Abstract
We report the cloning and catalytic activity of a β-carbonic anhydrase (CA, EC 4.2.1.1), isolated from the pathogenic protozoan Entamoeba histolytica, EhiCA. This enzyme has a high catalytic activity for the physiologic CO2 hydration reaction, with a kcat of 6.7 × 105 s−1 and a kcat/Km of 8.9 × 107 M−1 × s−1. An anion inhibition study of EhiCA with inorganic/organic anions and small molecules revealed that fluoride, chloride, cyanide, azide, pyrodiphosphate, perchlorate, tetrafluoroborate and sulfamic acid did not inhibit the enzyme activity, whereas pseudohalides (cyanate and thiocyanate), bicarbonate, nitrate, nitrite, diethyldithiocarbamate, and many complex inorganic anions showed inhibition in the millimolar range (KIs of 0.51–8.4 mM). The best EhiCA inhibitors were fluorosulfonate, sulfamide, phenylboronic acid and phenylarsonic acid (KIs in the range of 28–86 μM). Since β-CAs are not present in vertebrates, the present study may be useful for detecting lead compounds for the design of effective enzyme inhibitors, with potential to develop anti-infectives with alternative mechanisms of action.
Keywords: carbonic anhydrase, metalloenzymes, protozoan, Entamoeba histolytica, anions, inhibitor
1. Introduction
The carbonic anhydrases (CAs, EC 4.2.1.1) are enzymes that effectively catalyze the reaction between CO2 and water, yielding bicarbonate (HCO3−) and protons (H+). They are among the fastest catalysts known in nature [1,2,3]. CAs are multifunctional enzymes, which play a central role in different physiological, biochemical, and metabolic processes, such as acid-base homeostasis; respiratory gas exchange; electrolyte secretion; and biosynthesis of urea, glucose, fatty acids, and carbamoyl phosphate. They are also vital in ionic transport, muscular contraction (in vertebrates), and photosynthesis (in plants and algae). Seven distinct genetic families (i.e., the α, β, γ, δ, ζ, η, and θ class CAs) are known to date, with a wide distribution in organisms throughout the tree of life [4,5,6,7,8,9,10]. The CA classes do not share any significant sequence and structural identity, being a paradigmatic example of convergent evolution at the molecular level [1,2,3].
The first β-CA was discovered in 1939, but it took several decades until it was recognized as evolutionarily and structurally distinct from the previously studied CAs, those belonging to the α-class [11]. After the 1990s, many new β-CAs were discovered in the genomes of various organisms [11]. Based on current knowledge, these enzymes are found in photosynthetic organisms, eubacteria, yeasts, and Archaea [11,12,13]. Later on, it was discovered that they are also present in the genomes of insects, nematodes, and protozoans, but not in mammals [14]. Therefore, β-CAs are considered promising target enzymes for antiparasitic drugs [15,16,17]. The physiological significance of β-CAs is somehow ambiguous in most organisms studied so far, because frequently there are more than one enzyme class present [1,2,3,14,15,16,17,18]. However, β-CAs were shown to have important roles (for instance, in providing bicarbonate/CO2 for the photosynthetic enzyme Rubisco in the chloroplasts of many plants/algae [11,17,18]). Helicobacter pylori contains only one α- and one β-CA, whose inhibition with sulfonamides impairs the growth of the pathogen in vitro and in vivo [13,15]. The physiological relevance of β-CAs in many organisms, including protozoans belonging to the Amoebozoas, is yet to be discovered. However, in Leishmania spp., a β-class CA enzyme (LdcCA) was recently shown to be a potential drug target [19,20,21]. Indeed, the inhibition of protozoan β-CAs with sulfonamides, formulated as nanoemulsions, had a profound effect on the survival and growth of L. amazonensis and L. infantum, two species which provoke serious disease in the tropical and subtropical countries [21].
Entamoeba histolytica is a pathogenic protozoan human parasite causing amebiasis, which can be expressed as colitis or abscess of intestines or liver [22,23]. The common symptoms are diarrhea, colitis, and dysentery, but the majority of infections are asymptomatic [22,23]. E. histolytica is ingested with contaminated food or water as mature cysts, which excystate in the small intestine. The released trophozoites will then invade the large intestine [22]. E. histolytica is capable of lysing human tissues, killing immune effector cells by contact-dependent cytolysis [22,24]. The parasite has many virulence mechanisms, as it can adhere to host cells with multi-subunit GalGalNAc lectins, degrade the host extracellular matrix with cysteine proteases, and lyses target cells with amoebapores [25]. The invasive forms of the infection generally include cyst formation in the liver, which can lead to complications such as pleural effusion, due to the rupture of the cyst [22,26]. Rarely, they also disseminate through other extraintestinal organs (e.g., the brain or pericardium) [22,23]. Although there are effective medications for treating E. histolytica, therapies for the invasive forms produce many adverse side effects [22,23], and there are additional limitations to such therapies, among which is an increasing prevalence of resistance to commonly used drugs, which emphasizes the need for new drug targets against this protozoan [23,25]. Thus, we decided to clone and investigate in detail the β-CA present in this pathogenic protozoan. Here we report the cloning, purification, investigation of the catalytic activity, and the anion inhibition profile of the recombinant enzyme belonging to the β-class, identified in the genome of the pathogenic protozoan E. histolytica, denominated EhiCA.
2. Results and Discussion
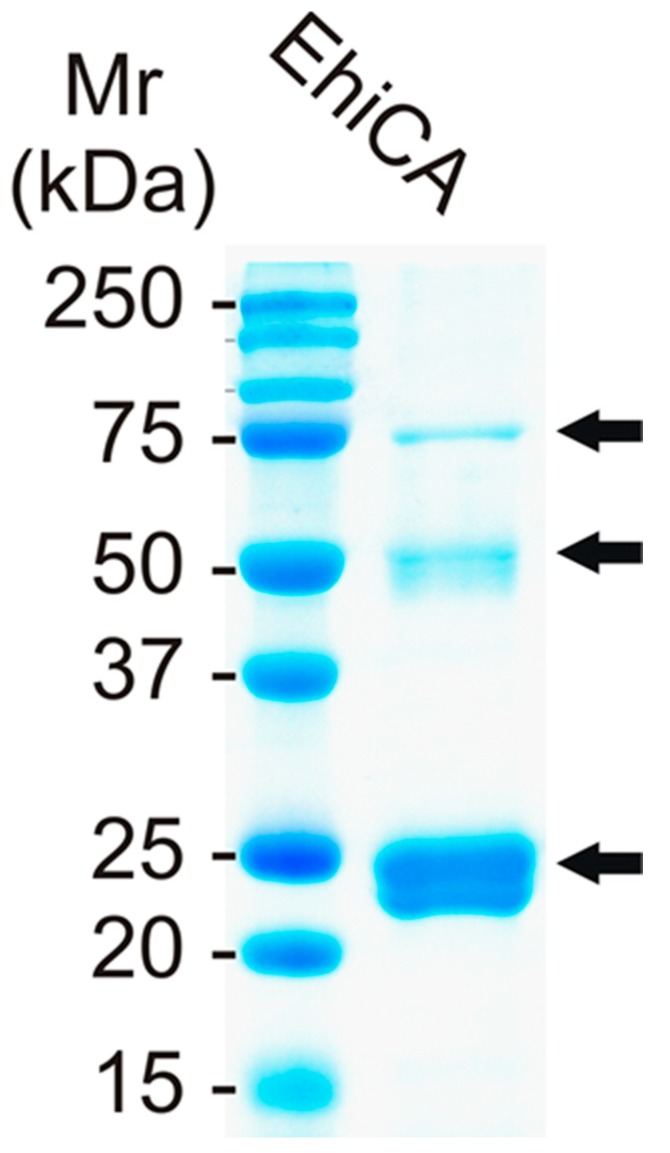
We produced the β-CA of E. histolytica, EhiCA, in the E. coli production system (see Experimental for details) as reported earlier for other CAs, such as hCA VII [19]. As a result, we obtained a 21 kDa protein, which was confirmed to be the right β-CA with mass spectrometry (MS) and SDS-PAGE (Figure 1). Furthermore, atomic absorption spectroscopy allowed us to determine the presence of one zinc ion per polypeptide chain (data not shown), which confirmed the MS data.
Figure 1.
SDS PAGE of the β-CA from E. histolytica (EhiCA) showing a 21/25 kDa doublet polypeptide and additional polypeptides of about 50 and 75 kDa (arrows). Left lane: Standard Mw markers. The dimer and the trimer of EhiCA are also seen (arrows), as reported for other β-CAs cloned and purified earlier [11,12,13,14,15].
We measured the catalytic activity of the recombinant EhiCA (for the CO2 hydration reaction) [27], comparing its kinetic parameters with those of other such enzymes, belonging to the α-class, such as hCA I and II, (h stands for human isoform). Table 1 shows that EhiCA has a significant catalytic activity (for the physiologic reaction, CO2 hydration to bicarbonate and protons), with a kcat of 6.7 × 105 s−1 and a kcat/Km of 8.9 × 107 M−1 × s−1, being thus 1.8 times more effective as a catalyst, compared to the slow human isoform hCA I (considering the kcat/Km values). Furthermore, like most enzymes belonging to the CA superfamily, EhiCA was inhibited by acetazolamide (AZA, 5-acetamido-1,3,4-thiadiazole-2-sulfonamide): A standard, clinically used sulfonamide CA inhibitor [1,2,3]. It can be observed that, similar to hCA I, EhiCA was inhibited in the high nanomolar range by this compound, with an inhibition constant KIs of 509 nM (Table 1).
Table 1.
Kinetic parameters for the CO2 hydration reaction catalyzed by the human cytosolic isozymes hCA I and II (α-class CAs) at 20 °C and pH 7.5 in 10 mM HEPES buffer and 20 mM Na2SO4, and the β-CA EhiCA form E. histolytica measured at 20 °C, pH 8.3 in 20 mM TRIS buffer and 20 mM NaClO4. Inhibition data with the clinically used sulfonamide acetazolamide (5-acetamido-1,3,4-thiadiazole-2-sulfonamide) are also provided [31].
| Enzyme | Activity Level | Class | kcat (s−1) | Km (mM) | kcat/Km (M−1 × s−1) | KI (Acetazolamide) (nM) |
|---|---|---|---|---|---|---|
| hCA I | moderate | α | 2.0 × 105 | 4.0 | 5.0 × 107 | 250 |
| hCA II | very high | α | 1.4 × 106 | 9.3 | 1.5 × 108 | 2 |
| EhiCA | high | β | (6.7 ± 0.2) × 105 | 7.5 ± 0.08 | (8.9 ± 0.1) × 107 | 509 |
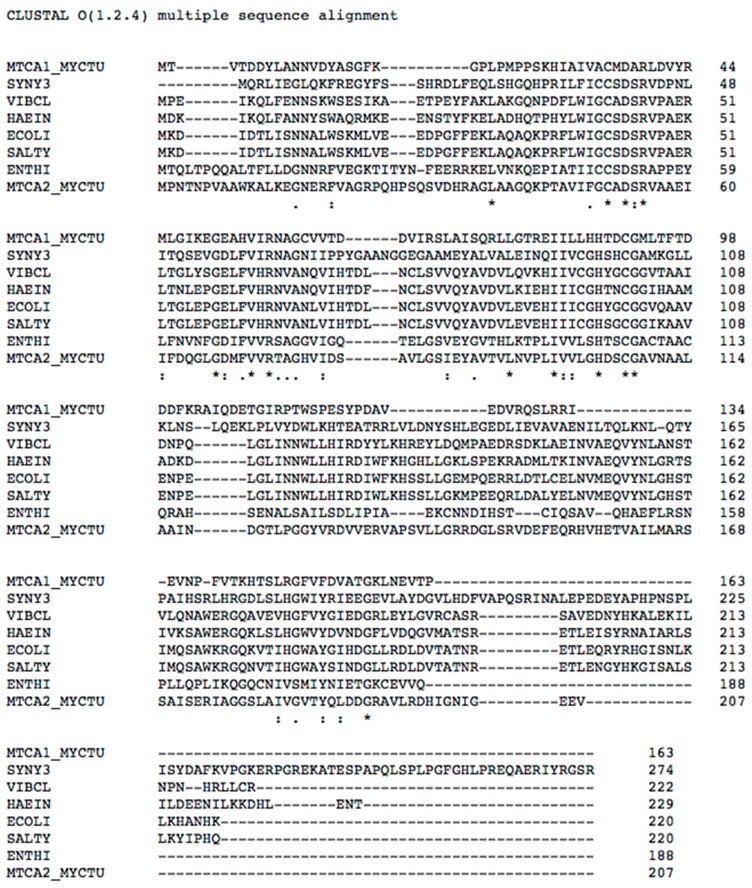
In order to rationalize the effective catalytic activity of EhiCA, we aligned the amino acid sequence of this protein with that of other β-CAs, such as those from the pathogenic bacteria Haemophilus influenzae, Vibrio cholerae, Escherichia coli, Salmonella typhimurium, two isoforms from Mycobacterium tuberculosis [11,27,28,29], and the cyanobacterium Synechocystis sp. PCC 6803 [30] (Figure 2).
Figure 2.
Multi-alignment of the amino acid sequences of the β-CAs from M. tuberculosis (isoform MTCA1_MYCTU), Synechocystis sp. (SYNY3), V. cholerae (VIBCL), H. influenzae (HAEIN), E. coli (ECOLI), S. typhimurium (SALTY), E. histolytica (ENTHI), and M. tuberculosis (isoform MTCA2_ MYCTU) [11,27,28,29,30]. Conserved amino acids depicted by an asterisk (*), semiconserved ones by (.) or (:).
As seen in Figure 2, EhiCA (as all β-CAs investigated to date) has the conserved three zinc(II) ligands, Cys50, His103, and Cys106 (the fourth ligand is presumably a water molecule/hydroxide ion), as well as the catalytic dyad constituted by the pair Asp52–Arg54 (also conserved in all enzymes belonging to this class) [11,12,13,14,15,27,28,29,30], which contributes to the enhancement of the nucleophilicity of the water coordinated to the metal ion. The presence of these conserved amino acids, and all the structural elements connected to them, may explain the catalytic activity of EhiCA reported in this paper (Table 1).
We also investigated the inhibition of EhiCA with a set of inorganic simple and complex anions, as well as small organic molecules known [11,12,13,14,15,27,28,29,30] to interact with CAs, such as diethyl-dithiocarbamate, sulfamide, sulfamic acid, phenyboronic and phenylphosphonic acid, among others (Table 2).
Table 2.
Inhibition constants of anionic inhibitors against the α-CA isoforms hCA II and hCA I, as well as the β-class protozoan enzyme EhiCA, for the CO2 hydration reaction at 20 °C [31].
| Inhibitor § | KI [mM] # | ||
|---|---|---|---|
| hCA II | hCA I | EhiCA | |
| F− | >300 | >300 | >100 |
| Cl− | 200 | 6.0 | >100 |
| Br− | 63 | 4.1 | 36.8 |
| I− | 26 | 0.3 | 7.4 |
| CNO− | 0.03 | 0.0007 | 0.77 |
| SCN− | 1.6 | 0.2 | 7.9 |
| CN− | 0.02 | 0.0005 | >100 |
| N3− | 1.51 | 0.0012 | >100 |
| HCO3− | 85 | 12 | 0.28 |
| CO32− | 73 | 15 | 2.4 |
| NO3− | 35 | 7.0 | 3.6 |
| NO2− | 63 | 8.4 | 1.7 |
| HS− | 0.04 | 0.0006 | 6.9 |
| HSO3− | 89 | 18 | 11.5 |
| SO42− | >200 | 63 | 21.6 |
| SnO32− | 0.83 | 0.57 | 0.51 |
| SeO42− | 112 | 118 | 6.0 |
| TeO42− | 0.92 | 0.66 | 0.61 |
| P2O74− | 48.50 | 25.8 | >100 |
| V2O74− | 0.57 | 0.54 | >100 |
| B4O72− | 0.95 | 0.64 | 0.29 |
| ReO4− | 0.75 | 0.11 | 7.1 |
| RuO4− | 0.69 | 0.10 | 7.0 |
| S2O82− | 0.084 | 0.11 | 8.4 |
| SeCN− | 0.086 | 0.085 | 0.87 |
| CS32− | 0.0088 | 0.0087 | 6.0 |
| Et2NCS2− | 3.1 | 0.00079 | 0.51 |
| ClO4− | >200 | >200 | >100 |
| BF4− | >200 | >200 | >100 |
| FSO3− | 0.46 | 0.79 | 0.086 |
| PF6− | >200 | >200 | >100 |
| CF3SO3− | >200 | >200 | >100 |
| NH(SO3)22− | 0.76 | 0.31 | 2.2 |
| H2NSO2NH2 | 1.13 | 0.31 | 0.028 |
| H2NSO3H | 0.39 | 0.021 | >100 |
| Ph-B(OH)2 | 23.1 | 38.6 | 0.047 |
| Ph-AsO3H2 | 49.2 | 31.7 | 0.038 |
§ As sodium salt; # Errors were in the range of 3–5% of the reported values, from three different assays.
The following observations can be made from the inhibition data shown in Table 2:
(i) The anions which did not show inhibitory activity against EhiCA were fluoride, chloride, and, surprisingly, cyanide and azide, which are highly effective inhibitors of α-CAs such as hCA I and II [32]; pyrodiphosphate and divanadate; perchlorate, tetrafluroborate, hexafluorophosphate, and triflate (which usually do not significantly inhibit any CA [32]); and, again surprisingly, sulfamic acid. All these compounds did not show significant inhibition up to a 100 mM concentration in the assay system.
(ii) The most effective EhiCA inhibitors were sulfamide (which is structurally highly similar to sulfamic acid, except that the pKa of the two compounds is highly different) [33,34] and fluorosulfonate, as well as phenylboronic acid and phenylarsonic acid, which showed KIs in the range of 28–86 µM (Table 2). As seen in Table 2, many of these small molecules/anions also act as inhibitors of hCA I and II, but with a rather different efficacy [33].
(iii) Several anions, such as cyanate, selenocyanate, bicarbonate, stannate, tellurate, tetraborate, and N,N-diethyl-dithiocarbamate were also sub-millimolar EhiCA inhibitors, with KIs in the range of 0.28–0.87 mM. Some of these compounds are typical metal complexing agents (cyanate, selenocyanate, N,N-diethyt-dithiocarbamate), and their propensity to bind the zinc ion in this β-CA explains these inhibitory activities. However, others, (among which are bicarbonate, stannate, tellurate, and tetraborate) show less affinity to act as metal complexing anions [32]. The inhibitory action of bicarbonate, one of the reaction products/substrates of the CA, is particularly interesting, possibly indicating that the enzyme is not acting as a highly efficient bicarbonate dehydratase, but instead that the CO2 hydratase activity might be crucial during the life cycle of this protozoan. However, this speculation needs careful validation.
(iv) Many anions acted as low millimolar EhiCA inhibitors. They include iodide, thiocyanate, carbonate, nitrate, nitrite, hydrogensulfide, selenite, perrhenate, perruthenate, peroxydisulfate, trithiocarbonate, and imidosulfonate (KIs in the range of 1.7–8.4 mM).
(v) Anions with a less effective inhibitory action against EhiCA were bromide, bisulfite, and sulfate, with KIs in the range of 11.5–365.8 mM (Table 1).
3. Materials and Methods
3.1. Vector Construction
We produced the EhiCA as a recombinant protein in E. coli. The DNA sequence was retrieved from UniProt, and modified for recombinant protein production. We provided the sequence of the insert, and the actual construction of the plasmid vector was performed by GeneArt (Invitrogen, Regensburg, Germany). The structure of the insert was specifically modified for production in E. coli. The insert was ligated into a modified plasmid vector, pBVboost.
3.2. Production of the Protein
The freeze-dried plasmid was prepared, according to manufacturer’s manual. Deep-frozen BL21 Star™ (DE3) cells (Invitrogen, Carlsbad, CA, USA) were slowly melted on ice. Once melted, 25 µL of the cell suspension and 1 µL of the plasmid solution were combined. The suspension was kept on ice for 30 min. Heat shock was performed by submerging the suspension-containing tube into 42 °C water for 30 s, and was then incubated on ice for 2 min. To the tube 125 µL of S.O.C Medium (Invitrogen, Carlsbad, CA, USA) was added, and the tube was incubated for 1 h with constant shaking (200 rpm) at 37 °C. Growth plates (gentamycin-LB medium, ratio 1:1000) were prewarmed at 37 °C for 40 min. Then, 20 µL and 50 µL of suspension was spread on two plates which were incubated overnight at 37 °C. A volume of 5 mL preculture was prepared by inoculating single colonies from growth plates onto an LB medium with gentamycin (ratio 1:1000). It was then incubated overnight at 37 °C with constant shaking (200 rpm). The production was executed according to pO-stat fed batch protocol, which is essentially as described in Määttä et al. [35]. There were some alterations to the previously described protocol: The fermentation medium did not contain glycerol, as the cell line used did not require it. The induction of the culture was performed with 1 mM IPTG, 12 h after starting the fermentation. Temperature was decreased to 25 °C at the time of the induction. Culturing was stopped after 12 h of the induction with the OD 34 (A600). The cells were collected by centrifugation, and the wet weight of the cell pellet was 303 g. The fermentation was performed by the Tampere facility of Protein Services (PS). The cell pellet (approximately 35 g) was suspended in 150 mL of binding buffer containing 50 mM Na2HPO4, 0.5 M NaCl, 50 mM imidazole, and 10% glycerol (pH 8.0), and the suspension was homogenized with an EmulsiFlex-C3 (AVESTIN, Ottawa, Canada) homogenizer. The lysate was centrifuged at 13,000× g for 15 min at 4 °C, and the clear supernatant was mixed with HisPur™ Ni-NTA Resin (Thermo Fisher Scientific, Waltham, MA, USA) and bound to the resin for 2 h at room temperature on the magnetic stirrer. Then resin was washed with the binding buffer and collected onto an empty column with an EMD Millipore™ vacuum filtering flask (Merck, Kenilworth, NJ, USA) and filter paper. The protein was eluted from the resin with 50 mM Na2HPO4, 0.5 M NaCl, 350 mM imidazole, and 10% glycerol (pH 7.0). The protein was re-purified with TALON® Superflow™ cobalt resin (GE Healthcare, Chicago, IL, USA). The eluted protein fractions were diluted with binding buffer (50 mM Na2HPO4, 0.5 M NaCl, and 10% glycerol pH 8.0), so that the imidazole concentration was under 10 mM. The protein binding and elution was performed as described above. The purity of the protein was determined with gel electrophoresis (SDS-PAGE), and visualized with PageBlue Protein staining solution (Thermo Fisher Scientific, Waltham, MA, USA). Protein fractions were pooled and concentrated with 10 kDa Vivaspin® Turbo 15 centrifugal concentrators (Sartorius™, Göttingen, Germany) at 4000× g at 4 °C. Buffer exchange in 50 mM TRIS (pH 7.5) was done using the same centrifugal concentrators. His-tag was cleaved from the purified protein by Thrombin CleanCleave Kit (Sigma-Aldrich, Saint Louis, MO, USA), according to manufacturer’s manual.
3.3. CA Activity and Inhibition Measurements
An Sx.18Mv-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used to assay the catalytic activity of various CA isozymes for the CO2 hydration reaction [31]. Phenol red (at a concentration of 0.2 mM) was used as indicator (working at the absorbance maximum of 557 nm). Following the CA-catalyzed CO2 hydration reaction, 10 mM Hepes (pH 7.5, for α-CAs) or TRIS (pH 8.3, for β-CAs) as buffers, and 0.1 M NaClO4 (for maintaining constant ionic strength), were used for a period of 10 s at 25 °C. The CO2 concentrations ranged from 1.7 to 17 mM, for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5–10% of the reaction were used for determining the initial velocity. The uncatalyzed rates were determined in the same manner, and subtracted from the total observed rates. Stock solutions of inhibitors (10 mM) were prepared in distilled and deionized water, and dilutions of up to 1 µM were done thereafter with the assay buffer. Enzyme and inhibitor solutions were pre-incubated together for 15 min (standard assay at room temperature) prior to assay, in order to allow for the formation of the enzyme–inhibitor complex. The inhibition constants were obtained by non-linear least-squares methods, using PRISM 3 and the Cheng–Prusoff equation [36,37,38].
4. Conclusions
In the search for alternative drug targets against anti-protozoan agents, we report the cloning and catalytic activity of a β-CA from Entamoeba histolytica, EhiCA, the etiological agent of diarrhea and amebic liver abscesses. This new enzyme has a high catalytic activity for the physiologic CO2 hydration reaction, with a kcat of 6.7 × 105 s−1 and a kcat/Km of 8.9 × 107 M−1 × s−1. An anion inhibition study of EhiCA with inorganic/organic anions and small molecules was performed, in order to detect interesting leads for effective inhibitors. Fluoride, chloride, cyanide, azide, pyrodiphosphate, perchlorate, tetrafluoroborate, and sulfamic acid did not inhibit the enzyme activity, whereas pseudohalides (cyanate and thiocyanate), bicarbonate, nitrate, nitrite, diethyldithiocarbamate, and many complex inorganic anions showed inhibition in the millimolar range (KIs of 0.51–8.4 mM). The best EhiCA inhibitors were fluorosulfonate, sulfamide, phenylboronic acid, and phenylarsonic acid (KIs in the range of 28–86 μM). Since β-CAs are not present in vertebrates, the present study may be useful for detecting lead compounds for the design of effective enzyme inhibitors, with potential to develop anti-infectives with alternative mechanisms of action.
Acknowledgments
The authors acknowledge the Tampere Facility of Protein Services (PS) for their service.
Author Contributions
S.H., S.B. and M.K. performed the experiments. S.P. and C.T.S. supervised the experiments and wrote the article. All authors contributed to the final version of the paper.
Funding
The work has been supported by grants from the Academy of Finland, the Sigrid Juselius Foundation, and the Jane and Aatos Erkko Foundation.
Conflicts of Interest
The authors do not declare conflict of interest.
Footnotes
Sample availability: Samples of the compounds described in the paper are available from the authors.
References
- 1.Supuran C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016;473:2023–2032. doi: 10.1042/BCJ20160115. [DOI] [PubMed] [Google Scholar]
- 2.Supuran C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 3.Neri D., Supuran C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011;10:767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 4.Capasso C., Supuran C.T. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzyme Inhib. Med. Chem. 2015;30:325–332. doi: 10.3109/14756366.2014.910202. [DOI] [PubMed] [Google Scholar]
- 5.Vullo D., Del Prete S., Di Fonzo P., Carginale V., Donald W.A., Supuran C.T., Capasso C. Comparison of the sulfonamide inhibition profiles of the β- and γ-carbonic anhydrases from the pathogenic bacterium Burkholderia pseudomallei. Molecules. 2017;22:421. doi: 10.3390/molecules22030421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrino E., Bua S., Mori M., Botta M., Murthy V.S., Vijayakumar V., Tamboli Y., Bartolucci G., Mugelli A., Cerbai E., et al. Novel sulfamide-containing compounds as selective carbonic anhydrase i inhibitors. Molecules. 2017;22:1049. doi: 10.3390/molecules22071049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saad A.E., Ashour D.S., Abou Rayia D.M., Bedeer A.E. Carbonic anhydrase enzyme as a potential therapeutic target for experimental trichinellosis. Parasitol. Res. 2016;115:2331–2339. doi: 10.1007/s00436-016-4982-9. [DOI] [PubMed] [Google Scholar]
- 8.Alterio V., Langella E., Viparelli F., Vullo D., Ascione G., Dathan N.A., Morel F.M., Supuran C.T., De Simone G., Monti S.M. Structural and inhibition insights into carbonic anhydrase CDCA1 from the marine diatom Thalassiosira weissflogii. Biochimie. 2012;94:1232–1241. doi: 10.1016/j.biochi.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Cau Y., Mori M., Supuran C.T., Botta M. Mycobacterial carbonic anhydrase inhibition with phenolic acids and esters: Kinetic and computational investigations. Org. Biomol. Chem. 2016;14:8322–8330. doi: 10.1039/C6OB01477A. [DOI] [PubMed] [Google Scholar]
- 10.Supuran C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017;12:61–88. doi: 10.1080/17460441.2017.1253677. [DOI] [PubMed] [Google Scholar]
- 11.Rowlett R.S. Structure and catalytic mechanism of the β-carbonic anhydrases. Biochim. Biophys. Acta Proteins Proteomics. 2010;1804:362–373. doi: 10.1016/j.bbapap.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Zolfaghari Emameh R., Barker H., Hytönen V.P., Tolvanen M.E.E., Parkkila S. Beta carbonic anhydrases: Novel targets for pesticides and anti-parasitic agents in agriculture and livestock husbandry. Parasites Vect. 2014;7:403. doi: 10.1186/1756-3305-7-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimori I., Onishi S., Takeuchi H., Supuran C.T. The α and β classes carbonic anhydrases from helicobacter pylori as novel drug targets. Curr. Pharm. Des. 2008;14:622–630. doi: 10.2174/138161208783877875. [DOI] [PubMed] [Google Scholar]
- 14.Syrjänen L., Parkkila S., Scozzafava A., Supuran C.T. Sulfonamide inhibition studies of the β carbonic anhydrase from Drosophila melanogaster. Bioorg. Med. Chem. Lett. 2014;24:2797–2801. doi: 10.1016/j.bmcl.2014.04.117. [DOI] [PubMed] [Google Scholar]
- 15.Supuran C.T., Capasso C. An overview of the bacterial carbonic anhydrases. Metabolites. 2017;7:56. doi: 10.3390/metabo7040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Supuran C.T., Capasso C. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. J. Enzym. Inhib. Med. Chem. 2016;31:1254–1260. doi: 10.1080/14756366.2016.1201479. [DOI] [PubMed] [Google Scholar]
- 17.Supuran C.T., Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin. Ther. Pat. 2018;28:745–754. doi: 10.1080/13543776.2018.1497161. [DOI] [PubMed] [Google Scholar]
- 18.Del Prete S., De Luca V., Capasso C., Supuran C.T., Carginale V. Recombinant thermoactive phosphoenolpyruvate carboxylase (PEPC) from Thermosynechococcus elongatus and its coupling with mesophilic/thermophilic bacterial carbonic anhydrases (CAs) for the conversion of CO2 to oxaloacetate. Bioorg. Med. Chem. 2016;24:220–225. doi: 10.1016/j.bmc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Bootorabi F., Jänis J., Smith E., Waheed A., Kukkurainen S., Hytönen V., Valjakka J., Supuran C.T., Vullo D., Sly W.S., et al. Analysis of a shortened form of human carbonic anhydrase VII expressed in vitro compared to the full-length enzyme. Biochimie. 2010;92:1072–1080. doi: 10.1016/j.biochi.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Vermelho A.B., Capaci G.R., Rodrigues I.A., Cardoso V.S., Mazotto A.M., Supuran C.T. Carbonic anhydrases from Trypanosoma and Leishmania as anti-protozoan drug targets. Bioorg. Med. Chem. 2017;25:1543–1555. doi: 10.1016/j.bmc.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 21.Da Silva Cardoso V., Vermelho A.B., Ricci Junior E., Almeida Rodrigues I., Mazotto A.M., Supuran C.T. Antileishmanial activity of sulphonamide nanoemulsions targeting the β-carbonic anhydrase from Leishmania species. J. Enzym. Inhib. Med. Chem. 2018;33:850–857. doi: 10.1080/14756366.2018.1463221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashmey N., Genta N., White N., Jr. Parasites and Diarrhea. I: Protozoans and Diarrhea. J. Travel Med. 1997;4:17–31. doi: 10.1111/j.1708-8305.1997.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 23.Meier A., Erler H., Beitz E. Targeting Channels and Transporters in Protozoan Parasite Infections. Front. Chem. 2018;6:88. doi: 10.3389/fchem.2018.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quach J., St-Pierre J., Chadee K. The future for vaccine development against Entamoeba histolytica. Hum. Vaccin. Immunother. 2014;10:1514–1521. doi: 10.4161/hv.27796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loftus B., Anderson I., Davies R., Alsmark U.C.M., Samuelson J., Amedeo P., Roncaglia P., Berriman M., Hirt R.P., Mann B.J., et al. The genome of the protist parasite Entamoeba histolytica. Nature. 2005;433:865–868. doi: 10.1038/nature03291. [DOI] [PubMed] [Google Scholar]
- 26.Hoffner R.J., Kilaghbian T., Esekogwu V.I., Henderson S.O. Common presentations of amebic liver abscess. Ann. Emerg. Med. 1999;34:351–355. doi: 10.1016/S0196-0644(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 27.Covarrubias A.S., Bergfors T., Jones T.A., Högbom M. Structural mechanics of the pH-dependent activity of beta-carbonic anhydrase from Mycobacterium tuberculosis. J. Biol. Chem. 2006;281:4993–4999. doi: 10.1074/jbc.M510756200. [DOI] [PubMed] [Google Scholar]
- 28.Nishimori I., Minakuchi T., Vullo D., Scozzafava A., Innocenti A., Supuran C.T. Carbonic anhydrase inhibitors. Cloning, characterization, and inhibition studies of a new beta-carbonic anhydrase from Mycobacterium tuberculosis. J. Med. Chem. 2009;52:3116–3120. doi: 10.1021/jm9003126. [DOI] [PubMed] [Google Scholar]
- 29.Ferraroni M., Del Prete S., Vullo D., Capasso C., Supuran C.T. Crystal structure and kinetic studies of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Acta Crystallogr. D Biol. Crystallogr. 2015;71:2449–2456. doi: 10.1107/S1399004715018635. [DOI] [PubMed] [Google Scholar]
- 30.McGurn L.D., Moazami-Goudarzi M., White S.A., Suwal T., Brar B., Tang J.Q., Espie G.S., Kimber M.S. The structure, kinetics and interactions of the β-carboxysomal β-carbonic anhydrase, CcaA. Biochem. J. 2016;473:4559–4572. doi: 10.1042/BCJ20160773. [DOI] [PubMed] [Google Scholar]
- 31.Khalifah R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971;246:2561–2573. [PubMed] [Google Scholar]
- 32.De Simone G., Supuran C.T. (In)organic anions as carbonic anhydrase inhibitors. J. Inorg. Biochem. 2012;111:117–129. doi: 10.1016/j.jinorgbio.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Abbate F., Supuran C.T., Scozzafava A., Orioli P., Stubbs M.T., Klebe G. Nonaromatic sulfonamide group as an ideal anchor for potent human carbonic anhydrase inhibitors: Role of hydrogen-bonding networks in ligand binding and drug design. J. Med. Chem. 2004;47:550–557. doi: 10.1021/jm011131t. [DOI] [PubMed] [Google Scholar]
- 34.Murray A.B., Aggarwal M., Pinard M., Vullo D., Patrauchan M., Supuran C.T., McKenna R. Structural Mapping of Anion Inhibitors to β-Carbonic Anhydrase psCA3 from Pseudomonas aeruginosa. ChemMedChem. 2018;13:2024–2029. doi: 10.1002/cmdc.201800375. [DOI] [PubMed] [Google Scholar]
- 35.Määttä J.A.E., Eisenberg-Domovich Y., Nordlund H.R., Hayouka R., Kulomaa M.S., Livnah O. Chimeric avidin shows stability against harsh chemical conditions—Biochemical analysis and 3D structure. Biotechnol. Bioengin. 2011;108:481–490. doi: 10.1002/bit.22962. [DOI] [PubMed] [Google Scholar]
- 36.Borras J., Scozzafava A., Menabuoni L., Mincione F., Briganti F., Mincione G., Supuran C.T. Carbonic anhydrase inhibitors: Synthesis of water-soluble, topically effective intraocular pressure lowering aromatic/heterocyclic sulfonamides containing 8-quinoline-sulfonyl moieties: Is the tail more important than the ring? Bioorg. Med. Chem. 1999;7:2397–2406. doi: 10.1016/S0968-0896(99)00190-X. [DOI] [PubMed] [Google Scholar]
- 37.Supuran C.T., Clare B.W. Carbonic anhydrase inhibitors—Part 57: Quantum chemical QSAR of a group of 1,3,4-thiadiazole-and 1,3,4-thiadiazoline disulfonamides with carbonic anhydrase inhibitory properties. Eur. J. Med. Chem. 1999;34:41–50. doi: 10.1016/S0223-5234(99)80039-7. [DOI] [Google Scholar]
- 38.Zimmerman S.A., Ferry J.G., Supuran C.T. Inhibition of the archaeal beta-class (Cab) and gamma-class (Cam) carbonic anhydrases. Curr. Top. Med. Chem. 2007;7:901–908. doi: 10.2174/156802607780636753. [DOI] [PubMed] [Google Scholar]