Abstract
A new triterpenoid saponin, named oleiferasaponin A2, was isolated and identified from Camellia oleifera defatted seeds. Oleiferasaponin A2 exhibited anti-hyperlipidemic activity on HepG2 cell lines. Further study of the hypolipidemic mechanism showed that oleiferasaponin A2 inhibited fatty acid synthesis by significantly down-regulating the expression of SREBP-1c, FAS and FAS protein, while dramatically promoting fatty acid β-oxidation by up-regulating the expression of ACOX-1, CPT-1 and ACOX-1 protein. Our results demonstrate that the oleiferasaponin A2 possesses potential medicinal value for hyperlipidemia treatment.
Keywords: Camellia oleifera, triterpenoid saponin, oleiferasaponin A2, hypolipidemic activity, fatty acid metabolism
1. Introduction
Hyperlipidemia is a common disease resulting from abnormal lipid metabolism, considered as one of the high-risk factors of inducing cardio-cerebrovascular disease. A large proportion of deaths is caused by cardiovascular disease around the world, more than twice as many deaths as cancer [1], which bring about heavy global medical burden and economic burden. The existing drugs used for lowering lipids are still with non-negligible side-effects and the current status of drug development is less than satisfactory [2,3]. There is an urgent need for safe and efficient anti-hyperlipidemic drugs. Plant extracts have been studied as therapeutic agents for hyperlipidemia [4,5,6]. Saponin is a vital class of natural products, widely used in daily life and production [7,8,9]. Twelve novel triterpenoid saponins have been extracted from Camellia oleifera Abel. seeds [10,11,12,13,14,15,16,17,18]. Camellia oleifera Abel. is widely cultivated throughout southern China as a commercial crop with abundant edible oil in its seeds. Almost 730,000 tons of defatted tea (Camellia oleifera) seeds with 10% saponin are yielded as residue in China annually [17,19], which is a big natural resource. The antimicrobial activity [10,20,21], antiallergic activity [22], antioxidant activity [23] and cytotoxic activity [14,15,24] of saponins have been reported. The research related to the hypolipidemic activity of saponin is scarce [25,26,27], and most hypolipidemic activity research is based on crude saponins [28,29].
Liver is an important organ for lipid metabolism. HepG2 cells not only produce lipids, but also breeds quickly, is easy to cultivate and has high stability. HepG2 cells possess similar cell characteristics to normal human liver cell. HepG2 cell is the representative cell model for studying lipid metabolism, widely used in lipid-regulating drug screening and mechanism research [30,31,32]. Fatty acid synthesis genes, e.g., SREBP-1c (sterol-regulatory element-binding protein-1c) [33,34], FAS (fatty acid synthase) [35], and ACC (acetyl-coenzyme A carboxylase) [36], and fatty acid oxidation genes, e.g., PPARα (peroxisone proliferators-activated receptor alpha) [37], ACOX-1 (acetyl coenzyme A oxidase-1) [38], and CPT-1 (carnitine palmitoyl transferase-1) [39], are often studied as key genes related to fatty acid metabolism.
In our recent study, we isolated a newly-discovered saponin, oleiferasaponin A2, from Camellia oleifera Abel. defatted seeds. The structure of oleiferasaponin A2 was identified by IR (Infrared Spectroscopy), HR-ESI-MS (High Resolution Electrospray Ionization Mass Spectrometry), NMR (Nuclear Magnetic Resonance) and GC-MS (Gas Chromatography-Mass Spectrometer). The anti-hyperlipidemic activity and mechanism study of oleiferasaponin A2 was investigated.
2. Results and Discussion
2.1. Isolation and Characterization of the Oleiferasaponin A2
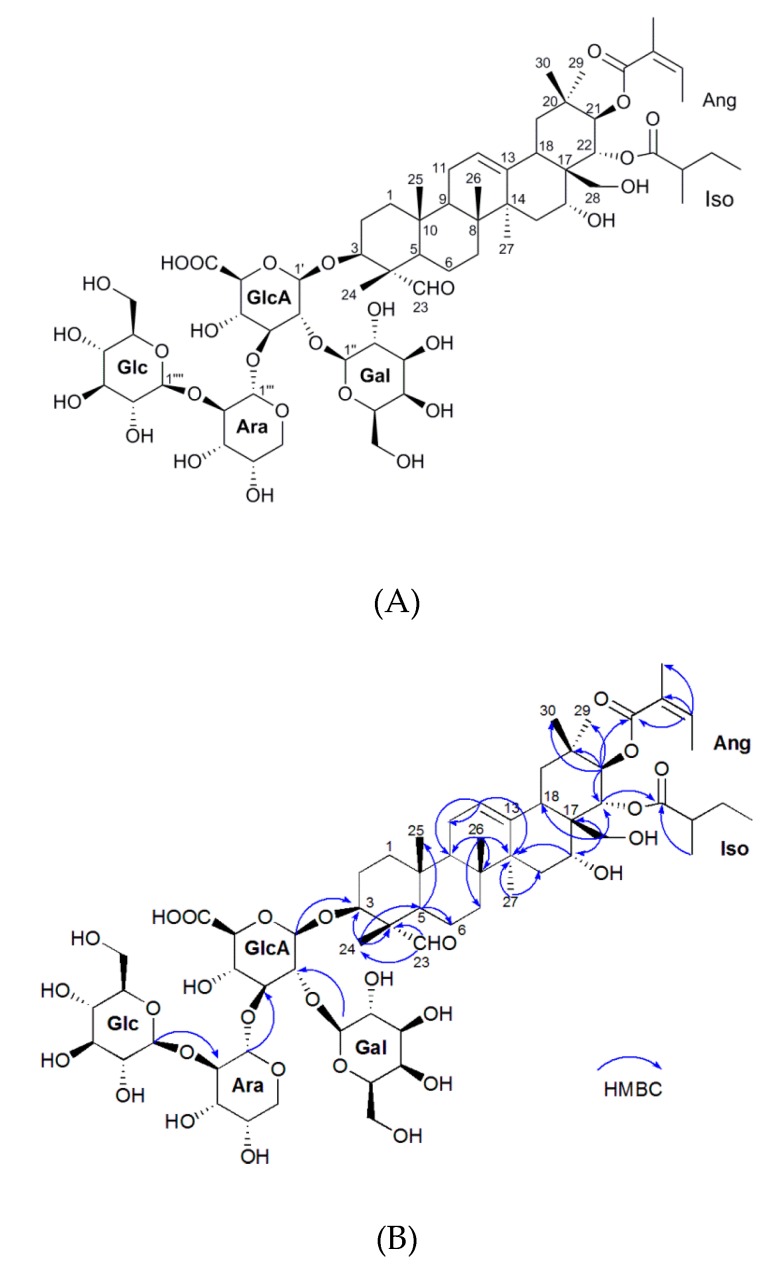
The structure of oleiferasaponin A2 was determined mainly by IR, NMR, HR-ESI-MS and GC-MS (Figure 1A, Table 1, and Figures S1–S10). The molecular formula of oleiferasaponin A2 is C63H99O28 deduced from the HR-ESI-MS [M-H]-ion at m/z 1302.6231 (Figure S3). The IR spectrum (cm−1) suggested absorption bands at 3378, 1716, 1618, 1088, and 1045 due to hydroxyl, carbonyl, olefinic, and ether functional groups, respectively (Figure S2). The NMR spectroscopic data of oleiferasaponin A2 is shown in Table 1 and Figures S4 and S5). The presence of signals at C-21 (δC 78.3, δH 5.94, d, 10.1) and C-22 (δC 73, δH 5.55, d, 10.1) indicate the existence of 21-angeloyl group and 22-isovaleric group, respectively (Table 1). The HMBC (1H detected heteronuclear Multiple Bond Correlation) spectrum (Figure S8) showed correlation between δH 5.94 (H-21) and δC 167.7 (Ang-C-1), δH 5.55 (H-22) and δC 177.4 (IsoA-C-1) (Figure 1B), which confirmed the presence of the Ang group linked to C-21 and Iso group linked to C-22. The NOESY (Nuclear Overhauser Effect Spectroscopy) spectrum (Figure S9) showed the cross peaks between H-22 at δH 5.55 and H-30 at δH 1.10, as well as those between H-16 at δH 4.00 and H-28 at δH 2.95, suggesting that H-22 and H-16 are both β-oriented, that is, iso groups at C-22 and 16-OH group are both α-orientations. The H-3 at δH 3.89 correlated with H-23 at δH 9.48 and H-21 at δH 5.94 correlated with H-29 at δH 0.88, indicating that the glycosidic chain group at C-3 and Ang group at C-21 are β-configured.
Figure 1.
(A) Structure of oleiferasaponin A2; and (B) Key HMBC correlations of oleiferasaponin A2.
Table 1.
NMR spectroscopic data for oleiferasaponin A2 (in methanol-d4).
| Position | δ C | δ H | Position | δ C | δ H |
|---|---|---|---|---|---|
| 1 | 38.0 | 1.14 m, 1.73 m | 21-O-Ang | ||
| 2 | 24.3 | 1.81 m, 2.05 m | Ang-1 | 167.7 | |
| 3 | 84.9 | 3.89 m | Ang-2 | 127.7 | |
| 4 | 55.0 | Ang-3 | 139.0 | 6.18 m | |
| 5 | 47.5 | 1.38 m | Ang-4 | 14.8 | 1.94 m |
| 6 | 19.8 | 0.96 m | Ang-5 | 19.7 | 1.87 s |
| 7 | 31.8 | 1.29 m, 1.64 m | 22-O-Iso | ||
| 8 | 39.9 | Iso-1 | 177.4 | ||
| 9 | 46.5 | 1.81 m | Iso-2 | 41.3 | 2.33 m |
| 10 | 35.6 | Iso-3 | 26.3 | 1.43 m, 1.65 m | |
| 11 | 23.3 | 1.98 m | Iso-4 | 10.8 | 0.89 m |
| 12 | 123.6 | 5.41, t, 3.7 | Iso-5 | 15.6 | 1.05, d, 6.8 |
| 13 | 141.6 | GlcA-1′ | 103.4 | 4.42, d, 7.7 | |
| 14 | 41.0 | GlcA-2′ | 76.8 | 3.77 m | |
| 15 | 33.4 | 1.37 m, 1.70 m | GlcA-3′ | 81.7 | 3.88 overlap |
| 16 | 68.1 | 4.00 m | GlcA-4′ | 69.1 | 3.84 overlap |
| 17 | 47.2 | GlcA-5′ | 75.5 | 3.56 m | |
| 18 | 39.4 | 2.65 m | GlcA-6′ | 177.4 | |
| 19 | 46.3 | 1.21 m, 2.69 m | Gal-1” | 101.3 | 5.04, d, 7.3 |
| 20 | 35.6 | Gal-2” | 72.1 | 3.50 m | |
| 21 | 78.3 | 5.94, d, 10.1 | Gal-3” | 73.4 | 3.76 m |
| 22 | 73.0 | 5.55, d, 10.1 | Gal-4” | 69.1 | 3.84 m |
| 23 | 209.4 | 9.48 s | Gal-5” | 74.8 | 3.30 m |
| 24 | 9.5 | 1.19 s | Gal-6” | 61.1 | 3.69 m |
| 25 | 15.1 | 2.00 s | Ara-1‴ | 100.2 | 5.03, d, 7.3 |
| 26 | 15.9 | 0.96 s | Ara-2‴ | 82.4 | 3.68 m |
| 27 | 26.4 | 1.53 s | Ara-3‴ | 69.6 | 3.53 m |
| 28 | 63.0 | 2.95 m, 3.77 m | Ara-4‴ | 68.1 | 4.00 m |
| 29 | 28.3 | 0.88 s | Ara-5‴ | 65.9 | 3.21 m, 3.97 m |
| 30 | 19.0 | 1.10 s | Glc-1‴′ | 106.2 | 4.53, d, 7.7 |
| Glc-2‴′ | 75.1 | 3.65 m | |||
| Glc-3‴′ | 73.6 | 3.51 overlap | |||
| Glc-4‴′ | 69.6 | 3.53 overlap | |||
| Glc-5‴′ | 76.4 | 3.36 m | |||
| Glc-6‴′ | 61.2 | 3.80 m |
1H (δ ppm; J in Hz; s, Single peak; d, Double peaks; m, multiple peaks) and 13C-NMR (δ ppm).
Acid hydrolysis and GC-MS analysis (Figure S10) of oleiferasaponin A2 revealed there are one unit each of d-glucuronic acid (GlcA), D-glucose (Glu), D-galactose (Gal) and l-arabinose (Ara). The HMBC experiment confirmed the position of the sugar components by the long-ranged correlations between the H-1′ of glucuronic acid and the C-3 of the aglycone, between the H-1′′ of galactose and C-2′ of glucuronic acid, between the H-1‴ of arabinose and C-3′ glucuronic acid, and between the H-1‴′ of glucose and C-2‴ of arabinose (Figure 1B). Based on the above spectroscopy analysis, the structure of novel oleiferasaponin A2 was elucidated to be 16α-hydroxy-21β-O-angeloyl-22α-O-isovaleric-28-dihydroxymethyleneolean-12-ene-3-O-[β-d-galactopyranosyl(1→2)]-[β-d-glucopyranosyl(1→2)-α-L-arabinopyranosyl(1→3)]-β-d-gluco-pyranosiduronic acid. Oleiferasaponin A2 with specific structure is a novel compound. The structure of oleiferasaponin A2 is composed of two parts, aglycone and sugar moieties, which is similar to oleiferasaponin A1, camelliasaponin B1 and camelliasaponin B2, except for C-21 angeloyl group and C-22 ovaleric group [13,40].
2.2. Hypolipidemic Activity of the Oleiferasaponin A2
2.2.1. Oleiferasaponin A2 Exhibited Hypolipidemic Activity on HepG2 Cell Lines
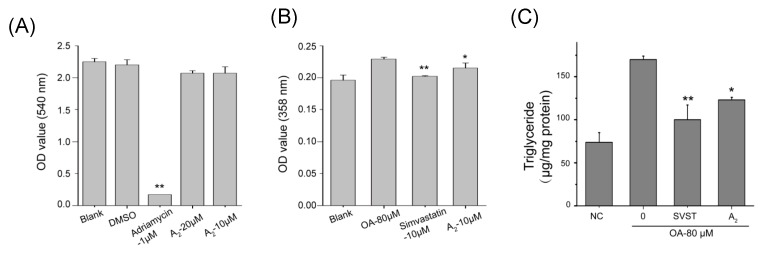
Oleiferasaponin A2 with concentration of 10 μM and 20 μM did not exhibit effective cytotoxic activity on HepG2 cell lines in proliferation bioassay (SRB) (Figure 2A). There are some reports indicating that the cinnamoyl group at C-22 and the free hydroxy group at C-28 play important roles in the anti-proliferative activity of oleanane-type saponins [14,17,41,42]. The cytotoxicity of saponins depends on the key group, as well as the combination of properties at both the aglycone and the sugar moieties. Comparing with amelliasaponin B1, which has been reported with obvious cytotoxicity, it seems that the angeloyl group at C-22, rather than C-21, may increase the cytotoxicity [14]. The C-21 angeloyl group and C-22 ovaleric group, as well as the combination of aglycone and sugar moieties make oleiferasaponin A2 without cytotoxic activity.
Figure 2.
(A) The effect of oleiferasaponin A2 on HepG2 cells proliferation; (B) the lipid lowering activity of oleiferasaponin A2 under oleic acid treatment; and (C) the content of triglyceride under OA inducement. NC, blank control without oleic acid; 0, model group with 80 μM oleic acid; SVST, group with 80 μM oleic acid and 10 μM simvastain; A2, group with 80 μM oleic acid and 10 μM oleiferasaponin A2. ** p < 0.01, * p < 0.05.
The results of preliminary screening of hypolipidemic activity demonstrated that 10 μM oleiferasaponin A2 (p = 0.027) and 10 μM simvastatin (p = 0.000) exhibited significant hypolipidemic activity on HepG2 cell lines (Figure 2B). The content of triglyceride was determined by detection kit (Figure 2C). Comparing with model group, 10 μM simvastatin (p = 0.000) and 10 μM (p = 0.012) oleiferasaponin A2 significantly inhibited the accumulation of triglyceride. Comparing the structure of oleiferasaponin A2 with Chakasaponin I–III [43], Floratheasaponins A–C and theasaponin E1 and E2 [27], we speculated the acyl groups at C21 and C22 may increase the anti-hyperlipidemic activity of oleanane-type saponins, especially, the angeloyl group at C-21.
2.2.2. Oleiferasaponin A2 Affected the Expression of Genes Related to Fatty Acid Metabolism
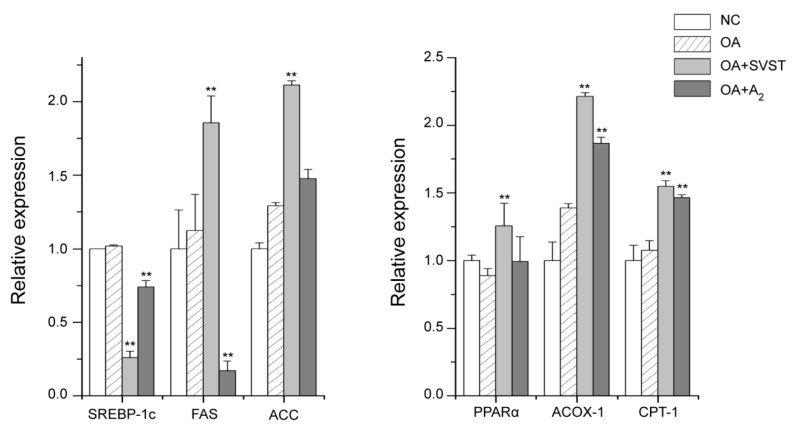
Fluorescent quantitative PCR assay made clear the regulation mechanism of oleiferasaponin A2 on the genes related to fatty acid metabolism (Figure 3). Oleiferasaponin A2 significantly inhibited the expression of fatty acid synthesis genes, including SREBP-1c (p = 0.000), FAS (p = 0.000) and greatly promoted the expression of fatty acid oxidation genes, including ACOX-1 (p = 000) and CPT-1 (p = 0.000). The relative expression of PPARα was promoted by oleiferasaponin A2 (p = 0.208), but without significant variations. Oleiferasaponin A2 can reduce lipid accumulation by inhibiting fatty acid synthesis and promoting fatty acid β-oxidation, which can explain the decrease of triglyceride content (Figure 2C). The expression of SREBP-1c (p = 0.000) was significantly inhibited by simvastatin. The relative expression of fatty acid synthesis genes, FAS (p = 0.005) and ACC (p = 0.000), and fatty acid β-oxidation genes, PPARα (p = 0.008), ACOX-1 (p = 0.000) and CPT-1 (p = 0.000), were significantly elevated by the simvastatin, which revealed the action mechanism of simvastatin. Simvastatin realized lipid lowering by inhibited SREBP-1c and promoting fatty acid β-oxidation. Comparing with simvastatin, oleiferasaponin A2 showed excellent inhibition action on the expression of FAS, which plays a key role in fatty acid synthesis.
Figure 3.
The relative expression of genes related to fatty acid metabolism on fatty acid synthesis genes (SREBP-1c, FAS and ACC) and fatty acid β-oxidation genes (PPARα, ACOX-1 and CPT-1). NC, blank control; OA, 80 μM oleic Acid; OA + SVST, 80 μM Oleic Acid + 10 μM simvastatin; OA + A2, 80 μM Oleic Acid + 10 μM Oleiferasapoin A2. Compared to the control, ** p < 0.01.
2.2.3. Oleiferasaponin A2 Affected the Expression of Proteins Related to Fatty Acid Metabolism
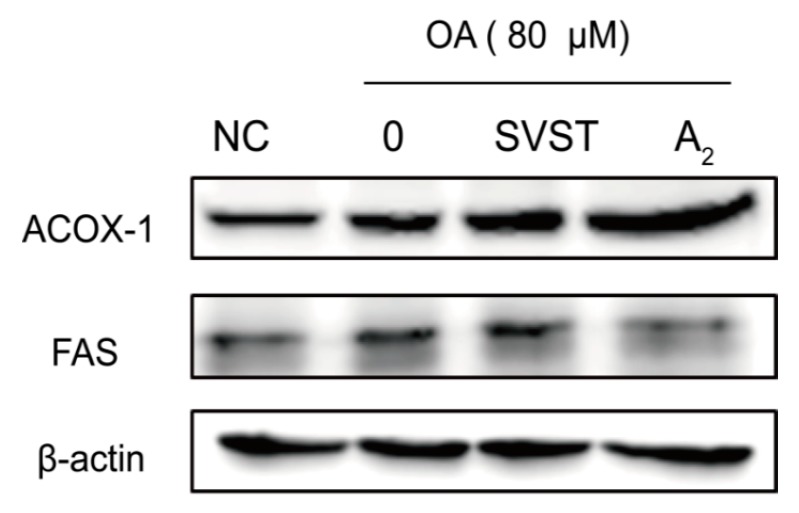
Western blotting results showed the effect of oleiferasaponin A2 and simvastatin on the proteins related to fatty acid metabolism (Figure 4). The expression of ACOX-1 was significantly promoted by 10 μM oleiferasaponin A2, which was superior to the promotion effect of 10 μM simvastatin. The expression of FAS was significantly inhibited by 10 μM oleiferasaponin A2, while promoted by 10 μM simvastatin. FAS is responsible for the formation of free fatty acids, such as palmitate from acetyl-CoA and malonyl-CoA [44]. ACOX-1 is the rate-limiting enzyme of the first dehydrogenation reaction in peroxysomal lipase oxygenation [45]. Therefore, FAS and ACOX-1 play an important role in fat deposition. Oleiferasaponin A2 performs lipid-lowering effect by reducing the fatty acid production and accelerating fatty acid oxidation.
Figure 4.
The relative expression of proteins related to fatty acid metabolism. NC, blank control without oleic acid; 0, model group with 80 μM oleic acid; SVST, group with 80 μM oleic acid and 10 μM simvastain; A2, group with 80 μM oleic acid and 10 μM oleiferasapoin A2; ACOX-1, fatty acid oxidation protein; FAS, fatty acid synthesis protein; β-actin, reference protein.
3. Materials and Methods
3.1. General
The following equipment was used: 3000-Da nanofiltration membrane (SJM, Hefei, China), AB-8 macroporous resin column (Bonc, Cangzhou, China), ordinary-phase silica gel column (200–300 mesh, Anhui Liangchen Silicon Material Co. Ltd., Huoshan, China) and a Varian Prostar HPLC instrument (Model 325) (Varian, Mulgrave, Australia). The HPLC purifications were run on Agilent 1260 HPLC (Agilent, Palo Alto, CA, USA). IR (infrared) spectra were recorded on Nicolet iN10 (Thermo Scientific Instrument Co., Boston, MA, USA) with KBr pellets. NMR spectra were measured on an AVANCE III (600 MHz) spectrometer (Bruker, Fallanden, Switzerland) using methanol-d4 as solvent g methanol-d4 (Sigma-Aldrich, St. Louis, MO, USA). HR-ESI-MS were determined on an Electrostatic Field Orbital Trap Mass Spectrometer (Thermo Scientific, Bremen, Germany) using an ESI source.
3.2. Plant Material
The Camellia oleifera Abel. defatted seeds were obtained from a manufacture factory in Shucheng, Anhui province, China, in October 2013. The plant material was identified by one of the authors (X.F.Z.), and was deposited in State Key Laboratory of Tea Plant Biology and Utilization, Anhui Agricultural University.
3.3. Extraction and Isolation
The defatted seed powder (10 kg) was extracted three times with methanol at 60 °C under reflux for 3 h each time. The extract solvent was evaporated under reduced pressure. Next, the concentrated solution (1.3 kg) was suspended in water and successively subjected to nanofiltration membrane, AB-8 macroporous resin column (Bonc, Cangzhou, Herbei, China) (chromatography with stepwise gradients of water and ethanol (100:0, 70:30, 30:70, and 0:100, v/v)), and ordinary-phase silica gel column [CHCl2:CH3OH:H2O (80:60:5, v/v)] to yield a high purity fraction (1.6 g). Further isolation and purification were performed by HPLC [MeOH: H2O (30:70)] to yield two purity fractions [Fr. 1 (0.07 g), Fr. 2 (0.21 g)], which were purified by HPLC [acetonitrile-0.2% AcOH: H2O (41:59, v/v)]. Then, the oleiferasaponin A2 (12.9 mg) was obtained from the second fraction. All sample extraction and isolation methods were according to Zhang et al. [13].
3.4. Acid Hydrolysis and GC-MS Analysis
The method of acid hydrolysis and GC-MS analysis was according to Zong et al. [14]. Oleiferasaponin A2 was dissolved in 1 M HCI (1 mL) for 3 h at 90 °C and extracted with chloroform, and then evaporated under N2 flow. The residue was dissolved in 0.2 mL pyridine containing L-cysteine methyl ester hydrochloride (10 mg/mL) and reacted at 70 °C for 1 h, and then evaporated under N2 flow again. After being concentrated, 0.2 mL trimethylsilylimidazole was added for derivatization reaction, and reacted at 70 °C for another 1 h. The reaction mixture was partitioned between n-hexane and water. The organic phase was analyzed by GC-MS (Agilent, Palo Alto, CA, USA) (injector temperature was 280 °C; the initial oven temperature was 160 °C for 1 min, linearly increased to 200 °C at 6 °C/min, further linearly increased to 280 °C at 3 °C/min and held for 5 min). The standard sugar samples were subjected to the same reaction and GC-MS conditions.
3.5. Cell Viability Assay
Human liver tumor cell (HepG2) lines were obtained from Qingdao Marine Biomedical Research Institute Limited by Share Ltd. Testing Center (Qingdao, China). Cells were cultured in DMEM complete medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin at 37 °C in a 5% CO2 humidified atmosphere. The culture medium was refreshed every other day. After 80% of the cells were fused, cells were kept in logarithmic phase by trypsinization and subculturing [14,16].
Human tumor cell lines in logarithmic phase were seeded in 96-well plates at 4 × 103 cells per well (180 μL per well), and incubated for 24 h. After 24 h, an additional complete medium with no additions (negative control), 0.1% DMSO (solvent control), 1 μM adriamycin (positive control), or 20 μM or 10 μM test saponins (sample control) was incubated for 72 h. Then, 50% (m/v) ice-cold trichloroacetic acid was added to the medium to fix cells. After staining by Sulforhodamine B, tris solution (150 μL per well) was added to culture medium. Absorbance values were measured at 540 nm using an enzyme-linked immunosorbent Reader. The inhibition rate of cell proliferation was calculated as: Inhibition rate (%) = [(OD540 (Negative control) − OD540 (Sample control))/OD540 (Negative control)] × 100% [14].
3.6. Preliminary Screening of Hypolipidemic Activity
HepG2 cells (1.2 × 103 per well) at logarithmic phase were seeded in 96-well plates (100 μL per well). After 70–80% fused, the medium was replaced with free-blood serum DMEM (80 μL per well), and starved for 12 h. Blank group with 20 μL free-blood serum DMEM, model group, SVST group and A2 group were added with 80 μM Oleic Acid (10 μL per well). Then, model group was supplemented with 10 μL serum-free medium, and 10 μM simvastatin and oleiferasaponin A2 were added to positive control and treatment group, respectively, incubated for 24 h. After incubating for 24 h, the medium was removed. Cells were washed with PBS once, fixed with 4% paraformaldehyde (80 μL per well) for 1 h, washed with PBS again and rinsed with 60% isopropanol for 10 min. Then, cells were stained with 60 μL 0.3% Oil Red O solution (Sigma O0625) for 1 h, washed with PBS for thrice, dissolved by DMSO (100 μL per well). The OD value (358 nm) was measured by ELIASA (SpectraMax i3) (Molecular devices, Shanghai, China).
3.7. Triglyceride Test
HepG2 cells (2.5 × 105 per well) at logarithmic phase were seeded in 6-well plates (2 mL per well). After 12 h, the medium was replaced with free-blood serum DMEM (2 mL per well), and starved for 12 h. Blank group was supplemented with 1 mL free-blood serum DMEM, and the other groups were added with 80 μM Oleic Acid (300 μL per well). Then, model group was supplemented with 700 μL serum-free medium, and 300 μL simvastatin and oleiferasaponin A2 (10 μM) were added to positive control and treatment group, respectively, and incubated for 24 h. After incubating 24 h, the medium was removed. Cells were washed with PBS twice. The content of triglyceride was tested according to K622-100 triglyceride test kit (BioVision, San Francisco, CA, USA) instructions.
3.8. Fluorescent Quantitative PCR
After incubating 24 h, for the medium was removed. Cells were washed with PBS twice. Trizol (500 μL per well) was added for cell lysis, then homogenized and placed at room temperature for 5 min. One hundred microliters of chloroform were added, then shook for 15 s, let stand for 3 min, and centrifuged at 12,000 r/min, 4 °C for 15 min. The supernatant was transferred to centrifuge tube, 250 μL of isopropanol were added, shook, let stand, and then centrifuged. The supernatant was removed, and the remaining was resuspended with 75% ethyl alcohol and centrifuged. Supernatant was removed and aired. Twenty microliters of DEPC were added for RNA hydrolysis, and the concentration determined. cDNA was obtained through reverse transcription reaction, and then the gene relative expression was tested by real-time fluorescent quantitative PCR (LightCycler 96, Roche Life Science, Basel, Switzerland) under following reaction systems: 12.5 μL SYBR Premix Ex Taq ll (Tli RNaseH Plus) (2×), 1.0 μL PCR Forward Primer (10 μM), 1.0 μL PCR Reverse Primer (10 μM), 2 μL cDNA solution, and 8.5 μL dH2O. Primer set used for q-PCR analysis was designed by Primer 3 (Table S1) (http:// Frodo.wi.mit.edu/cgi-bin/primer3-www.cgi).
3.9. Western Blot Analysis
After incubating for 24 h, the medium was removed. Cells were washed with PBS twice. Two hundred microliters of RIPA (containing 10 μL PMSF) were added for cell lysis. After being homogenized on ice for 30 min, the samples were centrifuged at 12,000 r/min for 5 min, and the supernatants were collected for Western blot analysis. The concentration of protein was measured by BCA kit (Thermo Fisher scientific, Waltham, MA, USA). The lysate containing SDS-PAGE protein loading buffer was placed in boiling bath for protein albumen metamorphism, centrifuged at 12,000 rpm for 2 min and the supernatant was kept at −80 °C for SDS-PAGE. Equal amounts of protein were subjected to SDS-PAGE (12% separating gel and 5% stacking gel), and then transferred to PVDF membranes, which was placed in sealing solution and blocked for 2 h at room temperature. The PVDF membranes was incubated and shocked overnight with primary antibodies (FAS:1:200; ACOX-1:1:1000), and washed with TBST for 3 times. After washing with TBST, the PVDF membranes were incubated with secondary antibodies (1:2000) for 2 h at room temperature and washed with TBST thrice. PVDF membranes with ECL reagent (regent A:regent B is 1:1) were incubated for 30 s, and then chemiluminescent signals were visualized and analyzed using scanned gel imaging systems (GIS-2008) (Analytik Jena AG, Jena, Germeny). The method of Western blot refers to Zong et al. [16].
4. Conclusions
Our present study reveals that oleiferasaponin A2, a novel compound from Camellia oleifera defatted seeds, can accomplish lipid-lowering of HepG2 cells. Oleiferasaponin A2 can down-regulate SREBP-1c and FAS expression and FAS protein expression, and up-regulate ACOX-1, CPT-1, and PPARα expression and ACOX-1 protein expression, which are related to fatty acid metabolism. Thus, we have primarily testified that oleiferasaponin A2 performs anti-hyperlipidemic effect by repressing fatty acid synthesis and accelerating fatty acid oxidation in vitro. The structure identification and anti-hyperlipidemic activity study of oleiferasaponin A2 will promote understanding of the structure–activity relationship of oleanane-type saponins and expedite drug development, while improving the utilization ratio of natural resources. In the future, animal experiments of hypolipidemic activity should be carried out, and the medicinal value of oleiferasaponin A2 should be excavated in more detail.
Supplementary Materials
HR-ESI-MS and NMR spectra data of oleiferasaponin A2 can be accessed.
Author Contributions
T.D. and X.Z. wrote the manuscript; X.Z., L.Z. and T.X. designed the experiments; T.D., S.Y. and X.L. performed the experiments; S.Y. contributed reagents and materials; and F.D., T.D. and X.Z. analyzed the data. All authors approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 31301601) and Open Fund of State Key Laboratory of Tea Plant Biology and Utilization (SKLTOF20150110). We sincerely acknowledge Fali Bai, Ying Yang and Juan Wu for technical assistance, and Fandong Kong for spectrum unfolding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Melanie N., Townsend N., Scarnorough P., Rayner M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014;35:2950–2959. doi: 10.1093/eurheartj/ehu299. [DOI] [PubMed] [Google Scholar]
- 2.Program L.C. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1985;253:635–636. doi: 10.1001/jama.1985.03350290037018. [DOI] [PubMed] [Google Scholar]
- 3.Shrestha S., Bhattarai B.R., Lee K.H., Cho H. Mono- and disalicylic acid derivatives: Ptp1b inhibitors as potential anti-obesity drugs. Bioorgan. Med. Chem. 2007;15:6535–6548. doi: 10.1016/j.bmc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S.B., Nasir A., Prabhu K.M., Murthy P.S., Dev G. Hypoglycemic and hypolipidemic effect of ethanolic extract of seeds of eugenia jambolana in alloxan induced diabetic rabbits. J. Ethnopharmacol. 2003;85:201–206. doi: 10.1016/S0378-8741(02)00366-5. [DOI] [PubMed] [Google Scholar]
- 5.Zou B., Li C.M., Chen J.Y., Dong X.Q., Zhang Y., Du J. High molecular weight persimmon tannin is a potent hypolipidemic in high-cholesterol diet fed rats. Food Res. Int. 2012;48:970–977. doi: 10.1016/j.foodres.2012.05.024. [DOI] [Google Scholar]
- 6.Xia D.Z., Yu X.F., Wang H.M., Ren Q.Y., Chen B.M. Anti-obesity and hypolipidemic effects of ethanolic extract from alpinia officinarum hance (zingiberaceae) in rats fed high-fat diet. J. Med. Food. 2010;13:785–791. doi: 10.1089/jmf.2009.1235. [DOI] [PubMed] [Google Scholar]
- 7.Jian H.L., Liao X.X., Zhu L.W., Zhang W.M., Jiang J.X. Synergism and foaming properties in binary mixtures of a biosurfactant derived from camellia oleifera abel and synthetic surfactants. J. Coll. Interface Sci. 2011;359:487–492. doi: 10.1016/j.jcis.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y.Z., Li M.A., Liu Z.C., Peng S.F., Chen X.L., Chen L.S., Wang X.N., Wang R. Molluscicidal effect of camellia oleifera saponin. J. Cent. South Univ. Technol. 2011;31:147–150. [Google Scholar]
- 9.Chen Y.F., Yang C.H., Chang M.S., Ciou Y.P., Huang Y.C. Foam properties and detergent abilities of the saponins from camellia oleifera. Int. J. Mol. Sci. 2010;11:4417–4425. doi: 10.3390/ijms11114417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo P.C., Lin T.C., Yang C.W., Lin C.L., Chen G.F., Huang J.W. Bioactive saponin from tea seed pomace with inhibitory effects against rhizoctonia solani. J. Agric. Food Chem. 2010;58:8618–8622. doi: 10.1021/jf1017115. [DOI] [PubMed] [Google Scholar]
- 11.Chen L., Chen J., Xu H. Sasanquasaponin from camellia oleifera abel. Induces cell cycle arrest and apoptosis in human breast cancer mcf-7 cells. Fitoterapia. 2013;84:123–129. doi: 10.1016/j.fitote.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Chen J.H., Wu H.Y., Bingchung L., Chang C.M.J., Tingting J., Wu L.C. Identification and evaluation of antioxidants defatted camellia oleifera seeds by isopropanol salting-out pretreatment. Food Chem. 2010;121:1246–1254. doi: 10.1016/j.foodchem.2010.01.015. [DOI] [Google Scholar]
- 13.Zhang X.F., Han Y.Y., Bao G.H., Ling T.J., Zhang L., Gao L.P., Xia T. A new saponin from tea seed pomace (camellia oleifera abel) and its protective effect on pc12 cells. Molecules. 2012;17:11721. doi: 10.3390/molecules171011721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zong J., Wang R., Bao G., Ling T., Zhang L., Zhang X., Hou R. Novel triterpenoid saponins from residual seed cake of camellia oleifera abel. Show anti-proliferative activity against tumor cells. Fitoterapia. 2015;104:7–13. doi: 10.1016/j.fitote.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Zong J.F., Peng Y.R., Bao G.H., Hou R.Y., Wan X.C. Two new oleanane-type saponins with anti-proliferative activity from camellia oleifera abel. Seed cake. Molecules. 2016;21:188–195. doi: 10.3390/molecules21020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zong J., Wang D., Jiao W., Zhang L., Bao G., Ho C.T., Hou R., Wan X. Oleiferasaponin c6 from the seeds of camellia oleifera abel.: A novel compound inhibits proliferation through inducing cell-cycle arrest and apoptosis on human cancer cell lines in vitro. RSC Adv. 2016;6:91386–91393. doi: 10.1039/C6RA14467E. [DOI] [Google Scholar]
- 17.Zhou H., Wang C.Z., Ye J.Z., Chen H.X. New triterpene saponins from the seed cake of camellia oleifera and their cytotoxic activity. Phytochem. Lett. 2014;8:46–51. doi: 10.1016/j.phytol.2014.01.006. [DOI] [Google Scholar]
- 18.Di T.M., Yang S.L., Du F.Y., Zhao L., Xia T., Zhang X.F. Cytotoxic and hypoglycemic activity of triterpenoid saponins from camellia oleifera abel. Seed pomace. Molecules. 2017;22:1562. doi: 10.3390/molecules22101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Y.Y., Cai H.Y., Wang L.S., Xia T., Xian-Feng D.U. Production of multi-enzyme enriched bio-feed from camellia oleifera seed cake in solid state fermentation by aspergillus niger. J. Anhui Agric. Univ. 2012;39:41–46. [Google Scholar]
- 20.Hu J.L., Nie S.P., Huang D.F., Chang L., Xie M.Y., Yin W. Antimicrobial activity of saponin—rich fraction from camellia oleifera cake and its effect on cell viability of mouse macrophage raw 264.7. J. Sci. Food Agric. 2012;92:2443–2449. doi: 10.1002/jsfa.5650. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X.F., Yang S.L., Han Y.Y., Zhao L., Lu G.L., Xia T., Gao L.P. Qualitative and quantitative analysis of triterpene saponins from tea seed pomace (camellia oleifera abel) and their activities against bacteria and fungi. Molecules. 2014;19:7568–7580. doi: 10.3390/molecules19067568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda H., Nakamura S., Fujimoto K., Moriuchi R., Kimura Y., Ikoma N., Hata Y., Muraoka O., Yoshikawa M. Medicinal flowers. Xxxi. Acylated oleanane-type triterpene saponins, sasanquasaponins i-v, with antiallergic activity from the flower buds of camellia sasanqua. Chem. Pharm. Bull. 2010;58:1617–1621. doi: 10.1248/cpb.58.1617. [DOI] [PubMed] [Google Scholar]
- 23.Hu J., Nie S., Huang D., Li C., Xie M. Extraction of saponin from camellia oleifera cake and evaluation of its antioxidant activity. Int. J. Food Sci. Technol. 2012;47:1676–1687. doi: 10.1111/j.1365-2621.2012.03020.x. [DOI] [Google Scholar]
- 24.Mu L.H., Huang C.L., Zhou W.B., Guo D.H., Liu P. Methanolysis of triterpenoid saponin from ardisia gigantifolia stapf. And structure–activity relationship study against cancer cells. Bioorgan. Med. Chem. Lett. 2013;23:6073–6078. doi: 10.1016/j.bmcl.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 25.Khan N., Akhtar M.S., Khan B.A., Braga V.D.A., Reich A. Antiobesity, hypolipidemic, antioxidant and hepatoprotective effects of achyranthes aspera seed saponins in high cholesterol fed albino rats. Arch. Med. Sci. Ams. 2015;11:1261–1271. doi: 10.5114/aoms.2015.56353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weng Y., Yu L., Cui J., Zhu Y.R., Guo C., Wei G., Duan J.L., Yin Y., Guan Y., Wang Y.H. Antihyperglycemic, hypolipidemic and antioxidant activities of total saponins extracted from aralia taibaiensis in experimental type 2 diabetic rats. J. Ethnopharmacol. 2014;152:553–560. doi: 10.1016/j.jep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Yoshikawa M., Morikawa T., Yamamoto K., Kato Y., Akifumi Nagatomo A., Matsuda H. Floratheasaponins a–c, acylated oleanane-type triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of the tea plant (camellia sinensis)1. J. Nat. Prod. 2005;68:1360–1365. doi: 10.1021/np0580614. [DOI] [PubMed] [Google Scholar]
- 28.Ijeh I., Inyang E., Egedigwe A., Ahaiwe U. Hypoglycaemic and hypolipidemic effects of crude saponins extracted from vernonia colorata in normoglycaemic albino rats (259.6) FASEB J. [DOI]
- 29.Song C., Yu Q., Li X., Jin S., Li S., Zhang Y., Jia S., Chen C., Xiang Y., Jiang H. The hypolipidemic effect of total saponins from kuding tea in high-fat diet-induced hyperlipidemic mice and its composition characterized by uplc-qtof-ms/ms. J. Food Sci. 2016;81:H1313–H1319. doi: 10.1111/1750-3841.13299. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y., Vermeer M.A., Bos W. Molecular structures of citrus flavonoids determine their effects on lipid metabolism in hepg2 cells by primarily suppressing apob secretion. J. Agric. Food Chem. 2011;59:4496–4503. doi: 10.1021/jf1044475. [DOI] [PubMed] [Google Scholar]
- 31.Sangkitikomol W., Rocejanasaroj A., Tencomnao T. Effect of moringa oleifera on advanced glycation end-product formation and lipid metabolism gene expression in hepg2 cells. Genet. Mol. Res. 2014;13:723–735. doi: 10.4238/2014.January.29.3. [DOI] [PubMed] [Google Scholar]
- 32.Niu Y., Lü N., Li Y., Zhao D., Sun C. Establishment of a model for evaluating hypolipidemic effect in hepg2 cells. J. Hyg. Res. 2010;39:155–158. [PubMed] [Google Scholar]
- 33.Kim K.Y., Park K.I., Lee S.G., Baek. S.Y., Lee E.H., Kim S.C., Kim S.H., Park S.G., Yu S.N., Oh T.W., et al. Deoxypodophyllotoxin in anthriscus sylvestris alleviates fat accumulation in the liver via amp-activated protein kinase, impeding srebp-1c signal. Chem.-Biol. Interact. 2018;294:151–157. doi: 10.1016/j.cbi.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Higuchi N., Kato M., Shundo Y., Tajiri H., Tanaka M., Yamashita N., Kohjima M., Kotoh K., Nakamuta M., Takayanagi R., et al. Liver x receptor in cooperation with srebp-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol. Res. 2008;38:1122–1129. doi: 10.1111/j.1872-034X.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- 35.Cabruja M., Momdino S., Tsai Y.T., Lara J., Gramajo H., Gago G. A conditional mutant of the fatty acid synthase unveils unexpected cross talks in mycobacterial lipid metabolism. Open Biol. 2017 doi: 10.1098/rsob.160277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenwood J., Bhat S., Huang X., Wang R., Paul D., Tong L., Saha A., Westlin W., Kapeller R., Harwood H. Acetyl-coa carboxylase inhibition by nd-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc. Nat. Acad. Sci. USA. 2016;113:E1796–E1805. doi: 10.1073/pnas.1520686113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge J., Miao J.J., Sun X.Y., Yu J.Y. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (ppar)-α/γ and attenuating endoplasmic reticulum stress in rats. J. Ethnopharmacol. 2016;189:238–249. doi: 10.1016/j.jep.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Jung Y.Y., Lee Y.K., Koo J.S. Expression of lipid metabolism-related proteins in breast phyllodes tumors. Neoplasma. 2016;63:254–262. doi: 10.4149/211_150715N393. [DOI] [PubMed] [Google Scholar]
- 39.Liu X.J., Duan N.N., Liu C.L., Niu C., Liu X.P., Wu J. Characterization of a murine nonalcoholic steatohepatitis model induced by high fat high calorie diet plus fructose and glucose in drinking water. Lab. Investing. J. Tech. Methods Pathol. 2018;98:1184–1199. doi: 10.1038/s41374-018-0074-z. [DOI] [PubMed] [Google Scholar]
- 40.Yoshikawa M., Harada E., Murakami T., Matsuda H., Yamahara J., Murakami N. Camelliasaponins b1, b2, c1 and c2, new type inhibitors of ethanol absorption in rats from the seeds of Camellia japonica L. Chem. Pharm. Bull. 1994;42:742–744. doi: 10.1248/cpb.42.742. [DOI] [PubMed] [Google Scholar]
- 41.Wang P., Ownby S., Zhang Z., Yuan W., Li S. Cytotoxicity and inhibition of DNA topoisomerase i of polyhydroxylated triterpenoids and triterpenoid glycosides. Bioorgan. Med. Chem. Lett. 2010;20:2790–2796. doi: 10.1016/j.bmcl.2010.03.063. [DOI] [PubMed] [Google Scholar]
- 42.Lee K.I., Choi S.U., Lee K.R. Triterpene saponins from pleurospermum kamtschaticum and their biological activity. Chem. Pharm. Bull. 2012;60:1011–1018. doi: 10.1248/cpb.c12-00274. [DOI] [PubMed] [Google Scholar]
- 43.Matsuda H., Hamao M., Nakamura S., Kon’I H., Murata M., Yoshikawa M. Medicinal flowers. Xxxiii. Anti-hyperlipidemic and anti-hyperglycemic effects of chakasaponins i-iii and structure of chakasaponin iv from flower buds of chinese tea plant (Camellia sinensis) Chem. Pharm. Bull. 2012;60:674–680. doi: 10.1248/cpb.60.674. [DOI] [PubMed] [Google Scholar]
- 44.Singh N., Singh H., Jagavelu K., Wahajuddin M., Hanif K. Fatty acid synthase modulates proliferation, metabolic functions and angiogenesis in hypoxic pulmonary artery endothelial cells. Eur. J. Pharmacol. 2017;815:462–469. doi: 10.1016/j.ejphar.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 45.NHammer C., El-Shabrawi Y., Schauer S., Hiden M., Berger J., Forss-Petter S., Winter E., Eferl R., Zechner R., Hoefler G. Cdna cloning and analysis of tissue-specific expression of mouse peroxisomal straight-chain acyl-coa oxidase. Eur. J. Biochem. 2010;267:1254–1260. doi: 10.1046/j.1432-1327.2000.01128.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.