Abstract
Arising within the neural tube between the cranial and trunk regions of the body axis, the vagal neural crest shares interesting similarities in its migratory routes and derivatives with other neural crest populations. However, the vagal neural crest is also unique in its ability to contribute to diverse organs including the heart and enteric nervous system. This review highlights the migratory routes of the vagal neural crest and compares them across multiple vertebrates. We also summarize recent advances in understanding vagal neural crest ontogeny and discuss the contribution of this important neural crest population to the cardiovascular system and endoderm-derived organs, including the thymus, lungs and pancreas.
Keywords: vagal neural crest, pancreas, lung, thymus, cardiac
1. Introduction
The neural crest is a transient embryonic population of multipotent stem cells unique to vertebrates. Arising within the dorsal neural tube along nearly the entire anterior-posterior axis of the embryo, neural crest cells undergo an epithelial-to-mesenchymal transition (EMT) to delaminate and embark upon distinct migratory routes toward their final destinations throughout the embryo (Gandhi and Bronner, 2018; Green et al., 2015; Martik and Bronner, 2017). During their migration, neural crest cells encounter extrinsic positional, patterning, and guidance cues that, together with intrinsic factors, contribute to their final fate decisions. Upon differentiation, they give rise to various types of specialized tissues, including craniofacial cartilage and bone, peripheral neurons and glia, pigment cells, and dental tissues, among other derivatives. Due to their remarkable multipotency and migratory ability, they have been extensively used as a model to better understand the process of EMT, cell migration and differentiation, and this knowledge has informed upon the etiology of neural crest derived birth defects and cancers.
During their migration, neural crest cells typically choose one of two major pathways after exiting the neural tube, migrating either dorsolaterally or ventrally (Harris and Erickson, 2007; Kulesa et al., 2010; Kuo and Erickson, 2010; Le Douarin and Kalcheim, 1999; Vega-Lopez et al., 2017). The majority of neural crest cells in the head, as well as late-migrating neural crest cells at spinal cord levels (destined to form melanocytes), take a dorsolateral pathway migrating between the overlying dorsal ectoderm and the underlying ventral mesoderm (Baker et al., 1997; Serbedzija et al., 1989). In contrast, neural crest cells that form more ventral derivatives (e.g. sympathetic and sensory ganglia, chromaffin cells of the adrenal medulla) predominantly migrate ventrally, either between the neural tube and somites, through the anterior sclerotome, or between adjacent somites (Bronner-Fraser, 1986; Serbedzija et al., 1990; Teillet et al., 1987).
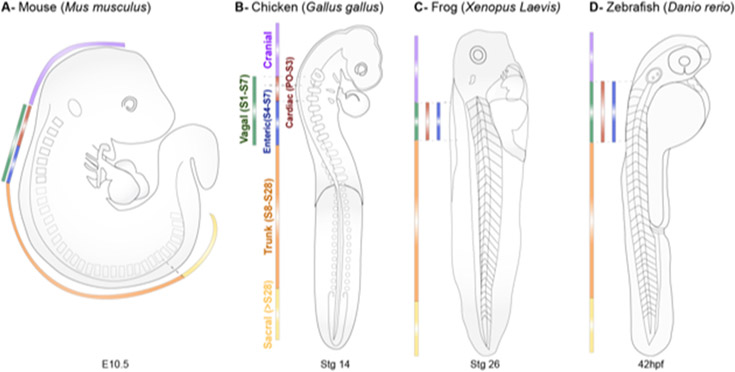
Although all neural crest cells have in common migratory ability and shared gene expression patterns, not all neural crest populations are the same. Based on the elegant quail-chick transplantation experiments of Le Douarin (1982) and colleagues, the neural crest can be subdivided along the anterior-posterior body axis into four subpopulations that differ in migration patterns and derivatives (Fig.1). From anterior to posterior, these are referred to as cranial, vagal, trunk, and sacral. These subpopulations arise sequentially as the embryo elongates along the anterior-posterior axis in time and space. The boundaries between these subpopulations have been best characterized in the chicken embryo using quail-chick grafts. The cranial neural crest originates from the neural tube anterior to the otic vesicle; the vagal neural crest emerges from the hindbrain region adjacent to somites 1-7; trunk neural crest arises from the neural tube adjacent to somites 8-27 (Fig.1A, B), while sacral neural crest arises posterior to somite 28 (Fig.1B). In chick and mouse, the vagal neural crest has been further divided into ‘cardiac’ (somites 1-3 and 1-4, respectively) and ‘enteric’ (somites 1-) subdivisions (Fig.1B).
Figure 1. The anterior-to-posterior regionalization of the neural crest.
Neural crest cells are regionalized into four major anterior-posterior axial levels along the dorsal neural tube of vertebrate embryos. Approximate boundaries of cranial (purple), vagal (green), trunk (orange), and sacral (yellow) neural crest populations are indicated for mouse (E10.5), chick (Hamburger-Hamilton 14), frog (Nieuwkoop-Faber 26), and zebrafish (42hpf) embryos. The cardiac (red) and enteric (blue) subpopulations of the vagal neural crest are also indicated across species.
While the boundaries between neural crest subpopulations appear to be approximately similar across vertebrates, there is some variation between species and increased complexity arising during the course of vertebrate evolution. In the frog, Xenopus laevis, vagal neural crest arise from somite 1 to somite 4 (Fig. 1C) (Epperlein et al., 1990; Krotoski et al., 1988; Lee and Saint-Jeannet, 2011; Sadaghiani and Thiebaud, 1987). In zebrafish, the vagal neural crest spans from immediately rostral to the otic vesicle to caudal to somite 6 (Sato and Yost, 2003) (Fig.1D), while the trunk neural crest region extends caudally from somite 6 (Raible et al., 1992; Vaglia and Hall, 2000). Moreover, a sacral neural crest population may not exist in zebrafish (Shepherd and Eisen, 2011). Similarly, the basal jawless vertebrate, lamprey, appears to entirely lack a vagal neural crest population (Green et al., 2017).
As a transition between the head and the trunk, the vagal neural crest gives rise to a rich variety of specialized tissue and cell types throughout the body. The vagal neural crest is the forerunner of structures such as the enteric nervous system (ENS), aspects of the cardiovascular system (Fig. 2) and other fundamental organ systems, such as the thymus and lung (Fig. 3). Much of what is known about the vagal neural crest has focused on formation of the ENS, about which there are many excellent recent reviews (Ganz, 2018; Nagy and Goldstein, 2017; Young et al., 2016). For this reason, here we focus on studies of vagal neural crest migration and contributions to less well-studied derivatives, with special emphasis on its early migratory routes, and diversification of vagal neural crest into cells of the thymus, cardiac tissues, and the lung and pancreas ganglia (Table 1). We also highlight recent advances in understanding the cellular and molecular mechanisms that regulate early vagal neural crest formation.
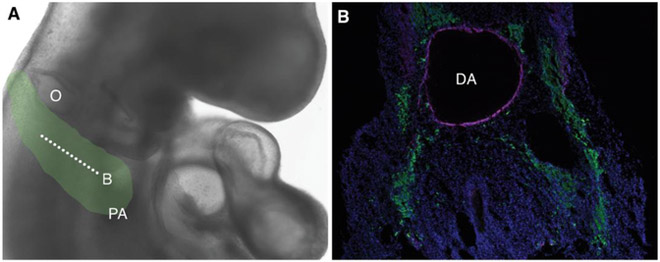
Figure 2. Cardiac neural crest migration.
(A) Cardiac neural crest cells derived from the post-otic region migrate towards the pharyngeal arches in a stage HH18 chick embryo. Green shading in (A) indicates path of cardiac neural crest. Cross section in (B) is indicated by dotted line. (B) Transverse section through a stage HH18 embryo shows neural crest immunostained for HNK-1 (green) migrating around the dorsal aorta (DA) to enter the pharyngeal arches. Nuclei are labeled with DAPI (blue); immunostaining for smooth muscle actin (magenta) lines the DA. O, otic; PA, pharyngeal arches; DA, dorsal aorta.
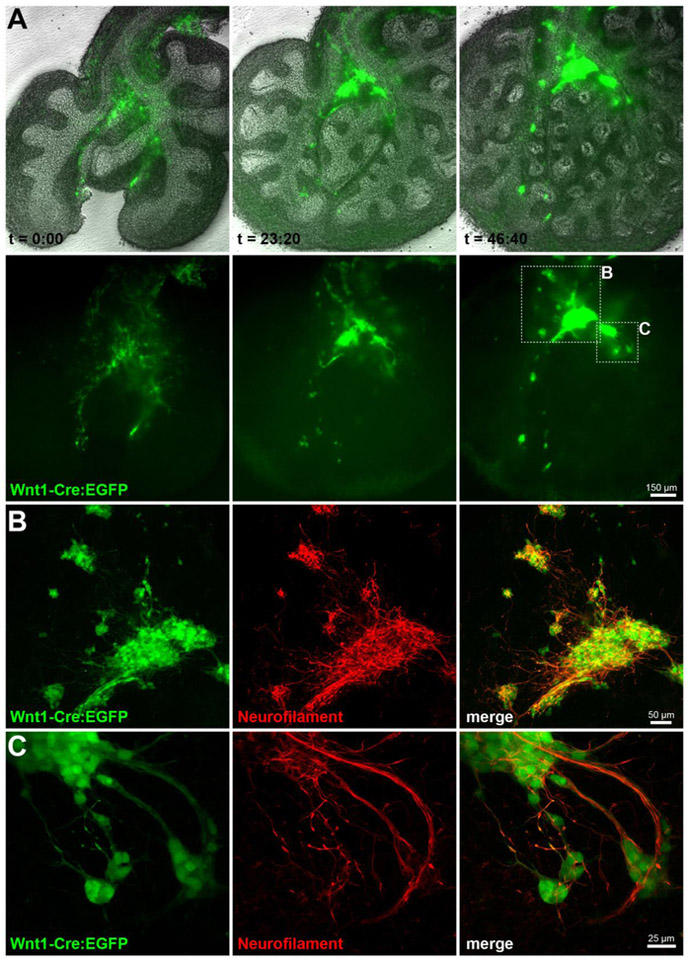
Figure 3. Lineage tracing reveals vagal neural crest migration into mouse lung buds.
(A) E11.5 Wnt-1Cre/ZEG mouse lungs were explanted and imaged at 20-minute intervals for 46 hours and 40 minutes, displaying extensive neural crest cell migration and coalescing into pulmonary ganglia. See also Supplemental Movie 1. (B-C) Explanted lungs were processed for immunostaining against neurofilament M, and imaged by confocal microscopy. EGFP positive cells extend a complex network of neurofilament positive fibers interconnecting the coalescing ganglia. (Images acquired at the 2014 Marine Biological Laboratory Embryology course, with the assistance of Dr. Angelo Iulianella and the mouse module).
Table 1:
Vagal neural crest contribution to various organ tissue types and their somite-level of origin.
| Organ contribution |
Tissue types | Species | Neural crest axial level | References | ||||
|---|---|---|---|---|---|---|---|---|
| Thymus | Mesenchyme of lobes, pericytes, smooth muscle | Chick, mouse, frog | post-otic, somite 1-5 | Lievre & Le Douarin (1975); Bockman & Kirby (1984); Kuratani & Bockman (1990); Kuratani & Bockman (1991); Foster et al. (2008); Muller et al. (2008); Lee et al.(2013) | ||||
| Thyroid, parathyroid |
Mesenchyme, pericytes |
Chick | post-otic, somite 1-5 | Lievre & Le Douarin (1975); Bockman & Kirby (1984); Maeda et al. (2016) | ||||
| Cardiac | Aorticopulmonary system, smooth muscle of aortic arches, ganglia | Chick, mouse |
post-otic, somite 1-3 (chick); somite 1-4 (mouse) |
Chan et al. (2004); Espinosa-Medina et al. (2017); Jiang et al., 2000; Kuo and Erickson (2011); Kirby and Stewart, 1983; Kirby et al. 1993., ; Kuratani and Kirby, 1991; Nishibatake et al. 1987 ; Phillips et al., 1987 |
||||
| Cardiac | Aortic arch arteries, | Frog | hyoid/branchial | Lee & Saint-Jeannet (2011) | ||||
| aortic sac | streams, somite 1-4 | |||||||
| Cardiac | Cardiomyocytes | Zebrafish | post-otic, somite 1-6 | Cavanaugh et al. (2015); Sato and Yost (2003) | ||||
| Lung | Ganglia | Human, mouse, chick | somite 1-7 | Burns & Delanlande ; (2005); Burns et al. (2008); Freem et al. (2010); Freem et al. (2012) | ||||
| Pancreas | Ganglia | Chick, mouse, rat | somite 1-7 | Fontaine et al (1977); Kirchgessner et al. (1992); Nekrep et al. (2008); Plank et al. (2011) | ||||
2. Early vagal neural crest migration
2.1. Vagal neural crest migratory routes in amniotes
The first descriptions of vagal neural crest migration and their importance for development of specific tissues were performed in amniote embryos, such as chicken and mice. Neural tube ablation experiments in chick embryos suggested that the neural crest arising from axial levels adjacent to somite levels 1-7 were the embryological source of enteric ganglia (Yntema and Hammond, 1945). Using interspecies quail-chick grafts, Le Douarin and colleagues then definitively demonstrated that neural crest cells emerging from the vagal region give rise to the enteric ganglia of the gut (Le Douarin and Teillet, 1973). Further anatomical evidence for the contribution of the vagal neural crest to the ENS came from antibody stainings that followed the progression over time of neural crest cells from the vagal region in chick (Tucker et al., 1986). Pachnis and colleagues subsequently extended this work to mouse; by labeling the neural tube with DiI to follow vagal neural crest lineages at E8.5 in mouse embryos, they showed that post-otic vagal neural crest arising adjacent to somite levels 1-4 give rise to both enteric ganglia and the anterior most sympathetic ganglia, the superior cervical ganglion (Durbec et al., 1996).
Precise neural tube ablations and transplantation experiments further described the regions from which ENS precursors arise in chick (Burns et al., 2000; Peters-van der Sanden et al., 1993), expanding our understanding of the importance of the vagal neural crest. Notably, neural crest arising from the anterior or posterior boundaries of the vagal region (i.e. somites 1-2 or somites 6-7) exhibited restricted contributions to the ENS of the gut, with derivatives only observed in the foregut or hindgut, respectively. In contrast, neural crest-derived from somite level 3-5 were observed at all levels of the gut (Burns et al., 2000). This observation has been corroborated using focal electroporation experiments where labeled neural crest cells of rostral origin colonized the rostral portions of the gut, while cells of more caudal origins migrated to caudal regions (Kuo and Erickson, 2011). Accordingly, selective loss of precise somite-level vagal regions (ex: 1-2, 3-5) leads to varying deficits in gut colonization, while total ablation of the vagal region— which leads to striking loss of enteric neurons in the gut— can be rescued by transplanting neural crest back from somite 3 into the neural tube, but not by crest from somite level 1 (Barlow et al., 2008). Interestingly, this suggests that neural crest cells from somite level 3 possess an intrinsic proliferative and/or migratory advantage over neural crest from somite level 1.
While early studies focused on evaluation of en masse neural crest migration from the vagal region, recent studies have provided clues regarding the particular paths undertaken by vagal neural crest cells in the early embryo. For example, precise GFP labeling via targeted electroporations in chicken embryos has shown that vagal neural crest originating from somite levels 1-4 commence migration towards and contribute to tissues in the heart and the ENS, while those arising from somite levels 4-7 contribute only to the ENS (Kuo and Erickson, 2011). At chicken stage HH10 (~E1.5), vagal crest from somite levels 1-4 follow a “dorsolateral” pathway of migration between the ectoderm and somites and migrate into the heart. By stage HH 13 (~E2), a subpopulation of vagal neural crest from somite levels 1-3 switch to a “ventral” path and, like neural crest from somite levels 5-7, follow the ventral pathway between the neural tube and dermomyotome, eventually giving rise to sensory ganglia, sympathetic ganglia and/or enteric ganglia. At stage HH21 (~E3.5), a final wave of migrating vagal neural crest cells take a dorsolateral route and primarily give rise to melanocytes (pigment cells) of the skin (Kuo and Erickson, 2011; Reedy et al., 1998). Though the core migration pathways are largely similar to chick, the dorsolateral and ventral migration occurs simultaneously in mouse, reflecting species-specific differences in timing and pathways of migration at the vagal level (Anderson et al., 2006; Chan et al., 2004).
Although the studies mentioned above have provided much information regarding vagal neural crest cell lineages, a more recent study further extends our understanding of early vagal neural crest cell development to reveal novel migratory routes. Espinosa-Medina and colleagues show that subpopulations of vagal neural crest cells partition along distinct migratory routes to give rise to parasympathetic ganglia, enteric ganglia, or the superior cervical ganglion in both chick and mouse (Espinosa-Medina et al., 2017). Notably, they show that the most rostral vagal neural crest (from somites 1-2) migrates along the vagus nerve in mouse embryos to invade the foregut. Collectively, Espinosa-Medina et al. describe fine sub-populations of “vagal” neural crest. First, there is a rostral circumpharyngeal neural crest population ranging from pre-otic level to somite levels 1-2, which migrates into the heart and 3rd branchial arch, or migrates as Schwann cell precursors along the vagus and give rise to the “parasympatho-enteric” lineage of cells (i.e. the parasympathetic and foregut enteric ganglia). Second, a cervical neural crest population from somite levels 3-7, gives rise to the “sympatho-enteric” lineage (i.e. the sympathetic ganglia and the majority of the ENS) (Espinosa-Medina et al., 2017).
The recent lineage tracing studies by Kuo and Erickson (2011) and Espinosa-Medina et al. (2017) provide somewhat conflicting reports regarding the somite levels that contribute to portions of the ENS and sympathetic chain in chick. Kuo and Erickson, using focal electroporation of fluorescent constructs, found that vagal neural crest from somites 1-2 reached only the more rostral level of the gut by ~E3.5, where the foregut splits into the esophagus and trachea/future lung buds, as well contributing to the sympathetic ganglia (Kuo and Erickson, 2011). In contrast, Espinosa-Medina and colleagues, using GFP isotopic grafts, found neural crest derived from the same axial level (somites 1-2) entered not only the esophagus, but also the stomach, with a few cells observed as distally as the colon by E7. Moreover, they did not observe contribution to the sympathetic chain (Espinosa-Medina et al., 2017). This discrepancy may be attributed to the different lengths of time embryos were allowed to develop between the two studies (E3.5 vs. E7), given that the fluorescence tracing from the focal electroporation technique employed by Kuo and Erickson is transient, and likely would not persist through the day E7 time point observed by Espinosa-Medina et al. Alternatively, whereas grafts contain many more labeled cells, the focal electroporations may only target subsets of cells at a given axial level, and thus, it is possible that the latter technique may not label enough cells to identify all derivatives and endpoints of migration. However, the precise and limited labeling of the focal electroporation experiments was able to elegantly demonstrate the timing of vagal neural crest pathway choice (i.e. dorsolateral versus ventral) and biological implications of migration pathway (i.e. heart versus gut derivatives).
2.2. Vagal neural crest migratory routes in anamniotes
In Xenopus laevis, vagal neural crest cells arise from the neural tube caudal to the otic vesicle (Fig.1C) (Epperlein et al., 1990; Krotoski et al., 1988; Sadaghiani and Thiebaud, 1987). Using orthotopic unilateral grafts and scanning electron microscopy, Sadaghiani and Thiebaud showed that vagal neural crest cells reside along the posterior hindbrain and anterior spinal cord, up to somite levels 1 and 2. Cells labeled from this region begin migration between the somites and neural tube around stage 25 and eventually migrate via a route through the “dorsal root fibers and the body of the vagus ganglion complex” by stage 40 (Sadaghiani and Thiebaud, 1987), suggesting that vagal neural crest cells migrate along the vagus nerve. Subsequently, the vagal neural crest cells migrate in the mesenchyme around the pronephric tubules and towards the ventral midline in order to invade the developing gut, later becoming enteric ganglia (Epperlein et al., 1990; Krotoski et al., 1988; Sadaghiani and Thiebaud, 1987).
In zebrafish, while we do not fully understand early migratory paths of vagal neural crest in as great detail as in the chick or mouse, lineage analysis for vagal/trunk neural crest cells during early stages of neural crest migration has provided some important clues into vagal neural crest migratory paths. A study by Vaglia and Hall has shown by precise DiI labeling that sub-populations of trunk neural crest, which the authors call “cardiac trunk”, “anterior trunk” and “posterior trunk”, differentially contribute to various structures in the zebrafish embryo; including pigment cells, enteric ganglia, ectomesenchyme of the aorta, dorsal root and sympathetic ganglia. “Cardiac trunk” neural crest encompass those arising adjacent to somites 1 to 3/5, while “anterior trunk” was defined as those from somites 6-8 and posterior trunk those born caudal to somite 8. Specifically, they noted that neural crest labeled from anterior trunk and posterior trunk levels contributed to enteric ganglia (Vaglia and Hall, 2000). In further support of the anterior-posterior boundary of vagal neural crest in zebrafish, neural crest that contribute to the zebrafish heart tissues originate from neural tube regions rostral to the otic vesicle and as far caudal as somite 6 (Fig.1D) (Li et al., 2003; Sato and Yost, 2003). Interestingly, these DiI lineage-tracing experiments during early zebrafish neural crest development suggest that neural crest born from somite levels 1-7 are broadly capable of giving rise to enteric ganglia and contributing to tissues in the heart.
Regardless of the details of early migratory pathways, it is clear that post-otic vagal neural crest cells give rise to neurons of the zebrafish ENS. By 32 hpf, enteric-fated vagal neural crest cells reside ventrally in a cluster immediately posterior to the left and right otic vesicles (Ganz et al., 2016; Shepherd and Eisen, 2011; Shepherd et al., 2004). Furthermore, visualization with broad neural crest gene markers like crestin, sox10, foxd3, tfap2a, and enteric progenitor markers phox2bb, ret and gfra1a/b label vagal neural crest en route to the gut (Barrallo-Gimeno et al., 2004; Knight et al., 2003; Luo et al., 2001; Shepherd et al., 2004; Stewart et al., 2006). These studies show that zebrafish vagal neural crest cells migrate medially from post-otic vagal domains toward the foregut entrance as two chains and migrate caudally along the left and right sides of the gut tube to fully colonize the hindgut by the third day of development (Dutton et al., 2001; Elworthy et al., 2005; Kelsh and Eisen, 2000; Olden et al., 2008; Shepherd and Eisen, 2011; Shepherd et al., 2001; Uribe and Bronner, 2015). It is not yet known if the post-otic vagal population of neural crest cells gives rise to tissues other than the ENS in zebrafish. Nonetheless, in situ hybridization suggests that a sub-population of phox2bb+ vagal neural crest cells localize near the dorsal aorta to become sympathoadrenal (SA) precursors of the superior cervical ganglion (Elworthy et al., 2005; Pei et al., 2013). It remains to be shown to what extent this subpopulation of vagal neural crest is similar to the “sympatho-enteric” neural crest that have been described in mouse and chick (Durbec et al., 1996; Espinosa-Medina et al., 2017).
Elaborating upon classical observations of the contributions arising from the vagal neural crest (Kuntz, 1910; Le Douarin and Teillet, 1973; Yntema and Hammond, 1954), recent studies have sought to more fully characterize tissues with vagal neural crest contributions, as well as refine the specific axial-levels from which they arise. In the following sections, we describe several major tissues to which the vagal neural crest contributes and their specific axial levels of origin: Thymus, Cardiovascular, Lung, and Pancreas. For further information regarding contributions of the vagal neural crest to the enteric nervous system of the gut, please see recent comprehensive reviews (Ganz, 2018; Nagy and Goldstein, 2017; Young et al., 2016).
3. Vagal neural crest contributions to the thymus
As an integral part of the immune system, the thymus is a lymphoid organ that primarily produces T-cells (Hollander and Peterson, 2009). The thymus forms as a diverticulum emanating from the endoderm of the 3rd and 4th pharyngeal pouches. By E5 in the chick, the thymic pouch becomes distinct and elongates along the jugular vein, by which time mesenchymal cells have surrounded and infiltrated it, fostering subsequent morphological development into a lobular structure (Le Douarin and Jotereau, 1975). Soon thereafter, lymphocyte progenitor cells migrate into the developing thymic mesenchyme in response to micro-environmental signals and undergo final maturation. Establishment of blood vessels enables immune cell entry to and exit from the thymus (Hollander and Peterson, 2009). In addition to immune cells, the mature thymus contains various specialized cells that support lymphoid cell development including connective mesenchyme, stromal cells, epithelial cells, blood vessels and pericytes (Muller et al., 2008).
Cell lineage analysis and ablation experiments support the idea that vagal neural crest cells contribute to the thymus and the neck glands in chick. Lievre and Le Douarin (1975) performed quail-chick grafting experiments to show that neural crest from post-otic to somite 5 levels were broadly able to contribute to mesenchyme of the glands in the neck, including the lingual gland in the tongue, and the pharynx glands consisting of the thymus, thyroid and parathyroid. In particular, they observed that the medulla of the thymic lobes, the parafollicular cells of the thyroid, pericytes surrounding the capillaries and connective tissue of the parathyroid glandular cells all are derived from vagal neural crest cells. A decade later, a series of studies provided further evidence of neural crest contribution and importance to the thymus. Bockman and Kirby (1984) observed that neural crest ablation from mid-otic to somite 5 levels drastically reduced the size of thymic tissue, thyroid and parathyroid glands, in addition to producing heart defects. Later, Kuratani and Bockman (1990) performed more precise ablations in chick, confined from mid-otic to somite 3 levels, to show that this precise axial level origin of neural crest was important for thymic development. Subsequently, they used quail-chick chimera to show that quail donor neural crest cells from somite 3 contributed to the thymus, as did neural crest near the hyoid and mesencephalic regions (pre-otic to somite 3) levels (Kuratani and Bockman, 1990, 1991).
Genetic lineage labeling in mice revealed a similar contribution of vagal neural crest cells to thymic tissue (Foster et al., 2008; Muller et al., 2008). Using Wnt1:Cre and Sox10:Cre lines to follow the fate of YFP+ neural crest cells throughout development, Foster and colleagues discovered that neural crest cells migrated into the thymic primordia at E12.5 and remained there throughout the gestational period, finally maturing in the adult (Foster et al., 2008). Of interest, they noted that thymic-resident YFP+ cells gave rise to pericytes and smooth muscle cells of the support tissue surrounding the thymic blood vessels, corroborating previous lineage studies in chick (Le Lievre and Le Douarin, 1975). Similarly, Muller and colleagues mapped the distribution of neural crest cells in the adult thymus using Sox10:Cre mice and found that the mesenchyme and pericytes that wrap blood vessels are neural crest-derived (Muller et al., 2008).
Characterizing thymic neural crest in Xenopus provided interesting insight, as Xenopus is a well described model of immune development (Pasquier et al., 1989; Robert and Cohen, 2011; Robert and Ohta, 2009). In Lee et al.’s (2013) study, RFP-labeled cardiac/vagal neural crest regions were carefully transplanted at stage 17 to unlabeled embryos of the same stage. Consistent with previous findings, they found that RFP+ vagal neural crest cells migrated and infiltrated the 2nd, 3rd, and 4th pharyngeal arches. By stages 41-47, labeled neural crest cells incorporated into the mesenchyme encompassing the thymic primordium (Lee and Saint-Jeannet, 2011; Lee et al., 2013; Manning and Horton, 1969). Interestingly, however, Lee et al.(2013) found that the labeled neural crest cells that contribute to the thymus do not express Hoxa3, which is expressed in the 3rd and 4th pharyngeal arches. This result implies that the thymic primordium in Xenopus originates from the 2nd pharyngeal arch, as opposed to the 3rd and 4th pharyngeal arches as in mouse and chick (Lee et al., 2013). Further, neural crest ablation experiments revealed that the Xenopus thymus was still specified and capable of T-cell maturation in the absence of neural crest cells. These results appear to be at odds with findings from mouse and chick; while neural crest cells appear to be essential for murine and avian thymic development, they are expendable to the Xenopus thymus. These findings suggest a differential role between Xenopus neural crest and other vertebrate models in thymus development and highlight the need for further investigation.
4. Vagal neural crest contributions to the cardiovascular tissue
4.1. Cardiac neural crest in amniotes
The cardiac subpopulation of the vagal neural crest contributes to multiple derivatives in the cardiovascular system, including the aorticopulmonary septum, the smooth muscle of the aortic arch arteries, and the cardiac ganglion (Bergwerff et al., 1998; Kirby et al., 1983; Kirby and Stewart, 1983). Following initial observations that neural crest arising from the vagal level populated the cardiac ganglion (Yntema and Hammond, 1947) and arterial walls from the 3rd, 4th, and 6th aortic arches (Le Lievre and Le Douarin, 1975), studies have focused on determining the axial level of origin and timing of the neural crest contribution to the cardiovascular system. Narrowly targeted extirpation experiments in chick established regionalization of the vagal neural crest, such that neural crest from somites 1-3 constituted an anterior portion of vagal neural crest that was responsible for generating the cardiac ganglion, whereas the more caudal portion from somites 4-7 migrated to the enteric ganglia (Kirby and Stewart, 1983). Closer examination of the heart following neural fold ablation between somites 1-3 uncovered septation defects, indicating that neural crest from this rostral vagal population form the aorticopulmonary septum within the cardiac outflow tract, earning the designation “cardiac” neural crest (Kirby et al., 1983; Kirby et al., 1985). Additional efforts to more clearly delineate the axial level of origin of the cardiac neural crest in chick revealed a more rostral contribution including the neural tube adjacent to the otic placode (Kirby et al., 1993; Nishibatake et al., 1987; Phillips et al., 1987). Subsequently, much attention was paid to describing the initial migration of the cardiac neural crest to the circumpharyngeal ridge, their ensuing routes to the aortic arch arteries and cardiac outflow tract, and the myriad of factors participating in these migratory events [for a comprehensive review, please see (Kirby and Hutson, 2010).
Recent evidence suggests that migration of the vagal neural crest population shares features of both cranial and trunk neural crest migration in terms of pathway choice. The cardiac region of neural crest, in particular, is able to enter both the dorsolateral and ventral pathways, depending on the time at which migration initiates. Lineage tracing via electroporation approaches to fluorescently label the vagal neural crest in chick found that cardiac neural crest cells initially exit the neural tube and enter the dorsolateral pathway (Kuo and Erickson, 2011), similar to the cranial neural crest. Based on analysis of cross sections through embryos, cardiac neural crest cells migrated dorsolaterally into pharyngeal arches 3, 4, and 6 (Fig. 2), and ultimately entered the outflow tract of the heart. This is consistent with prior work in chick using HNK-1 immunolabeling of migratory neural crest, showing that cardiac neural crest arising from post-otic to somite 3 level preferentially entered the dorsolateral pathway (Kuratani and Kirby, 1991).
Given that genetic perturbations in mouse produced many of the same abnormalities as those observed after cardiac neural crest extirpation experiments in birds (e.g. defects in aortic arch arteries, cardiac outflow tract) (Franz, 1989; Kurihara et al., 1995), it has been generally assumed that cardiac neural crest cells among amniotes share similar origins and derivatives. However, genetic lineage tracing experiments using Wnt1-Cre transgenic mice found that, while the mouse cardiac neural crest populated the outflow tract and arch arteries similar to chick, there was an additional observed contribution to the coronary arteries (Jiang et al., 2000). Further, there may be key differences between chick and mouse. First, focal cell lineage labeling in mouse embryos suggests that the murine cardiac neural crest arises between somites 1 through 4, without a more rostral contribution (Chan et al., 2004); in contrast, the chick cardiac neural crest region encompasses the level of the otic placode through the caudal limit of somite 3 (Kirby et al., 1993; Nishibatake et al., 1987; Phillips et al., 1987). Second, mouse cardiac neural crest cells did not appear to “pause” and form a circumpharyngeal crest, as described in chick embryos (Kuratani and Kirby, 1991). Finally, whereas chick cardiac neural crest predominantly migrates dorsolaterally (Kuo and Erickson, 2011; Kuratani and Kirby, 1991), mouse cardiac neural crest from somites 1 through 4 simultaneously migrated via dorsolateral, medial, and intersomitic pathways.
The establishment of the caudal limit of somite 3 as the boundary of the cardiac neural crest subpopulation in chick is largely based on ablation experiments (Kirby et al., 1983; Kirby et al., 1993; Kirby and Stewart, 1983; Kirby et al., 1985; Nishibatake et al., 1987), with corroboration from quail-chick grafting experiments (Phillips et al., 1987). Interestingly in chick, Kuo and Erickson (2011) observed neural crest arising from the level of somite 4 also entered the dorsolateral pathway and were found at the posterior of pharyngeal arch 6. Kuo and Erickson (2011) further noted that, while initial streams of vagal neural crest from the “cardiac” axial level entered the dorsolateral pathway, cells from the same axial level but exiting the neural tube later began to enter a ventral pathway. Interestingly, the cells that entered the ventral pathway ultimately contributed to the peripheral nervous system and enteric nervous system. Focal electroporation experiments performed at individual somite levels found that the more rostrally-derived cells populated rostral portions of the enteric nervous system (i.e. anterior foregut), whereas caudally-derived cells migrated to more distal portions of the developing gut (Kuo and Erickson, 2011). This is largely consistent with more recent GFP tissue grafting experiments in chick, though only cardiac level neural crest from somites 1 to 2 were found to contribute to both the heart structures and the enteric nervous system, whereas neural crest from somites 3 to 7 were found only in the ventral pathway, and only in sympathetic and enteric derivatives (Espinosa-Medina et al., 2017).
Despite these subtle differences in migratory behaviors, labeled cells from the mouse cardiac neural crest region similarly populated the 3rd, 4th, and 6th pharyngeal arches and eventually traversed to the outflow tract, though the major contribution to the outflow tract appeared to come from the level of somite 2 (Chan et al., 2004). It should be noted, however, that this study thoroughly examined the cardiac neural crest contribution from somites 1 through 4, and identified a contribution of vagal neural crest from somite 5 to the foregut. However, whether or not the level of somites 1-4 also contributed to the rostral portion of the gut [as described in chick (Kuo and Erickson, 2011)] has yet to be examined. Thus, further studies are needed to examine whether or not mammalian cardiac neural crest that enter the medial or intersomitic pathways may likewise contribute to the enteric nervous system, depending on pathway choice.
4.2. Cardiac neural crest in anamniotes
Though vertebrate heart development shares many common features in terms of morphogenetic movements, specification of the heart fields, and cardiac marker gene expression (Gessert and Kohl, 2009), the overall structure of the heart and accessory tissues varies between amniotes and anamniotes. As such, the cardiac population arises from a broader subsection of vagal neural crest and encompasses different derivatives in anamniotes, when compared to chick and mammals. In Xenopus, for example, a combination of ablation and grafting experiments recently established the cardiac neural crest as arising from the level of somites 1-4 and partially overlapping with caudal cranial neural crest (comprising the hyoid and branchial streams) (Lee and Saint-Jeannet, 2011). Furthermore, the frog heart is three-chambered and contains a single ventricle with incomplete septation of the outflow tract by a spiral valve (Kolker et al., 2000). Given the lack of an aorticopulmonary septum (which is cardiac neural crest-derived in amniotes), it is therefore not surprising that Xenopus cardiac neural crest does not contribute to the outflow tract. However, transplantation and lineage tracing experiments revealed cardiac neural crest contribution to the aortic arch arteries, as in amniotes, and the aortic sac, which lies through the spiral valve across from the outflow tract (Lee and Saint-Jeannet, 2011; Mohun et al., 2000). The transcription factor Ets1 was found to be important for development of the aortic arch arteries in Xenopus, as its perturbation caused defects in cardiac neural crest migration leading to arch artery malformation (Nie and Bronner, 2015). Interestingly, in humans Ets1 is critically important for cardiovascular development, such that deletions in its genomic region (associated with Jacobsen Syndrome) result in septation defects (Grossfeld et al., 2004). Similarly, Ets1 knockout mice exhibit similar congenital heart defects, thought to arise from defects in cardiac neural crest migration to the outflow tract (Gao et al., 2010; Ye et al., 2010).
Zebrafish, like Xenopus, have differences in heart structure compared to amniotes, and as such, differences in cardiac neural crest origin and derivative contribution. Like other vertebrates, zebrafish mesodermal precursors from the first heart field are specified on the left and right sides of the gastrulating embryo, then migrate inward to the midline and fuse to form the contractile heart tube. However, the zebrafish heart contains a single atrium and ventricle, and, like Xenopus, is not septated (Grant et al., 2017). Lineage tracing experiments found neural crest contribution to the heart, with the cardiac population arising from the first rhombomere of the hindbrain to the caudal limit of the sixth somite (Cavanaugh et al., 2015; Sato and Yost, 2003). The cardiac neural crest cells were observed migrating toward specified cardiac precursors, and adopted a myocardial cell fate when in proximity to the fused heart tube, becoming contractile cardiomyocytes populating the outflow tract. Subsequent targeted chemical ablation of the neural crest caused defects in development of the myocardium, both from the loss of neural crest-derived cardiomyocytes, and loss of cardiac progenitor contribution from the second heart field, suggesting that zebrafish cardiac neural crest interact with and/or recruit second heart field cells (Cavanaugh et al., 2015). An interaction of cardiac neural crest with the second heart field has been similarly described in both chick (Waldo et al., 2005) and Xenopus (Nie and Bronner, 2015). Thus, despite differences in regionalization and derivatives, useful comparisons can be drawn across species to identify genes important for cardiac neural crest migration and development.
5. Vagal neural crest contribution to the lung ganglia
The respiratory tract, encompassing the trachea and lungs, is endoderm-derived and develops as outgrowths of the foregut. During embryogenesis, the lung buds form in the ventral wall of the primitive gut tube in a region called the presumptive respiratory field (Motoyama et al., 1998; loannides et al., 2002; Sakiyama et al., 2003), which corresponds to the tracheal region of the foregut. A number of morphogenetic movements subsequently divide the future respiratory tract from the digestive tract to form a pair of primary lung buds, which elongate and branch extensively to give rise to the bronchial tree. Airway smooth muscle surrounds the epithelial buds and the bronchial tree and contracts to move lung fluid within the bronchial tubules during development, which is essential for stimulating further prenatal lung growth and myogenesis (Schittny et al., 2000).
An extensive network of ganglia that arises from vagal neural crest cells is closely associated with the airway smooth muscle in a range of species including chick (Burns and Delalande, 2005), mouse (Freem et al., 2010) and human (Burns et al., 2008). Vagal neural crest cells that have entered the foregut migrate tangentially into the primary lung buds. This subset of vagal neural crest subsequently differentiates into neurons and glia to form the autonomic airway ganglia in chicken (HH20), mouse (E10.5) and human (E8-10). In addition to the intrinsic innervation directly provided by the vagal neural crest, extrinsic pulmonary innervation arises from the jugular ganglia and dorsal root ganglia, both of which are populated by somatic sensory neurons that are of vagal neural crest origin (Baker, 2005; Kwong et al., 2008; Nassenstein et al., 2010; Springall et al., 1987; Yntema, 1944).
The first experimental demonstrations of vagal neural crest contribution to the intrinsic ganglia of the lung came from quail-chick interspecies grafting experiments, which determined the associated neurons and glia originated from the vagal neural crest region (Burns and Delalande, 2005). These observations were further supported by back transplantation of GFP tissues in chick (Freem et al., 2012) and correlated with immunohistochemical findings in human fetal lung tissue that contained airway smooth muscle closely associated with an extensive neural network consisting of neurons and glia, positive for the neural crest marker, p75 (Burns et al., 2008). These cells were also shown to express the neural crest specifier gene Sox10, and receptors RET and EdnrB in chick, while the Ret co-receptor, GFRα1, is expressed in the mesenchyme and in ganglia in both chick and human (Burns and Delalande, 2005; Burns et al., 2008), reminiscent of the gut mesenchyme through which enteric neural crest cells migrate. These findings suggest that vagal crest cells may use mechanisms to invade the lung buds similar to those of enteric precursors migrating into the gut (Burns and Delalande, 2005).
In mice, lineage-tracking experiments using Wnt1:Cre fluorescent reporter mice have confirmed contributions of vagal neural crest into the lung ganglia innervation (Freem et al., 2010) (Fig. 3, Supplemental Movie S1), similar to observations in chick (Freem et al.,2012; Freem et al., 2010). YFP-labeled vagal neural crest cells migrated into the mouse lung at embryonic day 10.5, and ultimately differentiated into the lung ganglia surrounding the bronchi and epithelial tubules. Organotypic cultures from the Wnt1:Cre reporter mice also revealed that these YFP-positive cells responded chemoattractively to the RET ligand GDNF, suggesting the RET signaling pathway is involved in neural crest pathfinding and development in the lung. However, examination of lungs from Ret−/− embryos showed no apparent defects in the extent of total lung innervation, though this may be due to compensation from extrinsic innervation, which may be RET-independent (Freem et al., 2010; Langsdorf et al., 2011). Together, these studies establish a vagal neural crest contribution to the lung ganglia across several species.
6. Vagal neural crest contribution to the pancreas ganglia
The pancreas plays a vital role in regulation of blood sugar levels via insulin and glucagon secretion into the bloodstream and regulates digestion via secretion of digestive enzymes into the intestine. Accordingly, it is composed of various types of specialized cells—including insulin-producing β-cells, glucagon-producing α-cells, somatostatin-producing δ-cells, polypeptide-producing cells and ghrelin-producing ε-cells (Gittes, 2009).
During development, the pancreas anlage form as bud evaginations of the foregut endoderm (Gittes, 2009). Ventral and dorsal pancreatic buds sprout along the lateral sides of the foregut endoderm into the surrounding mesenchyme, posterior to the entry point of neural crest cells into the foregut. In mouse, the sprouted buds first appear around E9.5 (Wessells and Cohen, 1967), by E11 in rat embryos (Richardson and Spooner, 1977), and by E3-E4 (HH18-23) in chick (Patten, 1951). The ventral and dorsal buds subsequently fuse into a single primordium.
The mature pancreas is highly innervated extrinsically by the vagus nerve, splanchnic nerve and sympathetic nervous system (Salvioli et al., 2002). While it has been known for many years that the pancreas also contains resident ganglia (Fujita, 1959), their origin from the vagal neural crest was established from studies spanning more than thirty years (Fontaine et al., 1977; Kirchgessner et al., 1992; Nekrep et al., 2008; Plank et al., 2011). Quail-chick grafting by Fontaine et al (1977) showed that vagal neural crest cells from somite levels 1-7 migrate and integrate into the developing pancreatic buds to give rise to the resident neurons in ganglia and Schwann cells surrounding nerve bundles. A subsequent study showed that vagal neural crest cells must first migrate into and through the foregut mesenchyme and then secondarily enter the pancreatic buds to integrate into the developing pancreas of the rat at ~ E12-E13 (Kirchgessner et al., 1992). In mouse embryos, the timing of arrival of neural crest cells occurs around E10 (Plank et al., 2011), as assayed by Wnt1:Cre lineage tracing. Similar lineage analyses revealed that neural crest cells invaded around the pancreatic islets, near α- and β-cells (Arntfield and van der Kooy, 2013; Shimada et al., 2012), where they give rise to neurons and Schwann cells surrounding the islets. Furthermore, it was found that Wnt1:Cre signal co-localized with Phox2b+ neuronal progenitors in the pancreas, consistent with a neural crest origin (Nekrep et al., 2008).
In addition to sharing a foregut entry point with enteric-fated vagal neural crest cells, pancreatic-bound vagal neural crest cells also appear to utilize similar gene batteries for neuronal differentiation. For example, loss of Phox2b led to a complete absence of neurons and glial cells in the pancreas, due to neural crest cell death and diminished migration into the foregut (Nekrep et al., 2008). Similarly, Foxd3 knockout leads to total loss of vagal neural crest cell migration into the pancreas and the absence of neurons, again suggesting that neural crest invasion of the pancreas is vital (Plank et al., 2011). In addition to Phox2b, neural crest cells that have newly arrived within the pancreas express Sox10, mirroring the sequence of gene expression seen in enteric progenitors along the gut (Nekrep et al., 2008).
In the mouse pancreas, all neuronal progenitors continue to express Phox2b during differentiation, while glial cells expressed Sox10. Moreover, similar to vagal neural crest migrating into the gut and lung buds, GDNF expression in the pancreas is required for neural crest colonization of the pancreas. GDNF is expressed within the pancreatic epithelium between E10.5-E15.5, coincident with neural crest entry into the developing pancreas. In addition, expression of other GDNF signaling pathway components, such as Ret and GFR α 1, was observed in the pancreas. Moreover, GDNF−/− mouse exhibit loss of neurons and glial cells in the pancreas (Munoz-Bravo et al., 2013), providing genetic evidence of GDNF’s requirement for neural crest contribution to the pancreas.
Taken together, these lineage studies in cardiac, lung, pancreas, and thymus highlight the important roles of the vagal neural crest for development of diverse organs. In particular, pancreatic- and lung-bound vagal neural crest cells first enter the foregut, then spread secondarily into the primitive pancreas or lung buds in order to become intrinsic ganglia. While the detailed studies mentioned here clearly reveal a neural crest contribution to the pancreas and lung ganglia, many questions remain: What controls their choice of migratory pathways to the gut tube versus pancreas, lung or thymic rudiments? What dictates their cell fate? Importantly, in light of recent findings of vagal nerve-associated neural crest cells that migrate into the gut and express markers of Schwann cell precursors [see review by (Furlan and Adameyko, 2018)], it will be important to decipher whether or not this subpopulation also plays a role in pancreas and/or lung ganglia development, and if they retain multipotent properties following colonization.
7. Molecular underpinnings of vagal neural crest development
Many conserved genes play key roles in formation and migration of vagal neural crest during development. Not surprisingly, key transcription factors of the neural crest gene regulatory network that are required for the specification of neural crest cells at other axial levels [reviewed in (Green et al., 2015; Martik and Bronner, 2017)] also are required at the vagal level. For example, loss of Sox10, Foxd3, Tfap2 α and Pax3 all exhibit reduction or loss of vagal neural crest numbers and defects in vagal neural crest-derived structures, such as the ENS and other elements of the peripheral nervous system (Carney et al., 2006; Kapur, 1999; Knight et al., 2003; Lang et al., 2000; Montero-Balaguer et al., 2006; Southard-Smith et al., 1998). Of interest, the transcription factor and BMP signaling target Sip1 (Smad-interacting protein-1) is expressed in the cranial and vagal neural tube and its expression is maintained in migrating neural crest cells (Delalande et al., 2008b; Rogers et al., 2013; Van de Putte et al., 2003). Sip1 mutation leads to neural tube closure and vagal neural crest formation defects in mice and zebrafish (Delalande et al., 2008b; Van de Putte et al., 2003). Subsequently, this results in loss of vagal neural crest cell numbers and defects including absence of enteric ganglia along variable lengths of the gut, as in Hirschsprung’s disease (Delalande et al., 2008b; Van de Putte et al., 2003; Wakamatsu et al., 2001). Sip1 is required for the proper expression of Sox10 and represses the expression of E-cadherin in mouse and chicken embryos (Rogers et al., 2013; Van de Putte et al., 2003). An intriguing possibility is that Sip1 loss alters neural crest cell migration via causing cell-cell adhesion defects, which may partially underlie some of the vagal-derived tissue defects observed in these phenotypes.
Other factors that influence vagal neural crest development include the Treacher Collins Syndrome-associated gene, Treacle ribosome biogenesis factor 1 (Tcof1), which has recently been implicated as a modifier of Pax3 in Hirschsprung’s disease. While Tcof1 +/− mice show reduced numbers of vagal neural crest cells and hypoganglionosis of the ENS, Tcof1+/−; Pax3+/− mice present with increased cell death of the vagal neural crest progenitor pool and colonic aganglionosis, demonstrating that Tcof1 and Pax3 synergistically function to control vagal neural crest cell numbers during development (Barlow et al., 2008). In addition, Pax3−/− mice exhibit enteric aganglionosis phenotype (Lang et al., 2000). Consistent with this, loss of pax3 in zebrafish results in a reduction of post-otic sox10+ vagal neural crest cells and gut aganglionosis (Minchin and Hughes, 2008). One important target of Pax3 in the vagal neural crest is the receptor tyrosine kinase RET proto-oncogene (Ret). Mechanistically, Pax3 synergistically acts with Sox10 to activate expression of Ret (Lang et al., 2000; Lang and Epstein, 2003), presumably acting in a vagal-specific gene regulatory battery.
Interestingly, Ret expression is not found at all axial levels, despite the importance of Pax3 and Sox10 in other neural crest cells. Ret expression is regionalized along the vagal neural tube in an anterior-posterior domain between rhombomeres 5/6 and somite 7, precisely along the vagal level neural tube (Robertson and Mason, 1995). Ret is then maintained in migrating vagal neural crest cells (Heanue and Pachnis, 2008; Iwashita et al., 2003; Robertson and Mason, 1995) as well as in vagal neural crest derivatives, such as the ENS and the sympathetic ganglia (Avantaggiato et al., 1994; Durbec et al., 1996; Pachnis et al., 1993). Ligands from the glial derived neurotrophic factor (GDNF) family activate Ret together with a coreceptor from the GFRα family [reviewed in (Takahashi, 2001)]; disruption of this signaling pathway results in decreased enteric colonization and a Hirschsprung’s phenotype (Durbec et al., 1996; Fernandez et al., 2008; Gui et al., 2013; Hearn et al., 1998; Ruiz-Ferrer et al., 2011; Salomon et al., 1996; Uesaka and Enomoto, 2010). Ectopic Ret activation potently enhances invasion of sacral neural crest cells into the colon in quail/chick chimeras (Delalande et al., 2008a). Together these results implicate Ret signaling as a critical regulator of vagal neural crest development.
Notably, the application of the natural vitamin A derivative, Retinoic Acid (RA), is sufficient to expand the domain of Ret expression rostrally in the developing neural tube in vivo (Robertson and Mason, 1995) and in culture (Simkin et al., 2013). While the 5’ upstream region of the Ret locus contains putative Retinoic Acid Response Elements (RARE) sequences, these failed to be activated in in vitro assays (Patrone et al., 1997). However, Ret can be co-activated by the transcription factors Hoxb5, Sox10, Pax3 and Nkx2-1 via conserved cis-regulatory elements (Lang et al., 2000; Lang and Epstein, 2003; Zhu et al., 2011; Zhu et al., 2014), raising the intriguing possibility that RA works indirectly through these transcription factors. From these studies, it is clear that Ret expression is controlled by multiple conserved inputs, highlighting the importance of this GRN during vagal neural crest development, migration, and evolution.
Consistent with the indirect effects of RA on Ret expression, the posterior Hox transcription factor, Hoxb5, is a target of RA signaling that regulates Ret expression (Fu et al., 2003; Kuratani and Wall, 1992; Lui et al., 2008; Wall et al., 1992). Hoxb5 binds to intron 1 in a highly conserved sequence of the Ret gene, leading to changes in histone modifications and enhanced Ret transcription (Zhu et al., 2014). Loss of Hoxb5 signaling in vagal neural crest cells leads to a cell autonomous downregulation of Ret expression, a decrease in colonization of the gut by enteric neural crest, and a Hirschsprung’s phenotype (Lui et al., 2008). Moreover, loss of function of Hoxb5 in all neural crest cells largely affects vagal neural crest derivatives causing increased apoptosis, reduced sympathetic and dorsal root ganglia, and ENS defects (Kam et al., 2014). Hence, it is clear that alterations at any level within the Ret gene regulatory network (Pax3, Sox10, Hoxb5, etc.) lead to detrimental changes in neural crest cell proliferation and survival, thereby affecting further development of vagal neural crest derivatives.
Vagal neural crest proliferation and survival are also influenced by other tissue-specific factors. For instance, the cell cycle-associated protein Geminin is expressed in migrating neural crest cells en route to the heart and gut at E10.5 in mouse, and in other neural crest-derived structures, such as the sympathetic ganglia and dorsal root ganglia. Following neural crest-specific loss of Geminin, a reduction in the vagal neural crest pool was observed that correlated with reduced proliferation and survival of enteric neural crest cells along the gut (Stathopoulou et al., 2016), leading to colonic aganglionosis. In zebrafish embryos, the transcription factor Meis3, a TALE-domain containing protein and known Hox/Pbx co-factor (Choe et al., 2009; Vlachakis et al., 2001), is expressed in migrating vagal neural crest cells en route to the gut and in the mesenchyme (Uribe and Bronner, 2015). Reduction of Meis3 led to decreased numbers of vagal neural crest that invaded the foregut as a result of reduced proliferation and inefficient migration along the gut, and consequently lead to colonic aganglionosis (Uribe and Bronner, 2015). In addition, loss of Meis and Pbx protein function led to severe vagal neural crest deficits and total gut aganglionosis (Uribe and Bronner, 2015), highlighting the importance of Hox/Pbx/Meis transcriptional control in vagal neural crest development.
In the mouse, knockout of the related gene, Meis2, results in hemorrhaging along with cardiac septation defects. Neural crest cell numbers are reduced in the cardiac outflow tract of Meis2−/− embryos, suggesting defects in cardiac neural crest cell migration, proliferation and/or survival (Machon et al., 2015). Moreover, Machon and colleagues noted that Meis2 was strongly expressed in the neural plate, neural tube and in migrating Tfap2+ neural crest cells between E8.5-10.5, suggesting an early functional role in vagal neural crest cells. Meis2 plays an autonomous role in the neural crest since conditional inactivation of Meis2 in Tfap2+ neural crest cells also led to defects in cardiac neural crest localization to the heart and malformed valves (Machon et al., 2015). These results suggest that Meis2 is functionally important during cardiac neural crest development and add to previous evidence that Pbx family proteins also play important roles during cardiac outflow tract formation in mouse (Stankunas et al., 2008). Collectively, these studies suggest that Meis/Pbx family proteins serve as regulators of vagal neural crest development. Notably, Meis family proteins are known to be downstream targets of the RA signaling pathway in multiple contexts (Mercader et al., 2005; Qin et al., 2002), raising the possibility that Meis proteins regulate vagal neural crest function through the activation of, or in parallel with, Ret transcription.
During early development, RA is fundamental for the survival and differentiation of vagal neural crest cells. Knockout of the gene responsible for most retinoic acid synthesis during embryonic development, Aldh1a2 (formerly Raldh2), results in impaired migration and survival of post-otic neural crest cells, complete loss of the enteric nervous system, and drastic malformations in septation of the cardiac outflow tract (Niederreither et al., 2003). Recently, zebrafish ret and meis3 have been placed epistatically downstream of RA signaling in vagal neural crest cells, as their ectopic expression was able to restore enteric neuron presence in the foregut and midgut of RA-deficient embryos (Uribe et al., 2018).
Taken together, these studies show that numerous neural crest transcription factors and signaling pathways play fundamental roles in early vagal neural crest ontogenesis. These function in combination with vagal tissue-specific factors to shape the final destiny of these cells into a myriad of vagal neural crest derivatives. The next important steps toward fully understanding the molecular pathways that define the vagal neural crest include formulation of a more complete vagal neural crest gene regulatory network, and testing the influences of the local microenvironment on neural crest migration and differentiation.
8. Future Perspectives
Together with a wealth of classical experiments, new discoveries are being made in elucidating vagal neural crest migratory pathways and tissue contributions, largely due to significant improvements in fluorescent and genetic lineage tracing methods. Across several species, these new techniques are serving to both corroborate and extend classical work, to precisely characterize migratory pathways and identify new signaling pathways and vagal neural crest derivatives.
A number of intriguing questions remain outstanding. First, what are the exact axial levels of origin of vagal neural crest cells that contribute to particular derivatives, such as the pancreas, thymus or the lung? Given the broad knowledge derived from classical experiments, new lineage tracing methods will improve perdurance of signal, while also allowing more precise and region-specific labeling, which will help to conclusively determine the precise origin and pathway choices of vagal neural crest derivatives. This may also help better understand various birth defects that originate due to problems in vagal neural crest development. Second, what are the unique and shared vagal crest derivatives across species? Further examination of species-specific differences in vagal neural crest contributions may provide insight into the whether and how vagal crest derivatives have expanded during the course of vertebrate evolution. Finally, what makes the vagal neural crest population unique and different from other neural crest populations? A more complete view of the gene regulatory network operating in the vagal neural crest will foster deeper understanding of the control and developmental potential of this fascinating cell population.
Supplementary Material
Supplemental Movie1 S1. Time lapse movie of stills from Fig. 3. Dissected lungs from transgenic Wnt1 -Cre/ZEG mouse embryos at E11.5 were imaged by live time lapse microscopy for approximately 48 hours. Over the course of branching morphogenesis, EGFP-labeled neural crest cells appear to migrate extensively through the lung mesenchyme. (Movie acquired at the 2014 Marine Biological Laboratory Embryology course, with the assistance of Dr. Angelo Iulianella and the mouse module).
Highlights.
Vagal neural crest cells originate from somite levels 1-7 in most jawed vertebrates
Vagal neural crest cells embark on diverse migratory routes
Vagal neural crest cells contribute to tissues in a spectrum of organs including the thymus, heart, and the pancreatic and lung ganglia.
Acknowledgments
We would like to thank Dr. Max Ezin for helpful discussion, Weiyi Tang for providing images in Figure 2, and Stephen Green for consultation on vagal neural crest. This work was supported by a Cancer Prevention & Research Institute of Texas (CPRIT) Recruitment grant to RAU, NIH DE024157 and HD037105 to MEB, and Ruth L. Kirschstein NRSAs F32DE026355, F32HD087026 and F32HD088022 to EJH, EK and MLP, respectively.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RB, Stewart AL, Young HM, 2006. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell and tissue research 323, 11–25. [DOI] [PubMed] [Google Scholar]
- Arntfield M, van der Kooy D, 2013. The adult mammalian pancreas contains separate precursors of pancreatic and neural crest developmental origins. Stem Cells Dev 22, 2145–2157. [DOI] [PubMed] [Google Scholar]
- Avantaggiato V, Dathan NA, Grieco M, Fabien N, Lazzaro D, Fusco A, Simeone A, Santoro M, 1994. Developmental expression of the RET protooncogene. Cell Growth Differ 5, 305–311. [PubMed] [Google Scholar]
- Baker C, 2005. The embryology of vagal sensory neurons, in: Undem BJ WD (Ed.), Advances in Vagal Afferent Neurobiology. CRC, Boca Raton, FL, pp. 3–26. [Google Scholar]
- Baker CV, Bronner-Fraser M, Le Douarin NM, Teillet MA, 1997. Early- and late-migrating cranial neural crest cell populations have equivalent developmental potential in vivo. Development 124, 3077–3087. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Wallace AS, Thapar N, Burns AJ, 2008. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development 135, 1681–1691. [DOI] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Holzschuh J, Driever W, Knapik EW, 2004. Neural crest survival and differentiation in zebrafish depends on mont blanc/tfap2a gene function. Development 131, 1463–1477. [DOI] [PubMed] [Google Scholar]
- Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC, 1998. Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circulation research 82, 221–231. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, 1986. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Developmental biology 115, 44–55. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Champeval D, Le Douarin NM, 2000. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Developmental biology 219, 30–43. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Delalande JM, 2005. Neural crest cell origin for intrinsic ganglia of the developing chicken lung. Developmental biology 277, 63–79. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Thapar N, Barlow AJ, 2008. Development of the neural crest-derived intrinsic innervation of the human lung. Am J Respir Cell Mol Biol 38, 269–275. [DOI] [PubMed] [Google Scholar]
- Carney TJ, Dutton KA, Greenhill E, Delfino-Machin M, Dufourcq P, Blader P, Kelsh RN, 2006. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development 133, 4619–4630. [DOI] [PubMed] [Google Scholar]
- Cavanaugh AM, Huang J, Chen JN, 2015. Two developmentally distinct populations of neural crest cells contribute to the zebrafish heart. Developmental biology 404, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Cheung CS, Yung KM, Copp AJ, 2004. Cardiac neural crest of the mouse embryo: axial level of origin, migratory pathway and cell autonomy of the splotch (Sp2H) mutant effect. Development 131, 3367–3379. [DOI] [PubMed] [Google Scholar]
- Choe SK, Lu P, Nakamura M, Lee J, Sagerstrom CG, 2009. Meis cofactors control HDAC and CBP accessibility at Hox-regulated promoters during zebrafish embryogenesis. Developmental cell 17, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delalande JM, Barlow AJ, Thomas AJ, Wallace AS, Thapar N, Erickson CA, Burns AJ, 2008a. The receptor tyrosine kinase RET regulates hindgut colonization by sacral neural crest cells. Developmental biology 313, 279–292. [DOI] [PubMed] [Google Scholar]
- Delalande JM, Guyote ME, Smith CM, Shepherd IT, 2008b. Zebrafish sip1a and sip1b are essential for normal axial and neural patterning. Developmental dynamics : an official publication of the American Association of Anatomists 237, 1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V, 1996. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development 122, 349–358. [DOI] [PubMed] [Google Scholar]
- Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN, 2001. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development 128, 4113–4125. [DOI] [PubMed] [Google Scholar]
- Elworthy S, Pinto JP, Pettifer A, Cancela ML, Kelsh RN, 2005. Phox2b function in the enteric nervous system is conserved in zebrafish and is sox10-dependent. Mechanisms of development 122, 659–669. [DOI] [PubMed] [Google Scholar]
- Epperlein HH, Krotoski D, Halfter W, Frey A, 1990. Origin and distribution of enteric neurones in Xenopus. Anat Embryol (Berl) 182, 53–67. [DOI] [PubMed] [Google Scholar]
- Espinosa-Medina I, Jevans B, Boismoreau F, Chettouh Z, Enomoto H, Muller T, Birchmeier C, Burns AJ, Brunet JF, 2017. Dual origin of enteric neurons in vagal Schwann cell precursors and the sympathetic neural crest. Proceedings of the National Academy of Sciences of the United States of America 114, 11980–11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez RM, Ruiz-Ferrer M, Lopez-Alonso M, Antinolo G, Borrego S, 2008. Polymorphisms in the genes encoding the 4 RET ligands, GDNF, NTN, ARTN, PSPN, and susceptibility to Hirschsprung disease. J Pediatr Surg 43, 2042–2047. [DOI] [PubMed] [Google Scholar]
- Fontaine J, Le Lievre C, Le Douarin NM, 1977. What is the developmental fate of the neural crest cells which migrate into the pancreas in the avian embryo? Gen Comp Endocrinol 33, 394–404. [DOI] [PubMed] [Google Scholar]
- Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D, Coles M, 2008. Contribution of neural crest-derived cells in the embryonic and adult thymus. Journal of immunology 180, 3183–3189. [DOI] [PubMed] [Google Scholar]
- Franz T, 1989. Persistent truncus arteriosus in the Splotch mutant mouse. Anat Embryol (Berl) 180, 457–464. [DOI] [PubMed] [Google Scholar]
- Freem LJ, Delalande JM, Campbell AM, Thapar N, Burns AJ, 2012. Lack of organ specific commitment of vagal neural crest cell derivatives as shown by back-transplantation of GFP chicken tissues. The International journal of developmental biology 56, 245–254. [DOI] [PubMed] [Google Scholar]
- Freem LJ, Escot S, Tannahill D, Druckenbrod NR, Thapar N, Burns AJ, 2010. The intrinsic innervation of the lung is derived from neural crest cells as shown by optical projection tomography in Wnt1-Cre;YFP reporter mice. J Anat 217, 651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Lui VC, Sham MH, Cheung AN, Tam PK, 2003. HOXB5 expression is spatially and temporarily regulated in human embryonic gut during neural crest cell colonization and differentiation of enteric neuroblasts. Developmental dynamics : an official publication of the American Association of Anatomists 228, 1–10. [DOI] [PubMed] [Google Scholar]
- Fujita T, 1959. Histological studies on the neuro-insular complex in the pancreas of some mammals. Zeitschrift für Zellforschung und Mikroskopische Anatomie 50, 94–109. [Google Scholar]
- Furlan A, Adameyko I, 2018. Schwann cell precursor: a neural crest cell in disguise? Developmental biology. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Bronner ME, 2018. Insights into neural crest development from studies of avian embryos. The International journal of developmental biology 62, 183–194. [DOI] [PubMed] [Google Scholar]
- Ganz J, 2018. Gut feelings: Studying enteric nervous system development, function, and disease in the zebrafish model system. Developmental dynamics : an official publication of the American Association of Anatomists 247, 268–278. [DOI] [PubMed] [Google Scholar]
- Ganz J, Melancon E, Eisen JS, 2016. Zebrafish as a model for understanding enteric nervous system interactions in the developing intestinal tract. Methods in cell biology 134, 139–164. [DOI] [PubMed] [Google Scholar]
- Gao Z, Kim GH, Mackinnon AC, Flagg AE, Bassett B, Earley JU, Svensson EC, 2010. Ets1 is required for proper migration and differentiation of the cardiac neural crest. Development 137, 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes GK, 2009. Developmental biology of the pancreas: a comprehensive review. Developmental biology 326, 4–35. [DOI] [PubMed] [Google Scholar]
- Grant MG, Patterson VL, Grimes DT, Burdine RD, 2017. Modeling Syndromic Congenital Heart Defects in Zebrafish. Curr Top Dev Biol 124, 1–40. [DOI] [PubMed] [Google Scholar]
- Green SA, Simoes-Costa M, Bronner ME, 2015. Evolution of vertebrates as viewed from the crest. Nature 520, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Uy BR, Bronner ME, 2017. Ancient evolutionary origin of vertebrate enteric neurons from trunk-derived neural crest. Nature 544, 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossfeld PD, Mattina T, Lai Z, Favier R, Jones KL, Cotter F, Jones C, 2004. The 11q terminal deletion disorder: a prospective study of 110 cases. American journal of medical genetics. Part A 129A, 51–61. [DOI] [PubMed] [Google Scholar]
- Gui H, Tang WK, So MT, Proitsi P, Sham PC, Tam PK, Ngan ES, Cherny SS, Garcia-Barcelo MM, 2013. RET and NRG1 interplay in Hirschsprung disease. Hum Genet 132, 591–600. [DOI] [PubMed] [Google Scholar]
- Harris ML, Erickson CA, 2007. Lineage specification in neural crest cell pathfinding. Developmental dynamics : an official publication of the American Association of Anatomists 236, 1–19. [DOI] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V, 2008. Ret isoform function and marker gene expression in the enteric nervous system is conserved across diverse vertebrate species. Mechanisms of development 125, 687–699. [DOI] [PubMed] [Google Scholar]
- Hearn CJ, Murphy M, Newgreen D, 1998. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Developmental biology 197, 93–105. [DOI] [PubMed] [Google Scholar]
- Hollander GA, Peterson P, 2009. Learning to be tolerant: how T cells keep out of trouble. J Intern Med 265, 541–561. [DOI] [PubMed] [Google Scholar]
- Iwashita T, Kruger GM, Pardal R, Kiel MJ, Morrison SJ, 2003. Hirschsprung disease is linked to defects in neural crest stem cell function. Science 301, 972–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM, 2000. Fate of the mammalian cardiac neural crest. Development 127, 1607–1616. [DOI] [PubMed] [Google Scholar]
- Kam MK, Cheung MC, Zhu JJ, Cheng WW, Sat EW, Tam PK, Lui VC, 2014. Perturbation of Hoxb5 signaling in vagal and trunk neural crest cells causes apoptosis and neurocristopathies in mice. Cell Death Differ 21, 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur RP, 1999. Early death of neural crest cells is responsible for total enteric aganglionosis in Sox10(Dom)/Sox10(Dom) mouse embryos. Pediatr Dev Pathol 2, 559–569. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Eisen JS, 2000. The zebrafish colourless gene regulates development of non-ectomesenchymal neural crest derivatives. Development 127, 515–525. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE, 1983. Neural crest cells contribute to normal aorticopulmonary septation. Science 220, 1059–1061. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Hutson MR, 2010. Factors controlling cardiac neural crest cell migration. Cell Adh Migr 4, 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML, Kumiski DH, Myers T, Cerjan C, Mishima N, 1993. Backtransplantation of chick cardiac neural crest cells cultured in LIF rescues heart development. Developmental dynamics : an official publication of the American Association of Anatomists 198, 296–311. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Stewart DE, 1983. Neural crest origin of cardiac ganglion cells in the chick embryo: identification and extirpation. Developmental biology 97, 433–443. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Turnage KL, Hays BM, 1985. Characterization of conotruncal malformations following ablation of "cardiac" neural crest. The Anatomical record 213, 87–93. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Adlersberg MA, Gershon MD, 1992. Colonization of the developing pancreas by neural precursors from the bowel. Developmental dynamics : an official publication of the American Association of Anatomists 194, 142–154. [DOI] [PubMed] [Google Scholar]
- Knight RD, Nair S, Nelson SS, Afshar A, Javidan Y, Geisler R, Rauch GJ, Schilling TF, 2003. lockjaw encodes a zebrafish tfap2a required for early neural crest development. Development 130, 5755–5768. [DOI] [PubMed] [Google Scholar]
- Kolker SJ, Tajchman U, Weeks DL, 2000. Confocal imaging of early heart development in Xenopus laevis. Developmental biology 218, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotoski DM, Fraser SE, Bronner-Fraser M, 1988. Mapping of neural crest pathways in Xenopus laevis using inter- and intra-specific cell markers. Developmental biology 127, 119–132. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Bailey CM, Kasemeier-Kulesa JC, McLennan R, 2010. Cranial neural crest migration: new rules for an old road. Developmental biology 344, 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz A, 1910. The development of the sympathetic nervous system in mammals. J. Comp. Neurol. Psychol 20, 211–258. [Google Scholar]
- Kuo BR, Erickson CA, 2010. Regional differences in neural crest morphogenesis. Cell Adh Migr 4, 567–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo BR, Erickson CA, 2011. Vagal neural crest cell migratory behavior: a transition between the cranial and trunk crest. Developmental dynamics : an official publication of the American Association of Anatomists 240, 2084–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani S, Bockman DE, 1990. Impaired development of the thymic primordium after neural crest ablation. The Anatomical record 228, 185–190. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Bockman DE, 1991. Capacity of neural crest cells from various axial levels to participate in thymic development. Cell and tissue research 263, 99–105. [DOI] [PubMed] [Google Scholar]
- Kuratani SC, Kirby ML, 1991. Initial migration and distribution of the cardiac neural crest in the avian embryo: an introduction to the concept of the circumpharyngeal crest. Am J Anat 191, 215–227. [DOI] [PubMed] [Google Scholar]
- Kuratani SC, Wall NA, 1992. Expression of Hox 2.1 protein in restricted populations of neural crest cells and pharyngeal ectoderm. Developmental dynamics : an official publication of the American Association of Anatomists 195, 15–28. [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, Oda H, Maemura K, Nagai R, Ishikawa T, Yazaki Y, 1995. Aortic arch malformations and ventricular septal defect in mice deficient in endothelin-1. J Clin Invest 96, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ, 2008. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol 295, L858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Chen F, Milewski R, Li J, Lu MM, Epstein JA, 2000. Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-ret. J Clin Invest 106, 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Epstein JA, 2003. Sox10 and Pax3 physically interact to mediate activation of a conserved c-RET enhancer. Human molecular genetics 12, 937–945. [DOI] [PubMed] [Google Scholar]
- Langsdorf A, Radzikinas K, Kroten A, Jain S, Ai X, 2011. Neural crest cell origin and signals for intrinsic neurogenesis in the mammalian respiratory tract. Am J Respir Cell Mol Biol 44, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N, 1982. The Neural Crest. Cambridge University Press, Cambridge. [Google Scholar]
- Le Douarin N, Kalcheim C, 1999. The neural crest, 2nd ed. Cambridge University Press, Cambridge, UK; ; New York, NY, USA. [Google Scholar]
- Le Douarin NM, Jotereau FV, 1975. Tracing of cells of the avian thymus through embryonic life in interspecific chimeras. J Exp Med 142, 17–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA, 1973. The migration of neural crest cells to the wall of the digestive tract in avian embryo. Journal of embryology and experimental morphology 30, 31–48. [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM, 1975. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. Journal of embryology and experimental morphology 34, 125–154. [PubMed] [Google Scholar]
- Lee YH, Saint-Jeannet JP, 2011. Cardiac neural crest is dispensable for outflow tract septation in Xenopus. Development 138, 2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Zdanowicz M, Young L, Kumiski D, Leatherbury L, Kirby ML, 2003. Cardiac neural crest in zebrafish embryos contributes to myocardial cell lineage and early heart function. Developmental dynamics : an official publication of the American Association of Anatomists 226, 540–550. [DOI] [PubMed] [Google Scholar]
- Lui VC, Cheng WW, Leon TY, Lau DK, Garcia-Barcelo MM, Miao XP, Kam MK, So MT, Chen Y, Wall NA, Sham MH, Tam PK, 2008. Perturbation of hoxb5 signaling in vagal neural crests down-regulates ret leading to intestinal hypoganglionosis in mice. Gastroenterology 134, 1104–1115. [DOI] [PubMed] [Google Scholar]
- Luo R, An M, Arduini BL, Henion PD, 2001. Specific pan-neural crest expression of zebrafish Crestin throughout embryonic development. Developmental dynamics : an official publication of the American Association of Anatomists 220, 169–174. [DOI] [PubMed] [Google Scholar]
- Machon O, Masek J, Machonova O, Krauss S, Kozmik Z, 2015. Meis2 is essential for cranial and cardiac neural crest development. BMC Dev Biol 15, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martik ML, Bronner ME, 2017. Regulatory Logic Underlying Diversification of the Neural Crest. Trends Genet. 33(10):715–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader N, Tanaka EM, Torres M, 2005. Proximodistal identity during vertebrate limb regeneration is regulated by Meis homeodomain proteins. Development 132, 4131–4142. [DOI] [PubMed] [Google Scholar]
- Minchin JE, Hughes SM, 2008. Sequential actions of Pax3 and Pax7 drive xanthophore development in zebrafish neural crest. Developmental biology 317, 508–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun TJ, Leong LM, Weninger WJ, Sparrow DB, 2000. The morphology of heart development in Xenopus laevis. Developmental biology 218, 74–88. [DOI] [PubMed] [Google Scholar]
- Montero-Balaguer M, Lang MR, Sachdev SW, Knappmeyer C, Stewart RA, De La Guardia A, Hatzopoulos AK, Knapik EW, 2006. The mother superior mutation ablates foxd3 activity in neural crest progenitor cells and depletes neural crest derivatives in zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists 235, 3199–3212. [DOI] [PubMed] [Google Scholar]
- Muller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson WD, Wegner M, Rodewald HR, 2008. Neural crest origin of perivascular mesenchyme in the adult thymus. Journal of immunology 180, 5344–5351. [DOI] [PubMed] [Google Scholar]
- Munoz-Bravo JL, Hidalgo-Figueroa M, Pascual A, Lopez-Barneo J, Leal-Cerro A, Cano DA, 2013. GDNF is required for neural colonization of the pancreas. Development 140, 3669–3679. [DOI] [PubMed] [Google Scholar]
- Nagy N, Goldstein AM, 2017. Enteric nervous system development: A crest cell's journey from neural tube to colon. Semin Cell Dev Biol 66, 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Nandigama R, Bettner W, Undem BJ, 2010. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol 588, 4769–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrep N, Wang J, Miyatsuka T, German MS, 2008. Signals from the neural crest regulate beta-cell mass in the pancreas. Development 135, 2151–2160. [DOI] [PubMed] [Google Scholar]
- Nie S, Bronner ME, 2015. Dual developmental role of transcriptional regulator Ets1 in Xenopus cardiac neural crest vs. heart mesoderm. Cardiovasc Res 106, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dolle P, 2003. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development 130, 2525–2534. [DOI] [PubMed] [Google Scholar]
- Nishibatake M, Kirby ML, Van Mierop LH, 1987. Pathogenesis of persistent truncus arteriosus and dextroposed aorta in the chick embryo after neural crest ablation. Circulation 75, 255–264. [DOI] [PubMed] [Google Scholar]
- Olden T, Akhtar T, Beckman SA, Wallace KN, 2008. Differentiation of the zebrafish enteric nervous system and intestinal smooth muscle. Genesis 46, 484–498. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F, 1993. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119, 1005–1017. [DOI] [PubMed] [Google Scholar]
- Patrone G, Puliti A, Bocciardi R, Ravazzolo R, Romeo G, 1997. Sequence and characterisation of the RET proto-oncogene 5' flanking region: analysis of retinoic acid responsiveness at the transcriptional level. FEBS Lett 419, 76–82. [DOI] [PubMed] [Google Scholar]
- Patten BM, 1951. The first heart beats and the beginning of the embryonic circulation. Am Sci 39, 225–243. [PubMed] [Google Scholar]
- Pei D, Luther W, Wang W, Paw BH, Stewart RA, George RE, 2013. Distinct neuroblastoma-associated alterations of PHOX2B impair sympathetic neuronal differentiation in zebrafish models. PLoS genetics 9, e1003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-van der Sanden MJ, Luider TM, van der Kamp AW, Tibboel D, Meijers C, 1993. Regional differences between various axial segments of the avian neural crest regarding the formation of enteric ganglia. Differentiation 53, 17–24. [DOI] [PubMed] [Google Scholar]
- Phillips MT, Kirby ML, Forbes G, 1987. Analysis of cranial neural crest distribution in the developing heart using quail-chick chimeras. Circulation research 60, 27–30. [DOI] [PubMed] [Google Scholar]
- Plank JL, Mundell NA, Frist AY, LeGrone AW, Kim T, Musser MA, Walter TJ, Labosky PA, 2011. Influence and timing of arrival of murine neural crest on pancreatic beta cell development and maturation. Developmental biology 349, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Cimildoro R, Kochhar DM, Soprano KJ, Soprano DR, 2002. PBX, MEIS, and IGF-I are potential mediators of retinoic acid-induced proximodistal limb reduction defects. Teratology 66, 224–234. [DOI] [PubMed] [Google Scholar]
- Raible DW, Wood A, Hodsdon W, Henion PD, Weston JA, Eisen JS, 1992. Segregation and early dispersal of neural crest cells in the embryonic zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists 195, 29–42. [DOI] [PubMed] [Google Scholar]
- Reedy MV, Faraco CD, Erickson CA, 1998. Specification and migration of melanoblasts at the vagal level and in hyperpigmented Silkie chickens. Developmental dynamics : an official publication of the American Association of Anatomists 213, 476–485. [DOI] [PubMed] [Google Scholar]
- Richardson KE, Spooner BS, 1977. Mammalian pancreas development: regeneration and differentiation in vitro. Developmental biology 58, 402–420. [DOI] [PubMed] [Google Scholar]
- Robertson K, Mason I, 1995. Expression of ret in the chicken embryo suggests roles in regionalisation of the vagal neural tube and somites and in development of multiple neural crest and placodal lineages. Mechanisms of development 53, 329–344. [DOI] [PubMed] [Google Scholar]
- Rogers CD, Saxena A, Bronner ME, 2013. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. The Journal of cell biology 203, 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ferrer M, Torroglosa A, Luzon-Toro B, Fernandez RM, Antinolo G, Mulligan LM, Borrego S, 2011. Novel mutations at RET ligand genes preventing receptor activation are associated to Hirschsprung's disease. J Mol Med (Berl) 89, 471–480. [DOI] [PubMed] [Google Scholar]
- Sadaghiani B, Thiebaud CH, 1987. Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Developmental biology 124, 91–110. [DOI] [PubMed] [Google Scholar]
- Salomon R, Attie T, Pelet A, Bidaud C, Eng C, Amiel J, Sarnacki S, Goulet O, Ricour C, Nihoul-Fekete C, Munnich A, Lyonnet S, 1996. Germline mutations of the RET ligand GDNF are not sufficient to cause Hirschsprung disease. Nat Genet 14, 345–347. [DOI] [PubMed] [Google Scholar]
- Salvioli B, Bovara M, Barbara G, De Ponti F, Stanghellini V, Tonini M, Guerrini S, Cremon C, Degli Esposti M, Koumandou M, Corinaldesi R, Sternini C, De Giorgio R, 2002. Neurology and neuropathology of the pancreatic innervation. JOP 3, 26–33. [PubMed] [Google Scholar]
- Sato M, Yost HJ, 2003. Cardiac neural crest contributes to cardiomyogenesis in zebrafish. Developmental biology 257, 127–139. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE, 1989. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development 106, 809–816. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Fraser SE, Bronner-Fraser M, 1990. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development 108, 605–612. [DOI] [PubMed] [Google Scholar]
- Shepherd I, Eisen J, 2011. Development of the zebrafish enteric nervous system. Methods in cell biology 101, 143–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd IT, Beattie CE, Raible DW, 2001. Functional analysis of zebrafish GDNF. Developmental biology 231, 420–435. [DOI] [PubMed] [Google Scholar]
- Shepherd IT, Pietsch J, Elworthy S, Kelsh RN, Raible DW, 2004. Roles for GFRalpha1 receptors in zebrafish enteric nervous system development. Development 131, 241–249. [DOI] [PubMed] [Google Scholar]
- Shimada K, Tachibana T, Fujimoto K, Sasaki T, Okabe M, 2012. Temporal and Spatial Cellular Distribution of Neural Crest Derivatives and Alpha Cells during Islet Development. Acta Histochem Cytochem 45, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin JE, Zhang D, Rollo BN, Newgreen DF, 2013. Retinoic acid upregulates ret and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut. PloS one 8, e64077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southard-Smith EM, Kos L, Pavan WJ, 1998. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet 18, 60–64. [DOI] [PubMed] [Google Scholar]
- Springall DR, Cadieux A, Oliveira H, Su H, Royston D, Polak JM, 1987. Retrograde tracing shows that CGRP-immunoreactive nerves of rat trachea and lung originate from vagal and dorsal root ganglia. J Auton Nerv Syst 20, 155–166. [DOI] [PubMed] [Google Scholar]
- Stankunas K, Shang C, Twu KY, Kao SC, Jenkins NA, Copeland NG, Sanyal M, Selleri L, Cleary ML, Chang CP, 2008. Pbx/Meis deficiencies demonstrate multigenetic origins of congenital heart disease. Circulation research 103, 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulou A, Natarajan D, Nikolopoulou P, Patmanidi AL, Lygerou Z, Pachnis V, Taraviras S, 2016. Inactivation of Geminin in neural crest cells affects the generation and maintenance of enteric progenitor cells, leading to enteric aganglionosis. Developmental biology 409, 392–405. [DOI] [PubMed] [Google Scholar]
- Stewart RA, Arduini BL, Berghmans S, George RE, Kanki JP, Henion PD, Look AT, 2006. Zebrafish foxd3 is selectively required for neural crest specification, migration and survival. Developmental biology 292, 174–188. [DOI] [PubMed] [Google Scholar]
- Takahashi M, 2001. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev 12, 361–373. [DOI] [PubMed] [Google Scholar]
- Teillet MA, Kalcheim C, Le Douarin NM, 1987. Formation of the dorsal root ganglia in the avian embryo: segmental origin and migratory behavior of neural crest progenitor cells. Developmental biology 120, 329–347. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Ciment G, Thiery JP, 1986. Pathways of avian neural crest cell migration in the developing gut. Developmental biology 116, 439–450. [DOI] [PubMed] [Google Scholar]
- Uesaka T, Enomoto H, 2010. Neural precursor death is central to the pathogenesis of intestinal aganglionosis in Ret hypomorphic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 5211–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe RA, Bronner ME, 2015. Meis3 is required for neural crest invasion of the gut during zebrafish enteric nervous system development. Mol Biol Cell 26, 3728–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe RA, Hong SS, Bronner ME, 2018. Retinoic acid temporally orchestrates colonization of the gut by vagal neural crest cells. Developmental biology 433, 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglia JL, Hall BK, 2000. Patterns of migration and regulation of trunk neural crest cells in zebrafish (Danio rerio). The International journal of developmental biology 44, 867–881. [PubMed] [Google Scholar]
- Van de Putte T, Maruhashi M, Francis A, Nelles L, Kondoh H, Huylebroeck D, Higashi Y, 2003. Mice lacking ZFHX1B, the gene that codes for Smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the etiology of Hirschsprung disease-mental retardation syndrome. Am J Hum Genet 72, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Lopez GA, Cerrizuela S, Aybar MJ, 2017. Trunk neural crest cells: formation, migration and beyond. The International journal of developmental biology 61, 5–15. [DOI] [PubMed] [Google Scholar]
- Vlachakis N, Choe SK, Sagerstrom CG, 2001. Meis3 synergizes with Pbx4 and Hoxb1b in promoting hindbrain fates in the zebrafish. Development 128, 1299–1312. [DOI] [PubMed] [Google Scholar]
- Wakamatsu N, Yamada Y, Yamada K, Ono T, Nomura N, Taniguchi H, Kitoh H, Mutoh N, Yamanaka T, Mushiake K, Kato K, Sonta S, Nagaya M, 2001. Mutations in SIP1, encoding Smad interacting protein-1, cause a form of Hirschsprung disease. Nat Genet 27, 369–370. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Hutson MR, Stadt HA, Zdanowicz M, Zdanowicz J, Kirby ML, 2005. Cardiac neural crest is necessary for normal addition of the myocardium to the arterial pole from the secondary heart field. Developmental biology 281, 66–77. [DOI] [PubMed] [Google Scholar]
- Wall NA, Jones CM, Hogan BL, Wright CV, 1992. Expression and modification of Hox 2.1 protein in mouse embryos. Mechanisms of development 37, 111–120. [DOI] [PubMed] [Google Scholar]
- Wessells NK, Cohen JH, 1967. Early Pancreas Organogenesis: Morphogenesis, Tissue Interactions, and Mass Effects. Developmental biology 15, 237–270. [DOI] [PubMed] [Google Scholar]
- Ye M, Coldren C, Liang X, Mattina T, Goldmuntz E, Benson DW, Ivy D, Perryman MB, Garrett-Sinha LA, Grossfeld P, 2010. Deletion of ETS-1, a gene in the Jacobsen syndrome critical region, causes ventricular septal defects and abnormal ventricular morphology in mice. Human molecular genetics 19, 648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema CL, 1944. Experiments on the origin of the sensory ganglia of the facial nerve in the chick. The Journal of Comparative Neurology 81, 147–167. [Google Scholar]
- Yntema CL, Hammond WS, 1945. Depletions and abnormalities in the cervical sympathetic system of the chick following extirpation of neural crest. J Exp Zool 100, 237–263. [DOI] [PubMed] [Google Scholar]
- Yntema CL, Hammond WS, 1947. The development of the autonomic nervous system. Biol Rev Camb Philos Soc 22, 344–359. [DOI] [PubMed] [Google Scholar]
- Yntema CL, Hammond WS, 1954. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol 101, 515–541. [DOI] [PubMed] [Google Scholar]
- Young HM, Stamp LA, McKeown SJ, 2016. ENS Development Research Since 1983: Great Strides but Many Remaining Challenges. Adv Exp Med Biol 891, 53–62. [DOI] [PubMed] [Google Scholar]
- Zhu J, Garcia-Barcelo MM, Tam PK, Lui VC, 2011. HOXB5 cooperates with NKX2–1 in the transcription of human RET. PloS one 6, e20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Kam MK, Garcia-Barcelo MM, Tam PK, Lui VC, 2014. HOXB5 binds to multi-species conserved sequence (MCS+9.7) of RET gene and regulates RET expression. The international journal of biochemistry & cell biology 51, 142–149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie1 S1. Time lapse movie of stills from Fig. 3. Dissected lungs from transgenic Wnt1 -Cre/ZEG mouse embryos at E11.5 were imaged by live time lapse microscopy for approximately 48 hours. Over the course of branching morphogenesis, EGFP-labeled neural crest cells appear to migrate extensively through the lung mesenchyme. (Movie acquired at the 2014 Marine Biological Laboratory Embryology course, with the assistance of Dr. Angelo Iulianella and the mouse module).