Abstract
The septin family of proteins have fascinated cell biologists for decades due to the elaborate architecture they adopt in different eukaryotic cells. Whether they exist as rings, collars, or gauzes in different cell types and at different times in the cell cycle illustrates a complex series of regulation in structure. While the organization of different septin structures at the cortex of different cell types during the cell cycle has been described to various degrees, the exact structure and regulation at the filament level is still largely unknown. Recent advances in fluorescent and electron microscopy, as well as work in septin biochemistry has allowed new insights into the aspects of septin architecture, remodeling, and function in many cell types. This mini review highlights many of the recent findings with an emphasis on the budding yeast model.
Keywords: septin, scaffold, diffusion barrier, cytokinesis, posttranslational modifications
Introduction
The septin genes (CDC3, CDC10, CDC11, and CDC12) were originally discovered in the classic Hartwell screen for mutants that affect the cell division cycle (CDC) in the budding yeast Saccharomyces cerevisiae (Hartwell 1971). The products of these genes were subsequently found to be GTP-binding proteins arranged into filaments, akin to a cytoskeleton, at the bud neck (Byers and Goetsch 1976). Another septin gene was later characterized, SHS1 (also originally termed SEP7), bringing the mitotic septin total to five in budding yeast (Carroll et al. 1998; Mino et al. 1998). The septins are highly conserved throughout eukaryotes with the exception of land plants (Pan et al. 2007) and are involved in numerous cell processes including cytokinesis, ciliogenesis, morphogenesis, mitosis, and exocytosis (Oh and Bi 2011; Mostowy and Cossart 2012; Dolat et al. 2014). Septins form heterooligomeric complexes that polymerize end-to-end into filaments. These filaments can be further organized into higher-order structures such as rings, hourglasses, and gauzes depending on cell types or cell cycle stages. These structures can serve as scaffolds at discrete cellular locations or membrane-bound diffusion barriers to spatially segregate cell compartments, or often a combination of both (Chant et al. 1995; Longtine et al. 1996; DeMarini et al. 1997; Barral et al. 2000; Takizawa et al. 2000). By remodeling the architecture of septin higher-order structures, cells are able to use the same set of proteins to achieve elaborate control of diverse cellular processes. While septin architecture has become increasingly clear due to recent developments in light and electron microscopy, the mechanisms controlling the assembly and remodeling of septin high-order structures still remain elusive. Recent work has begun to elucidate some of these key mechanisms and will be discussed here.
The septin blueprint: assembling the complexes, filaments, hourglasses, and rings
Septin protofilaments are composed of septin hetero-octamers, the “building blocks”, with the core subunits Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12 and the use of differential terminal subunits Cdc11 or Shs1, giving rise to two distinct septin hetero-oligomers (Bertin et al. 2008; Garcia et al. 2011). Expression of all five septins in E. coli gives rise to symmetric octamers capped with Cdc11 or Shs1 on both ends and never a combination of Cdc11 and Shs1 (Khan et al. 2018). Octamers capped with Cdc11 polymerize end-to-end to form linear filaments that are often paired like a railway track in vitro (Frazier et al. 1998; Bertin et al. 2008) (Fig. 1, upper left). In contrast, the octamers capped with Shs1 appear to polymerize end-to-end into single filaments and/or laterally associate with each other into curved bundles or rings in vitro (Garcia et al. 2011) (Fig. 1, upper left). This distinct property between the octamers might account for the double and single filaments observed in septin higher-order structures in vivo (Bertin et al. 2012; Ong et al. 2014). Much of the knowledge about septin complex formation and filament assembly has been inferred from the crystal structure of human septin complex SEPT2/6/7 and the proposed interactions between septin subunits during filament assembly (Sirajuddin et al. 2007; John et al. 2007; Bertin et al. 2008; Sellin et al. 2011). The crystal structure of the terminal yeast subunit Cdc11 was recently solved and illustrated that some of the interfaces once thought to mediate septin-septin interaction may be different (Brausemann et al. 2016). In fact, the conserved lysine in the GTP-binding P-loop of septins is an arginine in yeast Cdc11, calling into question the necessity of GTP binding and subsequently GTP hydrolysis of yeast septins in filament assembly. To address this, a recent study used Bimolecular Fluorescence Complementation (BiFC) in combination with the drug mycophenolic acid (MPA) to lower the cytosolic GDP and GTP levels to assess the step-wise association of septin subunits (Weems and McMurray 2017). It was discovered that under normal conditions, Cdc10 forms a homodimer before interacting with two Cdc11-Cdc12-Cdc3 trimers in high GTP conditions and Shs1-Cdc12-Cdc3 trimers in low GTP conditions (Weems and McMurray 2017). Cdc10 and Cdc12 were the only yeast septins previously shown to display GTP hydrolysis activity (Versele and Thorner 2004). Analysis of mutations leading to a loss of the guanine nucleotide binding in Cdc10 and Cdc12 by BiFC provided novel insights into septin complex formation. Without a bound nucleotide, Cdc10 loses the high propensity for homodimerization prior to interaction with Cdc3 (Weems and McMurray 2017). In this case, mutant Cdc10 interacts with Cdc3-Cdc12-Cdc11 (or Cdc3-Cdc12-Shs1) trimer to form a tetramer, and then two tetramers interact to form an octamer. This indicates that when the supposed optimal pathway for octamer formation is no longer available, the cells have developed secondary pathways for octamer formation to avoid detrimental effects. This explains the lack of a strong phenotype at low temperatures for the cdc10 mutation (Weems and McMurray 2017). This also defines an adaptive mechanism for the organism’s survival under different cellular GTP levels. For Cdc12, the nucleotide bound state was found to directly control the ability of Cdc11 (in high GTP) vs. Shs1 (in low GTP) to serve as the terminal subunit (Weems and McMurray 2017). Additionally, Shs1 was found to play a critical role in septin architecture formation in low GTP conditions as shs1Δ cells had relatively normal morphology and septin organization in normal glucose conditions, but were elongated and showed improper septin organization when cultured in glycerol (Weems and McMurray 2017). This, when coupled with the knowledge that altering the Cdc11:Shs1 ratios can change the septin ring diameter (Garcia et al. 2011; Weems and McMurray 2017), illustrates a complex mechanism for cells to regulate octamer formation to alter septin higher-order architecture.
Figure 1. The septin cycle in yeast: architecture, remodeling, and function.
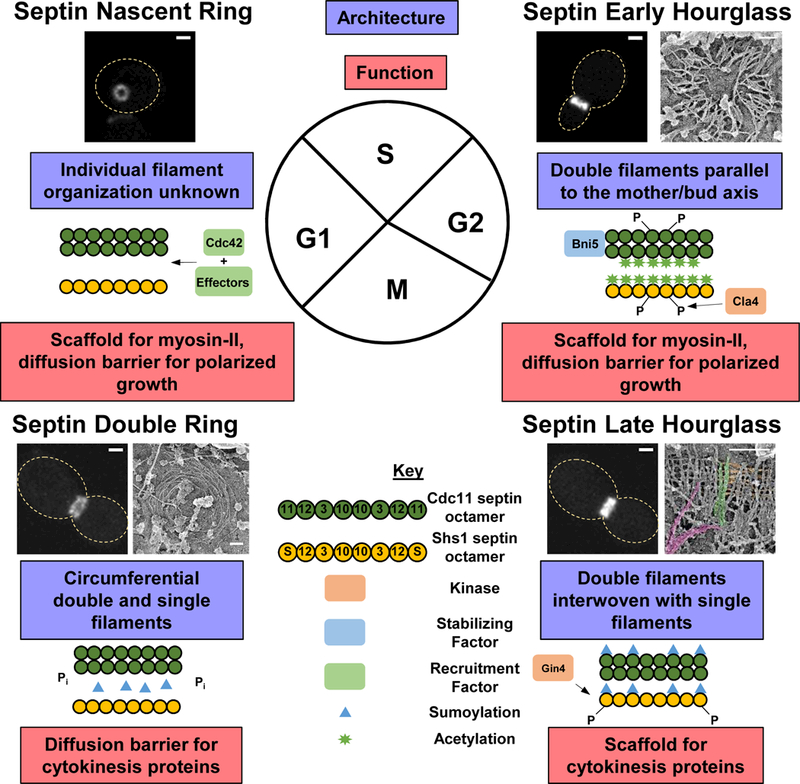
Starting from the upper left and going clockwise: the nascent septin ring forms in late G1 phase, after the launch of a new cell cycle, through Cdc42-controlled septin recruitment (light green boxes) and polarized exocytosis (not depicted here), and the cortical ring serves as a scaffold for myosin-II and a diffusion barrier for polarized growth. The exact filament structure is unknown, but most likely is composed of double filaments formed by Cdc11-capped octamers (green rods) and possibly single Shs1-capped octamers (yellow rods). Next, as the bud emerges and grows in S/G2, the septin structure expands to form an hourglass comprised of double filaments (green rods) that are potentially linked laterally by Shs1-capped octamers (yellow rods). This structure is regulated by interaction with Bni5 (blue box), phosphorylation by Cla4 (orange box), and acetylation (green stars), and the hourglass serves as a scaffold for myosin-II and a diffusion barrier for polarized growth. Third, as the cell enters anaphase, the septin hourglass exhibits a transitional structure that consists of double filaments (green rods in the cartoon and in the EM image) interwoven with single filaments (yellow rods in the cartoon and in the EM image) akin to a gauze-like structure. This transition is regulated by phosphorylation of Shs1 by Gin4 (orange box) and sumoylation of Cdc3, Cdc11, and Shs1 (blue triangles). Lastly, the septins adopt a double ring architecture at the onset of cytokinesis comprised of both double and single filaments arranged circumferentially at the bud neck. This organization is accompanied by a loss of sumoylation and phosphorylation of septins. This structure serves mainly as a diffusion barrier for cytokinesis proteins. In all cases, the light microscopy images are of cells expressing Cdc3-eGFP from its native promoter. The cell cycle timing was determined by bud size and/or spindle morphology. Scale bar, 1 μm. PREM images reproduced from Ong et al. 2014 with permission. Scale bars, 200 nm.
The first septin higher-order structure formed during the yeast cell cycle is a nascent septin ring at the presumed bud site (Fig. 1, upper left). The recruitment of septins to the presumed bud site depends on the small GTPase Cdc42, a master regulator of eukaryotic cell polarity (Cid et al. 2001b; Gladfelter et al. 2002; Caviston et al. 2003; Kadota et al. 2004; Iwase et al. 2006), and involves the function of its effectors Gic1 and Gic2 (Iwase et al. 2006; Sadian et al. 2013). Experimental and modeling studies indicate that a combination of feedback loops between and among Cdc42, septins, and exocytosis gives rise to the recruitment of septins to the presumed bud site, septin ring opening, and daughter cell identity (Okada et al. 2013). Despite these findings, the organization of septin filaments in the nascent septin ring remains unknown.
The second septin higher-order structure formed during the yeast cell cycle is an hourglass at the bud neck, which spans from bud emergence to anaphase (Fig. 1, upper right). The hourglass at the early budding stage, as revealed by platinum-replica electron microscopy (PREM), consists of a layer of membrane-associated, highly packed double filaments of 350 to 400 nm in length that are located at both sides of the bud neck and oriented along the mother-daughter axis (Ong et al. 2014). As Cdc11-capped octamers are rod-shaped and approximately 32-nm long, and have the propensity to form paired filaments in vitro (Frazier et al. 1998; Bertin et al. 2008), the early hourglass is likely mainly made of Cdc11-capped octamers, with 11-to-12 such building blocks constituting each of the paired filament. Shs1 localizes to the presumed bud site and the bud neck as other mitotic septins (Mino et al. 1998; Carroll et al. 1998; Dobbelaere et al. 2003). Shs1-capped octameric rods do not form filaments in vitro (Garcia et al. 2011), but might serve as a linker for the compaction of the double filaments in the early hourglass, a possibility that remains to be tested. The arrangement of double filaments seen by PREM in early budded cells is consistent with previous data obtained by polarized fluorescence microscopy (Vrabioiu and Mitchison 2006; DeMay et al. 2011b) and suggests a rigid structure that is supported by fluorescence-recovery-after-photobleaching (FRAP) analysis of septins in the early hourglass (Caviston et al. 2003; Dobbelaere et al. 2003). However, the mechanism of converting a relatively dynamic nascent septin ring prior to bud emergence to a rigid hourglass after bud emergence remains unknown.
The final septin structure witnessed in the yeast cell cycle is the double ring during cytokinesis. The transition from the hourglass to the double ring may initiate in anaphase and complete at the onset of cytokinesis. As seen by PREM, an array of paired filaments running axially along the mother-daughter axis and interconnecting single filaments running circumferentially to the mother-daughter axis make up a transitional gauze-like structure temporally between the hourglass and double ring (Fig. 1, lower right) (Ong et al. 2014). It is important to note that an earlier study found a gauze-like structure, which consists of circumferentially oriented paired filaments that are interwoven with perpendicularly oriented single filaments along the mother-daughter axis, to be the only hourglass structure in budded cells (Bertin et al. 2012). While this electron-tomography technique preserves the bud-neck shape for visualization (unlike PREM), the use of only asynchronous cell cultures and the fact that a thin layer of membrane-associated septin filaments is surrounded by electron-dense cytoplasm make it challenging to interpret the filament orientation and the timing of the hourglass appearance during the cell cycle. However, it remains possible that, in the PREM study, the circumferential single filaments are actually present in the “early hourglass”, but they are not stable enough to survive the sample-processing conditions. These single filaments might be stabilized by some factors in the “transitional hourglass” (Fig. 1, lower right), which enable their detection by PREM analysis. The double ring consists of both single and double filaments arranged circumferentially (Fig. 1, lower left) (Ong et al. 2014). Live-cell imaging coupled with FRAP, photoactivation, photoconversion, and super resolution microscopy provided a possible mechanism for the architecture change from the gauze-like structure to the double ring (Ong et al. 2014). This dramatic septin remodeling event likely involves rapid disassembly of the parallel double filaments in the transitional hourglass, and re-assembly of the recycled Cdc11-capped octamers into circumferential double filaments coupled with compaction of the circumferential single filaments into a double ring at the external edges of the transitional hourglass (Ong et al. 2014). The disassembly and reassembly of paired septin filaments in different orientations agrees with previous data obtained by both quantitative live imaging (Dobbelaere et al. 2003; Wloka et al. 2011) as well as polarized fluorescent microscopy in which a 90° change in septin positioning was observed (Vrabioiu and Mitchison 2006; DeMay et al. 2011a). Shs1 seems to play a particularly important role in the remodeling process. In shs1Δ cells, very few gauze-like structures and circumferential single filaments were observed, suggesting that Shs1-capped octamers might comprise the single filaments in the transitional structure (Ong et al. 2014). Despite the significant progress in dissecting detailed septin architecture during the cell cycle, the remodeling mechanism for the hourglass-to-double ring transition remains a central fascinating question in the septin field that will surely be addressed with much depth in the future.
Regulating the varieties of septin structures
As alluded to above, assembly and remodeling of the same septin subunits into drastically different higher-ordered structures during the cell cycle must be highly regulated in time and space. The regulation likely occurs through two mechanisms: septin binding proteins and post-translational modifications. Over 100 proteins are known to localize to the bud neck and interact with septins at certain times of the cell cycle (Gladfelter et al. 2001; McMurray and Thorner 2009). A recent proteomics study attempted to identify septin-associated proteins by affinity purification followed by mass spectrometry in yeast cells arrested at different stages of the cell cycle (Renz et al. 2016). This study found 83 septin-associated proteins with some interacting only with the septin complex during a discrete period of the cell cycle. For example, many proteins identified from α-factor-arrested cells represent novel binding partners such as ribosomal RNA processing proteins Nop1, New1, and Rlp7 and nuclear protein Cse1 (Renz et al. 2016). The authors argue that their methods preclude artifacts based on the identification of these interactors only in α-factor-arrested cells only arising in one sample set, confirmation of interactions using a modified and more stringent split-ubiquitin assay (Müller and Johnsson 2008; Moreno et al. 2014; Dünkler et al. 2015), and the use of SILAC labeling prior to mass spectrometry, which should limit artifacts (Ong et al. 2002). While these novel binding partners are potentially very interesting, their interactions with the septins at the bud neck require further investigation. This can be accomplished using a newly developed tripartite split-GFP system that can effectively evaluate the ability of a candidate protein for direct interaction with the septins at the bud neck (Finnigan et al. 2016). The biological significance of such interactions remains to be explored.
Other proteins with known biological functions in regulating septins were also found in the 2016 proteomics study. These include but are not limited to Bni5, Bud3, Bud4, and Gin4 (Renz et al. 2016). Bni5 was found to interact with septins in samples arrested at multiple phases of the cell cycle (Renz et al. 2016). Bni5 is essential for the localization of Myo1, the sole heavy chain of myosin-II in budding yeast, to the bud neck prior to cytokinesis by serving as a linker between the septins and the tail region of Myo1 (Fang et al. 2010; Finnigan et al. 2015). Bni5 dimerizes and regulates the spacing and organization of paired septin filaments by directly interacting with the terminal subunit Cdc11 (Patasi et al. 2015; Booth et al. 2016). Thus, Bni5 is thought to play a key role in stabilizing the septin hourglass. Bud3 and Bud4 were both found to be strong interactors of septins in later stages of the cell cycle (Renz et al. 2016). This finding is consistent with previous studies. Bud3 and Bud4 associate with the septin hourglass in mitosis and then with the double ring during and after cytokinesis (Chant et al. 1995; Sanders and Herskowitz 1996). Both proteins serve as a part of the landmark in directing axial budding (Chant et al. 1995; Sanders and Herskowitz 1996). Both proteins have also been implicated in septin organization (Gladfelter et al. 2005; Wloka et al. 2011; Guo et al. 2011; Eluere et al. 2012; Kang et al. 2013; McQuilken et al. 2017), with Bud4, the anillin-like protein, being involved in the hourglass-to-double ring transition (Wloka et al. 2011; McQuilken et al. 2017).
The kinase Gin4 was also found to strongly interact with the septins in hydroxyurea (S-phase) and cdc15-ts (late anaphase) arrested cells (Renz et al. 2016). Gin4 associates with the septins at the bud neck before cytokinesis and regulates septin organization in mitosis at least in part by phosphorylating Shs1 (Longtine et al. 1998; Mortensen et al. 2002; Asano et al. 2006). Gin4 binds directly to Cdc11 and Shs1, and these interactions may further activate Gin4 kinase activity in mitosis (Mortensen et al. 2002; Asano et al. 2006).
Gin4 is not the only kinase to regulate the septins by phosphorylation. The cyclin dependent kinase Cdc28 as well as the p-21 activated kinase Cla4 phosphorylate Cdc3 and Cdc10, respectively (Tang and Reed 2002; Dobbelaere et al. 2003; Versele and Thorner 2004; Kadota et al. 2004). In total, 59 phosphorylation events have been found to occur on the four septins Cdc3, Cdc10, Cdc11, and Shs1 (Hernández-Rodríguez and Momany 2012). These events are thought to regulate septin higher-order architecture by controlling the disassembly of old septin rings or stabilizing the septin hourglass (Tang and Reed 2002; Versele and Thorner 2004). Due to the plethora of phosphorylation events on septins from unknown kinases and the biological relevance of these phosphorylations being largely unknown (Hernández-Rodríguez and Momany 2012), regulation of septins by kinases remains a highly important topic to study in the field of septin biology.
Besides phosphorylation, septins are also posttranslationally modified by sumoylation and acetylation (Hernández-Rodríguez and Momany 2012; Alonso et al. 2015). Sumoylation of Cdc3, Cdc11, and Shs1 was observed in S. cerevisiae (Johnson and Blobel 1999; Takahashi et al. 2003). Cells carrying sumoylation-deficient alleles of these septin genes displayed no apparent phenotypes, except that ectopic septin rings were associated with the old cell division sites in addition to the bud neck (Johnson and Blobel 1999). This observation suggested that sumoylation of septins might control septin ring disassembly (Johnson and Blobel 1999). However, cells deleted for SIZ1, which encodes the bud neck-localized E3 ligase for sumoylation, failed to produce any phenotypes including formation of the ectopic septin rings, despite the fact that septin sumoylation was nearly abolished in the mutant (Johnson and Gupta 2001). Thus, the biological significance of septin sumoylation in yeast requires further investigation. In mammalian cells, expression of non-sumoylatable septins caused a dramatic defect in cytokinesis in a small, yet significant, population of cells (Ribet et al. 2017). This was attributed to a lack of clearance of large septin bundles from the intracellular bridge during cytokinesis (Ribet et al. 2017), which is strikingly similar to the failed disassembly of the ectopic rings formed by non-sumoylatable septins at the cell division sites in yeast. Together, these observations suggest that sumoylation may play a conserved role in septin filament disassembly, which presumably enables septin architectural remodeling such as the hourglass-to-double ring transition in yeast. A similar hourglass-to-double ring transition might also occur for mammalian septins during cytokinesis, which assume an hourglass-like structure during furrow ingression and then a double ring-like structure during abscission (Estey et al. 2010).
Regulation of septins by acetylation is probably the least studied posttranslational modification. All mitotic septins except Cdc11 are acetylated by the lysine acetyltransferase complex NuA4 (Mitchell et al. 2011). Septins became mislocalized during the nascent ring-to-hourglass transition in NuA4 complex deletion mutant strains, implying that acetylation of septins may regulate septin hourglass formation or its stability (Mitchell et al. 2011). The authors investigated how the loss of acetylation on specific septins could influence septin localization and function using a C-terminal truncation of Shs1 that loses a majority of acetylation sites. They conceded that many of their phenotypes (including elongated bud morphology and septin mislocalization) associated with this truncation allele are complicated by the fact that this region of Shs1 is also heavily phosphorylated and sumoylated (Egelhofer et al. 2008; Garcia et al. 2011; Mitchell et al. 2011; Hernández-Rodríguez and Momany 2012).
The functional significance of the septin architectures
The septins can serve as both scaffolds for proteins involved in cytokinesis and other processes and barriers for diffusion of membrane-associated proteins (Fig. 1). The septin ring at the presumptive bud site as well as the septin hourglass are required for the recruitment and retention of cytokinesis proteins at the bud neck either through direct interactions, for example, the F-BAR protein Hof1 (Oh et al. 2013; Meitinger et al. 2013) or through septin-binding proteins such as Bni5 that links septin filaments to the myosin-II heavy chain Myo1 (Fang et al. 2010; Schneider et al. 2013; Finnigan et al. 2015). Myo1 is required for actin ring formation (Bi et al. 1998). Thus, the septin ring and hourglass scaffold the assembly of the actomyosin ring (AMR) before cytokinesis (Bi et al. 1998; Schneider et al. 2013). The septin hourglass also acts as a scaffold for the morphogenesis checkpoint kinase Hsl1, which, in turn, regulates localization of the downstream component Hsl7 and the effector Wee1-like kinase Swe1 (an inhibitor of CDK1/mitotic cyclins) (Barral et al. 1999; Shulewitz et al. 1999; Longtine et al. 2000; Cid et al. 2001a). This checkpoint module (Hsl1-Hsl7-Swe1) is strategically assembled at the daughter side of the bud neck to monitor bud emergence and morphogenesis and it is activated and delays the entry into mitosis when the morphogenetic process is defective (Keaton and Lew 2006; Howell and Lew 2012). The septin hourglass also serves as a scaffold for many other processes such as bud-site selection and chitin ring synthesis at the mother side of the bud neck during early stage of budding (Longtine et al. 1996; Gladfelter et al. 2001; McMurray and Thorner 2009; Oh and Bi 2011). In animal cells, the septins provide a scaffold for myosin-II and its activating kinases during cytokinesis (Joo et al. 2007). Anillin-dependent organization of septin filaments at the division site also plays a critical role in the maturation of the midbody as well as its anchoring to the plasma membrane (Kechad et al. 2012; El Amine et al. 2013). These septin filaments also promote elongation of the intercellular bridge as well as targeting of the ESCRT-III component CHMP4B to the abscission site (Renshaw et al. 2014). Thus, through their ability to form different higher-order structures, septins are able to function as perfect scaffolds for protein machinery at distinct membranous localities in a variety of situations.
Evidence for the diffusion-barrier model of septin function also comes from work done primarily in budding yeast. During bud growth, the septin hourglass serves to compartmentalize the bud from the mother for membrane or membrane-associated proteins involved in the maintenance of cell polarity or organelle identity (Barral et al. 2000; Takizawa et al. 2000; Luedeke et al. 2005; Shcheprova et al. 2008). Even the nascent septin ring could function as a barrier to restrict Cdc42 activity and exocytosis within the lumen of the ring to control bud emergence and growth (Okada et al. 2013). During cytokinesis, the septin double ring is thought to function as a diffusion barrier that encapsulates diffusible cytokinesis proteins such as the exocyst component Sec3 and chitin synthase II (Chs2) at the division site (Dobbelaere and Barral 2004). The fact that only mild defects in cytokinesis are observed in cells lacking one side of the septin double ring or the entire structure has raised questions as to the necessity of this barrier function in yeast (Wloka et al. 2011). Additionally, Ist2, the marker used for inferring a septin-dependent diffusion barrier at the plasma membrane of the division site (Takizawa et al. 2000), was later found to be an endoplasmic-reticulum (ER) protein (Manford et al. 2012). The compartmentalization seen in the initial study is actually due to the presence of a septin-dependent diffusion barrier at the ER membrane (Manford et al. 2012). This diffusion barrier is critical for preventing the segregation of misfolded proteins from the mother to daughter compartment (Clay et al. 2014; Chao et al. 2014). Septins are also suggested to function as diffusion barriers in different mammalian cell processes such as compartmentalization of the sperm tail (Kissel et al. 2005; Ihara et al. 2005); the forming cilia (Hu et al. 2010), although caution should be taken for this interpretation (Palander et al. 2017); or during dendritic branching in neurons (Xie et al. 2007; Tada et al. 2007). This highlights the conservation of septin-based barriers beyond a specific model system.
Septins appear to play a critical role in modifying cell shape. In animal cells, this function likely occurs through an interaction with the actin cytoskeleton. In mammalian cells, septin filaments co-align with actin filaments in the stress fibers, and these filaments are interdependent for their assembly and organization (Kinoshita et al. 2002; Schmidt and Nichols 2004). HeLa cells show multiple plasma membrane abnormalities upon knockdown of septins in the presence of Latrunculin B, a drug that disrupts actin filaments (Mostowy et al. 2011). Drosophila septin complex crosslinks and bends actin filaments into ring-like structures in vitro and is required for maintaining cell shape during embryogenesis (Mavrakis et al. 2014). In yeast, it is still unclear if septins respond to or influence cell shape. Yeast cells containing a septin mutation exhibit an elongated bud morphology indicative of a role of the septins in regulating cell shape (Hartwell 1971; Adams and Pringle 1984). Yeast septins can also respond to changes in cell curvature as suggested in recent studies in which septin higher-order assembly favored positive curvature at the forming bud neck during bud emergence (Bridges et al. 2016; Kang et al. 2016). It is possible that septins can both detect and modulate cell shape based on their ability to form various higher-order structures that can be dynamically remodeled.
The most striking use of septins that incorporates all the functions discussed above is in the process of fungal pathogenesis. In species such as the opportunistic human pathogen Candida albicans and the rice blast fungus Magnaporthe oryzae, loss of septins or septin architecture results in a significant loss of pathogenicity (Warenda and Konopka 2002; Warenda et al. 2003; Dagdas et al. 2012; Momany and Talbot 2017). In M. oryzae, septins assemble into a ring structure at the base of an infectious peg that is used to penetrate the plant cell, and this septin ring scaffolds the assembly of a toroidal F-actin network that is required for pathogenesis (Dagdas et al. 2012). The septin ring also acts as a lateral diffusion barrier that prevents polarized growth factors from leaving the growing peg (Dagdas et al. 2012). Finally, the septin ring might sensor the membrane curvature and stabilize the neck of the penetration peg, thereby, restricting the protrusive structure to a single location (Dagdas et al. 2012; Momany and Talbot 2017). In summary, septins in different eukaryotic systems employ distinct architecture and dynamic remodeling to regulate diverse cell and developmental processes.
Concluding Remarks
The septins have been investigated since their discovery in the 1970s as critical regulators of cytokinesis, cell morphogenesis, and other processes. Key questions concerning the architecture, remodeling, and functions of the septin cytoskeleton are beginning to be answered largely due to the development of new imaging and gene-editing technologies. The budding yeast S. cerevisiae remains an effective model for defining new concepts and mechanisms for septin structure and function. For example, it is still unclear how various septin higher-order structures such as rings, hourglasses, and gauzes are assembled at the molecular and filament levels and how these structures are remodeled in time and space to perform distinct functions during the cell cycle. Continuing studies of septins in pathogenic fungi will help understand the cause and mechanism of infectious diseases in humans and plants. Septin studies in animal cells not only provide invaluable insights into the conservation of septin structure and function, but also lead to new discoveries regrading processes that are not shared by yeast such as cell migration. Septin studies in animal cells will also help understand the cause of septin-related diseases including male infertility, cancer, and neurodegenerative diseases.
Acknowledgments
The authors would like to thank members of the Bi lab, particularly Hiroki Okada, for insights into figure design. This work was supported by the National Institutes of Health grant GM116876 to E.B.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Adams AEM and Pringle JR. 1984. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol 98:934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Greenlee M, Matts J, Kline J, Davis KJ and Miller RK. 2015. Emerging roles of sumoylation in the regulation of actin, microtubules, intermediate filaments, and septins. Cytoskeleton (Hoboken) 72:305–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano S, Park JE, Yu LR, Zhou M, Sakchaisri K, Park CJ, Kang YH, Thorner J, Veenstra TD and Lee KS. 2006. Direct phosphorylation and activation of a Nim1-related kinase Gin4 by Elm1 in budding yeast. J Biol Chem 281:27090–8. [DOI] [PubMed] [Google Scholar]
- Barral Y, Parra M, Bidlingmaier S and Snyder M. 1999. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev 13:176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS and Snyder M. 2000. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell 5:841–51. [DOI] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Grob P, Park SS, Garcia G 3rd, Patanwala I, Ng HL, Alber T, Thorner J and Nogales E 2008. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci USA 105:8274–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Pierson J, Thai L, McDonald KL, Zehr EA, Garcia G 3rd, Peters P, Thorner J and Nogales E 2012. Three-dimensional ultrastructure of the septin filament network in Saccharomyces cerevisiae. Mol Biol Cell 23:423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E and Pringle JR. 1998. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol 142:1301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth EA, Sterling SM, Dovala D, Nogales E and Thorner J. 2016. Effects of Bni5 binding on septin filament organization. J Mol Biol 428:4962–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brausemann A, Gerhardt S, Schott A-K, Einsle O, Große-Berkenbusch A, Johnsson N and Gronemeyer T. 2016. Crystal structure of Cdc11, a septin subunit from Saccharomyces cerevisiae. Journal of structural biology 193:157–61. [DOI] [PubMed] [Google Scholar]
- Bridges AA, Jentzsch MS, Oakes PW, Occhipinti P and Gladfelter AS. 2016. Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J Cell Biol 213:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B and Goetsch L. 1976. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol 69:717–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CW, Altman R, Schieltz D, Yates JR and Kellogg D. 1998. The septins are required for the mitosis-specific activation of the Gin4 kinase. J Cell Biol 143:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Longtine M, Pringle JR and Bi E. 2003. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol Biol Cell 14:4051–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J, Mischke M, Mitchell E, Herskowitz I and Pringle JR. 1995. Role of Bud3p in producing the axial budding pattern of yeast. J Cell Biol 129:767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao JT, Wong AK, Tavassoli S, Young BP, Chruscicki A, Fang NN, Howe LJ, Mayor T, Foster LJ and Loewen CJ. 2014. Polarization of the endoplasmic reticulum by ER-septin tethering. Cell 158:620–32. [DOI] [PubMed] [Google Scholar]
- Cid VJ, Shulewitz MJ, McDonald KL and Thorner J. 2001a. Dynamic localization of the Swe1 regulator Hsl7 during the Saccharomyces cerevisiae cell cycle. Mol Biol Cell 12:1645–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid VJ, Adamikova L, Sanchez M, Molina M and Nombela C. 2001b. Cell cycle control of septin ring dynamics in the budding yeast. Microbiology 147:1437–50. [DOI] [PubMed] [Google Scholar]
- Clay L, Caudron F, Denoth-Lippuner A, Boettcher B, Buvelot Frei S, Snapp EL and Barral Y. 2014. A sphingolipid-dependent diffusion barrier confines ER stress to the yeast mother cell. Elife 3:e01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagdas YF, Yoshino K, Dagdas G, Ryder LS, Bielska E, Steinberg G and Talbot NJ. 2012. Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science 336:1590–95. [DOI] [PubMed] [Google Scholar]
- DeMarini DJ, Adams AEM, Fares H, De Virgilio C, Valle G, Chuang JS and Pringle JR. 1997. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol 139:75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMay BS, Noda N, Gladfelter AS and Oldenbourg R. 2011a. Rapid and quantitative imaging of excitation polarized fluorescence reveals ordered septin dynamics in live yeast. Biophys J 101:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMay BS, Bai X, Howard L, Occhipinti P, Meseroll RA, Spiliotis ET, Oldenbourg R and Gladfelter AS. 2011b. Septin filaments exhibit a dynamic, paired organization that is conserved from yeast to mammals. J Cell Biol 193:1065–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J and Barral Y. 2004. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science 305:393–96. [DOI] [PubMed] [Google Scholar]
- Dobbelaere J, Gentry MS, Hallberg RL and Barral Y. 2003. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev Cell 4:345–57. [DOI] [PubMed] [Google Scholar]
- Dolat L, Hu Q and Spiliotis ET. 2014. Septin functions in organ system physiology and pathology. Biol Chem 395:123–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dünkler A, Rösler R, Kestler HA, Moreno-Andrés D and Johnsson N. 2015. SPLIFF: A single-cell method to map protein-protein interactions in time and space. Vol. 1346; p 151–68. [DOI] [PubMed] [Google Scholar]
- Egelhofer TA, Villen J, McCusker D, Gygi SP and Kellogg DR. 2008. The septins function in G1 pathways that influence the pattern of cell growth in budding yeast. PLoS ONE 3:e2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Amine N, Kechad A, Jananji S and Hickson GRX. 2013. Opposing actions of septins and Sticky on anillin promote the transition from contractile to midbody ring. J Cell Biol 203:487–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluere R, Varlet I, Bernadac A and Simon MN. 2012. Cdk and the anillin homolog Bud4 define a new pathway regulating septin organization in yeast. Cell Cycle 11:151–8. [DOI] [PubMed] [Google Scholar]
- Estey MP, Di Ciano-Oliveira C, Froese CD, Bejide MT and Trimble WS. 2010. Distinct roles of septins in cytokinesis: SEPT9 mediates midbody abscission. J Cell Biol 191:741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Luo J, Nishihama R, Wloka C, Dravis C, Travaglia M, Iwase M, Vallen EA and Bi E. 2010. Biphasic targeting and cleavage furrow ingression directed by the tail of a myosin-II. J Cell Biol 191:1333–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnigan GC, Booth EA, Duvalyan A, Liao EN and Thorner J. 2015. The carboxy-terminal tails of septins Cdc11 and Shs1 recruit myosin-II binding factor Bni5 to the bud neck in Saccharomyces cerevisiae. Genetics 200:843–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnigan GC, Duvalyan A, Liao EN, Sargsyan A and Thorner J. 2016. Detection of protein-protein interactions at the septin collar in Saccharomyces cerevisiae using a tripartite split-GFP system. Mol Biol Cell 27:2708–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Wong ML, Longtine MS, Pringle JR, Mann M, Mitchison TJ and Field C. 1998. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J Cell Biol 143:737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia G 3rd, Bertin A, Li Z, Song Y, McMurray MA, Thorner J and Nogales E 2011. Subunit-dependent modulation of septin assembly: budding yeast septin Shs1 promotes ring and gauze formation. J Cell Biol 195:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter AS, Pringle JR and Lew DJ. 2001. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol 4:681–89. [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Kozubowski L, Zyla TR and Lew DJ. 2005. Interplay between septin organization, cell cycle and cell shape in yeast. J Cell Sci 118:1617–28. [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Bose I, Zyla TR, Bardes ESG and Lew DJ. 2002. Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J Cell Biol 156:315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Gong T and Gao XD. 2011. Identification of an amphipathic helix important for the formation of ectopic septin spirals and axial budding in yeast axial landmark protein Bud3p. PLoS One 6:e16744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH. 1971. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res 69:265–76. [DOI] [PubMed] [Google Scholar]
- Hernández-Rodríguez Y and Momany M. 2012. Posttranslational modifications and assembly of septin heteropolymers and higher-order structures. Curr Opin Microbiol 15:660–68. [DOI] [PubMed] [Google Scholar]
- Howell AS and Lew DJ. 2012. Morphogenesis and the cell cycle. Genetics 190:51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET and Nelson WJ. 2010. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329:436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O et al. 2005. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell 8:343–52. [DOI] [PubMed] [Google Scholar]
- Iwase M, Luo J, Nagaraj S, Longtine M, Kim HB, Haarer BK, Caruso C, Tong Z, Pringle JR and Bi E. 2006. Role of a Cdc42p effector pathway in recruitment of the yeast septins to the presumptive bud site. Mol Biol Cell 17:1110–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CM, Hite RK, Weirich CS, Fitzgerald DJ, Jawhari H, Faty M, Schlapfer D, Kroschewski R, Winkler FK, Walz T et al. 2007. The Caenorhabditis elegans septin complex is nonpolar. EMBO J 26:3296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ES and Blobel G. 1999. Cell cycle-regulated attachment of the ubiquitin-related protein SUMO to the yeast septins. J Cell Biol 147:981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ES and Gupta AA. 2001. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 106:735–44. [DOI] [PubMed] [Google Scholar]
- Joo E, Surka MC and Trimble WS. 2007. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev Cell 13:677–90. [DOI] [PubMed] [Google Scholar]
- Kadota J, Yamamoto T, Yoshiuchi S, Bi E and Tanaka K. 2004. Septin ring assembly requires concerted action of polarisome components, a PAK kinase Cla4p, and the actin cytoskeleton in Saccharomyces cerevisiae. Mol Biol Cell 15:5329–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Tsygankov D and Lew DJ. 2016. Sensing a bud in the yeast morphogenesis checkpoint: a role for Elm1. Mol Biol Cell 27:1764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang PJ, Hood-DeGrenier JK and Park HO. 2013. Coupling of septins to the axial landmark by Bud4 in budding yeast. J Cell Sci 126:1218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaton MA and Lew DJ. 2006. Eavesdropping on the cytoskeleton: progress and controversy in the yeast morphogenesis checkpoint. Curr Opin Microbiol 9:540–46. [DOI] [PubMed] [Google Scholar]
- Kechad A, Jananji S, Ruella Y and Hickson GRX. 2012. Anillin acts as a bifunctional linker coordinating midbody ring biogenesis during cytokinesis. Curr Biol 22:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Newby J and Gladfelter AS. 2018. Control of septin filament flexibility and bundling by subunit composition and nucleotide interactions. Mol Biol Cell 29:702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Field CM, Coughlin ML, Straight AF and Mitchison TJ. 2002. Self- and actin-templated assembly of Mammalian septins. Dev Cell 3:791–802. [DOI] [PubMed] [Google Scholar]
- Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR and Steller H. 2005. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell 8:353–64. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Fares H and Pringle JR. 1998. Role of the yeast Gin4p protein kinase in septin assembly and the relationship between septin assembly and septin function. J Cell Biol 143:719–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, Theesfeld CL, McMillan JN, Weaver E, Pringle JR and Lew DJ. 2000. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol Cell Biol 20:4049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C and Pringle JR. 1996. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol 8:106–19. [DOI] [PubMed] [Google Scholar]
- Luedeke C, Frei SB, Sbalzarini I, Schwarz H, Spang A and Barral Y. 2005. Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J Cell Biol 169:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manford AG, Stefan CJ, Yuan HL, Macgurn JA and Emr SD. 2012. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev Cell 23:1129–40. [DOI] [PubMed] [Google Scholar]
- Mavrakis M, Azou-Gros Y, Tsai F-C, Alvarado J, Bertin A, Iv F, Kress A, Brasselet S, Koenderink GH and Lecuit T. 2014. Septins promote F-actin ring formation by crosslinking actin filaments into curved bundles. Nat Cell Biol 16:322–34. [DOI] [PubMed] [Google Scholar]
- McMurray MA and Thorner J. 2009. Septins: molecular partitioning and the generation of cellular asymmetry. Cell Div 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuilken M, Jentzsch MS, Verma A, Mehta SB, Oldenbourg R and Gladfelter AS. 2017. Analysis of septin reorganization at cytokinesis using polarized fluorescence microscopy. Front Cell Dev Biol 5:42–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitinger F, Palani S, Hub B and Pereira G. 2013. Dual function of the NDR-kinase Dbf2 in the regulation of the F-BAR protein Hof1 during cytokinesis. Mol Biol Cell 24:1290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino A, Tanaka K, Kamei T, Umikawa M, Fujiwara T and Takai Y. 1998. Shs1p: a novel member of septin that interacts with Spa2p, involved in polarized growth in Saccharomyces cerevisiae. Biochem Biophys Res Comm 251:732–6. [DOI] [PubMed] [Google Scholar]
- Mitchell L, Lau A, Lambert J-P, Zhou H, Fong Y, Couture J-F, Figeys D and Baetz K. 2011. Regulation of septin dynamics by the Saccharomyces cerevisiae lysine acetyltransferase NuA4. PLoS ONE 6:e25336–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momany M and Talbot NJ. 2017. Septins focus cellular growth for host infection by pathogenic fungi. Front Cell Dev Biol 5:33–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno D, Neller J, Kestler HA, Kraus J, Dunkler A and Johnsson N. 2014. A fluorescent reporter for mapping cellular protein-protein interactions in time and space. Mol Syst Biol 9:647–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen EM, McDonald H, Yates J 3rd and Kellogg DR 2002. Cell cycle-dependent assembly of a Gin4-septin complex. Mol Biol Cell 13:2091–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S and Cossart P. 2012. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol 13:183–94. [DOI] [PubMed] [Google Scholar]
- Mostowy S, Janel S, Forestier C, Roduit C, Kasas S, Pizarro-Cerda J, Cossart P and Lafont F. 2011. A role for septins in the interaction between the Listeria monocytogenes invasion protein InlB and the Met receptor. Biophys J 100:1949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J and Johnsson N. 2008. Split-ubiquitin and the split-protein sensors: chessman for the endgame. Chembiochem: a Eur J Chem Biol 9:2029–38. [DOI] [PubMed] [Google Scholar]
- Oh Y and Bi E. 2011. Septin structure and function in yeast and beyond. Trends Cell Biol 21:141–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Schreiter J, Nishihama R, Wloka C and Bi E. 2013. Targeting and functional mechanisms of the cytokinesis-related F-BAR protein Hof1 during the cell cycle. Mol Biol Cell 24:1305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Leda M, Hanna J, Savage NS, Bi E and Goryachev AB. 2013. Daughter cell identity emerges from the interplay of Cdc42, septins, and exocytosis. Dev Cell 26:148–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong K, Wloka C, Okada S, Svitkina T and Bi E. 2014. Architecture and dynamic remodelling of the septin cytoskeleton during the cell cycle. Nat Commun 5:5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S-E, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A and Mann M. 2002. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Prot 1:376–86. [DOI] [PubMed] [Google Scholar]
- Palander O, El-Zeiry M and Trimble WS. 2017. Uncovering the roles of septins in cilia. Front Cell Dev Biol 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Malmberg RL and Momany M. 2007. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol 7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patasi C, Godocikova J, Michlikova S, Nie Y, Kacerikova R, Kvalova K, Raunser S and Farkasovsky M. 2015. The role of Bni5 in the regulation of septin higher-order structure formation. Biol Chem 396:1325–37. [DOI] [PubMed] [Google Scholar]
- Renshaw MJ, Liu J, Lavoie BD and Wilde A. 2014. Anillin-dependent organization of septin filaments promotes intercellular bridge elongation and CHMP4B targeting to the abscission site. Open Biol 4:130190–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz C, Oeljeklaus S, Grinhagens S, Warscheid B, Johnsson N and Gronemeyer T. 2016. Identification of cell cycle dependent interaction partners of the septins by quantitative mass spectrometry. Plos One 11:e0148340–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribet D, Boscaini S, Cauvin C, Siguier M, Mostowy S, Echard A and Cossart P. 2017. SUMOylation of human septins is critical for septin filament bundling and cytokinesis. J Cell Biol 216:4041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadian Y, Gatsogiannis C, Patasi C, Hofnagel O, Goody RS, Farkasovsky M and Raunser S. 2013. The role of Cdc42 and Gic1 in the regulation of septin filament formation and dissociation. Elife 2:e01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SL and Herskowitz I. 1996. The Bud4 protein of yeast, required for axial budding, is localized to the mother/bud neck in a cell cycle-dependent manner. J Cell Biol 134:413–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K and Nichols BJ. 2004. Functional interdependence between septin and actin cytoskeleton. BMC Cell Biol 5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Grois J, Renz C, Gronemeyer T and Johnsson N. 2013. Septin rings act as template for myosin higher-order structures and inhibit redundant polarity establishment. J Cell Sci 126:3390–400. [DOI] [PubMed] [Google Scholar]
- Sellin ME, Sandblad L, Stenmark S and Gullberg M. 2011. Deciphering the rules governing assembly order of mammalian septin complexes. Mol Biol Cell 22:3152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheprova Z, Baldi S, Frei SB, Gonnet G and Barral Y. 2008. A mechanism for asymmetric segregation of age during yeast budding. Nature (London) 454:728–34. [DOI] [PubMed] [Google Scholar]
- Shulewitz MJ, Inouye CJ and Thorner J. 1999. Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol Cell Biol 19:7123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, Weyand M, Stark H and Wittinghofer A. 2007. Structural insight into filament formation by mammalian septins. Nature (London) 449:311–5. [DOI] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D and Sheng M. 2007. Role of septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol 17:1752–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Toh-E A and Kikuchi Y. 2003. Comparative analysis of yeast PIAS-type SUMO ligases in vivo and in vitro. J Biochem 133:415–22. [DOI] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE and Vale RD. 2000. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290:341–44. [DOI] [PubMed] [Google Scholar]
- Tang CS and Reed SI. 2002. Phosphorylation of the septin Cdc3 in G1 by the Cdc28 kinase is essential for efficient septin ring disassembly. Cell Cycle 1:42–9. [PubMed] [Google Scholar]
- Versele M and Thorner J. 2004. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J Cell Biol 164:701–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabioiu AM and Mitchison TJ. 2006. Structural insights into yeast septin organization from polarized fluorescence microscopy. Nature (London) 443:466–69. [DOI] [PubMed] [Google Scholar]
- Warenda AJ and Konopka JB. 2002. Septin function in Candida albicans morphogenesis. Mol Biol Cell 13:2732–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenda AJ, Kauffman S, Sherrill TP, Becker JM and Konopka JB. 2003. Candida albicans septin mutants are defective for invasive growth and virulence. Infect Immun 71:4045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems A and McMurray M. 2017. The step-wise pathway of septin heterooctamer assembly in budding yeast. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloka C, Nishihama R, Onishi M, Oh Y, Hanna J, Pringle JR, Krauss M and Bi E. 2011. Evidence that a septin diffusion barrier is dispensable for cytokinesis in budding yeast. Biol Chem 392(8–9):813–29. [DOI] [PubMed] [Google Scholar]
- Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P and Kiebler MA. 2007. The GTP-binding protein septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol 17:1746–51. [DOI] [PubMed] [Google Scholar]


