Abstract
Proteins associated to the PI3K/AKT/mTOR signaling pathway are widely used targets for cancer treatment, and in recent years they have also been evaluated as putative targets in trypanosomatids parasites, such as Trypanosoma cruzi. Here, we performed a virtual screening approach to find candidates that can bind regions on or near the Pleckstrin homology domain of an AKT-like protein in T. cruzi. The compounds were also evaluated in vitro. The in silico and experimental results allowed us to identify a set of compounds that can potentially alter the intracellular signaling pathway through the AKT-like kinase of the parasite; among them, a derivative of the pyrazolopyridine nucleus with an IC50 of 14.25 ± 1.00 μM against amastigotes of T. cruzi. In addition, we built a protein–protein interaction network of T. cruzi to understand the role of the AKT-like protein in the parasite, and look for additional proteins that can be postulated as possible novel molecular targets for the rational design of compounds against T. cruzi.
Keywords: Trypanosoma cruzi, protein kinase B, pleckstrin homology domain, drug discovery
1. Introduction
Annually, around 40,000 new cases of people with Chagas disease are reported. This pathology is caused by a flagellated protozoan called Trypanosoma cruzi, which is transmitted to humans through a triatomine insect that functions as its vector. This parasite has two obligatory phases in the host, a replicative intracellular form called amastigote and a non-reproductive blood form called trypomastigote. Some of the effects of Chagas disease are alterations in the immune response, cardiomyopathy, and gastrointestinal disease. Two antiparasitic drugs, nifurtimox and benznidazole are used for treatment, but these have shown deficiencies associated to their toxicity and low effectiveness in the chronic phase, as well as reported cases of drug resistance [1,2,3]. Therefore, it is necessary to search for new, effective, and safe pharmacological alternatives against Chagas disease.
One strategy to design novel inhibitors is through the identification of novel molecular targets. For example, a subgroup of Ser/Thr kinases proteins called AGC kinases has been described as crucial for the regulation, growth, survival, differentiation, metabolism, and proliferation of cells. The protein kinase B (PKB or AKT) is part of this group [4,5]. There are numerous studies of the inhibition of PKB proteins and those present in the PI3K/AKT/mTOR signaling pathway, in order to control malignant cells and find better therapeutic alternatives for different types of cancer [6,7,8,9]. Some proteins from the PI3K/AKT/mTOR pathway are also studied for the control of some parasitic pathology. Recently, we reported our studies of recognition and inhibition of the Ld-RAC/AKT-like protein in Leishmania panamensis, suggesting that the AKT-like protein in Leishmania spp. could be a promising therapeutic target for treatments against Leishmaniasis [10,11,12,13].
The first report of PKB proteins in T. cruzi was made by Pascuccelli et al. in 1999. This protein is released in the three stages of the parasite life cycle, epimastigote (form present in the insect vector), amastigote, and trypomastigote, making it a promising drug target for the treatment of T. cruzi [5,14]. This protein contains in its amino terminus region a highly conserved domain called a Pleckstrin homology domain (PH), which briefly regulates the transport of crucial substrates (PI2P and PI3P) for the effective downstream signaling of proteins present in the PI3K/AKT/mTOR pathway. Therefore, this domain can be used to potentially control the PKB protein activity [15,16].
In recent years, computational methodologies have been used for drug discovery purposes in different diseases. Approaches such as virtual screening and molecular simulations, among others, allow the prediction of possible interactions between ligands and a receptor [17,18]. Here, we implemented computational tools to identify new compounds with possible preferences for pockets near the PH domain present in AKT-like proteins of T. cruzi. In addition, we built a potential protein–protein interaction network to understand the possible effects of inhibiting this protein in the parasite, and the identification of additional potential targets. Finally, we validated through in vitro assays some of the novel compounds with promising anti-Chagas activity.
2. Results
2.1. Modeling and Prediction of Pockets in the AKT-Like Protein of T. cruzi
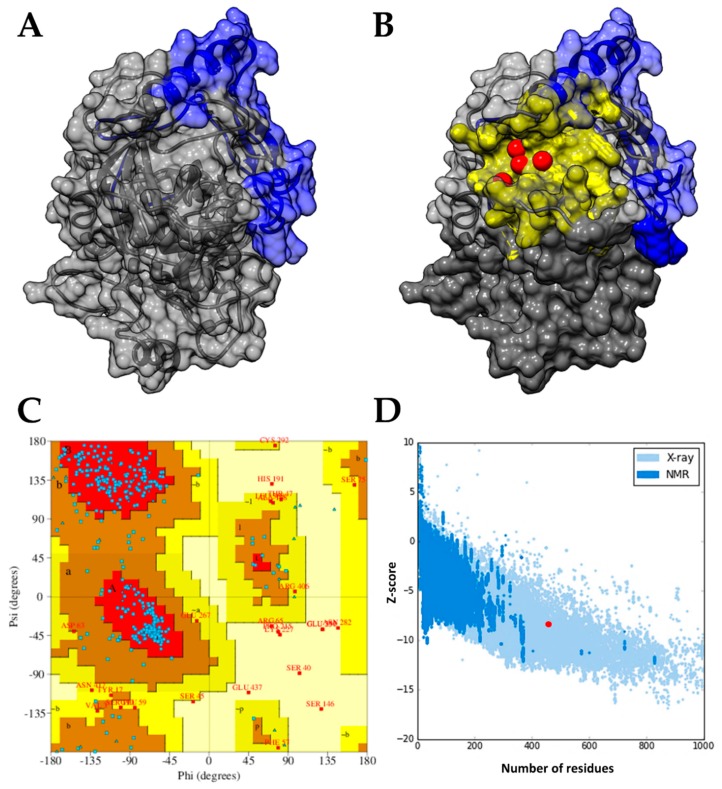
The model obtained for the TcAKT-like protein showed conserved structural folding between protein kinases. It contains the PH domain, which has a length of 99 amino acids, from Ser2 to Pro100 (Figure 1A). The stereochemistry of the model was examined with the Ramachandran plot, which suggests that 95% of the residues are in the favorable and allowed regions (Figure 1C). The overall quality of the model was evaluated with the Z-score. Figure 1D shows the Z-score values for all proteins determined experimentally with X-ray and NMR spectroscopy; the value obtained for the TcAKT-like protein model was −8.42, which is within the range of typical values for structures experimentally determined of the same amino acid length. These results suggest adequate folding and acceptable structural quality to continue with the drug discovery pipeline.
Figure 1.
(A) Structural model chosen of the TcAKT-like protein; the Pleckstrin homology (PH) domain is shown in blue, while the rest of the protein is shown in gray in a schematic way. (B) Selected drug pockets located near the PH domain of TcAKT-like predicted by the PockDrug (yellow surface) and metaPocket (red spheres) tools. (C) Analysis of the stereochemistry (angles Ψ and Φ) of the model selected for the TcAKT-like protein; 95% of the residues are in favored and allowed regions. (D) Evaluation of the overall quality of the TcAKT-like model; the model presented a Z-score (−8.42, highlighted with a red dot) similar to the structures of the same size resolved experimentally.
To detect possible pockets located near or on the PH domain of the TcAKT-like protein, the PockDrug and metaPocket web servers were used. The metaPocket results are the consensus of eight prediction tools, and in our case suggest a high probability that the pocket regions detected are potential ligand-binding sites. Four of the five predicted pockets were found located in the same superficial slit of the protein, near to the PH domain. These pockets are shown as red spheres in Figure 1B and represent the center of each predicted pocket [19]. The PockDrug web server allows the characterization of the predicted pockets and introduces a Druggability score. For the selected pocket, we obtained a Druggability score of 0.86 (from 0 to 1), suggesting a high probability of being a ligand-binding site in the TcAKT-like protein. The pocket predicted by PockDrug that is closer to the PH domain is shown in Figure 1B. This pocket is composed of approximately 57 residues, with a volume, diameter, and radius of 6620 Å3, 36.4 Å, and 18.2 Å, respectively.
2.2. Virtual Screening and Ligand Property Predictions
After the virtual screening, we obtained the best 1000 compounds, and the first 8 were selected for in silico structure activity relationship analysis and validation using in vitro assays. In addition, the compounds were subjected to a second molecular docking program as stated in the Methods section. The results suggest a similar trend in the affinity of the compounds for the predicted binding site, sharing also a similar ranking (Table 1). Figure 2 shows all the compounds docked to the TcAKT-like protein in the pocket region near the PH domain.
Table 1.
Predicted scores and possible toxicological risks of compounds selected against Trypanosoma cruzi.
| Compound | Predicted Scores (kcal/mol) | Predicted Toxicity | |
|---|---|---|---|
| AutoDock Vina | SwissDock | ||
| UBMC-1 | −10.6 | -8.61 | Positive |
| UBMC-2 | −10.3 | −8.33 | Positive |
| UBMC-3 | −10.0 | −7.41 | Positive |
| UBMC-4 | −9.9 | −8.41 | Positive |
| UBMC-5 | −9.8 | −7.9 | Negative |
| UBMC-6 | −9.8 | −7.63 | Negative |
| UBMC-7 | −9.7 | −7.07 | Positive |
| UBMC-8 | −9.3 | −7.27 | Negative |
| Benznidazole | −6.2 | −6.48 | Positive |
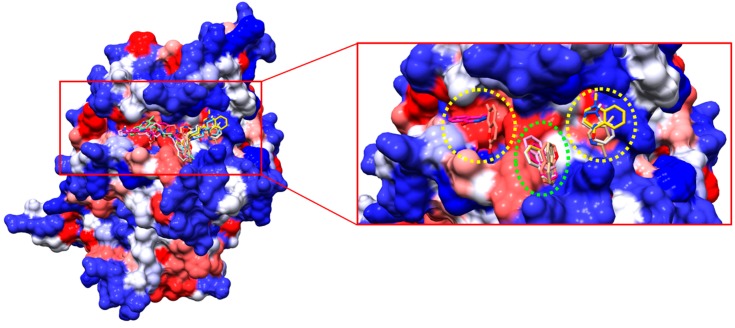
Figure 2.
Selected compounds of the virtual screening coupled in the predicted binding site for the TcAKT-like protein (left). Similar structural fragments between the coupled compounds. The naphthalene fragment could function as an anchor for each compound (right).
Visual inspection allowed us to find shared fragments among all the docked compounds. For example, compounds with heterocycles and aromatic rings with heteroatoms were mostly docked in the region demarcated in yellow circles (Figure 2). Groups such as naphthalene were docked in the region demarcated in green. This information can be taken as a starting point for further studies on the rational design of drugs based on the structure of the receptor.
Regarding the prediction of toxicological risk, an extensive report is available in the Supplementary information (Table S1), and a summary report is available in Table 1. The toxicity prediction results suggest that compounds UBMC-5, UBMC-6, and UBMC-8 would not present toxicological risks in future in vitro or in vivo experiments, while the other compounds are potentially associated with toxicological risks.
2.3. Results of the In Vitro Assay
The eight compounds evaluated had different effective IC50 concentrations, highlighting the compound UBMC-6 with 14.25 ± 1 μM, followed by the compound UBMC-8 with 18.26 ± 1.30 μM and UBMC-7 with 19.44 ± 0.35 μM; these three compounds had an effective concentration with high activity. However, their cytotoxicity (LC50) was considered high. The compounds UBMC-1 and UBMC-3 presented an IC50 of 34.92 ± 3.87 and 37.53 ± 2.65 μM, respectively, with moderate activity and high cytotoxicity, except for the compound UBMC-3 that was considered potentially noncytotoxic. Finally, compounds UBMC-2, UBMC-4, and UBMC-5 presented IC50 concentrations of 62.44 ± 4.62, 72.13 ± 8.58, and 69.82 ± 9.23 μM, respectively, which are the ones with the lowest activity and high cytotoxicity.
Although the order of the compounds according to the in vitro results (Table 2, from lowest to highest IC50 value) does not match the order of molecular docking results (Table 1, from lowest to highest binding score predicted in kcal/mol), all the compounds evaluated had in vitro activities below 75 μM. It should also be mentioned that the differences between the scores predicted by molecular docking are not large enough to correlate them to their in vitro activities, as evidenced with compound UBMC-6 that reported better in vitro activities than compounds such as UBMC-3, UBMC-4, and UBMC-5.
Table 2.
Results of the in vitro assays of the chosen compounds with possible anti-T. cruzi activity.
| Compound | IC50 (µM) a | LC50 (µM) b | SI c |
|---|---|---|---|
| UBMC-6 | 14.25 ± 1 | 34.11 ± 3.14 | 2.39 |
| UBMC-8 | 18.26 ± 1.30 | 55.2 ± 2.25 | 3.02 |
| UBMC-7 | 19.44 ± 0.35 | 62.3 ± 5.63 | 3.2 |
| UBMC-1 | 34.92 ± 3.87 | 61.66 ± 456 | 1.76 |
| UBMC-3 | 37.53 ± 2.65 | 197.9 ± 7.84 | 5.27 |
| UBMC-2 | 62.44 ± 4.62 | 44.5 ± 2.88 | 0.71 |
| UBMC-5 | 69.82 ± 9.23 | 43.25 ± 7.53 | 0.61 |
| UBMC-4 | 72.13 ± 8.58 | 70.88 ± 6.33 | 0.98 |
| Benznidazole | 16.61 ± 4 | >200 (µg/mL) | 12.04 |
| Doxorrubicin | NA d | 3.91 ± 0.40 | NC e |
a IC50 inhibitory concentration of T. cruzi amastigote; b LC50 lethal concentration on human monocyte-derived macrophages (hMDMD); c SI selectivity index between hMDMD and amastigote; d NA not applicable; e NC not calculated. Data represent the mean value ± standard deviation
2.4. Role of the AKT-Like Protein in a Reconstructed Protein–Protein Interaction Network of T. cruzi
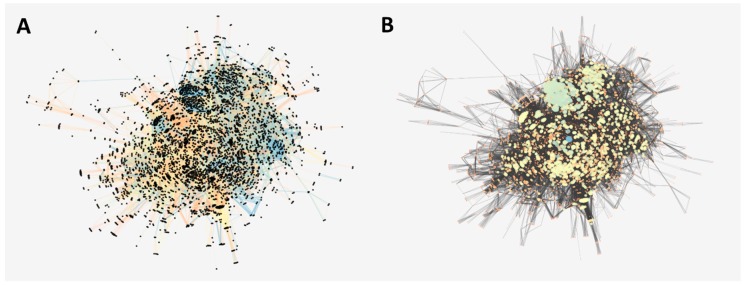
In order to understand, from a biological perspective, the role of the TcAKT-like protein, we reconstructed as much as possible the complete interactome of T. cruzi. We obtained a total 19,242 T. cruzi proteins derived from its whole-genome shotgun (WGS) genome project [20]. Then, we looked for all the inferred and validated interactions. Those with scores higher than 700 were filtered (Figure 3A). After that, the network represented 4910 proteins with 132,905 interactions.
Figure 3.
(A) Interaction network of T. cruzi strain CL Breiner. The nodes (proteins) are presented in black and the score associated with the interaction is presented in colors, blue being more reliable than orange. (B) Interaction network of T. cruzi strain CL Breiner. The axes (interactions) are represented in black and on a scale of orange (less connected) to blue (more connected) the nodes (proteins) of the network. The size of the circle indicates its degree of connectivity (degree).
The network reports a scale-free topology, indicating that highly connected proteins are located in the central region, as indicated in Figure 3B. After analyzing the degree of connectivity by number of nodes, we found that this distribution had a linear tendency behavior with a negative slope, with a R2 of 0.735 (unpublished figure). Although this is not a high value compared to other networks (probably due to lack of coverage), it is a sufficient condition to proceed with posterior topological calculations. We also observed a large number of nodes with a high degree of connectivity (Figure 3B).
2.5. Identification of Proteins Potentially Involved in the AKT-Like Pathway from the Parasite
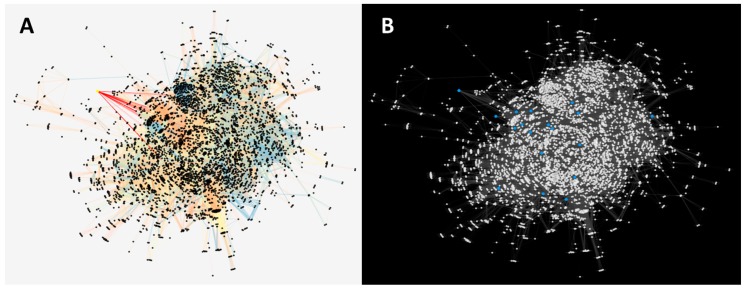
After obtaining a list of human proteins associated to the PI3K/AKT/mTOR pathway, we mapped them with proteins included in the protein–protein interaction network of T. cruzi, including TcAKT-like (Figure 4A). The complete results are reported in Supplementary Table S3. Topological properties were calculated, where the TcAKT-like yielded a degree value of 89 and a betweenness centrality of 0.002, which can be associated to a pleiotropic nature. The other proteins were also analyzed and visualized as blue nodes in Figure 4B. The proteins with their respective calculations of degrees of connectivity (degree) and betweenness centrality are reported in Table S4.
Figure 4.
(A) Protein–protein interaction network with the AKT-like protein signaled in yellow, where the axes that connect with the closest neighboring nodes (located in the center of the network) are highlighted in red. (B) Protein–protein interaction network with proteins represented as white nodes, and in blue are those that were mapped with the PI3K/AKT/mTOR pathway of human proteins.
Most of the proteins reported connectivity data within the mean and are also relatively central to certain pathways within the network. Particularly, we found two orthologs of the AKT3 and AKT2 human proteins, where one of them (highlighted in bold in Table S4) presents the conserved regions of the predicted AKT-like (including the PH domain), with an additional zinc finger domain, which can be also potentially explored as another allosteric site of the protein in T. cruzi. The topological calculation, after excluding the proteins associated with ribosomal units let us obtain a ranking of 30 proteins with the highest values. This list (Supplementary Table S5) provides interesting information for finding new pharmacological targets against T. cruzi. Among them, we found DNA topoisomerase 2 proteins [21], GMP synthase [22] and dihydrofolate reductase–thymidylate synthase [23], which have been previously studied as molecular targets for the treatment of Chagas disease.
3. Discussion
In recent years, there has been interest in postulating drug targets in parasites that has been related to gene expression, cytoskeleton, membrane integrity, intermediates in metabolism, and intracellular signaling, which is the case of the AKT-like protein in T. cruzi. There is information that shows the relationship of proteins such as PI3K and AKT with the survival and defense (anti-apoptotic effect) in trypanosomatid parasites [10,24,25]. Commonly, the AKT proteins or proteins within the PI3K/AKT/mTOR signaling pathway are the target of study for the development of pharmacological therapies against several types of cancer, since it has been demonstrated that these proteins participate in biological processes which are key for the cell, such as growth, differentiation, and survival [26]. The PH domain of the AKT proteins plays a crucial role, since it is responsible for the identification and transport of lipid substrates as phosphatidylinositol bisphosphate (PI2P) and phosphatidylinositol triphosphate (PI3P) produced by PI3K protein. These substrates are necessary for the correct intracellular signaling downstream. Therefore, AKT becomes a mediator of the activity of PI3K and is key for the control of this type of intracellular pathways [27,28].
Three PKB or AKT proteins have been found in humans with high sequence identity among them, and linked to molecular functions involved in metabolism, apoptosis, and proliferation. It has also been described that there are diverse cellular locations of these proteins in mice and humans, suggesting different routes of action [29,30]. To date, there is no information of AKT orthologs in T. cruzi. However, based on the above information, and the fact that we found one AKT-like protein different (UniProtKB ID Q4DL90) to the AKT-like protein suggested by us (UniProtKB ID Q4D6D3) [11], we propose that in T. cruzi, isoforms of this type of proteins could exist that can be associated with different cellular processes within the parasite. In addition, both proteins belong to different sections of the parasite genome (contigs) that has not been completely assembled, but with enough differences to consider them two individual gene products [31]. For example, the two sequences have an overall 45% of identity, but both shared the same functional domains (PH domain, kinase domain, and AGC kinase domain). However, part of the divergence is related to a zinc fingers domain found in one of the AKT-like proteins (UniProtKB ID Q4DL90) (Figure S1). This domain is categorized as a transporter of PI3P and key factor for the synthesis of ribosomal RNA, thus becoming regulatory factors of transcription processes in eukaryotic cells [32,33]. Something interesting is that zinc fingers domains are not present in any of the three human AKT proteins, and the sequence found in TcAKT-like has a maximum identity of 41% with zinc fingers domains of humans. The above clearly opens the possibility to venture into the rational design of compounds that take advantage of these differences between the parasite and the human.
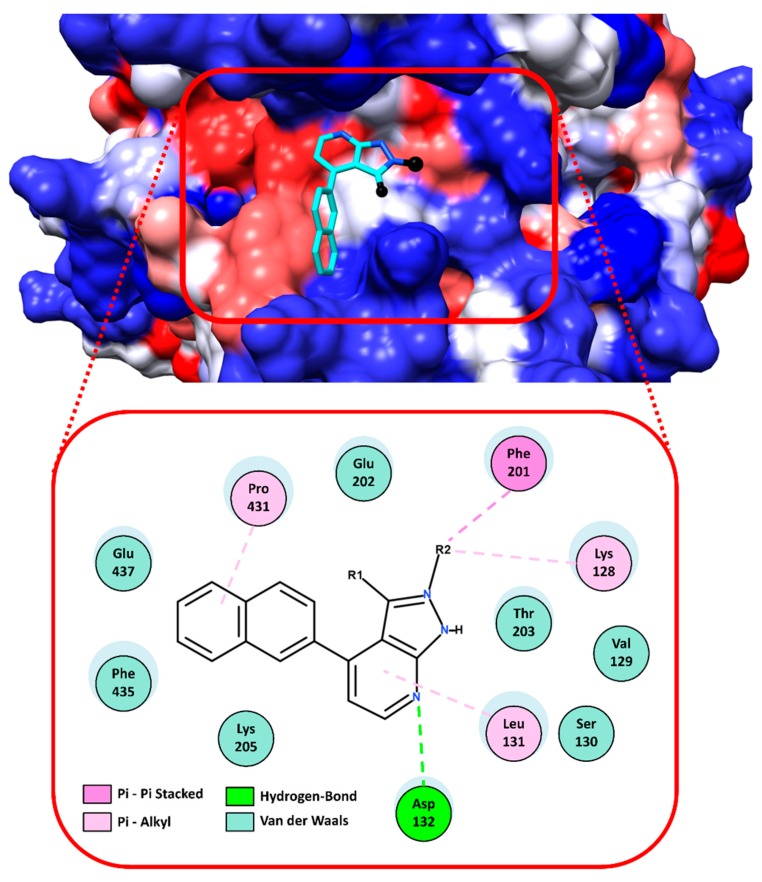
Regarding the virtual screening approach, it allowed us to select a group of eight compounds with potential affinity for a pocket close to the PH domain of the TcAKT-like protein (UniProtKB ID Q4D6D3). According to the in vitro results reported in Table 2, the interactions and the structural activity relationship of the UBMC-6 compound were analyzed, since it was the compound that presented the best activity against amastigotes of T. cruzi. The 2D interactions of the compound UBMC-6 are shown in Figure 5. The fragments R1 and R2 correspond to hydrophilic and aromatic rings substituted with heteroatoms groups, respectively. These fragments would facilitate the generation of hydrophobic interactions, Pi interactions, and possible hydrogen bonds. It is important to clarify that the UBMC-6 scaffold is shared with other compounds from the list, but their chemical structures are not depicted because they are currently subjected to additional SAR studies and patent evaluations.
Figure 5.
Interactions between the UBMC-6 compound and the TcAKT-like protein. In the upper part, the docking of the compound in the predicted binding site is observed in 3D. The compound UBMC-6 is shown in cyan color and its fragments called R1 and R2 are shown in black; the protein is shown on a hydrophobic surface, where red is hydrophobic and blue is hydrophilic. In the lower part, the 2D interactions between the UBMC-6 compound and the TcAKT-like protein are shown. The blue shadows on the amino acids represent that they are or not on the surface of the protein; the larger the size, the more exposed it is.
The compound UBMC-6 has in its structure a pyrazolopyridine core that have been previously assayed against T. cruzi amastigotes, with an IC50 of 10.47 μM [34]. In addition, its structural analogue pyrazolopyrimidine has presented promising effects against apicomplexan parasites, such as Neospora caninum and Toxoplasma gondii, acting on the protein kinase CDPK in both cases [35,36]. Some analog compounds of the pyrazolopyridine nucleus have already been used in therapies against cancers, targeting the PH domain of the AKT proteins [37]. These data suggest that the compound UBMC-6 could be a clear candidate for the study of new derivatives based on its structure. As a complementary objective of this investigation, the biological activity against Leishmania braziliensis of the selected compounds was also evaluated. Interestingly, the compound UBMC-6 was also the one with the best results (i.e., IC50 of 18.71 μM). The results of these in vitro tests are summarized in Table S2. We are aware that the experimental validations were measured on cells and not directly on the recombinant protein. However, this is a first validation result against the parasite that will be crucial for further experiments able to demonstrate the direct interaction of the compounds with the AKT-like protein of T. cruzi and L. braziliensis.
Another conclusion of the docked compounds is that while they do not interact directly with any residue of the PH domain, they do interact with residues from a pocket close to it. These interactions could cause possible allosteric inhibition of the TcAKT-like protein. The discovery of possible allosteric sites opens the opportunity to design new inhibitors against TcAKT-like, increasing the specificity and reducing the classic risks of drug promiscuity. It is evident that more robust molecular studies, both experimental and computational, are necessary to clarify the possible effect of UBMC-6 on the TcAKT-like protein.
Finally, regarding other potential molecular targets from the protein–protein interaction network of T. cruzi, we can highlight the topoisomerase 2 protein, supported also by previous studies of this protein as a molecular target for antibacterial and anticancer treatment [38,39]. They have also been inhibited by compounds derived from the quinolone core, such as ciprofloxacin, levofloxacin, norfloxacin, and moxifloxacin, among others [40,41,42]. Interestingly, these compounds with antibacterial activity have shown to be promising to explore possible antiparasitic treatments [43,44].
4. Materials and Methods
4.1. Structural Modeling of the AKT-Like Protein of T. cruzi
The structural model of the AKT protein was obtained by a threading modeling approach using the I-TASSER web server (https://zhanglab.ccmb.med.umich.edu/I-TASSER/) [45]. The amino acid sequence of the T. cruzi AKT-like protein (TcAKT-like) was obtained from UniProtKB with id Q4D6D3 [11]. The quality of the structural model was inspected using PDBsum (http://www.ebi.ac.uk/thornton-srv/databases/pdbsum/Generate.html) [46] and ProSA–web (https://prosa.services.came.sbg.ac.at/prosa.php) [47]. The metaPocket 2.0 (http://projects.biotec.tu-dresden.de/metapocket/) [19] and PockDrug (http://pockdrug.rpbs.univ-paris-diderot.fr) [48] web servers were used to predict possible cavities in the protein structure, prioritizing those located near or on their Pleckstrin Homology domain (PH). The predicted cavities were used as reference for the virtual screening.
4.2. Virtual Screening and Molecular Docking Simulations
The virtual screening was performed using the DrugDiscovery@TACC web portal (https://drugdiscovery.tacc.utexas.edu) [17] of the Texas Advanced Computing Center (TACC). The selected compound library contains ~640,000 drug-like molecules previously filtered. The receptor and the ligand structures were prepared following the protocol of software Autodock Tools v1.5.6 [49], which consists of adding polar hydrogens, calculating partial charges, and for the ligands, assigning rotatable bonds to confer flexibility. The screening was configured to search ligand poses in a box of 26 Å3 that covers the PH domain.
The first 8 compounds were selected for performing an additional molecular docking with the Swiss-Dock web server (http://www.swissdock.ch/) [50]. The two scoring functions from AutoDock Vina [51] and SwissDock were used in order to improve the predicted affinities and correlate them with the in vitro tests results.
4.3. In Silico Prediction of Toxicological Risks
Predictions of some toxicological risks for the selected compounds were made using the software DataWarrior v4.7.2 [52] and the web servers ProToxII (http://tox.charite.de/protox_II/) [53] and CarcinoPred-EL (http://ccsipb.lnu.edu.cn/toxicity/CarcinoPred-EL) [54]. The predicted toxic risks were Irritant, Mutagenic, Reproductive, Tumorigenic, Carcinogenic, Cytotoxic, and Immunotoxic effects. Compounds without any risk after the prediction of toxicity were classified as Negative, and those with at least one risk of toxicity were classified as Positive. Those compounds with a Negative classification have a higher probability to be effective in subsequent in vitro and in vivo experiments.
4.4. In Vitro Assay of the Antitrypanosomal Activity of the UBMC Compounds
The compounds were purchased from Molport (https://www.molport.com). The molecules were renamed using the UBMC tag in increasing order and solubilized in DMSO at a stock concentration of 20 mM.
A human promonocitic U937 line was cultivated in RPMI-1640 medium with 10% fetal bovine serum (FBS) at a concentration of 0.25 × 106 cells/mL; the cells were transformed into macrophages by being incubated with phorbol 12-myristate 13-acetate (PMA) at 0.1 µg/mL. To adhere the cells, 0.1 mL of cells was added in 96-well dishes. Adherence was carried out for 72 h, and then epimastigote were added in the stationary phase of the Tulahuen strain producing β-galactosidase, at 5 parasite/cellU937 concentrations for 24 h at 37 °C with 5% CO2. To calculate the IC50 (Table 2), different concentrations of the compounds to evaluate and Benznidazole were added at an initial concentration of 20 µg/mL and 6 serial dilutions were made as a control of the effectiveness of the experiment, in addition to the control of the viability of the parasites (cells without drug treatment), from the blank of the culture medium (medium without cells), in duplicate. The cells were incubated for 72 h at 37 °C with 5% of CO2. All medium was removed and 100 µL of substrate for β-galactosidase diluted in PBS (chlorophenol β-d-galactopyranoside-CPRG network at 100 mM and 0.1% Nonidet P-40) was added to each well and incubated at 37 °C for 3 h. The colorimetric reaction was read at 570 nm and optical densities (OD) for each experimental condition were registered. The in vitro antitrypanosomal activity was defined by the reduction of the amount of amastigotes in infected cells (percentage of infection) following formula: (%) inhibition of infection = 1 − [(OD treated cells/OD untreated cells) × 100], where the OD of untreated cells corresponds to 100% of the infection. The OD of the blank was subtracted from the culture medium. The half inhibitory concentration (IC50) was calculated by the Probit method using the % of inhibition for each concentration [55].
The antitrypanosomal activity of each compound was rated according to its IC50. Thus, IC50 values <25 µM showed high activity, whereas IC50 > 25 µM and < 50 µM had moderated activity, and IC50 > 50 µM had low activity.
4.5. In Vitro Assay of Cytotoxicity of UBMC Compounds
The cytotoxicity of the compounds was evaluated according to the ability to kill macrophages derived from human monocytes (hMDM) by the macrophage-to-myofibroblast transition (MMT) method, following the procedures performed by Pastrana Restrepo et al. [56]. The hMDM were obtained from 50 mL of desfribrinated whole blood from healthy donors. These samples were mixed in a 1:1 ratio with Dulbecco PBS free of calcium and magnesium (DPBS). The mixture was centrifuged in a Ficoll Hypaque 1077 density gradient in a 1:3 ratio (blood–Ficoll) for separation of mononuclear cells, centrifuging at 2000 rpm for 20 min at 37 °C. The mononuclear cell layer was separated and these cells were washed twice with a solution of DPBS centrifuged at 13000 rpm for 10 min. After the last wash, the cells were resuspended in an RPMI medium with 10% autologous serum at a concentration of 0.3 × 106 cells/mL. Then, 1 mL of cells was placed in each well of 24-well culture dishes and incubated at 37 °C, 5% CO2 for 72 h to allow the differentiation of monocytes to macrophages. For the MTT test, the hMDM were adjusted to a concentration of 0.5 × 106 cells/mL in the RPMI medium supplemented with 10% FBS, and the concentration of the compound in the culture was adjusted (four serial dilutions starting at 200 μM). Amphotericin B and Doxorubicin were used as controls for the determination of cytotoxicity. Each experiment was performed in triplicate.
In this case, the in vitro cytotoxicity was defined by the decrease in the cell viability and growth, obtained from the OD for each experimental condition, using the following formula: (%) viability inhibition = 1 − [(OD treated cells/OD untreated cells) × 100], where the OD of untreated cells corresponds to 100% of the viability. Growth inhibition percentage data obtained for each experimental condition were used to calculate the half lethal concentration (LC50) by the Probit analysis [55].
The cytotoxicity of each compound was rated according to its LC50. Thus, LC50 values <100 μM were considered highly cytotoxic, whereas LC50 > 100 μM and < 200 μM were considered moderately cytotoxic and LC50 > 200 μM were considered as potentially noncytotoxic.
4.6. Reconstruction of the Protein–Protein Interaction Network of T. cruzi
A set of proteins corresponding to the CL Brener strain of T. cruzi was obtained from the UniProtKB database [57]. In parallel, information about protein–protein interactions in T. cruzi and species related to the genus trypanosomatidae (Trypanosoma and Leishmania) was downloaded from multiple repositories associated to the Intact database [58]. Interactions directly implicated with T. cruzi or previously predicted as interologs of other species were added directly to the network. For the other species, an ortholog inference analysis was performed to predict the presence of these interactions in T. cruzi. The Cytoscape v3.6.1 software was used for the visualization of the interaction networks [59]. In addition, the degree of connectivity by number of nodes was analyzed to study the scale-free topology of the built network.
4.7. Detection of Orthologs between T. cruzi and Those Reported in the PI3/AKT/mTOR Pathway
Given the important role of the AKT protein in signaling pathways in the host and in the enzymatic machinery provided by the parasite, a mapping of the proteins reported in the PI3K/AKT/mTOR pathway with the set of T. cruzi CL Breiner strain proteins was carried out. The pathway components were obtained from the KEGG database [60]. The comparison between sequences was done with a Best Reciprocal Hits protocol using the BLAST algorithm. Additionally, we calculated different topological metrics, including the degree of connectivity for each node, using the Network Analyzer plugin of Cytoscape v3.6.1 [59]. We also identified and suggested other proteins within the PI3K/AKT/mTOR signaling pathway that interact closely with the AKT protein of T. cruzi, and consequently, can be considered as molecular targets for further drug discovery studies against T. cruzi.
5. Conclusions
The computational structure-based strategy followed in this work was essential to identify a possible pocket near the PH domain of the TcAKT-like protein, and subsequently identify compounds able to interact on the site, which were evaluated through in vitro tests. These findings could facilitate the design of effective compounds against trypanosomatids such as T. cruzi in future research. Although most of the compounds presented considerable cytotoxicity on host cells, compounds with better activities and low cytotoxicity can be taken as a starting point for the rational design of therapeutic agents against Chagas disease. In addition, based on the analysis of protein–protein interaction, we can conclude that the TcAKT-like protein of T. cruzi is a promising candidate to be a molecular target for this and similar parasites.
Acknowledgments
Authors thank L.M. Yepes for technical support.
Abbreviations
| PKB | Protein kinase B |
| RAC | Related to A- and C-kinases |
| PH | Pleckstrin homology domain |
| PI2P | Phosphatidylinositol bisphosphate |
| PI3P | Phosphatidylinositol trisphosphate |
| TcAKT-like | AKT-like protein of Trypanosoma cruzi |
| OD | Optical density |
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/19/12/3951/s1.
Author Contributions
The authors R.O., C.R.-R., M.M.-V., and R.E.V.-M. participated in the design of the research. R.O. and C.R.-R. performed computational experiments. S.M.R. performed in vitro experiments. R.O., C.R.R., M.M.-V., and R.E.V.-M. participated in the analysis of results and wrote the manuscript. All authors read and approved the final version of this manuscript.
Funding
This research was funded by COLCIENCIAS grant number (111571250689) and Universidad Santiago de Cali grant number (DGI-912-621116-C9).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kim J.H., Ryu W., Shim H., Park H. Development of new and selective Trypanosoma cruzi trans-sialidase inhibitors from sulfonamide chalcones and their derivatives. Chembiochem. 2009;10:2475–2479. doi: 10.1002/cbic.200900108. [DOI] [PubMed] [Google Scholar]
- 2.Khare S., Liu X., Stinson M., Rivera I., Groessl T., Tuntland T., Yeh V., Wen B., Molteni V., Glynne R., et al. Antitrypanosomal treatment with benznidazole is superior to posaconazole regimens in mouse models of chagas disease. Antimicrob. Agents Chemother. 2015;59:6385–6394. doi: 10.1128/AAC.00689-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabelli S.B., McLellan J.S., Montalvetti A., Oldfield E., Docampo R., Amzel L.M. Structure and mechanism of the farnesyl diphosphate synthase from Trypanosoma cruzi: Implications for drug design. Proteins. 2005;62:80–88. doi: 10.1002/prot.20754. [DOI] [PubMed] [Google Scholar]
- 4.Fayard E., Tintignac L.A., Baudry A., Hemmings B.A. Protein Kinase B/Akt at a Glance. J. Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- 5.Bachmaier S., Boshart M. Protein Phosphorylation in Parasites: Novel Targets for Antiparasitic Intervention. Willey; Hoboken, NJ, USA: 2013. Kinetoplastid AGC kinases; pp. 99–122. [Google Scholar]
- 6.Mabuchi S., Kuroda H., Takahashi R., Sasano T. The PI3K/AKT/MTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 2015;137:173–179. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson K.M., Anderson N.G. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 2002;14:381–395. doi: 10.1016/S0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 8.Prêtre V., Wicki A. Inhibition of Akt and other AGC kinases: A target for clinical cancer therapy? Semin. Cancer Biol. 2018;48:70–77. doi: 10.1016/j.semcancer.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Leroux A.E., Schulze J.O., Biondi R.M. AGC Kinases, Mechanisms of regulation and innovative drug development. Semin. Cancer Biol. 2018;48:1–17. doi: 10.1016/j.semcancer.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Tirado-Duarte D., Marín-Villa M., Ochoa R., Blandón-Fuentes G., Soares M.J., Robledo S.M., Varela-Miranda R.E. The Akt-like kinase of Leishmania panamensis: As a new molecular target for drug discovery. Acta Trop. 2018;177:171–178. doi: 10.1016/j.actatropica.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Varela R.E.M., Ochoa R., Muskus C.E., Muro A., Mollinedo F. Identification of a RAC/AKT-like gene in Leishmania parasites as a putative therapeutic target in leishmaniasis. Parasit. Vectors. 2017;10:1–10. doi: 10.1186/s13071-017-2379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva M.C., Davoli-Ferreira M., Medina T.S., Sesti-Costa R., Silva G.K., Lopes C.D., Cardozo L.E., Gava F.N., Lyroni K., Dias F.C., et al. Canonical PI3Kγ signaling in myeloid cells restricts Trypanosoma cruzi infection and dampens chagasic myocarditis. Nat. Commun. 2018;9:1513. doi: 10.1038/s41467-018-03986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Márquez J.D.R., Ana Y., Baigorrí R.E., Stempi C.C., Cerban F.M. Mammalian target of rapamycin inhibition in Trypanosoma cruzi-infected macrophages leads to an intracellular profile that is detrimental for infection. Front. Immunol. 2018;9:313. doi: 10.3389/fimmu.2018.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascuccelli V., Labriola C., Téllez-Iñón M.T., Parodi A.J. Molecular and biochemical characterization of a protein kinase B from Trypanosoma cruzi. Mol. Biochem. Parasitol. 1999;102:21–33. doi: 10.1016/S0166-6851(99)00076-6. [DOI] [PubMed] [Google Scholar]
- 15.Scheffzek K., Welti S. Pleckstrin homology (PH) like domains—Versatile modules in protein-protein interaction platforms. FEBS Lett. 2012;586:2662–2673. doi: 10.1016/j.febslet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Ahad A.M., Zuohe S., Du-Cuny L., Moses S.A., Zhou L.L., Zhang S., Powis G., Meuillet E.J., Mash E.A. Development of sulfonamide AKT PH domain inhibitors. Bioorganic Med. Chem. 2011;19:2046–2054. doi: 10.1016/j.bmc.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viswanathan U., Tomlinson S.M., Fonner J.M., Mock S.A., Watowich S.J. Identification of a novel inhibitor of dengue virus protease through use of a virtual screening drug discovery web portal. J. Chem. Inf. Model. 2014;54:2816–2825. doi: 10.1021/ci500531r. [DOI] [PubMed] [Google Scholar]
- 18.Sliwoski G., Kothiwale S., Meiler J., Lowe E.W. Computational methods in drug discovery. Pharmacol. Rev. 2013;66:334–395. doi: 10.1124/pr.112.007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z., Li Y., Lin B., Schroeder M., Huang B. Identification of cavities on protein surface using multiple computational approaches for drug binding site prediction. Bioinformatics. 2011;27:2083–2088. doi: 10.1093/bioinformatics/btr331. [DOI] [PubMed] [Google Scholar]
- 20.Luchtan M. TcruziDB: An integrated Trypanosoma cruzi genome resource. Nucleic Acids Res. 2004;32:344–346. doi: 10.1093/nar/gkh049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balaña-Fouce R., Álvarez-Velilla R., Fernández-Prada C., García-Estrada C., Reguera R.M. Trypanosomatids topoisomerase re-visited. new structural findings and role in drug discovery. Int. J. Parasitol. Drugs Drug Resist. 2014;4:326–337. doi: 10.1016/j.ijpddr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q., Leija C., Rijo-ferreira F., Chen J., Cestari I., Tu B.P., Phillips M.A. GMP synthase is essential for viability and infectivity of Trypanosoma brucei despite a redundant purine salvage pathway. Mol. Microbiol. 2016;97:1006–1020. doi: 10.1111/mmi.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senkovich O., Schormann N., Chattopadhyay D. Structures of Dihydrofolate Reductase-Thymidylate Synthase of Trypanosoma cruzi in the Folate-Free State and in Complex with Two Antifolate Drugs, Trimetrexate and Methotrexate. Acta Crystallogr. Sect. D Biol. 2009;65:704–716. doi: 10.1107/S090744490901230X. [DOI] [PubMed] [Google Scholar]
- 24.Wilkowsky S.E., Barbieri M.A., Stahl P., Isola E.L.D. Trypanosoma Cruzi: Phosphatidylinositol 3-kinase and protein kinase b activation is associated with parasite invasion. Exp. Cell Res. 2001;264:211–218. doi: 10.1006/excr.2000.5123. [DOI] [PubMed] [Google Scholar]
- 25.Chuenkova M.V., Furnari F.B., Cavenee W.K., Pereira M.A. Trypanosoma cruzi trans-sialidase: A potent and specific survival factor for human schwann cells by means of phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. USA. 2001;98:9936–9941. doi: 10.1073/pnas.161298398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X., Dai D., Chen B., Tang H., Xie X., Wei W. Efficacy of PI3K/AKT/MTOR pathway inhibitors for the treatment of advanced solid cancers: A literature-based meta-analysis of 46 randomised control trials. PLoS ONE. 2018;13:1–18. doi: 10.1371/journal.pone.0192464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahadevan D., Powis G., Mash E.A., George B., Gokhale V.M., Zhang S., Shakalya K., Du-Cuny L., Berggren M., Ali M.A., et al. Discovery of a novel class of AKT pleckstrin homology (PH) domain inhibitors. Mol. Cancer Ther. 2008;7:2621–2632. doi: 10.1158/1535-7163.MCT-07-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa B., Dangate M., Vetro M., Donvito G., Gabrielli L., Amigoni L., Cassinelli G., Lanzi C., Ceriani M., de Gioia L., et al. Synthetic sulfoglycolipids targeting the serine–threonine protein kinase Akt. Bioorganic Med. Chem. 2016;24:3396–3405. doi: 10.1016/j.bmc.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Osaki M., Oshimura M., Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 30.Franke T.F. Intracellular signaling by Akt: Bound to be specific. Sci. Signal. 2008;1:1–5. doi: 10.1126/scisignal.124pe29. [DOI] [PubMed] [Google Scholar]
- 31.Logan-Klumpler F.J., de Silva N., Boehme U., Rogers M.B., Velarde G., McQuillan J.A., Carver T., Aslett M., Olsen C., Subramanian S., et al. GeneDB-an annotation database for pathogens. Nucleic Acids Res. 2012;40:98–108. doi: 10.1093/nar/gkr1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albà M.M. Zinc-finger domains in metazoans: Evolution gone wild. Genome Biol. 2017;18:168. doi: 10.1186/s13059-017-1307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cassandri M., Smirnov A., Novelli F., Pitolli C., Agostini M., Malewicz M., Melino G., Raschellà G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017;3:17071. doi: 10.1038/cddiscovery.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvador R.R.S., Bello M.L., Barreto I.R.L., Vera M.A.F., Muri E.M.F., de Albuquerque S., Dias L.R.S. New carbohydrazide derivatives of 1H-Pyrazolo[3,4-b]Pyridine and trypanocidal activity. An. Acad. Bras. Cienc. 2016;88:2341–2348. doi: 10.1590/0001-3765201620160087. [DOI] [PubMed] [Google Scholar]
- 35.Ojo K.K., Reid M.C., Siddaramaiah L.K., Müller J., Winzer P., Zhang Z., Keyloun K.R., Vidadala R.S.R., Merritt E.A., Hol W.G.J., et al. Neospora caninum calcium-dependent protein kinase 1 is an effective drug target for neosporosis therapy. PLoS ONE. 2014;9:e92929. doi: 10.1371/journal.pone.0092929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutaganira F.U., Barks J., Dhason M.S., Wang Q., Lopez M.S., Long S., Radke J.B., Jones N.G., Maddirala A.R., Janetka J.W., et al. Inhibition of calcium dependent protein kinase 1 (CDPK1) by pyrazolopyrimidine analogs decreases establishment and reoccurrence of central nervous system disease by Toxoplasma gondii. J. Med. Chem. 2017;60:9976–9989. doi: 10.1021/acs.jmedchem.7b01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nitulescu G.M., Margina D., Juzenas P., Peng Q., Olaru O.T., Saloustros E., Fenga C., Spandidos D.A., Libra M., Tsatsakis A.M. Akt Inhibitors in cancer treatment: The long journey from drug discovery to clinical use. Int. J. Oncol. 2016;48:869–885. doi: 10.3892/ijo.2015.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan T., Sankhe K., Suvarna V., Sherje A., Patel K., Dravyakar B. DNA gyrase inhibitors: Progress and synthesis of potent compounds as antibacterial agents. Biomed. Pharmacother. 2018;103:923–938. doi: 10.1016/j.biopha.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 39.Ortega J.-A., Riccardi L., Minniti E., Borgogno M., Arencibia J.M., Greco M.L., Minarini A., Sissi C., de Vivo M. Pharmacophore hybridization to discover novel topoisomerase II poisons with promising antiproliferative activity. J. Med. Chem. 2017;61:1375–1379. doi: 10.1021/acs.jmedchem.7b01388. [DOI] [PubMed] [Google Scholar]
- 40.Naeem A., Badshah S.L., Muska M., Ahmad N., Khan K. The current case of quinolones: Synthetic approaches and antibacterial activity. Molecules. 2016;21:268. doi: 10.3390/molecules21040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocha-Roa C., Cossio-Pérez R., Molina D., Patiño J., Cardona N. In silico study of moxifloxacin derivatives with possible antibacterial activity against a resistant form of DNA gyrase from Porphyromonas gingivalis. Arch. Oral Biol. 2018;95:30–39. doi: 10.1016/j.archoralbio.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Paul M., Gafter-Gvili A., Fraser A., Leibovici L. The anti-cancer effects of quinolone antibiotics? Eur. J. Clin. Microbiol. Infect. Dis. 2007;26:825–831. doi: 10.1007/s10096-007-0375-4. [DOI] [PubMed] [Google Scholar]
- 43.Dalhoff A. Antiviral, antifungal, and antiparasitic activities of fluoroquinolones optimized for treatment of bacterial infections: A puzzling paradox or a logical consequence of their mode of action? Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:661–668. doi: 10.1007/s10096-014-2296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martins-Duarte E.S., Dubar F., Lawton P., Da Silva C.F., Soeiro M.D.N.C., de Souza W., Biot C., Vommaro R.C. Ciprofloxacin derivatives affect parasite cell division and increase the survival of mice infected with Toxoplasma gondii. PLoS ONE. 2015;10:1–23. doi: 10.1371/journal.pone.0125705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008;9:1–8. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laskowski R.A., Jabłońska J., Pravda L., Vařeková R.S., Thornton J.M. PDBsum: Structural summaries of PDB entries. Protein Sci. 2018;27:129–134. doi: 10.1002/pro.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiederstein M., Sippl M.J. ProSA-Web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:407–410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borrel A., Regad L., Xhaard H., Petitjean M., Camproux A.C. PockDrug: A model for predicting pocket druggability that overcomes pocket estimation uncertainties. J. Chem. Inf. Model. 2015;55:882–895. doi: 10.1021/ci5006004. [DOI] [PubMed] [Google Scholar]
- 49.Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., Olson A.J. Automated docking using a lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- 50.Grosdidier A., Zoete V., Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39:270–277. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sander T., Freyss J., Von Korff M., Rufener C. Datawarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015;55:460–473. doi: 10.1021/ci500588j. [DOI] [PubMed] [Google Scholar]
- 53.Banerjee P., Eckert A.O., Schrey A.K., Preissner R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018;46:W257–W263. doi: 10.1093/nar/gky318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L., Ai H., Chen W., Yin Z., Hu H., Zhu J., Zhao J., Zhao Q., Liu H. CarcinoPred-EL: Novel models for predicting the carcinogenicity of chemicals using molecular fingerprints and ensemble learning methods. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-02365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finney D.J. Statistical Method in Biological Assay. Charles Griffin and Company; London, UK: 1952. [Google Scholar]
- 56.Pastrana Restrepo M., Galeano Jaramillo E., Martínez Martínez A., Robledo Restrepo S. Synthesis and trypanocide activity of chloro-l-tyrosine and bromo-l-tyrosine derivatives. Med. Chem. Res. 2018;27:2454. doi: 10.1007/s00044-018-2249-y. [DOI] [Google Scholar]
- 57.Apweiler R., Bairoch A., Wu C.H., Barker W.C., Boeckmann B., Ferro S., Gasteiger E., Huang H., Lopez R., Magrane M., et al. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2004;32:D115–D119. doi: 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hermjakob H., Montecchi-Pilazzi L., Lewington C., Mudali S., Kerrien S., Orchard S., Vingron M., Roechert B., Roepstorff P., Valencia A., et al. IntAct: An open source molecular interaction database. Nucleic Acids Res. 2004;32:D452–D455. doi: 10.1093/nar/gkh052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanehisa M., Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.