Abstract
HIV-associated neurocognitive disorders (HAND) occur in ~50% of HIV infected individuals despite combined antiretroviral therapy. Transmigration into the CNS of CD14+CD16+ monocytes, particularly those that are HIV infected and express increased surface chemokine receptor CCR2, contributes to neuroinflammation and HAND. To examine whether in HIV infected individuals CCR2 on CD14+CD16+ monocytes serves as a potential peripheral blood biomarker of HAND, we examined a cohort of 45 HIV infected people. We correlated CCR2 on CD14+CD16+ monocytes with cognitive status, proton magnetic resonance spectroscopy (1H-MRS) measured neurometabolite levels, and peripheral blood mononuclear cell (PBMC) HIV DNA copies. We determined that CCR2 was increased specifically on CD14+CD16+ monocytes from people with HAND (median [interquartile range (IQR)]) (63.3 [51.6, 79.0]), compared to those who were not cognitively impaired (38.8 [26.7, 56.4]) or those with neuropsychological impairment due to causes other than HIV (39.8 [30.2, 46.5]). CCR2 was associated with neuronal damage, based on the inverse correlation of CCR2 on CD14+CD16+ monocytes with total N-Acetyl Aspartate (tNAA)/total Creatine (tCr) (r2 = 0.348, p = 0.01) and Glutamine-Glutamate (Glx)/tCr (r2 = 0.356, p = 0.01) in the right and left caudate nucleus, respectively. CCR2 on CD14+CD16+ monocytes also correlated with PBMC HIV DNA copies (ρ = 0.618, p = 0.02) that has previously been associated with HAND. These findings suggest that CCR2 on CD14+CD16+ monocytes may be a peripheral blood biomarker of HAND, indicative of increased HIV infected CD14+CD16+ monocyte entry into the CNS that possibly increases the macrophage viral reservoir and contributes to HAND.
Keywords: HAND; CD14+CD16+ Monocytes; CCR2, MRI/MRS; HIV; ddPCR
Introduction
HIV-associated neurocognitive disorders (HAND) are present in up to 50% of HIV infected individuals in the combined antiretroviral therapy (cART) era, despite cART adherence and widespread suppression of plasma viral loads (Heaton et al., 2010; Cysique and Brew, 2011; Saylor et al., 2016; Gott et al., 2017). On neuroimaging, low level neuroinflammation and progressive neuronal damage may be seen in cART treated cohorts. Neuronal changes are postulated to mediate cognitive dysfunction (Kaul et al., 2001; Ellis et al., 2007; Harezlak et al., 2011; Gongvatana et al., 2013). Central nervous system (CNS) inflammation and damage is mediated, in part, by HIV infection of the brain, which occurs within 4–8 days after peripheral infection, and by transmigration of monocytes into the CNS (Witwer et al., 2009; Burdo et al., 2010; Valcour et al., 2012; Campbell et al., 2014). More specifically, the mature CD14+CD16+ monocyte subset is strongly associated with HAND (Pulliam et al., 1997; Fischer-Smith et al., 2001; Valcour et al., 2010; Burdo et al., 2011; Williams et al., 2012; Williams et al., 2013). CD14+CD16+ monocytes can be infected by HIV and these cells preferentially transmigrate across the blood brain barrier (BBB), and some of these cells may differentiate into macrophages within the brain parenchyma and potentially contribute to continued viral (re-)seeding of the CNS viral reservoir (Veenstra et al., 2017). There are no biomarkers or therapeutics for HAND currently in clinical use. However, since neurocognitive testing requires specialized expertise, is laborious, and cannot definitively assign a causal mechanism for clinical deficits (Tedaldi et al., 2015), a peripheral biomarker that identifies those at risk of HAND would be invaluable.
The entry of mature CD14+CD16+ monocytes into the CNS is mediated by increased levels of chemokines in the brains of HIV infected individuals despite cART, including the chemokine (C-C motif) ligand 2 (CCL2) (Eugenin et al., 2006; Kamat et al., 2012; Williams et al., 2013). The chemokine receptor for CCL2 on monocytes is CCR2 (Williams et al., 2014). Our laboratory showed previously that CCR2 is increased on the CD14+CD16+ monocyte subset in the periphery from those with HAND, and that these cells from people with HAND transmigrate in much higher numbers across the blood brain barrier (BBB) to CCL2 than do cells from HIV infected people who are not cognitively impaired (NI) (Williams et al., 2014). In addition, we showed that HIV infected CD14+CD16+ monocyte transmigration is preferentially blocked by cenicriviroc, a CCR2/CCR5 dual antagonist (Veenstra et al., 2017). This suggests that HAND may be mediated, in part, by increased surface CCR2 on HIV infected CD14+CD16+ monocytes, providing a mechanism that promotes the entry of these cells into the CNS. This may result in an increase in HIV infected macrophages within the CNS, possibly contributing to the reseeding of viral reservoirs and the development of HAND.
To characterize further CCR2 on CD14+CD16+ monocytes as a potential biomarker specific for HAND, we examined CCR2 on CD14+CD16+ monocytes in the peripheral blood of HIV infected individuals from the Manhattan HIV Brain Bank (MHBB) cohort who were all prescribed cART, and were NI, had HAND, or had neuropsychological impairment due to causes other than HIV (NPI-O). We performed proton magnetic resonance spectroscopy (1H-MRS) studies on a smaller group of individuals from the cohort in a pilot study to determine whether there were direct associations between neurometabolite levels and surface CCR2 on peripheral CD14+CD16+ monocytes. In addition, increased HIV DNA in peripheral blood mononuclear cells (PBMC) has been associated with HAND with some studies proposing that the primary source of HIV DNA in PBMC are CD14+CD16+ monocytes (Shiramizu et al., 2005; Kusao et al., 2012; Cysique et al., 2015). To examine the relationship between HAND, CCR2 on CD14+CD16+ monocytes, and PBMC HIV DNA, we performed association studies between the aforementioned variables that were characterized in our cohort.
Materials and Methods
Study subjects and sample collection
All study participants were HIV infected, prescribed cART, and were part of the MHBB cohort (U24MH100931). Enrolled participants were older than 18 years of age and their demographic and virologic characteristics are presented in Table 1. Participant’s cART regimen descriptions, as well as year of, and years from, diagnosis and commencement of cART are detailed in Table 2. All individuals gave written, informed consent for the provision of blood for the purposes of research. The Mount Sinai Program for the Protection of Human Subjects Institutional Review Board, and The Institutional Review Board at the Albert Einstein College of Medicine approved the protocols under which these samples were obtained and utilized. A subset of study participants underwent neuroimaging as part of a Mount Sinai Brain Imaging Center-sponsored pilot project, and gave written, informed consent for MRI/1H-MRS procedures. Neuroimaging protocols were approved under a separate pilot protocol by The Mount Sinai Program for the Protection of Human Subjects Institutional Review Board. Blood samples from all individuals assessed for cognitive status were drawn on the same day as neuropsychological testing. Neuropsychological testing was performed as described previously (Byrd et al., 2013; Robbins et al., 2014). For the neuroimaging substudy, blood samples were drawn on the day of the radiologic procedure; the neuroimaging was performed within a one month period of the neuropsychological testing.
Table 1.
Demographic and clinical characteristics
| MHBB cohort characteristics | Overall (N = 45) Median [IQR] or n (%) |
|---|---|
| Age (yr) | 56 [47, 65] |
| Gender | |
| Female | 24 (53.3%) |
| Male | 21 (46.7%) |
| Race | |
| Black | 18 (40.0%) |
| Hispanic | 18 (40.0%) |
| White | 9 (20.0%) |
| Years from HIV diagnosis | 21 [3, 30] |
| Years from starting cART | 15 [0, 24] |
| Years between HIV diagnosis and starting cART | 4 [0 , 23] |
| HAND | 21 (46.7%) |
| CD4 T cell count (cells/mm3) | 475 [0, 989] |
| Nadir CD4 T cell count (cells/mm3) | 67 [0, 261] |
| Plasma HIV RNA (log10 copies/ml) | 1.4 [1.4, 2.1] |
| Viral load undetectable (<20 copies/ml) | 19 (42.2%) |
| Viral load >20 - <400 copies/ml | 20 (44.4%) |
| Viral load >400 copies/ml | 6 (13.3%) |
Table 2.
Description of cART regimens and HIV and HAND diagnoses
| Study Participant | HAND Diagnosis | cART at Draw | Regimen Base | PI y/n | Yr Dx | Year Started Any ART | Time From Diagnosis | Time From Starting ART | Time From Diagnosis to Starting ART |
|---|---|---|---|---|---|---|---|---|---|
| 1 | No Impairment | Truvada (viread, emtriva), raltegravir | INT | no | 1996 | 1996 | 16 | 16 | 0 |
| 2 | MCMD | epzicom (ziagen, epivir), rit darunavir, raltegravir | PI, INT | yes | 1991 | 1992 | 21 | 20 | 1 |
| 3 | No Impairment | atripla (viread, emtriva, efavirenz) | NNRTI | no | 1984 | 1988 | 28 | 24 | 4 |
| 4 | MCMD | epivir, abacavir, raltegravir | INT | no | 1992 | 1997 | 20 | 15 | 5 |
| 5 | HAD | Epzicom (ziagen, epivir), atazanavir | PI | yes | 1993 | 1998 | 19 | 14 | 5 |
| 6 | ANI | tenofovir, rit darunavir, raltegravir | PI, INT | yes | 1992 | 1993 | 20 | 19 | 1 |
| 7 | MCMD | Epzicom (ziagen, epivir), efavirenz | NNRTI | no | 1994 | 1996 | 18 | 16 | 2 |
| 8 | HAD | truvada (viread, emtriva), rit atazanavir | PI | yes | 1991 | 2010 | 21 | 2 | 19 |
| 9 | No Impairment | truvada (viread, emtriva), rit darunavir | PI | yes | 1993 | 1997 | 19 | 15 | 4 |
| 10 | MCMD | Truvada (viread, emtriva), raltegravir | INT | no | 2002 | 2003 | 10 | 9 | 1 |
| 11 | NPI-O | non-compliant | none | no | 1987 | 2010 | 25 | 2 | 23 |
| 12 | NPI-O | non-compliant | none | no | 1985 | 1990 | 27 | 22 | 5 |
| 13 | MCMD | atripla (viread, emtriva, efavirenz) | NNRTI | no | 1991 | 2003 | 21 | 9 | 12 |
| 14 | NPI-O | truvada (viread, emtriva), rit atazanavir | PI | yes | 1985 | 2004 | 27 | 8 | 19 |
| 15 | ANI | epivir, abacavir, raltegravir | INT | no | 1999 | 2000 | 14 | 13 | 1 |
| 16 | MCMD | abacavir, kaletra (rit lopinivir) | PI | yes | 1990 | 1993 | 23 | 20 | 3 |
| 17 | MCMD | truvada (viread, emtriva), raltegravir, rit fosamprenavir | PI, INT | yes | 1998 | 1999 | 15 | 14 | 1 |
| 18 | No Impairment | epivir, rit darunavir, raltegravir | PI, INT | yes | 1990 | 2005 | 23 | 8 | 15 |
| 19 | NPI-O | epzicom (ziagen, epivir), rit darunavir | PI | yes | 1997 | 2000 | 16 | 13 | 3 |
| 20 | NPI-O | Truvada (viread, emtriva), raltegravir | INT | no | 2010 | 2011 | 3 | 2 | 1 |
| 21 | HAD | epzicom (ziagen, epivir), rit darunavir | PI | yes | 1994 | 1997 | 19 | 16 | 3 |
| 22 | No Impairment | Truvada (viread, emtriva), raltegravir | INT | no | 1991 | 1995 | 22 | 18 | 4 |
| 23 | NPI-O | combivir (azt, 3tc), kaletra (rit ritonivir), efavirenz | PI, NNRTI | yes | 1985 | 2002 | 28 | 11 | 17 |
| 24 | No Impairment | complera (emtricitabine, tenofovir, rilpivirine), rit darunavir, raltegravir | PI, INT, NNRTI | yes | 1986 | 1991 | 27 | 22 | 5 |
| 25 | No Impairment | atripla (viread, emtriva, efavirenz) | NNRTI | no | 1999 | 1999 | 14 | 14 | 0 |
| 26 | NPI-O | truvada (viread, emtriva), abacavir, rit darunavir | PI | yes | 1999 | 2003 | 14 | 10 | 4 |
| 27 | NPI-O | truvada (viread, emtriva), rit darunavir, raltegravir | PI, INT | yes | 1998 | 1998 | 15 | 15 | 0 |
| 28 | NPI-O | Epzicom (ziagen, epivir), atazanavir | PI | yes | 1985 | 1995 | 28 | 18 | 10 |
| 29 | No Impairment | non-compliant | None | no | 1991 | 2008 | 22 | 5 | 17 |
| 30 | No Impairment | epivir, rit darunavir, raltegravir, maraviroc | PI, INT, fusion | yes | 1988 | 1993 | 25 | 20 | 5 |
| 31 | HAND / mcmd | combivir (azt, 3tc), rit atazanavir | PI | yes | 1989 | 1997 | 26 | 18 | 8 |
| 32 | HAND / mcmd | Complera (emtricitabine, tenofovir, rilpivirine) | NNRTI | no | 1992 | 1994 | 23 | 21 | 2 |
| 33 | HAND / mcmd | 3tc. Abacavir, efavirenz | NNRTI | no | 1989 | 2000 | 26 | 15 | 11 |
| 34 | No Impairment | emtricitabine, tenofovir, rit atazanavir | PI | yes | |||||
| 35 | HAND / mcmd | truvada (viread, emtriva), rit atazanavir | PI | yes | 2000 | 2001 | 15 | 14 | 1 |
| 36 | HAND / HAD | truvada (viread, emtriva), raltegravir | INT | no | 1999 | 2000 | 16 | 15 | 1 |
| 37 | No Impairment | emtricitabine, tenofovir, raltegravir | INT | no | |||||
| 38 | HAND / HAD | Complera (emtricitabine, tenofovir, rilpivirine), dolutegravir | NNRTI, INT | no | 1994 | 2015 | 21 | 0 | 21 |
| 39 | NPI-O | tenofovir, triumeq (abacavir, dolutegravir, 3tc) | INT | no | 1990 | 1997 | 25 | 18 | 7 |
| 40 | No Impairment | truvada (viread, emtriva), rit atazanavir | PI | yes | 2000 | 2000 | 15 | 15 | 0 |
| 41 | No Impairment | truvada (viread, emtriva), rit atazanavir, raltegravir | PI, INT | yes | 1992 | 1992 | 23 | 23 | 0 |
| 42 | No Impairment | epivir, rit darunavir, etravirine, raltegravir | PI, NNTRI, INT | yes | 1991 | 1997 | 24 | 18 | 6 |
| 43 | HAND / MCMD | truvada (viread, emtriva), kaletra (rit lopinivir) | PI | yes | 1985 | 2000 | 30 | 15 | 15 |
| 44 | HAND / ANI | Epzicom (ziagen, epivir), dolutegravir | INT | no | 1999 | 2000 | 16 | 15 | 1 |
| 45 | HAND / HAD | epzicom (ziagen, epivir), rit atazanavir | PI | yes | 1990 | 1997 | 25 | 18 | 7 |
Sample processing and flow cytometry
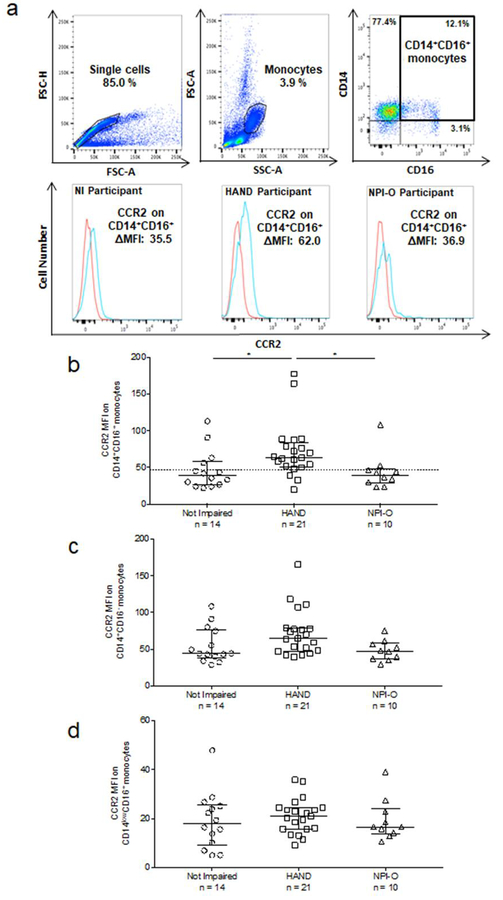
Blood samples collected in EDTA-coated collection tubes were processed within 2–4 hours of blood draw. Whole blood was layered onto Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) for density gradient centrifugation after which PBMC were obtained. PBMC were immediately stained for flow cytometry, and if there were enough PBMC, 106 cells were pelleted and stored at −80°C until HIV DNA analysis by droplet digital PCR (ddPCR) was performed. PBMC were stained using fluorochrome coupled antibodies to human CD14 (BD; clone M5E2), CD16 (BD; clone 3G8), and CCR2 (R&D; clone 48607) or isotype matched control antibodies. This staining was performed according to a protocol described previously (Williams et al., 2014). Briefly, PBMC (2 × 105 in 100 μl) were added to FACS tubes, spun, and media aspirated. CCR2 or isotype matched control antibody was added in 50 μl FACS buffer (1% BSA in PBS) for 5 minutes, prior to addition of antibodies to CD14 and CD16. Cells were stained for 30 minutes in the dark on ice, washed once, and fixed with 2% paraformaldehyde in PBS. At least 10,000 monocytes, or 1,000 CD14+CD16+ monocytes, were acquired with the BD FACSCantoII flow cytometer. Analysis was performed using FlowJo software (v. 10.0.8, TreeStar, Ashland, OR). Forward and side scatter were used to determine monocyte gating. Monocytes were defined as CD14 positive, and monocyte subsets were then distinguished according to CD14 and CD16 expression. The monocyte subsets were identified as CD14+CD16−, CD14+CD16+, and CD14lowCD16+. Mean fluorescence intensity (MFI) for CCR2 was calculated after subtracting the isotype matched control antibody MFI from the CCR2 MFI for each monocyte subset. An example of gating for monocyte subsets and quantification of CCR2 MFI on CD14+CD16+ monocytes from NI, HAND and NPI-O study participants is shown in Fig 1a.
Fig. 1. HIV infected people with HAND have increased CCR2 on CD14+CD16+ monocytes.
. Surface CCR2 on three major monocyte subsets in PBMC from 45 subjects was determined by staining freshly isolated PBMC for human CD14, CD16, and CCR2, or with isotype matched control antibodies. Blood was drawn and PBMC isolated on the same day as neuropsychological assessment, which determined cognitive status. Cognitive status was determined as NI, HAND, or NPI-O. (a). Single cells were selected for analysis and forward- and side-scatter area (FSC-A and SSC-A) characteristics were used to determine monocyte gating. Monocyte subsets were then identified based on staining with isotype matched control, CD14 and CD16 antibodies. CD16 and the level of CD14 expression were used to gate for monocyte subsets, with high expression of both identifying CD14+CD16+ monocytes. CCR2 MFI was calculated by subtracting the MFI of the IgG2b isotype matched control antibody (red line) from the MFI of CCR2 (blue line) for each monocyte subset. (b). CCR2 MFI on CD14+CD16+ monocytes by cognitive status. (c). CCR2 MFI on CD14+CD16- monocytes by cognitive status. (d). CCR2 MFI on CD14lowCD16+ monocytes by cognitive status. Each data point is one individual subject, and data are represented as median ± IQR. Significance was determined by Kruskal Wallis test (p < 0.01) with multiple comparison’s post-hoc testing. * p < 0.05.
Neuropsychological testing
Functional status questionnaires and neuropsychological tests sensitive to HIV-associated cognitive deficits that examine a broad range of cognitive domains were administered to study participants. Tests used and their normative data are detailed as published previously (Byrd et al., 2013). Scores for each test were converted to T-scores, after performance adjustment for the effects of age, education, sex, and ethnicity, where appropriate. Individuals were then assigned a cognitive diagnosis as NI or cognitively impaired, as previously described (Williams et al., 2014). Study participants who demonstrated cognitive impairment and had pre-existing or co-occurring conditions that accounted for cognitive dysfunction, for example, traumatic brain injury, severe depression, CNS opportunistic disease, or metabolic abnormality, were, after extensive examination, assigned the diagnosis of NPI-O. The remaining cognitively impaired participants were assigned a diagnosis of HAND (Antinori et al., 2007). In the current sample, low levels of literacy, CNS insult (history of cerebrovascular incident and traumatic brain injury), and recent illicit substance use accounted for the 10 cases of NPI-O. Participants with cognitive impairment who had only HIV infection and not any potentially confounding condition were assigned a HAND diagnosis.
Neuroimaging
A subset of 18 study participants underwent MRI/1H-MRS using a 3T scanner (Skyra, Siemens Healthcare, Erlangen, Germany) using the vendor-provided head coil. The 1H-MRS/MRI protocol included: a) Sagittal T1-weighted Magnetization-Prepared Rapid Gradient-Echo (MP-RAGE): (TE/TI/TR=1.99/1000/2400 ms, voxel size: 1 mm3); b) Sagittal 3D T2- weighted space sequence (TE/TR=566/ /3200 ms, voxel size: 1 mm3). T1 MPRAGE images were resliced in axial and coronal directions for chemical shift imaging (CSI) volume of interest (VOI) placement; c) Multivoxel 2D-CSI 1H-MRS was performed on an axial 15-mm thick slice at the level of the head of the caudate nucleus using a point-resolved spectroscopy (PRESS) sequence (TE/TR=30/1700 ms, FOV: 160×160 mm2 matrix 16 × 16, voxel size 10 × 10 × 15 mm3, 1024 data points, spectral width of 1200 Hz). Vendor provided semi-automatic shimming procedure was performed prior to the spectroscopic acquisition. The position of the CSI slice was carefully chosen to anatomically comparable position. Spectra were obtained from voxels of 1.5 cm3 in the following regions: deep frontal white matter, head of caudate bilaterally; midline frontal gray matter.
Neuroimaging post-processing and metabolite quantification
For each participant undergoing neuroimaging, spectra from the above listed brain regions were fitted and metabolite amplitudes were evaluated using the time domain linear combination software LCModel (version 6.2) (Provencher, 2001) and expressed in arbitrary units (a.u.). A quality control was applied to each voxel and the spectra were included in the analysis only if the signal-to-noise ratio (SNR) was equal to or greater than 5 and Cramer-Rao lower bounds (CRLBs) was equal to or smaller than 25% of the estimated concentration. The following metabolites were calculated per voxel: total N-Acetyl-Aspartate (tNAA), total choline (tCho), total creatine (tCr), myoinositol (mI), and glutamine and glutamate (Glx). Ratios of tNAA/tCr, tCho/tCr, mI/tCr, and Glx/tCr were measured and used for the correlative studies.
Droplet digital PCR (ddPCR)
HIV DNA was quantified in PBMC. PBMC were isolated from whole blood as described in Sample Processing, after which DNA was extracted from 106 cells using the QIAamp DNA Blood Mini Kit according to the manufacturer’s protocol (Qiagen, Valencia, CA, USA). Analysis of HIV DNA was done using ddPCR. The ddPCR assay was performed in a Bio-Rad QX-100 system as described previously (Strain et al., 2013), using a primer/probe set for HIV Gag: HIV Gag F: ‘5-TCAGCCCAGAAGTAATACCCATGT-3’, HIV Gag R: ‘5-CACTGTGTTTAGCATGGTGTTT-3’, and HIV probe: 5’-ATTATCAGAAGGAGCCACCCCACAAGA-3’ (Integrated DNA Technologies, Coralville, Iowa, USA).
Statistical analysis
Statistical analyses were performed using STATA/IC 14.2 for Mac (College Station, TX, USA). Kruskal-Wallis test was performed with Dunn’s multiple comparison post hoc test to examine CCR2 on monocyte subsets between cognitive statuses, and race, and to examine differences in race between cognitive status. Kruskal-Wallis test with Dunn’s multiple comparison post hoc test was also performed to examine differences between cognitive status and the following: percent of CD14+CD16+ monocytes that were CCR2+, years from HIV diagnosis, years from beginning cART, or years between HIV diagnosis and beginning cART. Mann Whitney U test was performed to examine CCR2 on CD14+CD16+ monocytes by sex, and detection of viral load, and to examine HIV DNA copies per 106 PBMC by cognitive status dichotomized in NI/NPI-O and HAND, as well as to examine cohort characteristics by cognitive status dichotomized in NI/NPI-O and HAND. Spearman’s rank correlation coefficient was used to examine the relationship between CCR2 on CD14+CD16+ monocytes and plasma viral load (log10 copies/ml), current CD4+ T cell counts (cell/μl), nadir CD4+ T cell counts (cell/μl), HIV DNA copies per 106 PBMC, years from HIV diagnosis, year from beginning cART, and years between HIV diagnosis and beginning cART. Pearson’s correlation coefficient was used to examine the relationship between MRI /1H-MRS markers and CCR2 on CD14+CD16+ monocytes, CD14+CD16− monocytes (%), and CD14+CD16+ monocytes (%).
Results
Characteristics of study participants
The demographic and clinical characteristics of HIV infected individuals from the MHBB cohort who participated in this study are in Table 1. The 45 study subjects had a median age of 56, with 24 participants (53.3%) being female and 21 being male (46.7%) with median years since HIV diagnosis of 21. All individuals in the cohort had longstanding histories of cART, with 96% on cART at the time of blood draw for this study (3 were non-compliant) with median years from beginning any cART treatment of 15 and median years between HIV diagnosis and time of beginning cART treatment of 4. Further information and descriptions of study participant’s cART regimens, year of HIV diagnosis and year of beginning cART can be found in Table 2. The median plasma viral load was just above the limit of detection (19 copies/ml) at 24 copies per ml (log10 copies/ml: 1.4). While 59% of individuals had a detectable plasma viral load, only 28.9% had loads greater than 100 copies/ml. HAND was diagnosed in 21 of the participants (46.7%). We found no difference between cognitive status diagnosis and years from HIV diagnosis (data not shown), years from beginning cART (data not shown), or years between HIV diagnosis and beginning cART (data not shown).
CCR2 is increased on CD14+CD16+ monocytes specifically from HIV infected individuals with cognitive impairment due to HIV
In a prior study, our laboratory demonstrated that surface CCR2 is increased on CD14+CD16+ monocytes in PBMC from HIV infected individuals with HAND, independent of cART status, viral load, nadir CD4+ T cell or current CD4+ T cell counts (Williams et al., 2014). To determine whether this effect was specific to impairment due to HIV related events, we examined CCR2 on CD14+CD16+ monocytes in PBMC from an additional 24 HIV infected individuals in the MHBB cohort that were NI, NPI-O, or had HAND for a total sample size of 45.
The gating strategy for monocytes and their subsets, as described in the Methods, is shown in Figure 1a. We determined that there was a significant increase in CCR2 MFI on CD14+CD16+ monocytes (median [interquartile range (IQR)]) from those with HAND (63.3 [51.6, 79.0]) compared to those that were NI (38.8 [26.7, 56.4]; Kruskal-Wallis test, p < 0.01; Dunn’s multiple comparison post-hoc, p < 0.05), or those that were NPI-O (39.8 [30.2, 46.5]; Dunn’s multiple comparison post-hoc, p < 0.05) (Fig. 1b). The CCR2 MFI on CD14+CD16+ monocytes was above 47 for 18 (85.7%) individuals with HAND, and below 47 for 10 (71.4%) individuals that were NI and 8 (80%) of the individuals with NPI-O. Thus, there was a “threshold of impairment” represented by the lowest CCR2 MFI on peripheral blood CD14+CD16+ monocytes that was indicative of HAND. CCR2 MFI’s at, or above, this threshold level distinguish HIV infected individuals with HAND from those that are NI or NPI-O. In this study, CCR2 MFI levels at, or above, 47 on CD14+CD16+ monocytes was the “threshold of impairment”. We did not find a significant difference among the three cognitive diagnoses with CCR2 on CD14+CD16- monocytes (Fig. 1c), or CCR2 on CD14lowCD16+ monocytes (Fig. 1d). We also did not find significance in the percentage of CD14+CD16+ monocytes that express CCR2 between the HAND, NI and NPI-O populations (data not shown).
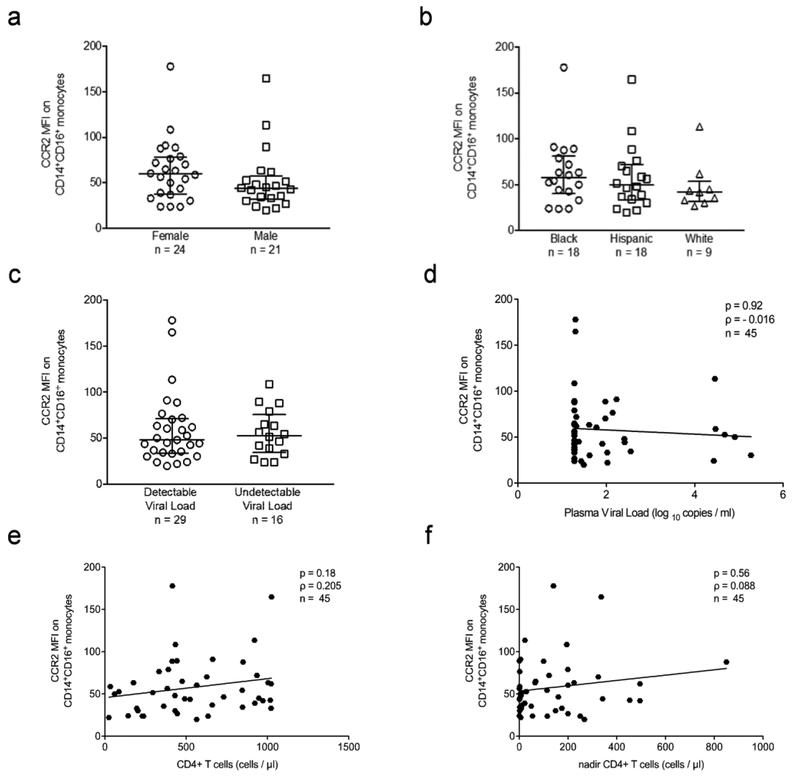
The expression of CCR2 on CD14+CD16+ monocytes was not significantly associated with gender (Fig. 2a), ethnicity (Fig. 2b), detectable (>19 copies/ml) viral load (Fig. 2c), viral load quantification (Fig. 2d), CD4+ T cell counts (Fig. 2e), nadir CD4+ T cell counts (Fig. 2f), cART treatment (data not shown), years from HIV diagnosis to CCR2 analysis (data not shown), years from cART treatment to CCR2 analysis (data not shown), or years between HIV diagnosis and beginning of cART (data not shown). Thus, while CCR2 on CD14+CD16+ monocytes in PBMC from HIV infected individuals, specifically distinguished those with HAND from those who are NI or who are impaired due to causes other than HIV (NPI-O), it did not appear to be related to routinely measured immunovirologic indices. Moreover, the increase in CCR2 was independent of other demographic and clinical data assessed, providing additional support for the specificity of CCR2 on CD14+CD16+ monocytes as a biomarker for HAND.
Fig. 2. CCR2 on CD14+CD16+ monocytes does not vary by demographic and HIV clinical variables.
. Surface CCR2 on CD14+CD16+ monocytes was determined by staining freshly isolated PBMC for human CD14, CD16, and CCR2, or with isotype matched control antibodies. CCR2 MFI was calculated by subtracting the MFI of the IgG2b isotype matched control staining on CD14+CD16+ monocytes from the MFI of CCR2 on CD14+CD16+ monocytes. Each data point is one individual subject. (a). CCR2 on CD14+CD16+ monocytes by sex (female: n = 24, male: n = 21). (b). CCR2 on CD14+CD16+ monocytes by ethnicity (Black: n = 18, Hispanic: n = 18, White: n = 9). (c). CCR2 on CD14+CD16+ monocytes by detection of viral load (detectable: n = 29, undetectable: n = 16). (d). Spearman’s rank correlation between CCR2 on CD14+CD16+ monocytes and viral load (log10) (n = 45, p = 0.92, ρ = −0.016). (e). Spearman’s rank correlation between CCR2 on CD14+CD16+ monocytes and current CD4+ T cell counts (cells/μl) (n = 45, p = 0.18, ρ = 0.205). (f). Spearman’s rank correlation between CCR2 on CD14+CD16+ monocytes and nadir CD4+ T cell counts (cells/μl) (n = 45, p = 0.56, ρ = 0.088). (a–c). Data are represented as median ± IQR. Significance was determined by Mann Whitney U test. Data were not significant (p > 0.05).
Brain imaging cohort characteristics
To characterize further CCR2 on CD14+CD16+ monocytes as a biomarker for neurobiologic processes associated with HAND, we performed additional analyses with 18 of the 45 individuals in this study who were also undergoing MRI/1H-MRS in a pilot neuroimaging study. Thus, we were able to perform association studies between cognitive status, CCR2 on peripheral CD14+CD16+ monocytes, proton magnetic resonance spectroscopy (1H-MRS) measured neurometabolite levels, and PBMC HIV DNA copies in a subgroup of the participants in our study. The demographic and clinical characteristics of this subgroup are shown in Table 3. As the NI and NPI-O group had very similar CCR2 MFI values on their CD14+CD16+ monocytes, data from participants from these groups were combined for further analyses. There were no significant differences in the demographic and clinical characteristics between the NI/NPI-O subjects and the subjects who had HAND. Of the 18 individuals participating in the neuroimaging pilot study, 9 (50%) were opioid users. These 9 individuals were relatively equally distributed across the NI/NPI-O and HAND groups (not shown, p = 0.64), and it did not affect the outcome of our additional analyses.
Table 3.
Brain imaging cohort characteristics stratified by cognitive status
| Cohort Characteristics | Overall (N = 18) Median [IQR] or n (%) | NI / NPI-O (N=7) Median [IQR] or n (%) | HAND (N=11) Median [IQR] or n (%) | p value |
|---|---|---|---|---|
| Age(yr) | 56 [52, 59] | 56 [53, 62] | 56 [51, 59] | 0.78 |
| Gender | 0.62 | |||
| Female | 14 (77.8%) | 5 (71.4%) | 9 (81.8%) | |
| Male | 4 (22.2%) | 2 (28.6%) | 2 (18.2%) | |
| Race | 0.31 | |||
| Black | 7 (38.9%) | 2 (28.6%) | 5 (45.5%) | |
| Hispanic | 6 (32.3%) | 2 (28.6%) | 3 (36.4%) | |
| White | 5 (27.8%) | 3 (42.9%) | 2 (18.2%) | |
| Years from HIV diagnosis | 24 [15, 30] | 24 [15, 30] | 23 [16, 26] | 0.84 |
| cART | 18 (100.0%) | 7 (100.0%) | 11 (100.0%) | >0.99 |
| Years from starting cART | 17 [0, 23] | 18 [15, 23] | 15 [14, 21] | 0.19 |
| Years between HIVdiagnosis and starting cART | 4 [0, 21] | 6 [0, 10] | 2 [1, 15] | 0.52 |
| CD4 T cell count (cells/ul) | 662 [399, 922] | 660 [399, 947] | 702 [294, 922] | 0.96 |
| Nadir CD4 T cell count (cells/ul) | 63 [8, 225] | 12 [2, 77] | 114 [10, 387] | 0.09 |
| Plasma viral load (log10 copies/ml) | 1.3 [1.3, 1.8] | 1.3 [1.3, 1.6] | 1.3 [1.3, 2.0] | 0.73 |
| viral load undetectable | 10 (55.6%) | 4 (57.1%) | 6 (54.5%) | 0.92 |
1H-MRS-derived neuronal damage is greater in those with increased CCR2 on CD14+CD16+ monocytes
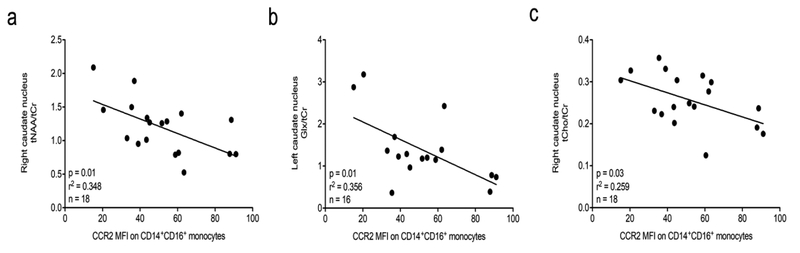
Three regions of brain (deep frontal white matter, head of caudate bilaterally; midline frontal gray matter) were analyzed. We found, using Pearson’s correlation coefficient, that there was an inverse association between CCR2 MFI on CD14+CD16+ monocytes and tNAA/tCr in the right caudate nucleus (B = −32.16, r2 = 0.348, p = 0.01) (Table 4 and Fig. 3a). In the left caudate nucleus, we found an inverse association between CCR2 MFI on CD14+CD16+ monocytes and Glx/tCr (B = −17.0, r2 = 0.356, p = 0.01) (Table 4 and Fig. 3b). This indicates that with higher CCR2 on peripheral blood CD14+CD16+ monocytes, there is increased impairment of 1H-MRS metabolites specific for neuronal damage in the BG. For Glx/tCr we lack two measures in the left caudate nucleus (n=16 versus n=18 for tNAA/tCr and tCho/tCr in the right caudate nucleus) because we were unable to identify Glx measures reliably. There was also a significant inverse association between CCR2 on CD14+CD16+ monocytes and tCho/tCr in the right caudate nucleus (B = −180.01, r2 = 0.259. p = 0.03) (Table 4 and Fig. 3c). As tCho/tCr is a marker indicative of cell proliferation and inflammation (Mohamed et al., 2010; Harezlak et al., 2014), and neuroinflammation is thought to be part of HAND (Campbell et al., 2014; Saylor et al., 2016), this association was opposite to what we expected. This may be related to longterm cART and the low level chronic inflammation that is more likely characteristic of this cohort. A more detailed analysis of these data is included in the Discussion. There were no other associations between 1H-MRS data and CCR2 MFI on CD14+CD16+ monocytes (Table 4).
Table 4.
Pearson’s correlation between 1H-MRS measured metabolites and CCR2 on CD14+CD16+ monocytes
| tNAA/tCr | tCho/tCr | mI/tCr | Glx/tCr | |
|---|---|---|---|---|
| Right Caudate Nucleus | B: −32.16 | B: −180.01 | B: −24.57 | B: −0.66 |
| r2: 0.348 | r2: 0.259 | r2: 0.082 | r2: 0.000 | |
| P: 0.01 | P: 0.03 | P: 0.27 | P: 0.95 | |
| Right Frontal White Matter | B: −2.83 | B: −3.49 | B: −25.94 | B: −8.99 |
| r2: 0.005 | r2: 0.000 | r2: 0.126 | r2: 0.073 | |
| P: 0.80 | P: 0.94 | P: 0.18 | P: 0.29 | |
| Midline Frontal Gray Matter | B: 19.44 | B: −40.41 | B: 12.70 | B: 8.88 |
| r2: 0.150 | r2: 0.019 | r2: 0.115 | r2: 0.080 | |
| P: 0.17 | P: 0.59 | P: 0.22 | P: 0.31 | |
| Left Caudate Nucleus | B: −13.16 | B: 95.41 | B: 3.87 | B: −17.0 |
| r2: 0.024 | r2: 0.035 | r2: 0.037 | r2: 0.356 | |
| P: 0.54 | P: 0.46 | P: 0.45 | P: 0.01 | |
| Left Frontal Whiter Matter | B: 8.31 | B: 27.80 | B: 0.53 | B: 0.98 |
| r2: 0.013 | r2: 0.069 | r2: 0.000 | r2: 0.000 | |
| P: 0.69 | P: 0.31 | P: 0.97 | P: 0.93 | |
Fig. 3. 1H-MRS markers of neuronal health correlate inversely with CCR2 on CD14+CD16+ monocytes.
. Freshly isolated PBMC were stained for human CD14, CD16, and CCR2, or with isotype matched control antibodies. CCR2 MFI was calculated by subtracting the MFI of the IgG2b isotype matched control staining on CD14+CD16+ monocytes from the MFI of CCR2 on CD14+CD16+ monocytes. MRI/1H-MRS imaging was performed within 1–2 weeks of blood draw. Pearson’s correlation coefficient was used to determine the coefficient of determination (r2). Each data point is one individual subject. (a). Inverse correlation of NAA/Cr in the right caudate nucleus with CCR2 on CD14+CD16+ monocytes (n = 18, p = 0.01, r2 = 0.348). (b). Inverse correlation of Glx/Cr in the left caudate nucleus with CCR2 on CD14+CD16+ monocytes (n = 16, p = 0.01, r2 = 0.356). (c). Inverse correlation of tCho/tCr in the right caudate nucleus with CCR2 on CD14+CD16+ monocytes (n = 18, p = 0.03, r2 = 0.259).
In addition, we found associations between decreased tNAA/tCr in the BG and the percentage of total peripheral monocytes that were CD14+CD16+ (Table 5); in contrast, the percentage of CD14+CD16− monocytes was associated with increased tNAA/tCr in the caudate nucleus (not shown). CD14+CD16+ monocytes are important in HAND (Pulliam et al., 1997; Fischer-Smith et al., 2001; Valcour et al., 2010; Burdo et al., 2011; Williams et al., 2012; Williams et al., 2013), thus, these data indicate that an increased presence of these monocytes in the periphery may, in part, contribute to neuronal damage. As CCR2 on CD14+CD16+ monocytes was associated with neuronal damage as well, and with HAND, it suggests that this monocyte subset, and more specifically CCR2 on CD14+CD16+ monocytes, is related to the CNS pathology that is associated with HAND.
Table 5.
Pearson’s correlation between 1H-MRS measured metabolites and CD14+CD16+ monocytes (%, of total monocytes)
| tNAA/tCr | tCho/tCr | mI/tCr | Glx/tCr | |
|---|---|---|---|---|
| Right Caudate Nucleus | B: −13.42 | B: −19.83 | B: 8.99 | B: −1.42 |
| r2: 0.324 | r2: 0.017 | r2: 0.066 | r2: 0.006 | |
| P: 0.01 | P: 0.61 | P: 0.32 | P: 0.75 | |
| Right Frontal White Matter | B: −0/95 | B: −25.75 | B: −0.77 | B: −1.27 |
| r2: 0.005 | r2: 0.106 | r2: 0.001 | r2: 0.011 | |
| P: 0.80 | P: 0.19 | P: 0.91 | P: 0.69 | |
| Midline Frontal Gray Matter | B: −2.18 | B: 24.05 | B: −0.68 | B: 2.94 |
| r2: 0.018 | r2: 0.052 | r2: 0.003 | r2: 0.081 | |
| P: 0.65 | P: 0.38 | P: 0.85 | P: 0.30 | |
| Left Caudate Nucleus | B: −17.33 | B: −59.00 | B: 4.00 | B: 1.19 |
| r2: 0.220 | r2: 0.072 | r2: 0.208 | r2: 0.013 | |
| P: 0.05 | P: 0.28 | P: 0.06 | P: 0.67 | |
| Left Frontal Whiter Matter | B: −0.60 P | B: 16.57 | B: 6.06 | B: −1.41 |
| r2: 0.000 | r2: 0.187 | r2: 0.110 | r2: 0.006 | |
| P: 0.94 | P: 0.08 | P: 0.21 | P: 0.77 | |
Increased PBMC HIV DNA is associated with increased CCR2 on CD14+CD16+ monocytes
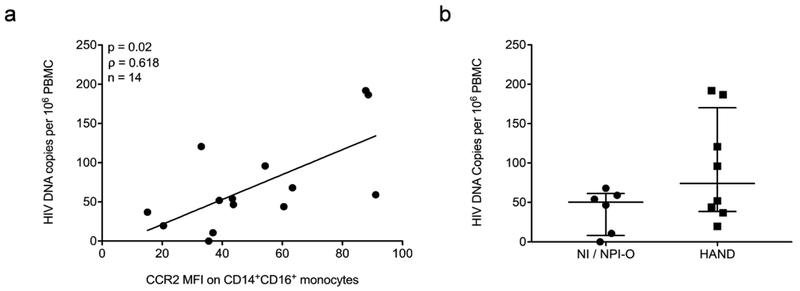
Increased HIV DNA in PBMC has also been associated with HAND, with CD14+CD16+ monocytes proposed to be the major source of HIV DNA (Shiramizu et al., 2005; Kusao et al., 2012; Cysique et al., 2015). To determine whether in our study CCR2 on CD14+CD16+ monocytes was also associated with increased PBMC HIV DNA, we examined the number of HIV DNA copies per 106 PBMC from 14 of the 18 individuals from whom we were able to obtain sufficient cells for HIV DNA analyses. We performed a Spearman’s rank correlation between CCR2 on CD14+CD16+ monocytes and the number of HIV DNA copies per 106 PBMC and found a significant association between the two variables (ρ = 0.618, p = 0.02) (Fig. 4a). In addition, we found that there was a trend to a higher number of HIV DNA copies per 106 PBMC (median [IQR]) in those with HAND (74 [39, 170]) as compared to those infected individuals that are NI/NPI-O (50 [8, 61]), but this was not statistically significant (Fig. 4b). These data suggest that in those that have increased CCR2 on CD14+CD16+ monocytes, and thus likely HAND, an increase in peripheral HIV DNA, proposed to correlate with the levels of infected peripheral CD14+CD16+ monocytes (Shiramizu et al., 2005; Kusao et al., 2012), may also contribute to the development of cognitive deficits.
Fig. 4. PBMC HIV DNA is associated with CCR2 on CD14+CD16+ monocytes, and is increased in some people with HAND.
. PBMC were isolated from fresh peripheral blood samples, and 106 cells collected, pelleted, and stored until DNA was isolated and HIV DNA was quantified. HIV DNA was quantified using ddPCR. CCR2 MFI on CD14+CD16+ monocytes was determined by subtracting the MFI of the IgG2b isotype matched control staining on CD14+CD16+ monocytes from the MFI of CCR2 on CD14+CD16+ monocytes in freshly isolated PBMC stained for human CD14, CD16, and CCR2, or with isotype matched control antibodies. Each data point is one individual subject. (a). Spearman’s rank correlation associated HIV DNA copies per 106 PBMC with CCR2 MFI on CD14+CD16+ monocytes (n = 14, ρ = 0.618, p = 0.02). (b). HIV DNA copies per 106 PBMC from HIV infected people that are not impaired (NI) and NPI-O (n = 6), or are diagnosed with HAND (n = 8). Data are represented as median ± IQR. Significance was determined by Mann Whitney U test (p > 0.05).
Discussion
It is recommended for all HIV infected people to be screened for HAND at initial diagnosis and at subsequent appropriate intervals (Mind Exchange Working, 2013). This is because HAND, even at subclinical presentation, could pose a risk for lower cART adherence, a decreased quality of life, and an increased risk of reduced life span (McArthur and Smith, 2013). HAND is diagnosed by neuropsychologists after an extensive cognitive analysis consisting of a large battery of neuropsychological tests and examination of activities of daily living, as described in detail previously (Antinori et al., 2007). However, the identification of HAND in a different clinical setting may be difficult due to several reasons. The severity of HAND is decreased in the cART era as compared to pre-cART (Saylor et al., 2016), and symptoms may be subclinical, making it challenging to identify those with HAND. Also, there is a balance between the ease of screening measures and their sensitivity; in general, those measures readily applicable to clinic settings have reduced sensitivity, while more sensitive measures require specialized expertise in cognitive testing (McArthur and Smith, 2013). A recent study showed that the majority of HIV infected people with long-term viral suppression experience subclinical slow cognitive decline in psychomotor speed and executive function, typical neurocognitive domains affected during HAND progression that could affect an individual’s quality of life (Gott et al., 2017). Therefore, it would be valuable for practitioners to be able to test all HIV infected people for their risk of HAND. Screening tools for HAND that analyze cognitive function have been recently developed that shorten the time and cost of neuropsychological testing, but these depend on the test-taker’s ability to use a smartphone/tablet, and rely on testing competence (Robbins et al., 2014). The development of an easy accessible biomarker that can be assessed at frequent intervals, in a fast and standardized manner, is therefore essential. In this study, we showed that CCR2 on CD14+CD16+ monocytes may be such a biomarker, as it is increased on this monocyte subpopulation in the peripheral blood specifically in infected individuals who have HAND, while CCR2 is lower in those with NPI-O or those who are not impaired (NI). In addition, we determined that increased CCR2 on CD14+CD16+ monocytes is associated with neuronal damage and PBMC HIV DNA that have both been associated with HAND in other studies.
We found that CCR2 on CD14+CD16+monocytes does not change with gender, ethnicity, viral load, current or nadir CD4+ T cell counts, cART, years from HIV diagnosis to CCR2 analysis, years from beginning cART to CCR2 analysis, and years between HIV diagnosis and beginning cART. In a prior study from our laboratory it was shown that increased CCR2 did not associate with other comorbid conditions, including substance use, diabetes, and liver disease (Williams et al., 2014). Additionally, we now demonstrate that increased CCR2 is also not associated with neurocognitive deficits due to causes other than HIV (NPI-O). This suggests that CCR2 as a biomarker might specifically identify individuals at risk for cognitive deficits due to HAND. With professional neuropsychological assessment, the interrater reliability (IRR) for detecting cognitive impairment in people with HIV is very high, however, the IRR for distinguishing HAND from NPI-O is very low (Woods et al., 2004). Our study suggests that CCR2 may possibly be of use in the diagnosis of NPI-O, as we found that CCR2 remains below the threshold of impairment for 80% of individuals with NPI-O. As this study was performed in a cross-sectional manner, it is important to confirm this finding with longitudinal studies with a larger sample size that include subjects who are believed to have NPI-O. We examined one NPI-O subject twice over a period of 27 months, and found that at both time points CCR2 was below the CCR2 threshold of impairment (not shown). This suggests that CCR2 may assist in NPI-O diagnosis, and that it could be a reliable marker for frequent testing.
A prior study associated monocyte subsets with MRI/MRS neuroimaging during acute HIV infection. It showed a positive correlation between mI/Cr in the BG and absolute number of CD14+CD16- monocytes (Lentz et al., 2011). This monocyte subset is not believed to be the major cell type to enter the CNS during HIV infection. They also found an inverse correlation between NAA in the BG and the CD14+CD16+ monocyte subset (Lentz et al., 2011). This monocyte population can be infected with HIV (Ellery et al., 2007), transmigrates preferentially across the BBB into the CNS (Buckner et al., 2011; Williams et al., 2013), and is found within the CNS of infected individuals (Fischer-Smith et al., 2001). This correlation between NAA/Cr and CD14+CD16+ monocytes was also shown in acute SIV infection, in the frontal white matter (Campbell et al., 2011). We now found, in a cohort with chronic HIV infection, that the percentage of CD14+CD16+ monocytes was inversely associated with tNAA/tCr and Glx/tCr in the right and left caudate nucleus, respectively, in agreement with the previous study. In contrast, we showed that the population of monocytes that is suggested to contribute only minimally to HAND, the CD14+CD16− monocyte subset (Williams et al., 2012), associated positively with tNAA/tCr, meaning the higher the number of CD14+CD16− monocytes the better the neuronal health. Thus, our data indicate that the CD14+CD16+ monocyte subset specifically is associated with neuronal damage in the BG, connecting the peripheral monocyte subset that has been shown to be key to HAND pathogenesis, to neuronal damage. Moreover, this is the first time to our knowledge, that a surface marker on peripheral immune cells isolated from HIV infected individuals is associated with neuronal damage detected by 1H-MRS. The association between CCR2 on CD14+CD16+ monocytes and tNAA/tCr in the right caudate nucleus, and Glx/tCr in the left caudate nucleus, reinforces the potential of CCR2 as a biomarker for HAND, as it is not only associated with neuropsychological diagnosis of impairment, but also with neuronal damage.
While the inverse correlation of tCho/tCr with CCR2 on CD14+CD16+ monocytes in the right caudate nucleus was unexpected, it may be that an increase in tCho/tCr is associated more with acute rather than chronic neuroinflammation, as it is a well established marker in glioblastoma. Moreover, data from another group suggest that tCho may be decreased by cART, while it is increased in cART-naïve HIV infected individuals (Zahr et al., 2014). The majority of HIV infected individuals in our study were prescribed cART, and have been living with HIV for a median of 21 years. As such, the inverse correlation found in this study may reflect low level chronic neuroinflammation and cART.
An increase in peripheral HIV DNA content may indicate that a higher number of infected CD14+CD16+ monocytes will transmigrate across the BBB into the CNS. In the presence of cART, cells with viral DNA may still produce early viral proteins, including Tat, that in the CNS lead to release of inflammatory and toxic mediators and subsequent neuronal damage (Weiss et al., 1999; Eugenin et al., 2003; Norman et al., 2008; Fields et al., 2015). The association between HAND and HIV DNA in PBMC, proposed to be localized predominately in CD14+CD16+ monocytes in some of these studies, has been established by several laboratories and in different cohorts (Shiramizu et al., 2005; Valcour et al., 2010; Kusao et al., 2012; Cysique et al., 2015). Therefore, the association we identified between PBMC HIV DNA and CCR2 on CD14+CD16+ monocytes is of high interest. CCR2 on CD14+CD16+ monocytes is associated with HAND and with increased CD14+CD16+ monocyte transmigration across the BBB. Thus, it suggests that a higher HIV DNA content in PBMC in those with increased CCR2 on CD14+CD16+ monocytes may result in increased infected macrophages within the CNS as a consequence of the transmigration of infected peripheral CD14+CD16+ monocytes across the BBB, perhaps contributing to the reseeding of CNS viral reservoirs and subsequent neuronal damage and HAND.
This study underscores that CCR2 on CD14+CD16+ monocytes may be a useful biomarker for HAND. The advantage of a biological biomarker in the peripheral blood is that it is easily accessible, and that it can be examined frequently. Moreover, it can be easily tested by flow cytometry, similar to assessment of CD4+ T cell counts. It may assist neuropsychologists in distinguishing NPI-O cases from HAND. Frequent evaluation of CCR2 may identify those who should be examined with a more thorough assessment by a neuropsychologist. Based on the findings of the neuropsychologist, HIV treatment and patient support may be adjusted. Although this study only examined individuals from one cohort, and performed neuroimaging and PBMC HIV DNA analysis on a small and heterogeneous subset of individuals, our study further reinforced that CD14+CD16+ monocytes are essential to HAND, due to the significant associations of this monocyte subset with neuronal damage detected by neuroimaging, and because of their potential to contribute to increased viral entry into the CNS (Veenstra et al., 2017). The association between PBMC HIV DNA and CCR2 on CD14+CD16+ monocytes, and PBMC HIV DNA and HAND in individuals prescribed cART further underscores that HIV infected CD14+CD16+ monocytes may contribute to HAND in the cART era. Future therapeutics that could reduce CNS entry of CD14+CD16+ monocytes may then assist in reducing and preventing HAND.
Acknowledgements
The authors thank MHBB staff and patients who generously contributed their time to the study. This study was funded by National Institutes of Health U24MH100931 (MHBB) (S.M.), R01MH075679 (J.W.B.), R21MH102113–01A1 (J.W.B), R01MH090958 (J.W.B.), R01MH112391 (T.M.C., J.W.B.), R01NS077869 (J.E.C), R01AI127142 (J.E.C.), P30AI124414 (ERC CFAR) (M.V., R.L-R., T.M.C., J.W.B.), Mount Sinai Institute for NeuroAIDS Disparities (R25 MH080663) (R.L-R.), MSTP Training Grant at Albert Einstein College of Medicine (5T32GM007288) (R.L-R.), TL1TR001072 (Einstein-Montefiore CTSA) (M.V.), pilot research grant Icahn School of Medicine Brain Imaging Center (S.M.), National MS Society RG 5120A3/1 (M.I.), and eCLIPSE fellowship (M.V.), Burroughs Wellcome Foundation Grant program “Unifying Population and Laboratory Science”.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Antinori A et al. (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW (2011) Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol 267:109–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC (2010) Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 6:e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC (2011) Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 204:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DA, Robinson-Papp J, Mindt MR, Mintz L, Elliott K, Lighty Q, Morgello S, Manhattan HIVBB (2013) Isolating cognitive and neurologic HIV effects in substance-dependent, confounded cohorts: a pilot study. J Int Neuropsychol Soc 19:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JH, Burdo TH, Autissier P, Bombardier JP, Westmoreland SV, Soulas C, Gonzalez RG, Ratai EM, Williams KC (2011) Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PLoS One 6:e18688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JH, Ratai EM, Autissier P, Nolan DJ, Tse S, Miller AD, Gonzalez RG, Salemi M, Burdo TH, Williams KC (2014) Anti-alpha4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog 10:e1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ (2011) Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol 17:176–183 [DOI] [PubMed] [Google Scholar]
- Cysique LA, Hey-Cunningham WJ, Dermody N, Chan P, Brew BJ, Koelsch KK (2015) Peripheral blood mononuclear cells HIV DNA levels impact intermittently on neurocognition. PLoS One 10:e0120488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM (2007) The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol 178:6581–6589 [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 8:33–44 [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW (2003) MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem 85:1299–1311 [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 26:1098–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Eleuteri S, Campos S, Serger E, Trejo M, Kosberg K, Adame A, Spencer B, Rockenstein E, He JJ, Masliah E (2015) HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci 35:1921–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J (2001) CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 7:528–541 [DOI] [PubMed] [Google Scholar]
- Gongvatana A, Harezlak J, Buchthal S, Daar E, Schifitto G, Campbell T, Taylor M, Singer E, Algers J, Zhong J, Brown M, McMahon D, So YT, Mi D, Heaton R, Robertson K, Yiannoutsos C, Cohen RA, Navia B, Consortium HIVN (2013) Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J Neurovirol 19:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gott C, Gates T, Dermody N, Brew BJ, Cysique LA (2017) Cognitive change trajectories in virally suppressed HIV-infected individuals indicate high prevalence of disease activity. PLoS One 12:e0171887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B, Consortium HIVN (2011) Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 25:625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Cohen R, Gongvatana A, Taylor M, Buchthal S, Schifitto G, Zhong J, Daar ES, Alger JR, Brown M, Singer EJ, Campbell TB, McMahon D, So YT, Yiannoutsos CT, Navia BA, Consortium HIVN (2014) Predictors of CNS injury as measured by proton magnetic resonance spectroscopy in the setting of chronic HIV infection and CART. J Neurovirol 20:294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D (2012) Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr 60:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988–994 [DOI] [PubMed] [Google Scholar]
- Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, Velasco VN, Marshall A, Whitenack N, Shikuma C, Valcour V (2012) Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci 24:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz MR, Kim WK, Kim H, Soulas C, Lee V, Venna N, Halpern EF, Rosenberg ES, Williams K, Gonzalez RG (2011) Alterations in brain metabolism during the first year of HIV infection. J Neurovirol 17:220–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur J, Smith B (2013) Neurologic Complications and Considerations in HIV-Infected Persons. Curr Infect Dis Rep 15:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mind Exchange Working G (2013) Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the mind exchange program. Clin Infect Dis 56:1004–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MA, Barker PB, Skolasky RL, Selnes OA, Moxley RT, Pomper MG, Sacktor NC (2010) Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magn Reson Imaging 28:1251–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Reynolds HM, Kiebala M, De Mesy Bentley KL, Trejo M, Volsky DJ, Maggirwar SB, Dewhurst S, Masliah E, Gelbard HA (2008) HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS One 3:e3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW (2001) Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14:260–264 [DOI] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS (1997) Unique monocyte subset in patients with AIDS dementia. Lancet 349:692–695 [DOI] [PubMed] [Google Scholar]
- Robbins RN, Brown H, Ehlers A, Joska JA, Thomas KG, Burgess R, Byrd D, Morgello S (2014) A Smartphone App to Screen for HIV-Related Neurocognitive Impairment. J Mob Technol Med 3:23–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat Rev Neurol 12:234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V (2005) Circulating proviral HIV DNA and HIV-associated dementia. AIDS 19:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, Spina CA, Woelk CH, Richman DD (2013) Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One 8:e55943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedaldi EM, Minniti NL, Fischer T (2015) HIV-associated neurocognitive disorders: the relationship of HIV infection with physical and social comorbidities. Biomed Res Int 2015:641913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J, Group RSS (2012) Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 206:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Shiramizu BT, Shikuma CM (2010) HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol 87:621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra M, Leon-Rivera R, Li M, Gama L, Clements JE, Berman JW (2017) Mechanisms of CNS Viral Seeding by HIV(+) CD14(+) CD16(+) Monocytes: Establishment and Reseeding of Viral Reservoirs Contributing to HIV-Associated Neurocognitive Disorders. MBio 8:e01280–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Nath A, Major EO, Berman JW (1999) HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol 163:2953–2959 [PubMed] [Google Scholar]
- Williams DW, Eugenin EA, Calderon TM, Berman JW (2012) Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol 91:401–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW (2014) CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol Neuroinflamm 1:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, Berman JW (2013) Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One 8:e69270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer KW, Gama L, Li M, Bartizal CM, Queen SE, Varrone JJ, Brice AK, Graham DR, Tarwater PM, Mankowski JL, Zink MC, Clements JE (2009) Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One 4:e8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK (2004) Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 26:759–778 [DOI] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Sullivan EV, Pfefferbaum A (2014) Imaging neuroinflammation? A perspective from MR spectroscopy. Brain Pathol 24:654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]