Abstract
Bacillus amyloliquefaciens FZB42 is a plant growth-promoting rhizobacteria that stimulates plant growth, and enhances resistance to pathogens and tolerance of salt stress. Instead, the mechanistic basis of drought tolerance in Arabidopsis thaliana induced by FZB42 remains unexplored. Here, we constructed an exopolysaccharide-deficient mutant epsC and determined the role of epsC in FZB42-induced drought tolerance in A. thaliana. Results showed that FZB42 significantly enhanced growth and drought tolerance of Arabidopsis by increasing the survival rate, fresh and dry shoot weights, primary root length, root dry weight, lateral root number, and total lateral root length. Coordinated changes were also observed in cellular defense responses, including elevated concentrations of proline and activities of superoxide dismutase and peroxidase, decreased concentrations of malondialdehyde, and accumulation of hydrogen peroxide in plants treated with FZB42. The relative expression levels of drought defense-related marker genes, such as RD29A, RD17, ERD1, and LEA14, were also increased in the leaves of FZB42-treated plants. In addition, FZB42 induced the drought tolerance in Arabidopsis by the action of both ethylene and jasmonate, but not abscisic acid. However, plants inoculated with mutant strain epsC were less able to resist drought stress with respect to each of these parameters, indicating that epsC are required for the full benefit of FZB42 inoculation to be gained. Moreover, the mutant strain was less capable of supporting the formation of a biofilm and of colonizing the A. thaliana root. Therefore, epsC is an important factor that allows FZB42 to colonize the roots and induce systemic drought tolerance in Arabidopsis.
Keywords: Bacillus amyloliquefaciens FZB42, biofilm, drought stress, epsC, exopolysaccharides, phytohormone
1. Introduction
Tissue dehydration imposed by drought can cause irreversible cellular damage, with a consequential loss in the economic yield of crop plants [1]. A number of breeding approaches are being actively pursued to promote the drought tolerance of many leading crop species. An alternative approach to drought mitigation relies on the presence of certain plant growth-promoting rhizobacteria (PGPR) [2,3,4,5]. Improving the tolerance of drought stress in plants using PGPR and understanding the molecular mechanisms involved can help to address strategic needs and complement classical genetic selection strategies.
Studies have shown that PGPR can promote the growth and development of host plants as well as eliciting induced systemic tolerance to reduce the susceptibility of plants to drought conditions [6,7,8,9,10]. It has been established that their presence affects the host’s metabolism, in particular by boosting its production of osmoprotectants, promoting photosynthesis, and increasing the activity of reactive oxygen species (ROS) scavenging enzymes [11,12,13]. Other studies have determined that their presence regulates a number of phytohormones, such as abscisic acid (ABA), salicylic acid (SA), jasmonic acid (JA), and ethylene (ET), thereby influencing many plant signaling networks, including those involved in the abiotic stress response [14,15,16,17].
Bacterial cells proliferate and associate into multicellular structure, form robust floating pellicle in standing medium referred as biofilm at the air–liquid interface [18,19]. Biofilms can protect member cells from adverse environmental stresses [20]. PGPR form biofilms after being inoculated in the rhizosphere of plants [21,22]. The formation of PGPR biofilm is a complex process, which is regulated by multiple genes. It has been revealed that degU, abrB, and resE regulate the formation of B. amyloliqueliciens SQR9 biofilm [23,24,25]. B. amyloliquefaciens FZB42 collagen-like proteins encoding genes, including clpA, clpB, clpC, and clpD, have been reported to be crucial for biofilm formation and adhesion to plant roots [26].
Many microbes, especially those belonging to certain bacterial and fungal species, secrete exopolysaccharides, large carbohydrate polymers formed by the concatenation of monosaccharides through glycosidic bonds [27]. These compounds have been shown to be protective of plants challenged by abiotic stress [28,29]. For instance, in soybean plants exposed to salinity stress, the exopolysaccharides generated by certain Pseudomonas spp. act to reduce the translocation of Na+ ions from the soil into the root [30]. Consortia of PGPR and their respective exopolysaccharides have been suggested as conferring a greater benefit with respect to drought tolerance compared with inoculation using PGPR alone [31]. Moreover, exopolysaccharides are a large component of bacterial biofilms [32]. Thus, in terms of the beneficial effects of PGPR on their interactions with plants, it is hardly surprising that exopolysaccharides are of great significance in improving the ability of plants to resist drought stress.
Strain FZB42 of the gram-positive bacterium, Bacillus amyloliqueliciens, has been shown to promote plant growth by increasing the supply of mineral nutrition to the plant, while also boosting the plants’ level of resistance to some pathogens and their tolerance of salinity stress [33,34,35]. However, to the best of our knowledge, no previous studies have evaluated the effects of FZB42 on drought stress in Arabidopsis and study the underlying molecular mechanisms involved.
Here, a comparison between the impact of inoculation with wild type FZB42 and an epsC mutant strain that is incapable of producing exopolysaccharides on the drought tolerance of model plant Arabidopsis was observed [36]. We found that FZB42 could induce drought tolerance in Arabidopsis via ethylene and jasmonate-mediated pathways and the epsC mutant had a decreased capacity for inducing drought tolerance. Thus, we suggest that exopolysaccharides encoding gene epsC plays a crucial role in allowing FZB42 to improve the tolerance of drought by Arabidopsis.
2. Results
2.1. Epsc Contributes to the Ameliorative Effect of B. amyloliquefaciens on the Drought Tolerance of A. thaliana
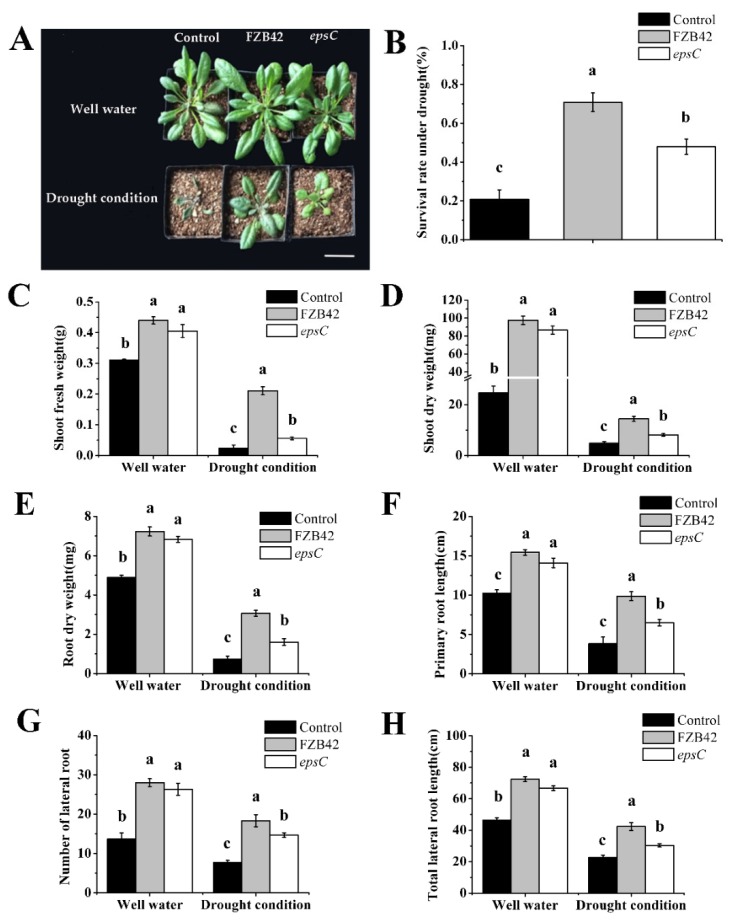
Under well-watered conditions, inoculation with either wild type FZB42 or the epsC mutant, the shoot biomass of A. thaliana seedlings increased significantly (Figure 1A,C,D). Furthermore, the benefit to the plants’ performance was similar for both strains of B. amyloliquefaciens (Figure 1C,D). Similarly, we found that treatment with FZB42 and the epsC mutant significantly increased root dry weight, primary root length, lateral root number, and total lateral root length in Arabidopsis compared with the non-inoculated plants (p < 0.05), and there were no significant differences between the FZB42 and epsC mutant treatments (Figure 1E–H).
Figure 1.
The effect of inoculation with either wild type FZB42 or the epsC mutant on the performance of A. thaliana seedlings. Appearance of the plants. Scale bar, 2 cm (A), seedling survival rate under drought stress (B), shoot fresh weight (C), shoot dry weight (D), root dry weight (E), primary root length (F), lateral root number (G), total lateral root length (H). Values in (B) through (H) are shown as means, with the whiskers representing the standard error (SE, n = 18). Different letters above each column indicate statistically significant (p < 0.05) differences in mean performance.
After drought treatment, inoculation with FZB42 and the epsC mutant in the rhizosphere of Arabidopsis significantly improved survival rate, increased shoot dry weight and fresh weight, affected root system, including increased root dry weight, primary root length, lateral root number, and total lateral root length in Arabidopsis seedlings compared with the control treatment (p < 0.05) (Figure 1B–H). However, the benefit was less marked when the epsC mutant was used for the inoculation.
2.2. The Presence of EpsC Promoted Antioxidant Metabolism in Droughted A. thaliana Plants
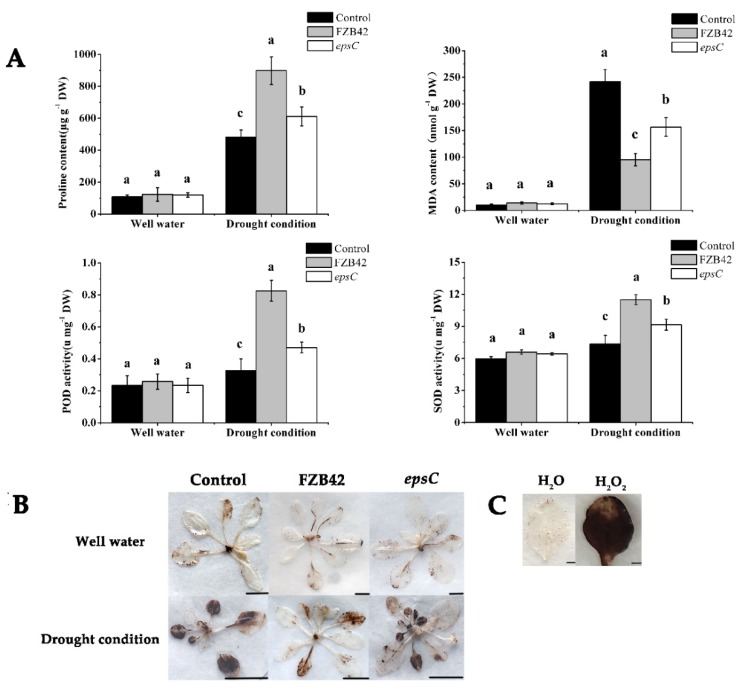
Under well-watered conditions, inoculation with either wild type FZB42 or the epsC mutant had no effect on the concentrations in the leaf of either proline or malondialdehyde (MDA), nor did it influence the activity of either peroxidase (POD) or superoxide dismutase (SOD) (Figure 2A). Under drought stress conditions, the application of FZB42 and the epsC mutant greatly increased the proline contents and the activities of SOD and POD compared with the control (p < 0.05) (Figure 2A). However, inoculation with the epsC mutant significantly decreased the proline content and the activities of SOD and POD compared with the FZB42 treatment (p < 0.05) (Figure 2A). In addition, the MDA content decreased significantly after inoculation with FZB42 and the epsC mutant compared with the control treatment (p < 0.05) (Figure 2A). However, a greater increase in leaf MDA content was observed in plants that had been inoculated with the epsC mutant than those that had been inoculated with the wild type strain (Figure 2A) (p < 0.05) (Figure 2A).
Figure 2.
The biochemical response of the A. thaliana leaf to drought stress. The concentration of proline and malondialdehyde (MDA), and the activity of superoxide dismutase (SOD) and peroxidase (POD) (A), 3,3-diaminobenzidine staining revealing the accumulation of hydrogen peroxide in well-watered and droughted plants inoculated with either wild type FZB42 or the epsC mutant. Scale bar, 1 cm (B), 3,3-diaminobenzidine staining revealing the accumulation of hydrogen peroxide in wild type Arabidopsis leaves treated with H2O or 20 mM H2O2. Scale bars, 1 mm (C). Values in (A) are shown as means, with the whiskers representing the standard error (SE, n = 18). Different letters above each column indicate statistically significant (p < 0.05) differences in mean performance.
We then detected the accumulation of hydrogen peroxide in the leaves under well-watered conditions by DAB (3,3-diaminobenzidine) staining. The accumulation of hydrogen peroxide in the leaves of non-treated plants was similar as in the leaves of plants treated with either the wild type or the mutant strain of B. amyloliquefaciens (Figure 2B,C). However, the accumulation of hydrogen peroxide in the leaves of non-inoculated plants under drought stress was more substantial than in the leaves of plants inoculated with either the wild type or the mutant strain of B. amyloliquefaciens; and the level was higher in the epsC mutant-inoculated ones than in the wild type-inoculated ones. (Figure 2B,C).
2.3. The Presence of EpsC Influenced Stomatal Control from Detached Leaves in Droughted A. thaliana Plants
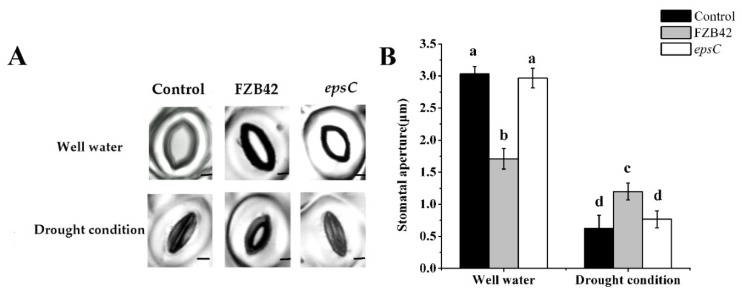
A comparison between the leaves of well-watered plants derived from the three inoculation treatments (non-inoculated and inoculated with either the wild type or the mutant strain of B. amyloliquefaciens) suggested that stomatal aperture was smaller in the leaf of plants inoculated with the wild type strain than in those of plants exposed to either of the other two treatments: Stomatal aperture in the leaf of non-inoculated plants was indistinguishable from that seen in the leaf of the mutant strain-inoculated ones (Figure 3). The effect of moisture stress was to greatly reduce stomatal aperture, irrespective of the inoculation treatment. Somewhat unexpectedly, stomatal aperture in the leaf of plants inoculated with the wild type strain was significantly greater than in the leaf of plants exposed to either of the two other treatments; just as in the well-watered plants, stomatal aperture did not respond to inoculation with the mutant strain (Figure 3).
Figure 3.
The physiological response of the A. thaliana leaf to drought stress. Appearance of stoma. Scale bar, 2.5 µm (A), and stomatal aperture in detached leaves of plants inoculated with either wild type FZB42 or the epsC mutant (B). Values are shown as means, with the whiskers representing the standard error (SE, n = 18). Different letters above each column indicate statistically significant (p < 0.05) differences in mean performance.
2.4. The Presence of EpsC Altered the Transcript Levels of Stress-Responsive Genes in Arabidopsis Plants
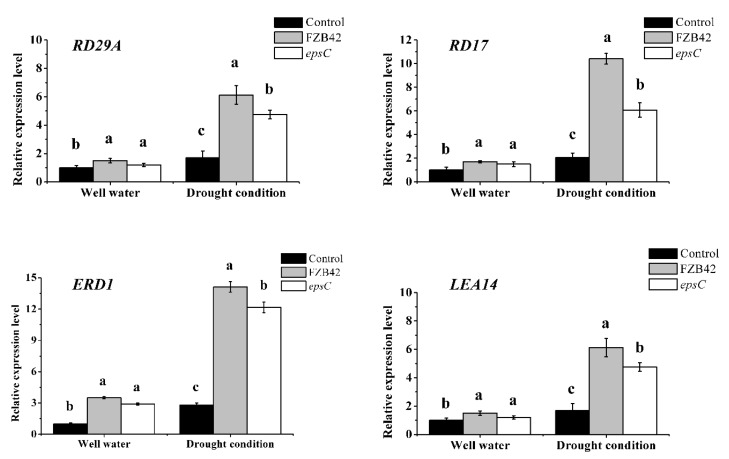
The transcription levels of stress-responsive marker genes, RD29A, RD17, ERD1, and LEA14, in the leaves were assessed using qRT-PCR (Figure 4). All of these genes are involved in the typical response to drought. Compared with the expression level of the genes in control Arabidopsis plants (without bacterial inoculation), the abundance of each of the transcripts were significantly higher in Arabidopsis plants inoculated with FZB42 or the epsC mutant (p < 0.05) (Figure 4). Of note was that the extent of the induction was uniformly greater in the leaf of plants inoculated with the wild type strain FZB42 than in leaves from plants inoculated with the mutant strain (Figure 4).
Figure 4.
The relative expression levels of drought-responsive marker genes in the leaves of A. thaliana. A qRT-PCR assay was used to estimate the expression levels of the stress response-associated marker genes, RD29A, RD17, ERD1, and LEA14, in drought plants inoculated with either wild type FZB42 or the epsC mutant. Values are shown as means, with the whiskers representing the standard error (SE, n = 18). Different letters above each column indicate statistically significant (p < 0.05) differences in mean performance.
2.5. The Additional Drought Tolerance Induced by Inoculation with FZB42 Involved the Action of both Ethylene and Jasmonate
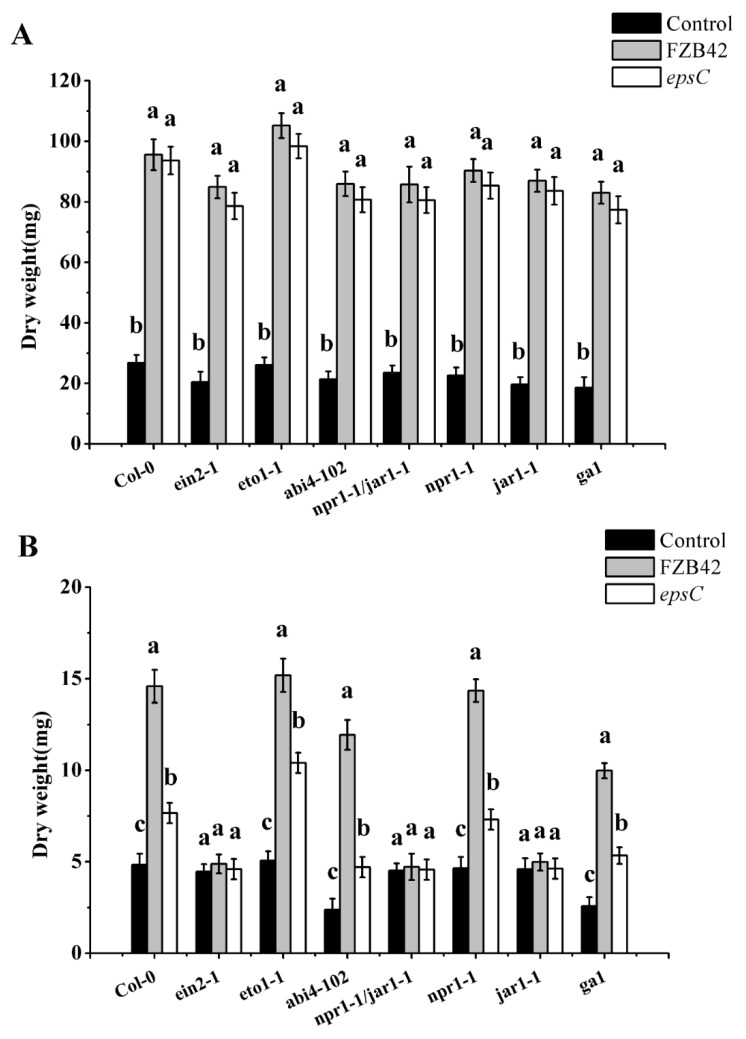
A series of established A. thaliana mutants involving altered responses to the major phytohormones were then tested for their interaction with FZB42 and the epsC mutant: The mutants were ein2-1 (ethylene insensitive), eto1-1 (ethylene over-producer), jar1-1 (jasmonate insensitive), abi4-102 (ABA insensitive), ga1 (gibberellin-responsive male-sterile dwarf), npr1-1 (salicylic acid insensitive), and the npr1-1/jar1-1 double mutant. When treated with wild type FZB42 and the epsC mutant cells under the well-watered condition, all the mutant plants exhibited a significantly higher dry weight than untreated plants. Furthermore, the dry weight of mutant plants was similar for both strains of B. amyloliquefaciens (Figure 5A).
Figure 5.
The effect of inoculation with either wild type FZB42 or the epsC mutant on the accumulation of dry matter by well-watered and drought seedlings of Arabidopsis mutants ein2-1 (ethylene insensitive), eto1-1 (ethylene over-producer), jar1-1 (jasmonate insensitive), abi4-102 (ABA insensitive), ga1 (gibberellin-responsive male-sterile dwarf), npr1-1 (salicylic acid insensitive), and the npr1-1/jar1-1 double mutant. Dry weight of Arabidopsis mutants under well-watered condition (A), dry weight of Arabidopsis mutants under drought condition (B). Values are shown as means, with the whiskers representing the standard error (SE, n = 18). Different letters above each column indicate statistically significant (p < 0.05) differences in mean performance.
However, when eto1-1, abi4-102, npr1-1, and ga1 plants were inoculated with wild type FZB42 and the epsC mutant cells, the plants exhibited a significantly higher dry weight under drought condition than did the equivalent non-inoculated plants. In contrast, the shoot dry weight of ein2-1, jar1-1, and the npr1-1/jar1-1 double mutant did not change when inoculated with either strain (Figure 5B).
2.6. The Absence of epsC Impaired Biofilm Formation and Compromised the Colonization of Arabidopsis Roots
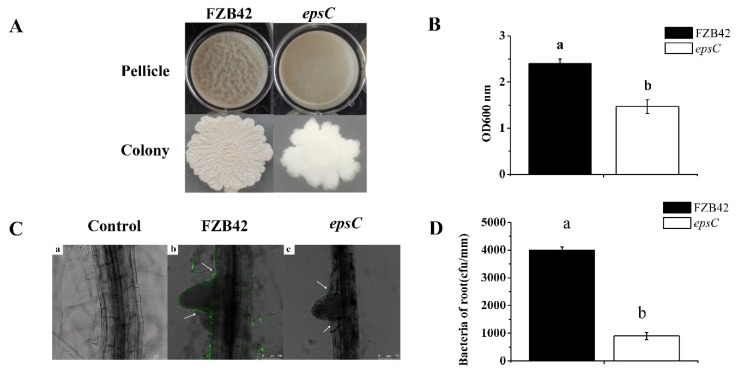
To confirm the roles of epsC in biofilm formation by FZB42, we determined the biofilm formation activities of the wild type and epsC mutant strain. The wild type and epsC mutant strain produced colonies with different shapes and pellicle characteristics on biofilm growth-specific LBGM medium (Lysogeny broth supplemented with 1% (v/v) glycerol and 0.1 mM MnSO4) (Figure 6A). The wild type strain was more effective than the mutant strain in forming a biofilm. Furthermore, the crystal violet staining procedure was used to quantify the amount of biofilm formed confirmed the superiority of the wild type strain (p < 0.05) (Figure 6B).
Figure 6.
Biofilm formation by wild type FZB42 and the epsC mutant. The upper panel illustrates the formation of a pellicle by cells cultured at 30 °C for three days on LBGM medium (Lysogeny broth supplemented with 1% (v/v) glycerol and 0.1 mM MnSO4); the lower panel illustrates colony morphology (A), quantitative spectrophotometric assay of biofilms stained with crystal violet (B), confocal laser scanning microscopy analysis of root colonization. Scale bar, 100 µm (C), adherence capacities of wild type and epsC mutant to the A. thaliana root (D). Values in (B) and (D) are shown as means, with the whiskers representing the standard error (SE, n = 12). Different letters above each column indicate statistically significant (p < 0.05) differences in mean performance.
Inoculation with GFP-labeled strains made it possible to monitor the progress of root colonization via confocal laser scanning microscopy (CLSM). This analysis demonstrated that the wild type strain was able to colonize the entire root surface, including the lateral roots, while the mutant strain exhibited a weaker capacity to colonize (Figure 6C). Consistently, in seedlings co-cultivated with B. amyloliquefaciens strains for seven days, the adherence capacity of mutant strain epsC was significantly lower compared with that of wild type FZB42 (p < 0.05) (Figure 6D).
2.7. Composition of the Exopolysaccharides Produced by B. amyloliquefaciens FZB42
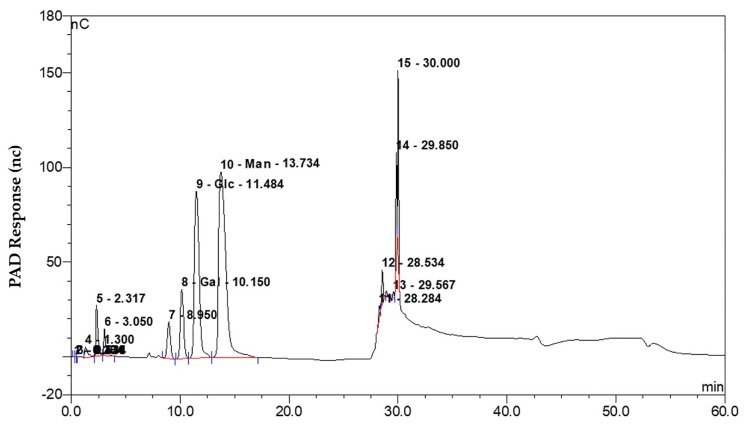
Investigating the monosaccharide compositions of exopolysaccharides is important for establishing their functional relationships. Applying high performance anion exchange chromatography to reveal the composition of the exopolysaccharides produced by FZB42 cells showed that it was composed of the three monosaccharides mannose, galactose, and glucose. The molar ratio of these three compounds was 19.0:2.3:1.0 (Figure 7).
Figure 7.
High performance anion exchange chromatography analysis of the monosaccharide composition of exopolysaccharides formed by wild type FZB42 cells. Peaks 8, 9, and 10 correspond to, respectively, galactose, glucose, and mannose. Red line represented the base line. Additional signals occurring between 28 and 32 min were system peaks.
3. Discussion
Efforts to improve the tolerance of plants to drought have focused on a range of traits, most notably their capacity to access water from the soil by either enlarging their root system or by enhancing the production of osmolytes, and by the control of stomatal aperture. Here, it has been shown that the drought response of A. thaliana was boosted by colonization with B. amyloliquefaciens FZB42 (Figure 1). Similar results were obtained after inoculating foxtail millet with Pseudomonas fluorescens [37], wheat with Bacillus subtilis LDR2 [38], and Arabidopsis and maize with Mitsuaria sp. and Burkholderia sp. [39] under water deprivation conditions. A universal component of the plant response to drought stress is the accumulation of ROS, compounds that are cytotoxic if allowed to build up to an excessive level, and which therefore need to be neutralized [40,41]. Elevated levels of proline and high activities of POD and SOD in plants may be correlated with enhanced stress tolerance [42,43,44]. The presence of certain Bacillus sp. is known to support the ability of moisture-stressed plants to increase their cellular content of the osmolyte proline and the activity of ROS scavenging enzymes, such as POD and SOD [45,46], which was precisely the consequence of inoculating A. thaliana with wild type FZB42 (Figure 2A). At the same time, the inoculation reduced the cellular production of MDA, a marker of cell membrane damage [14,47], as similarly observed when droughted A. thaliana plants were inoculated with Azospirillum brasilense [48]. The accumulation of hydrogen peroxide was also lower in the leaf of plants inoculated with FZB42 (Figure 2B). The implication is that FZB42 cells are able to mitigate against lipid peroxidation, thereby helping to retain the integrity of the cell membranes in the leaf of droughted A. thaliana seedlings. The stomatal aperture is a major factor that contributes to drought tolerance [49,50,51]. In our investigation, under well-watered conditions, a negative effect of FZB42 inoculation was observed on stomatal conductance compared with the non-inoculated control. However, the application of FZB42 to Arabidopsis increased the stomatal aperture under drought conditions (Figure 3). The result is supported by a stronger stomatal closure inhibition after inoculation of soybean, Platycladus, and maize with Bacillus sp. [52,53,54]. Therefore, inoculation with FZB42 had beneficial effects on seedling growth in Arabidopsis even when plants were exposed to severe drought stress condition.
The products of many genes have been implicated as contributing to the survival of A. thaliana plants challenged with moisture deficiency [55]. The transcriptional outcome of some of these of inoculating with FZB42 was for RD29A, RD17, ERD1, and LEA14 all to be up-regulated (Figure 4), as has similarly been observed in cognate studies [56,57,58,59]. Considering the physiological changes, this indicated FZB42 could induce systemic drought tolerance in A. thaliana. While the direct molecular basis of the FZB42 effect is unknown, it is likely that phytohormone-mediated processes are involved, since these are central to the plant abiotic stress response [60,61,62]. Several studies have shown that the expression of RD29A, RD17, ERD1, and LEA14 are associated with drought tolerance in A. thaliana in an ABA independent way [63,64]. Consistently, the drought response (in terms of dry matter accumulation) of a set of key A. thaliana mutants suggested that the FZB42 did not act through pathways controlled by either ABA, gibberellin, or salicylic acid, but jasmonate and ethylene (Figure 5B). The non-involvement of ABA was also supported by the stomatal movement data, which showed that stomatal aperture in droughted plants was greater in FZB42-inoculated plants than in non-inoculated ones. However, all mutant plants inoculated with wild type FZB42 cells under the well-watered condition exhibited a significantly higher dry weight than did the equivalent non-inoculated plants (Figure 5A). This suggests the growth-promoting effects and drought tolerance induction of FZB42 on plants may not be the same signaling pathway. Thus, the underlying molecular processes responsible for enhancing cell viability and growth still need to be elucidated.
Microbial exopolysaccharides have been suggested as being able to promote plant growth, along with improving tolerance/resistance to both abiotic and biotic stress [29]. When the B. subtilis sacB gene was expressed in Nicotiana tabacum and in Beta vulgaris, in both cases, there was a measurable improvement in the plants’ tolerance of drought [65,66], thought to be engendered by an alteration in fructan metabolism. The fructans act to protect plants from dehydration by interacting directly with the cell membrane. When certain rhizobacteria are genetically modified to over-produce trehalose, their ability to support the survival of the host plant under conditions of severe water limitation appears to be bolstered [67]. Rhizobium sp. cells impaired for the ability to produce extracellular polysaccharide are less able than wild type cells to buffer their plant host against oxidative stress. Here, the effect of removing the capacity to generate exopolysaccharides strongly compromised the ability of B. amyloliquefaciens to protect A. thaliana from drought stress: Compared to those seedlings inoculated with the wild type FZB42 strain, seedlings treated with the mutant strain showed a lower survival rate, accumulated less biomass, and suffered from more extensive oxidative stress. Nevertheless, the performance of these seedlings was still superior to that of non-inoculated seedlings, which implied that the improved drought tolerance imparted by wild type cells did not just result from the presence of the bacterial exopolysaccharides. It is known in this context that certain volatile organic compounds can also mediate plant abiotic stress tolerance [68,69]. Moreover, the beneficial effect was not lost in the jar1-1 mutant or the ein2-1 and eto1-1 mutant, which suggests that the epsC in FZB42 might not participate in the regulation of the jasmonate and ethylene pathway in Arabidopsis (Figure 5B).
Exopolysaccharides represent a major component of bacterial biofilms [70,71,72]. Biofilms are usually produced by a multicellular assemblage and are critical to both adhesion and colonization [73,74]. It has been documented that biofilms help microbes tolerate adverse environmental conditions and that they are required to maintain a long-term interaction between the microbe and its plant host [75,76]. Here, it was demonstrated that the product of B. amyloliquefaciens epsC mediated biofilm formation and was influential in the process of root colonization (Figure 6). Hence, we suggest that epsC is important for the colonization of the rhizosphere of Arabidopsis and the induction of drought tolerance by FZB42. Given that epsC is responsible for the production of exopolysaccharides, we hypothesize that exopolysaccharides may comprise a microbe-associated molecular pattern that is recognized by plants to induce drought tolerance. The exopolysaccharide produced by FZB42 were shown to be composed of galactose, glucose, and mannose (Figure 7). However, peak 7 could not be identified according to the known monosaccharide, and needs further research. This composition likely determines the structure of the exopolysaccharide, which may ultimately influence its functionality. The focus of this research is expected to shift towards investigating how exopolysaccharides are perceived by the plants and how the plant’s defense response is activated post recognition.
4. Materials and Methods
4.1. Plant Materials
The experiments were based on the Col-0 ecotype of A. thaliana. In addition to wild type, the following mutant lines were also assessed for their response to drought stress: Ein2-1 (CS3071), eto1-1 (CS3072), jar1-1 [77], abi4-102 (CS3837), ga1 (CS3103), npr1-1 (CS3726), and the npr1-1/jar1-1 double mutant [78] were provided by Nicole K. Clay. The reference stock numbers of Arabidopsis mutant lines are deposited in (https://www.arabidopsis.org/abrc/catalog/mutant_seed_28.html. 16 November 2018).
4.2. Bacterial Strains and Plasmids
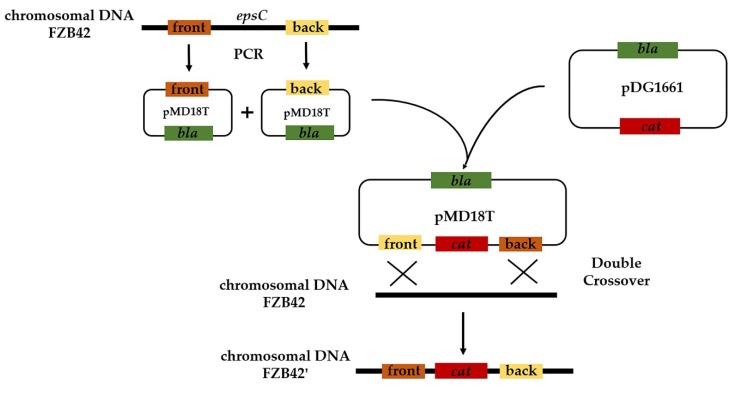
The bacterial strains used were B. amyloliquefaciens FZB42 (BGSC 10A6), a newly created epsC mutant (ΔepsC::Cmr) and Escherichia coli DH5α. All bacteria were cultured at 37 °C in Luria broth (LB) medium. The three plasmids deployed were pDG1661, containing Chlor(R) (BGSC ECE112); pMD18-T, containing Amp(R) (TaKaRa, Kusatsu, Japan), and pMD18-epsC, a derivative of pMD18-T (Figure 8).
Figure 8.
Construction of epsC double-crossover deletion mutants.
4.3. Construction of an epsC Mutant in FZB42
The epsC gene was disabled via double crossover homologous recombination. Two fragments of the intact gene were PCR-amplified from a template of wild type FZB42 chromosomal DNA using ExTaq DNA polymerase (TaKaRa, Kusatsu, Japan) and the primer pairs, respectively, epsC front-F/R and epsC back-F/R (Table 1). The amplicons were each inserted into pMD18-T, using its HindIII and BamHI; SphI and XbaI cloning sites, and the resulting engineered plasmids introduced into wild type FZB42; transgenic cells were selected on LB (Lysogeny broth) agar plates containing 50 µg/mL chloramphenicol and verified using a PCR targeting a chloramphenicol resistance gene sequence. Wild type FZB42 cells were rendered competent using an established protocol [79], applying the following modifications: Briefly, bacteria were grown overnight in LB medium at 37 °C (200 rpm) and diluted 1:50 in 20 mL of SPI (Spizizen’s minimal medium). The bacteria were then incubated at 37 °C with vigorous shaking (200 rpm) until the middle of the exponential growth period (OD600 = 1.3). The culture was then diluted (1:10) in 6 mL SPII (SPI medium supplemented with 1% (v/v) 50 mM CaCl2 and 250 mM MgCl2). The cells were then incubated for another 1.5 h at 100 rpm. Next, 60 μL of 10 mmol/L EGTA (ethylene glycol tetraacetic acid) was added to the medium, before shaking for another 10 min. The culture was divided into equal volumes and no more than 5 μL pMD18-epsC plasmid DNA was added. After incubation at 37 °C with shaking at 100 rpm for 0.5 h, 1 mL of LB medium containing a sublethal concentration (0.1 μg/mL) of chloramphenicol was added. The cells were cultured with vigorous shaking for 90 min and plated onto selective agar plates. The pMD18T-epsC plasmid was also transferred into a derivative of FZB42 harboring GFP [80].
Table 1.
Primers used in this study.
| Primer | Sequence (5′–3′) | Size of DNA Sequence (bp) | Gene |
|---|---|---|---|
| epsC front-F | CCCAAGCTTCGTTGTCCTGAATGATCCGT | 539 | epsC front |
| epsC front-R | CGGGATCCGGAGAACCCGTCAAAATCGTC | …… | …… |
| epsC back-F | ACATGCATGCGATTTCCCGCGGAAGAAACG | 559 | epsC back |
| epsC back-R | CTAGTCTAGACCAATACGGGGTGTTCCACA | …… | …… |
| Chlor-F | CGGGATCCTAGAAGCTTATCGAATTCTCATG | 1250 | chlor |
| Chlor-R | ACATGCATGCAAGGAGATGGCGCCCAAC | …… | …… |
4.4. Analysis of the Monosaccharide Composition FZB42 Exopolysaccharide
Exopolysaccharides were prepared from wild type FZB42 cells following Liu et al. [81]. Briefly, marine LB medium was incubated with 2% inoculum at 30 °C and 200 rpm for 2 days. Next, the exopolysaccharides in the supernatant from the culture were precipitated with chilled absolute ethanol. The precipitate was dissolved in distilled water. Proteins in the exopolysaccharide solution were removed using the Sevag method [82], and small molecular carbohydrates were removed by dialyzing the exopolysaccharide solution against distilled water. The polysaccharide content of the exopolysaccharide solution was determined using the phenol-sulfuric acid method.
The monosaccharide composition was analyzed by HPAEC (ICS5000, Dionex, Sunnyvale, CA, USA) [83]. Ten milligrams of exopolysaccharide were hydrolyzed with 2 mL of 2 mol/L trifluoroacetic acid at 110 °C for 12 h. The hydrolyzate was co-concentrated repeatedly with methanol to dryness and then filtered through a 0.45 μm nylon filter. The standard sugars were prepared in the same manner. The samples were analyzed on a Carbo Pac PA20 column (ID 3 mm × 150 mm) (Dionex, Sunnyvale, CA, USA) with pulsed amperometric detection using the gradient elution procedure with H2O–250 mM NaOH–1.0 M NaAc as the mobile phase. The column was eluted at a flow rate of 0.5 mL/min and the injection volume of the sample was 20 μL.
4.5. Biofilm Formation and Growth of FZB42 and the epsC Mutant
Biofilms were generated from both wild type FZB42 and epsC mutant cells, following the protocol given by Zhao et al. [26], applying the following modifications: Briefly, cells were cultured at 37 °C and grown to OD600 = 1.4, before diluting to 1:100 in LBGM liquid medium containing chloramphenicol in a 12-well plate and then incubating at 30 °C for 48 h. LBGM is composed of LB with supplementation of 1% glycerol (v/v) and 100 μM MnSO4 [84]. To determine the colony morphology, 5 μL of cells were plated onto LBGM solid medium and incubated at 30 °C for 72 h. To quantify biofilm growth, we applied the crystal violet staining method in 96-well polystyrene plates (Thermo Fisher Scientific, Waltham, MA, USA). The culture (1:100) was added to 100 μL of LB liquid medium in each well and incubated at 30 °C for 24 h, before staining with 40 μL of 0.25% crystal violet for 15 min and washing three times with phosphate-buffered saline. Next, 200 μL of 95% ethanol was added to dissolve the biofilm for 15 min at room temperature. The biofilm formation in each well was quantified by measuring the OD600 using a spectrophotometer.
4.6. Inoculation of A. thaliana with B. amyloliquefaciens
Seed was germinated on solidified Murashige & Skoog medium (MS medium) for one week, after which the seedlings were transplanted into either clean soil or soil inoculated with ~107 CFU·mL−1 of either wild type FZB42 or the epsC mutant. After 3 days of transplantation, the plants were subjected to experimental treatments for three weeks. A. thaliana plants were raised in a chamber delivering a 16 h photoperiod, with the temperature set to 22 ± 2 °C during the light phase and 20 ± 2 °C during the dark phase.
4.7. Adherence to and Colonization of A. thaliana Roots by Wild Type FZB42 and the epsC Mutant Cells
A. thaliana seedlings were raised in 12 well microtiter plates containing half strength MS, and inoculated with ~106 CFU·mL−1 B. amyloliqueliciens. To quantify colonization of the A. thaliana seedlings, the roots were removed from seven day old seedlings, then vortexed for 5 s in an Eppendorf tube containing 1 mL sterile saline; the sample was then transferred to a fresh tube containing 1 mL sterile saline and vortexed for a further 20 s. The solution was then diluted three times with saline (1:10, 1:100, 1:1000) and 100 µL samples were plated on plates. After an overnight incubation at 37 °C, a count was taken of the number of B. amyloliqueliciens colonies formed. Root colonization was monitored by immersing A. thaliana seedling roots for seven days in ~106 CFU mL−1 of either wild type FZB42 or the epsC mutant cells, after which the roots were rinsed in sterile saline and imaged by confocal laser scanning microscopy (488 nm emission).
4.8. Stomatal Aperture Measurement
A. thaliana seedlings were inoculated with either epsC mutant or wild type FZB42, and grown under well-watered or drought stress conditions by withholding water. After three weeks, rosette leaves were sampled. The leaf surface imprint method was used to assess stomatal aperture, as described previously [50].
4.9. Physiological Assays
SOD activity in the leaf was determined following [85] and that of POD using the guaiacol oxidation method [86]. Leaf proline content was obtained from ethanolic extracts prepared by homogenizing ~100 mg tissue in 1 mL 70% ethanol [87], while that of MDA was determined using a reaction based on 2-thiobarbituric acid, as described by [88]. The accumulation of hydrogen peroxide in A. thaliana leaves was detected by staining with 3,3-diaminobenzidine, as described elsewhere [89] with minor modifications: Leaf samples were vacuum-infiltrated with 0.1 mg·mL−1 3,3-diaminobenzidine in 50 mM Tris-acetate buffer (pH 5.0), and incubated at room temperature in the dark for 48 h. The reactivity of 3,3-diaminobenzidine with H2O2 in the experimental conditions was confirmed by infiltrating rosettes leaves for 48h with the 3,3–diaminobenzidine solution buffer in the presence and absence of 20 mM H2O2.
4.10. Quantitative Real Time PCR (qRT-PCR) Analysis
Total RNA was isolated from A. thaliana seedlings using the Tri-Reagent (Sigma, St. Louis, MO, USA), and treated with DNase to remove DNA contaminants. The first cDNA strand was synthesized from a 1 µg aliquot of the resulting RNA by priming with oligo dT in a 20 µL reaction based on a PrimeScriptTM RT reagent Kit, following the manufacturer’s protocol. The resulting cDNA was diluted in four volumes of deionized water for use as a qRT-PCR template. The qRT-PCRs were based on SYBR® Premix Ex Taq ™ II (TaKaRa, Japan), with each sample represented by three replicates. The ACT2 sequence (GenBank: AT3G18780) was chosen as the reference. The relevant primers were chosen according to [64,90]. The cycling regime comprised an initial denaturation of 95 °C/30 s, 40 cycles of 95 °C/ 5 s, 60 °C/30 s, followed by melt curve analysis at 95 °C for 15 S, 60 °C for 1 min, and 95 °C for 15 s. Transcript abundances were calculated using the 2−ΔΔCt method [91] and are expressed in the form of mean ± standard error. A one-way analysis of variance was performed to identify significant differences in transcript abundance between plants (either non-stressed or drought-stressed) inoculated with wild type FZB42 or the epsC mutant. Means were compared using Duncan’s multiple range test, applying a significance threshold of 0.05, carried out with routines implemented in SPSS software v16.0.
Acknowledgments
The Arabidopis mutant lines were kindly provided by Nicole K. Clay (Yale University, New Haven, CT, USA).
Author Contributions
X.L. and S.-F.L.: methodology; X.L.: data curation; X.L.: writing and original draft preparation; X.L., L.Y., X.Z., Y.-B.Z., Z.-K.X. and R.-Y.W.: review and editing; R.-Y.W.: funding acquisition.
Funding
This study was supported by the Development and application of key technologies for green prevention and control of native Chinese herbal medicines in Gansu, China (No. 55Y855Z11) and Application of bio-environmental sand-fixation technology in combating desertification (No. HHS-TSS-STS-1505).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chaves M.M., Maroco J.P., Pereira J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- 2.Lugtenberg B., Kamilova F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- 3.Vessey J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- 4.Bhattacharyya P.N., Jha D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microb. Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 5.He A.-L., Niu S.-Q., Zhao Q., Li Y.-S., Gou J.-Y., Gao H.-J., Suo S.-Z., Zhang J.-L. Induced salt tolerance of perennial ryegrass by a novel bacterium strain from the rhizosphere of a desert shrub Haloxylon ammodendron. Int. J. Mol. Sci. 2018;19:469. doi: 10.3390/ijms19020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Loon L.C. Plant responses to plant growth-promoting bacteria. Eur. J. Plant Pathol. 2007;119:243–254. doi: 10.1007/s10658-007-9165-1. [DOI] [Google Scholar]
- 7.Richardson A.E., Barea J.-M., McNeill A.M., Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009;321:305–339. doi: 10.1007/s11104-009-9895-2. [DOI] [Google Scholar]
- 8.Yang J., Kloepper J.W., Ryu C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14:1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Paul D., Lade H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014;34:737–752. doi: 10.1007/s13593-014-0233-6. [DOI] [Google Scholar]
- 10.Wang C.-J., Yang W., Wang C., Gu C., Niu D.-D., Liu H.-X., Wang Y.-P., Guo J.-H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE. 2012;7:e52565. doi: 10.1371/journal.pone.0052565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadeem S.M., Ahmad M., Zahir Z.A., Javaid A., Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014;32:429–448. doi: 10.1016/j.biotechadv.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Kohler J., Hernández J.A., Caravaca F., Roldán A. Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct. Plant Biol. 2008;35:141–151. doi: 10.1071/FP07218. [DOI] [PubMed] [Google Scholar]
- 13.Calvo-Polanco M., Sánchez-Romera B., Aroca R., Asins M.J., Declerck S., Dodd I.C., Martínez-Andújar C., Albacete A., Ruiz-Lozano J.M. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 2016;131:47–57. doi: 10.1016/j.envexpbot.2016.06.015. [DOI] [Google Scholar]
- 14.Wilkinson S., Davies W.J. ABA-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell Environ. 2002;25:195–210. doi: 10.1046/j.0016-8025.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- 15.Figueiredo M.V., Burity H.A., Martínez C.R., Chanway C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008;40:182–188. doi: 10.1016/j.apsoil.2008.04.005. [DOI] [Google Scholar]
- 16.Tiwari S., Prasad V., Chauhan P.S., Lata C. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front. Plant Sci. 2017;8:1510. doi: 10.3389/fpls.2017.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glick B.R., Cheng Z., Czarny J., Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. In: Bakker P.A.H.M., Raaijmakers J.M., Bloemberg G., Höfte M., Lemanceau P., Cooke B.M., editors. New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research. 1st ed. Springer; Dordrecht, The Netherlands: 2007. pp. 329–339. [Google Scholar]
- 18.Branda S.S., Vik A., Friedman L., Kolter R. Biofilms: The matrix revisited. Trends Microbiol. 2005;13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Davey M.E., O’toole G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000;64:847–867. doi: 10.1128/MMBR.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart P.S., Franklin M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008;6:199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 21.Zhang N., Wang D., Liu Y., Li S., Shen Q., Zhang R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil. 2014;374:689–700. doi: 10.1007/s11104-013-1915-6. [DOI] [Google Scholar]
- 22.Timmusk S., Grantcharova N., Wagner E.G.H. Paenibacillus polymyxa invades plant roots and forms biofilms. Appl. Environ. Microbiol. 2005;71:7292–7300. doi: 10.1128/AEM.71.11.7292-7300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu M., Xu Z., Li X., Li Q., Zhang N., Shen Q., Zhang R. Comparative proteomics analysis of Bacillus amyloliquefaciens SQR9 revealed the key proteins involved in in situ root colonization. J. Proteome Res. 2014;13:5581–5591. doi: 10.1021/pr500565m. [DOI] [PubMed] [Google Scholar]
- 24.Xu Z., Zhang R., Wang D., Qiu M., Feng H., Zhang N., Shen Q. Enhanced control of cucumber wilt disease by Bacillus amyloliquefaciens SQR9 through altering the regulation of its DegU phosphorylation. Appl. Environ. Microbiol. 2014;80:2941–2950. doi: 10.1128/AEM.03943-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng J., Wang Y., Li J., Shen Q., Zhang R. Enhanced root colonization and biocontrol activity of Bacillus amyloliquefaciens SQR9 by abrB gene disruption. Appl. Microbiol. Biotechnol. 2013;97:8823–8830. doi: 10.1007/s00253-012-4572-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X., Wang Y., Shang Q., Li Y., Hao H., Zhang Y., Guo Z., Yang G., Xie Z., Wang R. Collagen-like proteins (ClpA, ClpB, ClpC, and ClpD) are required for biofilm formation and adhesion to plant roots by Bacillus amyloliquefaciens FZB42. PLoS ONE. 2015;10:e0117414. doi: 10.1371/journal.pone.0117414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John A., Leigh D.L.C. Exopolysaccharides in plant-bacterial interactions. Annu. Rev. Microbiol. 1992;46:307–346. doi: 10.1146/annurev.mi.46.100192.001515. [DOI] [PubMed] [Google Scholar]
- 28.Ashraf M., Hasnain S., Berge O., Mahmood T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fert. Soils. 2004;40:157–162. doi: 10.1007/s00374-004-0766-y. [DOI] [Google Scholar]
- 29.Sandhya V., Grover M., Reddy G., Venkateswarlu B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fert. Soils. 2009;46:17–26. doi: 10.1007/s00374-009-0401-z. [DOI] [Google Scholar]
- 30.Kasotia A., Varma A., Tuteja N., Choudhary D.K. Amelioration of soybean plant from saline-induced condition by exopolysaccharide producing Pseudomonas-mediated expression of high affinity K+-transporter (HKT1) gene. Curr. Sci. 2016;111:1961–1967. doi: 10.18520/cs/v111/i12/1961-1967. [DOI] [Google Scholar]
- 31.Naseem H., Bano A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014;9:689–701. doi: 10.1080/17429145.2014.902125. [DOI] [Google Scholar]
- 32.Jennings L.K., Storek K.M., Ledvina H.E., Coulon C., Marmont L.S., Sadovskaya I., Secor P.R., Tseng B.S., Scian M., Filloux A. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA. 2015;112:11353–11358. doi: 10.1073/pnas.1503058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Idris E.E., Iglesias D.J., Talon M., Borriss R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant Microbe Interact. 2007;20:619–626. doi: 10.1094/MPMI-20-6-0619. [DOI] [PubMed] [Google Scholar]
- 34.Liu S., Hao H., Lu X., Zhao X., Wang Y., Zhang Y., Xie Z., Wang R. Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Sci. Rep. 2017;7:10795. doi: 10.1038/s41598-017-11308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie S., Jiang H., Ding T., Xu Q., Chai W., Cheng B. Bacillus amyloliquefaciens FZB42 repressed plant miR846 to induce systemic resistance via jasmonic acid-dependent signaling pathway. Mol. Plant Pathol. 2017;19:1612–1623. doi: 10.1111/mpp.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X.H., Koumoutsi A., Scholz R., Eisenreich A., Schneider K., Heinemeyer I., Morgenstern B., Voss B., Hess W.R., Reva O., et al. Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007;25:1007–1014. doi: 10.1038/nbt1325. [DOI] [PubMed] [Google Scholar]
- 37.Niu X., Song L., Xiao Y., Ge W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018;8:2580. doi: 10.3389/fmicb.2017.02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnawal D., Bharti N., Pandey S.S., Pandey A., Chanotiya C.S., Kalra A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plantarum. 2017;161:502–514. doi: 10.1111/ppl.12614. [DOI] [PubMed] [Google Scholar]
- 39.Huang X.-F., Zhou D., Lapsansky E.R., Reardon K.F., Guo J., Andales M.J., Vivanco J.M., Manter D.K. Mitsuaria sp. and Burkholderia sp. from Arabidopsis rhizosphere enhance drought tolerance in Arabidopsis thaliana and maize (Zea mays L.) Plant Soil. 2017;419:523–539. doi: 10.1007/s11104-017-3360-4. [DOI] [Google Scholar]
- 40.Xiong J.-L., Li J., Wang H.-C., Zhang C.-L., Naeem M.S. Fullerol improves seed germination, biomass accumulation, photosynthesis and antioxidant system in Brassica napus L. under water stress. Plant Physiol. Biochem. 2018;129:130–140. doi: 10.1016/j.plaphy.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Xiong H., Yu J., Miao J., Li J., Zhang H., Wang X., Liu P., Zhao Y., Jiang C., Yin Z. Natural variation in OsLG3 increases drought tolerance in rice by inducing ROS scavenging. Plant Physiol. 2018;178:451–467. doi: 10.1104/pp.17.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada M., Morishita H., Urano K., Shiozaki N., Yamaguchi-Shinozaki K., Shinozaki K., Yoshiba Y. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005;56:1975–1981. doi: 10.1093/jxb/eri195. [DOI] [PubMed] [Google Scholar]
- 43.Wang W.-B., Kim Y.-H., Lee H.-S., Kim K.-Y., Deng X.-P., Kwak S.-S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009;47:570–577. doi: 10.1016/j.plaphy.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Wang S., Liang D., Li C., Hao Y., Ma F., Shu H. Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol. Biochem. 2012;51:81–89. doi: 10.1016/j.plaphy.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Gururani M.A., Upadhyaya C.P., Baskar V., Venkatesh J., Nookaraju A., Park S.W. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J. Plant Growth Regul. 2013;32:245–258. doi: 10.1007/s00344-012-9292-6. [DOI] [Google Scholar]
- 46.Ngumbi E., Kloepper J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 2016;105:109–125. doi: 10.1016/j.apsoil.2016.04.009. [DOI] [Google Scholar]
- 47.Del Rio D., Stewart A.J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Cohen A.C., Bottini R., Pontin M., Berli F.J., Moreno D., Boccanlandro H., Travaglia C.N., Piccoli P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plantarum. 2015;153:79–90. doi: 10.1111/ppl.12221. [DOI] [PubMed] [Google Scholar]
- 49.Van Ha C., Leyva-González M.A., Osakabe Y., Tran U.T., Nishiyama R., Watanabe Y., Tanaka M., Seki M., Yamaguchi S., Van Dong N. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA. 2014;111:851–856. doi: 10.1073/pnas.1322135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W., Nguyen K.H., Chu H.D., Van Ha C., Watanabe Y., Osakabe Y., Leyva-González M.A., Sato M., Toyooka K., Voges L., et al. The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana. PLoS Genet. 2017;13:e1007076. doi: 10.1371/journal.pgen.1007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z., Wang F., Hong Y., Huang J., Shi H., Zhu J.K. Two chloroplast proteins suppress drought resistance by affecting ROS production in guard cells. Plant Physiol. 2016;172:2491–2503. doi: 10.1104/pp.16.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prudent M., Salon C., Souleimanov A., Emery R.N., Smith D.L. Soybean is less impacted by water stress using Bradyrhizobium japonicum and thuricin-17 from Bacillus thuringiensis. Agron. Sustain. Dev. 2015;35:749–757. doi: 10.1007/s13593-014-0256-z. [DOI] [Google Scholar]
- 53.Ali L., Khalid M., Asghar H.N., Asgher M. Scrutinizing of rhizobacterial isolates for improving drought resilience in maize (Zea mays) Int. J. Agric. Biol. 2017;19:1054–1064. doi: 10.17957/IJAB/15.0387. [DOI] [Google Scholar]
- 54.Liu F., Xing S., Ma H., Du Z., Ma B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013;97:9155–9164. doi: 10.1007/s00253-013-5193-2. [DOI] [PubMed] [Google Scholar]
- 55.Shinozaki K., Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- 56.Gontia-Mishra I., Sapre S., Sharma A., Tiwari S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 2016;18:992–1000. doi: 10.1111/plb.12505. [DOI] [PubMed] [Google Scholar]
- 57.Montero-Barrientos M., Hermosa R., Cardoza R.E., Gutierrez S., Nicolas C., Monte E. Transgenic expression of the Trichoderma harzianum hsp70 gene increases Arabidopsis resistance to heat and other abiotic stresses. J. Plant Physiol. 2010;167:659–665. doi: 10.1016/j.jplph.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 58.Pandey V., Ansari M.W., Tula S., Yadav S., Sahoo R.K., Shukla N., Bains G., Badal S., Chandra S., Gaur A. Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta. 2016;243:1251–1264. doi: 10.1007/s00425-016-2482-x. [DOI] [PubMed] [Google Scholar]
- 59.Ali F., Bano A., Fazal A. Recent methods of drought stress tolerance in plants. Plant Growth Regul. 2017;82:363–375. doi: 10.1007/s10725-017-0267-2. [DOI] [Google Scholar]
- 60.Raghavendra A.S., Gonugunta V.K., Christmann A., Grill E. ABA perception and signalling. Trends Plant Sci. 2010;15:395–401. doi: 10.1016/j.tplants.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Chini A., Grant J.J., Seki M., Shinozaki K., Loake G.J. Drought tolerance established by enhanced expression of the CC–NBS–LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. Plant J. 2004;38:810–822. doi: 10.1111/j.1365-313X.2004.02086.x. [DOI] [PubMed] [Google Scholar]
- 62.Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015;20:219–229. doi: 10.1016/j.tplants.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Singh D., Laxmi A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015;6:895. doi: 10.3389/fpls.2015.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Golan Y., Shirron N., Avni A., Shmoish M., Gepstein S. Cytokinins induce transcriptional reprograming and improve arabidopsis plant performance under drought and salt stress conditions. Front. Environ. Sci. 2016;4:63. doi: 10.3389/fenvs.2016.00063. [DOI] [Google Scholar]
- 65.Pilon-Smits E.A., Terry N., Sears T., van Dun K. Enhanced drought resistance in fructan-producing sugar beet. Plant Physiol. Biochem. 1999;37:313–317. doi: 10.1016/S0981-9428(99)80030-8. [DOI] [Google Scholar]
- 66.Pilon-Smits E.A., Ebskamp M.J., Paul M.J., Jeuken M.J., Weisbeek P.J., Smeekens S.C. Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol. 1995;107:125–130. doi: 10.1104/pp.107.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forni C., Duca D., Glick B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil. 2017;410:335–356. doi: 10.1007/s11104-016-3007-x. [DOI] [Google Scholar]
- 68.Ledger T., Rojas S., Timmermann T., Pinedo I., Poupin M.J., Garrido T., Richter P., Tamayo J., Donoso R. Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 2016;7:1838. doi: 10.3389/fmicb.2016.01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X.-M., Zhang H. The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front. Plant Sci. 2015;6:774. doi: 10.3389/fpls.2015.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fazli M., Almblad H., Rybtke M.L., Givskov M., Eberl L., Tolker-Nielsen T. Regulation of biofilm formation in P. seudomonas and B. urkholderia species. Environ. Microbiol. 2014;16:1961–1981. doi: 10.1111/1462-2920.12448. [DOI] [PubMed] [Google Scholar]
- 71.Rinaudi L.V., González J.E. The low-molecular-weight fraction of exopolysaccharide II from Sinorhizobium meliloti is a crucial determinant of biofilm formation. J. Bacteriol. 2009;191:7216–7224. doi: 10.1128/JB.01063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramey B.E., Koutsoudis M., von Bodman S.B., Fuqua C. Biofilm formation in plant–microbe associations. Curr. Opin. Microbiol. 2004;7:602–609. doi: 10.1016/j.mib.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 73.Flemming H.-C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 74.Hori K., Matsumoto S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010;48:424–434. doi: 10.1016/j.bej.2009.11.014. [DOI] [Google Scholar]
- 75.Kumar M., Mishra S., Dixit V., Kumar M., Agarwal L., Chauhan P.S., Nautiyal C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.) Plant Signal. Behav. 2016;11:e1071004. doi: 10.1080/15592324.2015.1071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Danhorn T., Fuqua C. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 2007;61:401–422. doi: 10.1146/annurev.micro.61.080706.093316. [DOI] [PubMed] [Google Scholar]
- 77.Staswick P.E., Su W., Howell S.H. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA. 1992;89:6837–6840. doi: 10.1073/pnas.89.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clarke S.M., Cristescu S.M., Miersch O., Harren F.J., Wasternack C., Mur L.A. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol. 2009;182:175–187. doi: 10.1111/j.1469-8137.2008.02735.x. [DOI] [PubMed] [Google Scholar]
- 79.Anagnostopoulos C., Spizizen J. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan B., Chen X.H., Budiharjo A., Bleiss W., Vater J., Borriss R. Efficient colonization of plant roots by the plant growth promoting bacterium Bacillus amyloliquefaciens FZB42, engineered to express green fluorescent protein. J. Biotechnol. 2011;151:303–311. doi: 10.1016/j.jbiotec.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 81.Liu C., Lu J., Lu L., Liu Y., Wang F., Xiao M. Isolation, structural characterization and immunological activity of an exopolysaccharide produced by Bacillus licheniformis 8-37-0-1. Bioresour. Technol. 2010;101:5528–5533. doi: 10.1016/j.biortech.2010.01.151. [DOI] [PubMed] [Google Scholar]
- 82.Yang H., Wu Y., Gan C., Yue T., Yuan Y. Characterization and antioxidant activity of a novel polysaccharide from Pholidota chinensis Lindl. Carbohydr. Polym. 2016;138:327–334. doi: 10.1016/j.carbpol.2015.11.071. [DOI] [PubMed] [Google Scholar]
- 83.Quigley M.E., Englyst H.N. Determination of the uronic acid constituents of non-starch polysaccharides by high-performance liquid chromatography with pulsed amperometric detection. Analyst. 1994;119:1511–1518. doi: 10.1039/an9941901511. [DOI] [PubMed] [Google Scholar]
- 84.Gao T., Greenwich J., Li Y., Wang Q., Chai Y. The bacterial tyrosine kinase activator TkmA contributes to biofilm formation largely independent of the cognate kinase PtkA in Bacillus subtilis. J. Bacteriol. 2015 doi: 10.1128/JB.00438-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hodges D.M., Forney C.F. The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J. Exp. Bot. 2000;51:645–655. doi: 10.1093/jexbot/51.344.645. [DOI] [PubMed] [Google Scholar]
- 86.Meloni D.A., Oliva M.A., Martinez C.A., Cambraia J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003;49:69–76. doi: 10.1016/S0098-8472(02)00058-8. [DOI] [Google Scholar]
- 87.Demiral T., Türkan I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005;53:247–257. doi: 10.1016/j.envexpbot.2004.03.017. [DOI] [Google Scholar]
- 88.Dhindsa R.S., Plumb-Dhindsa P., Thorpe T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981;32:93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- 89.Wang X., Huang W., Yang Z., Liu J., Huang B. Transcriptional regulation of heat shock proteins and ascorbate peroxidase by CtHsfA2b from African bermudagrass conferring heat tolerance in Arabidopsis. Sci. Rep. 2016;6:28021. doi: 10.1038/srep28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qin F., Sakuma Y., Tran L.-S.P., Maruyama K., Kidokoro S., Fujita Y., Fujita M., Umezawa T., Sawano Y., Miyazono K.-I. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress–responsive gene expression. Plant Cell. 2008;20:1693–1707. doi: 10.1105/tpc.107.057380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]