Abstract
Objectives: UBC® Rapid Test measures soluble fragments of cytokeratins 8 and 18 in urine. We present results of a multicenter study using an updated version of UBC® Rapid Test in bladder cancer patients, patients with urinary bladder cancer positive history, and healthy controls. Material and Methods: In total 530 urine samples have been included in this study. Clinical urine samples were used from 242 patients with tumors of the urinary bladder (134 non-muscle-invasive low-grade tumors (NMI-LG), 48 non-muscle-invasive high-grade tumors (NMI-HG), and 60 muscle-invasive high-grade tumors (MI-HG)), 62 patients with non-evidence of disease (NED), and 226 healthy controls. Urine samples were analyzed by the UBC® Rapid point-of-care (POC) assay and evaluated by Concile Omega 100 POC Reader. All statistical analyses have been performed using R version 3.2.3. Results: Elevated levels of UBC® Rapid Test in urine are higher in patients with bladder cancer in comparison to the control group (p < 0.001). The sensitivity for the whole bladder cancer cohort was 53.3% (positive predictive value (PPV) 90.2%, negative predictive value (NPV) 65.2%) and was 38.8% (PPV 78.8%, NPV 72.1%) for non-muscle-invasive low-grade bladder cancer; 75.0% (PPV 72.0%, NPV 94.7%) for non-muscle-invasive high-grade bladder cancer and 68.3% (PPV 74.6%, NPV 91.8%) for muscle-invasive high-grade bladder cancer. The specificity for the statistical calculations was 93.8%. The cut-off value (10 µg/L) was evaluated for the whole patient cohort. The area under the curve of the quantitative UBC® Rapid Test using the optimal threshold obtained by receiver operating characteristics (ROC) analysis was 0.774. Elevated values of UBC® Rapid Test in urine are higher in patients with high-grade bladder cancer in comparison to low-grade tumors and the healthy control group. Conclusions: UBC® Rapid Test has potential to be a clinically valuable urinary protein biomarker for detection of high-grade bladder cancer patients and could be added in the management of NMI-HG tumors. UBC® Rapid results generated in both study centers in the present multicenter study are very similar and reproducible. Furthermore UBC® Rapid Test is standardized and calibrated and thus independent of used batch of test as well as study site.
Keywords: bladder cancer, tumor markers, urinary based diagnostics
1. Introduction
In Europe bladder cancer (BCa) is the fifth most frequent cancer. Its incidence rate was 151,200 and its annual mortality rate was 51,400 cases in 2012 [1]. Around 30% of bladder cancer patients suffered from muscle-invasive bladder cancer (MIBC) at the time of first diagnosis [2]. Radical cystectomy (RC) is the gold standard to treat patients with MIBC.
Non-muscle invasive high-grade bladder cancer has a particularly high rate of recurrence and will progress to muscle-invasive disease. The ideal urine-soluble marker should be used for primary diagnosis, follow-up, and screening of high-risk populations; replacing cystoscopy during follow-up or decreasing the number of control cystoscopies during follow-up would be a worthwhile goal. Due to its contact with urine, malignant cells are shed into the urine, and this urine contains the carcinogens producing the malignancy. Some of these urinary based tests have a higher specificity and sensitivity than classical urine cytology and could be important for screening and case findings [3].
Intermediate filaments of the cytoskeleton of epithelial cells containing cytokeratins are often overexpressed in urothelial tumors. In humans twenty different cytokeratins have been identified, and cytokeratins 8, 18, and 19 are known to be important in urothelial cells [4]. The expressions of cytokeratins such as 8, 18, and 19 are higher in urothelial cells and may be elevated because of a higher cell turnover rate [5,6]. Immunohistochemical features of urothelial dysplasia include aberrant cytokeratin 20 expression at different levels of the urothelium, however, there is also usually overexpression of p53 and high Ki-67 index [7].
UBC® Rapid Test is based upon an immunochromatographic method and measures fragments of cytokeratin 8 and 18 qualitatively. The measured levels are lower in low-grade tumors and benign urological diseases [8,9]. Cytokeratins 8 and 18 are soluble in urine and can be detected quantitatively with monoclonal antibodies using sandwich ELISA as well as UBC® Rapid assay with a photometric reader. It is important to highlight that this version of UBC® Rapid Test is a modified and updated version of fast cytokeratin determination in urine in comparison to the assay introduced 15 years ago. Furthermore this new version of UBC® Rapid Test is used in combination with a reader to quantitate the signal quite comparably with an ELISA assay, but it is a point-of-care (POC) assay. Previous UBC Rapid assays were only assays for visual evaluation of results.
In the last publication of our group we had a focus on carcinoma in situ (CIS), and we could show excellent results for UBC® Rapid Test for detecting CIS [10]. Regarding these facts, it is mandatory to include new tests into bladder cancer diagnostics, specifically a test that could detect flat, high-risk tumors difficult to detect in cystoscopy would be a step to ameliorate the finding of these tumors. The aim of this multicenter study is to report the final results with the highest number of measured samples for UBC® Rapid Test and to evaluate the usefulness of UBC® Rapid Test in patients with urinary bladder cancer with a focus on non-muscle invasive high-grade (NMI-HG) tumors and compare with healthy individuals.
2. Results
A total of 530 patients were included in the study; 242 with confirmed bladder cancer, 62 with non-evidence of disease (NED), and 226 healthy controls with no history of bladder cancer. The median age of the study population was 73 (range 26–98) years. Of these patients, 391 (73.8%) were men and 139 (26.3%) were women. Among the 242 patients with confirmed bladder cancer, 134 had non-muscle-invasive low-grade (NMI-LG), 48 had NMI-HG, and 60 had muscle-invasive high-grade (MI-HG) BCa; 182 (75.2%) had non-muscle-invasive bladder cancer (pTa and pT1 tumors), 60 (24.8%) had stage pT2–4. Carcinoma in situ (CIS) was detected in 23 cases (9.5%). A detailed analysis of the CIS patients in this study had already been published [10].
The number of patients and healthy controls are listed in Table 1 for study center I (HELIOS Hospital Bad Saarow) and study center II (Lukaskrankenhaus Neuss). Both groups enrolled a similar number of patients in the study. Table 2 shows all relevant data for center 1 and center 2 separately.
Table 1.
Patient characteristics and results of UBC® Rapid. Abbreviations: non-muscle-invasive low grade (NMI-LG), non-muscle-invasive high grade (NMI-HG), muscle-invasive high grade (MI-HG), non-evidence of disease (NED), positive predictive value (PPV), negative predictive value (NPV).
| NMI-LG | NMI-HG | MI-HG | NED | Control | p-Value | |
|---|---|---|---|---|---|---|
| n | 134 | 48 | 60 | 62 | 226 | |
|
Age Mean (SD) Median Range |
71.0 (11.9) 73.5 26–92 |
73.8 (10.6) 75 51–94 |
73.6 (10.0) 74.5 52–98 |
70.8 (11.4) 72 46–88 |
68.9 (12.2) 71 31–93 |
0.036 |
|
Sex Female (%) Male (%) |
34 (25.4) 100 (74.6) |
6 (12.5) 42 (87.5) |
19 (31.7) 41 (68.3) |
10 (16.1) 52 (83.9) |
70 (31.0) 156 (69.0) |
0.021 |
| Diabetes | 25 (18.7%) | 8 (16.7%) | 12 (20%) | 10(16.1%) | 34 (15%) | 0.849 |
| Erythrocyte (urine dipstick) | 83 (61.9%) | 44 (91.7%) | 58 (96.7%) | 38 (61.3%) | 73 (32.3%) | <0.001 |
|
Leucocytes (urine dipstick) Mean (SD) Median Range |
84.6 (172.6) 0 0–500 |
109.9 (167.6) 25 0–500 |
229.7 (228.1) 100 0–500 |
127.8 (206.6) 20 0–500 |
64.3 (150.3) 0 0–500 |
<0.001 |
| Nitrite pos. | 5 (3.7%) | 5 (10.4%) | 8 (13.3%) | 3 (4.8%) | 12 (5.3%) | 0.085 |
| Cystoscopy | 100 (74.6%) | 33 (68.8%) | 42 (70%) | 49 (79%) | 36 (15.9%) | <0.001 |
|
UBC (µg/L) Mean (SD) Median Range |
30.9 (63.4) 6.4 5–300 |
95.5 (104.6) 46 5–300 |
66.9 (90.1) 24.85 5–300 |
10 (9.79) 5 5–56.5 |
7.7 (13.92) 5 5–166 |
<0.001 |
| Sensitivity Specificity PPV NPV |
38.8% 93.8% 78.8% 72.1% |
75.0% 93.8% 72.0% 94.6% |
68.3% 93.8% 74.6% 94.6% |
22.6% 93.8% 50% 81.5% |
Table 2.
Patient characteristics and results of UBC® Rapid separated for center 1 and center 2.
| NMI-LG | NMI-HG | MI-HG | NED | Control | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Center 1 | Center 2 | Center 1 | Center 2 | Center 1 | Center 2 | Center 1 | Center 2 | Center 1 | Center 2 | Center 1 | Center 2 | |
| n | 78 | 56 | 26 | 22 | 25 | 35 | 30 | 32 | 78 | 148 | ||
|
Age Mean (SD) Median Range |
71.1 (11.6) 74 33–90 |
70.8 (12.4) 72 26–92 |
74.9 (11.6) 78.5 52–94 |
72.5 (9.4) 75 51–92 |
74.2 (11.0) 75 52–98 |
73.2 (9.3) 74 53–88 |
73.0 (8.8) 73.5 46–88 |
68.8 (13.1) 70.5 46–88 |
67.6 (12.64) 69.5 31–86 |
69.6 (11.94) 71.5 33–93 |
0.042 | 0.554 |
|
Gender Female (%) Male (%) |
20 (25.6) 58 (74.4) |
14 (25.0) 42 (75.0) |
4 (15.4) 22 (84.6) |
2 (9.1) 20 (90.9) |
6 (24.0) 19 (76.0) |
13 (37.1) 22 (62.9) |
3 (10.0) 27 (90.0) |
7 (21.9) 25 (78) |
23 (29.5) 55 (70.5) |
47 (31.8) 101 (68.2) |
0.220 | 0.127 |
| Diabetes | 22 (28.2%) | 3 (5.4%) | 6 (23.1) | 2 (9.1%) | 6 (24%) | 6 (17.1%) | 5 (16.7%) | 5 (15.6%) | 13 (16.7%) | 21 (14.2%) | 0.469 | 0.337 |
| Erythrocyte pos. | 45 (57.7%) | 38 (67.9%) | 24 (92.3%) | 20 (91.0%) | 24 (96.0%) | 34 (97.1%) | 17 (56.7%) | 21 (65.6%) | 34 (43.6%) | 39 (26.4%) | <0.001 | <0.001 |
|
Leucocytes Mean (SD) Median Range |
79.17 (165.2) 0 0–500 |
92.39 (183.8) 0 0–500 |
142.3 (182.04) 100 0–500 |
71.6 (143.4) 25 0–500 |
249.0 (229.41) 100 0–500 |
215.4 (229.5) 100 0–500 |
142.50 (220.1) 25 0–500 |
113.55 (195.2) 0 0–500 |
76.6 (165.60) 0 0–500 |
57.7 (141.68) 0 0–500 |
<0.001 | <0.001 |
| Nitrite pos. | 3 (3.8%) | 2 (3.6%) | 4 (5.1%) | 1 (1.8%) | 2 (8%) | 6 (17.1%) | 1 (3.3%) | 2 (6.3%) | 3 (3.8%) | 9 (6.1%) | 0.199 | 0.185 |
| Cystoscopy | 47 (60.3%) | 53 (94.6%) | 11 (42.3%) | 22 (100%) | 11 (44%) | 31 (88.6%) | 20 (66.7%) | 29 (90.6%) | 16 (20.5%) | 20 (13.5%) | <0.001 | <0.001 |
|
UBC [µg/L] Mean (SD) Median Range |
21.1 (43.7) 6.5 5–300 |
44.6 (81.9) 6.2 5–300 |
83.9 (94.8) 41.4 5–300 |
109.3 (115.8) 59.4 5–300 |
61.4 (80.9) 28.9 5–300 |
70.9 (97.1) 20.7 5–300 |
7.48 (7.06) 5 5–39.1 |
12.37 (11.4) 6.5 5–56.5 |
7.8 (13.9) 5 5–121 |
7.6 (14.0) 5 5–166 |
<0.001 | <0.001 |
| Sensitivity Specificity PPV NPV |
38.5% 92.3% 83.3% 60.0% |
39.3% 94.6% 73.3% 80.5% |
73.1% 92.3% 76.0% 91.1% |
77.3% 94.6% 68.0% 96.6% |
68.0% 92.3% 73.9% 90.0% |
68.6% 94.6% 75.0% 92.7% |
6.7% 92.3% 25% 72% |
37.5% 94.6% 60% 87.5% |
||||
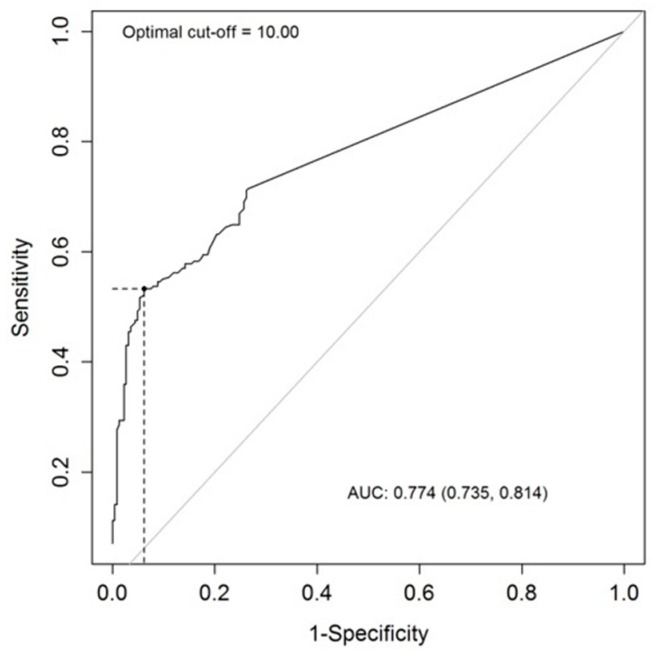
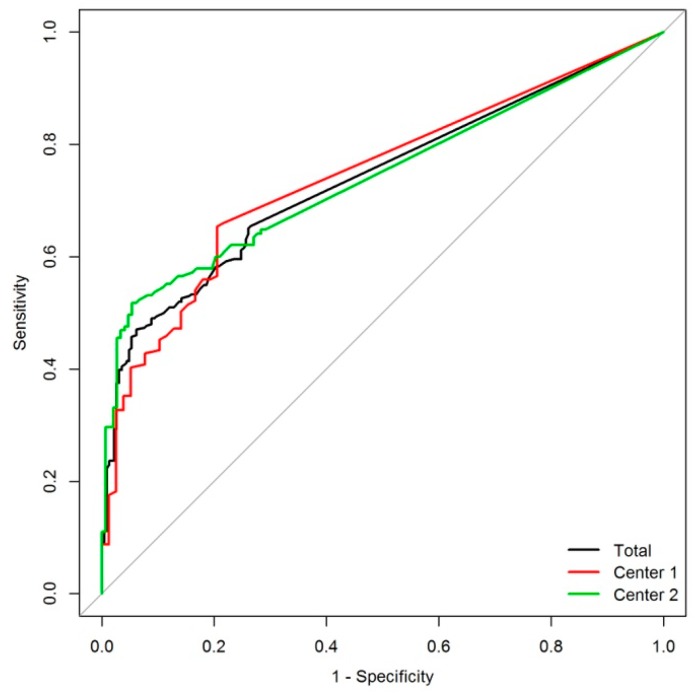
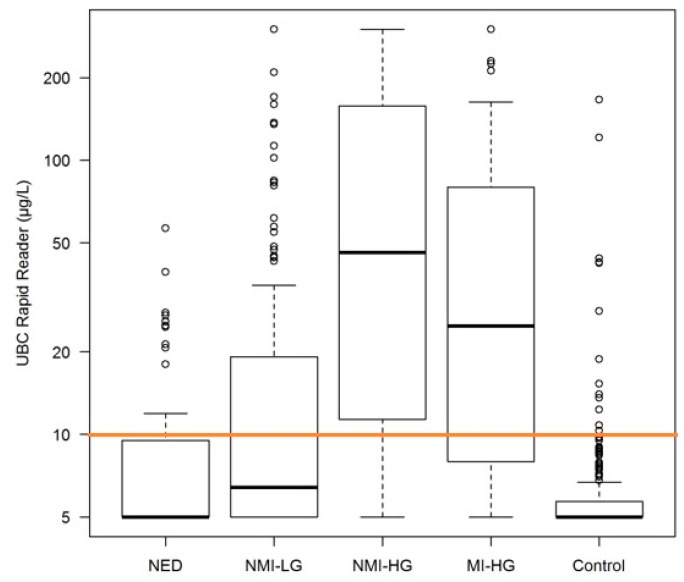
We could show that elevated concentrations of UBC® Rapid Test are detectable in urine of bladder cancer patients (Table 1 and Table 2). Elevated levels of UBC® Rapid Test in urine are higher in patients with bladder cancer in comparison to the control group. In 134 NMI-LG tumors the mean value of UBC® Rapid Test was 30.9 µg/L, for NMI-HG tumors 95.5 µg/L, for MI-HG tumors 66.9 µg/L, for NED patients 10.0 µg/L, and for the healthy individuals 7.7 µg/L. Elevated levels of UBC® Rapid Test in urine are statistically significantly higher in patients with bladder cancer in comparison to the control group (p < 0.0001). The high-risk group showed a markedly higher UBC® Rapid signal than the low-risk group. The area under the curve (AUC) of the quantitative UBC® Rapid Test using the optimal threshold obtained by receiver operating characteristics curve (ROC) analysis (cut-off 10.0 µg/L) was 0.774 as shown in Figure 1. ROC analyses of patients from center 1, center 2, and all patients together is shown in Figure 2, and demonstrated very similar outcomes. Figure 3 shows the distribution of UBC® Rapid values in boxplots in the different patient groups (overall p < 0.001). It shows also that most of the elevated values are definitely higher than the cut-point, especially for NMI-HG tumors.
Figure 1.
Analysis of the predictive ability—receiver operating (ROC) curve analysis for UBC® Rapid at cut-off value 10.0 µg/L with AUC 0.774 for the whole population.
Figure 2.
Analysis of the predictive ability—ROC curve analysis for UBC® Rapid at cut-off value 10.0 µg/L for the center 1 (red), center 2 (green), and whole population (black). p-value for comparison between centers = 0.874.
Figure 3.
Box plot for non-evidence of disease (NED), non-muscle-invasive low grade (NMI-LG), non-muscle-invasive high grade (NMI-HG), muscle-invasive high grade (MI-HG), control. Orange line for cut-off at 10 µg/L.
Sensitivity was calculated as 38.8% for NMI-LG, 75.0% for NMI-HG, and 68.3% for MI-HG bladder cancer, and the UBC® Rapid specificity was 93.8% for all calculations.
Data of sensitivity, specificity, positive, and negative predictive values using a cut-off 10.0 µg/L for UBC® Rapid Test including the 95% confidence interval are also listed in Table 1 and Table 2.
The data, which were generated in the two centers separately and reported in Figure 2, show impressively that ROC analysis is very similar and the sensitivity and specificity in both centres demonstrate no significant differences (Table 2). In the clinical data base it is obvious that center 1 has a higher rate of patients with diabetes and the rate of cystoscopies is higher in center 2. Though the rate of nitrite positive urine samples in center 2 is higher, the mean value of leucocytes is similar in both centers. Nevertheless, the results for UBC© Rapid Test are very similar in both centers demonstrating the robustness and stability of UBC® Rapid Test POC assay.
3. Discussion
The main purpose of this multi-center study was to evaluate the clinical usefulness of UBC® Rapid Test for diagnosis of bladder cancer with a specific focus on patients with NMI-HG tumors of the urinary bladder compared with healthy individuals. The results of the present study show that cytokeratin concentrations determined by UBC® Rapid Test measured by POC reader are statistically significant for patients with bladder cancer and healthy controls. Values of UBC® Rapid Test in high-grade tumors are significantly higher than in low-grade tumors, NED patients, and healthy individuals. The AUC as a parameter of diagnostic quality was calculated with 0.774. UBC® Rapid Test determined quantitatively could be applied to determine the risk for bladder cancer, but also the risk of having a high-grade tumor with increased risk for recurrence. The need for quantitative urinary markers like UBC® Rapid Test had also been published before [11]. The results of this study are showing again high values for UBC® Rapid Test especially for patients with high-grade bladder cancer [9,12,13,14]. Following from a previously published study with a high number of samples, the results of this study show that this test could be useful for combination in a diagnostic panel for patients of the high-risk group for bladder cancer. In previous reports of UBC® Rapid, a sensitivity of 65% and a specificity of 92% was calculated [15,16]. In the study of Mian et al. [16] only the older version UBC® Rapid with only visual evaluation was available. In our study, the new version of UBC® Rapid Test as cytokeratin assay was used; an improved lateral flow method resulted in a clearer test and control bands to evaluate. In this study UBC® Rapid Test was used with the Omega 100 reader to quantify the results.
Data presented in newer UBC® Rapid studies reported a cut-off of 12.3 µg/L, a sensitivity, specificity, PPV, and NPV of 60.7%, 70.1%, 46.8%, and 79.3%, respectively, and with an AUC of 0.68 [9]. According to other previous reported UBC® Rapid studies in the literature, the sensitivity of the qualitative UBC® Rapid Test ranged from 46.2% to 78.4%, and its specificity from 82.4% to 97.4% [9,12,16,17,18,19,20]. These data concur with our own results, whereas the sensitivity was low with 38.8% for detecting non-muscle invasive low-grade tumors. It increased to 75% for non-muscle invasive high-grade tumors and 68.3% for muscle-invasive tumors at a specificity of 93.8%. Therefore, it achieved the highest sensitivity of a single urinary marker test for detecting high-grade bladder cancer. The diagnostic accuracy of the quantitative UBC® Rapid Test POC system has been assessed in just five studies, which reported a sensitivity of 46.6% to 64.5% and a specificity ranging from 70.1% to 86.3%, respectively [9,12,17,19,20]. In this multicenter study, we could measure a high number of samples and the results for UBC© Rapid Test are very similar in both study centers. There is no significant difference in the ROC analyses, showing that the test is very stable and reproducible.
Currently, many different urinary POC test systems are available on the market, permitting non-invasive and rapid determination of urinary markers. Their diagnostic accuracy, however, is mostly controversially discussed in a small number of studies [21,22]. The sensitivities are usually higher than those reported for urinary cytology alone, but at a lower specificity [5,22]. Nevertheless, additional costs of urinary markers in surveillance protocols are ultimately not justified [23].
According to EAU guidelines, the examination of voided urine to detect cancer cells by cytology has a high sensitivity in high-grade tumors and CIS [2]. The major limitations for cytology are that specimens could be hampered by low cellular yield, urinary tract infections, stones, or intravesical instillations. Regardless, experienced readers can exceed specificity of up to 90% [5,24]. However, negative cytology does not exclude a tumor. As method cytology is subjective, on the other side UBC® Rapid Test is an objective method that is standardized and reproducible [22].
The use of those urinary markers for routine follow-up is not recommended in clinical practice by current guidelines and remains a debated issue [22,25]. The use of urine markers is only recommended as an adjunct to cystoscopy in current guidelines [26,27,28]. Other tests like Fluorescence in situ hybridization (FISH) and immunocytology have shown improved sensitivity compared with cytology [29,30,31]. These tests are complex and difficult to perform and they require specialized laboratory facilities.
It is common that new urine tests are compared with the results of cytology. Across a large number of studies, the results varied a lot. Sensitivity for G1-tumors is lower than 30%, for G2-tumors around 60%, and for G3-tumors 90%. Specificity is around 90–95% [32]. In the study of Ritter et al., UBC® Rapid Test was also compared with cytology, showing better results for UBC® Rapid Test [9]. One limitation of our study is that we had no comparison to cytology, mainly due to the focus on high-grade bladder cancer. But it is also known that urinary cytology is of limited diagnostic value for detecting low-grade bladder tumors compared to high-grade tumors (up to 84% [33]). In the reported study by Pichler et al., the sensitivity of bladder wash cytology was only 21.4%; the sensitivity of high-grade tumors can reach sensitivities of up to 84% [33]. Urinary cytology had been evaluated in many studies previously, and sensitivities and specificities are limited by the experience of the pathologists. This well-known fact has also been reported in recent references. Furthermore, it could also be interesting to include a combination of different tumor markers into BCa diagnostics. How to combine UBC® Rapid with other markers has been shown by Gleichenhagen et al. [13]. In this study a combination of UBC® Rapid and survivin increased the sensitivity to 66% with a specificity of 95%. For high-grade tumors, the combination showed a sensitivity of 82% and a specificity of 95%. A combination of both assays confirmed the benefit of using marker panels.
In contrast to dichotomized urinary tests, the quantitative character of the UBC® Rapid Test enables risk stratification for bladder cancer based on the absolute UBC® Rapid Test value. UBC® Rapid Test might not only contribute to improved detection of bladder cancer, but also to improved prediction of high-risk tumors. This has also been shown for other quantitative protein-based urinary tests [34]. An approach to objectify risk stratification should include a number of different parameters including quantitative UBC® Rapid Test, grade of haematuria, smoking status, age, and gender for developing a nomogram [35].
Of course, there are many other markers on the market, and genetic testing looks especially promising. Regardless, at the moment these markers are a rapid diagnostic tool too complicated to be included into basic and fast diagnostics. Currently, we still must stick to the proteins when we discuss quick testing. However, the “ideal urine-based marker” for detecting bladder cancer recurrence during surveillance would be rapid, non-invasive, and easy to perform and interpret. Furthermore, the assay should possess not only a high specificity to reduce superfluous cystoscopies on oncological follow-up, but also a high sensitivity so that no patient with low-grade and high-grade bladder cancer will be missed [34].
4. Materials and Methods
4.1. Patients
For this prospective study, 530 urine samples from bladder cancer patients and healthy controls have been collected between January 2014 and October 2015 at the Department of Urology, HELIOS, Hospital Bad Saarow (study center 1), and Lukas Hospital Neuss (study center 2), Germany. The study was approved by the local Institutional Review Board of national Medical Association Brandenburg (AS 147(bB)/2013). All patients with confirmed bladder cancer underwent cystoscopy, bladder ultrasound, and transurethral resection of bladder tumor in case of abnormal findings. Exclusion criteria were any kind of mechanical manipulation (cystoscopy, transrectal ultrasound, and catheterization) within 10 days before urine sampling. Other exclusion criteria were benign prostate enlargement, urolithiasis, other tumor diseases, severe infections, and pregnancy. All these criteria could influence the test to produce false positive results. Less than 10% of the possible study cohort had to be excluded based on exclusion criteria.
4.2. Procedure
Voided urine samples were collected in a sterile plastic container and subsequently processed. Urine samples were analyzed by the UBC® Rapid Test (Concile GmbH, Freiburg/Breisgau, Germany). All tests were carried out as recommended by the manufacturer’s instructions. The presence of a test band after 10 minutes of incubation was checked. After visual evaluation, the test cartridges were analyzed by the photometric point-of-care (POC) system Concile Omega 100 reader (Concile GmbH, Freiburg/Breisgau, Germany) for quantitative analysis. The cut-off value used for calculation of statistical parameters was based upon the evaluation of the receiver operating characteristics curve (ROC) and defined as 10.0 µg/L. The Omega 100 reader illuminates the test field with a complementary colored light to reduce interference in the analysis. The built-in charge-coupled device–matrix sensor takes a photograph of the light reflected, which is analyzed by the device.
4.3. Statistical Analysis
All statistical analyses have been performed using R version 3.2.3 (R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/). Data are presented descriptively using means and standard deviations for numerical variables and absolute and relative frequencies for categorical variables. Comparison between groups at baseline has been performed using analysis of variance (ANOVA) for numerical variables and chi-square tests for categorical variables.
The predictive ability of UBC® Rapid Test measurements to detect bladder cancer was evaluated using Receiver Operating Characteristics (ROC) analysis, where the optimal cut-point was determined using the Youden index [36]. Sensitivity, specificity, positive, and negative predictive value was then calculated for the optimal cut-off and presented with exact 95% confidence intervals.
4.4. Ethics
The study was performed according to the Declaration of Helsinki. The study was approved by the local Institutional Review Board of National Medical Association Brandenburg (No. AS 147(bB)/2013 dated by 17 November 2013). Written and informed consent was obtained from each participant.
5. Conclusions
Elevated values of UBC® Rapid Test in urine are higher in patients with non-muscle invasive high-grade bladder cancer in comparison to low-grade tumors and the healthy control group. Sensitivity for non-muscle invasive high-grade tumors is very high with 75% at a specificity of 93.8%. Thus, UBC® Rapid Test has the potential to be a more sensitive and specific urinary protein biomarker to identify patients with high-grade tumors that are difficult to detect in cystoscopy. Results for UBC® Rapid Test in both study centers of the present multicenter study are very similar and reproducible. UBC® Rapid Test is standardized and calibrated, and thus independent of use, batch of test, as well as study site. UBC® Rapid Test should be added in the diagnostics and follow-up for NMI-HG tumors of urinary bladder cancer, though cystoscopy is still an important part of monitoring of bladder cancer.
Acknowledgments
We thank the staff of the Urological Departments at HELIOS Hospital Bad, Saarow and Lukas Hospital Neuss, Germany, for their excellent help while collecting the samples. Statistical calculations have been performed by Marcus Thuresson from Statisticon, Uppsala, Sweden. The test systems were sponsored by Concile GmbH, Freiburg/Breisgau, Germany, and IDL Biotech AB, Bromma, Sweden.
Author Contributions
T.H.E. and H.G. conceived and designed the experiments; T.H.E., C.A. and D.B. performed the experiments; T.H.E. and C.S. analyzed the data; S.W., S.H. and T.O. contributed analysis tools; T.H.E. wrote the paper.
Funding
The author(s) disclosed receipt of the following financial support for the research: The test systems were sponsored by the local distributor Concile GmbH, Freiburg/ Breisgau, Germany; the main company is IDL Biotech AB, Bromma, Sweden.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ferlay J., Steliarova-Foucher E., Lortet-Tieulent J., Rosso S., Coebergh J.W., Comber H., Forman D., Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Witjes J.A., Comperat E., Cowan N.C., de Santis M., Gakis G., Lebret T., Ribal M.J., Van der Heijden A.G., Sherif A. EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur. Urol. 2014;65:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Lotan Y., Roehrborn C.G. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: Results of a comprehensive literature review and meta-analyses. Urology. 2003;61:109–118. doi: 10.1016/S0090-4295(02)02136-2. [DOI] [PubMed] [Google Scholar]
- 4.Southgate J., Harnden P., Trejdosiewicz L.K. Cytokeratin expression patterns in normal and malignant urothelium: A review of the biological and diagnostic implications. Histol. Histopathol. 1999;14:657–664. doi: 10.14670/HH-14.657. [DOI] [PubMed] [Google Scholar]
- 5.Lokeshwar V.B., Habuchi T., Grossman H.B., Murphy W.M., Hautmann S.H., Hemstreet G.P., III, Bono A.V., Getzenberg R.H., Goebell P., Schmitz-Drager B.J., et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology. 2005;66:35–63. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 6.Siracusano S., Niccolini B., Knez R., Tiberio A., Benedetti E., Bonin S., Ciciliato S., Pappagallo G.L., Belgrano E., Stanta G. The simultaneous use of telomerase, cytokeratin 20 and CD4 for bladder cancer detection in urine. Eur. Urol. 2005;47:327–333. doi: 10.1016/j.eururo.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Hodges K.B., Lopez-Beltran A., Davidson D.D., Montironi R., Cheng L. Urothelial dysplasia and other flat lesions of the urinary bladder: Clinicopathologic and molecular features. Hum. Pathol. 2010;41:155–162. doi: 10.1016/j.humpath.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder G.L., Lorenzo-Gomez M.F., Hautmann S.H., Friedrich M.G., Ekici S., Huland H., Lokeshwar V. A side by side comparison of cytology and biomarkers for bladder cancer detection. J. Urol. 2004;172:1123–1126. doi: 10.1097/01.ju.0000134347.14643.ab. [DOI] [PubMed] [Google Scholar]
- 9.Ritter R., Hennenlotter J., Kuhs U., Hofmann U., Aufderklamm S., Blutbacher P., Deja A., Hohneder A., Gerber V., Gakis G., et al. Evaluation of a new quantitative point-of-care test platform for urine-based detection of bladder cancer. Urol. Oncol. 2014;32:337–344. doi: 10.1016/j.urolonc.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Ecke T.H., Weiss S., Stephan C., Hallmann S., Barski D., Otto T., Gerullis H. UBC® Rapid Test for detection of carcinoma in situ for bladder cancer. Tumour Biol. 2017;39:1010428317701624. doi: 10.1177/1010428317701624. [DOI] [PubMed] [Google Scholar]
- 11.Shariat S.F., Casella R., Wians F.H., Jr., Ashfaq R., Balko J., Sulser T., Gasser T.C., Sagalowsky A.I. Risk stratification for bladder tumor recurrence, stage and grade by urinary nuclear matrix protein 22 and cytology. Eur. Urol. 2004;45:304–313. doi: 10.1016/j.eururo.2003.10.020. author reply 313. [DOI] [PubMed] [Google Scholar]
- 12.Ecke T.H., Arndt C., Stephan C., Hallmann S., Lux O., Otto T., Ruttloff J., Gerullis H. Preliminary Results of a Multicentre Study of the UBC Rapid Test for Detection of Urinary Bladder Cancer. Anticancer Res. 2015;35:2651–2655. [PubMed] [Google Scholar]
- 13.Gleichenhagen J., Arndt C., Casjens S., Meinig C., Gerullis H., Raiko I., Bruning T., Ecke T., Johnen G. Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine. Int. J. Mol. Sci. 2018;19:226. doi: 10.3390/ijms19010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Styrke J., Henriksson H., Ljungberg B., Hasan M., Silfverberg I., Einarsson R., Malmstrom P.U., Sherif A. Evaluation of the diagnostic accuracy of UBC® Rapid in bladder cancer: A Swedish multicentre study. Scand. J. Urol. 2017;51:293–300. doi: 10.1080/21681805.2017.1313309. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Carbayo M., Herrero E., Megias J., Mira A., Soria F. Initial evaluation of the new urinary bladder cancer rapid test in the detection of transitional cell carcinoma of the bladder. Urology. 1999;54:656–661. doi: 10.1016/S0090-4295(99)00195-8. [DOI] [PubMed] [Google Scholar]
- 16.Mian C., Lodde M., Haitel A., Vigl E.E., Marberger M., Pycha A. Comparison of the monoclonal UBC-ELISA test and the NMP22 ELISA test for the detection of urothelial cell carcinoma of the bladder. Urology. 2000;55:223–226. doi: 10.1016/S0090-4295(99)00383-0. [DOI] [PubMed] [Google Scholar]
- 17.Hakenberg O.W., Fuessel S., Richter K., Froehner M., Oehlschlaeger S., Rathert P., Meye A., Wirth M.P. Qualitative and quantitative assessment of urinary cytokeratin 8 and 18 fragments compared with voided urine cytology in diagnosis of bladder carcinoma. Urology. 2004;64:1121–1126. doi: 10.1016/j.urology.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Carbayo M., Herrero E., Megias J., Mira A., Espasa A., Chinchilla V., Soria F. Initial evaluation of the diagnostic performance of the new urinary bladder cancer antigen test as a tumor marker for transitional cell carcinoma of the bladder. J. Urol. 1999;161:1110–1115. doi: 10.1016/S0022-5347(01)61604-5. [DOI] [PubMed] [Google Scholar]
- 19.Babjuk M., Kostirova M., Mudra K., Pecher S., Smolova H., Pecen L., Ibrahim Z., Dvoracek J., Jarolim L., Novak J., et al. Qualitative and quantitative detection of urinary human complement factor H-related protein (BTA stat and BTA TRAK) and fragments of cytokeratins 8, 18 (UBC rapid and UBC IRMA) as markers for transitional cell carcinoma of the bladder. Eur. Urol. 2002;41:34–39. doi: 10.1016/S0302-2838(01)00015-X. [DOI] [PubMed] [Google Scholar]
- 20.Pichler R., Tulchiner G., Fritz J., Schaefer G., Horninger W., Heidegger I. Urinary UBC Rapid and NMP22 Test for Bladder Cancer Surveillance in Comparison to Urinary Cytology: Results from a Prospective Single-Center Study. Int. J. Med. Sci. 2017;14:811. doi: 10.7150/ijms.19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou R., Gore J.L., Buckley D., Fu R., Gustafson K., Griffin J.C., Grusing S., Selph S. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015;163:922–931. doi: 10.7326/M15-0997. [DOI] [PubMed] [Google Scholar]
- 22.Van Rhijn B.W., van der Poel H.G., van der Kwast T.H. Urine markers for bladder cancer surveillance: A systematic review. Eur. Urol. 2005;47:736–748. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Kamat A.M., Karam J.A., Grossman H.B., Kader A.K., Munsell M., Dinney C.P. Prospective trial to identify optimal bladder cancer surveillance protocol: Reducing costs while maximizing sensitivity. BJU Int. 2011;108:1119–1123. doi: 10.1111/j.1464-410X.2010.10026.x. [DOI] [PubMed] [Google Scholar]
- 24.Raitanen M.P., Aine R., Rintala E., Kallio J., Rajala P., Juusela H., Tammela T.L. Differences between local and review urinary cytology in diagnosis of bladder cancer. An interobserver multicenter analysis. Eur. Urol. 2002;41:284–289. doi: 10.1016/S0302-2838(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 25.Babjuk M., Bohle A., Burger M., Capoun O., Cohen D., Comperat E.M., Hernandez V., Kaasinen E., Palou J., Roupret M., et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017;71:447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 26.Babjuk M., Oosterlinck W., Sylvester R., Kaasinen E., Bohle A., Palou-Redorta J., Roupret M. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur. Urol. 2011;59:997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Sturgeon C.M., Duffy M.J., Hofmann B.R., Lamerz R., Fritsche H.A., Gaarenstroom K., Bonfrer J., Ecke T.H., Grossman H.B., Hayes P., et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin. Chem. 2010;56:e1–e48. doi: 10.1373/clinchem.2009.133124. [DOI] [PubMed] [Google Scholar]
- 28.Hall M.C., Chang S.S., Dalbagni G., Pruthi R.S., Seigne J.D., Skinner E.C., Wolf J.S., Jr., Schellhammer P.F. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J. Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Banek S., Schwentner C., Tager D., Pesch B., Nasterlack M., Leng G., Gawrych K., Bonberg N., Johnen G., Kluckert M., et al. Prospective evaluation of fluorescence-in situ-hybridization to detect bladder cancer: Results from the UroScreen-Study. Urol. Oncol. 2013;31:1656–1662. doi: 10.1016/j.urolonc.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Friedrich M.G., Hellstern A., Hautmann S.H., Graefen M., Conrad S., Huland E., Huland H. Clinical use of urinary markers for the detection and prognosis of bladder carcinoma: A comparison of immunocytology with monoclonal antibodies against Lewis X and 486p3/12 with the BTA STAT and NMP22 tests. J. Urol. 2002;168:470–474. doi: 10.1016/S0022-5347(05)64660-5. [DOI] [PubMed] [Google Scholar]
- 31.Van Rhijn B.W., Catto J.W., Goebell P.J., Knuchel R., Shariat S.F., van der Poel H.G., Sanchez-Carbayo M., Thalmann G.N., Schmitz-Drager B.J., Kiemeney L.A. Molecular markers for urothelial bladder cancer prognosis: Toward implementation in clinical practice. Urol. Oncol. 2014;32:1078–1087. doi: 10.1016/j.urolonc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Ecke T.H. Focus on urinary bladder cancer markers: A review. Ital. J. Urol. Nephrol. 2008;60:237–246. [PubMed] [Google Scholar]
- 33.Yafi F.A., Brimo F., Steinberg J., Aprikian A.G., Tanguay S., Kassouf W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol. Oncol. 2015;33:66.e25–66.e31. doi: 10.1016/j.urolonc.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Shariat S.F., Karam J.A., Lotan Y., Karakiewizc P.I. Critical evaluation of urinary markers for bladder cancer detection and monitoring. Rev. Urol. 2008;10:120–135. [PMC free article] [PubMed] [Google Scholar]
- 35.Lotan Y., Capitanio U., Shariat S.F., Hutterer G.C., Karakiewicz P.I. Impact of clinical factors, including a point-of-care nuclear matrix protein-22 assay and cytology, on bladder cancer detection. BJU Int. 2009;103:1368–1374. doi: 10.1111/j.1464-410X.2009.08360.x. [DOI] [PubMed] [Google Scholar]
- 36.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]