walking are completely different. So are the exploratory movements required to perceive these relations. Thus, experience with each developing action system teaches infants to perceive novel affordances for that particular action system. Put a different way, infants do not really learn to move. Instead, to borrow Harlow’s (1949) notion, they are learning to learn to move. Infants learn to perceive affordances at each moment, with their current body and skills in the current environment and for the current task.
Exploration for Action and Action for Exploration
Exploration and action are reciprocal functions. Perceptual information specifies the current constraints on action and the current opportunities for action. Exploratory movements, in turn, generate the requisite information for perceptual systems (Gibson 1988).
Exploration in the service of action refers to the information-gathering activities required to guide actions adaptively in real time. Infants must generate, detect, and use perceptual information about current body–environment relations to determine which actions are possible and which are not. Peripheral vision is the least costly form of exploration. The information gathered is essentially free because the eyes are parked in front of the body. All other forms of exploration take effort. Infants can collect visual information from a distance, but haptic information arises only from direct contact. Alternative strategies are more costly still and emerge from testing various options. Given the varying cost of exploratory movements, infants do not produce every type of exploratory activity on every encounter with an object or obstacle. Instead, exploratory activity sequentially ramps up from less to more costly forms of exploration as more information is needed. Information generated by earlier forms of exploration instigates the exploration that follows (Kretch & Adolph 2017).
Obstacle navigation provides a useful illustration. The ramping-up process begins when infants detect depth cues from a distance. While approaching a bridge, head-mounted eye tracking shows that experienced walking infants give the obstacle a brief glance (Kretch & Adolph 2017). On wide bridges, infants walk straight across. However, on narrow bridges, a quick look causes infants to slow down and modify their gait. Infants may engage in more costly haptic exploration by probing the bridge with a foot and taking tiny test steps at the edge (similar to the cluster of footprints at the brink of the steep slope in Figure 1b). If touching suggests that the bridge is passable, then infants go. If walking feels perilous, then infants use an alternative strategy (e.g., crawling) or avoid going. When an alternative is not readily available, infants test various options by shifting from one position to another. In some cases, multiple shifts lead to the discovery of a new strategy, such as backing feet first into a precipice (Adolph 1997). In other cases, testing the options convinces infants to stay put. They may move partway onto the obstacle and then change their mind and return to the starting platform.
If infants fail to generate the appropriate exploratory behaviors, or fail to use the resulting perceptual information, then actions are not adaptive. Sometimes, infants fail to explore because the affordance-relevant forces cannot be detected from a distance (Adolph & Robinson 2015). Notably, information about friction and rigidity requires exploration via direct contact. Haptic exploration of object friction or rigidity involves some cost but little risk. However, during locomotion, haptic exploration of surface friction or rigidity is both costly and risky. To mitigate the risk, walkers must modify their gait prior to touching the obstacle. However, visual information about a shiny Teflon surface, undulating waterbed, or lumpy foam pit is not sufficient to elicit gait modifications during approach. Shine, for example, is not a reliable visual cue for a slippery surface (Joh et al. 2006). Thus, infants (like older children and adults) step straight onto a slippery patch of ground, squishy waterbed, or soft foam pit—and fall (Adolph et al. 2010a, Gibson et al. 1987, Joh & Adolph 2006). Similarly, infants misadjust their grip if they fail to perceive that an object is slippery or deformable and miscalculate the lift force needed for a surprisingly heavy or lightweight object (Adolph & Robinson 2015).
Action in the service of exploration refers to behaviors that generate information about the self and the world. Action is itself a perceptual system. We know the world by moving in it (Thelen 1995, 2000). Infants do not simply see—they look by turning their eyes, their head, and often their whole body (Gibson 1988). They generate information about unseen properties such as friction, rigidity, weight, and substance by fingering, shaking, banging, and rotating (Bushnell & Boudreau 1998, Fontenelle et al. 2007). They generate information about the surface layout and spatial surroundings via changes in posture and locomotion.
Although infants can and do engage in deliberate, goal-directed exploration, opportunities for learning are largely incidental in everyday life. Of course, infants in laboratory experiments deliberately explore objects to learn about surprising or ambiguous properties (Sim & Xu 2017, Stahl & Feigenson 2015) and readily navigate obstacles to get to a toy or caregiver on the other side. However, during natural activity, infants spontaneously produce immense amounts of manual and locomotor exploration with no clear objective. During each hour of free play, walking infants interact with objects 50% of the time and average more than 40 bouts of object carrying (Karasik et al. 2011, 2012; Logan et al. 2015). However, most object engagements involve touching and carrying with no apparent goal (Karasik et al. 2011, 2012). Similarly, during an hour of free play, infants average 2,400 walking steps, travel the distance of eight American football fields, and visit most of the available area and ground surfaces (Adolph et al. 2012). They produce a large proportion of short bouts (30–50% involve only 1–3 steps), and a few very long bouts (30–155 steps). Most paths are curved and involve steps in every direction (Cole et al. 2016, Lee et al. 2018). However, infants rarely move toward a person, place, or thing. Most trips do not end at any recognizable destination (Cole et al. 2016). Instead, infants mostly take steps in place or stop walking in the middle of the floor.
MOTOR DEVELOPMENT IS ENCULTURATED
Developmental psychology is largely a science of lonely children. In most studies of cognitive, perceptual, and motor development, researchers observe children in isolation. Although caregivers are typically present, they sit quietly while their children complete a task. Experimenters are also typically present, but their social interactions adhere to a predetermined protocol, or they do not interact at all.
Researchers in motor development are particularly guilty of ignoring social and cultural influences on skill acquisition. Most papers focus on embodied movement and are replete with images such as that in Figure 1a: a solitary infant coping with physical forces (e.g., while walking, standing, sitting, or reaching). A smaller body of work focuses on embedded action and includes images such as that in Figure 1b: a lone infant deciding whether to cross an adjustable obstacle (or how to grasp a rod at various orientations, etc.). In both types of work, researchers routinely use social information to persuade infants to reach, crawl, and walk (the experimenter smiles and cheers infants’ efforts; caregivers wait at the far side of the obstacle), but social influences are treated as a constant, like furniture or lighting.
However, outside the laboratory, motor development is not a lonely enterprise, and social influences are not a constant. Infants grow up in both a physical and a social environment. Indeed, motor development—like all development—is shaped by social and cultural factors. Motor development is enculturated. Social interactions are an important impetus for movement: Infants’ first steps are likely into their caregiver’s open arms, as illustrated in Figure 1c; infants’ actions with objects are typically enmeshed in interactions with caregivers. Motor behaviors must be flexibly geared to the people as well as the places and things in the environment, and social information from caregivers is important for adaptively guiding actions. Caregivers also support and constrain motor behavior by structuring the physical environment in which motor skills develop. Cultural norms, in turn, influence the social interactions, child-rearing practices, and home environments that provide the backdrop for motor skill acquisition.
Social Influences
From the start, caregivers encourage and discourage their infants’ actions. In the first few months after birth, infants can control an abundance of voluntary movements in their eyes, face, and head (Adolph & Berger 2015). Infants exploit and develop these motor skills during face-to-face interactions with caregivers. They move eyes and head to look at or away from their caregiver (Beebe et al. 2016). They move cheeks and mouth to produce smiles and pouts, lip smacks and puckers, mouth openings and tongue protrusions, and eye widening and brow furrows (Oster 2005). They move mouth and throat to produce coos, babbles, and cries (Wilson et al. 2008). Infants and caregivers are acutely sensitive to each other’s facial gestures and vocalizations and use this social information to update their own actions in real time (Beebe et al. 2016). In response to their caregiver’s greeting, infants vocalize, smile, and direct their gaze toward the caregiver’s face (Tronick et al. 1978). When the time-locked contingencies are disrupted, for example, if their caregiver’s responses are delayed or caregivers are unresponsive, infants stop vocalizing and smiling, and they avert their gaze and turn their heads away (Tronick et al. 1978).
Opportunities to see and be seen depend on real-time and developmental changes in infants’ posture (Franchak et al. 2018). Most laboratory studies of dyadic interaction limit caregiver and infant mobility by seating the partners face to face. This seating assignment facilitates contingent interactions. However, during everyday activity, head cameras reveal that, at 1 to 2 months of age, caregivers’ faces are only in view for 15 min out of every hour (Jayaraman et al. 2015). Face time decreases to 5 min per hour by 11 months of age. Presumably, the decrease results from changes in where caregivers place premobile infants and in infants’ developing motor skills (Frank et al. 2013). After infants become mobile and caregivers let them roam free, both partners’ real-time posture limits the availability of the other’s face. Head-mounted eye tracking shows that crawling infants cannot see their standing caregiver’s face, even if they strain to lift their heads as high as possible (Franchak et al. 2018, Kretch et al. 2014). Walking infants rarely lift their heads or turn their eyes to look at their caregiver’s face (only doing so 5% of the time) because they are too busy playing with toys and running around the room (Franchak et al. 2018).
As illustrated by the footprints in Figure 1c, in a laboratory playroom, infants and caregivers often play within arms’ reach. The infant’s walking bouts vary in length, with frequent starts and stops, curved paths, and omnidirectional steps. The caregiver’s path is synchronized in time and space with the infant’s path. However, as infants gain greater mobility, they travel farther from their caregivers (Rheingold & Eckerman 1970, Thurman & Corbetta 2017), and social information influences exploration of the larger environment. Caregivers are more likely to admonish and restrain infants’ movements after they become independently mobile (Green et al. 1980). Caregivers’ frightened facial expressions or vocalizations curtail infants’ proximity to and manual exploration of novel objects, whereas positive social messages lead to greater exploration (Feinman et al. 1992).
Infants also take caregivers’ social messages into account when navigating obstacles, but only when perceptual information for risk is uncertain. At the edge of an ambiguously risky drop-off (30 cm high) on the visual cliff, experienced crawlers cross if their caregivers pose positive facial and vocal expressions but avoid crossing if caregivers look fearful (Moller et al. 2014, Sorce et al. 1985, Vaish & Striano 2004). On a full-size (90 cm high) visual cliff, they ignore their caregivers’ entreaties to cross, and when there is no apparent drop-off, infants cross regardless of caregivers’ social messages (Dahl et al. 2013, Sorce et al. 1985). Similarly, when navigating real cliffs and slopes, experienced walkers defer to their caregiver’s advice only when successful navigation is uncertain; they ignore discouraging messages at safe increments and likewise ignore encouraging messages at risky increments (Karasik et al. 2016, Tamis-LeMonda et al. 2008). While wearing slippery, Teflon-soled shoes that make formerly safe slopes risky, experienced walkers update their perception of their abilities and defer to social information from caregivers at the new, much shallower region of uncertainty (Adolph et al. 2010c). In fact, walking is so treacherous in the slippery shoes that, even on 0–10° slopes, infants walk when caregivers say “go” and avoid when caregivers say “no.”
Caregivers also literally support infants’ interactions with the outside world. Before infants can sit or walk well on their own, they rely on caregivers to provide the missing postural control and strength. Infants gain control of their trunk, vertebra by vertebra, from head to hip (Saavedra et al. 2012). Caregivers are sensitive to their infant’s developing postural skills and spontaneously support their infant’s trunk at appropriate levels (Duncan et al. 2018). By providing just the right amount of support, caregivers facilitate infants’ reaching and manual actions (Rachwani et al. 2015). Caregivers also support infants’ first walking steps by holding their infant’s hands or trunk and walking them around the room. With adults holding their hands, new walkers improve postural stability, fall less often, and display more mature gait patterns (Ivanenko et al. 2005).
Cultural Influences
For decades, cross-cultural researchers from Western, industrialized countries have reported the remarkable ways that motor development in far-away places and seemingly exotic cultures differs from the standard account in Western journal articles, textbooks, and parenting guides (for reviews, see Adolph & Robinson 2015, Adolph et al. 2010b, Bril 2018, Super 1976). Nonetheless, most research on motor development (and motor behavior more broadly) ignores cultural influences and treats developmental processes and outcomes as if they were universal (Bril 2018). Indeed, the World Health Organization (WHO) published standards (imperatives all infants should meet) rather than norms (describing a given population) for the sequence and timing of infant postural and locomotor milestones (Martorell et al. 2006).
Many child-rearing practices are so pervasive and seemingly trivial that people take them for granted—for example, how caregivers position infants for sleep, dress and toilet them, hold and carry them, and bathe and exercise them. However, because infants’ movements are embodied and embedded, historical and cultural differences in child-rearing practices have a profound influence on which motor skills children acquire, the sequence and ages at which children acquire them, and the subsequent developmental outcomes (Adolph & Robinson 2015, Adolph et al. 2010b).
Historical changes in sleep position affect rolling, crawling, and other prone skills. In 1992, the American Academy of Pediatrics recommended that caregivers place infants on their backs to sleep (instead of on their bellies) to reduce the incidence of sudden infant death syndrome (Kattwinkel et al. 1992). This flip in child-rearing practices delays prone skills in back sleepers relative to belly sleepers (Davis et al. 1998). Presumably, back sleepers have fewer opportunities to use their arms to bear weight and lift their torso in the crib. To counteract delays, pediatricians now recommend that caregivers place infants on their stomachs while the infants are awake. The more daily tummy-time infants experience, the earlier the onset of prone skills (Dudek-Shriber & Zelazy 2007).
Similarly, historical changes in clothing affect the development of prone skills. In the late 1800s, when US infants wore long dresses, most log-rolled or hitched instead of crawling to avoid tangling their legs and feet in their garments (Trettien 1900). Climate-induced changes in clothing also affect motor development. In colder weather, caregivers dress infants in heavier clothes and more layers than they do in warmer weather. The extra bulk delays the onset of rolling and crawling relative to infants who wear less clothing (Benson 1993, Hayashi 1992).
Something as basic as toileting can have profound influences on motor development. Caregivers in Central Asia lay infants supine in a gahvora cradle and bind them from neck to toe; infants are toileted with an external catheter that drains waste through a hole in the bottom of the cradle (Save the Children 2011). In rural China, where water is scarce, caregivers toilet infants by lying them supine in bags of sand to absorb waste (Mei 1994). Although both methods keep infants clean and dry, the restrictive bindings and heavy sand constrain infants’ movements, causing delays in gross motor skills relative to WHO standards.
Moreover, cultural differences in handling and bathing can affect infant motor development. Although most Western caregivers believe that newborns are fragile and should be handled with care, some African and Caribbean cultures consider rough handling and deliberate exercise to be necessary for healthy motor development. As part of their daily bathing routine, caregivers hold young infants by an arm, an ankle, or even the head. They toss infants into the air and catch them. Starting from the newborn period, caregivers train sitting by propping infants upright and encouraging them to resist gravity. Caregivers also encourage newborn upright stepping movements to train walking. In these natural experiments, infants sit and walk at younger ages than non-exercised infants, and some skip crawling altogether (Hopkins & Westra 1988, 1990; Super 1976). Similarly, in true experiments with random assignment, a few weeks of daily practice with upright stepping results in early walking onset in Western infants (Zelazo et al. 1972). A few weeks of daily postural training leads to faster improvements in prone and sitting skills and earlier onset of crawling and walking (Lobo & Galloway 2012).
Beyond infancy, caregivers’ customs and expectations continue to shape motor development (Adolph & Robinson 2015, Adolph et al. 2010b, Bril 2018). For example, Tarahumara children in the Sierra Madre Mountains run long distances as part of everyday activity and sport. By the time they are adults, they can run the distance of several back-to-back marathons (Devine 1985). Similarly, in cultures that practice persistence hunting, adults chase game for hours or days until their prey drop from exhaustion (Devine 1985). Children in Nepal and girls in some African cultures begin practicing load carriage at young ages as part of work and play. As adults, Nepalese porters and African women carry tremendous loads (70–200% of their body weight) for great distances, over rough terrain, and up and down steep mountainsides (Bastien et al. 2016, Minetti et al. 2006). They can freeload up to 20% of their body weight without increased energy expenditure (Bastien et al. 2005, Heglund et al. 1995). Other cultural practices impair walking skill. Until the 1920s, Chinese women bound their daughters’ feet to make them marriageable and attractive to members of their culture. For 1,000 years, this cultural preference forced girls to learn to walk on tiny, foreshortened feet (Ebrey 1999).
MOTOR DEVELOPMENT IS ENABLING
In most developmental psychology textbooks, motor development is an early, isolated chapter, cordoned off from the rest of the topics. But motor development should not be quarantined. It is foundational for all the chapters that come after. In fact, developmental psychology has a long history of linking infant motor development to improvements in perceptual and cognitive abilities. Piaget (1952) most famously proposed that infant motor behavior is the launch point for cognitive development, and that sensorimotor exploration is integral to the developmental process. Similarly, Gibson (1988) and Thelen (1995, 2000) argued that developing motor skills instigate and guide infants’ learning about objects, surfaces, people, and events. Moreover, they suggested that motor development both promotes and demands improvements in behavioral flexibility because new motor skills provide new opportunities for action but also require new solutions.
Motor development is enabling—it engenders new opportunities for learning and doing that can instigate cascades of development in far-flung domains (Adolph & Robinson 2015, Campos et al. 2000). However, the availability of opportunities for learning does not guarantee that learning occurs. The causal links between infant motor skill acquisition and the development of perceptual, cognitive, and social abilities are not always obvious. Multiple pathways can lead to the same developmental outcome, and often, multiple factors cooperate to push development toward a particular outcome. Thus, in many cases, new motor skills are neither necessary nor sufficient for driving more distant developmental achievements.
Figure 1d illustrates a compelling example of the opportunities enabled by motor development. The transition from crawling to walking provides infants with increased visual access to people, places, and things, and thereby enables new opportunities for learning. Crawling infants mostly see the ground in front of their hands, but when infants sit or stand up, the whole room and its contents swoop into view (Kretch et al. 2014). The higher vantage point enjoyed by walkers expands their field of view and frees up their hands for carrying and exploring objects. Indeed, opportunities to visually explore the larger layout grow with each new achievement in posture and locomotion: The limited views glimpsed from caregivers’ arms expand to self-selected vistas after infants can sit up and pull to a stand (Franchak et al. 2018, Kretch & Adolph 2015). These panoramic scenes, in turn, become a hands-on, step-by-step appreciation of what is around the corner or in the next room after infants become independently mobile (Gibson 1988, Rheingold & Eckerman 1970). Infants sometimes capitalize on opportunities for learning and sometimes do not, but nevertheless, the opportunities are available.
New Opportunities for Exploration and Learning
The ability to reach and to grasp enables new opportunities for visual, manual, and oral exploration of objects. With the advent of prehension, infants can hold objects in front of their eyes, rotate objects to see the backsides, finger the edges and surfaces, and squeeze and mouth objects to feel their texture and rigidity (Bushnell & Boudreau 1998, Needham 2016, Soska & Adolph 2014). Training interventions can jumpstart object exploration by experimentally boosting infants’ prehension skills. Before 3-month-olds can retrieve objects on their own, they can retrieve objects with the help of sticky Velcro mittens that transform a messy swat into a functional grasp of Velcro-covered toys (Needham et al. 2002). Compared to infants in control groups who did not wear sticky mittens or whose training objects had sham Velcro tabs, infants who received a few minutes of sticky-mittens training every day for 2 weeks demonstrated more visual attention to objects and more deliberate swats (with the mittens off). Trained infants also spend more time manually exploring objects placed in their bare hands (Libertus & Needham 2010). Even more remarkable, sticky-mittens training at 3 months of age results in more bare-handed visual–manual object exploration 12 months later (Libertus et al. 2016).
The ability to sit independently enables new opportunities for object prehension and exploration. Consequently, manual skills become more advanced with sitting experience (Rachwani et al. 2015, Soska et al. 2010). Normally, the benefits of sitting await the development of postural control. However, presitters and tripod sitters (who prop themselves on their arms with their legs outstretched) can reach for objects if their posture is supported artificially (Rachwani et al. 2015). Moreover, support at the chest facilitates more skilled reaching and more mature patterns of muscle activation compared to support at the hips. Experimental support of sitting also allows presitters to engage in more coordinated visual–manual–oral object exploration compared to prone and supine postures (Soska & Adolph 2014). Sitting is most conducive to visual, manual, and oral exploration because the hands are free and in view. Infants look less at objects while lying supine because they must fight gravity to hold objects at eye level. Bimanual exploration is depressed while lying prone because the arms are occupied with propping up the chest and keeping balance.
The development of independent mobility enables infants to venture farther away from their caregivers and farther into the environment (Campos et al. 2000). Before infants can crawl or walk on their own, moving around the world is literally in the hands of someone else. After they acquire mobility, infants’ newfound autonomy facilitates interactions with people, places, and objects beyond arms’ reach. With each week of mobility, locomotor exploration increases: Infants take more steps, travel greater distances, cover more area, and engage in more interactions with objects and caregivers (Adolph et al. 2012, Clearfield 2011, Green et al. 1980, Thurman & Corbetta 2017).
However, walking has notable advantages over crawling. Compared with experienced crawlers, novice walkers go more, see more, do more, and play more (Adolph & Tamis-LeMonda 2014). Novice walkers take twice as many steps, travel three times the distance, and spend more time in motion than crawlers (Adolph et al. 2012). As illustrated in Figure 1d, walking infants’ new upright posture brings more things into view than crawling (Kretch et al. 2014). Both crawlers and walkers appear to recognize the disadvantages of a prone posture. Crawlers spend more time in sitting and upright positions (standing and cruising) than prone, and walkers rarely revert to prone postures (Franchak et al. 2018). Although crawlers can carry objects, walkers do so more frequently. Walking infants also interact with distant objects and caregivers more often, and direct more pointing and waving gestures to their caregivers, than crawlers (Clearfield 2011; Green et al. 1980; Karasik et al. 2011, 2012).
Developmental Cascades
The developmental story does not end with enhanced perceptual–motor skills or new opportunities for object and locomotor exploration. These achievements are only the beginning. Motor skill acquisition can instigate cascades of development that are so far-flung from motor behavior and so far removed in time that, on the surface, they hardly seem connected at all. For example, who would have suspected that sitting skill and object exploration at 5 months of age are related to children’s intelligence scores at 4 and 10 years of age and to school achievement at 14 years of age (Bornstein et al. 2013)?
Decades after Piaget (1952) first proposed that sensorimotor exploration drives cognition, the literature is now replete with descriptions of developmental cascades from infant motor skill to other domains of development. For example, object exploration skills lead to developmental improvements in cross-modal perception of object properties (Eppler 1995), discrimination of object boundaries (Needham 2000), and mental rotation of objects (Mohring & Frick 2013). A few weeks of postural and object training facilitate infants’ understanding of means–ends relations—infants repeatedly flip a switch to turn on a distant musical spinning toy (Lobo & Galloway 2008). Improvements in the ability to grasp objects—whether naturally occurring or via sticky-mittens training—promotes infants’ understanding of other people’s intention to grasp objects (Daum et al. 2011, Sommerville et al. 2005).
Similarly, the advent of locomotor skills enables developmental changes in other psychological domains. Like manual skills, the ability to crawl facilitates mental rotation abilities in a visual test (Schwarzer et al. 2013). Crawling promotes more flexible memory retrieval and transfer (how to push a button to operate a new toy) after a 24-h delay (Herbert et al. 2007). Crawling experience leads to increased anger in response to arm restraint (Roben et al. 2012), and both crawling and walking experience facilitate infants’ use of landmarks to find their hidden caregivers (Clearfield 2004).
How does one get from here to there: from grasping to intentions, from crawling to mental rotation, and so on? Despite an abundance of evidence that motor skill acquisition leads to developmental changes in other domains, in most cases, researchers have only identified the starting and ending points. Without identifying the causal mechanisms, they can only speculate about the intermediate links. The task of connecting the dots is complicated because multiple developmental pathways can lead to the same endpoint and multiple factors typically contribute to development.
The task is difficult but not insurmountable. For example, sitting experience facilitates improvements in three-dimensional form perception (Soska et al. 2010). How does this occur? Infants with more sitting experience spontaneously produce exploratory behaviors that reveal the backsides of objects—looking at objects while fingering, rotating, and transferring them from hand to hand. These exploration skills, in turn, predict longer looking at displays of rotating objects that reveal an unexpectedly hollow rather than complete, three-dimensional shape.
Through a surprising cascade of events, crawling may be linked to respiratory infections and asthma (Wu et al. 2018). What is the link? Crawling and walking movements momentarily churn up dust and debris from the carpet, and some types of airborne particles are detrimental if inhaled. Because crawlers’ breathing zone is so close to the floor (Kretch et al. 2014), they are more likely to inhale suspended particulate matter than are walking infants or adults (Wu et al. 2018). The particulates, which can include allergens related to respiratory infections and asthma, land deep in the lower airways of the respiratory system because infants tend to breathe through their mouths, rather than their noses.
A particularly intriguing cascade traces crawling experience to avoidance at the edge of a drop-off. What happens in this case? The obvious culprit—prior falls from a high place—is not related to avoidance (Adolph 1997, Kretch & Adolph 2013a). Novice crawlers and walkers plunge over the edge regardless of prior injuries from falling. The actual mechanisms are far less dramatic. One type of mechanism involves accumulated experience with varying body–environment relations and the acquisition of exploratory movements that generate information for affordances (Adolph & Robinson 2015). A less obvious pathway involves sensitivity to peripheral optic flow (Campos et al. 2000). Infants with a few weeks of experience moving themselves through the environment—whether crawling on their own or operating a joystick to move a baby go-cart—are more sensitive to peripheral optic flow than are precrawling infants (Dahl et al. 2013). Specifically, infants with crawling or go-cart experience adjust their sitting posture in response to a so-called moving room (a three-walled mini-room on wheels or suspended from the laboratory ceiling). Forward movement of the side walls simulates the visual information experienced as the body moves forward (similar to the false perception of self-motion when an adjacent car or train starts moving). Postural responses, in turn, predict avoidance of the apparent drop-off on the visual cliff (Dahl et al. 2013). Presumably, the distant floor of the drop-off disrupts the expected pattern of optic flow and alerts infants that something is amiss.
In a similarly unforeseen cascade, the onset of walking is associated with increases in infants’ receptive and productive vocabulary (Walle & Campos 2014). Why is this the case? One cascading pathway involves carrying. Walkers carry objects more frequently than crawlers (Karasik et al. 2012). As a result of more frequent carrying, walkers are also more likely to initiate social interactions by carrying objects to their caregivers and by directing caregivers’ attention to particular objects (Clearfield 2011, Karasik et al. 2011). Compared with stationary bids for attention, moving bids (regardless of whether initiated by crawlers or walkers) make caregivers more likely to respond with action directives such as “open it” (Karasik et al. 2014). Thus, walking facilitates carrying, carrying promotes new types of social interactions, and these social interactions shape infants’ language input.
CONCLUSIONS: MOTOR DEVELOPMENT IS ESSENTIAL TO PSYCHOLOGY
We began this review by asking what infant motor development has to do with psychology. We conclude that motor development is essential to the study of psychology. Motor development both requires and facilitates the development of basic psychological functions. Relegating motor behavior to Cinderella status in psychology has a certain irony. After all, motor development is really behavioral development. All infant behaviors—looking, reaching, walking, talking—are motor behaviors. Thus, for researchers who view psychology as the study of behavior, motor skill acquisition is the stuff of the science. For researchers who view psychology as the study of mind, infants’ motor behaviors (e.g., looking time, reaching, vocalizations, facial expressions) provide access to their percepts, intentions, thoughts, and feelings. The peripheral status of motor behavior belies its central role in psychological research.
Throughout this review, we argue for the primacy of behavioral flexibility—the ability to do what needs to be done to accomplish the goals of everyday life. Indeed, flexibility is the hallmark of skill (Adolph & Robinson 2015, Bernstein 1996). Adaptive action requires the right solution for the current situation. Movements cannot be repeated in the same way in every situation because bodies, environments, and tasks are in continual flux. Novelty and variability are the rule, not the exception. Thus, as illustrated by the footprints in Figure 1a–c, motor behavior can only be interpreted in context. The infant’s footprint patterns on the shallow slope in Figure 1b resemble the experienced walker’s path on flat ground in Figure 1a, whereas the expert gait modifications on the steep slope in Figure 1b resemble the novice walker’s footprints in Figure 1a. Similarly, footprint patterns during spontaneous free play in Figure 1c can resemble both novice and experienced walking in the standard gait assessment in Figure 1a. In sum, infants’ short, variable steps can be (a) a marker of poor skill in a novice walker, (b) evidence of gait modifications in an experienced walker approaching an obstacle, or (c) emblematic of natural locomotion in both novice and experienced walkers during free play with a caregiver.
The organization of our review into sections on the embodied, embedded, enculturated, and enabling aspects of motor behavior reflects historical and disciplinary divisions among researchers. In fact, all four aspects are emblematic of infant motor development. All motor behaviors are embodied and embedded: Movements only occur in a body in a physical environment, and changes in either component alter the possibilities for action. Most (perhaps all) motor skills are enculturated and enabling: Infants’ actions are supported and constrained by social partners. Growing up in a crib, a sling, or bound in a gahvora cradle surely affects intentional and spontaneous movements. New motor skills open up more of the world and thereby enable new opportunities for exploration and learning. Reciprocally, new motor skills make new demands on perceiving, planning, problem solving, memory, and so on.
We conclude with a final suggestion for why motor development should be of general interest to researchers in psychology. Infant skill acquisition provides a unique window into processes of change. In contrast to the covert nature of perception, cognition, emotion, and other psychological functions, motor behaviors are out in the open and are directly accessible to observation. Moreover, researchers can observe change over multiple nested time scales. Changes in the trajectory of an eye movement or a step that occur within milliseconds are nested within a series of eye and leg movements that take place over seconds and minutes. These changes, in turn, are nested within changes in the speed, accuracy, and efficiency of looking and walking movements that play out over longer time scales of days, weeks, months, and years. Indeed, the study of motor development has a long history of exploiting the directly observable nature of infants’ motor actions to illuminate general processes of developmental change (for reviews, see Adolph & Berger 2006; Adolph & Robinson 2015; Bertenthal & Clifton 1998; Thelen 1995, 2000).
FUTURE ISSUES.
To better understand embodied action, researchers must experimentally alter infants’ bodies and document the effects of naturally occurring variations (e.g., obesity, disability) on motor skill acquisition and implementation.
A significant gap in the literature on embedded action concerns infants’ perception of affordances for manual actions. More studies that parametrically scale relevant features of the environment (e.g., object size, shape, orientation, location) are needed.
Research on social and cultural influences on infant action should expand beyond mothers to other significant social partners, including fathers, siblings, peers, and pets.
Given that most psychological research focuses on only a small segment of the world’s population, more work is needed on the influence of child-rearing practices and home environments on motor skill acquisition.
In addition to reporting cultural differences in the ages of skill onset, new work on enculturated action should examine which actions are acquired, the form of these actions, and their developmental trajectories.
Despite a large literature showing that new motor skills enable new opportunities for learning, experimental studies are required to test whether, when, and how infants exploit these benefits.
An important goal for future research is the identification and understanding of the intermediary mechanisms that underlie developmental cascades.
ACKNOWLEDGMENTS
Work on this review was supported by the grants from the National Institute of Child Health and Human Development (NICHD; grants R37-HD033486 and R01-HD086034), the National Science Foundation (NSF; grant BCS-1528831), and the LEGO Foundation to K.E.A. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, NSF, or LEGO Foundation. We are grateful to Dima Amso, Mark Blumberg, William Fabricius, Rick Gilmore, Lana Karasik, Amy Needham, Marjorie Rhodes, and members of the New York University Infant Action Lab for their insightful comments. We thank Orit Herzberg for her wonderful figure illustrations and design.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adolph KE. 1997. Learning in the development of infant locomotion. Monogr. Soc. Res. Child Dev 62(3):1–140 [PubMed] [Google Scholar]
- Adolph KE. 2000. Specificity of learning: why infants fall over a veritable cliff. Psychol. Sci 11:290–95 [DOI] [PubMed] [Google Scholar]
- Adolph KE, Avolio AM. 2000. Walking infants adapt locomotion to changing body dimensions. J. Exp. Psychol. Hum. Percept. Perform 26:1148–66 [DOI] [PubMed] [Google Scholar]
- Adolph KE, Berger SE. 2006. Motor development In Handbook of Child Psychology, Vol. 2: Cognition, Perception, and Language, ed. Kuhn D, Siegler RS, pp. 161–213. New York: Wiley; 6th ed. [Google Scholar]
- Adolph KE, Berger SE. 2015. Physical and motor development In Development Science: An Advanced Textbook, ed. Bornstein MH, Lamb ME, pp. 261–333. New York: Psychol. Press. 7th ed. [Google Scholar]
- Adolph KE, Berger SE, Leo AJ. 2011. Developmental continuity? Crawling, cruising, and walking. Dev. Sci 14:306–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Cole WG, Komati M, Garciaguirre JS, Badaly D, et al. 2012. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychol. Sci 23:1387–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Joh AS, Eppler MA. 2010a. Infants‘ perception of affordances of slopes under high and low friction conditions. J. Exp. Psychol. Hum. Percept. Perform 36:797–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Karasik LB, Tamis-LeMonda CS. 2010b. Motor skills. In Handbook of Cultural Development Science, Vol. 1: Domains of Development Across Cultures, ed. Bornstein MH, pp. 61–88. New York: Taylor and Francis [Google Scholar]
- Adolph KE, Karasik LB, Tamis-LeMonda CS. 2010c. Using social information to guide action: infants‘ locomotion over slippery slopes. Neural Netw. 23:1033–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Robinson SR. 2015. Motor development In Handbook of Child Psychology and Developmental Science, Vol. 2, ed. L Liben, Muller U, pp. 114–57. New York: Wiley; 7th ed. [Google Scholar]
- Adolph KE, Tamis-LeMonda CS. 2014. The costs and benefits of development: the transition from crawling to walking. Child Dev. Perspect 8:187–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Tamis-LeMonda CS, Ishak S, Karasik LB, Lobo SA. 2008. Locomotor experience and use of social information are posture specific. Dev. Psychol 44:1705–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Vereijken B, Denny MA. 1998. Learning to crawl. Child Dev. 69:1299–312 [PubMed] [Google Scholar]
- Barrett TM, Davis EF, Needham AW. 2007. Learning about tools in infancy. Dev. Psychol 43:352–68 [DOI] [PubMed] [Google Scholar]
- Bastien GJ, Schepens B, Willems PA, Heglund NC. 2005. Energetics of load carrying in Nepalese porters. Science 308:1755. [DOI] [PubMed] [Google Scholar]
- Bastien GJ, Willems PA, Schepens B, Heglund NC. 2016. The mechanics of head-supported load carriage by Nepalese porters. J. Exp. Biol 219:3626–34 [DOI] [PubMed] [Google Scholar]
- Beebe B, Messinger DS, Bahrick LE, Margolis A, Buck KA, Chen H. 2016. A systems view of mother-infant face-to-face communication. Dev. Psychol 52:556–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JB. 1993. Season of birth and onset of locomotion: theoretical and methodological implications. Infant Behav. Dev 16:69–81 [Google Scholar]
- Bernstein NA. 1996. On dexterity and its development In Dexterity and Its Development, ed. Latash ML, Turvey MT, pp. 3–244. Mahwah, NJ: Lawrence Erlbaum Assoc. [Google Scholar]
- Bertenthal BI. 1996. Origins and early development of perception, action, and representation. Annu. Rev. Psychol 47:431–59 [DOI] [PubMed] [Google Scholar]
- Bertenthal BI, Clifton RK. 1998. Perception and action In Handbook of Child Psychology, Vol. 2: Cognition, Perception, and Language, ed. Kuhn D, Siegler RS, pp. 51–102. New York: Wiley; 5th ed. [Google Scholar]
- Bisi MC, Stagni R. 2015. Evaluation of toddler different strategies during the first six-months of independent walking: a longitudinal study. Gait Posture 41:574–79 [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Dooley JC. 2017. Phantom limbs, neuroprosthetics, and the developmental origins of embodiment. Trends Neural Sci. 40:603–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Hahn CS, Suwalsky JTD. 2013. Physically developed and exploratory young infants contribute to their own long-term academic achievement. Psychol. Sci 24:1906–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril B 2018. Action, movement, and culture: Does culture shape movement? Kinesiol. Rev 7:79–87 [Google Scholar]
- Bril B, Dupuy L, Dietrich G, Corbetta D. 2015. Learning to tune the antero-posterior propulsive forces during walking: a necessary skill for mastering upright locomotion in toddlers. Exp. Brain Res 233:2903–12 [DOI] [PubMed] [Google Scholar]
- Burnay C, Cordovil R. 2016. Crawling experience predicts avoidance of real cliffs and water cliffs: insight from a new paradigm. Infancy 21:677–84 [Google Scholar]
- Bushnell EW, Boudreau JP. 1998. Exploring and exploiting objects with the hands during infancy In The Psychobiology of the Hand: Clinics in Developmental Medicine, ed. Connolly KJ, pp. 144–61. London: MacKeith Press [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, Witherington DC. 2000. Travel broadens the mind. Infancy 1:149–219 [DOI] [PubMed] [Google Scholar]
- Chang CL, Kubo M, Buzzi U, Ulrich B. 2006. Early changes in muscle activation patterns of toddlers during walking. Infant Behav. Dev 29:175–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clearfield MW. 2004. The role of crawling and walking experience in infant spatial memory. J. Exp. Child Psychol 89:214–41 [DOI] [PubMed] [Google Scholar]
- Clearfield MW. 2011. Learning to walk changes infants‘ social interactions. Infant Behav. Dev 34:15–25 [DOI] [PubMed] [Google Scholar]
- Cole WG, Gill SV, Vereijken B, Adolph KE. 2014. Coping with asymmetry: how infants and adults walk with one elongated leg. Infant Behav. Dev 37:305–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole WG, Lingeman JM, Adolph KE. 2012. Go naked: Diapers affect infant walking. Dev. Sci 15:783–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole WG, Robinson SR, Adolph KE. 2016. Bouts of steps: the organization of infant exploration. Dev. Psychobiol 58:341–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly KJ, Dalgleish M. 1989. The emergence of a tool-using skill in infancy. Dev. Psychol 25:894–912 [Google Scholar]
- Dahl A, Campos JJ, Anderson DI, Uchiyama I, Witherington DC, et al. 2013. The epigenesis of wariness of heights. Psychol. Sci 24:1361–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum MM, Prinz W, Aschersleben G. 2011. Perception and production of object-related grasping in 6-month-olds. J. Exp. Child Psychol 108:810–18 [DOI] [PubMed] [Google Scholar]
- Davis BE, Moon RY, Sachs HC, Ottolini MC. 1998. Effects of sleep position on infant motor development. Pediatrics 102:1135–40 [DOI] [PubMed] [Google Scholar]
- Devine J 1985. The versatility of human locomotion. Am. Anthropol 87:550–70 [Google Scholar]
- Dominici N, Ivanenko YP, Lacquaniti F. 2007. Control of foot trajectory in walking toddlers: adaptation to load changes. J. Neurophysiol 97:2790–801 [DOI] [PubMed] [Google Scholar]
- Dudek-Shriber L, Zelazy S. 2007. The effects of prone positioning on the quality and acquisition of developmental milestones in four-month-old infants. Pediatr. Phys. Ther 19:48–55 [DOI] [PubMed] [Google Scholar]
- Duncan K, Goodworth A, Da Costa CSN, Wininger M, Saavedra S. 2018. Parent handling of typical infants varies segmentally across development of postural control. Exp. Brain Res 236:645–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey P 1999. Gender and sinology: shifting Western interpretations of footbinding, 1300–1890. Late Imp. China 20:1–34 [DOI] [PubMed] [Google Scholar]
- Eppler MA. 1995. Development of manipulatory skills and the deployment of attention. Infant Behav. Dev 18:391–405 [Google Scholar]
- Feinman S, Roberts D, Hsieh KF, Sawyer D, Swanson D. 1992. A critical review of social referencing in infancy In Social Referencing and the Construction of Reality in Infancy, ed. Feinman S, pp. 15–54. New York: Plenum Press [Google Scholar]
- Fontenelle SA, Kahrs BA, Neal SA, Newton AT, Lockman JJ. 2007. Infant manual exploration of composite substrates. J. Exp. Child Psychol 98:153–67 [DOI] [PubMed] [Google Scholar]
- Fowler ES, Glinski LP, Reiser CA, Horton VK, Pauli RM. 1997. Biophysical bases for delayed and aberrant development in young children with achondroplasia. Dev. Behav. Pediatr 18:143–50 [DOI] [PubMed] [Google Scholar]
- Franchak JM, Adolph KE. 2012. What infants know and what they do: perceiving possibilities for walking through openings. Dev. Psychol 48:1254–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchak JM, Adolph KE. 2014. Affordances as probabilistic functions: implications for development, perception, and decisions for action. Ecol. Psychol 26:109–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchak JM, Kretch KS, Adolph KE. 2018. See and be seen: infant-caregiver social looking during freely mobile play. Dev. Sci 21:e12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MC, Simmons K, Yurovsky D, Pusiol G. 2013. Developmental and postural changes in children’s visual access to faces In Proceedings of the 35th Annual Meeting of the Cognitive Science Society, ed. Knauff M, Pauen M, Sebanz N Wachsmuth I, pp. 454–59. Austin, TX: Cogn. Sci. Soc. [Google Scholar]
- Garciaguirre JS, Adolph KE, Shrout PE. 2007. Baby carriage: infants walking with loads. Child Dev. 78:664–80 [DOI] [PubMed] [Google Scholar]
- Gesell A 1929. Maturation and infant behavior pattern. Psychol. Rev 36:307–19 [Google Scholar]
- Gesell A 1946. The ontogenesis of infant behavior In Manual of Child Psychology, ed. Carmichael L, pp. 295–331. New York: Wiley [Google Scholar]
- Gibson EJ. 1982. The concept of affordances in development: the renascence of functionalism. In The Concept of Development: The Minnesota Symposia on Child Psychology, Vol. 15, ed. Collins WA, pp. 55–81. Mahwah, NJ: Lawrence Erlbaum Assoc. [Google Scholar]
- Gibson EJ. 1988. Exploratory behavior in the development of perceiving, acting, and the acquiring of knowledge. Annu. Rev. Psychol 39:1–41 [Google Scholar]
- Gibson EJ, Riccio G, Schmuckler MA, Stoffregen TA, Rosenberg D, Taormina J. 1987. Detection of the traversability of surfaces by crawling and walking infants. J. Exp. Psychol. Hum. Percept. Perform 13:533–44 [DOI] [PubMed] [Google Scholar]
- Gill SV, Adolph KE, Vereijken B. 2009. Change in action: how infants learn to walk down slopes. Dev. Sci 12:888–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Gustafson GE, West MJ. 1980. Effects of infant development on mother-infant interactions. Child Dev. 51:199–207 [PubMed] [Google Scholar]
- Hallemans A, De Clercq D, Aerts P. 2006. Changes in 3D joint dynamics during the first 5 months after the onset of independent walking: a longitudinal follow-up study. Gait Posture 24:270–79 [DOI] [PubMed] [Google Scholar]
- Harlow HF. 1949. The formation of learning sets. Psychol. Rev 56:51–65 [DOI] [PubMed] [Google Scholar]
- Hayashi K 1992. The influence of clothes and bedclothes on infants‘ gross motor development. Dev. Med. Child Neurol 34:557–58 [DOI] [PubMed] [Google Scholar]
- Heglund NC, Willems PA, Penta M, Cavagna GA. 1995. Energy-saving gait mechanics with head-supported loads. Nature 375:52–54 [DOI] [PubMed] [Google Scholar]
- Herbert J, Gross J, Hayne H. 2007. Crawling is associated with more flexible memory retrieval by 9-month-old infants. Dev. Sci 10:183–89 [DOI] [PubMed] [Google Scholar]
- Hopkins B, Westra T. 1988. Maternal handling and motor development: an intracultural study. Genet. Soc. Gen. Psychol. Monogr 114:379–408 [PubMed] [Google Scholar]
- Hopkins B, Westra T. 1990. Motor development, maternal expectations, and the role of handling. Infant Behav. Dev 13:117–22 [Google Scholar]
- Ivanenko YP, Dominici N, Cappellini G, Dan B, Cheron G, Lacquaniti F. 2004. Development of pendulum mechanism and kinematic coordination from the first unsupported steps in toddlers. J. Exp. Biol 207:3797–810 [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Dominici N, Cappellini G, Lacquaniti F. 2005. Kinematics in newly walking toddlers does not depend upon postural stability. J. Neurophysiol 94:754–63 [DOI] [PubMed] [Google Scholar]
- Jayaraman W, Fausey CM, Smith LB. 2015. The faces in infant-perspective scenes change over the first year of life. PLOS ONE 10:e0123780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh AS, Adolph KE. 2006. Learning from falling. Child Dev. 77:89–102 [DOI] [PubMed] [Google Scholar]
- Joh AS, Adolph KE, Campbell MR, Eppler MA. 2006. Why walkers slip: Shine is not a reliable cue for slippery ground. Percept. Psychophys 68:339–52 [DOI] [PubMed] [Google Scholar]
- Kahrs BA, Jung WP, Lockman JJ. 2014. When does tool use become distinctively human? Hammering in young children. Child Dev. 85:1050–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Adolph KE, Tamis-LeMonda CS, Zuckerman A. 2012. Carry on: spontaneous object carrying in 13-month-old crawling and walking infants. Dev. Psychol 48:389–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. 2011. Transition from crawling to walking and infants‘ actions with objects and people. Child Dev. 82:1199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. 2014. Crawling and walking infants elicit different verbal responses from mothers. Dev. Sci 17:388–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. 2016. Decisions at the brink: locomotor experience affects infants‘ use of social information on an adjustable drop-off. Front. Psychol 7:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattwinkel J, Brooks J, Myerberg D. 1992. Positioning and SIDS. Pediatrics 89:1120–261503575 [Google Scholar]
- Keen R 2011. The development of problem solving in young children: a critical cognitive skill. Annu. Rev. Psychol 62:1–21 [DOI] [PubMed] [Google Scholar]
- Kingsnorth S, Schmuckler MA. 2000. Walking skill versus walking experience as a predictor of barrier crossing in toddlers. Infant Behav. Dev 23:331–50 [Google Scholar]
- Kopp CB. 2011. Development in the early years: socialization, motor development, and consciousness. Annu. Rev. Psychol 62:165–87 [DOI] [PubMed] [Google Scholar]
- Kretch KS, Adolph KE. 2013a. Cliff or step? Posture-specific learning at the edge of a drop-off. Child Dev. 84:226–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretch KS, Adolph KE. 2013b. No bridge too high: Infants decide whether to cross based on the probability of falling not the severity of the potential fall. Dev. Sci 16:336–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretch KS, Adolph KE. 2015. Active vision in passive locomotion: real-world free viewing in infants and adults. Dev. Sci 18:736–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretch KS, Adolph KE. 2017. The organization of exploratory behaviors in infant locomotor planning. Dev. Sci 20:e12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretch KS, Franchak JM, Adolph KE. 2014. Crawling and walking infants see the world differently. Child Dev. 85:1503–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl M 1992. Further observations on diurnal variation in standing height. Ann. Hum. Biol 19:87–90 [DOI] [PubMed] [Google Scholar]
- Lampl M 1993. Evidence of saltatory growth in infancy. Am. J. Hum. Biol 5:641–52 [DOI] [PubMed] [Google Scholar]
- Lampl M, Thompson AL. 2007. Growth chart curves do not describe individual growth biology. Am. J. Hum. Biol 19:643–53 [DOI] [PubMed] [Google Scholar]
- Lee DK, Cole WG, Golenia L, Adolph KE. 2018. The cost of simplifying complex developmental phenomena: a new perspective on learning to walk. Dev. Sci 21:e12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertus K, Joh AS, Needham AW. 2016. Motor training at 3 months affects object exploration 12 months later. Dev. Sci 19:1058–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertus K, Needham AW. 2010. Teach to reach: the effects of active versus passive reaching experiences on action and perception. Vis. Res 50:2750–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MA, Galloway JC. 2008. Postural and object-oriented experiences advance early reaching, object exploration, and means-end behavior. Child Dev. 79:1869–90 [DOI] [PubMed] [Google Scholar]
- Lobo MA, Galloway JC. 2012. Enhanced handling and positioning in early infancy advances development throughout the first year. Child Dev. 83:1290–302 [DOI] [PubMed] [Google Scholar]
- Logan SW, Schreiber MA, Lobo MA, Pritchard B, George L, Galloway JC. 2015. Real-world performance: physical activity, play, and object-related behaviors of toddlers with and without disabilities. Pediatr. Phys. Ther 27:433–41 [DOI] [PubMed] [Google Scholar]
- Martorell R, Onis M, Martines J, Black M, Onyango A, Dewey KG. 2006. WHO motor development study: windows of achievement for six gross motor development milestones. Acta Paediatr. 95(S450):86–95 [DOI] [PubMed] [Google Scholar]
- McGraw MB. 1935. Growth: A Study of Johnny and Jimmy. New York: Appleton-Century Crofts [Google Scholar]
- Mei J 1994. The Northern Chinese custom of rearing babies in sandbags: implications for motor and intellectual development In Motor Development: Aspects of Normal and Delayed Development, ed. van Rossum JHA, Laszlo JI, pp. 41–48. Amsterdam: VU Uitgeverij [Google Scholar]
- Meltzoff AN. 1988. Infant imitation after a 1-week delay: long-term memory for novel acts and multiple stimuli. Dev. Psychol 24:470–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti AE, Formenti F, Ardigo LP. 2006. Himalayan porter’s specialization: metabolic power, economy, efficiency, and skill. Proc. R. Soc. Lond. B 273:2791–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohring W, Frick A. 2013. Touching up mental rotation: effects of manual experience on 6-month-old infants‘ mental object rotation. Child Dev. 84:1554–65 [DOI] [PubMed] [Google Scholar]
- Moller EL, Majdandzic M, Bogels SM. 2014. Fathers‘ versus mothers‘ social referencing signals in relation to infant anxiety and avoidance: a visual cliff experiment. Dev. Sci 17:1012–28 [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN. 1993. The Developing Human: Clinically Oriented Embryology. Philadelphia, PA: W.B. Saunders; 5th ed. [Google Scholar]
- Needham AW. 2000. Improvements in object exploration skills may facilitate the development of object segregation in early infancy. J. Cogn. Dev 1:131–56 [Google Scholar]
- Needham AW. 2016. Learning About Objects in Infancy. Abingdon, UK: Routledge [Google Scholar]
- Needham AW, Barrett T, Peterman K. 2002. A pick-me-up for infants‘ exploratory skills: Early simulated experiences reaching for objects using “sticky” mittens enhances young infants‘ object exploration skills. Infant Behav. Dev 25:279–95 [Google Scholar]
- Oster H 2005. The repertoire of infant facial expressions: an ontogenetic perspective In Emotional Development: Recent Research Advances, ed. Nadel J, Muir D, pp. 261–92. Oxford, UK: Oxford Univ. Press [Google Scholar]
- Patrick SK, Noah JA, Yang JF. 2012. Developmental constraints of quadrupedal coordination across crawling styles in human infants. J. Neurophysiol 107:3050–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J 1952. The Origins of Intelligence in Children. New York: Int. Univ. Press [Google Scholar]
- Piek JP, Carman R. 1994. Developmental profiles of spontaneous movements in infants. Early Hum. Dev 39:109–26 [DOI] [PubMed] [Google Scholar]
- Rachwani J, Santamaria V, Saavedra S, Woollacott MH. 2015. The development of trunk control and its relation to reaching in infancy: a longitudinal study. Front. Hum. Neurosci 9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachwani J, Soska KC, Adolph KE. 2017. Behavioral flexibility in learning to sit. Dev. Psychobiol 59:937–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheingold HL, Eckerman CO. 1970. The infant separates himself from his mother. Science 168:78–83 [DOI] [PubMed] [Google Scholar]
- Roben CK, Bass AJ, Moore GA, Murray-Kolb L, Tan PZ, et al. 2012. Let me go: the influences of crawling experience and temperament on the development of anger expression. Infancy 17:558–77 [DOI] [PubMed] [Google Scholar]
- Rochat P, Goubet N, Senders SJ. 1999. To reach or not to reach? Perception of body effectivities by young infants. Infant Child Dev. 8:129–48 [Google Scholar]
- Rosenbaum DA. 2005. The Cinderella of psychology: the neglect of motor control in the science of mental life and behavior. Am. Psychol 60:308–17 [DOI] [PubMed] [Google Scholar]
- Saavedra SL, van Donkelaar P, Woollacott MH. 2012. Learning about gravity: segmental assessment of upright control as infants develop independent sitting. J. Neurophysiol 108:2215–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save the Children. 2011. Harmful traditional practices in Tajikistan. Rep., Save the Children, London: http://www.ohchr.org/Documents/HRBodies/CEDAW/HarmfulPractices/SavetheChildren.pdf [Google Scholar]
- Schmuckler MA. 1996. Development of visually guided locomotion: barrier crossing by toddlers. Ecol. Psychol 8:209–36 [Google Scholar]
- Schwarzer G, Freitag C, Buckel R, Lofruthe A. 2013. Crawling is associated with mental rotation ability by 9-month-old infants. Infancy 18:432–41 [Google Scholar]
- Shapiro L 2011. Embodied Cognition. Abingdon, UK: Routledge [Google Scholar]
- Sim ZL, Xu F. 2017. Infants preferentially approach and explore the unexpected. Br. J. Dev. Psychol 35:596–608 [DOI] [PubMed] [Google Scholar]
- Smitsman AW, Corbetta D. 2010. Action in infancy: perspectives, concepts, and challenges In The Wiley-Blackwell Handbook of Infant Development, Vol. 1, ed. Bremner JG, Wachs TD, pp. 167–203. New York: Wiley; 2nd ed. [Google Scholar]
- Snapp-Childs W, Corbetta D. 2009. Evidence of early strategies in learning to walk. Infancy 14:101–16 [DOI] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, Needham AW. 2005. Action experience alters 3-month-old infants‘ perception of others‘ actions. Cognition 96: B1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce JF, Emde RN, Campos JJ, Klinnert MD. 1985. Maternal emotional signaling: its effects on the visual cliff behavior of 1-year-olds. Dev. Psychol 21:195–200 [Google Scholar]
- Soska KC, Adolph KE. 2014. Postural position constrains multimodal object exploration in infants. Infancy 19:138–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soska KC, Adolph KE, Johnson SP. 2010. Systems in development: motor skill acquisition facilitates three-dimensional object completion. Dev. Psychol 46:129–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl AE, Feigenson L. 2015. Observing the unexpected enhances infants‘ learning and exploration. Science 348:91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Super CM. 1976. Environmental effects on motor development: the case of “African infant precocity”. Dev. Med. Child Neurol 18:561–67 [DOI] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Adolph KE, Lobo SA, Karasik LB, Dimitropoulou KA, Ishak S. 2008. When infants take mothers‘ advice: 18-month-olds integrate perceptual and social information to guide motor action. Dev. Psychol 44:734–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E 1994. Three-month-old infants can learn task-specific patterns of interlimb coordination. Psychol. Sci 5:280–85 [Google Scholar]
- Thelen E 1995. Motor development: a new synthesis. Am. Psychol 50:79–95 [DOI] [PubMed] [Google Scholar]
- Thelen E 2000. Motor development as foundation and future of developmental psychology. Int. J. Behav. Dev 24:385–97 [Google Scholar]
- Thelen E, Corbetta D, Kamm K, Spencer JP, Schneider K, Zernicke RF. 1993. The transition to reaching: mapping intention and intrinsic dynamics. Child Dev. 64:1058–98 [PubMed] [Google Scholar]
- Thelen E, Skala K, Kelso JAS. 1987. The dynamic nature of early coordination: evidence from bilateral leg movements in young infants. Dev. Psychol 23:179–86 [Google Scholar]
- Theveniau N, Boisgontier MP, Verieras S, Olivier I. 2014. The effects of clothes on independent walking in toddlers. Gait Posture 39:659–61 [DOI] [PubMed] [Google Scholar]
- Thurman SL, Corbetta D. 2017. Spatial exploration and changes in infant-mother dyads around transitions in infant locomotion. Dev. Psychol 53:1207–21 [DOI] [PubMed] [Google Scholar]
- Trettien AW. 1900. Creeping and walking. Am. J. Psychol 12:1–57 [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. 1978. The infant’s response to entrapment between contradictory messages in face-to-face interaction J. Am. Acad. Child Adolesc. Psychiatry 17:1–13 [DOI] [PubMed] [Google Scholar]
- Vaish A, Striano T. 2004. Is visual reference necessary? Contributions of facial versus vocal cues in 12-month-olds‘ social referencing behavior. Dev. Sci 7:261–69 [DOI] [PubMed] [Google Scholar]
- Van Dam M, Hallemans A, Truijen S, Segers V, Aerts P. 2011. A cross-sectional study about the relationship between morphology and kinematic parameters in children between 15 and 36 months. Gait Posture 34:159–63 [DOI] [PubMed] [Google Scholar]
- Vereijken B, Pedersen AV, Storksen JH. 2009. Early independent walking: a longitudinal study of load perturbation effects. Dev. Psychobiol 51:374–83 [DOI] [PubMed] [Google Scholar]
- von Hofsten C 2004. An action perspective on motor development. Trends Cogn. Sci 8:266–72 [DOI] [PubMed] [Google Scholar]
- von Hofsten C 2007. Action in development. Dev. Sci 10:54–60 [DOI] [PubMed] [Google Scholar]
- Walle EA, Campos JJ. 2014. Infant language development is related to the acquisition of walking. Dev. Psychol 50:336–48 [DOI] [PubMed] [Google Scholar]
- Wilson EM, Green JR, Yunusova YY, Moore CA. 2008. Task specificity in early oral motor development. Semin. Speech Lang 29:257–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherington DC, Campos JJ, Anderson DI, Lejeune L, Seah E. 2005. Avoidance of heights on the visual cliff in newly walking infants. Infancy 7:285–98 [DOI] [PubMed] [Google Scholar]
- Wu T, Taubel M, Holopainen R, Viitanen AK, Vainiotalo S, et al. 2018. Infant and adult inhalation exposure to resuspended biological particulate matter. Environ. Sci. Technol 52:237–47 [DOI] [PubMed] [Google Scholar]
- Yonas A, Hartman B. 1993. Perceiving the affordance of contact in four- and five-month-old infants. Child Dev. 64:298–308 [DOI] [PubMed] [Google Scholar]
- Zelazo PR, Zelazo NA, Kolb S. 1972. “Walking” in the newborn. Science 176:314–15 [DOI] [PubMed] [Google Scholar]


