Abstract
Over the past two decades, public health has focused on the identification of environmental chemical factors that are able to adversely affect hormonal function, known as endocrine disruptors (EDs). EDs mimic naturally occurring hormones like estrogens and androgens which can in turn interfere with the endocrine system. As a consequence, EDs affect human reproduction as well as post and pre-natal development. In fact, infants can be affected already at prenatal level due to maternal exposure to EDs. In particular, great attention has been given to those chemicals, or their metabolites, that have estrogenic properties or antagonistic effects on the activity of androgen or even inhibiting their production. These compounds have therefore the potential of interfering with important physiological processes, such as masculinization, morphological development of the urogenital system and secondary sexual traits. Animal and in vitro studies have supported the conclusion that endocrine-disrupting chemicals affect the hormone-dependent pathways responsible for male gonadal development, either through direct interaction with hormone receptors or via epigenetic and cell-cycle regulatory modes of action. In human populations, epidemiological studies have reported an overall decline of male fertility and an increased incidence of diseases or congenital malformations of the male reproductive system. The majority of studies point towards an association between exposure to EDs and male and/or female reproductive system disorders, such as infertility, endometriosis, breast cancer, testicular cancer, poor sperm quality and/or function. Despite promising discoveries, a causal relationship between the reproductive disorders and exposure to specific toxicants has yet to be established, due to the complexity of the clinical protocols used, the degree of occupational or environmental exposure, the determination of the variables measured and the sample size of the subjects examined. Despite the lack of consistency in the results of so many studies investigating endocrine-disrupting properties of many different classes of chemicals, the overall conclusion points toward a positive association between exposure to EDs and reproductive system. Future studies should focus on a uniform systems to examine human populations with regard to the exposure to specific EDs and the direct effect on the reproductive system.
Keywords: Endocrine disruptors, Infertility, Sexual development, Male health, Seminal parameters
Introduction
Endocrine disruptors (EDs) are exogenous chemical entities or mixtures of compounds that interfere with any aspect of hormone action responsible for the maintenance of homeostasis and the regulation of developmental processes. The research conducted in the field of EDs has increased considerably over the last two decades, due to their potentially adverse effects on human health, supported by increasing experimental evidence in the areas of developmental biology and environmental toxicology. More specifically, it is well known that chemicals interfering with hormonal pathways can seriously affect human reproduction. Several studies have demonstrated a significant decrease in fertility biomarkers, notably sperm counts, in human populations that have been exposed to EDs [1–4]. The toxic effects of EDs have resulted in the restriction of their use in countries where evidence of extensive exposure is wide [5]. In some westernized countries, the use of certain EDs has been banned. However, in some cases the human exposure to EDs is inevitable, when such chemicals are used in occupational activities or are widely dispersed across the environment. The daily used products like pesticides, plastic items containing bisphenol A and phthalates, flame retardants, personal care products containing antimicrobials, heavy metals and perfluoroalkyls are regularly being manufactured in the industries. These are some of the most potential candidates of endocrine disruptors. From these industries, chemicals are easily released into the environment for example through leaching into the soil and water. These are then taken up by microorganisms, algae and plants which are then taken up by animals. After this, endocrine disruptors find their way in the food chain from the animals to finally into human being [6].
Over the past two decades, public health has focused on the identification of environmental chemical factors that are able to adversely affect hormonal function [7]. EDs mimic naturally occurring hormones like estrogens and androgens which can in turn interfere with the endocrine system. EDs are highly heterogeneous and can be classified according to their origins in: i) Natural and artificial hormones (e.g. fitoestrogens, 3-omegafatty acids, contraceptive pills and thyroid medicines); (ii) drugs with hormonal side effects (e.g.naproxen, metoprololand clofibrate); (iii) industrial and household chemicals (e.g. phthalates, alkylphenoletoxilate detergents, plasticizers, solvents) and (iv) side products of industrial and household processes (e.g. polycyclic aromatic hydrocarbons, dioxins, pentachlorobenzene). Given their widespread diffusion and environmental exposure not only limited to professional activities, this review will focus only on the 3rd class of EDs.
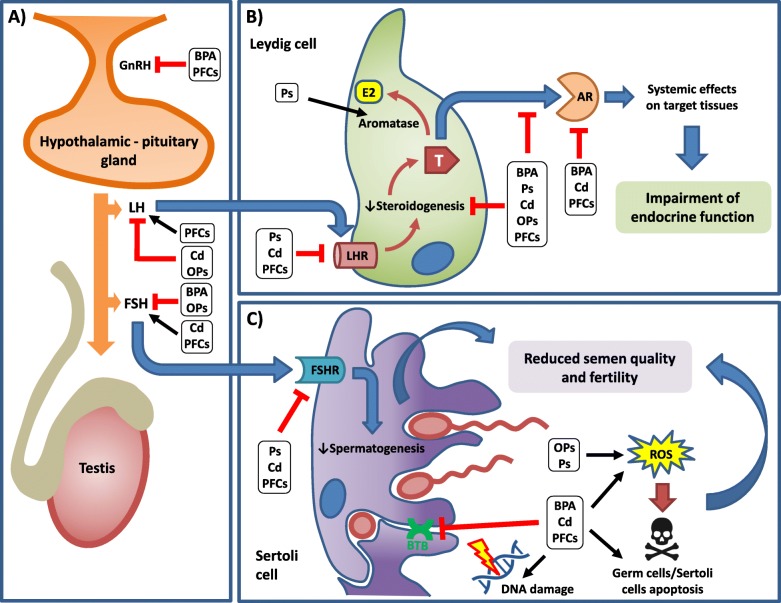
EDs exert their toxicity by interfering with the normal hormonal homeostatic mechanisms that promote growth and development of tissues. The classical action with respect to the reproductive system involves interference of EDs with hormone binding to the corresponding receptor, notably the androgen receptor (AR) or the estrogen receptor (ER). Following binding to a receptor the ED can trigger two types of responses: a hormonal response that is termed an agonistic effect, and/or a lack of hormonal response that is termed an antagonistic action. In addition to the hormone-related receptors, EDs act on enzymes involved in steroidogenesis and the metabolism of hormones [8] (Fig. 1).
Fig. 1.
Schematic representation of endocrine disruptors’ (EDs) effects on male fertility and the related mechanisms of toxicity. Results from both pre-clinical and clinical studies are summarized for each ED. If proposed effects are common to more EDs, they are reported together within black squares. Black arrows refer to stimulatory pathways. Red arrows with blunt ends represent inhibitory regulation. Hypotalamic-pituitary regulation of testicular function is impaired by most EDs (a). Within the testis, gonadotropins stimulates steroidogenesis in Leydig cells (b) and spermatogenesis in Sertoli cells (c). Overall, EDs disrupt endocrine function by reducing testosterone release or its activity on target tissues. In addition, EDs can reduce semen quality by directly impairing cell structure/viability or indirectly by interfering with hormonal patwhays. GnRH: Gonadotropin-releasing hormone; LH: Lutehinizing Hormone; FSH: Follicle-Stimulating Hormone; T: testosterone; AR: Androgen Receptor; FSHR: FSH Receptor; LHR: LH Receptor; E2: Estradiol; ROS: Reactive Oxygen Species; BTB: Blood-Testis Barrier; BPA: Bisphenol A; Ps: Phtalathes; Cd: Cadmium; Ops: Organophosphate pesticides; PFCs: Perfluoroalkyl Compounds
The effects of EDs on the male reproductive system are notably attributed to the interactions of these chemicals with the normal production and/or function of steroid hormones that are responsible for the initiation of prostate development and the masculinization of the Wolffian ducts in order to form the epididymis, seminal vesicles, and vas deferens [9]. The inhibition of the enzymes 5α-reductase and aromatase by EDs is one of the main mechanisms responsible for the adverse effects noted, as 5α-reductase is required for the conversion of the androgens to DHT [10], whereas aromatase catalyses the metabolism of androgens to oestrogen [9].
As consequence, EDs affect human reproduction as well as human post and pre-natal development. In fact, infants can be affected already at prenatal level due to maternal exposure to ED (reviewed in [11]). Epidemiological studies have reported an overall decline of male fertility and an increased incidence of diseases or congenital malformations of the male reproductive system [12]. Specifically, it has been observed a decreased sperm count in semen over time which inversely correlates with the incidence of diseases such as testis cancer, cryptorchidism and hypospadias [13]. This trend, known as testis dysgenesis syndrome, was first reported in 1992 by a Danish study that found a 50% decrease in sperm count in the male population across the 1938–1992 period [14]. These reports alarmed both general population and public authorities. In particular, great attention has been given to those chemicals, or their metabolites, that have estrogenic properties or antagonistic effects on the activity of androgen or even inhibiting their production.
These compounds have therefore the potential of interfering with important physiological processes, such as masculinization, morphological development of the urogenital system and secondary sexual traits [15].
The current review discusses the detrimental effects of EDs exposure on male health and fertility, by providing an overview of experimental pre-clinical studies on animal models and humans, when available, and by reporting epidemiological observational studies in humans.
Phthalates
There are numerous substances with a recognized anti-androgenic effect, from air and ground pollutants to plasticizers. In the latter category, phthalates (Ps) are the most investigated compounds as they are employed in virtually all industrial applications and consumer products as additives. Ps are inexpensive synthetic chemicals and have been widely used as plasticizers in a broad range of industrial and commercial products [16, 17]. The most commonly used phthalates are di-(2-ethylhexyl) phthalate (DEHP), di-n-butyl phthalate (DBP), diethyl phthalate (DEP), and benzylbutyl phthalate (BzBP). More than 75% of DEHP produced worldwide is used in plastic products. The other Ps are largely used in personal care products like foams, shampoos, dyes, lubricants, and food packaging materials [18]. Since these compounds are not covalently-bound polymers, their exposure to heat over time has the potential to favour their migration into food [19]. Human exposure to environmental pollutants from foodstuff poses health risk for the general population. Plasticizers such as phthalate esters, because of their anti-androgen and estrogen-like activity, are indicated as major endocrine disruptors. Both in vitro and in vivo toxicology studies have demonstrated their endocrine disrupting potential in model organisms, with endpoints such as antiandrogen effects, reproductive abnormalities, testicular lesions and reduced sperm production [20] (Fig. 1). However, dose ranges used for traditional reproductive toxicological studies were much higher than those observed in human epidemiological studies. Therefore, it is not surprising that these studies do not entirely align with the human studies. Controversially, in vitro and in vivo toxicology studies with low exposures to Ps were linked to decreased semen quality and male infertility in animals, as well as to decreased androgen production and steroidogenesis [21–30]. Ps have mostly shown the antiandrogen effect on testicular function during steroid formation [31–33]. Several in vitro or in vivo studies also showed that Ps, as well as their metabolites (e.g., DEHP/MEHP, DBP/ MBP) have stimulatory effects at low doses through inducing the production of progesterone, testosterone, steroidogenesis-related proteins and gene expression [23, 24, 26, 27, 29, 30]. The adverse effects of Ps on sperm quality were confirmed by ex vivo studies, where spermatozoa were incubated in vitro and exposed with high concentrations of phthalates [34]. It was reported that the sperm motility was decreased and that cytotoxicity was caused at long-term exposures (> 3 days) of human semen samples to the metabolite DEHP [34]. In parallel DHEP has been shown to inhibit testosterone production, when cultured in vitro with explants derived from human testes [35].
Epidemiological studies report an association between Ps exposure and altered seminal parameters have been reported [36](Table 1). It is important to note that exposure of infants to Ps is mainly due to both maternal exposure and breastfeeding. In fact, breastmilk levels of the phthalate metabolites are positively associated with maternal diet and water consumption. In Korea, breast feed infants exceeded the reference daily dose of DEHP by 8% and of DBP by 6% [37]. More recently, the urinary levels of Ps metabolites were related to infertile biomarkers and infertility in Chinese men [38].
Table 1.
Summary of epidemiological observational studies on the effects of EDs exposure on semen quality and endocrine function in humans
| EDs | Population | Design | Main Findings | Ref. |
|---|---|---|---|---|
| Ps | 168 men from subfertile couples | CC | Decreased sperm motility and concentration | [39] |
| 463 male partners of subfertile couples | C | Decreased sperm concentration and mobility | [42] | |
| 150 men | C | Decreased sperm concentration | [43] | |
| 379 men from an infertility clinic | CC | Increased DNA damage | [40] | |
| 65 asthenospermic, 65 oligoasthenospermic, 50 fertile males | CC | Decreased sperm motility | [34] | |
| 425 men from an infertility clinic | P | Decreased testosterone, estradiol,and free androgen index | [44] | |
| BPA | 42 occupationally exposed and 42 occupationally nonexposed men | CS | Lower FSH in occupationally exposed men. No differences in LH and fT. | [79] |
| 307 men from general population | CS | No associations with E2, SHBG, and fT. Associated with higher T. | [80] | |
| 167 men attending a fertility clinic | P | Associated with lower inhibin B and LH and higher FSH. No relationship with T, SHBG, E2, fT, T3, T4, and TSH. | [133] | |
| 190 men attending a fertility clinic | CS | Associated with lower sperm concentration, normal morphology and motility. No association with total sperm count. Associated with higher sperm DNA damage. | [74] | |
| 315 fertile men from prenatal clinics | CS | Associated with lower FAI and FAI:LH and higher SHBG. No association with semen parameters, FSH, LH, T, inhibin B, and fT. | [75] | |
| 218 occupationally exposed and nonexposed men | P | Associated with lower sperm concentration, total count, normal motility and vitality in all men. Associated with lower sperm concentration, normal motility and vitality in occupationally exposed men. Associated with lower sperm concentration in occupationally nonexposed men. No association with ejaculate volume and morphology. | [76] | |
| 149 male partners of couples undergoing IVF treatments | P | Associated with lower total sperm count, concentration, and vitality. No association with other semen quality parameters. | [5] | |
| 308 young men from general population | CS | Associated with lower progressive motility. No association with other semen quality parameters. Associated with higher T, LH, E2, and fT. No association with FSH, inhibin B, and SHBG. | [77] | |
| 418 male partners of couples trying to become pregnant | P | Associated with lower % sperm DNA fragmentation. No association with semen quality parameters | [78] | |
| OPs | 94 cases and 95 controls | CC | Decreased sperm concentration and motility | [92] |
| 31 sprayers and 80 controls | CC | Pesticide sprayers had significantly reduced seminal volume, percentage of motility, percentage of sperm with normal morphology, serum LH and T levels, increased time of liquefaction, seminal pH, percentage of immature sperm morphology, | [93] | |
| 32 cases, 46 internal controls, 22 external controls | CC | Sperm motion parameters; sperm progression and beat cross frequency in the exposure group were decreased significantly compared with the internal and the external control groups. | [94] | |
| PFCs | 105 men from general population | R | Association of PFOS and PFOA with abnormal sperm morphology; no association between other PFCs and semen parameters or reproductive hormones | [116] |
| 256 non-exposed adult men attending infertility clinic | CS | No association with semen parameters; positive correlation of serum PFOA and PFOS with LH | [110] | |
| 604 fertile men from general population | C | No association between PFCs and apoptotic markers or reproductive hormones emerged; slight increase in SHBG and DNA fragmentation with increased PFOA exposure | [119] | |
| 588 partners of pregnant women | C | Association between PFOS and abnormal sperm morphology; positive association between PFOA and semen motility | [118] | |
| 247 healthy men from general population | CS | PFOS levels were negatively associated with testosterone; negative association between PFHpS and sperm motility; other PFCs were not significantly associated with semen quality or reproductive hormones | [134] | |
| 169 men from an exposed pregnancy cohort | R | Association between PFOA and reduced sperm concentration and total sperm count; association between PFOA and increased levels of LH and FSH; no association between PFOS and any of the measured parameters | [114] | |
| 501 couples discontinuing contraception | C | Association with abnormal sperm morphology and sperm immaturity for at least 2 PFCs combined | [117] | |
| 59 male patients attending the Centre for Couple Sterility | P | Significant increase in alterations of sperm parameters in PFC-positive subjects; disomy and diploidy rates were significantly increased in PFC-positive males; sperm DNA fragmentation index resulted significantly increased in PFC-positive subjects | [120] | |
| Cd | 60 Infertile patients and 40 ferile controls | C | Significant negative correlation was observed between serum Cd level and total sperm count, sperm viability, sperm motility and normal sperm morphology. A positive correlation was also observed between seminal plasma Cd and FSH. | [135] |
| 140 Infertile patients, 15 Sperm donors and 35 Unselected males | C | The percentage of motile sperm and sperm concentration correlated inversely with seminal plasma cadmium among the infertility patients | [136] | |
| 73 infertile patients and 46 fertile controls | CS | A negative association between seminal cadmium concentration and sperm concentration and sperm motility was found | [129] | |
| 61 infertile patients | CC | There was a significant positive association between the percentage of immotile sperms and seminal plasma levels of cadmium. | [137] | |
| 149 environmentally-exposed males | CS | Significant negative correlation between blood plasma levels of cadmium and normal sperm morphology. No correlation was found between cadmium and other seminal parameters. | [138] | |
| 56 environmentally-exposed males | CS | Significant negative correlation between seminal plasma cadmium levels and total sperm count and sperm concentration. No association was found between cadmium and other seminal parameters | [139] | |
| 219 infertile patients | CS | No significant association was found between cadmium exposure and seminal parameters | [140] | |
| 123 infertile patients | CS | Serum cadmium was significantly associated with a decrease in testis size and an increase in serum estradiol, FSH and testosterone | [141] | |
| 27 occupationally-exposed workers and 45 sperm donors | CS | The concentrations of cadmium did not show any correlation with parameters of semen analysis. | [142] | |
| 1052 men attending fertility clinics | CS | Urinary levels of cadmium were significantly inversely associated with progressive sperm motility and total motility | [143] | |
| 587 men from the general population | CS | Inverse associations between Cd and semen volume, progressive motility and sperm morphology were found across the whole group | [144] |
EDs: Endocrine Disruptors; Ps: Phtalathes; BPA: Bisphenol A; Ops: Organophosphate pesticides; PFCs: Perfluoroalkyl Substances; CC: Case-Control study; CS: Cross-Sectional study; C: Cohort study; P: Prospective Study; R: Retrospective study
Studies that were conducted in human populations corroborated the in vitro findings and suggested that exposure to phthalate metabolites is correlated with lower motility of spermatozoa in men from subfertile couples [39]. The DNA damage induced in spermatozoa, the sperm motility and the morphology of the spermatozoa were weakly associated with the exposure to Ps [40–43], whereas with regard to the disruption of the hormonal function, an inverse association between MEHP exposure and testosterone and oestradiol levels was reported [44].
Data available on the effect of Ps on male reproductive health is limited, largely confined to specific cases of infertility [45]. Ps are rapidly metabolized and excreted in urine and feces and therefore the assessment of exposure to Ps in human relies on the measurement of urinary concentrations of phthalate metabolites. However, little or even no attention is given to the possible accumulation of un-metabolized Ps in different tissues [46]. This evidence rises some concerns about the appropriateness of parameters employed as index of exposure to contaminants, in particular for those substances like Ps that, showing specific tissue accumulation, may exert risk associated to long term exposures [32]. To this regard, quantification of both parent compound and corresponding metabolites in specific body fluids may represent an informative parameter with better correlation with clinical parameters [33].
Bisphenol A
In addition to phthalates, human exposure to the ED Bisphenol A (BPA) affects endocrine-reproductive function in males (Fig. 1). BPA is a high production-volume chemical that is widely used in the manufacture of consumer products such as polycarbonate plastics, epoxy resin liners of canned foods, some dental sealants and composites, and thermal receipts [47]. Due to its widespread use in consumer products, exposure to BPA is ubiquitous. In the United States, more than 90% of urine samples obtained from participants in the 2003–2004 and 2011–2012 National Health and Nutrition Examination Survey (NHANES) had BPA concentrations above the limit of detection [48, 49]. Exposure to BPA has garnered concern and regulatory attention over the past decade owing to its potential endocrine disrupting effects. Specifically, in vitro studies have shown that BPA binds to ERα and ERβ, producing weak estrogenic activity [50, 51]. BPA has also been cited for its ability to bind to the AR, peroxisome proliferator–activated receptor γ, and thyroid hormone receptor in experimental animal studies [52]. For example, doses below the present lowest observed adverse effect level (LOAEL; < 50 mg/kg) for BPA were associated with decreased sperm counts [53–57], impaired sperm motility [53, 55, 56, 58], and increased sperm DNA damage [55, 58–65]. In addition, doses below the present LOAEL for BPA were related to decreased testosterone levels [56, 63, 66–68]. Most animal studies concluded that BPA was a testicular toxicant [69–72]. There are differences across studies related to methodologic aspects such as dose, exposure route, timing, and outcomes measured (reviewed in [73]).
In humans, there is a growing body of literature exploring the associations between male urinary BPA concentrations and semen quality parameters, DNA damage, and reproductive hormones [5, 74–80] (Table 1) and a few studies on paternal urinary BPA concentrations and markers of couple fecundity and fertility such as time to pregnancy and live birth [28, 81]. Only six studies have explored the relationship between urinary BPA concentrations and semen parameters, and two of these studies also examined the association with sperm DNA damage [74, 78]. In the only prospective study to date, Li et al. explored the association of urinary BPA concentrations on semen parameters among 218 factory workers from four regions in China [76]. Their study found a negative association between urinary BPA concentrations and sperm concentration, total sperm count, sperm vitality, and sperm motility. The epidemiologic literature investigating the endocrine disrupting effects of BPA on male reproductive hormones also is limited and presents heterogeneous results. To date, one study has explored this association among men occupationally exposed to BPA [79], two studied the association among men from the general population [77, 80], and two studies investigated this association among either fertile men or subfertile men from a fertility clinic [74, 75]. The association of male urinary BPA concentrations with couple reproductive outcomes was recently assessed in two studies. Using the EARTH study cohort consisting of subfertile couples undergoing fertility treatment at MGH, Dodge et al. examined the associations of paternal urinary BPA concentrations with fertilization, embryo quality, implantation, and live birth among 218 couples who underwent 195 intrauterine inseminations and 211 in vitro fertilization cycles [81]. No associations between paternal urinary BPA concentrations and reproductive outcomes following fertility treatment were found. The association of paternal urinary BPA concentrations with couple reproductive outcomes was also investigated in the LIFE study of 501 couples discontinuing contraception with the intention of becoming pregnant. Similarly to the study among fertility clinic patients, Buck-Louis et al. did not find association between paternal urinary BPA concentrations and time to pregnancy [28], but interestingly higher paternal urinary BPA concentrations were significantly associated with fewer male births. Although the epidemiologic literature on this topic is growing, the evidence supporting an association between urinary BPA concentrations and male reproductive health in humans remains limited and inconclusive (Table 1). Several methodologic differences could explain discrepancies between human studies. First, studies included different study populations, including fertile males who may be less susceptible to the effects of BPA than would sub-fertile men. Second, the distribution of urinary BPA concentrations varied across studies. If there is a nonlinear association between BPA exposure and markers of reproductive health then we may not find consistent results across study populations with markedly different exposure levels. However, it is worth noting that contradictory results were found even among populations with similar urinary concentrations. Moreover, if exposure to BPA is not constant (within-individual variability is known to exist), the time window of BPA exposure captured in cross-sectional studies (e.g., the last 24 h) may not be the biologically relevant exposure window (e.g., the last 90 days for spermatogenesis). Third, all studies measured adult exposure, but none considered early life exposure (e.g., prenatal or peripubertal windows) which may be more sensitive to effects of BPA. Finally, residual confounding factors correlated with BPA exposure and semen quality were not accounted for.
Organophosphate pesticides
Organophosphate (OPs) pesticides are one of the most widely used class of pesticides for agricultural purposes [82]. They are metabolized by xenobiotic metabolizing enzymes, notably the cytochrome P450 (CYP) and the Paraoxonase (PON) families of enzymes and are therefore not persistent in the environment [83]. The exposure to OPs is assessed by the detection of their corresponding secondary metabolites notably the dialkyl phosphates in biological matrices such as urine [83–85]. The exposure of humans to OPs can be either professional (chemical plant workers, agricultural workers) or environmental (through soil and water contamination). Organochlorine compounds such as DDT and dioxins are not metabolized by the human body and accumulate for a long period of time [8]. In addition, such compounds appear to be a lot more persistent in the environment compared to organophosphorus compounds. Agonistic effects of MTX, an organochlorine pesticide used as an insecticide that was indented to replace DDT, have been reported for the estrogen receptor subtypes ERα and ERβ, whereas an opposite response was noted for the AR [86–88]. Thiophosphates, a class of organophosphorous pesticides, inhibit P450 enzymes namely, CYP3A4 and CYP1A2 that are involved in the metabolism of estrone and testosterone in the liver [89]. At the molecular level, EDs can affect the expression of steroid and sex hormone related enzymes by inducing their corresponding transcription, via binding to nuclear receptors. Notably, OPs and dioxins have been documented to bind with considerable potency to the aryl hydrocarbon receptor (AhR) that induces the expression of CYP1 genes that in turn metabolizes estradiol (E2) to hydroxylated derivatives [90]. Specific adverse effects on the male reproductive system have been reported to occur by organochlorine pesticides, such as endosulfan and DDT due to the disruption of the hypothalamic–pituitary testes axis and the direct interaction with the sex steroid receptors in the target tissue [91] (Fig. 1). The occupational exposure to pesticides increases the risk of morphological abnormalities in the sperm of farm workers that includes a decline in sperm count and a decreased percentage of viable sperms. OP pesticides such as parathion and methyl parathion can decrease the concentration of the sperm by damaging the seminiferous epithelium, while it has been suggested that pesticide exposure affects sex accessory glands that may also reduce the seminal volume [92, 93]. The exposure to pesticides reduces the seminal volume, increases the seminal pH and increases the abnormal sperm head morphology [93]. In addition, Lifeng et al. demonstrated that sperm motility could be affected by a limited number of pyrethroid pesticides, such as fenvalerate [94]. However, most studies were cross-sectional and due to different participation rates and lack of information on time dimension of the cause–effect relationship cannot be supported. Further controlled studies are needed to make sure about the effects of pesticides on male infertility. Although animal studies confirmed an impact of these chemicals on reproductive health, it should be noted that rats are more sensitive to the effects of pesticide exposure in comparison to humans. Moreover, synergistic, and potentiating effects of multiple chemicals are rarely explored in toxicological research. In spite of several studies and laboratory researches, no consistent view exists on the role of chronic pesticide exposure on semen parameters at present [91].
Perfluoroalkyl compounds
Perfluoroalkyl compounds (PFCs) are a class of organic molecules characterized by fluorinated hydrocarbon chains extensively used in industry and consumer products including oil and water repellents, coatings for cookware, carpets and textiles. PFCs possess unique physical chemical properties due to their amphiphilic structures and their strong carbonfluorine bonds. Therefore, long chain PFCs are non-biodegradable and bioaccumulate in the environment [95, 96]. PFCs have been found in humans and in the global environment and their toxicity, environmental fate, and sources of human exposure have been a major subject of research. Currently 23 PFCs are available, which includes perfluorooctanoic acid (PFOA) and perfluooctane sulfonate (PFOS), which are the predominant forms in human and environmental samples. However, the stability that makes PFCs desirable for commercial use, also entails that they are environmental contaminants due to their resistance to various modes of degradation [97].
Both in vitro and animal studies on PFCs toxicity have shown a detrimental effect of PFOA and PFOS on testicular function, by the alteration of steroidogenic machinery and subsequent defect of spermatogenesis [98–102]. Among the endocrine effects of PFOS in particular, it should be emphasized that this compound can affect the hypothalamic–pituitary axis activity [103, 104] (Fig. 1). It is also able to exert its toxicity at testicular level [105], as reported in rats [103, 106] and in testis models [107]. According to a recent study on male rats [108], high doses of PFOS orally administered for 28 days seem to modify the relative gene and protein receptor expressions of several hormones of the reproductive axis (GnRH, LH, FSH and testosterone) (Fig. 1).
Various PFCs compounds have been found in human serum [109], seminal fluid [110], breast milk [111] and even umbilical cord [112], suggesting a life-long exposure to PFCs in humans, from foetal stages until the adult life. In addition to their persistence, PFOA and PFOS have been shown to induce severe health consequences, such as neonatal mortality, neurotoxicity and immunotoxicity: PFCs act as endocrine disruptors on the foetus and newborns, leading to developmental defects [113]. This has led to strict regulation of PFOA and PFOS use in industrial processes, as the compounds were added to the Annex B of the Stockholm Convention on Persistent Organic Pollutants. In addition to health concerns on the impact of these substances on foetal development, epidemiological studies have focused also on the relationship between PFCs and human fertility, but recent research has focused mostly on female fecundity. In humans, in utero exposure to PFOA was associated later in adult life with lower sperm concentration and total sperm count and with higher levels of luteinizing hormone and follicle-stimulating hormone [114] (Table 1).
Besides the impact of PFCs on the professionally-exposed populations, recent evidence of pollution from chemical industries producing PFCs have emerged also in the general population from at least four different area worldwide: Mid-Ohio valley in the USA, Dordrecht area in Netherlands, Shandong district in China, and Veneto region in Italy. In the latter, the population at risk includes the cities of Vicenza, Padova and Verona, with 350.000–400.000 potentially exposed subjects, in an area of approximately 150 km2 and the consequent contamination of water and food [115]. Despite strong evidence pointing towards a negative role of PFCs on male reproductive function, to date few evidence are available on the actual effect of these substances on seminal parameters in men, with conflicting results [110, 116, 117]. Two cross-sectional studies reported negative associations of PFOS, or high PFOA and PFOS combined, with the proportion of morphologically normal spermatozoa in adult men [116, 118]. Furthermore, in a study of men attending an in vitro fertilization clinic, Raymer et al. [110] reported that luteinizing hormone (LH) and free testosterone significantly and positively correlated with plasma levels of PFOA, although PFOA was not associated with semen quality. Conflicting results are reported also for the association between PFCs and sperm DNA quality, although a significant trend is evident for increased DNA fragmentation in exposed men [117, 119, 120]. In infertile males, PFOS levels were higher than fertile counterparts, together with a higher gene expressions of estrogen receptor (ER) α, ERβ and androgen receptor (AR) [121, 122], suggesting that PFCs activity might be linked also to the genetic expression of sex hormones nuclear receptors. With respect to Androgen Receptor (AR), PFOS and PFOA induce a decrease of the protein expression of this receptor in the hypothalamus and pituitary gland as well as in the testis (Reviewed in [123]). These findings clearly suggest an antiandrogenic potential of PFCs and given the growing evidence suggesting a link between AR disruptors and disorders of male health [124, 125], PFCs effect on AR and consequent derangement of hypothalamic-pituitary axis should be a major concern. However it should be noted that there was a lack of consistent results among the investigated outcomes (Table 1). Subtle associations between higher PFOS and lower testosterone or abnormal semen morphology cannot be excluded. In conclusion, in men, there is little evidence of an association between PFAS exposure and semen quality or levels of reproductive hormones. As is the case for many epidemiological studies, causality cannot be definitively established in these studies, largely because of their cross-sectional design. However the consistency of findings in pre-clinical studies strongly suggests a causal relationship for some endpoints. Some effects are similar to associations seen in humans, whereas other effects cannot be extrapolated to humans given differences in toxicokinetics across species of these chemicals.
Cadmium
Heavy metals have also been recognized as likely inducers of testicular damage and, to this regard, the toxicity of Cadmium (Cd) as environmental contaminant has been known for several decades. Some industrial activities, such as melting and welding of metals, as well as municipal waste incineration are processes that contribute in the release of heavy metals in the environment. Among environmentally exposed population, tobacco smokers are the most exposed subjects, since tobacco leaves accumulate large amounts of Cd, making tobacco smoke the main source of Cd in smokers. Although the mechanisms of testicular toxicity exerted by heavy metals are still under investigation, the permeation through the blood-testis barrier is acknowledged as a fundamental process [126]. Like plasticizers, heavy metals widely employed, in industry as well as in food and dietary supplements [127–129]. Heavy metals can interfere a the different stages of spermatogenesis resulting in either decrease in sperm count or abnormal increase in sperm counts, sperm DNA damage, and impaired sperm motility [130](Fig. 1). Redox active heavy metals are also found to increase the levels of reactive oxygen species, leading to oxidative stress, induction of DNA damage and apoptosis of spermatozoa together with disruption of the blood-testis barrier and further damaging spermatogenesis [131](Fig. 1). Among heavy metals, Cd has been repeatedly proven to induce reproductive toxicity in the male, which has been extensively reviewed elsewhere [132]. Briefly, experimental studies in animal models strongly support the hypothesis that Cd affects male reproductive function, including spermatogenesis and semen quality, as well as endocrine function (Fig. 1). Indeed, Cd induces severe structural damage to testis vascular endothelium, which ultimately results in necrosis of the testis, and impaired spermatogenesis and testis endocrine function, and affects the BTB integrity, which might lead to susceptibility to toxicity and to the development of autoimmunity against germ cells. Moreover, Cd might induce inflammation and apoptosis within the testis, by means of direct effects on inflammation mediators, and on pro-apoptotic and anti-apoptotic factors, and by interfering with signalling pathways of calcium and cyclic AMP. In addition, Cd exerts targeted effects on selected cell populations of the testis, which include direct cytotoxicity and functional impairment of Sertoli and Leydig cells, and oxidative stress in both somatic and germ cells, mainly by means of mimicry mechanisms and interference with antioxidative activity. Moreover, Cd induces epigenetic modifications in Leydig cells and testis of Cd-treated animals, which might potentially determine an impairmentof semen quality, although these changes were not directly linked to reproductive dysfunction. Lastly, Cd treatment determines a direct disturbance of the hypothalamus-pituitary-gonadal axis (Fig. 1), which might determine the impairment of spermatogenesis and endocrine function (reviewed in [132]). Conversely, evidence from clinical studies is less consistent (Table 1). Observational studies in both environmental and occupational exposed males suggest that Cd have a detrimental effect on semen quality, and can alter endocrine function. Nevertheless, some studies failed to identify differences between Cd exposed and non-exposed subjects, probably due to small-sized study populations, and lack of control for potential confounding variables (reviewed in [132]). Therefore, additional well-designed observational studies, as well as further experimental research in humans, are required to eliminate inconsistencies, and to confirm the effects of Cd on human male reproductive function.
Conclusions
Endocrine disruptors include a class of chemicals that can potentially cause harmful effects to the male and female reproductive systems. In addition to the classical action of EDs that includes the agonism and/or antagonism with hormone and nuclear receptors, the last decade of scientific research has given significant scientific advances in the field of molecular biology that confirmed endocrine disruption by several compounds by interfering with the cell cycle, the apoptotic machinery and the epigenetic regulation of the target cells [8]. However, action mechanisms should not be generally extrapolated since each chemical has different routes to interfere with endocrine activity. Among the EDCs considered in this Review, there is strong experimental evidence of antagonism with hormone nuclear receptors (AR and/or ER) only for heavy metals, in particular Cadmium, whereas weaker evidence is reported for PFCs, BPA and OPs. The modulation of the downstream genes involved in the steroidogenic machinery is another possible target of EDCs (Fig. 1), leading to decreased androgen production and altered spermatogenesis, as reported for Ps, BPA, OPs and to a less extent PFCs.
However, epidemiological studies have shown controversial and inconsistent results (Table 1). This discrepancy can be attributed to several factors that could affect the outcome of the studies, notably to the complexity of the clinical protocols used, the degree of occupational or environmental exposure, the selection of the target group under investigation, the determination of the variables measured and the sample size of the subjects examined. With regard to the male reproductive system, the contribution of geographical and seasonal variation of the semen parameters must be considered. The majority of the epidemiological studies that have examined chemical exposure and the associated semen quality deterioration are cross-sectional. A longitudinal design would be preferable in studies of semen quality. Despite the lack of consistency in the results of so many studies investigating endocrine-disrupting properties of many different classes of chemicals, the overall conclusion points toward a positive association between exposure to EDs and reproductive system.
Despite methodological differences, major concerns are raised by these chemicals, and fertility evaluation is preferred particularly in specific professionally-exposed male workers. In the general population, public health programmes should lead toward a case-by-case investigation of the chemicals depending on environmental (i.e. water, soil and food) pollution. In particular, heavy metals such as Cadmium, pesticides and BPA raise most concerns to male fertility, with strong evidence linking environmental exposure to reduced semen quality parameters (i.e. concentration, total count, viability, motility) and even increased miscarriage rate in females. Evidence of such effects for phthalates and PFCs is less consistent, also due to the relatively recent interest of the scientific community in the effects of these chemicals on male fertility, and for these reasons more studies are clearly needed. Future studies should focus on a uniform system of the investigation of human populations with regard to the exposure to specific EDs and the direct effect on the reproductive system. In addition, the use of advanced molecular biology techniques that are employed to evaluate DNA damage in spermatozoa should be included.
Acknowledgements
Not applicable.
Funding
No funding.
Availability of data and materials
Not applicable.
Authors’ contributions
ADN and CF conceived and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Safe S. Endocrine disruptors and falling sperm counts: lessons learned or not! Asian J Androl. 2013;15:191–194. doi: 10.1038/aja.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slutsky M, Levin JL, Levy BS. Azoospermia and Oligospermia among a large cohort of DBCP applicators in 12 countries. Int J Occup Environ Health. 1999;5:116–122. doi: 10.1179/oeh.1999.5.2.116. [DOI] [PubMed] [Google Scholar]
- 3.Perry MJ. Effects of environmental and occupational pesticide exposure on human sperm: a systematic review. Hum Reprod Update. 2008;14:233–242. doi: 10.1093/humupd/dmm039. [DOI] [PubMed] [Google Scholar]
- 4.Jouannet P, Wang C, Eustache F, Kold-Jensen T, Auger J. Semen quality and male reproductive health: The controversy about human sperm concentration decline. 2001. pp. 333–344. [DOI] [PubMed] [Google Scholar]
- 5.Knez J. Endocrine-disrupting chemicals and male reproductive health. Reprod BioMed Online. 2013;26:440–448. doi: 10.1016/j.rbmo.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Kabir ER, Rahman MS, Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol. 2015;40:241–258. doi: 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Holmes D. Endocrine disruptors cause toxic fallout. Lancet Diabetes Endocrinol. 2013;1:93–94. doi: 10.1016/S2213-8587(13)70057-3. [DOI] [PubMed] [Google Scholar]
- 8.Sifakis S, Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ Toxicol Pharmacol. 2017;51:56–70. doi: 10.1016/j.etap.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney MF, Hasan N, Soto AM, Sonnenschein C. Environmental endocrine disruptors: effects on the human male reproductive system. Rev Endocr Metab Disord. 2015;16:341–357. doi: 10.1007/s11154-016-9337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 11.Katsikantami I, Sifakis S, Tzatzarakis MN, Vakonaki E, Kalantzi O-I, Tsatsakis AM, et al. A global assessment of phthalates burden and related links to health effects. Environ Int. 2016;97:212–236. doi: 10.1016/j.envint.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Nordkap L, Joensen UN, Blomberg Jensen M, Jørgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355:221–230. doi: 10.1016/j.mce.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Thankamony A, Pasterski V, Ong KK, Acerini CL, Hughes IA. Anogenital distance as a marker of androgen exposure in humans. Andrology. 2016;4:616–625. doi: 10.1111/andr.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juul A, Almstrup K, Andersson A-M, Jensen TK, Jørgensen N, Main KM, et al. Possible fetal determinants of male infertility. Nat Rev Endocrinol. 2014;10:553–562. doi: 10.1038/nrendo.2014.97. [DOI] [PubMed] [Google Scholar]
- 16.Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, et al. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003;111:1148–1151. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Wu Q, Kannan K. Phthalate metabolites in urine from China, and implications for human exposures. Environ Int. 2011;37:893–898. doi: 10.1016/j.envint.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Weck J, Sundaram R, Goldstone AE, Louis GB, Kannan K. Urinary concentrations of phthalates in couples planning pregnancy and its association with 8-hydroxy-2′-deoxyguanosine, a biomarker of oxidative stress: longitudinal investigation of fertility and the environment study. Environ Sci Technol. 2014;48:9804–9811. doi: 10.1021/es5024898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skinner MK. Endocrine disruptors in 2015: epigenetic transgenerational inheritance. Nat Rev Endocrinol. 2015;12:68–70. doi: 10.1038/nrendo.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meeker JD, Ferguson KK. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011-2012. J Clin Endocrinol Metab. 2014;99:4346–4352. doi: 10.1210/jc.2014-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao A-M, Man X-M, Guo X-J, Dong H-B, Wang F-Q, Sun H, et al. Effects of di-n-butyl phthalate on male rat reproduction following pubertal exposure. Asian J Androl. 2011;13:702–709. doi: 10.1038/aja.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloom MS, Whitcomb BW, Chen Z, Ye A, Kannan K, Buck Louis GM. Associations between urinary phthalate concentrations and semen quality parameters in a general population. Hum Reprod. 2015;30:2645–2657. doi: 10.1093/humrep/dev219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan J, Traore K, Li W, Amri H, Huang H, Wu C, et al. Molecular mechanisms mediating the effect of mono-(2-Ethylhexyl) phthalate on hormone-stimulated steroidogenesis in MA-10 mouse tumor Leydig cells. Endocrinology. 2010;151:3348–3362. doi: 10.1210/en.2010-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunnarsson D, Leffler P, Ekwurtzel E, Martinsson G, Liu K, Selstam G. Mono-(2-ethylhexyl) phthalate stimulates basal steroidogenesis by a cAMP-independent mechanism in mouse gonadal cells of both sexes. Reproduction. 2008;135:693–703. doi: 10.1530/REP-07-0460. [DOI] [PubMed] [Google Scholar]
- 25.Han X, Cui Z, Zhou N, Ma M, Li L, Li Y, et al. Urinary phthalate metabolites and male reproductive function parameters in Chongqing general population, China. Int J Hyg Environ Health. 2014;217:271–278. doi: 10.1016/j.ijheh.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, Dong C, Chen M, Lu J, Han X, Qiu L, et al. Low-dose monobutyl phthalate stimulates steroidogenesis through steroidogenic acute regulatory protein regulated by SF-1, GATA-4 and C/EBP-beta in mouse Leydig tumor cells. Reprod Biol Endocrinol. 2013;11:72. doi: 10.1186/1477-7827-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Hu Y, Dong C, Lu H, Zhang C, Hu Q, et al. Vimentin-Mediated Steroidogenesis Induced by Phthalate Esters: Involvement of DNA Demethylation and Nuclear Factor κB. Delmas D, editor. PLoS One. 2016;11:e0146138. doi: 10.1371/journal.pone.0146138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol a, phthalates, and couple fecundity: the longitudinal investigation of fertility and the environment (LIFE) study. Fertil Steril. 2014;101:1359–1366. doi: 10.1016/j.fertnstert.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savchuk I, Söder O, Svechnikov K. Mono-2-Ethylhexyl phthalate stimulates androgen production but suppresses mitochondrial function in mouse Leydig cells with different steroidogenic potential. Toxicol Sci. 2015;145:149–156. doi: 10.1093/toxsci/kfv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CHEN X, LIU YN, ZHOU QH, LENG L, CHANG Y, TANG NJ. Effects of low concentrations of Di-(2-ethylhexyl) and mono-(2-ethylhexyl) phthalate on steroidogenesis pathways and apoptosis in the murine Leydig tumor cell line MLTC-1. Biomed Environ Sci. 2013;26:986–989. doi: 10.3967/bes2013.034. [DOI] [PubMed] [Google Scholar]
- 31.Dees JH, Gazouli M, Papadopoulos V. Effect of mono-ethylhexyl phthalate on MA-10 Leydig tumor cells. Reprod Toxicol. 2001;15:171–187. doi: 10.1016/S0890-6238(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 32.Fiandanese N, Borromeo V, Berrini A, Fischer B, Schaedlich K, Schmidt J-S, et al. Maternal exposure to a mixture of di(2-ethylhexyl) phthalate (DEHP) and polychlorinated biphenyls (PCBs) causes reproductive dysfunction in adult male mouse offspring. Reprod Toxicol. 2016;65:123–132. doi: 10.1016/j.reprotox.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pant N, Pant A, Shukla M, Mathur N, Gupta Y, Saxena D. Environmental and experimental exposure of phthalate esters: the toxicological consequence on human sperm. Hum Exp Toxicol. 2011;30:507–514. doi: 10.1177/0960327110374205. [DOI] [PubMed] [Google Scholar]
- 35.Desdoits-Lethimonier C, Albert O, Le Bizec B, Perdu E, Zalko D, Courant F, et al. Human testis steroidogenesis is inhibited by phthalates. Hum Reprod. 2012;27:1451–1459. doi: 10.1093/humrep/des069. [DOI] [PubMed] [Google Scholar]
- 36.Hauser R, Sokol R. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult male. Fertil Steril. 2008;89:e59–e65. doi: 10.1016/j.fertnstert.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Lee J, Park J, Kim H-J, Cho G, Kim G-H, et al. Concentrations of phthalate metabolites in breast milk in Korea: estimating exposure to phthalates and potential risks among breast-fed infants. Sci Total Environ. 2015;508:13–19. doi: 10.1016/j.scitotenv.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Wang H, Tian M, Zhang J, Panuwet P, D’Souza PE, et al. Phthalate metabolites related to infertile biomarkers and infertility in Chinese men. Environ Pollut. 2017;231:291–300. doi: 10.1016/j.envpol.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, et al. Phthalate exposure and human semen parameters. Epidemiology. 2003;14:269–277. [PubMed] [Google Scholar]
- 40.Hauser R, Meeker JD, Singh NP, Silva MJ, Ryan L, Duty S, et al. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod. 2007;22:688–695. doi: 10.1093/humrep/del428. [DOI] [PubMed] [Google Scholar]
- 41.Duty SM, Calafat AM, Silva MJ, Brock JW, Ryan L, Chen Z, et al. The relationship between environmental exposure to phthalates and computer-aided sperm analysis motion parameters. J Androl. 2004;25:293–302. doi: 10.1002/j.1939-4640.2004.tb02790.x. [DOI] [PubMed] [Google Scholar]
- 42.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Bao H, Liu F, Zhang J, Shen H. Phthalates exposure of Chinese reproductive age couples and its effect on male semen quality, a primary study. Environ Int. 2012;42:78–83. doi: 10.1016/j.envint.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Meeker JD, Calafat AM, Hauser R. Urinary metabolites of Di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J Androl. 2009;30:287–297. doi: 10.2164/jandrol.108.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kay VR, Bloom MS, Foster WG. Reproductive and developmental effects of phthalate diesters in males. Crit Rev Toxicol. 2014;44:467–498. doi: 10.3109/10408444.2013.875983. [DOI] [PubMed] [Google Scholar]
- 46.Rusyn I, Peters JM, Cunningham ML. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol. 2006;36:459–479. doi: 10.1080/10408440600779065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehrlich S, Calafat AM, Humblet O, Smith T, Hauser R. Handling of thermal receipts as a source of exposure to bisphenol a. JAMA. 2014;311:859. doi: 10.1001/jama.2013.283735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol a and 4-tertiary-Octylphenol: 2003–2004. Environ Health Perspect. 2007;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol a (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, et al. Bisphenol a interacts with the estrogen receptor α in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142:203–214. doi: 10.1016/S0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 51.Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 52.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol a in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobrzyńska MM, Radzikowska J. Genotoxicity and reproductive toxicity of bisphenol a and X-ray/bisphenol a combination in male mice. Drug Chem Toxicol. 2013;36:19–26. doi: 10.3109/01480545.2011.644561. [DOI] [PubMed] [Google Scholar]
- 54.Tainaka H, Takahashi H, Umezawa M, Tanaka H, Nishimune Y, Oshio S, et al. Evaluation of the testicular toxicity of prenatal exposure to bisphenol a based on microarray analysis combined with MeSH annotation. J Toxicol Sci. 2012;37:539–548. doi: 10.2131/jts.37.539. [DOI] [PubMed] [Google Scholar]
- 55.Tiwari D, Vanage G. Mutagenic effect of bisphenol a on adult rat male germ cells and their fertility. Reprod Toxicol. 2013;40:60–68. doi: 10.1016/j.reprotox.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Salian S, Doshi T, Vanage G. Perinatal exposure of rats to bisphenol a affects the fertility of male offspring. Life Sci. 2009;85:742–752. doi: 10.1016/j.lfs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Qiu L-L, Wang X, Zhang X, Zhang Z, Gu J, Liu L, et al. Decreased androgen receptor expression may contribute to spermatogenesis failure in rats exposed to low concentration of bisphenol a. Toxicol Lett. 2013;219:116–124. doi: 10.1016/j.toxlet.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Minamiyama Y, Ichikawa H, Takemura S, Kusunoki H, Naito Y, Yoshikawa T. Generation of reactive oxygen species in sperms of rats as an earlier marker for evaluating the toxicity of endocrine-disrupting chemicals. Free Radic Res. 2010;44:1398–1406. doi: 10.3109/10715762.2010.510523. [DOI] [PubMed] [Google Scholar]
- 59.Chitra K, Latchoumycandane C, Mathur P. Induction of oxidative stress by bisphenol a in the epididymal sperm of rats. Toxicology. 2003;185:119–127. doi: 10.1016/S0300-483X(02)00597-8. [DOI] [PubMed] [Google Scholar]
- 60.Liu C, Duan W, Li R, Xu S, Zhang L, Chen C, et al. Exposure to bisphenol a disrupts meiotic progression during spermatogenesis in adult rats through estrogen-like activity. Cell Death Dis. 2013;4:e676. doi: 10.1038/cddis.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rashid H, Ahmad F, Rahman S, Ansari RA, Bhatia K, Kaur M, et al. Iron deficiency augments bisphenol A-induced oxidative stress in rats. Toxicology. 2009;256:7–12. doi: 10.1016/j.tox.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 62.Wu H-J, Liu C, Duan W-X, Xu S-C, He M-D, Chen C-H, et al. Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat Res Toxicol Environ Mutagen. 2013;752:57–67. doi: 10.1016/j.mrgentox.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 63.D’Cruz SC, Jubendradass R, Mathur PP. Bisphenol a induces oxidative stress and decreases levels of insulin receptor substrate 2 and glucose transporter 8 in rat testis. Reprod Sci. 2012;19:163–172. doi: 10.1177/1933719111415547. [DOI] [PubMed] [Google Scholar]
- 64.Kabuto H, Hasuike S, Minagawa N, Shishibori T. Effects of bisphenol a on the metabolisms of active oxygen species in mouse tissues. Environ Res. 2003;93:31–35. doi: 10.1016/S0013-9351(03)00062-8. [DOI] [PubMed] [Google Scholar]
- 65.Anjum S, Rahman S, Kaur M, Ahmad F, Rashid H, Ansari RA, et al. Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food Chem Toxicol. 2011;49:2849–2854. doi: 10.1016/j.fct.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 66.Salian S, Doshi T, Vanage G. Neonatal exposure of male rats to bisphenol a impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology. 2009;265:56–67. doi: 10.1016/j.tox.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 67.El-Beshbishy HA, Aly HAA, El-Shafey M. Lipoic acid mitigates bisphenol A-induced testicular mitochondrial toxicity in rats. Toxicol Ind Health. 2013;29:875–887. doi: 10.1177/0748233712446728. [DOI] [PubMed] [Google Scholar]
- 68.Xi W, Lee CKF, Yeung WSB, Giesy JP, Wong MH, Zhang X, et al. Effect of perinatal and postnatal bisphenol a exposure to the regulatory circuits at the hypothalamus–pituitary–gonadal axis of CD-1 mice. Reprod Toxicol. 2011;31:409–417. doi: 10.1016/j.reprotox.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 69.Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE. Gestational and Lactational exposure to Ethinyl estradiol, but not bisphenol a, decreases androgen-dependent reproductive organ weights and Epididymal sperm abundance in the male long Evans hooded rat. Toxicol Sci. 2008;102:371–382. doi: 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi K, Ohtani K, Kubota H, Miyagawa M. Dietary exposure to low doses of bisphenol a: effects on reproduction and development in two generations of C57BL/6J mice. Congenit Anom (Kyoto) 2010;50:159–170. doi: 10.1111/j.1741-4520.2010.00279.x. [DOI] [PubMed] [Google Scholar]
- 71.LaRocca J, Boyajian A, Brown C, Smith SD, Hixon M. Effects of in utero exposure to bisphenol a or diethylstilbestrol on the adult male reproductive system. Birth Defects Res Part B Dev Reprod Toxicol. 2011;92:526–533. doi: 10.1002/bdrb.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, et al. Two-generation reproductive toxicity study of dietary bisphenol a in CD-1 (Swiss) mice. Toxicol Sci. 2008;104:362–384. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- 73.Mínguez-Alarcón L, Hauser R, Gaskins AJ. Effects of bisphenol a on male and couple reproductive health: a review. Fertil Steril. 2016;106:864–870. doi: 10.1016/j.fertnstert.2016.07.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, et al. Semen quality and sperm DNA damage in relation to urinary bisphenol a among men from an infertility clinic. Reprod Toxicol. 2010;30:532–539. doi: 10.1016/j.reprotox.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mendiola J, Jørgensen N, Andersson A-M, Calafat AM, Ye X, Redmon JB, et al. Are environmental levels of bisphenol a associated with reproductive function in fertile men? Environ Health Perspect. 2010;118:1286–1291. doi: 10.1289/ehp.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li D-K, Zhou Z, Miao M, He Y, Wang J, Ferber J, et al. Urine bisphenol-A (BPA) level in relation to semen quality. Fertil Steril. 2011;95:625–30-4. [DOI] [PubMed]
- 77.Lassen TH, Frederiksen H, Jensen TK, Petersen JH, Joensen UN, Main KM, et al. Urinary bisphenol a levels in young men: association with reproductive hormones and semen quality. Environ Health Perspect. 2014;122(5):478–84. [DOI] [PMC free article] [PubMed]
- 78.Goldstone AE, Chen Z, Perry MJ, Kannan K, Louis GMB. Urinary bisphenol a and semen quality, the LIFE study. Reprod Toxicol. 2015;51:7–13. doi: 10.1016/j.reprotox.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanaoka T, Kawamura N, Hara K, Tsugane S. Urinary bisphenol a and plasma hormone concentrations in male workers exposed to bisphenol a diglycidyl ether and mixed organic solvents. Occup Environ Med. 2002;59:625–628. doi: 10.1136/oem.59.9.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galloway T, Cipelli R, Guralnik J, Ferrucci L, Bandinelli S, Corsi AM, et al. Daily bisphenol a excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ Health Perspect. 2010;118:1603–1608. doi: 10.1289/ehp.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dodge LE, Williams PL, Williams MA, Missmer SA, Toth TL, Calafat AM, et al. Paternal urinary concentrations of parabens and other phenols in relation to reproductive outcomes among couples from a fertility clinic. Environ Health Perspect. 2015;123:665–671. doi: 10.1289/ehp.1408605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koureas M, Tsakalof A, Tzatzarakis M, Vakonaki E, Tsatsakis A, Hadjichristodoulou C. Biomonitoring of organophosphate exposure of pesticide sprayers and comparison of exposure levels with other population groups in Thessaly (Greece) Occup Environ Med. 2014;71:126–133. doi: 10.1136/oemed-2013-101490. [DOI] [PubMed] [Google Scholar]
- 83.Androutsopoulos VP, Hernandez AF, Liesivuori J. A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology. 2013;307:89–94. doi: 10.1016/j.tox.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 84.Sokoloff K, Fraser W, Arbuckle TE, Fisher M, Gaudreau E, LeBlanc A, et al. Determinants of urinary concentrations of dialkyl phosphates among pregnant women in Canada — results from the MIREC study. Environ Int. 2016;94:133–140. doi: 10.1016/j.envint.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 85.Omoike OE, Lewis RC, Meeker JD. Association between urinary biomarkers of exposure to organophosphate insecticides and serum reproductive hormones in men from NHANES 1999–2002. Reprod Toxicol. 2015;53:99–104. doi: 10.1016/j.reprotox.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: structure-activity studies. Mol Pharmacol. 2000;58:852–858. doi: 10.1124/mol.58.4.852. [DOI] [PubMed] [Google Scholar]
- 87.Waters KM, Safe S, Gaido KW. Differential gene expression in response to Methoxychlor and estradiol through ERalpha, ERbeta, and AR in reproductive tissues of female mice. Toxicol Sci. 2001;63:47–56. doi: 10.1093/toxsci/63.1.47. [DOI] [PubMed] [Google Scholar]
- 88.Mrema EJ, Rubino FM, Brambilla G, Moretto A. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 2013;307:74–88. doi: 10.1016/j.tox.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 89.Usmani KA, Cho TM, Rose RL, Hodgson E. Inhibition of the human liver microsomal and human cytochrome P450 1A2 and 3A4 metabolism of estradiol by deployment-related and other chemicals. Drug Metab Dispos. 2006;34:1606–1614. doi: 10.1124/dmd.106.010439. [DOI] [PubMed] [Google Scholar]
- 90.Park S-A, Lee M-H, Na H-K, Surh Y-J. 4-Hydroxyestradiol induces mammary epithelial cell transformation through Nrf2-mediated heme oxygenase-1 overexpression. Oncotarget. 2017;8:164–178. doi: 10.18632/oncotarget.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. Occupational exposure to pesticides and consequences on male semen and fertility: a review. Toxicol Lett. 2014;230:146–156. doi: 10.1016/j.toxlet.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 92.Perry MJ, Venners SA, Chen X, Liu X, Tang G, Xing H, et al. Organophosphorous pesticide exposures and sperm quality. Reprod Toxicol. 2011;31:75–79. doi: 10.1016/j.reprotox.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yucra S, Rubio J, Gasco M, Gonzales C, Steenland K, Gonzales GF. Semen quality and reproductive sex hormone levels in Peruvian pesticide sprayers. Int J Occup Environ Health. 2006;12:355–361. doi: 10.1179/oeh.2006.12.4.355. [DOI] [PubMed] [Google Scholar]
- 94.Lifeng T, Shoulin W, Junmin J, Xuezhao S, Yannan L, Qianli W, et al. Effects of fenvalerate exposure on semen quality among occupational workers. Contraception. 2006;73:92–96. doi: 10.1016/j.contraception.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 95.Conder JM, Hoke RA, De Wolf W, Russell MH, Buck RC. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ Sci Technol. 2008;42:995–1003. doi: 10.1021/es070895g. [DOI] [PubMed] [Google Scholar]
- 96.Steenland K, Zhao L, Winquist A. A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA) Occup Environ Med. 2015;72:373–380. doi: 10.1136/oemed-2014-102364. [DOI] [PubMed] [Google Scholar]
- 97.Giesy JP, Kannan K. Perfluorochemical surfactants in the environment. Environ Sci Technol. 2002;36:146A–152A. doi: 10.1021/es022253t. [DOI] [PubMed] [Google Scholar]
- 98.Biegel LB, Liu RCM, Hurtt ME, Cook JC. Effects of ammonium Perfluorooctanoate on Leydig-cell function: in vitro, in vivo, and ex vivo studies. Toxicol Appl Pharmacol. 1995;134:18–25. doi: 10.1006/taap.1995.1164. [DOI] [PubMed] [Google Scholar]
- 99.Shi Z, Zhang H, Liu Y, Xu M, Dai J. Alterations in gene expression and testosterone synthesis in the testes of male rats exposed to Perfluorododecanoic acid. Toxicol Sci. 2007;98:206–215. doi: 10.1093/toxsci/kfm070. [DOI] [PubMed] [Google Scholar]
- 100.Wan HT, Zhao YG, Wong MH, Lee KF, Yeung WSB, Giesy JP, et al. Testicular signaling is the potential target of Perfluorooctanesulfonate-mediated subfertility in male Mice1. Biol Reprod. 2011;84:1016–1023. doi: 10.1095/biolreprod.110.089219. [DOI] [PubMed] [Google Scholar]
- 101.Zhang H, Lu Y, Luo B, Yan S, Guo X, Dai J. Proteomic analysis of mouse testis reveals perfluorooctanoic acid-induced reproductive dysfunction via direct disturbance of testicular steroidogenic machinery. J Proteome Res. 2014;13:3370–3385. doi: 10.1021/pr500228d. [DOI] [PubMed] [Google Scholar]
- 102.Kang JS, Choi JS, Park JW. Transcriptional changes in steroidogenesis by perfluoroalkyl acids (PFOA and PFOS) regulate the synthesis of sex hormones in H295R cells. Chemosphere. 2016;155:436–443. doi: 10.1016/j.chemosphere.2016.04.070. [DOI] [PubMed] [Google Scholar]
- 103.López-Doval S, Salgado R, Pereiro N, Moyano R, Lafuente A. Perfluorooctane sulfonate effects on the reproductive axis in adult male rats. Environ Res. 2014;134:158–168. doi: 10.1016/j.envres.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 104.Pereiro N, Moyano R, Blanco A, Lafuente A. Regulation of corticosterone secretion is modified by PFOS exposure at different levels of the hypothalamic–pituitary–adrenal axis in adult male rats. Toxicol Lett. 2014;230:252–262. doi: 10.1016/j.toxlet.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Qiu L, Zhang X, Zhang X, Zhang Y, Gu J, Chen M, et al. Sertoli cell is a potential target for perfluorooctane sulfonate-induced reproductive dysfunction in male mice. Toxicol Sci. 2013;135:229–240. doi: 10.1093/toxsci/kft129. [DOI] [PubMed] [Google Scholar]
- 106.Jensen AA, Leffers H. Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl. 2008;31:161–169. doi: 10.1111/j.1365-2605.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol. 2013;47:10619–10627. doi: 10.1021/es401905e. [DOI] [PubMed] [Google Scholar]
- 108.López-Doval S, Salgado R, Lafuente A. The expression of several reproductive hormone receptors can be modified by perfluorooctane sulfonate (PFOS) in adult male rats. Chemosphere. 2016;155:488–497. doi: 10.1016/j.chemosphere.2016.04.081. [DOI] [PubMed] [Google Scholar]
- 109.Olsen GW, Lange CC, Ellefson ME, Mair DC, Church TR, Goldberg CL, et al. Temporal trends of Perfluoroalkyl concentrations in American red Cross adult blood donors, 2000–2010. Environ Sci Technol. 2012;46:6330–6338. doi: 10.1021/es300604p. [DOI] [PubMed] [Google Scholar]
- 110.Raymer JH, Michael LC, Studabaker WB, Olsen GW, Sloan CS, Wilcosky T, et al. Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) and their associations with human semen quality measurements. Reprod Toxicol. 2012;33:419–427. doi: 10.1016/j.reprotox.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kubwabo C, Kosarac I, Lalonde K. Determination of selected perfluorinated compounds and polyfluoroalkyl phosphate surfactants in human milk. Chemosphere. 2013;91:771–777. doi: 10.1016/j.chemosphere.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 112.Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, et al. Trans-placental transfer of thirteen Perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol. 2011;45:7465–7472. doi: 10.1021/es202408a. [DOI] [PubMed] [Google Scholar]
- 113.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 114.Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Kristensen SL, Halldorsson TI, et al. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect. 2013;121:453–458. doi: 10.1289/ehp.1205118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ingelido AM, Abballe A, Gemma S, Dellatte E, Iacovella N, De Angelis G, et al. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto region. Italy Environ Int. 2018;110:149–159. doi: 10.1016/j.envint.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 116.Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebæk NE, Jørgensen N. Do Perfluoroalkyl compounds impair human semen quality? Environ Health Perspect. 2009;117:923–927. doi: 10.1289/ehp.0800517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Louis GMB, Chen Z, Schisterman EF, Kim S, Sweeney AM, Sundaram R, et al. Perfluorochemicals and human semen quality: the LIFE study. Environ Health Perspect. 2015;123:57–63. doi: 10.1289/ehp.1307621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toft G, Jönsson BAG, Lindh CH, Giwercman A, Spano M, Heederik D, et al. Exposure to perfluorinated compounds and human semen quality in arctic and European populations. Hum Reprod. 2012;27:2532–2540. doi: 10.1093/humrep/des185. [DOI] [PubMed] [Google Scholar]
- 119.Specht IO, Hougaard KS, Spanò M, Bizzaro D, Manicardi GC, Lindh CH, et al. Sperm DNA integrity in relation to exposure to environmental perfluoroalkyl substances - A study of spouses of pregnant women in three geographical regions. Reprod Toxicol. 2012;33(4):577–83. [DOI] [PubMed]
- 120.Governini L, Guerranti C, De Leo V, Boschi L, Luddi A, Gori M, et al. Chromosomal aneuploidies and DNA fragmentation of human spermatozoa from patients exposed to perfluorinated compounds. Andrologia. 2015;47:1012–1019. doi: 10.1111/and.12371. [DOI] [PubMed] [Google Scholar]
- 121.La Rocca C, Alessi E, Bergamasco B, Caserta D, Ciardo F, Fanello E, et al. Exposure and effective dose biomarkers for perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in infertile subjects: preliminary results of the PREVIENI project. Int J Hyg Environ Health. 2012;215:206–211. doi: 10.1016/j.ijheh.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 122.La Rocca C, Tait S, Guerranti C, Busani L, Ciardo F, Bergamasco B, et al. Exposure to endocrine disruptors and nuclear receptors gene expression in infertile and fertile men from Italian areas with different environmental features. Int J Environ Res Public Health. 2015;12:12426–12445. doi: 10.3390/ijerph121012426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Foresta C, Tescari S, Di Nisio A. Impact of perfluorochemicals on human health and reproduction: a male’s perspective. J Endocrinol Investig. 2018;41(6):639–645. doi: 10.1007/s40618-017-0790-z. [DOI] [PubMed] [Google Scholar]
- 124.Luccio-Camelo DC, Prins GS. Disruption of androgen receptor signaling in males by environmental chemicals. J Steroid Biochem Mol Biol. 2011;127:74–82. doi: 10.1016/j.jsbmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paoli D, Giannandrea F, Gallo M, Turci R, Cattaruzza MS, Lombardo F, et al. Exposure to polychlorinated biphenyls and hexachlorobenzene, semen quality and testicular cancer risk. J Endocrinol Investig. 2015;38:745–752. doi: 10.1007/s40618-015-0251-5. [DOI] [PubMed] [Google Scholar]
- 126.Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wirth JJ, Mijal RS. Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med. 2010;56:147–167. doi: 10.3109/19396360903582216. [DOI] [PubMed] [Google Scholar]
- 128.Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, et al. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals. 2010;23:769–782. doi: 10.1007/s10534-010-9343-z. [DOI] [PubMed] [Google Scholar]
- 129.Pant N, Kumar G, Upadhyay AD, Patel DK, Gupta YK, Chaturvedi PK. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ Sci Pollut Res. 2014;21:11066–11074. doi: 10.1007/s11356-014-2986-5. [DOI] [PubMed] [Google Scholar]
- 130.Rana SVS. Perspectives in endocrine toxicity of heavy metals—a review. Biol Trace Elem Res. 2014;160:1–14. doi: 10.1007/s12011-014-0023-7. [DOI] [PubMed] [Google Scholar]
- 131.Carette D, Perrard M-H, Prisant N, Gilleron J, Pointis G, Segretain D, et al. Hexavalent chromium at low concentration alters Sertoli cell barrier and connexin 43 gap junction but not claudin-11 and N-cadherin in the rat seminiferous tubule culture model. Toxicol Appl Pharmacol. 2013;268:27–36. doi: 10.1016/j.taap.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 132.de Angelis C, Galdiero M, Pivonello C, Salzano C, Gianfrilli D, Piscitelli P, et al. The environment and male reproduction: the effect of cadmium exposure on reproductive functions and its implication in fertility. Reprod Toxicol. 2017;73:105–127. doi: 10.1016/j.reprotox.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 133.Meeker JD, Calafat AM, Hauser R. Urinary bisphenol a concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol. 2010;44:1458–1463. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Joensen UN, Veyrand B, Antignac JP, Blomberg Jensen M, Petersen JH, Marchand P, et al. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum Reprod. 2013;28:599–608. doi: 10.1093/humrep/des425. [DOI] [PubMed] [Google Scholar]
- 135.Akinloye O, Arowojolu AO, Shittu OB, Anetor JI. Cadmium toxicity: a possible cause of male infertility in Nigeria. Reprod Biol. 2006;6:17–30. [PubMed] [Google Scholar]
- 136.Benoff S, Hauser R, Marmar JL, Hurley IR, Napolitano B, Centola GM. Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers) Mol Med. 2009;15:248–262. doi: 10.2119/molmed.2008.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mendiola J, Moreno JM, Roca M, Vergara-Juárez N, Martínez-García MJ, García-Sánchez A, et al. Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: a pilot study. Environ Health. 2011;10(1):6. [DOI] [PMC free article] [PubMed]
- 138.Telisman S, Cvitković P, Jurasović J, Pizent A, Gavella M, Rocić B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect. 2000;108:45–53. doi: 10.1289/ehp.0010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu D-X, Shen H-M, Zhu Q-X, Chua L, Wang Q-N, Chia S-E, et al. The associations among semen quality, oxidative DNA damage in human spermatozoa and concentrations of cadmium, lead and selenium in seminal plasma. Mutat Res Toxicol Environ Mutagen. 2003;534:155–163. doi: 10.1016/S1383-5718(02)00274-7. [DOI] [PubMed] [Google Scholar]
- 140.Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, et al. Cadmium, Lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect. 2008;116:1473–1479. doi: 10.1289/ehp.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jurasovi J, Cvitkovi P, Pizent A, Colak B, Telišman S. Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. Biometals. 2004;17:735–743. doi: 10.1007/s10534-004-1689-7. [DOI] [PubMed] [Google Scholar]
- 142.Hovatta O, Venalainen ER, Kuusimaki L, Heikkila J, Hirvi T, Reima I. Aluminium, lead and cadmium concentrations in seminal plasma and spermatozoa, and semen quality in Finnish men. Hum Reprod. 1998;13:115–119. doi: 10.1093/humrep/13.1.115. [DOI] [PubMed] [Google Scholar]
- 143.Wang Y-X, Sun Y, Feng W, Wang P, Yang P, Li J, et al. Association of urinary metal levels with human semen quality: A cross-sectional study in China. Environ Health. 2016;91:51–59. [DOI] [PubMed]
- 144.Li Y, Wu J, Zhou W, Gao E. Association between environmental exposure to cadmium and human semen quality. Int J Environ Health Res. 2016;26:175–86. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.