Summary:
Objectives.
This study investigated the effects of cigarette smoke exposure on the pig larynx using an inhalation chamber. Specifically, we compared the effects of cigarette smoke exposure from either 3 cigarettes per day (3cd) or 15 cigarettes per day (15cd) for 20 days.
Study design.
In vivo prospective design.
Methods.
Female pigs were exposed via an inhalation chamber to cigarette smoke (3R4F research cigarettes) from 3cd (n = 6) or 15cd (n = 6) for 20 days. Outcomes included histopathology of vocal fold and airway tissues; gene expression of interleukins, TNF-α, and VEGF; protein levels of TNF-α and IL-6; and number of coughs recorded in the chamber.
Results.
Pigs exposed to cigarette smoke from 15cd exhibited mild vocal fold edema as compared to the 3cd group on histopathological evaluation. There was also minimal inflammation of nasal and tracheal tissue characterized by presence of more granulocytes in the 15cd group compared to the 3cd group. Cough frequency was significantly greater for the 15cd group compared to the 3cd group.
Conclusions.
A custom-designed large animal inhalation chamber successfully challenged pigs repeatedly, to varying levels of cigarette smoke. Future studies will combine such low levels of smoke exposure with other common challenges such as acid reflux to understand the multifactorial causation of laryngeal pathologies.
Keywords: Pigs, Cigarette smoke, Vocal folds, Inflammation, In vivo
INTRODUCTION
Cigarette smoke is concomitant with a multitude of vocal pathologies.1,2 Sources of cigarette smoke exposure are classified as first-hand, second-hand, or third-hand. Primary users of inhaled tobacco products are classified as first-hand smokers. Second-hand smoke or passive smoke is the exposure to the output of a burning tobacco product. Third-hand smoke arises from the exposure to areas and surfaces that are contaminated from second-hand smoke (National Cancer Institute; NCI, 2017). The inflammatory effects of these afore-mentioned sources of cigarette smoke are being increasingly recognized3,4 although data specific to vocal fold inflammation is limited. The objective of this study was to investigate the inflammatory effects of repeated cigarette smoke exposure delivered via an inhalation chamber, on porcine vocal folds in vivo.
The effects of smoke exposure on laryngeal cells, tissue, and mucus have been examined.5–7 These research studies investigated one-time exposures, cells in isolation, or animal models that have suboptimal vocal fold anatomy and function compared with human beings. We utilized the pig model in this study because porcine vocal folds have similar TaggedPgeometry8 as compared to human vocal folds, and are a well-established model for the study of wound healing and inflammation.9–11
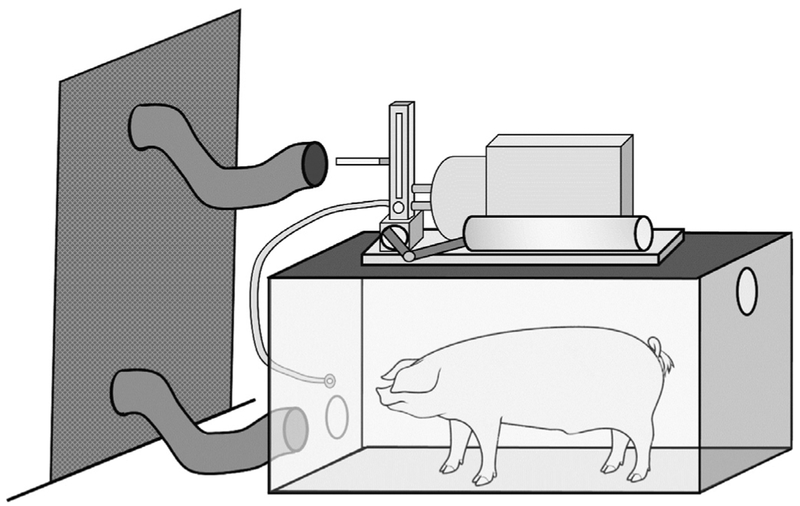
To address our study objective, we custom-designed an inhalation chamber (Figure 1) that could accommodate an awake, mature pig to voluntarily enter the chamber. Twelve pigs were exposed to either cigarette smoke from 3 cigarettes per day (3cd) or 15 cigarettes per day (15 cd), for 20 days in this chamber. We hypothesized that the 15cd group would demonstrate altered vocal fold epithelial morphology, inflammation, and increased cough frequency as compared to the 3cd group. Research data suggest that repeated short-duration cigarette smoke exposures can induce airway inflammation.12–14 Such findings raise questions about the influence of cigarette smoke in making airway tissues vulnerable to other environmental or systemic threats such as pollution or hypersensitive conditions. Whether repeated, short-duration exposures to cigarette smoke also inflame the healthy vocal folds is not known. We were therefore interested in evaluating any potential inflammatory effects of small amounts of cigarette smoke exposure over a short timeline on vocal fold tissue.
FIGURE 1.
Schematic of the smoking chamber.
MATERIALS AND METHODS
Animals
The Purdue Animal Care and Use Committee approved this protocol (#1112000344). Six adult female Sinclair mini pigs (Sus scrofa) were exposed to 3cd for 5 days per week for a total of 4 consecutive weeks (total = 60 cigarettes). Likewise, six adult female Sinclair mini pigs were exposed to 15cd for 5 days a week for 4 consecutive weeks (total = 300 cigarettes). At the end of the 20 day exposure period, the animals were humanely euthanized. The larynx was excised and hemisected. The entire mid-membranous vocal fold from one hemisected larynx was used for histopathological evaluation. The mid-membranous vocal fold (epithelium and lamina propria) of the contralateral vocal fold was used for gene and protein studies. Additionally, airway tissues directly impacted by inhalation of cigarette smoke (ie, nasal conchae, trachea, and lung) were also collected for histologic examination.
Inhalation chamber and cigarette smoke exposure
Pigs were trained via positive reinforcement to voluntarily enter the custom-designed inhalation chamber (Figure 1) for cigarette smoke exposure. The cigarette smoke was comprised of both primary cigarette smoke and smoke exhaled by the animal while it was in the chamber (NCI, 2017). These research grade cigarettes (3R4F) were obtained from the Center for Tobacco Reference Products (University of Kentucky). The exposure chamber (4′×2′×2′) contained a mouthpiece attached to a KONG that delivered cigarette smoke to the animal via a respiration machine (Harvard Apparatus, Boston, Massachusetts) mounted on the roof of the chamber. The respiration machine mimics the normal breathing rate of the pig (12 breaths per minute)15 and delivers the smoke to the interior chamber where the pig is trained to target the mouthpiece delivering the cigarette smoke.
On each of the 20 days, the pig voluntarily entered the chamber three times per day. During each of these three sessions a cigarette was loaded onto tubing connected to the respiration machine and ignited. Pigs in the 3cd group were exposed to smoke from one cigarette per session while pigs in the 15 cd groups were exposed to smoke from five cigarettes per session. At the conclusion of every session, the remaining smoke in the chamber was purged via a local exhaust duct connected to the chamber. This duct ultimately released the smoke to the exterior of the building, away from building air intakes. All procedures were approved by the Department of Radiological and Environmental Management at Purdue University.
Histopathology evaluation
A 6 mm punch biopsy was used to obtain the vocal fold sample from the hemisected larynx. The biopsied sample was fixed in 10% neutral buffered formalin and embedded in paraffin blocks. Tissue from the nasal conchae, cranial one third of trachea and right cranial lung lobe were excised and similarly fixed and paraffin-embedded. Four-micrometer sections of all histology tissues were stained with haematoxylin and eosin according to standard protocols and interpreted based on standard histopathological morphology by a blinded veterinary pathologist (author AD).
Gene expression
Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was applied to quantify the mRNA level of genes encoding inflammatory cytokines. Total RNA was isolated and purified using NucleoSpin RNA Isolation & Purification Kit (Takara, Mountain View, California). The total RNA was reverse transcribed into cDNA using the Bio-Rad iScript cDNA Synthesis Kit (Biorad, Hercules, California). The qPCR was run in the CFX connect Real-Time PCR Detection System (Bio-Rad, Hercules, California). Primers targeting IL-1α, IL-1β, IL-1R1, IL-6, IL-12A, TNF-α, VEGF, and β-actin were designed using Primer316 and purchased from Integrated DNA Technologies (Coral-ville, Iowa). The primer sequences used in this study were: forward primer (5-TCGCA ACTTC CTGTG ACTCT AA-3) and reverse primer (5-TTCCC AGAAG AAGAG GAGAC TG-3) for IL-1α; forward primer (5-ACATG GAGAA GCGAT TTGTC TT-3) and reverse primer (5-GCTTT TGTTC TGCTT GAGAG GT-3) for IL-1β; forward primer (5-GTTCT CCCAC TAGCA TTTCA CC-3) and reverse primer (5-ATGAG AGGAG GATTT CTGGT CA-3) for IL-1R1; forward primer (5-ATAAG CTGCA GTCAC AGAAC GA-3) and reverse primer (5-ATTAT CCGAA TGGCC CTCAG-3) for IL-6; forward primer (5-AAGGC CAAAC AAACC CTAGA AT-3) and reverse primer (5-TGGCT AGTTC AAGTG GTAAG CA-3) for IL-12A; forward primer (5-GTTTT CCTCA CTCAC ACCAT CA-3) and reverse primer (5-AAAAT AGACC TGCCC AGATT CA-3) for TNF-α; forward primer (5-ATGAG CTTCC TACAG CACAA CA-3) and reverse primer (5-TTTGC AGGAA CATTT ACACG TC-3) for VEGF; forward primer (5-ATGCT TCTAG GCGGA CTGTT AG-3) and reverse primer (5-ACCTT CACCG TTCCA GTTTT TA-3) for β-actin. Each of the samples were assessed in duplicate with iTaq Universal SYBR Green Supermix Kit (Bio-Rad, Hercules, California) according to the protocol established previously in our laboratory.17 The cycle time values of the target genes were normalized with the cycle time values of reference gene, β-actin. Then the relative expressions of genes in 15cd group were normalized to the average of the 3cd group after setting the 3cd as 100%.
Protein levels
Enzyme-linked immunosorbent assays (ELISA) were used to quantify protein levels of TNF-α and IL-6 from vocal fold tissue homogenates (Porcine TNFα ELISA Kit and Swine IL-6 ELISA Kit, Thermo Scientific, Frederick, Maryland) following manufacturer directions. The value of absorbance was measured on an ELISA plate reader (Spec-traMax M2e, Molecular Devices, Sunnyvale, California) at 450 nm. Concentration of TNF-α or IL-6 in the supernatant was determined using a standard curve. The concentration for each supernatant was normalized to tissue weight to calculate the level of TNF-α or IL-6 in the tissue. The relative levels of TNF-α and IL6 were normalized to the average level of the 3cd group.
Cough frequency
The number of coughs produced in the chamber during each session were counted manually by the experimenters and recorded in a notebook. The coughs per session were summed across each week of exposure.
Statistical analysis
Analyses were completed using IBM SPSS (v. 20, Chicago, Illinois). Independent t tests were used for gene expression and protein level studies. A Mann Whitney U test was used to compare cough frequency. The alpha level was set to 0.05. Histopathological evaluations are reported descriptively.
RESULTS
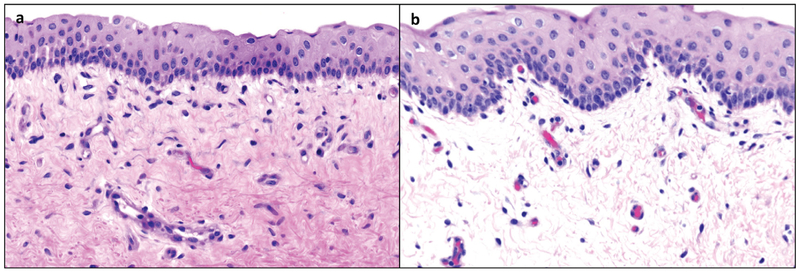
Significant gross laryngeal findings were identified in half of the 15cd group. Three of these six pigs had increased amounts of viscous mucus with rare flecks of opaque material collecting at the ventral floor of the larynx and in the laryngeal ventricles. These findings were not observed in the 3cd group. Histological evaluation of the vocal folds by a veterinary pathologist (author AD) revealed that the vocal fold superficial lamina propria of the 15cd exposure group had mild edema characterized by separation of collagen fibrils by nonstaining material as compared to the 3cd group (Figure 2). Additional histological evaluation of other airway tissues revealed that the nasal lamina propria in four of the six 15cd group had vascular congestion and increased numbers of eosinophils and neutrophils intermixed with the mononuclear inflammatory population. There were more granulocytes (neutrophils and eosinophils) mixed with the mononuclear population in tracheal tissue of 15cd compared to 3cd group. The pulmonary bronchi were devoid of any observable inhaled particulate and lung histology was similar in both groups.
FIGURE 2.
Photomicrographs of vocal fold tissues. Representative vocal fold exposed to 3 cigarettes per day is depicted in (a). Representative vocal fold exposed to 15 cigarettes per day is depicted in (b). The vocal fold superficial lamina propria of the 15 cigarettes per day group developed observable separation of collagen fibers suggesting edema.
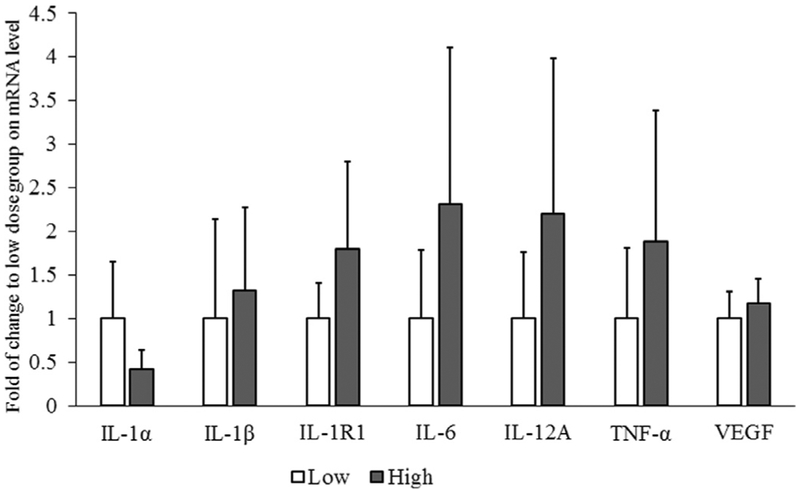
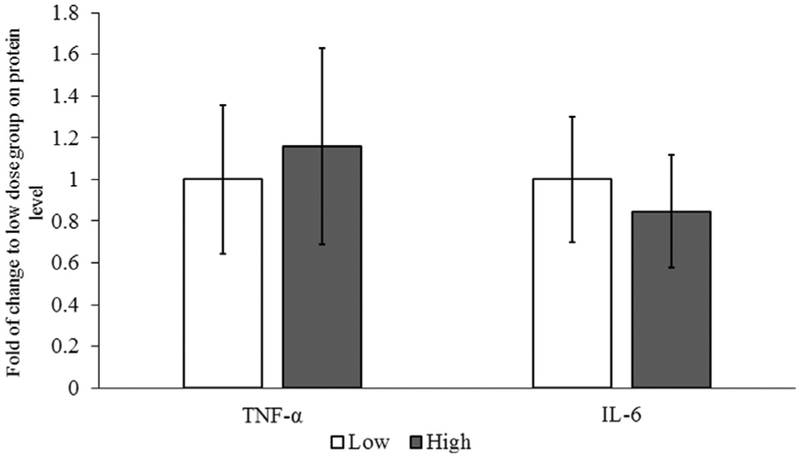
Gene expression data are depicted in Figure 3. Though the level of IL-1β, IL-1R1, IL-6, IL12A, and TNF-α mRNA showed a tendency for increase in the 15cd group, the mRNA levels were not significantly different in the 3cd and 15cd groups. Protein data depicted in Figure 4 did not differ between the 3cd and 15cd groups. Finally, cough frequency was significantly greater for the 15cd exposure (range: 13‒24 coughs) as compared to the 3cd group (range: 1‒6 coughs; (P < 0.05).
FIGURE 3.
Expression of genes encoding cytokines. Relative expressions of genes in 15 cigarettes per day group normalized to 3 cigarettes per day (3cd) group after setting the 3cd as 100%. No differences in vocal fold gene expression were detected between 3cd and 15 cigarettes per day animals.
FIGURE 4.
Synthesis of cytokine proteins. Animals in the 15 cigarettes per day group had no difference in protein synthesis of TNF-α and IL-6 compared to 3 cigarettes per day animals.
DISCUSSION
To our knowledge, this is the first in vivo pig study investigating the effects of cigarette smoke on the larynx. The novelty of this study is imparted by the use of a large animal challenged with low levels of cigarette smoke (not a laboratory derivative such as cigarette smoke solution) to investigate its impact on vocal folds specifically. We hypothesized that second-hand and third-hand smoke exposure from 15cd would induce inflammation characterized by upregulated transcript levels of genes mediating inflammation, increased protein levels of inflammatory cytokines, coughing, and morphological markers of inflammation when compared to exposure from 3cd. We designed an inhalation chamber to accommodate an awake, mature pig. The use of an awake and unstressed animal enables an investigation of the inflammatory mechanism without confounding factors that hinder well-being. We found that animals in the 15cd group presented with more coughs as compared to the 3cd group. Histological evaluation of the vocal folds revealed mild edema in the 15cd group. Edema is a common indicator of inflammation, but this morphologic change was not manifested at gene transcript levels.
It is widely accepted that cigarette smoke exposure induces inflammation.18,19 Therefore, common, well-characterized pro-inflammatory genes (TNF-α, IL-6, IL-12, IL-1α, IL-1R, VEGF) that have been investigated in prior cigarette smoke exposure studies19–22 were examined here. TNF-α and IL-6 were selected as they mediate the cellular phases of inflammation by modulating cell migration, phagocytosis, and immune response.23 IL-12 stimulates the differentiation of naïve T-cells into type 1 T helper cells involved in cell mediated immunity.24 IL-1R resides on T-lymphocytes and transmits the proinflammatory signal of IL-1α which is upregulated in acute inflammation.25 IL-1β synthesis is also induced by cigarette smoke in human beings.26 VEGF is an important angiogenic factor produced by epithelial cells that induces vascular permeability, a feature of acute inflammation, and Reinke’s edema.27
The lack of change in gene expression is noteworthy in this context, especially in light of mild changes observed during histological evaluation. Interestingly, cough frequency was different between the two groups with more coughs in the 15cd group. It is possible that the coughing itself contributed to the histological changes (eg, edema). Examining gene transcript levels for other inflammatory markers,19 markers of oxidative stress,28,29 and changes in mucin expression30 will be included in future studies. Our future studies will also incorporate next generation sequencing to cast a wider net on possible genetic changes due to the cigarette smoke.
It is possible that our exposure duration or frequency was too short to elicit changes in vocal fold tissue since it has been shown that the severity of histological changes in the larynx is related to the number of cigarettes smoked.31 Other studies investigated cigarette smoke products in varying duration and frequency. Rabbit exposure to cigarette smoke for 3 months (10 minutes each time, twice per day) showed epithelial hyperplasia with disturbed stratification of epithelium in vocal folds.32 Increased expression of TNFα and IL6, along with Muc5ac was detected in the subglottic mucosal and submucosa following the cigarette smoke solution exposure in rats for 4 weeks.30 Our 20 day timeline spread over 4 weeks may have obfuscated any changes, or perhaps the vocal folds tissue composition precludes similar changes as those seen in the subglottis. Published reports on the effects of cigarette smoke document changes after very short exposures ranging from 1 to 4 days, or more chronic exposures, over 6 months.33,34 Future studies will investigate a more standard exposure paradigm that replicates what smokers inhale on average (one pack of 20 cigarettes).
This study is a programmatic extension of previous research in our lab involving pigs as an animal model,35 and sets the groundwork to investigate multifactorial disease processes related to laryngeal pathology. Apart from the anatomic and biomechanical similarity to human larynges,8,36,37 pigs are highly trainable.38 The availability of disease models of pigs39,40 open up additional avenues to investigate the interaction of lung disease and vocal fold pathology as well as developmental laryngeal pathologies and to determine how comorbidities contribute to the development of laryngeal disease.
CONCLUSIONS
This study extends the growing body of literature surrounding cigarette smoke and vocal fold inflammation. Here, awake pigs were exposed to second-hand and third-hand smoke from 3cd and 15cd. The exposure to smoke from 15cd induced mild vocal fold edema. Future extensions of this work will investigate similar low dosages of cigarette smoke exposure but over a longer timeline and with additional challenges to further understand underlying mechanisms of vocal fold inflammation from pollutant exposure.
Acknowledgments
We gratefully thank David McMillan for the design and construction of the inhalation chamber and Betsy Pray for her help in developing diagrams. We would also like to thank Jessica Roller and Nathan Garrison for their expertise in pig handling and data collection, and members of the ADDL Histology laboratory and the Clinical Discovery Laboratory. Details of the chamber and animal exposure were presented in a scientific poster at Experimental Biology in Chicago, Illinois in April 2017.
Funding: Funding was provided from R01DC011759 (National Institutes of Health and National Institute on Deafness and other Communication Disorders).
REFERENCES
- 1.Gonzalez J, Carpi A. Early effects of smoking on the voice: a multidimensional study. Med Sci Monitor. 2004;10:CR649–CR656. [PubMed] [Google Scholar]
- 2.Lee L, Stemple J, Geiger D, et al. Effects of environmental tobacco smoke on objective measures of voice production. Laryngoscope. 1999;109:1531–1534. [DOI] [PubMed] [Google Scholar]
- 3.Gruer L, Hart C, Watt G. After 50 years and 200 papers, what can the Midspan cohort studies tell us about our mortality? Public Health. 2017;142:186–195. [DOI] [PubMed] [Google Scholar]
- 4.e Silva S, In acia S, Bowder P, et al. A simple and rapid method for the determination of nicotine in third-hand smoke by liquid chromatography and its application for the assessment of contaminated outdoor communal areas. Drug Testing Anal. 2016;8:676–681. [DOI] [PubMed] [Google Scholar]
- 5.Mouadeb D, Belafsky P, Birchall M, et al. The effects of allergens and tobacco smoke on the laryngeal mucosa of guinea pigs. Otolaryngol Head Neck Surg. 2009;140:493–497. [DOI] [PubMed] [Google Scholar]
- 6.Berchtold C, Coughlin A, Kasper Z, et al. Paracrine potential of fibro-blasts exposed to cigarette smoke extract with vascular growth factor induction. Laryngoscope. 2013;123:2228–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leão H, Zettler C, Cambruzzi E, et al. The effects of passive smoking on laryngeal and tracheal mucosa in male wistar rats during growth: an experimental study. J Voice. 2017;31:126.e19–126.e24. [DOI] [PubMed] [Google Scholar]
- 8.Stevens K, Thomson S, Jett e M, et al. Quantification of porcine vocal fold geometry. J Voice. 2015;19(1):060305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King S, Woo J, Tang S, et al. Macrophage response to allogeneic adi-pose tissue-derived stromal cells in hyaluronan-based hydrogel in a porcine vocal fold injury model. Ann Otol Rhinol Laryngol. 2017; 126:463–477. [DOI] [PubMed] [Google Scholar]
- 10.Woodson G Developing a porcine model for study of vocal fold scar. JVoice. 2012;26:706–710. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau B, Tateya I, Lim X, et al. Investigation of anti-hyaluronidase treatment on vocal fold wound healing. J Voice. 2006;20:443–451. [DOI] [PubMed] [Google Scholar]
- 12.Martins TL, Campos KKD, Araújo NPDS, et al. Extrapulmonary effects of temporal exposure to cigarette smoke. Toxicol Ind Health. 2017;33:717–725. [DOI] [PubMed] [Google Scholar]
- 13.Carter CA, Misra M, Maronpot RR. Tracheal morphologic and protein alterations following short-term cigarette mainstream smoke exposure to rats. J Toxicol Pathol. 2012;25:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura M, Wada H, Honda K, et al. Clarithromycin ameliorates pulmonary inflammation induced by short term cigarette smoke exposure in mice. Pulm Pharmacol Ther. 2015;35:60–66. [DOI] [PubMed] [Google Scholar]
- 15.Plumb DC. Plumb’s Veterinary Drub Handbook; Ames, IA: BlackwellPublishing Professional; 2005. [Google Scholar]
- 16.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Zheng W, Sivasankar MP. Acute acrolein exposure induces impairment of vocal fold epithelial barrier function. PloS One. 2016; 11(9):e0163237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thibeault SL, Rees L, Pazmany L, et al. At the crossroads: mucosal immunology of the larynx. Mucosal immunol. 2009;2:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branski R, Zhou H, Kraus D, Sivasankar M. The effects of cigarette smoke condensate on vocal fold transepithelial resistance and inflamma-tory signaling in vocal fold fibroblasts. Laryngoscope. 2011;121:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiels MS, Katki HA, Freedman ND, et al. Cigarette smoking and variations in systemic immune and inflammation markers. JNCI. 2014;106(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halappanavar S, Nikota J, Wu D, et al. IL-1 receptor regulates microRNA-135b expression in a negative feedback mechanism during cigarette smoke induced in flammation. J Immunol. 2013;190:3679–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sopori M Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. [DOI] [PubMed] [Google Scholar]
- 23.Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of Disease, Professional Edition E-Book. Elsevier Health Sciences; 2014. [Google Scholar]
- 24.Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/inter-leukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. [DOI] [PubMed] [Google Scholar]
- 25.Cohen I, Rider P, Carmi Y, et al. Differential release of chromatin-bound IL-1α discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci U S A. 2010;107:2574–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuschner WG, D’Alessandro A, Wong H, et al. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J. 1996;9:1989–1994. [DOI] [PubMed] [Google Scholar]
- 27.Sato K, Hirano M, Nakashima T. Electron microscopic and immunohistochemical investigation of Reinke’s edema. Ann Otol Rhinol Laryngol. 1999;108:1068–1072. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Fang R, Peterson A, et al. The protective role of autophagy in human vocal fold fibroblasts under cigarette smoke extract exposure: a new insight into the study of Reinke’s edema. ORL. 2016;78:26–35. [DOI] [PubMed] [Google Scholar]
- 29.Branski RC, Saltman B, Sulica L, et al. Cigarette smoke and reactive oxygen species metabolism: implications for the pathophysiology of Reinke’s edema. Laryngoscope. 2009;119:2014–2018. [DOI] [PubMed] [Google Scholar]
- 30.Ueha R, Ueha S, Kondo K, et al. Laryngeal mucus hypersecretion is exacerbated after smoking cessation and ameliorated by glucocorticoid administration. Toxicol Lett. 2017;265:140–146. [DOI] [PubMed] [Google Scholar]
- 31.Marcotullio D, Magliulo G, Pezone T. Reinke’s edema and risk factors: clinical and histopathologic aspects. Am J Otolaryngol. 2002; 23:81–84. [DOI] [PubMed] [Google Scholar]
- 32.Gaafar HA, Al-Mansour AH. The effect of cigarette smoke on the vocal cord mucosa of the rabbit: an electron microscopic study. J Laryngol Otol. 1981;95:721–729. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Vlahos R, Bozinovski S, et al. Effect of short-term cigarette smoke exposure on body weight, appetite and brain neuropeptide Y in mice. Neuropsychopharmacology. 2005;30:713–719. [DOI] [PubMed] [Google Scholar]
- 34.Coggins CR. An updated review of inhalation studies with cigarette smoke in laboratory animals. Int J Toxicol. 2007;26:331–338. [DOI] [PubMed] [Google Scholar]
- 35.Durkes A, Sivasankar MP. In vivo investigation of acidified pepsin exposure to porcine vocal fold epithelia. Laryngoscope. 2016;126:E12–E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birchall MA, Bailey M, Barker EV, et al. Model for experimental revascularized laryngeal allotransplantation. Br J Surg. 2002;89:1470–1475. [DOI] [PubMed] [Google Scholar]
- 37.Jiang JJ, Raviv JR, Hanson DG. Comparison of the phonation-related structures among pig, dog, white-tailed deer, and human larynges. Ann Otol Rhinol Laryngol. 2001;110:1120–1125. [DOI] [PubMed] [Google Scholar]
- 38.Durkes A, Sivasankar MP. A method to administer agents to the larynx in an awake large animal. J Speech Lang Hear Res. 2017;60:3171–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Tang XX, Vargas Buonfiglio LG, et al. Electrolyte transport properties in distal small airways from cystic fibrosis pigs with implications for host defense. Am J Physiol Lung Cell Mol Physiol. 2016;310: L670–L679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adam RJ, Abou Alaiwa MH, Bouzek DC, et al. Postnatal airway growth in cystic fibrosis piglets. J Appl Physiol. 2017;123(3):526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]