Abstract
Low temperatures have adverse impacts on plant growth, developmental processes, crop productivity and food quality. It is becoming clear that Ca2+ signaling plays a crucial role in conferring cold tolerance in plants. However, the role of Ca2+ involved in cold stress response needs to be further elucidated. Recent studies have shown how the perception of cold signals regulate Ca2+ channels to induce Ca2+ transients. In addition, studies have shown how Ca2+ signaling and its cross-talk with nitric oxide (NO), reactive oxygen species (ROS) and mitogen-activated protein kinases (MAPKs) signaling pathways ultimately lead to establishing cold tolerance in plants. Ca2+ signaling also plays a key role through Ca2+/calmodulin-mediated Arabidopsis signal responsive 1 (AtSR1/CAMTA3) when temperatures drop rapidly. This review highlights the current status in Ca2+ signaling-mediated cold tolerance in plants.
Keywords: plants, calcium signaling, cold stress response
1. Introduction
Cold is a major environmental factor that limits plant growth and reduces productivity and quality [1]. Under low temperature conditions, plants exhibit a variety of cold-induced physiological and biochemical responses, such as production of reactive oxygen species (ROS), changes in membrane lipid composition and changes in osmolytes [1,2]. Low temperatures also induce the expression of certain cold regulated (COR) genes, such as responsive to desiccation 29 (RD29A), COR15a, and kinase (KIN1), in order to stabilize membranes against freezing-induced injury [1]. The dehydration response element-binding protein (DREB1) or CRT/DRE-binding factors are a family of closely related AP2/ERF transcription factors that regulate the COR gene expression [3], and c-repeat binding factors (CBFs) are themselves upregulated by cold through CBF expression 1 (ICE1) [4,5].
Plant response to cold stress is a complex process. This response has been reported to require several signal pathways such as oxidative pathway, mitogen-activated protein kinases (MAPKs), phytohormone, abscisic acid (ABA), as well as Arabidopsis response regulators (ARRs) [6,7,8,9]. Ca2+, as a second messenger, is known to be involved in a variety of biological processes in eukaryotic cells, including playing a critical role in cold stress response in plants [8,10,11]. Plants perceive cold stimulus by sensing the changes in cytoplasmic membrane via cold signal sensors, such as chilling tolerance divergence 1 (COLD1) [12]. Cold signal transduction involves the activation of Ca2+ channels and/or Ca2+ pumps to induce Ca2+ influx (Ca2+ signature) in plant cells [13,14]. Ca2+ signals triggered by cold stimulus are relayed by Ca2+ sensors, such as calmodulins (CaMs), CaM-like proteins (CMLs), Ca2+-dependent protein kinases (CPKs/CDPKs), and calcineurin B-like proteins (CBLs) [8,14,15,16]. These Ca2+ sensors, together with other components in Ca2+ signaling, decode Ca2+ signals into downstream signaling events, such as phosphorylation, transcriptional reprogramming, activation of MAPKs cascade, as well as the accumulation of ROS or nitric oxide (NO) [17,18], suggesting that Ca2+ signaling plays a key role in mediating plant response to cold stress.
2. Cold Stress-Induced Calcium Transients
Changes in Ca2+ transients are an early event in plant response to diverse environmental signals. The specific characteristics of the Ca2+ transients are defined as Ca2+ signatures, including amplitude, duration, and frequency [19]. It is well documented that microbial signals induce specific Ca2+ signatures in the root hair [20,21]. For example, the fungal symbiotic microbe (mycorrhizae) triggers a different Ca2+ signature, as compared to bacterial symbiotic microbe (rhizobacteria) [10,22,23,24]. Similarly, low temperatures also trigger Ca2+ transients in plants [25,26]. Aequorin-based Ca2+ imaging revealed that low temperature (0 °C) induced a rapid and transient Ca2+ influx in whole seedlings of Arabidopsis thaliana [27]. Transient Ca2+ changes triggered by cold stress were also observed by using another Ca2+ sensor; Yellow Cameleon (YC3.6) [28]. The patch-clamp technique provided evidence of a predicted nonselective Ca2+-permeable cation channel in Arabidopsis mesophyll cells, and this Ca2+ channel is activated by cold stress [25].
3. Membrane Proteins Involved in the Perception of Cold Stress Signal Trigger Ca2+ Transients
Perception of environmental stimuli is considered an upstream event involved in inducing Ca2+ transients. Plants use diverse cytoplasmic membrane-localized receptor proteins/kinases in the perception and transduction of developmental and environmental signals. Although numerous receptor proteins/kinases (RKs) or receptor-like proteins/kinases (RLKs) have been identified in the perception of specific pathogens or hormones, few RKs or RLKs have been observed in the perception of cold stress signals in plants. Previous studies have indicated that plants could perceive cold signals through the physical change or damage of the cellular membranes [29,30]. For example, low temperatures (0–10 °C) cause the reduction of fluidity in the cellular phospholipid membrane, while chilling stress results in plasma membrane rigidity [31]. Supporting evidence is that dimethyl sulfoxide (DMSO) triggers plant cell membrane rigidification and activates a cold response signaling pathway even when plants are grown at normal temperatures, while benzyl alcohol, as a fluidizer of cell membrane, suppresses a cold response signaling pathway when plants are grown at low temperatures [8]. It is a reasonable assumption that the damage to plant membranes leads to ion leakage that induces Ca2+ changes under low temperature conditions. However, the perception, transduction, and the ultimate response to cold signals are complex and not clearly understood.
Another assumption is that the accumulation of ROS triggered by cold stress results in Ca2+ transients in plant cells. Low temperatures cause the production of ROS, and enhanced ROS induces Ca2+ transients, although the mechanisms are not clear [32,33]. Previous reports identify that respiratory burst oxidase homologs (RBOHs) and encoding plasma membrane NADPH oxidases are crucial for ROS burst in plant response to diverse environmental stresses, including cold stress [34,35,36]. Further study suggests that soybean genes regulated by cold 2 (AtSRC2) interacts with N-terminal of AtRBOHF and activates Ca2+-mediated NADPH oxidase activity of AtRBOHF in response to cold stress [37]. These observations suggest that the cross-talk between Ca2+ signaling and ROS signaling plays a role in the perception of cold stimulus.
A recent study identified a specific receptor, COLD1, involved in the recognition of cold stress signal in rice. COLD1 is a plasma membrane (PM)- and endoplasmic reticulum (ER)-localized transmembrane protein which senses cold signals and induces cold stress tolerance in plants [38]. The cold1 knock-out mutants display cold sensitivity as compared to WT, when grown at 4 °C for 96 h; while the cold1-overexpressed plants display cold tolerance under similar conditions. In addition, Ca2+ transients triggered by cold stress are compromised in cold1 mutants as compared to WT. However, the hierarchy of Ca2+ transient changes and COLD1 in plant cold stress signaling pathway is unclear [12,39]. One reasonable explanation is that COLD1 encodes a cation permeable channel or a subunit of calcium channel.
In addition, a recent study revealed that two putative Ca2+-permeable mechanosensitive channels, mid1-complementing activity 1 (MCA1) and MCA2, are involved in cold-induced Ca2+ transients in Arabidopsis [13]. MCA1 and MCA2 are PM-localized cation channels, and MCA1 interacts with MCA2 to form a homotetramer to regulate Ca2+ increase in the cytoplasm [40,41,42]. Ca2+ influx triggered by cold stress is compromised in mca1 mca2 double mutant plants. Also, this double mutant plant displays cold sensitivity as compared to wild-type (WT). Further study revealed that MCA1 and MCA2 regulated CBF-independent cold signaling in plants, although the mechanism remains unclear [13].
4. Ca2+ Signaling-Mediated Phosphorylation Events Involved in Cold Stress Response
4.1. Ca2+/CaM-Regulated Receptor-Like Kinases
It has been reported that Ca2+/CaM-regulated receptor-like kinases 1 (CRLK1), encoding a PM-associated serine/threonine kinase regulated by Ca2+ signaling, plays a critical role in plant response to cold stress [43,44,45,46]. crlk1 knock-out mutant plants display enhanced sensitivity as compared to WT at freezing temperatures. The induction of cold-responsive genes triggered by cold stress, such as CBF1, RD29A and COR15a, were suppressed in crlk1 plants as compared to WT. In addition, low temperature (4 °C) and 10 mM H2O2 treatments induced the expression of CRLK1 protein. These observations suggest that CRLK1 positively regulates cold response in Arabidopsis. Further studies suggest that Ca2+/CaM is required for the activation of CRLK1 kinase. The CRLK1 kinase activity increased with an increase in CaM concentration in the presence of Ca2+, while CaM antagonist, CPZ, suppressed the CaM-stimulated CRLK1 kinase activity. Furthermore, the CaM-binding domain in the C-terminal of CRLK1 (residues 369–390) is required for CaM-stimulated kinase activity, and only the specific CaM isoform, such as potato calmodulin 1 (PCM1) and not PCM6, is responsible for the activation of CRLK1. These observations suggest that there is a Ca2+ signaling-mediated cold response pathway regulated by CRLK1 [43,44].
4.2. Ca2+/CaM-Mediated CRLK and MAPK Cascade Response to Cold Stress
MAPK cascades are evolutionarily conserved in eukaryotic organisms [9,47,48,49]. Previous studies have suggested that MAPK signaling pathways play a positive role in the regulation of cold response in plants, since mkk2 mutant plants are more sensitive to cold stress as compared to WT, while mkk2-overexpressed mutant plants display enhanced cold tolerance [45,46]. Additionally, the transcriptional expression of CBF was induced in mkk2-overexpressing mutant plants even at normal temperatures. Further study revealed that low temperature activates Ca2+ signal-mediated CRLK1, which subsequently phosphorylated and activated MEKK1, an upstream component in MAPK cascade of MKK2 [43,44,45,46]. Activation of MKK2 by MEKK1 induced phosphorylation of MPK4 and MPK6. These results suggest that Ca2+ signaling plays a key role in MAPK-mediated plant response to cold stress. A recent study revealed that MPK3 and MPK6 negatively regulate cold tolerance in plants through the phosphorylation of the inducer of CBF expression 1 (ICE1), which subsequently induces ICE1 degradation [50]. However, CRLK1 and CRLK2 repress the activation of MPK3 and MPK6 during cold stress, although the molecular mechanism is not clear. One reasonable assumption is that CRLKs regulates MEKK1-MKK1/2-MPK4 cascade (MEKK1 is reported to be phosphorylated by CRLK1 [44]), while MEKK1-MKK1/2-MPK4 cascade negatively regulates MPK4/MPK6 cascade [51]. In addition, as mentioned above, Ca2+ signaling also positively regulates MAPKs-mediated plant response to cold stress through CPKs.
4.3. Ca2+-Dependent Protein Kinases
CPKs are reported to be involved in Ca2+-mediated signal transductions by connecting cold stress-triggered Ca2+ transients to downstream phosphorylation events [52,53,54,55]. In rice, OsCPK27 was identified as a positive regulator of cold tolerance. The transcriptional expression of OsCPK27 was greatly induced by cold stress (4 °C, 12 h) and oscpk27-silenced mutant plants displayed compromised cold tolerance as compared to WT [56]. Further study revealed that OsCPK27 induces cold response involving ROS, NO, and MAPKs pathways. At low temperatures, accumulations of ROS and NO was attenuated in oscpk27-silenced mutant plants. In addition, the activation of MAPK cascades, such as MPK1/2, was repressed in oscpk27-silenced mutant plants [56]. Another study revealed that OsCPK24 regulated low temperature-triggered production of ROS through direct phosphorylation and suppression of thioltransferase activity of glutaredoxin 10 (OsGrx10), which acts as a glutathione-dependent thioltransferase in ROS signaling pathways [57]. In addition, OsCPK17 was involved in cold stress and induced the accumulation of NO through its putative substrate, nitrate reductase 1 (OsNR1), involving NO metabolism [52]. OsCPK17 has been reported to confer cold tolerance through the activation of PM-localized water transport plasma membrane intrinsic protein 2-1 (OsPIP2;1) [58].
4.4. CBL and CIPK
CBLs, as Ca2+ signal sensors, also relay cold stress-triggered Ca2+ transients into phosphorylation events through their interaction with CBL-interacting protein kinases (CIPKs) [59]. CBLs and CIPKs play crucial roles in abiotic and biotic stresses [59,60,61]. In Arabidopsis, the transcriptional expressions of CBL1 and CIPK7 were induced at 4 °C, 3 h and 12 h, respectively [62]. In addition, cbl1 knock-out mutant plants displayed increased cold sensitivity. In addition, the inductions of cold response genes, such as COR15a and KIN1, were suppressed in cbl1. Furthermore, CBL1 is found to interact with CIPK7 in the presence of Ca2+ in vitro and in vivo. These observations indicate that CIPK7, together with CBL1, are required for cold tolerance. In rice, the transcriptional expression of OsCIPK1, OsCIPK3 and OsCIPK9 were induced at low temperatures. Further study revealed that oscipk3-overexpressing mutant plants are more tolerant to cold stress as compared to WT, indicating that OsCIPK3 would be a positive regulator in cold tolerance [63]. A recent investigation of the entire signal networks of CBLs-CIPKs in response to cold stress in Cassava. MeCBL2, MeCBL4, MeCBL5, MeCBL9, and MeCBL10 were induced by cold stress in roots, while only MeCBL5 was induced by cold stress in leaves. In addition, MeCIPK7, MeCIPK10, and MeCIPK13 were induced by cold stress in roots, while MeCIPK4, MeCIPK12, and MeCIPK14 were induced by cold stress in leaves [64]. These observations suggest tissue-specific functions of CBLs-CIPKs in cold tolerance. However, how CIPKs and their substrates are involved in plant response to cold stress is not clear.
5. AtSR1/CAMTA3-Mediated Transcriptional Reprogramming
Ca2+/CaM-mediated transcription factors relay Ca2+ transients triggered by cold stress to transcriptional reprogramming. Arabidopsis signal responsive 1 (AtSR1), also known as calmodulin-binding transcriptional activator 3 (CAMTA3), is well documented as a CaM-mediated transcription factor (TF) to regulate gene expressions by binding to the “CGCG” DNA-binding motif in their promoter region [65,66,67]. AtSR1 positively regulates plant cold tolerance, since atsr1 knock-out mutant plants display increased sensitivity to cold stress as compared to WT. The transcriptional expression of CBF1 was suppressed in atsr1 at low temperatures, and the promoter of CBF1 contains the AtSR1-recognized DNA motif [68]. These observations indicate that AtSR1 regulates CBF1-mediated signaling pathway during cold stress. Furthermore, accumulated salicylic acid (SA) in atsr1 had no impact on cold tolerance, but SA plays a key role in transcriptional reprogramming at low temperatures [69,70]. Promoter analysis of wound-induced genes revealed a rapid stress response DNA element (RSRE), CGCGTT. In addition, promoter activity assay revealed that luciferase activity level triggered by cold stress was compromised in atsr1 as compared to WT [71], indicating AtSR1-mediate cold tolerance through the regulation of genes containing RSRE in their promoters. An earlier study indicated that AtSR1 positively regulates the transcriptional expression of CBF2 through binding to “CCGCGT” motif in its promoter region [68]. In addition, heptahelical protein 2 (HHP2) was identified to interact with AtSR1; HHP2, including HHP1 and HHP3, was induced by R2R3-type MYB transcription factor, MYB96, at low temperatures [72]. These results suggest that Ca2+ signaling regulates transcriptome response to cold stress through AtSR1.
A recent study revealed that AtSR1, together with CAMTA5, regulated CBFs only in response to a rapid reduction in temperatures, but not to a gradual reduction in temperatures (3 °C/10 min) [73]. Interestingly, unlike circadian clock associated 1 (CCA1), which only mediates cold response during the daytime, AtSR1 regulates cold response during both day and night-time [73], although the underlying mechanism is not clear. Another recent study revealed that the first IQ motifs in AtSR1 (residues 850–875) are required for its activity [74]. Additionally, post-translational modifications, such as phosphorylation or dephosphorylation, would play an important role in AtSR1-mediated signaling response to environmental stimulus. Two putative phosphorylation sites in AtSR1, S454, and S964, were identified [75]. Functional tests revealed that camta1 camta3 double mutants complemented with mutated AtSR1 protein, S454A and S964A (in the phosphorylation sites of AtSR1), were partially restored to WT; and the repression of the biosynthesis of SA in the mutants was impaired, indicating that phosphorylation is required for the full function of AtSR1 [74].
It has been documented that AtSR1 is a defense repressor and is degraded to induce SA-mediated immune response during pathogen attack [67,76,77,78]. Interestingly, SA-mediated signaling pathways were also observed to be activated in plants subjected to long-term cold treatments (4 °C, one or two weeks) [79,80]. However, the activation of the SA signaling pathway was not caused by the degradation of AtSR1 protein. In contrast, AtSR1 protein accumulates under low temperatures [74]. These observations indicate that plants may possess multiple pathways to overcome AtSR1 suppression of the SA signaling pathway, in order to establish appropriate response to specific stress, although the molecular mechanism remains unclear.
6. Concluding Remarks and Future Directions
In recent years, the mechanism of plant cold stress tolerance and genes involved in the cold stress signaling network have been intensively investigated. Several signal pathways are involved in cold stress signaling. Perception of cold stimulus is considered to be the earliest event in triggering the induction of Ca2+ transients in plant cells [12]. A variety of Ca2+ channels and/or Ca2+ pumps are involved in creating cold stress-triggered Ca2+ transients, which are relayed and deciphered by various Ca2+ sensors to induce the changes in gene expression and eventually cold tolerance in plants [12,13]. Much progress has been made in the understanding of several Ca2+-mediated signal networks, such as Ca2+-CBL-CIPK and Ca2+-CaM-AtSR1/CAMTA3 [62,73,74]. Figure 1 illustrates the current status of Ca2+-mediated signaling in cold stress response. It should be noted that we attempted to cover as many studies on calcium-regulated cold signaling as possible. However, in this short communication we had to restrict ourselves to some recent highlights. Plant cold tolerance is a complex process involving a variety of signal transduction pathways. It is still not clear how Ca2+-mediated signaling interacts with other signaling pathways and coordinates cold response in plants. Furthermore, it is a challenge to dissect the role of Ca2+ signals in cold acclimation, and to determine whether cold stress induces Ca2+ transients in the nucleus. Emerging technologies, such as more sensitive Ca2+ imaging, -omics and gene editing approaches will be powerful tools to answer these questions.
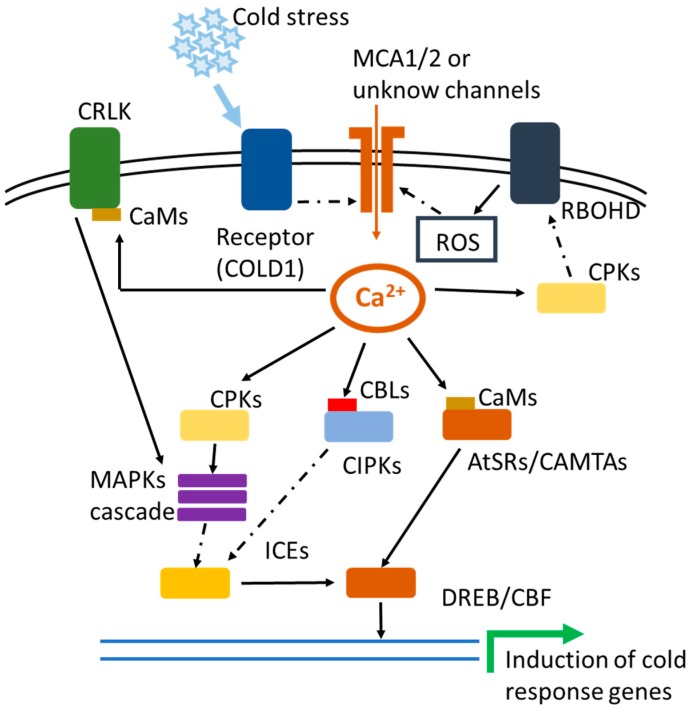
Figure 1.
Calcium signaling-mediated plant response to cold stress in Arabidopsis. The perception of cold stimulus is the first step in the activation of cold tolerance in plants. Plants sense cold signals through the recognition of the changes in cellular membrane by cold stress, or cold signals sensor, COLD1. The perception of cold stimulus activates cold responsive Ca2+ channels (MCA1 and MCA2) or other unknown Ca2+ channels and/or pumps to induce Ca2+ transients, also knowns as Ca2+ signals or signatures. The cold stress-induced Ca2+ transient changes in plant cell and the expression of AtSRC2 subsequently facilitate the production of ROS, through the activation of Ca2+-mediated NADPH oxidase activity of AtRBOHF. Enhanced ROS further activates Ca2+ channels and/or pumps for inducing Ca2+ transients to form a positive feedback. CPKs also relay cold-triggered Ca2+ signals into phosphorylation and activation of the MAPK cascade. In addition, the MAPK cascade is activated by CRLK1 or CRLK2 through the interaction with calmodulin (CaM) in response to cold stress. The MAPK signaling pathway triggered by cold stress suppresses the degradation of ICE1 to establish cold tolerance in plants. CaM also relays cold stress-triggered changes into transcriptional reprogramming via AtSR1/CAMTA3. Solid arrows represent physical interaction and/or positive regulation (induction). Dashed arrows represent mechanism that is unclear.
Acknowledgments
Our thanks to Lorie Mochel for her help with manuscript preparation.
Abbreviations
| ABA | abscisic acid |
| ARRs | Arabidopsis response regulators |
| AtSRs/CAMTAs | Arabidopsis signal responsive genes/calmodulin-binding transcriptional activators |
| CaMs | calmodulins |
| CBFs | c-repeat binding factors |
| CBLs | calcineurin B-like proteins |
| CIPKs | CBL-interacting protein kinases |
| CMLs | CaM-like proteins |
| COLD | chilling tolerance divergence |
| COR | cold regulated |
| CPKs/CDPKs | Ca2+-dependent protein kinases |
| CRLK | Ca2+/CaM-regulated receptor-like kinases |
| DREB | dehydration response element-binding protein |
| ICE | inducer of CBF expression |
| MAPKs | mitogen-activated protein kinase |
| MEKKs/MKKs | mapk/erk kinase kinases |
| MPKs | map kinases |
| NO | nitric oxide |
| RBOHs | respiratory burst oxidase homologs |
| RKs | receptor proteins/kinases |
| RLKs | receptor-like proteins/kinases |
| ROS | reactive oxygen species |
| SA | salicylic acid |
| TF | transcription factor |
Author Contributions
All three authors (P.Y., T.Y. and B.W.P. have contributed in preparing this review. B.W.P. served as the corresponding author and project leader.
Funding
This work was supported by a US National Science Foundation grant (1021344), National Institute of Food and Agriculture project WNP00321 and USDA-ARS Grant No. 8042-43000-015-00D.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Thomashow M.F. PLANT COLD ACCLIMATION: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 2.Browse J., Xin Z. Temperature sensing and cold acclimation. Curr. Opin. Plant Biol. 2001;4:241–246. doi: 10.1016/S1369-5266(00)00167-9. [DOI] [PubMed] [Google Scholar]
- 3.Shinozaki K., Yamaguchi-Shinozaki K., Seki M. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 2003;6:410–417. doi: 10.1016/S1369-5266(03)00092-X. [DOI] [PubMed] [Google Scholar]
- 4.Chinnusamy V., Ohta M., Kanrar S., Lee B.-H., Hong X., Agarwal M., Zhu J.-K. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003;17:1043–1054. doi: 10.1101/gad.1077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee B.-H., Henderson D.A., Zhu J.-K. The Arabidopsis Cold-Responsive Transcriptome and Its Regulation by ICE1. Plant Cell. 2005;17:3155–3175. doi: 10.1105/tpc.105.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon J., Kim J. Arabidopsis response Regulator1 and Arabidopsis histidine phosphotransfer Protein2 (AHP2), AHP3, and AHP5 function in cold signaling. Plant Physiol. 2013;161:408–424. doi: 10.1104/pp.112.207621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon J., Kim N.Y., Kim S., Kang N.Y., Novák O., Ku S.-J., Cho C., Lee D.J., Lee E.-J., Strnad M., et al. A Subset of Cytokinin Two-component Signaling System Plays a Role in Cold Temperature Stress Response in Arabidopsis. J. Biol. Chem. 2010;285:23371–23386. doi: 10.1074/jbc.M109.096644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J.-K. Abiotic Stress Signaling and Responses in Plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danquah A., de Zelicourt A., Colcombet J., Hirt H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014;32:40–52. doi: 10.1016/j.biotechadv.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Zipfel C., Oldroyd G.E.D. Plant signalling in symbiosis and immunity. Nature. 2017;543:328–336. doi: 10.1038/nature22009. [DOI] [PubMed] [Google Scholar]
- 11.Yuan P., Du L., Poovaiah B. Ca2+/Calmodulin-Dependent AtSR1/CAMTA3 Plays Critical Roles in Balancing Plant Growth and Immunity. Int. J. Mol. Sci. 2018;19:1764. doi: 10.3390/ijms19061764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y., Dai X., Xu Y., Luo W., Zheng X., Zeng D., Pan Y., Lin X., Liu H., Zhang D., et al. COLD1 Confers Chilling Tolerance in Rice. Cell. 2015;160:1209–1221. doi: 10.1016/j.cell.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 13.Mori K., Renhu N., Naito M., Nakamura A., Shiba H., Yamamoto T., Suzaki T., Iida H., Miura K. Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci. Rep. 2018;8 doi: 10.1038/s41598-017-17483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkins K.A., Matthus E., Swarbreck S.M., Davies J.M. Calcium-Mediated Abiotic Stress Signaling in Roots. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan P., Jauregui E., Du L., Tanaka K., Poovaiah B.W. Calcium signatures and signaling events orchestrate plant–microbe interactions. Curr. Opin. Plant Biol. 2017;38:173–183. doi: 10.1016/j.pbi.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Shi S., Li S., Asim M., Mao J., Xu D., Ullah Z., Liu G., Wang Q., Liu H. The Arabidopsis Calcium-Dependent Protein Kinases (CDPKs) and Their Roles in Plant Growth Regulation and Abiotic Stress Responses. Int. J. Mol. Sci. 2018;19:1900. doi: 10.3390/ijms19071900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z., Jia Y., Ding Y., Shi Y., Li Z., Guo Y., Gong Z., Yang S. Plasma Membrane CRPK1-Mediated Phosphorylation of 14-3-3 Proteins Induces Their Nuclear Import to Fine-Tune CBF Signaling during Cold Response. Mol. Cell. 2017;66:117–128.e5. doi: 10.1016/j.molcel.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Reddy A.S.N., Ali G.S., Celesnik H., Day I.S. Coping with Stresses: Roles of Calcium- and Calcium/Calmodulin-Regulated Gene Expression. Plant Cell. 2011;23:2010–2032. doi: 10.1105/tpc.111.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plieth C. Calcium: Just another regulator in the machinery of life? Ann. Bot. 2005;96:1–8. doi: 10.1093/aob/mci144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aldon D., Mbengue M., Mazars C., Galaud J.-P. Calcium Signalling in Plant Biotic Interactions. Int. J. Mol. Sci. 2018;19:665. doi: 10.3390/ijms19030665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilroy S., Suzuki N., Miller G., Choi W.-G., Toyota M., Devireddy A.R., Mittler R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends. Plant Sci. 2014;19:623–630. doi: 10.1016/j.tplants.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Hines P.J. Calcium signals the making of symbiosis. Science. 2016;352:1071. doi: 10.1126/science.352.6289.1071-e. [DOI] [Google Scholar]
- 23.Charpentier M., Sun J., Martins T.V., Radhakrishnan G.V., Findlay K., Soumpourou E., Thouin J., Véry A.-A., Sanders D., Morris R.J., et al. Nuclear-localized cyclic nucleotide–gated channels mediate symbiotic calcium oscillations. Science. 2016;352:1102–1105. doi: 10.1126/science.aae0109. [DOI] [PubMed] [Google Scholar]
- 24.Poovaiah B.W., Du L. Calcium signaling: Decoding mechanism of calcium signatures. New Phytol. 2018;217:1394–1396. doi: 10.1111/nph.15003. [DOI] [PubMed] [Google Scholar]
- 25.Carpaneto A., Ivashikina N., Levchenko V., Krol E., Jeworutzki E., Zhu J.-K., Hedrich R. Cold Transiently Activates Calcium-Permeable Channels in Arabidopsis Mesophyll Cells. Plant Physiol. 2007;143:487–494. doi: 10.1104/pp.106.090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight H., Trewavas A.J., Knight M.R. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon J., Kim J. Cold stress signaling networks in Arabidopsis. J. Plant Biol. 2013;56:69–76. doi: 10.1007/s12374-013-0903-y. [DOI] [Google Scholar]
- 28.Krebs M., Held K., Binder A., Hashimoto K., Den Herder G., Parniske M., Kudla J., Schumacher K. FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 2012;69:181–192. doi: 10.1111/j.1365-313X.2011.04780.x. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi D., Li B., Nakayama T., Kawamura Y., Uemura M. Plant plasma membrane proteomics for improving cold tolerance. Front. Plant Sci. 2013;4 doi: 10.3389/fpls.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robards A.W., Clarkson D.T. Effects of chilling temperatures on root cell membranes as viewed by freeze-fracture electron microscopy. Protoplasma. 1984;122:75–85. doi: 10.1007/BF01279439. [DOI] [Google Scholar]
- 31.Miura K., Furumoto T. Cold Signaling and Cold Response in Plants. Int. J. Mol. Sci. 2013;14:5312–5337. doi: 10.3390/ijms14035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo X., Liu D., Chong K. Cold signaling in plants: Insights into mechanisms and regulation. J. Int. Plant Biol. 2018;60:745–756. doi: 10.1111/jipb.12706. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki N., Mittler R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006;126:45–51. doi: 10.1111/j.0031-9317.2005.00582.x. [DOI] [Google Scholar]
- 34.Keller T., Damude H.G., Werner D., Doerner P., Dixon R.A., Lamb C. A Plant Homolog of the Neutrophil NADPH Oxidase gp91phox Subunit Gene Encodes a Plasma Membrane Protein with Ca2+ Binding Motifs. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L., Li M., Yu L., Zhou Z., Liang X., Liu Z., Cai G., Gao L., Zhang X., Wang Y., et al. The FLS2-Associated Kinase BIK1 Directly Phosphorylates the NADPH Oxidase RbohD to Control Plant Immunity. Cell Host Microbe. 2014;15:329–338. doi: 10.1016/j.chom.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Akamatsu A., Wong H.L., Fujiwara M., Okuda J., Nishide K., Uno K., Imai K., Umemura K., Kawasaki T., Kawano Y., et al. An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 Module Is an Essential Early Component of Chitin-Induced Rice Immunity. Cell Host Microbe. 2013;13:465–476. doi: 10.1016/j.chom.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Kawarazaki T., Kimura S., Iizuka A., Hanamata S., Nibori H., Michikawa M., Imai A., Abe M., Kaya H., Kuchitsu K. A low temperature-inducible protein AtSRC2 enhances the ROS-producing activity of NADPH oxidase AtRbohF. Biochim. Biophys. Acta. 2013;1833:2775–2780. doi: 10.1016/j.bbamcr.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 38.Manishankar P., Kudla J. Cold Tolerance Encoded in One SNP. Cell. 2015;160:1045–1046. doi: 10.1016/j.cell.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 39.Gough N.R. Rice that tolerates a chill. Sci. Signal. 2015;8:ec76. doi: 10.1126/scisignal.aab2152. [DOI] [Google Scholar]
- 40.Nakagawa Y., Katagiri T., Shinozaki K., Qi Z., Tatsumi H., Furuichi T., Kishigami A., Sokabe M., Kojima I., Sato S., et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA. 2007;104:3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shigematsu H., Iida K., Nakano M., Chaudhuri P., Iida H., Nagayama K. Structural Characterization of the Mechanosensitive Channel Candidate MCA2 from Arabidopsis thaliana. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0087724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakano M., Iida K., Nyunoya H., Iida H. Determination of Structural Regions Important for Ca2+ Uptake Activity in Arabidopsis MCA1 and MCA2 Expressed in Yeast. Plant Cell Physiol. 2011;52:1915–1930. doi: 10.1093/pcp/pcr131. [DOI] [PubMed] [Google Scholar]
- 43.Yang T., Chaudhuri S., Yang L., Du L., Poovaiah B.W. A Calcium/Calmodulin-regulated Member of the Receptor-like Kinase Family Confers Cold Tolerance in Plants. J. Biol. Chem. 2010;285:7119–7126. doi: 10.1074/jbc.M109.035659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang T., Ali G.S., Yang L., Du L., Reddy A.S.N., Poovaiah B.W. Calcium/calmodulin-regulated receptor-like kinase CRLK1 interacts with MEKK1 in plants. Plant Signal. Behav. 2010;5:991–994. doi: 10.4161/psb.5.8.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furuya T., Matsuoka D., Nanmori T. Membrane rigidification functions upstream of the MEKK1-MKK2-MPK4 cascade during cold acclimation in Arabidopsis thaliana. FEBS Lett. 2014;588:2025–2030. doi: 10.1016/j.febslet.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 46.Furuya T., Matsuoka D., Nanmori T. Phosphorylation of Arabidopsis thaliana MEKK1 via Ca2+ signaling as a part of the cold stress response. J. Plant Res. 2013;126:833–840. doi: 10.1007/s10265-013-0576-0. [DOI] [PubMed] [Google Scholar]
- 47.Su J., Yang L., Zhu Q., Wu H., He Y., Liu Y., Xu J., Jiang D., Zhang S. Active photosynthetic inhibition mediated by MPK3/MPK6 is critical to effector-triggered immunity. PLOS Biol. 2018;16 doi: 10.1371/journal.pbio.2004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frei dit Frey N., Garcia A.V., Bigeard J., Zaag R., Bueso E., Garmier M., Pateyron S., de Tauzia-Moreau M.-L., Brunaud V., Balzergue S., et al. Functional analysis of Arabidopsis immune-related MAPKs uncovers a role for MPK3 as negative regulator of inducible defences. Genome Biol. 2014;15:R87. doi: 10.1186/gb-2014-15-6-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjornson M., Benn G., Song X., Comai L., Franz A.K., Dandekar A., Drakakaki G., Dehesh K. Distinct roles for MAPK signaling and CAMTA3 in regulating the peak time and amplitude of the plant general stress response. Plant Physiology. 2014;166:988–996. doi: 10.1104/pp.114.245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao C., Wang P., Si T., Hsu C.-C., Wang L., Zayed O., Yu Z., Zhu Y., Dong J., Tao W.A., et al. MAP Kinase Cascades Regulate the Cold Response by Modulating ICE1 Protein Stability. Dev. Cell. 2017;43:618–629. doi: 10.1016/j.devcel.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y., Zhou J. MAPping Kinase Regulation of ICE1 in Freezing Tolerance. Trends Plant Sci. 2018;23:91–93. doi: 10.1016/j.tplants.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Almadanim M.C., Alexandre B.M., Rosa M.T.G., Sapeta H., Leitão A.E., Ramalho J.C., Lam T.T., Negrão S., Abreu I.A., Oliveira M.M. Rice calcium-dependent protein kinase OsCPK17 targets plasma membrane intrinsic protein and sucrose-phosphate synthase and is required for a proper cold stress response. Plant Environ. 2017;40:1197–1213. doi: 10.1111/pce.12916. [DOI] [PubMed] [Google Scholar]
- 53.Matschi S., Hake K., Herde M., Hause B., Romeis T. The Calcium-Dependent Protein Kinase CPK28 Regulates Development by Inducing Growth Phase-Specific, Spatially Restricted Alterations in Jasmonic Acid Levels Independent of Defense Responses in Arabidopsis. Plant Cell. 2015;27:591–606. doi: 10.1105/tpc.15.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boudsocq M., Sheen J. CDPKs in immune and stress signaling. Trends in plant science. 2013;18:30–40. doi: 10.1016/j.tplants.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H., Liu W.-Z., Zhang Y., Deng M., Niu F., Yang B., Wang X., Wang B., Liang W., Deyholos M.K., et al. Identification, expression and interaction analyses of calcium-dependent protein kinase (CPK) genes in canola (Brassica napus L.) BMC Genom. 2014;15:211. doi: 10.1186/1471-2164-15-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lv X., Li H., Chen X., Xiang X., Guo Z., Yu J., Zhou Y. The role of calcium-dependent protein kinase in hydrogen peroxide, nitric oxide and ABA-dependent cold acclimation. J. Exp. Bot. 2018;69:4127–4139. doi: 10.1093/jxb/ery212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y., Xu C., Zhu Y., Zhang L., Chen T., Zhou F., Chen H., Lin Y. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice. J. Integr. Plant Biol. 2018;60:173–188. doi: 10.1111/jipb.12614. [DOI] [PubMed] [Google Scholar]
- 58.Kumar K., Mosa K.A., Chhikara S., Musante C., White J.C., Dhankher O.P. Two rice plasma membrane intrinsic proteins, OsPIP2;4 and OsPIP2;7, are involved in transport and providing tolerance to boron toxicity. Planta. 2014;239:187–198. doi: 10.1007/s00425-013-1969-y. [DOI] [PubMed] [Google Scholar]
- 59.Mao J., Manik S.M.N., Shi S., Chao J., Jin Y., Wang Q., Liu H. Mechanisms and Physiological Roles of the CBL-CIPK Networking System in Arabidopsis thaliana. Genes. 2016;7:62. doi: 10.3390/genes7090062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinl S., Kudla J. The CBL–CIPK Ca2+-decoding signaling network: Function and perspectives. New Phytol. 2009;184:517–528. doi: 10.1111/j.1469-8137.2009.02938.x. [DOI] [PubMed] [Google Scholar]
- 61.Luan S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14:37–42. doi: 10.1016/j.tplants.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Huang C., Ding S., Zhang H., Du H., An L. CIPK7 is involved in cold response by interacting with CBL1 in Arabidopsis thaliana. Plant Sci. 2011;181:57–64. doi: 10.1016/j.plantsci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Xiang Y., Huang Y., Xiong L. Characterization of Stress-Responsive CIPK Genes in Rice for Stress Tolerance Improvement. Plant Physiol. 2007;144:1416–1428. doi: 10.1104/pp.107.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mo C., Wan S., Xia Y., Ren N., Zhou Y., Jiang X. Expression Patterns and Identified Protein-Protein Interactions Suggest That Cassava CBL-CIPK Signal Networks Function in Responses to Abiotic Stresses. Front. Plant Sci. 2018;9 doi: 10.3389/fpls.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan P., Tanaka K., Du L., Poovaiah B.W. Calcium Signaling in Plant Autoimmunity: A Guard Model for AtSR1/CAMTA3-Mediated Immune Response. Mol. Plant. 2018;11:637–639. doi: 10.1016/j.molp.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 66.Du L., Ali G.S., Simons K.A., Hou J., Yang T., Reddy A.S.N., Poovaiah B.W. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature. 2009;457:1154–1158. doi: 10.1038/nature07612. [DOI] [PubMed] [Google Scholar]
- 67.Galon Y., Nave R., Boyce J.M., Nachmias D., Knight M.R., Fromm H. Calmodulin-binding transcription activator (CAMTA) 3 mediates biotic defense responses in Arabidopsis. FEBS Lett. 2008;582:943–948. doi: 10.1016/j.febslet.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 68.Doherty C.J., Van Buskirk H.A., Myers S.J., Thomashow M.F. Roles for Arabidopsis CAMTA Transcription Factors in Cold-Regulated Gene Expression and Freezing Tolerance. Plant Cell. 2009;21:972–984. doi: 10.1105/tpc.108.063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y., Park S., Gilmour S.J., Thomashow M.F. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013;75:364–376. doi: 10.1111/tpj.12205. [DOI] [PubMed] [Google Scholar]
- 70.Scott I.M., Clarke S.M., Wood J.E., Mur L.A.J. Salicylate Accumulation Inhibits Growth at Chilling Temperature in Arabidopsis. Plant Physiol. 2004;135:1040–1049. doi: 10.1104/pp.104.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benn G., Wang C.-Q., Hicks D.R., Stein J., Guthrie C., Dehesh K. A key general stress response motif is regulated non-uniformly by CAMTA transcription factors. Plant J. 2014;80:82–92. doi: 10.1111/tpj.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee H.G., Seo P.J. The MYB96–HHP module integrates cold and abscisic acid signaling to activate the CBF–COR pathway in Arabidopsis. Plant J. 2015;82:962–977. doi: 10.1111/tpj.12866. [DOI] [PubMed] [Google Scholar]
- 73.Kidokoro S., Yoneda K., Takasaki H., Takahashi F., Shinozaki K., Yamaguchi-Shinozaki K. Different Cold-Signaling Pathways Function in the Responses to Rapid and Gradual Decreases in Temperature. Plant Cell. 2017;29:760–774. doi: 10.1105/tpc.16.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim Y.S., An C., Park S., Gilmour S.J., Wang L., Renna L., Brandizzi F., Grumet R., Thomashow M. CAMTA-Mediated Regulation of Salicylic Acid Immunity Pathway Genes in Arabidopsis Exposed to Low Temperature and Pathogen Infection. The Plant Cell. 2017;29:2465–2477. doi: 10.1105/tpc.16.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones A.M.E., MacLean D., Studholme D.J., Serna-Sanz A., Andreasson E., Rathjen J.P., Peck S.C. Phosphoproteomic analysis of nuclei-enriched fractions from Arabidopsis thaliana. J. Proteom. 2009;72:439–451. doi: 10.1016/j.jprot.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L., Du L., Shen C., Yang Y., Poovaiah B.W. Regulation of plant immunity through ubiquitin-mediated modulation of Ca2+–calmodulin–AtSR1/CAMTA3 signaling. Plant J. 2014;78:269–281. doi: 10.1111/tpj.12473. [DOI] [PubMed] [Google Scholar]
- 77.Poovaiah B.W., Du L., Wang H., Yang T. Recent Advances in Calcium/Calmodulin-Mediated Signaling with an Emphasis on Plant-Microbe Interactions. Plant Physiol. 2013;163:531–542. doi: 10.1104/pp.113.220780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fromm H., Finkler A. Repression and De-repression of Gene Expression in the Plant Immune Response: The Complexity of Modulation by Ca2+ and Calmodulin. Mol. Plant. 2015;8:671–673. doi: 10.1016/j.molp.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 79.Miura K., Tada Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014;5 doi: 10.3389/fpls.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kurepin L., Dahal K., Savitch L., Singh J., Bode R., Ivanov A., Hurry V., Hüner N. Role of CBFs as Integrators of Chloroplast Redox, Phytochrome and Plant Hormone Signaling during Cold Acclimation. Int. J. Mol. Sci. 2013;14:12729–12763. doi: 10.3390/ijms140612729. [DOI] [PMC free article] [PubMed] [Google Scholar]