Abstract
A series of 2-aryl-3-hydroxy-6-iodo-4H-chromen-4-ones substituted at the 7-position with a halogen atom (X = F, Cl and Br) or methoxy group and their corresponding 4-substituted 2-hydroxy-5-iodochalcone precursors were evaluated in vitro for inhibitory effect against acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and β-secretase (BACE1) activities. Although moderate inhibitory effect was observed for the chalcones against AChE, derivatives 2h, 2j and 2n exhibited significant inhibitory effect against BChE and BACE-1. The 2-aryl-7-fluoro-8-iodoflavonols 3b and 3c, on the other hand, exhibited increased activity and selectivity against AChE and reduced effect on BACE-1. The flavonols 3h, 3i, 3k, 3l and 3p exhibited moderate inhibitory effect against AChE, but significant inhibition against BChE. Compounds 2j and 3l exhibited non-competitive mode of inhibition against BACE-1. Molecular docking predicted strong interactions with the protein residues in the active site of BACE-1 implying these compounds bind with the substrate. Similarly docking studies predicted interaction of the most active compounds with both CAS and PAS of either AChE or BChE with mixed type of enzyme inhibition confirmed by kinetic studies.
Keywords: 4-substituted 2-hydroxy-5-iodochalcones, 7-substituted 6-iodoflavonols, X-ray, cholinesterases, BACE-1, molecular docking
1. Introduction
The chalcones [1] and the corresponding chromone-based derivatives [2] are common structural components in various compounds with anti-Alzheimer properties. Alzheimer’s disease (AD) is one of the most severe forms of dementia characterized by progressive loss of memory, cognitive impairment or inability to learn and abnormal behavior, which tends to affect the elderly people throughout the world [3]. The World Health Organization has predicted that neurodegenerative diseases will overtake cancer to become the second most prevalent cause of death after cardiovascular diseases [4,5]. Strategies currently pursued for the treatment of AD include inhibiting cholinesterases, targeting amyloid-β (Aβ) peptides and metal-Aβ complexes [6]. The use of acetylcholinesterase (AChE) inhibitors is still one of the most efficient approaches for the treatment of the symptoms of Alzheimer’s disease. The main physiological function of AChE is the splitting of acetylcholine (ACh), a mediator of cholinergic synapses during the transduction of nerve impulses [7]. Four of the six drugs currently approved by the U.S. Food and Drug Administration (FDA) for treating AD, namely, tacrine, donepezil, rivastigmine, and galantamine increase the amount of acetylcholine neurotransmitter in the brain by inhibiting the action of AChE [8,9]. However, their efficiency as inhibitors at alleviating AD symptoms is modest and these drugs have also been found to lead to side effects such as toxicity, short half-life, and non-selective inhibition of acetylcholinesterase [8,9]. Another example of a cholinesterase is butyrylcholinesterase (BChE), which is synthesized in the liver and its main function is to hydrolyze ester-containing drugs and to scavenge cholinesterase inhibitors including potent organophosphorus nerve agents before they reach their synaptic targets [10]. BChE inhibition has been found to result in improved cognitive potential with elevated levels of ACh in the brain [11]. Inhibition of BChE becomes more relevant as the disease progresses, since the activity of AChE gradually decreases. AChE and BChE are thus important as therapeutic targets in early stages and advanced stages of the AD. High levels of AChE or BChE, on the other hand, have been found to have a role in amyloid beta(Aβ)-peptide aggregation during the early stages of senile plaque formation as well as in other pathological characteristics of AD [12,13]. Studies confirmed that β-site amyloid precursor protein cleaving enzyme 1 (BACE1) plays a significant role in the cleavage of amyloid precursor protein (APP), which leads to the production of α,β-peptide [14]. Overproduction of Aβ by BACE1 results in toxic fibrils causing neurodegeneration, one of the major causes of histological hallmarks of Alzheimer’s disease [15] and this makes this enzyme another target for AD treatment.
Synthetic chalcones and chromone- or flavone-based compounds have demonstrated beneficial effects against Alzheimer’s disease in a variety of cell culture and animal models [16]. We became interested in the design and synthesis of halogenated chalcones and flavonol derivatives for further studies of biological activity as potential anti-Alzheimer agents. This interest was prompted by the fact that the presence of halogen atom/s in a molecule hold the promise of effective drug design. They increase the membrane permeability and hence improve oral absorption to fill hydrophobic cavities in the protein binding site, they facilitate the blood–brain barrier crossing, and also prolong the lifetime of the drug [17]. Moreover, the strong electron-withdrawing effect of the halogen atoms help in forming hydrogen and/or halogen bonds that help to stabilize the interactions of drug molecules with their protein targets [18,19,20,21]. Based on these considerations, we designed and synthesized series of the 2-hydroxychalcones substituted with iodine at position-5 and another halogen atom (X = F, Cl, Br) or methoxy group at position 4 of ring-A and transform them into the corresponding 7-halogeno/methoxy substituted 6-iodo-3-flavonol derivatives. The main aim was to evaluate both groups of compounds for their ability to inhibit AChE and/or BChE activities as well as β-secretase (BACE-1) activity through enzymatic assays. The experimental results are complemented with molecular docking studies into the active sites of these enzymes to predict the hypothetical protein–ligand binding modes.
2. Results and Discussion
2.1. Chemistry
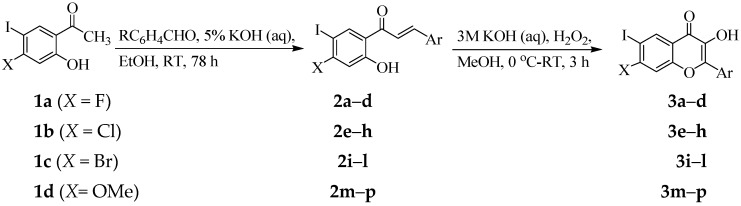
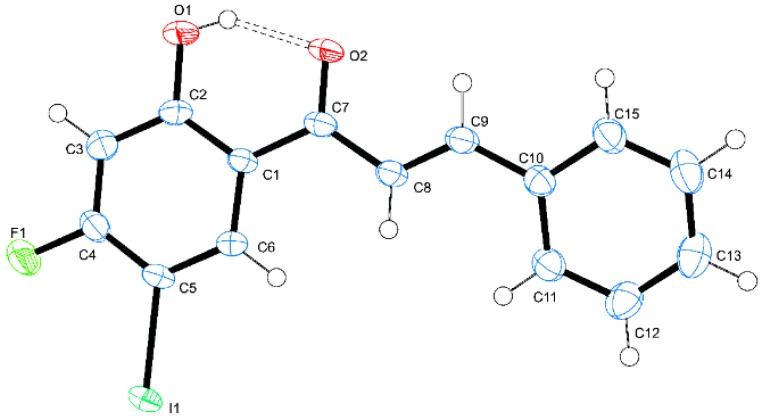
The synthesis of the chalcones of our interest and their conversion into the corresponding 3-flavonol derivatives have been achieved as shown in Scheme 1 and the substitution patterns on the A and B rings of these compounds are represented in Table 1. The 4-substituted 2-hydroxy-5-iodoacetophenones 1 substituted at the 4-position with different halogen atoms (X = F, 1a; Cl, 1b; Br, 1c) or methoxy group (1d) were prepared via iodination of the corresponding 4-substituted 2-hydroxyacetophenones with N-iodosuccinimide in the presence of an equivalent amount of p-toluenesulfonic acid in acetonitrile at room temperature (RT) for 14 h. The 4-substituted 2-hydroxy-5-iodoacetophenones 1a–d were easily distinguished from their corresponding precursors by the presence of a set of singlets in the aromatic region corresponding to H-3 and H-6, respectively. These compounds were, in turn, subjected to the base-mediated Claisen-Schmidt aldol condensation with benzaldehyde derivatives to afford the corresponding 4-halogeno/methoxy substituted 2-hydroxy-5-iodochalcones 2a–p. Their 1H-NMR spectra revealed the presence of group of signals in the aromatic region and a singlet significantly downfield beyond δ 13.00 ppm for the intramolecularly hydrogen bonded hydroxyl proton. The chalcone nature of compounds 2a–p was confirmed by the presence of a set of doublets around δ = 7.40 ppm and 7.90 ppm with coupling constant value (Jtrans) of 15.5 Hz, which correspond to the α-H and β-H, respectively. The E-geometry of the α,β-unsaturated framework was further confirmed by the single crystal X-ray diffraction (XRD) structure of 2a (Figure 1) [22]. The XRD structure of this compound revealed the presence of strong intramolecular hydrogen bonding between the carbonyl and hydroxyl groups (O(1)-H(1)...O(2) = 1.77 Å). We decided to transform the chalcone ortho-hydroxy-trans-α,β-unsaturated carbonyl framework into a geometrically constraint flavonol scaffold. Consequently, we subjected the chalcone derivatives 2a–p to hydrogen peroxide in the presence of 3 M aqueous potassium hydroxide in methanol at 0 °C to room temperature (RT) for 3 h to afford the corresponding 3-flavonol derivatives 3a–p. The 1H-NMR spectra of the latter compounds lack the set of doublets corresponding to the olefinic protons observed in the spectra of the corresponding substrates. Moreover, the singlet for the 3-OH resonates significantly upfield in the region δ 9.00–10.20 ppm compared to that of the corresponding substrates. The structure of these compounds was distinctly confirmed by single crystal X-ray diffraction (XRD) analysis of compound 3h (Figure 2) [23]. The molecule crystalized in the orthorhombic space group Pbca and there are two molecules in the unit cell. We observed the intramolecular hydrogen bond intuitively between the carbonyl oxygen and hydroxyl groups of the two molecules (O(2)-H(2)...O(1) = 2.39 and 2.03 Å, respectively.
Scheme 1.
Synthesis of the 2-hydroxy-5-iodochalcones 2a–p and their flavonol derivatives 3a–p.
Table 1.
Substitution pattern for compounds 2a–p and 3a–p.
| Ar | Designation of X for 2a–p | |||
| C6H5- | F (2a) | Cl (2e) | Br (2i) | OCH3 (2m) |
| 4-FC6H4- | F (2b) | Cl (2f) | Br (2j) | OCH3 (2n) |
| 4-ClC6H4- | F (2c) | Cl (2g) | Br (2k) | OCH3 (2o) |
| 4-MeOC6H4- | F (2d) | Cl (2h) | Br (2l) | OCH3 (2p) |
| Ar | Designation of X for 3a–p | |||
| C6H5- | F (3a) | Cl (3e) | Br (3i) | OCH3 (3m) |
| 4-FC6H4- | F (3b) | Cl (3f) | Br (3j) | OCH3 (3n) |
| 4-ClC6H4- | F (3c) | Cl (3g) | Br (3k) | OCH3 (3o) |
| 4-MeOC6H4- | F (3d) | Cl (3h) | Br (3l) | OCH3 (3p) |
Figure 1.
Oak Ridge Thermal Ellipsoid Plot (ORTEP) diagram (50% probability level) of 2a. For clarity, hydrogen atoms are not labeled.
Figure 2.
ORTEP diagram (50% probability level) of 3h.
With the chalcones 2a–p and their geometrically constraint flavonol derivatives 3a–p in hand, we proceeded to evaluate them for potential inhibitory effects against the human acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities as well as inhibitory effect against β-secretase (BACE-1) activity.
2.2. Biology
Compounds 2a–p and 3a–p were grouped into four series each, namely, a–d (X = F), e–h (X = Cl), i–l (X = Br) and m–p (X = OCH3) with the substituent Ar variable and representing C6H5-, 4-FC6H4, 4-ClC6H4- and 4-MeOC6H4-, respectively, as shown in Table 2 and Table 3. The structure activity relationship (SAR) of compounds 2a–p and 3a–p has been rationalized with respect to the nature of substituent on the aryl substituent (Ar) and the 4-position of the relatively flexible enone framework (chalcone) or 7-position of the rigid chromenone moiety (flavonol), respectively. The compounds were first evaluated for inhibitory effect against the AChE and BChE activities in cell-free assays using the anti-Alzheimer drug, donepezil, as a reference standard. The inhibitory effects are expressed as means of IC50 values (the concentration that inhibits ChE by 50%) in µM from triplicate runs.
Table 2.
AChE and BChE inhibitory activities of flavonols 2a–p.
| Compound | IC50 (µM) | SI | ||
|---|---|---|---|---|
| AChE | BChE | BChE/AChE | AChE/BChE | |
| 2a | 38.78 ± 0.01 | 28.06 ± 0.10 | 0.72 | 1.38 |
| 2b | 16.60 ± 0.03 | 19.73 ± 0.05 | 1.19 | 0.84 |
| 2c | 10.50 ± 0.08 | 14.58 ± 0.14 | 1.39 | 0.72 |
| 2d | 10.95 ± 0.30 | 8.49 ± 0.05 | 0.78 | 1.29 |
| 2e | 10.64 ± 0.07 | 25.19 ± 0.30 | 2.37 | 0.46 |
| 2f | 9.57 ± 0.20 | 17.86 ± 0.08 | 1.87 | 0.54 |
| 2g | 11.00 ± 0.06 | >100 | - | - |
| 2h | 11.72 ± 0.07 | 4.17 ± 0.15 | 0.36 | 2.81 |
| 2i | 58.14 ± 0.21 | 14.11 ± 0.11 | 0.24 | 4.12 |
| 2j | 10.98 ± 0.03 | 5.73 ± 0.05 | 0.52 | 1.92 |
| 2k | 11.56 ± 0.05 | 10.44 ± 0.09 | 0.90 | 1.11 |
| 2l | 10.51 ± 0.03 | 15.25 ± 0.01 | 1.45 | 0.69 |
| 2m | 10.01 ± 0.09 | 9.52 ± 0.03 | 0.95 | 1.05 |
| 2n | 9.38 ± 0.07 | 5.91 ± 0.05 | 0.63 | 1.59 |
| 2o | 11.07 ± 0.12 | 11.63 ± 0.17 | 1.05 | 0.95 |
| 2p | 10.96 ± 0.03 | 4.89 ± 0.02 | 0.45 | 2.24 |
| Donepezil | 4.77 ± 0.06 | 6.04 ± 0.03 | 1.27 | 0.79 |
The values are given as mean ± standard deviation (SD) in three independent experiments. SI (Selectivity Index): the AChE selectivity index is defined as IC50 (BChE)/IC50 (AChE) and that of BChE as IC50 (AChE)/IC50 (BChE) affinity ratios.
Table 3.
AChE and BChE inhibitory activities of flavonols 3a–p.
| Compound | IC50 (µM) | SI | ||
|---|---|---|---|---|
| AChE | BChE | BChE/AChE | AChE/BChE | |
| 3a | 29.71 ± 0.01 | 27.01 ± 0.06 | 0.91 | 1.10 |
| 3b | 3.23 ± 0.02 | 29.78 ± 0.04 | 9.22 | 0.11 |
| 3c | 3.45 ± 0.07 | 37.51 ± 0.08 | 10.8 | 0.09 |
| 3d | 58.18 ± 0.03 | 29.41 ± 0.04 | 0.50 | 1.98 |
| 3e | 47.85 ± 0.04 | 6.64 ± 0.03 | 0.14 | 7.21 |
| 3f | 38.39 ± 0.02 | 18.17 ± 0.10 | 0.47 | 2.11 |
| 3g | 59.10 ± 0.05 | 7.69 ± 0.01 | 0.13 | 7.69 |
| 3h | 14.33 ± 0.03 | 5.72 ± 0.01 | 0.40 | 2.51 |
| 3i | 9.96 ± 0.01 | 5.76 ± 0.04 | 0.57 | 1.73 |
| 3j | 11.16 ± 0.01 | 27.01 ± 0.07 | 2.42 | 0.41 |
| 3k | 29.24 ± 0.03 | 5.25 ± 0.01 | 0.18 | 5.57 |
| 3l | 28.99 ± 0.02 | 4.88 ± 0.01 | 0.17 | 5.94 |
| 3m | 9.25 ± 0.09 | 10.50 ± 0.02 | 1.14 | 0.88 |
| 3n | 6.15 ± 0.01 | 7.93 ± 0.06 | 1.29 | 0.76 |
| 3o | 55.27 ± 0.04 | 13.71 ± 0.14 | 0.24 | 4.03 |
| 3p | 7.19 ± 0.02 | 3.29 ± 0.03 | 0.45 | 2.18 |
| Donepezil | 4.79 ± 0.05 | 6.04 ± 0.03 | 1.26 | 0.79 |
The values are given as mean ± standard deviation (SD) in three independent experiments. SI (Selectivity Index): the AChE selectivity index is defined as IC50 (BChE)/IC50 (AChE) and that of BChE as IC50 (AChE)/IC50 (BChE) affinity ratios.
2.2.1. Inhibitory Effect of Chalcones 2a–p against AChE and BChE Activities
With the exception of the less active chalcones 2a (IC50 = 38.78 µM) and 2p (IC50 = 58.14 µM), all of the other derivatives exhibited moderate 2b (IC50 = 16.60 µM) to significant inhibitory effect against the AChE activity with the IC50 values ranging from 9.38–11.56 µM. These IC50 values are twice higher than that of donepezil (IC50 = 4.79 µM). By contrast, some of the chalcones in the series 2a–p were found to be generally more inhibiting towards the BChE activity when compared to donepezil. Within the series 2a–d, only compound 2d substituted with fluorine atom at positon 4 of ring-A and a 4-(methoxyphenyl) group at the β-carbon was found to exhibit significant inhibitory effect against BChE activity (IC50 = 8.49 µM) when compared to donepezil (IC50 = 6.04 µM). A combination of the 4-chloro atom on ring-A and a strongly pi-electron donating 4-methoxyphenyl group at the β-carbon of 2h resulted in increased inhibitory effect against the BChE activity (IC50 = 4.17 µM) when compared to the reference standard. The presence of a chlorine atom at position-4 of ring-A of compounds 2i–l resulted in significant inhibitory effect against BChE activity in this series. A combination of the 4-bromo substituent on ring-A and a 4-fluorophenyl group at the β-position of 2j, for example, resulted in increased inhibitory activity against BChE (IC50 = 5.73 µM). Increased inhibitory activity was also observed for compounds 2n and 2p within the series 2m–p substituted with a 4-methoxy group at the 4-positon of ring-A and the observed trend in IC50 values is as follows: 4.89 (2p) > 5.91 (2n) > 9.52 (2m) > 11.63 µM (2o). The anti-cholinesterase activity which is in the order, OCH3 > F > H > Cl, is consistent with the decreasing propensity of the 2-(4-substituted phenyl) ring for pi-electron delocalization into the α,β-unsaturated framework, respectively. A combination of the lipophilic 4-methoxyphenyl group and a fluorine (2d), chlorine (2h) or methoxy (2p) substituent at the 4-position of ring-A seem to be more favorable for inhibitory effect against the BChE activity. Likewise, a combination of 4-fluorophenyl group at the β-carbon and a bromine atom (2j) or methoxy group (2n) at position 4 of ring-A imparted increased inhibitory effect against BChE activity when compared to donepezil. The most active chalcone derivatives against the BChE tend to be moderately inhibiting against AChE. Their modest selectivity values in our view may make them potential dual inhibitors against both enzymes.
2.2.2. Inhibitory Effect of Flavonols 3a–p against AChE and BChE Activities
The 3-flavonol derivatives 3a–p were also evaluated for inhibitory effect against the two enzymes and the results are represented in Table 3. From the results of the analysis of inhibitory effects of these chromenone derivatives against AChE activity (Table 3), it is evident that a combination of a fluorine atom at the 7-position of ring-A and a 2-(4-fluorophenyl) group in 3b (IC50 = 3.23 µM) or 2-(4-chlorophenyl) ring in 3c (IC50 = 3.35 µM) has a vital role in the inhibition of AChE when compared to donepezil (IC50 = 4.79 µM). These compounds tend to be selective towards AChE since they presented reduced anti-BChE activity. The strong electron-withdrawing substituents of fluorine and chlorine at the para position of the 2-aryl group of 3b and 3c are probably responsible for the observed increased AChE inhibition in comparison to 3a and 3d. This observation is in line with the literature precedent, which showed that the 4-halogenophenyl substituted compounds exhibited increased inhibitory effect against AChE activity when compared to the unsubstituted analogues [24]. Enhanced inhibitory effect due to halogen substituents has also been observed in various series of AChE inhibitors and is explained in terms of additional hydrogen bonding or dipole-dipole interactions, and/or increased van der Waals interactions [25]. The presence of chlorine atom at the 7-position of ring-A of 3e–h resulted in significantly reduced inhibitory effect for these compounds against AChE activity. The presence of a bromine atom at the 7- position of ring-A of compounds 3i–l, on the other hand, imparted moderate to significant inhibitory effect against the AChE activity and the observed trend in activity is in the order, 3i (H) > 3j (F) > 3l (OCH3) > 3k (Cl), with the following trend in IC50 values: 9.96 > 11.16 > 28.99 > 29.24 µM, respectively. It seems the presence of a bulky 2-aryl group on the 7-bromo-6-iodochromenone framework is not favorable for inhibitory effect against the AChE activity as observed for compounds 3k and 3l. The 7-methoxyflavonol derivative 3o substituted with a 2-(4-chlorophenyl) group on the chromenone framework was found to be the least active (IC50 = 55.27 µM) within the series 3m–p. The other derivatives within this series were found to be relatively more inhibiting and the observed trend in inhibitory activity is as follows: 3n (6.15 µM) > 3p (7.15 µM) > 3m (9.25 µM).
The enzyme inhibitory assays of the flavonol derivatives 3a–p against BChE activity revealed that the 7-fluoro substituted derivatives 3a–d are generally less or not active against this enzyme’s activity. However, the presence of chlorine at position 7 of ring-A in compounds 3e–h resulted in moderate to significant inhibitory effect against the BChE activity and the trend in IC50 values is as follows: 3h (5.72 µM) > 3e (6.64 µM) > 3g (7.69 µM) > 3f (18.17 µM). The inhibitory effect in the case of the derivatives within this series seems to be influenced by the electron-donating inductive effect of the substituent at the para position of the 2-aryl ring in the order, -OCH3 > H > Cl > F. The trend in inhibitory effect against the BChE for the 7-bromo-6-iodoflavonol derivatives 3i–l, on the other hand, is as follows: 3l (-OCH3, 4.88 µM) > 3k (Cl, 5.25 µM) > 3i (H, 5.76 µM) > 3j (F, 27.01 µM). Larger substituents at the para position of the 2-aryl ring in this series have therefore exhibited better activities in comparison to those with the smaller size. The inhibitory effect of compounds 3m–p against BChE activity appear to be influenced by the propensity of the 2-aryl ring for pi-electron delocalization into the chromenone framework (4-CH3OC6H4- > 4-FC6H4- > C6H5- > 4-ClC6H4-) and the trend in IC50 values and therefore inhibitory effect is as follows: 3p (3.29 µM) > 3n (7.93 µM) > 3m (10.51 µM) > 3o (13.71 µM). The presence of the strongly pi-electron donating 4-CH3OC6H4- group at the 2-position of the chromenone framework appears to be favorable for increased inhibitory effect against BChE activity. Though strongly inhibiting against this enzyme’s activity, compound 3p has modest selectivity and therefore potential to also act as a dual inhibitor against both AChE and BChE activities. Dual inhibition of AChE and BChE has become an efficient strategy to alleviate AD’s symptoms with minimal or no side effects [26].
2.2.3. Inhibitory Activities of Compounds 2h, 2j, 2n, 2p, 3b, 3c, 3l and 3p against BACE-1
β-Secretase (BACE1) is the key enzyme in Aβ peptide generation and anti-Aβ aggregation, and inhibition of its activity represents a promising approach for the treatment of Alzheimer’s disease. We decided to evaluate the most active chalcones (2h, 2j, 2n and 2p) and flavonols (3b, 3c, 3l and 3p) for inhibitory effect against β-site amyloid precursor protein cleaving enzyme 1 (BACE1) using quercetin as a reference standard (Table 4). Quercetin previously showed significant inhibitory effect against BACE-1 and reduced the levels of Aβ in neurons [27]. The strongly inhibiting chalcone derivatives 2h, 2j and 2n against BChE activity exhibited moderate to significant inhibitory effect against BACE-1 when compared to quercetin (IC50 = 12.66 µM) with IC50 values of 13.82, 4.70 and 25.07, respectively. The presence of 4-bromo atom and 4-fluorophenyl group on the ortho-hydroxy-trans-α,β-unsaturated carbonyl framework of 2j resulted in increased inhibitory effect against BACE-1 (IC50 = 4.70 µM) activity, more so than the geometrically constraint flavonol derivatives. Compound 2j which also exhibited increased inhibitory effect against BChE activity has potential to serve as a dual inhibitor of these enzymes. Although chalcone 2p exhibited increased inhibitory effect against BChE, this compound was found to be poorly inhibiting against BACE-1 activity with IC50 value of 50.79 µM. The flavonol derivatives 3b and 3c, which exhibited increased inhibitory effect and selectivity against AChE activity, on the other hand, were found to exhibit moderate inhibitory effect against BACE-1 with IC50 values of 32.19 and 19.69 µM, respectively. Significant inhibitory effect against BACE-1 was also observed for compound 3l (IC50 = 15.74 µM), which also exhibited significant inhibitory effect against BChE activity. The most active flavonol 3p against BChE was found to be less inhibiting against BACE-1 activity when compared to the reference standard with IC50 = 22.44 µM.
Table 4.
IC50 values for the inhibitory effect of 2h, 2j, 2n, 2p, 3b, 3c, 3l, 3p against BACE-1 activity.
| Compound | BACE-1 |
|---|---|
| 2h | 13.82 ± 0.03 |
| 2j | 4.703 ± 0.06 |
| 2n | 25.07 ± 0.1 |
| 2p | 70.79 ± 0.2 |
| 3b | 32.18 ± 0.15 |
| 3c | 19.69 ± 0.05 |
| 3l | 15.74 ± 0.12 |
| 3p | 22.44 ± 0.07 |
| Quercetin | 12.66 ± 0.02 |
2.2.4. Enzyme Kinetic Analysis of the Most Active Compounds for ChEs and BACE-1
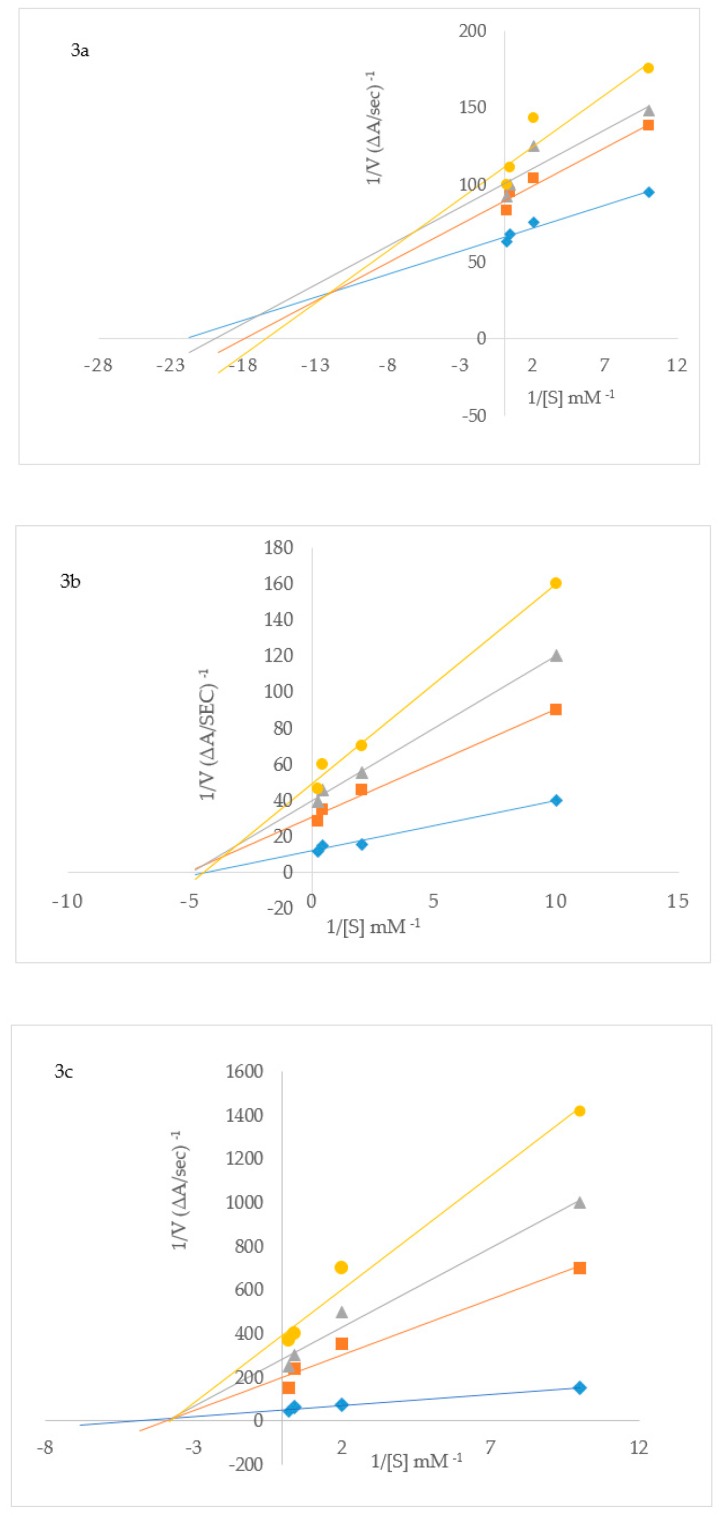
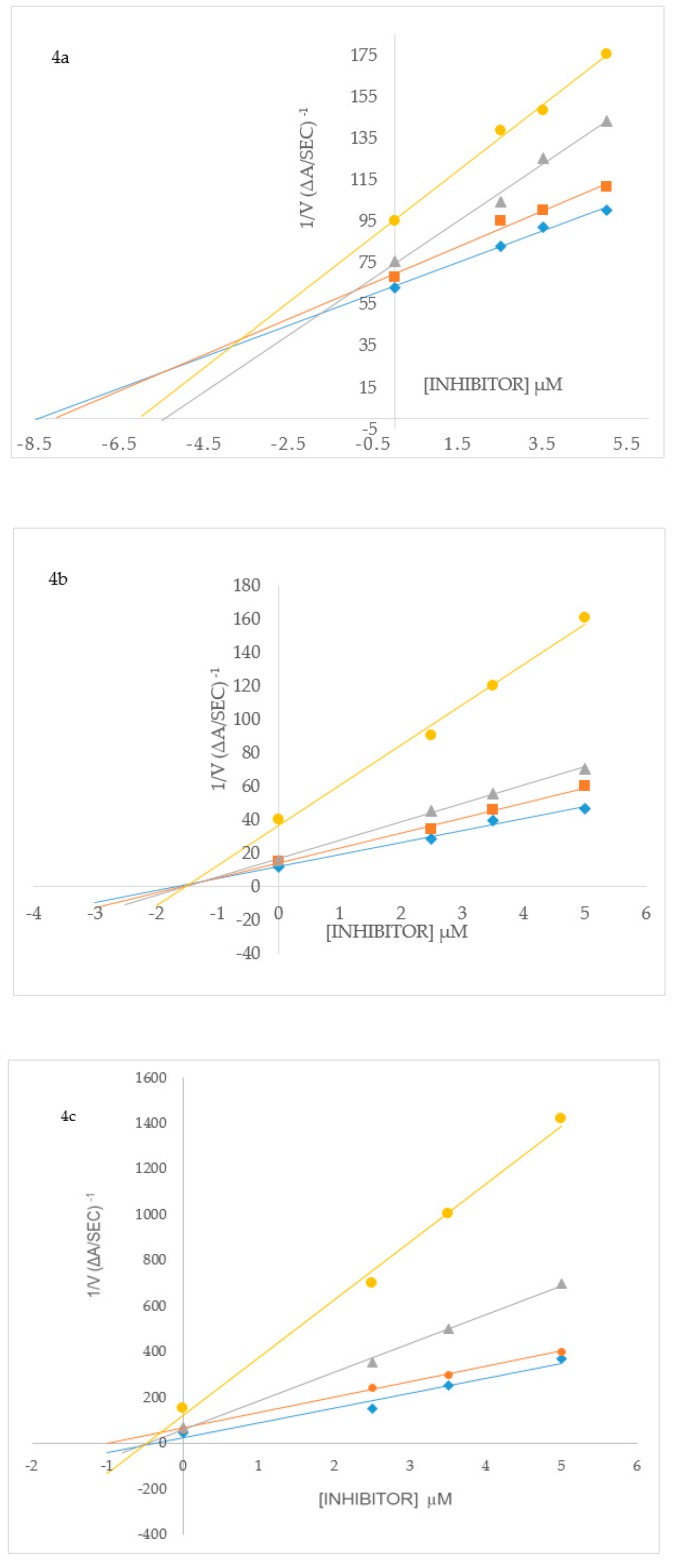
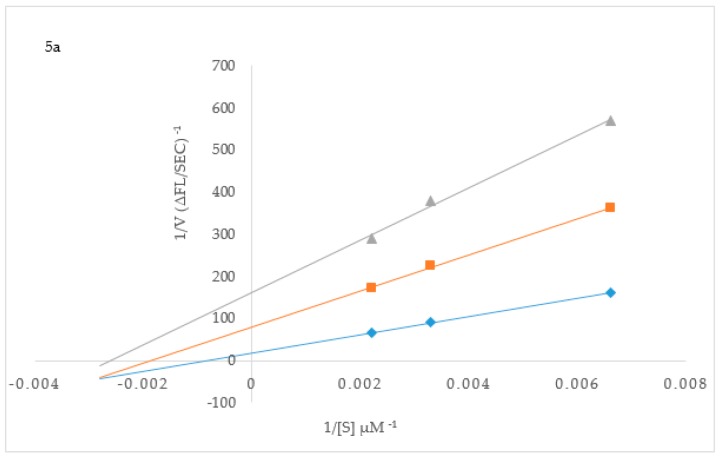
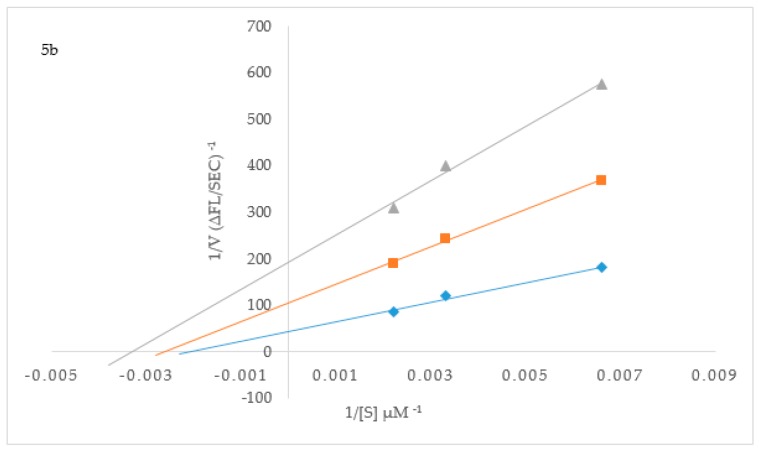
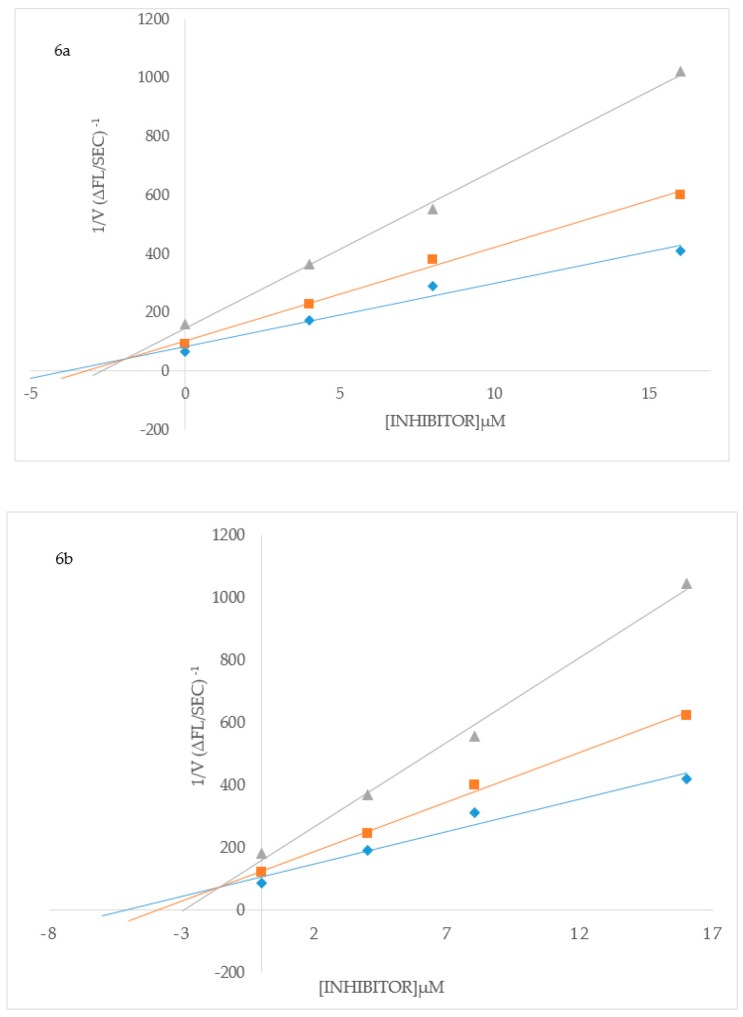
To elucidate the mechanisms of ChE and BACE-1 inhibition, kinetic studies of enzyme activity were performed. Compound 3b which exhibited the highest inhibitory effect and selectivity against AChE activity and the highest active compounds 2h and 3p against BChE activity were selected for the kinetic study on increasing concentrations (0.1, 0.5, 2.5 and 5 µM) of the substrates, acetylthiocholine iodide (ATChI) and butyrylthiocholine iodide (BTChI), respectively. The linear Lineweaver–Burk equation of the Michaelis–Menten was applied to evaluate the inhibition profile and the plots of inverse of the velocity of the reaction versus inverse of the substrate concentration are represented in Figure 3. Molecules that bind only to the catalytic active site (CAS) or only to the peripheral anionic site (PAS) of the cholinesterase enzymes (AChE or BChE) are classified as competitive or non-competitive inhibitors, respectively [28]. If molecules can bind AChE or BChE at CAS or PAS sites and to additional sites on the enzymes, while still allowing the substrates AChI or BChI to bind then their mode of inhibition is considered to be mixed type [28]. Plot of inverse of the velocity of the reaction versus inverse of the substrate concentration (Figure 3) suggests that 3b (Figure 3a) and 3p (Figure 3c) decrease the Vmax of AChE and BChE respectively, and increase the Michaelis constant (Km) of the enzymes. The Km values for 3b at 0–5.0 µM are 0.05 and 0.06, whereas for compound 3p at the same concentration are 0.17 and 0.30, respectively. These compounds presumably also bind to other sites of the enzyme and also interact with the protein residues in the functional sites, CAS and PAS, of BChE. The Michaelis constant (Km) value for 2h (Km = 0.22; Figure 3b), on the other hand, remained relatively unchanged during inhibition of AChE while Vmax decreases with increasing inhibitor concentration. This suggests non-competitive mode of inhibition against BChE activity where the compound binds BChE modulating the enzymes structure and therefore ability to catalyze the reaction while not affecting the substrates ability to bind the enzyme. Inhibition constants (Ki) were determined by interpreting the Dixon plots (Figure 4). For 3b the intercept of the straight lines or the Ki value was determined to be 4.34 ± 1.1 µM (Figure 4a). An intercepting set of straight lines on the x-axis of the Dixon plot (Figure 4b) confirms that 2h is a non-competitive inhibitor of BChE. Similarly the Ki values for 2h and 3p are 1.36 ± 0.17 and 0.46 ± 0.16, respectively (Figure 4b,c). The lower the Ki value the better the inhibitor, therefore 3p is the best inhibitor of BChE among the two series of compounds. Based on the IC50 value of the chalcone 2j and flavanol derivative 3l against BACE-1 activity, we evaluated them for their inhibition mode toward this enzyme (Figure 5). Both compounds displayed a mixed mode of inhibition with Km values affected in the presence of increasing inhibitor (4 and 8 µM) and absence of inhibitor as can be seen from the x-intercepts for both compounds in Figure 5. The Vmax values decreased with increasing inhibitor present for 2j and 3l. Dixon plots (Figure 6) confirmed the mixed mode of inhibition as the intercept for all the lines occurred above the x axis. The intercept points represent the Ki values to be 3.04 ± 2.31 and 1.21 ± 0.66 for 2j and 3l respectively, making 3l the better inhibitor of BACE-1.
Figure 3.
Lineweaver–Burk plots for inhibition of AChE by 3b (a) and BChE by 2h (b) and 3p (c). Blue symbols and fitted straight lines represent enzyme activity in the absence of inhibitor, while orange (2.5 µM), grey (3.5 µM) and yellow (5 µM) represent various concentrations of inhibitor.
Figure 4.
Dixon plots for inhibition of AChE by 3b (a) and BChE by 2h (b) and 3p (c). Blue symbols and fitted straight lines represent enzyme activity with 5 mM substrate, while orange (2.5 mM), grey (0.5 mM) and yellow (0.1 mM) represent various concentrations of substrate.
Figure 5.
Lineweaver–Burk plots for inhibition of BACE-1 by 2j (a) and 3l (b), respectively. Blue symbols and fitted straight lines represent enzyme activity in the absence of inhibitor, while orange (4 µM) and grey (8 µM) represent various concentrations of inhibitor.
Figure 6.
Dixon plots for inhibition of BACE-1 by 2j (a) and 3l (b), respectively. Blue symbols and fitted straight lines represent enzyme activity in the presence of 450 nM substrate and orange and grey, 300 nM and 150 nM substrate, respectively.
Molecular docking studies were performed on compounds 2a–p and 3a–p into the active sites of AChE, BChE and BACE-1 to predict their hypothetical protein–ligand binding mode.
2.3. Molecular Docking Studies into AChE and BChE Active Sites
In order to figure out the plausible protein-ligand interactions at molecular level and to rationalize the structure activity relationship (SAR), we docked both the chalcones 2a–p and flavonols 3a–p into the active pockets of AChE and BChE against donepezil (refer to Figures S2 and S3 in the Supplementary Material for their docking poses). The two groups of compounds are represented herein by the docking poses of the most active compounds from each series, namely, compounds 2f and 3b against AChE (Figure 7) and 2h and 3p against BChE (Figure 8), respectively. The active site of AChE has been reported to consist of the catalytic triad (Ser200, His440, Glu327), oxyanion hole (OH, Gly118, Gly119, Ala201), anionic subsite (Trp84, Tyr121, Glu199, Gly449, Ile444), acyl binding pocket (Trp233, Phe288, Phe290, Phe292, Phe330, Phe331) and peripheral anionic subsite (Asp72, Tyr121, Ser122, Trp279, Phe331, Tyr334), which are known to be buried at the bottom of a 20 Å deep aromatic cleft [29]. The catalytic triad of BChE (PDB code: 1P0I), on the other hand, consists of serine (Ser198), histidine (His438) and glutamate (Glu325), which are interconnected by hydrogen bonds [30].
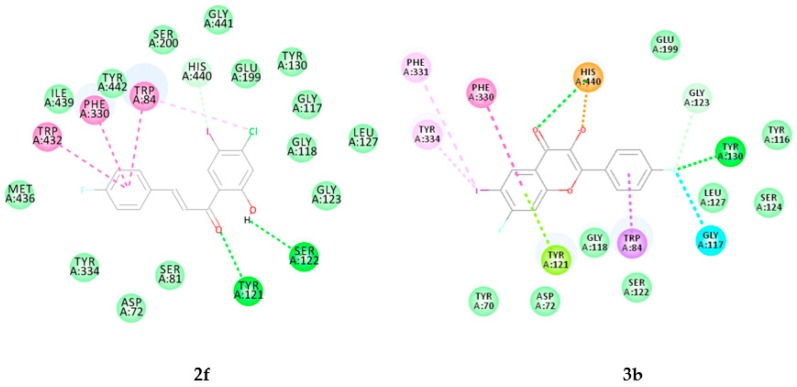
Figure 7.
Docking poses of 2f and 3b into the active site of AChE. Interactions are color coded, bright green represents conventional hydrogen bonds, light green- van der Waals interactions, very light green- carbon hydrogen bonds, orange- salt bridges, blue- halogen bonds, dark pink- pi–pi interactions, light pink- pi-alkyl interactions, purple- pi-sigma interactions and green yellow- pi lone pair interactions.
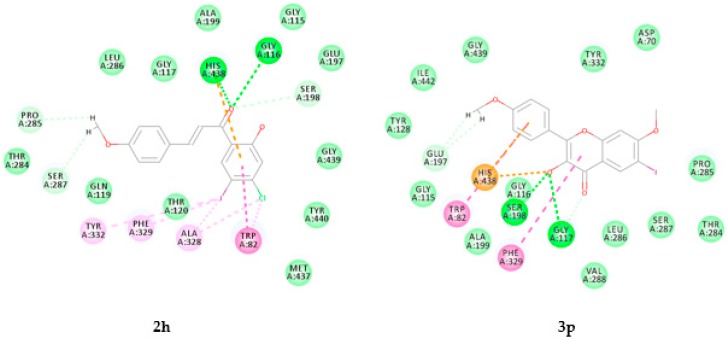
Figure 8.
Docking poses of compounds 2h and 3P into the active site of BChE. Interactions are color coded, bright green represents conventional hydrogen bonds, light green- van der Waals interactions, very light green- carbon hydrogen bonds, orange- pi carbon and attractive charge interactions, dark pink- pi–pi interactions and light pink- pi-alkyl and alkyl interactions.
2.3.1. Molecular Docking of Compounds 2f and 3b into AChE Active Site
Donepezil used as a reference standard in the experimental studies has previously been found to interact with both the catalytic active site (CAS) and the peripheral anionic site (PAS) tryptophans via ring-stacking interactions [31]. The docking pose of compound 2f shown in Figure 9, revealed the presence of two relatively weak hydrogen bond (Hb) interactions detected between the carbonyl and hydroxyl group of 2f with tyrosine-121 (Tyr121; Hb distance = 2.80 Å) and serine-122 (Ser122; Hb distance = 2.62 Å) on the peripheral anionic and anionic subsites, respectively. The molecule penetrates the aromatic cleft of the AChE through ring B and its entrance into the aromatic cleft was supported by π-π interaction with phenylalanine (Phe330) in the acyl binding pocket. These hydrophobic interactions are generated by the close contacts between the non-polar amino acid side chains of the enzyme and the lipophilic groups of 2f. Although the chalcone derivatives 2a–p interact with the protein residues in the peripheral anionic and acyl binding pocket of AChE, molecular docking predicted no interactions with any of the protein residues in the catalytic triad residues of AChE. The observed inhibitory effect against the AChE activity for compounds 2 is probably due to their binding to the enzyme’s peripheral anionic site.
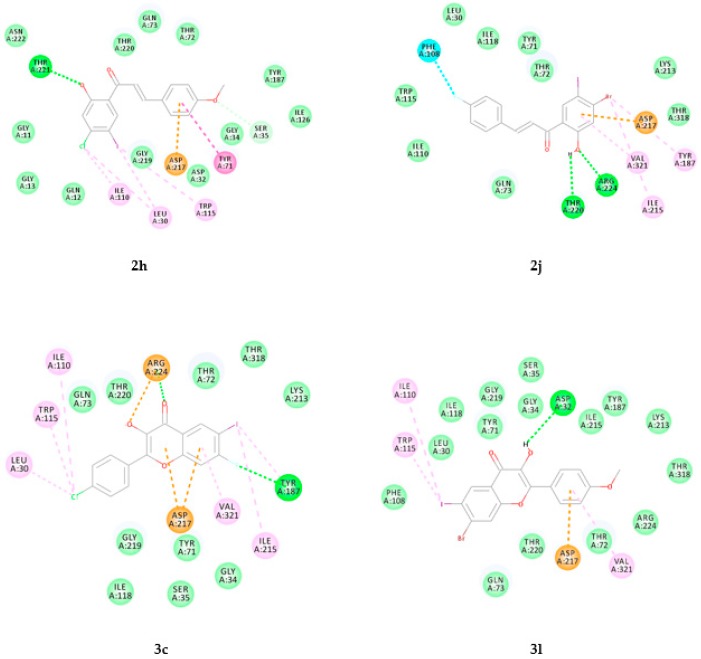
Figure 9.
Docking poses of 2h, 2j, 3c and 3l into BACE-1. Interactions are color coded, bright green represents conventional hydrogen bonds, light green- van der Waals interactions, very light green- carbon hydrogen bonds, orange- pi-anion and attractive charge interactions, blue- halogen bonds, dark pink- pi–pi interactions and light pink- pi–alkyl and alkyl interactions.
The docking pose of 3b with AChE (Figure 7) within the series 3a–p reveals the involvement of the carbonyl group in hydrogen bonding with histidine-440 (His440, Hb distance = 2.73 Å) of the catalytic triad, which also forms a salt bridge with oxygen of the hydroxyl group. The fluorine atom on the 2-aryl ring is involved in hydrogen bonding with Tyr130 (Hb distance = 2.18 Å) and halogen bond interaction with Gly117. Pi-pi stacking and T-shaped interactions exist between the fused benzo ring and the protein residue, Phe330, in the acyl binding pocket. Ring-A is also involved in pi-lone pair with Tyr121 while its iodine atom is involved in pi-sigma interactions with the protein residues Phe331 and Tyr334. Noncovalent interactions (hydrogen bond, van der Waals, charge transfer, pi-stacking, etc.) play significant roles in the stability of the protein-ligand complexes. The interactions of compounds 3b (Figure 7) and 3c (see Figure S2.20 in the Supplementary Material) with these protein residues and with His440 of the catalytic triad probably account for their observed increased inhibitory effect and selectivity against the AChE activity. The predicted interaction of these compounds with protein residues in the CAS and PAS is consistent with the observed mixed inhibitory mode.
The predicted interaction of compounds 3b and 3c with some of the protein residues of the catalytic triad is consistent with their increased inhibitory effect and selectivity against this enzyme, while still potentially allowing substrate binding. The kinetic analysis results, suggest that these compounds exhibit a mixed type of inhibition consistent with their predicted interactions with both functional sites, CAS and PAS, as well as its acyl binding pocket.
2.3.2. Molecular Docking of Compounds 2h and 3p into the Active Site of BChE
Figure 8 shows the most active compounds from both series, namely, 2h and 3p docked into BChE. The docking pose of compound 2h predicts the presence of weak hydrophobic interactions (alkyl, pi-alkyl and pi-pi T shaped) with the protein residues Trp82 and Ala328 located at the choline binding site (or cation-π site) Hydrophobic interactions are also predicted between this compound and Tyr332 of the peripheral anionic site (PAS) as well as Phe329 in the acyl binding pocket of BChE. Intermolecular hydrogen bonding interaction is predicted between the carbonyl oxygen and Gly116 with hydrogen bond (Hb) distance of 2.98 Å. Ring-A and the carbonyl group of 2h form pi-cation interaction and weak hydrogen bonding interaction (Hb distance = 2.54 Å) with the protein residue His438 in the catalytic triad. A weak carbon hydrogen bond interaction has also been predicted between the carbonyl group of this compound and Ser198 (Hb distance = 2.97 Å) in the catalytic triad. The observed increased activity and selectivity of compound 2h for this enzyme probably result from the interaction of this compound with the protein residues His438 and Ser198 of the catalytic triad. Docking of the flavonol derivatives 3a–p into the active site of BChE (refer to Figures S3.18–3.33 in the Supplementary Material for the docking poses) also revealed the involvement of His438. Their docking poses represented herein by that of the most active compound 3p (Figure 8), revealed the presence of pi-cation interaction and attractive charge interaction (pi-anion) of His438 with the 2-aryl substituent and oxygen atom of the 3-hydroxyl group, respectively. The C-ring of this compound is involved in hydrophobic pi–pi T-shaped interactions with Phe329 and similar association exist between its aryl substituent and Trp82 inside the primary binding site. Its hydroxyl oxygen atom is involved in weak hydrogen bond interaction with Gly117 (Hb distance = 2.86 Å) and the protein residue, Ser198 (Hb distance = 3.03 Å) of the catalytic triad.
The predicted strong interactions of compounds 2h and 3p with protein residues in the acyl binding pocket or choline binding site as well as those of CAS and PAS of BChE are consistent with the observed mixed kinetic type of inhibition for these compounds. Such interaction with both protein residues of CAS and PAS accounts for the observed increased inhibitory effect and moderate selectivity against BChE activity (see Table 2).
2.3.3. Docking of Chalcones (2h, 2j, 2n and 2p) and Flavonols (3b, 3c, 3l and 3p) into BACE-1
The docking poses of 2h, 2j, 3c and 3l into BACE-1 (PDB code: 4D8C) are represented in Figure 9 and those of compounds 2n, 2p, 3b and 3p have been included as Figure S4 in the Supplementary Information. These compounds are involved in electrostatic and hydrophobic interactions with the protein residues in the active site of BACE-1. The hydroxyl group of 2h is also involved in hydrogen bonding interaction with Thr221 (Hb distance = 2.0 Å). Two hydrogen bond interactions are predicted between oxygen and hydrogen atoms of the hydroxyl group of 2j with Arg224 (Hb distance = 2.12 Å) and Thr220 (Hb distance = 2.62 Å), respectively. There is also a halogen bond interaction between the fluorine atom of ring-B and the protein residue, Phe108. Ring-A is involved in pi-anion interaction with Asp217 and Asp457. These interactions may account for the observed increased inhibitory effect of 2j against this enzyme’s activity. Two hydrogen bond interactions are predicted between the carbonyl oxygen of 3c with Arg224 (Hb distance = 2.04 Å) and between fluorine atom on the fused benzo ring and Tyr187 (Hb distance = 2.90 Å). The protein residue, Arg224, also forms a salt bridge with the hydroxyl oxygen while the fused benzo and heterocyclic rings form a pi–cation interaction with Asp217. The hydroxyl group of the chalcone derivative 3l is involved in hydrogen bond interaction with Asp32 (Hb distance = 2.22 Å) of the catalytic aspartic acid dyad.
3. Materials and Methods
3.1. General
The melting point values were recorded on a Thermocouple digital melting point apparatus (Mettler Toledo LLC, Columbus, OH, USA) and are uncorrected. The IR spectra, on the other hand, were recorded as powders using a Bruker VERTEX 70 FT-IR Spectrometer (Bruker Optics, Billerica, MA, USA) with a diamond ATR (attenuated total reflectance) accessory by using the thin-film method. For column chromatography, we employed Merck kieselgel 60 (0.063–0.200 mm) as stationary phase (Merck KGaA, Frankfurt, Germany). NMR spectra were obtained as DMSO-d6 solutions on a Varian Mercury 300 MHz NMR spectrometer (Varian Inc., Palo Alto, CA, USA) or Agilent 500 MHz NMR spectrometer (Agilent Technologies, Oxford, UK) and the chemical shifts are quoted relative to TMS peak. Low- and high-resolution mass spectra were recorded at an ionization potential of 70 eV using Micromass Autospec-TOF (double focusing high resolution) instrument (Waters Corp., Milford, MA, USA).
3.2. Typical Procedure for the Synthesis of the 4-Substituted 2-Hydroxy-5-Iodoacetophenones 1a–d
A stirred mixture of a 4-substituted 2-hydroxyacetophenone derivative (1 equiv.) and p-toluenesulfonic acid (1.2 equiv.) in acetonitrile (25 mL/mmol of ketone) at 0 °C was treated with N-iodosuccinimide (1 equiv.) over 5 min. The mixture was then allowed to stir at room temperature (RT) for 14 h and then quenched with an ice-cold saturated aqueous solution of sodium thiosulphate. The precipitate was filtered and recrystallized to afford pure 4-substituted 2-hydroxy-5-iodoacetophenone derivative as a solid. The following acetophenone derivatives were prepared in this fashion.
1-(4-F.luoro-2-hydroxy-5-iodophenyl)ethanone (1a)
Solid (8.81 g, 90 %), mp. 87–88 °C; ATR γmax (cm−1) 450, 544, 781, 1146, 1202, 1468, 1631; 1H-NMR (DMSO-d6) δ (ppm) 2.53 (3H, s, -CH3), 6.62 (1H, d, J = 9.3 Hz, H-3), 8.01 (1H, d, J = 7 Hz, H-6), 12.40 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 28.7, 70.2 (d, 2JCF = 27.5 Hz), 105.2 (d, 2JCF = 26.3 Hz), 120.8 (d, 4JCF = 2.4 Hz), 142.3 (d, 3JCF = 5.77 Hz), 163.0 (d, 3JCF = 12.6 Hz), 163.4 (d, 1JCF = 249.6 Hz), 202.3; HRMS (ES): MH+ found 340.8518. C8H7O2FI+ requires 340.8535.
1-(4-C.hloro-2-hydroxy-5-iodophenyl)ethanone (1b)
Solid (7.39 g, 85%), mp. 143–145 °C; ATR γmax (cm−1) 440, 519, 794, 968, 1204, 1309,1452, 1634; 1H-NMR (DMSO-d6) δ (ppm) 2.58 (3H. s, -CH3), 7.17 (1H, s, H-3), 8.18 (1H, s, H-6), 11.72 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 29.2, 86.0, 118.7, 123.0, 141.9, 143.8, 160.6, 201.6; HRMS (ES): MH+ found 294.9037. C8H5IO235ClI+ requires 294.9023.
1-(4-B.romo-2-hydroxy-5-iodophenyl)ethanone (1c)
Solid (6.90 g, 88%), mp. 157–158 °C; ATR γmax (cm−1) 448, 620, 780, 1203, 1280, 1446, 1630; 1H-NMR (DMSO-d6) δ (ppm) 2.56 (3H, s, -CH3), 7.33 (1H, s, H-3), 8.15 (1H, s, H-6), 11.70 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 29.2, 83.2, 99.9, 116.0, 141.1, 163.8, 165.3, 201.8; HRMS (ES): MH+ found 338.8518. C8H5IO279BrI+ requires 338.8535.
1-(2-H.ydroxy-5-iodo-4-methoxyphenyl)ethanone (1d)
Solid (7.04 g, 80%), mp.156–157 °C (Lit. [32]. 161 °C), ATR γmax (cm−1) 438, 563, 751, 1175, 1220, 1442, 1619 cm−1; 1H-NMR (DMSO-d6) δ (ppm) 2.53 (3H, s, -CH3), 3.88 (3H, s, -OCH3), 6.38 (1H, s, H-3), 8.05 (1H, s, H-6), 12.67 (1H, s, -OH); cm−1; 13C-NMR (DMSO-d6) δ (ppm) 26.3, 56.7, 73.2, 100.0, 115.9, 141.1, 163.8, 165.3, 201.8; HRMS (ES): MH+ found 292.9518. C9H10O3I+ requires 292.9521.
3.3. Typical Procedure for the Synthesis of the 2-hydroxy-5-iodo-4-methoxychalcones 2a–p
A mixture of 1 (1 equiv.) and benzaldehyde derivative (1.2 equiv.) in the presence of 5% KOH (15 mL/mmol of 1) in ethanol (30 mL/mmol of 1) was stirred at room temperature (RT) for 78 h. The mixture was acidified dropwise with concentrated hydrochloric acid and the resultant precipitate was filtered off, washed with cold water and then recrystallized from ethanol to afford compound 2a–p. The following compounds were prepared in this fashion.
(E)-1-(4-Fluoro-2-hydroxy-5-iodophenyl)-3-phenylpropenone (2a)
Solid (5.32 g, 81%), mp.138–139 °C; ATR γmax (cm−1) 492, 579, 645, 730, 831, 973, 1042, 1156, 1200, 1278, 1342, 1477, 1560, 1635, 2361, 3027, 3062; 1H-NMR (DMSO-d6) δ (ppm) 6.72 (1H, d, J = 9.3 Hz, H-3), 7.43-7.44 (3H, m, H-4′ and H-2′,6′), 7.46 (1H, d, Jtrans = 16.0 Hz, H-8), 7.65 (2H, t, J = 3.6 Hz, H-3′,5′), 7.90 (1H, d, Jtrans = 16.0 Hz, H-7), 8.20 (1H, d, J = 7.2 Hz, H-6), 13.10 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 67.9 (d, 2JCF = 26.3 Hz), 105.4 (d, 2JCF = 25.2 Hz), 118.8, 128.6 (d, 3JCF = 17.2 Hz), 128.8 (2 × C), 131.0, 133.8, 140.0 (d, 4JCF = 4.5 Hz), 146.3, 165.3 (d, 1JCF = 254.1 Hz), 165.6 (d, 3JCF = 5.7 Hz), 191.2; HRMS (ES): MH+ found 368.9767. C15H11O2FI+ requires 368.9774.
(E)-1-(4-Fluoro-2-hydroxy-5-iodophenyl)-3-(4-fluorophenyl)propenone (2b)
Solid (5.30 g, 77%), mp. 125–126 °C; ATR γmax (cm−1) 485, 509, 731, 766, 806, 949, 1014, 1158, 1196, 1286, 1428, 1596, 1636, 2912, 3000; 1H-NMR (DMSO-d6) δ (ppm) 6.75 (1H, d, J = 9.3 Hz, H-3), 7.15 (2H, t, J = 8.4 Hz, H-3′,5′), 7.42 (1H, d, Jtrans = 15.3 Hz, H-8), 7.65 (2H, dd, J = 5.4 Hz and 9 Hz, H-2′,6′), 7.93 (1H, d, Jtrans = 15.6 Hz, H-7), 8.24 (1H, d, J = 6.9 Hz, H-6), 13.12 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 68.3 (d, 2JCF = 26.3 Hz), 105.4 (d, 2JCF = 26.3 Hz), 114.7, 115.0, 116.4 (d, 2JCF = 21.8 Hz), 118.9, 130.5 (d, 4JCF = 3.45 Hz), 131.0 (d, 3JCF = 8.0 Hz), 140.3 (d, 4JCF = 4.6 Hz), 145.4, 164.5 (d, 1JCF = 252.0 Hz), 166.1 (d, 3JCF = 17.2 Hz), 191.4; HRMS (ES): MH+ found 386.9879. C15H10O2F2I+requires 386.9918.
(E)-3-(4-chlorophenyl)-1-(4-fluoro-2-hydroxy-5-iodophenyl)prop-2-en-1-one (2c)
Solid (6.04 g, 84%), mp.146–147 °C; ATR γmax (cm−1) 472, 548, 760, 852, 1092, 1283, 1321, 1424, 1592, 1670, 2559, 2838; 1H-NMR (DMSO-d6) δ (ppm) 6.75 (1H, d, J = 8.7 Hz, H-3), 7.42 (2H, d, J = 8.0 Hz, H-3′,5′), 7.46 (1H, d, Jtrans = 15.5 Hz, H-8), 7.61 (2H, d, J = 8.0 Hz, H-2′,6′), 7.82 (1H, d, Jtrans = 15.5 Hz, H-7), 8.22 (1H, d, J = 6.6 Hz, H-6), 13.08 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 68.4 (d, 2JCF = 26.3 Hz), 106.0 (d, 2JCF = 26.3 Hz), 119.6, 129.3 (d, 3JCF = 16.0 Hz), 130.0, 131.6, 132.6, 137.4, 140.3 (d, 4JCF = 4.5 Hz), 145.2, 165.1 (d, 1JCF = 254.2 Hz), 166.0 (d, 3JCF = 13.7 Hz), 191.3; HRMS (ES): MH+ found 402.9385. C15H10O2F35ClI+ requires 402.9398.
(E)-1-(4-Fluoro-2-hydroxy-5-iodophenyl)-3-(4-methoxyphenyl)propenone (2d)
Solid (5.20 g, 73%), mp. 154–155 °C; ATR γmax (cm−1) 448, 513, 534, 579, 664, 800, 1042, 1168, 1194, 1256, 1509, 1553, 1634, 2840, 2936; 1H-NMR (DMSO-d6) δ (ppm) 3.87 (3H, s, -OCH3), 6.75 (1H, d, J = 9.6 Hz, H-3), 6.95 (2H, d, J = 8.7 Hz, H-3′,5′), 7.36 (1H, d, Jtrans = 15.0 Hz, H-8), 7.64 (2H, d, J = 8.7 Hz, H-2′,6′), 7.90 (1H, d, Jtrans = 15.3 Hz, H-7), 8.23 (1H, d, J = 7.2 Hz, H-6), 13.30 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 55.5, 68.2 (d, 2JCF = 27.5 Hz), 105.8 (d, 2JCF = 25.2 Hz), 114.6, 116.6, 119.3, 126.9, 130.9, 140.1 (d, 4JCF = 5.7 Hz), 146.6, 162.4, 165.6 (d, 1JCF = 252.1 Hz), 166.0 (d, 3JCF = 13.7 Hz), 191.5; HRMS (ES): MH+ found 398.9895. C16H13O3FI+ requires 398.9893.
(E)-1-(4-Chloro-2-hydroxy-5-iodophenyl)-3-phenylpropenone (2e)
Solid (5.12 g, 79%), mp. 150–151 °C; ATR γmax (cm−1) 476, 523, 684, 970, 1188, 1556, 1633, 3064; 1H-NMR (DMSO-d6) δ (ppm) 7.12 (1H, s, H-3) 7.44 (2H, d, J = 4 Hz, H-3′,5′), 7.50 (1H, d, Jtrans = 15 Hz, H-8), 7.68 (2H, dd, J = 3.6 and 7.0 Hz, H-2′,6′), 7.94 (1H, d, Jtrans = 15 Hz, H-7), 8.30 (1H, s, H-6), 12.82 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 88.2, 119.1, 119.6, 120.6, 128.9, 129.1, 131.4, 134.1, 140.3, 145.8, 146.9, 163.7, 191.9; HRMS (ES): MH+ found 380.9484. C15H11O235ClI+ requires 384.9492.
(E)-1-(4-Chloro-2-hydroxy-5-iodophenyl)-3-(4-fluorophenyl)propenone (2f)
Solid (5.90 g, 87%), mp. 144–145 °C; ATR γmax (cm−1) 469, 507, 825, 1029, 1157, 1187, 1507, 1562, 1639, 3075; 1H-NMR (DMSO-d6) δ (ppm) 7.14 (2H, t, J = 8.5 Hz, H-3′,5′), 7.12 (1H, s, H-3) 7.41 (1H, d, Jtrans = 15.5 Hz, H-8), 7.67 (2H, t, J = 7 Hz, H-2′,6′), 7.90 (1H, d, Jtrans = 15.5 Hz, H-7), 8.27 (1H, s, H-6), 12.76 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 84.2, 116.3 (d, 2JCF = 21.75 Hz), 118.8, 118.9, 119.7, 120.6, 130.5 (d, 4JCF = 2.75 Hz), 130.9 (d, 3JCF = 8.6 Hz), 140.2, 145.6, 145.9, 163.8, 164.6 (d, 1JCF = 251.2 Hz), 191.7; HRMS (ES): MH+ found 402.9390. C15H10O235ClIF+ requires 402.9398.
(E)-1-(4-Chloro-2-hydroxy-5-iodophenyl)-3-(4-chlorophenyl)propenone (2g)
Solid (5.65 g, 80%), mp. 190–191 °C; ATR γmax (cm−1) 490, 795, 813, 824, 1025, 1171, 1551, 1635, 3075; 1H-NMR (DMSO-d6) δ (ppm) 7.20 (1H, s, H-3) 7.43 (2H, d, J = 7.5 Hz, H-3′,5′), 7.47 (1H, d, Jtrans = 16.0 Hz, H-8), 7.62 (2H, d, J = 8.0 Hz, H-2′,6′), 7.90 (1H, d, Jtrans = 15.5 Hz, H-7), 8.30 (1H, s, H-6), 12.72 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 84.3, 119.6, 119.7, 120.6, 129.5, 130.1, 132.6, 137.4, 140.2, 145.4, 146.0, 163.8, 191.6; HRMS (ES): MH+ found 420.0472. C15H10O235Cl2I+requires 420.0484.
(E)-1-(4-Chloro-2-hydroxy-5-iodophenyl)-3-(4-methoxyphenyl)propenone (2h)
Solid (5.10 g, 73%), mp. 152–153 °C; ATR γmax (cm−1) 517, 633, 824, 990, 1170, 1244, 1548, 1602, 1623, 3074; 1H-NMR (DMSO-d6) δ (ppm) 3.87 (3H. s, -OCH3), 7.00 (2H, d, J = 8.5 Hz, H-3′,5′), 7.16 (1H, s, H-3), 7.36 (1H, d, Jtrans = 15.5 Hz, H-8), 7.64 (2H, d, J = 9.0 and 8.7 Hz, H-2′,6′ ), 7.88 (1H, d, Jtrans = 15.5 Hz, H-7), 8.30 (1H, s, H-6), 12.97 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 55.5, 84.1, 114.6, 116.5, 119.6, 120.8, 127.0, 131.0, 140.2, 145.5, 146.9, 162.5, 163.8, 191.8; HRMS (ES): MH+ found 414.9586. C16H13O335ClI+ requires 414.9598.
(E)-1-(4-Bromo-2-hydroxy-5-iodophenyl)-3-phenylpropenone (2i)
Solid (4.92 g, 78%), mp. 145–146 °C; ATR γmax (cm−1) 484, 624, 733, 819, 975, 1190, 1323, 1555, 1638, 3025; 1H-NMR (DMSO-d6) δ (ppm) 7.36 (1H, s, H-3), 7.45 (3H, m, Ar), 7.50 (1H, d, Jtrans = 15.0 Hz, H-8), 7.65 (2H, d, J = 7.0 Hz, H-2′,6′), 7.93 (1H, d, Jtrans = 15.0 Hz, H-7), 8.28 (1H, s, H-6), 12.74 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 87.2, 119.1, 121.0, 123.2, 129.0, 129.1, 131.4, 134.2, 137.5, 140.0, 147.0, 163.3, 192.1; HRMS (ES): MH+ found 426.8840. C15H11O279BrI+ requires 426.8831.
(E)-1-(4-Bromo-2-hydroxy-5-iodophenyl)-3-(4-fluorophenyl)propenone (2j)
Solid (5.45 g, 83%), mp. 169–170 °C; ATR γmax (cm−1) 462, 489, 505, 824, 1185, 1198, 1508, 1556, 1638, 3075; 1H-NMR (DMSO-d6) δ (ppm) 7.14 (2H, t, J = 7.8 Hz, H-3′,5′), 7.37 (1H, s, H-3), 7.41 (1H, d, Jtrans = 15.0 Hz, H-8), 7.68 (2H, d, J = 7.8 Hz, H-2′,6′), 7.90 (1H, d, Jtrans = 15.0 Hz, H-7), 8.26 (1H, s, H-6), 12.70 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 87.2, 116.3 (d, 2JCF = 21.8 Hz), 118.8 (d, 4JCF = 2.75 Hz), 120.9, 123.2, 130.5, 130.9 (d, 3JCF = 9.5 Hz), 137.6, 140.0, 145.6, 163.3, 164.6 (d, 1JCF = 252 Hz), 191.9; HRMS (ES): MH+ found 444.8745. C15H10O2F79BrI+ requires 444.8736.
(E)-1-(4-Bromo-2-hydroxy-5-iodophenyl)-3-(4-chlorophenyl)propenone (2k)
Solid (5.41 g, 80%), mp. 185–187 °C; ATR γmax (cm−1) 416, 501, 794, 817, 1024, 1340, 1489, 1552, 1636, 3074; 1H-NMR (DMSO-d6) δ (ppm) 7.37 (1H, s, H-3) 7.42 (2H, d, J = 7.5 Hz, H-3′,5′), 7.53 (1H, d, Jtrans = 15.5 Hz, H-8), 7.61 (2H, d, J = 8.0 Hz, H-2′,6′ ), 7.88 (1H, d, Jtrans = 15.5 Hz, H-7), 8.26 (1H, s, H-6), 12.70 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 87.3, 119.5, 120.9, 123.2, 129.4, 130.0, 132.6, 137.5, 137.7, 140.0, 145.4, 163.3, 191.8; HRMS (ES): MH+ found 460.8444. C15H11O235Cl79BrI+ requires 460.8441.
(E)-1-(4-Bromo-2-hydroxy-5-iodophenyl)-3-(4-methoxyphenyl)propenone (2l)
Solid (5.06 g, 75%), mp. 150–151 °C; ATR γmax (cm−1) 489, 559, 624, 825, 1021, 1169, 1507, 1546, 1623, 2919; 1H-NMR (DMSO-d6) δ (ppm) 3.87 (3H. s, -OCH3), 6.95 (2H, d, J = 8.5 Hz, H-3′,5′), 7.35 (1H, s, H-3), 7.36 (1H, d, Jtrans = 15.5 Hz, H-8), 7.63 (2H, d, J = 8.0 Hz, H-2′,6′), 7.91 (1H, d, Jtrans = 15.5 Hz, H-7), 8.26 (1H, s, H-6), 12.90 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 55.5, 87.1, 114.6, 116.5, 121.1, 123.1, 127.0, 131.0, 137.1, 140.0, 146.9, 162.5, 163.3, 191.9; HRMS (ES): MH+ found 458.9099. C16H13O379BrI+ requires 458.9093.
(E)-1-(2-Hydroxy-5-iodo-4-methoxyphenyl)-3-phenylprop-2-en-1-one (2m)
Solid (4.68 g, 72%), mp. 141–143 °C; ATR γmax (cm−1) 559, 664,836, 1176, 1214, 1287, 1558, 1603, 1626; 1H-NMR (DMSO-d6) δ (ppm) 3.89 (3H. s, -OCH3), 6.42 (1H, s, H-3), 7.43 (2H, d, J = 7.5 Hz, H-3′,5′), 7.50 (1H, d, Jtrans = 15.5 Hz, H-8), 7.65 (2H, d, J = 7.5 Hz, H-2′,6′ ), 7.88 (1H, d, Jtrans = 15.5 Hz, H-7), 8.22 (1H, s, H-6), 13.42 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 56.7, 73.4, 100.2, 116.1, 119.6, 120.0, 128.8, 129.0, 131.0, 134.5, 140.0, 145.3, 163.8, 166.7, 191.0; HRMS (ES): MH+ found 380.9984. C16H13O3I+ requires 380.9988.
(E)-3-(4-Fluorophenyl)-1-(2-hydroxy-5-iodo-4-methoxyphenyl)prop-2-en-1-one (2n)
Solid (5.31 g, 78%), mp. 197–199 °C; ATR γmax (cm−1) 507, 534, 831, 1159, 1208, 1356, 1597, 1632; 1H-NMR (DMSO-d6) δ (ppm) 3.93 (3H. s, -OCH3), 6.46 (1H, s, H-3), 7.14 (2H, t, J = 8.7 Hz, H-3′,5′), 7.43 (1H, d, Jtrans = 15.5 Hz, H-8), 7.67 (2H, d, J = 8.7 Hz, H-2′,6′), 7.86 (1H, d, Jtrans = 15.5 Hz, H-7), 8.23 (1H, s, H-6), 12.37 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 56.8, 73.4, 100.3, 116.3 (d, 2JCF = 21.8 Hz), 119.3 (d, 4JCF = 2.3 Hz), 130.7 (d, 3JCF = 8.0 Hz), 140.0, 144.0, 163.9, 164.3 (d, 1JCF = 252.0 Hz), 166.8, 190.8; HRMS (ES): MH+ found 398.9878. C16H12O3FI+ requires 398.9893.
(E)-3-(4-Chlorophenyl)-1-(2-hydroxy-5-iodo-4-methoxyphenyl)prop-2-en-1-one (2o)
Solid (5.68 g, 80%), mp. 200–201 °C; ATR γmax (cm−1) 430, 565, 798, 829, 1044, 1213, 1560, 1632; 1H-NMR (DMSO-d6) δ (ppm) 3.93 (3H. s, -OCH3), 6.46 (1H, s, H-3), 7.42 (2H, d, J = 8.1 Hz, H-3′,5′), 7.48 (1H, d, Jtrans = 15.5 Hz, H-8), 7.61 (2H, d, J = 8.7 Hz, H-2′,6′ ), 7.85 (1H, d, Jtrans = 15.5 Hz, H-7), 8.23 (1H, s, H-6), 12.34 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 56.8, 73.4, 100.3, 116.1, 120.1, 129.3, 130.0, 133.0, 136.9, 140.0, 143.9, 163.9, 166.8, 190.7; HRMS (ES): MH+ found 414.9582. C16H12O335ClI+ requires 414.9598.
(E)-1-(2-Hydroxy-5-iodo-4-methoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (2p)
Solid (4.91 g, 70%), mp. 185–187 °C (Lit. [33] 182–184 °C); ATR γmax (cm−1) 559, 664, 821, 1176, 1214, 1287, 1510, 1603, 1626; 1H-NMR (DMSO-d6) δ (ppm) 3.87 (3H. s, -OCH3), 3.93 (3H. s, -OCH3), 6.46 (1H, s, H-5), 6.96 (2H, d, J = 8.4 Hz, H-3′,5′), 7.36 (1H, d, Jtrans = 15.3 Hz, H-8), 7.64 (2H, d, J = 8.7 Hz, H-2′,6′), 7.88 (1H, d, Jtrans = 15.6 Hz, H-7), 8.25 (1H, s, H-6), 13.53 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 55.5, 56.7, 73.2, 100.3, 114.5, 116.3, 117.2, 127.3, 130.6, 139.9, 145.3, 162.1, 163.7, 166.7, 191.0; HRMS (ES): MH+ found 411.0098. C17H15O4I+ requires 411.0095.
3.4. Typical Procedure for the Synthesis of the 7-substituted 2-aryl-3-hydroxy-6-iodochromen-4-ones 3a–p
A mixture of 2a (0.50 g 1.17 mmol) and 3 M KOH (15 mL) in ethanol (20 mL) was stirred at RT for 15 min and then placed in an ice-bath. Hydrogen peroxide (5 mL) was added slowly to the reaction mixture and stirring was continued for 30 min. The ice-bath was removed and the reaction mixture was stirred at RT for 3 h and then poured into a mixture of crushed ice and conc. HCl. The organic phase was extracted with chloroform and the combined organic layers were dried over anhydrous MgSO4. The salt was filtered off and the solvent was evaporated on a rotary evaporator under reduced pressure. The residue was purified by column chromatography on silica gel to afford 3 as a yellow solid. Compounds 3a–p were prepared in this fashion.
7-Flu.oro-3-hydroxy-6-iodo-2-phenyl-4H-chromen-4-one (3a)
Solid (0.42 g, 80%), mp. 275–276 °C; ATR γmax (cm−1) 499, 577, 654, 782, 841, 1105, 1204, 1369, 1442, 1590, 1609, 3075; 1H-NMR (DMSO-d6) δ (ppm) 7.52 (3H, m, H-4′ and H-3′,5′), 7.78 (1H, d, J = 8.7 Hz, H-8), 8.16 (2H, d, J = 6.3 Hz, H-2′,6′), 8.44 (1H, d, J = 7.2 Hz, H-5), 9.83 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 79.6 (d, 2JCF = 27.5 Hz), 106.0 (d, 2JCF = 28.5 Hz), 120.7, 128.0, 129.0, 130.5 (d, 4JCF = 2.3 Hz), 131.3, 136.0 (d, 3JCF = 4.5 Hz), 139.6, 146.4, 155.7 (d, 3JCF = 13.7 Hz), 163.7 (d, 1JCF = 247.3 Hz), 171.7; HRMS (ES): MH+ found 382.9575. C15H9O3FI+ requires 382.9580.
7-Flu.oro-2-(4-fluorophenyl)-3-hydroxy-6-iodo-4H-chromen-4-one (3b)
Solid (0.38 g, 73%), mp. 330–331 °C; ATR γmax (cm−1) 502, 553, 650, 836, 1158, 1214, 1392, 1496, 1557, 1585, 2361, 2986, 3073; 1H-NMR (DMSO-d6) δ (ppm) 7.10 (2H, d, J = 8.7 Hz, H-3′,5′), 7.80 (1H, d, J = 8.1 Hz, H-8), 8.14 (2H, d, J = 9.0 Hz, H-2′,6′), 8.44 (1H, d, J = 7.0 Hz, H-5), 9.70 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 79.4, 105.5 (d, 2JCF = 26.2 Hz), 115.3 (d, 2JCF = 24.0 Hz), 116.4 (d, 2JCF = 21.75 Hz), 122.3, 129.5 (d, 3JCF = 8.5 Hz), 131.6 (d, 4JCF = 2.9 Hz), 132.2 (d, 3JCF = 8.5 Hz), 141.5 (d, 4JCF = 2.8 Hz), 144.5, 164.2 (d, 1JCF = 248.4 Hz), 164.6, 165.3 (d, 1JCF = 249.4 Hz); HRMS (ES): MH+ found 401.1101. C15H8O3F2I+ requires 401.1223.
2-(4-C.hlorophenyl)-7-fluoro-3-hydroxy-6-iodo-4H-chromen-4-one (3c)
Solid (0.41 g, 79%), mp. 225–226 °C; ATR γmax (cm−1) 469, 634, 654, 831, 1011, 1089, 1218, 1391, 1450, 1551, 1586, 2361, 3072, 3095; 1H-NMR (DMSO-d6) δ (ppm) 7.12 (2H, d, J = 7.5 Hz, H-3′,5′), 7.72 (1H, d, J= 8.7 Hz, H-3), 7.94 (2H, d, J = 8.1 Hz, H-2′,6′), 8.25 (1H, d, J = 8.0 Hz, H-5); 13C-NMR (DMSO-d6) δ (ppm) 79.5 (d, 2JCF = 27.4 Hz), 106.0 (d, 2JCF = 29.8 Hz), 114.5 (2×C), 120.8, 123.6, 129.8, 135.8, 138.7, 146.7, 155.2 (d, 3JCF = 13.7 Hz), 161.0, 163.5 (d, 1JCF = 247.4 Hz), 171.4; HRMS (ES): MH+ found 416.9173. C15H8O3F35ClI+ requires 416.9191.
7-Flu.oro-3-hydroxy-6-iodo-2-(4-methoxyphenyl)-4H-chromen-4-one (3d)
Solid (0.43 g, 84 %), mp. 261–262 °C; ATR γmax (cm−1) 492, 568, 659, 1034, 1177, 1205, 1255, 1388, 1450, 1558, 1595, 2360, 2837, 2935, 3092; 1H-NMR (DMSO-d6) δ (ppm) 3.76 (3H, s, -OCH3), 7.02 (2H, d, J = 8.1 Hz, H-3′,5′), 7.71 (1H, d, J = 8.1 Hz, H-8), 8.12 (2H, d, J = 8.7 Hz, H-2′,6′), 8.35 (1H, d, J = 7.2 Hz, H-5), 9.61 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 55.8, 79.3 (d, 2JCF = 27.5 Hz), 106.0 (d, 2JCF = 28.6 Hz), 114.4, 120.7, 123.6 (d, 3JCF = 13.7 Hz), 128.0, 129.7, 135.8, 138.5 (d, 3JCF = 11.4 Hz), 146.7, 155.5 (d, 3JCF = 13.7 Hz), 160.9, 163.4 (d, 1JCF = 247.3 Hz), 171.3; HRMS (ES): MH+ found 412.9690. C16H11O4FI+ requires 412.9686.
7-Chl.oro-3-hydroxy-6-iodo-2-phenyl-4H-chromen-4-one (3e)
Solid (0.39 g, 75%), mp. 303–304 °C; ATR γmax (cm−1) 468, 486, 505, 686, 765, 948, 1012, 1207, 1432, 1549, 1592, 1605, 2323, 2912, 3000, 3054; 1H-NMR (DMSO-d6) δ (ppm) 7.23 (3H, d, J = 6.9 Hz, H-4′ and H-3′,5′), 7.93 (2H, d, J = 5.4 Hz, H-2′,6′), 8.02 (1H, s, H-8), 8.21 (1H, s, H-5), 9.64 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 91.3, 110.0, 119.2, 112.5, 125.7, 126.9, 128.2, 135.0, 136.6, 139.3, 144.6, 153.7, 177.4; HRMS (ES): MH+ found 398.9275, C15H9O335ClI+ requires 398.9285.
7-Chl.oro-2-(4-fluorophenyl)-3-hydroxy-6-iodo-4H-chromen-4-one (3f)
Solid (0.36 g, 70%), mp. 330–331 °C; ATR γmax (cm−1) 459, 501, 540, 666, 736, 836, 948, 1014, 1098, 1158, 1222, 1402, 1433, 1560, 1578, 1634, 2911, 3004, 3058; 1H-NMR (DMSO-d6) δ (ppm) 7.42 (2H, t, J = 9.0 Hz, H-3′,5′), 8.18 (1H, s, H-8), 8.23 (2H, t, J = 6.0 Hz, H-2′,6′), 8.57 (1H, s, H-5), 10.02 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 94.1, 116.1 (d, 2JCF = 21.8 Hz), 119.9, 122.2,, 127.9 (d, 4JCF = 2.75 Hz), 130.7 (d, 3JCF = 8.5 Hz), 136.2, 140.0, 142.3, 145.5, 154.7, 163.2 (d, 1JCF = 248.3 Hz), 171.7; HRMS (ES): MH+ found 416.9181. C15H8O3F35ClI+ requires 416.9191.
7-Chl.oro-2-(4-chlorophenyl)-3-hydroxy-6-iodo-4H-chromen-4-one (3g)
Solid (0.35 g, 67%), mp. 247–248 °C; ATR γmax (cm−1) 484, 509, 636, 806, 949, 1013, 1207, 1434, 1449, 1550, 1593, 1635, 2911, 3025, 3060; 1H-NMR (DMSO-d6) δ (ppm) 6.67 (2H, d, J = 8.1 Hz, H-3′,5′), 7.20 (1H, s, H-8), 7.34 (2H, d, J = 7.5 Hz, H-2′,6′ ), 7.55 (1H, s, H-5), 10.09; 13C-NMR (DMSO-d6) δ (ppm) 94.6, 120.0, 122.2, 129.1, 129.8, 130.4, 135.2, 136.2, 140.2, 142.4, 145.0, 154.7, 171.8; HRMS (ES): MH+ found 432.8890. C15H8O335Cl2I+ requires 432.8895.
7-Chl.oro-3-hydroxy-6-iodo-2-(4-methoxyphenyl)-4H-chromen-4-one (3h)
Solid (0.32 g, 62%), mp. 252–253 °C; ATR γmax (cm−1) 506, 635, 732, 767, 806, 949, 1013, 1114, 1259, 1450, 1594, 1610, 2339, 2913, 3005, 3027, 3059; 1H-NMR (DMSO-d6) δ (ppm) 3.32 (1H, s, -OCH3), 6.57 (2H, d, J = 6.0 Hz, H-3′,5′), 7.60 (1H, s, H-8), 7.67 (2H, d, J = 6.3 Hz, H-2′,6′ ), 8.00 (1H, s, 5-H); 13C-NMR (DMSO-d6) δ (ppm) 55.6, 94.2, 114.4, 119.7, 122.2, 123.7, 130.0, 131.3, 136.0, 141.8, 146.5, 154.4, 160.9, 171.5; HRMS (ES): MH+ found 428.9379. C16H11O435ClI+ requires 428.9391.
7-Bro.mo-3-hydroxy-6-iodo-2-phenyl-4H-chromen-4-one (3i)
Solid (0.40 g, 77%), mp. 190–191 °C; ATR γmax (cm−1) 468, 685, 765, 1126, 1205, 1372, 1429, 1549, 1607, 1682, 2149, 3079, 3278; 1H-NMR (DMSO-d6) δ (ppm) 7.23 (4H, d, J = 7.8 Hz, H-8, H-4′ and H-3′,5′), 7.91 (2H, d, J = 9.0 Hz, H-2′,6′), 8.18 (1H, s, H-5), 9.58 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 97.4, 122.4, 123.1, 128.1, 128.9, 130.5, 131.3, 134.2, 135.8, 139.9, 146.0, 154.3, 171.8; HRMS (ES): MH+ found 444.0754. C15H9O379BrI+ requires 444.0777.
7-Bro.mo-2-(4-fluorophenyl)-3-hydroxy-6-iodo-4H-chromen-4-one (3j)
Solid (0.37 g, 72%), mp. 180–181 °C; ATR γmax (cm−1) 436, 500, 626, 697, 788, 861, 1097, 1201, 1240, 1431, 1594, 1666, 2927, 3076; 1H-NMR (DMSO-d6) δ (ppm) 7.42 (2H, t, J = 8.7 Hz, H-3′,5′), 8.26 (2H, d, J = 6.0 Hz, H-2′,6′), 8.32 (1H, s, H-8), 8.48 (1H, s, H-5), 10.02 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 94.5, 116.1 (d, 2JCF = 21.75 Hz), 119.9, 122.2, 127.9 (d, 4JCF = 2.3 Hz), 130.6 (d, 3JCF = 9.15 Hz), 136.2, 139.7, 142.3, 145.5, 154.7, 163.2 (d, 1JCF = 248.5 Hz), 171.8; HRMS (ES): MH+ found 460.8680. C15H8O3F79BrI+ requires 460.8696.
7-Bro.mo-2-(4-chlorophenyl)-3-hydroxy-6-iodo-4H-chromen-4-one (3k)
Solid (0.42 g, 81%), mp. 282–283 °C; ATR γmax (cm−1) 497, 590, 635, 834, 1093, 1110, 1206, 1430, 1449, 1548, 1590, 1601, 1653, 3082, 3250; 1H-NMR (DMSO-d6) δ (ppm) 7.62 (2H, d, J = 8.0 Hz, H-3′, 5′), 8.15 (1H, s, H-8), 8.20 (2H, d, J = 7.0 Hz, H-2′,6′), 8.49 (1H, s, H-5), 10.09 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 97.9, 122.7, 123.5, 129.4, 130.1, 130.5, 134.7, 135.5, 136.2, 140.5, 145.2, 154.6, 172.2; HRMS (ES): MH+ found 476.8386. C15H8O335Cl79BrI+ requires 476.8390.
7-Bro.mo-3-hydroxy-6-iodo-2-(4-methoxyphenyl)-4H-chromen-4-one (3l)
Solid (0.38 g, 74%), mp. 201–202 °C; ATR γmax (cm−1) 435, 499, 625, 696, 787, 901, 1096, 1200, 1311, 1430, 1456, 1590, 1661, 2867, 2924, 3046; 1H-NMR (DMSO-d6) δ (ppm) 3.40 (3H, s, -OCH3), 6.64 (2H, d, J = 6.3 Hz, H-3′,5′), 6.91 (1H, s, H-8), 7.72 (2H, d, J = 7.5 Hz, H-2′,6′ ), 8.00 (1H, s, H-5), 9.28 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 55.8, 97.4, 114.5, 23.1, 130.0, 134.0, 135.8, 139.0, 140.9, 146.6, 154.3, 161.1, 161.3, 171.5; HRMS (ES): MH+ found 472.8863. C16H11O479BrI+ requires 472.8885.
3-Hyd.roxy-6-iodo-7-methoxy-2-phenyl-4H-chromen-4-one (3m)
Solid (0.39 g, 75%), mp. 233–234 °C; ATR γmax (cm−1) 459, 659, 829, 1034, 1175, 1213, 1263, 1451, 1599, 2359, 2838, 3095; 1H-NMR (DMSO-d6) δ (ppm) 3.93 (3H, s, -OCH3), 7.20 (1H, s, H-5), 7.34 (3H, t, J = 7.5 Hz, H-3′,5′ and h-5′), 8.30 (1H, s, H-8), 8.53 (2H, d, J = 7.5 Hz, H-2′,6′); 13C-NMR (DMSO-d6) δ (ppm) 57.5, 81.7, 100.2, 117.9, 125.4, 126.5, 127.3, 128.2, 128.5, 135.2, 135.4, 143.7, 155.7, 160.0; HRMS (ES): MH+ found 394.9775. C16H12O4I+ requires 394.9780.
2-(4-F.luorophenyl)-3-hydroxy-6-iodo-7-methoxy-4H-chromen-4-one (3n)
Solid (0.42 g, 82%), mp. > 350 °C; ATR γmax (cm−1) 469, 632, 667, 825, 1009, 1038, 1087, 1207, 1263, 1443, 1477, 1548, 1588, 2360, 2936, 2966, 3095; 1H-NMR (DMSO-d6) δ (ppm) 3.92 (3H, s, -OCH3), 7.14 (1H, s, H-5), 7.31 (2H, d, J = 7.2 Hz, H-3′,5′), 8.30 (1H, s, H-8), 8.62 (2H, d, J = 7.5 Hz, H-2′,6′); 13C-NMR (DMSO-d6) δ (ppm) 55.8, 97.4, 114.5, 123.1, 130.0, 134.0, 135.8, 139.0, 140.9, 146.6, 154.3, 161.1, 161.3, 171.5; HRMS (ES): MH+ found 413.15766. C16H11O4FI+ requires 413.1574.
2-(4-C.hlorophenyl)-3-hydroxy-6-iodo-7-methoxy-4H-chromen-4-one (3o)
Solid (0.38 g, 74%), mp 258–259 °C; ATR γmax (cm−1) 484, 635, 662, 831, 1089, 1172, 1212, 1262, 1446, 1480, 1552, 1598, 2360, 3105, 3199; 1H-NMR (DMSO-d6) δ (ppm) 3.95 (3H, s, -OCH3), 7.28 (1H, s, H-8),7.52 (2H, d, J = 6.0 Hz, H-3′,5′), 8.28 (2H, d, J = 6.0 Hz, H-2′,6′), 8.33 (1H, s, H-5), 9.86 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 57.8, 83.8, 100.4, 114.3, 117.2, 127.8, 128.8, 129.0, 131.1, 134.0, 135.1, 143.7, 156.5, 161.5; MH+ found 428.9385, C16H11O435ClI+. requires 428.9391.
3-Hyd.roxy-6-iodo-7-methoxy-2-(4-methoxyphenyl)-4H-chromen-4-one (3p)
Solid (0.39 g, 76%), mp. 230–231 °C; ATR γmax (cm−1) 498, 567, 661, 787, 1041, 1206, 1251, 1434, 1603, 1651, 2495, 2836, 2937, 3039; 1H-NMR (DMSO-d6) δ (ppm) 3.83 (3H, s, -OCH3), 3.97 (3H, s, -OCH3), 7.10 (2H, d, J = 9.0 Hz, H-3′,5′), 7.35 (1H, s, H-5), 8.16 (2H, d, J = 8.7 Hz, H-2′,6′), 8.34 (1H, s, H-8), 8.52 (1H, s, -OH); 13C-NMR (DMSO-d6) δ (ppm) 55.8, 57.8, 84.0, 100.5, 114.5, 117.4, 123.9, 129.6, 135.0, 138.4, 145.8, 156.6, 160.8, 161.6, 171.4; HRMS (ES): MH+ found 424.9887. C17H14O5I+, requires 424.9886.
3.5. In Vitro Cholinesterase (AChE and BChE) Inhibition Assays
Cholinesterase activities were assayed at 25 °C by the Ellman’s method [34], which involves following the hydrolysis of acetylthiocholine iodide (ACh) or butyrylthiocholine iodide (BCh) by monitoring formation of 5-thio-2-nitrobenzoate from 5-5′-dithiobis(2-nitrobenzoic acid) spectrophotometrically at 412 nm. The solution of the test compound 2 or 3 was prepared in DMSO (20 mL) and diluted in 50 mM tris buffer (pH 7.7) to obtain the final concentrations (10–100 µM) for assay. All the experiments were performed in triplicate in a 96-well plate reader at controlled temperature (37 °C). 70 µL of tris buffer (C4H11NO3, 50 mM, pH 7.7), 9.0 µL of the test compound solution and 1.0 µL of the respective enzyme were added sequentially to each well. The assay mixture was preincubated for 20 min at room temperature (RT), and then 10 µL of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, 3 mM in buffer) and 10 µL of ACh (5 mM in buffer) were added to each well. The absorbances were determined spectrometrically at 412 nm on a Varioskan flash spectrophotometer (Thermo Scientific, Waltham, MA, USA). The IC50 and standard deviation values were determined graphically using Graph Pad Prism.
3.6. In Vitro BACE-1 Inhibitory Assays
The inhibitory properties of chalcones (2h, 2j, 2n and 2p) or flavonols (3b, 3c, 3l and 3p) on BACE-1 were evaluated by a fluorescence resonance energy transfer (FRET) assay (Pan Vera) with a recombinant baculovirus-expressed BACE-1 and a specific substrate (Rh-EVNLDAEFK-Quencher) according to manufacturer instructions. A mixture of human recombinant BACE-1 (1.0 U/mL), the substrate (75 µM in 50 mM ammonium bicarbonate), and test compound dissolved in an assay buffer (50 mM sodium acetate, pH 4.5), was incubated for 60 min at 25 °C in a 96-well plate. The increase in fluorescence intensity produced by substrate hydrolysis was observed on a fluorescence microplate reader with an excitation wavelength of 545 and emission wavelength 590 nm. The inhibition ratio was calculated using the following equation:
| Inhibition (%) = [1 − (S − S0)/(C − C0)] × 100 | (1) |
where C is the fluorescence of control (enzyme, assay buffer, and substrate) after 60 min of incubation, C0 is the fluorescence of control at time 0, S is the fluorescence of tested samples (enzyme, sample solution, and substrate) after 60 min of incubation, and S0 the fluorescence of the tested samples at time 0.
3.7. Kinetic Studies against ChEs and BACE-1
3.7.1. Kinetic Evaluation of 2h, 3b and 3p against ChEs
The experiment was performed using different inhibitor (2h, 3b or 3p) concentrations (0, 2.5, 3.5 and 5 µM) and substrate (acetylthiocholine iodide (ACh) or butyrylthiocholine iodide (BCh) concentrations (0.1, 0.5, 2.5 and 5 µM). The reaction was carried out in triplicate and the absorbences were measured at 412 nm after every 1 s. A linear graph of the absorbences against time were plotted to obtain the velocity. Finally, the lineweaver burk plot (reciprocal of the velocity versus reciprocal of the subtrate concentrations) was plotted. as well as the Dixon plot (reciprocal of the velocity versus inhibitor concentration). The inhibition constant Ki, was determined by calculating the straight line equations and identifying the point at which the lines would intersect.
3.7.2. Kinetic Evaluation of 2j and 3l against BACE-1
The kinetics assays were conducted according to the manufacturer’s instructions similarly to the inhibitory assay except that change in fluorescence over time was monitored at 590 nm without the addition of the stop solution. Each substrate (0.15, 0.30, 0.45 µM) and compound (0, 4, 8 and 16 µM) concentration pair were prepared in triplicate and analyzed. The Lineweaver Burk and Dixon plots were plotted as described in Section 3.7.1.
3.8. Molecular Docking
Discovery studio and specifically the CDOCKER module was used to investigate interactions of compounds with AChE, BChE and BACE-1. The protein structures exported from the Protein Data Bank (PDB) for AChE were, 1E66 and 1GQR, and for BChE was 1P0I. The PDB structure used for docking of compounds 2h, 2p, 3b and 3p to BACE-1 was 4D8C. The compounds were drawn in discovery studio and docked into AChE, BChE or BACE-1 binding sites. The binding sites used to dock compounds represented co-crystalized ligand or substrate location and all other parameters were maintained as defaults for the docking. Prior to docking both protein and compound structures were prepared using the prepare protein and prepare ligand protocols in discovery studio, respectively. Docking resulted in at least ten poses per compound docked to each protein structure, the best scoring pose without unfavorable interactions was selected and represented as 2D plots using discovery studio.
4. Conclusions
The chalcone derivatives 2h, 2j, 2n and 2p exhibited moderate inhibitory effect against AChE activity when compared to donepezil, but increased inhibitory effect and selectivity against BChE. These compounds were also found to exhibit moderate to significant inhibitory effect against BACE-1. Compounds 2h and 2j in particular have potential to act as dual inhibitors against BChE and BACE-1 activities. The flavonol derivatives 3b and 3c appear to be more selective against AChE activity and exhibit significantly reduced inhibitory effect against BChE and moderate effect against BACE-1 activity. The flavonol derivatives 3k, 3l and 3p, on the other hand, tend to be more selective against BChE activity with modest inhibitory effect against AChE. Moderate and significant inhibitory effect were observed for the flavonols 3p and 3l against BACE-1 activity, respectively. Molecular docking studies into AChE, BChE and BACE-1 binding sites suggested electrostatic and hydrophobic interactions as the key factors that stabilize the enzyme-ligand complexes. The results of the kinetic studies of 2j and 3l on BACE-1 indicate a mixed mode of inhibition. The ortho-hydroxy-trans-α,β-unsaturated carbonyl framework makes the chalcones prepared in this investigation potential metal chelating pharmacophores that could widen their spectrum of biological activities. Selectivity against BChE has been suggested to be crucial with relation to inflammation, oxidative stress, and lipid metabolism [35]. Some of these chalcones and flavonol derivatives represent suitable candidates for further studies of biological activity as anti-inflammatory and/or antioxidant agents.
Acknowledgments
We are grateful to the University of South Africa and the National Research Foundation for financial assistance. We also thank the University of Stellenbosch Central Analytical Facility (CAF) and the University of the Witwatersrand for mass spectrometric and X-ray data, respectively.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/19/12/4112/s1.
Author Contributions
M.J.M. coordinated the study, reviewed the literature, interpreted the data and results, has conceptualized and written the manuscript. E.N.A. carried out the synthesis, acquired and analyzed the spectral data and performed the enzyme assays under the supervision of S.G. who performed molecular docking and contributed in the interpretation of the corresponding data.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hasan A., Khan M., Sher M., Maharvi G.M., Nawaz S.A., Choudhary M.I., Atta-Ur-Rahman, Supuran C.T. Synthesis and inhibitory potential towards acetylcholinesterase, butyrylcholinesterase and lipoxygenase of some variably substituted chalcones. J. Enz. Inh. Med. Chem. 2005;20:41–47. doi: 10.1080/14756360400015231. [DOI] [PubMed] [Google Scholar]
- 2.Jalili-Balej L., Babaei E., Abdpour S., Bukhari S.N.A., Oroumadi A., Ramazani A., Sharifzadeh M., Abdollahi M., Khoobi M. A review on flavonoid-based scaffolds as multi-target-directed ligands (MTDLs) for Alzheimer’s disease. Eur. J. Med. Chem. 2018;152:570–589. doi: 10.1016/j.ejmech.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Emmerzaal T.L., Kiliaan A.J., Gustafson D.R. 2003–2013: A decade of body mass index, Alzheimer’s disease, and dementia. J. Alzheimer’s Dis. 2015;43:739–755. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- 4.Gammon K. Neurodegenerative disease: Brain windfall. Nature. 2014;515:299–300. doi: 10.1038/nj7526-299a. [DOI] [PubMed] [Google Scholar]
- 5.Durães F., Pinto M., Sousa E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals. 2018;11:44. doi: 10.3390/ph11020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fosso M.Y., LeVine Rd H., Green K.D., Tsodikov O.V., Garneau-Tsodikova S. Effects of structural modifications on the metal binding, anti-amyloid activity, and cholinesterase inhibitory activity of chalcones. Org. Biomol. Chem. 2015;13:9418–9426. doi: 10.1039/C5OB01478F. [DOI] [PubMed] [Google Scholar]
- 7.Chuiko G., Podgornaya V., Zhelnin Y. Acetylcholinesterase and butyrylcholinesterase activities in brain and plasma of freshwater teleosts: Cross-species and cross-family differences. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003;135:55–61. doi: 10.1016/S1096-4959(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto H., Yamanishi Y., Limura H., Kawakami Y. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr. Med. Chem. 2000;7:303–317. doi: 10.2174/0929867003375191. [DOI] [PubMed] [Google Scholar]
- 9.McGleenon B.M., Dynan K.B., Passmore A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999;48:471–480. doi: 10.1046/j.1365-2125.1999.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raveh L., Grauver E., Grunwald J., Cohen E., Ashani Y. The stoichiometry of protection against soman and VX toxicity in monkeys pretreated with human butyrylcholinesterase. Toxicol. Appl. Pharm. 1997;145:43–53. doi: 10.1006/taap.1997.8160. [DOI] [PubMed] [Google Scholar]
- 11.Yu S.Q., Holloway H.W., Utsuki T., Brossi A., Greig N.H. Synthesis of novel phenserine-based-selective inhibitors of butyrylcholinesterase for Alzheimer’s disease. J. Med. Chem. 1999;42:1855–1861. doi: 10.1021/jm980459s. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Ayllon M.S., Small D.H., Avila J., Saez-Valero J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: Cross-talk with P.-tau and β-amyloid. Front. Mol. Neurosci. 2011;22:1–9. doi: 10.3389/fnmol.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillozet A.L., Smiley J.F., Mash D.C., Mesulam M.-M.C. Butyrycholinesterase in the life cycle of amyloid plaques. Ann. Neurol. 1997;42:909–918. doi: 10.1002/ana.410420613. [DOI] [PubMed] [Google Scholar]
- 14.Youn K., Park J.-H., Lee J., Jeong W.-S., Ho C.-T., Jun M. The identification of biochanin A as a potent and selective β-site app-cleaving enzyme 1 (Bace1) inhibitor. Nutrients. 2016;8:637. doi: 10.3390/nu8100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang J.E., Cho J.K., Curtis-Long M.J., Ryu H.W., Kim J.H., Kim H.J., Yuk H.K., Kim D.W., Park K.H. Inhibitory evaluation of sulfonamide chalcones on β-secretase and acylcholinesterase. Molecules. 2013;18:140–153. doi: 10.3390/molecules18010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaspar A., Matos M.J., Garrido J., Uriarte E., Borges F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014;114:4960–4992. doi: 10.1021/cr400265z. [DOI] [PubMed] [Google Scholar]
- 17.Rahman A., Ali M.T., Shawan M.M.A.K., Sarwar M.G., Khan M.A.K., Halim M.A. Halogen-directed drug design for Alzheimer’s disease: A combined density functional and molecular docking study. SpringerPlus. 2016;5:1346–1359. doi: 10.1186/s40064-016-2996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y., Shi T., Wang Y., Yang H., Yan X., Luo X., Jiang H., Zhu W. Halogen bonding—A novel interaction for rational drug design? J. Med. Chem. 2009;52:2854–2862. doi: 10.1021/jm9000133. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y., Liu Y., Xu Z., Li H., Liu H., Zhu W. Halogen bonding for rational drug design and new drug discovery. Expert Opin. Drug Discov. 2012;7:375–383. doi: 10.1517/17460441.2012.678829. [DOI] [PubMed] [Google Scholar]
- 20.Wilcken R., Zimmermann M.O., Lange A., Joerger A.C., Boeckler F.M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem. 2012;56:1363–1388. doi: 10.1021/jm3012068. [DOI] [PubMed] [Google Scholar]
- 21.Kolář M., Hobza P., Bronowska A. Plugging the explicit σ-holes in molecular docking. Chem. Comm. 2013;49:981–983. doi: 10.1039/C2CC37584B. [DOI] [PubMed] [Google Scholar]
- 22.CCDC 1880847. [(accessed on 28 November 2018)]; Available online: www.ccdc.cam.ac.uk/data_request/cif.
- 23.CCDC 1880859. [(accessed on 28 November 2018)]; Available online: www.ccdc.cam.ac.uk/data_request/cif.
- 24.Andersson C.D., Forsgren N., Akfur C., Allgardsson A., Berg L., Engdahl C., Qian W., Ekström F., Linusson A. Divergent structure-activity relationships of structurally similar acetylcholinesterase inhibitors. J. Med. Chem. 2013;56:7615–7624. doi: 10.1021/jm400990p. [DOI] [PubMed] [Google Scholar]
- 25.Rampa A., Bartolini M., Proccoli L., Naldi M., Iriepa I., Moraleda I., Belluti F., Gobbi S., Tarozzi A., Bisi A. Exploiting the chalcone scaffold to develop multifunctional agents for Alzheimer’s disease. Molecules. 2018;23:1902. doi: 10.3390/molecules23081902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballard C.G. Advances in the treatment of Alzheimer’s disease: Benefits of dual cholinesterase inhibition. Eur. Neurol. 2002;47:64–70. doi: 10.1159/000047952. [DOI] [PubMed] [Google Scholar]
- 27.Shimmyo Y., Kihara T., Akaike A., Niidome T., Sugimoto H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta. 2008;1780:819–825. doi: 10.1016/j.bbagen.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Wang B., Mai Y.C., Li Y., Hou J.Q., Huang S.L., Ou T.M., Tan J.H., An L.K., Li D., Gu L.Q., et al. Synthesis and evaluation of novel rutaecarpine derivatives and related alkaloids derivatives as selective acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2010;45:1415–1423. doi: 10.1016/j.ejmech.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 29.Khan M.T.H. Molecular interactions of cholinesterases inhibitors using in silico methods: Currents status and future prospects. New Biotech. 2009;25:331–346. doi: 10.1016/j.nbt.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Nicolet Y., Lockridge O., Masson P., Fontecilla-Camps J.C., Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- 31.Wang J., Wang Z.M., Li X.M., Li F., Wu J.-J., Kong L.-Y., Wang X.-B. Synthesis and evaluation of multi-target-directed ligands for the treatment of Alzheimer’s disease based on the fusion of donepezil and melatonin. Bioorg. Med. Chem. 2016;24:4324–4338. doi: 10.1016/j.bmc.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Shah M.V., Sethna S. Chromones and fluvones. Part, I. Iodination of 5- and 7-hydroxy -2-methylchromone. J. Chem. Soc. 1959:2676–2678. doi: 10.1039/jr9590002676. [DOI] [Google Scholar]
- 33.Ali S.M., Mohd I. Selective nuclear iodination of 2′-hydroxychalcones with iodine monochloride under basic conditions. Chem. Ind. 1986;12:426–427. doi: 10.1002/chin.198706150. [DOI] [Google Scholar]
- 34.Arduini F., Errico I., Amine A., Micheli L., Palleschi G., Moscone D. Enzymatic spectrophotometric method for aflatoxin B detection based on acetylcholinesterase inhibition. Anal. Chem. 2007;79:3409–3415. doi: 10.1021/ac061819j. [DOI] [PubMed] [Google Scholar]
- 35.Sridhar G.R., Rao A.A., Srinivas K., Nirmala G., Lakshmi G., Suryanarayna D., Rao P.V.N., Kaldhar D.G.S.V.G.L., Kumar S.V., Devi T.U., et al. Butyrylcholinesterase in metabolic syndrome. Med. Hypotheses. 2010;75:648–651. doi: 10.1016/j.mehy.2010.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.