Abstract
Objective:
Evaluate the impact of clinicopathologic characteristics and adjuvant treatment on survival outcomes in early stage uterine carcinosarcoma patients.
Methods:
We performed a retrospective cohort study of women with stage I or II uterine carcinosarcoma at our institution between March 1990 and June 2016. All pathology had been reviewed and confirmed by gynecologic pathologists. Data were extracted from the electronic medical record. Descriptive and comparative statistics were used to compare clinicopathologic characteristics. Univariable and multivariable analyses were performed for survival outcomes.
Results:
140 patients were identified. Median age was 67 years (range: 36–91). Median follow-up was 39.1 months (2.9–297.4). The majority of patients had stage IA (67%) versus stage IB (21%) or stage II (11%) disease. The majority of patients (63%) received adjuvant treatment: vaginal brachytherapy only (14%); whole pelvic radiation therapy only (16%); chemotherapy only (n=13, 9%); combination chemotherapy and vaginal brachytherapy (15%); combination chemotherapy and whole pelvic radiation (9%). 52 patients (37%) received no adjuvant therapy. Median overall survival (OS) was 48.0 months (95% CI 32.7–80.9). On multivariable analysis for OS, advancing age (HR 1.05, 95% CI 1.03–1.08, p<0.001), higher stage (stage IB: HR 1.64, 95% CI 0.91–2.95, p=0.10; stage II: HR 3.04, 95% CI 1.51–6.13, p=0.002), and the presence of a rhabdomyosarcoma component (HR 1.66, 95% CI 1.02–2.70, p=0.04) were significantly associated with worse OS.
Conclusions:
Advancing age, stage, and the presence of a rhabdomyosarcoma component were all associated with worse OS in patients with early stage uterine carcinosarcoma. New treatment algorithms should incorporate factors aside from stage alone.
INTRODUCTION
Among endometrial cancer patients, high grade endometrioid and non-endometrioid histology subsets have significantly worse outcomes [1–5]. Among these higher risk histologies, uterine carcinosarcoma remains one of the most aggressive subtypes [3, 6, 7]. Even when uterine carcinosarcomas are diagnosed at an early stage (i.e., FIGO stage I or II), 5-year overall survival rates have been reported to be as low as 30% [3].
Uterine carcinosarcomas are still relatively rare, representing fewer than 5% of new endometrial cancer diagnoses [6, 8]. Thus, prognostic factors and treatment recommendations are based upon small, retrospective studies. Across studies, FIGO stage at diagnosis has been the most consistent predictor of poor prognosis [3, 8–10]. As a result, the National Comprehensive Cancer Network guidelines for uterine carcinosarcoma remain vague, and are largely based on disease stage [11]. Several studies have identified higher risk attributes including advanced age, larger uterine size, and presence of a heterologous sarcomatous component [7, 9]. Fewer studies, however, have attempted an in depth investigation of the impact of pathology characteristics including specific heterologous elements in early stage uterine carcinosarcomas.
The purpose of this study was to identify factors in early stage uterine carcinosarcoma that portend a worse prognosis. The primary objective was to evaluate the impact of demographic, clinical, and histologic characteristics on overall survival. Secondary objectives were to assess the impact of those characteristics on recurrence-free survival, and to characterize and compare the current treatment approaches for women diagnosed with early stage carcinosarcoma of the uterus.
METHODS
Patient Population
Following Institutional Review Board approval (protocol RCR 05–0005), a single institution, retrospective cohort study was performed at the University of Texas MD Anderson Cancer Center on patients with early stage carcinosarcoma of the uterus diagnosed between March 1990 and June 2016. All patients were surgically managed, and none received neoadjuvant chemotherapy. Patients were considered to be early stage if they had presumed International Federation of Gynecology and Obstetrics (FIGO) Stage IA, IB, or II disease. All pathology was reviewed by the gynecologic pathologists at our institution to confirm histology. Patients were excluded if they did not have primary surgical management. Staging lymphadenectomy was performed at the discretion of the treating physician. Patients were included in survival analysis if they had any post-operative follow-up contact with MD Anderson Cancer Center.
Data Collection and Statistical Analyses
Demographic, clinical, and pathology data were obtained by review of the electronic medical record. All data were collected and managed using a secure REDCap electronic database [12]. Descriptive statistics were used for demographic and clinical characteristics of the cohort. Comparative statistics including Student’s t-test or Wilcoxon rank-sum test, and chi-squared or Fisher’s exact tests were used for continuous or categorical variables, respectively. Overall survival and recurrence-free survival analyses were performed using the Kaplan-Meier product-limit estimator. A log-rank test was used to compare across specified demographic and clinical characteristics. Overall survival was measured from the date of diagnosis until the date of last contact or the date of death. Recurrence-free survival was measured from the date of diagnosis until the date of diagnosed recurrence of disease or date of death. Multivariable analyses for overall survival and recurrence-free survival were performed using a Cox proportional hazards regression analysis. All statistical analysis was performed using Stata/MP v15.0 (College Station, TX).
RESULTS
There were 140 patients included in this analysis. Baseline demographic and clinicopathologic characteristics are listed in Table 1. The median age was 67 years (range 36– 91), the median body mass index (BMI) was 30 kg/m2 (range 17–73), and most patients (71%) were of white race. The most common prior cancer diagnosis was breast cancer, with 15% of women having been previously diagnosed. Only one patient (1%) had received prior pelvic radiation, and 13 patients (9%) had previously received tamoxifen. In terms of pathology characteristics, 77 patients (56%) had a preoperative biopsy demonstrating carcinosarcoma. The majority of patients were FIGO stage IA (67%) at diagnosis. One hundred five patients (74%) underwent staging pelvic lymphadenectomy (median of 8 lymph nodes removed among the 99 lymphadenectomy patients with lymph node number data available, range 1–38), and 69 patients (49%) underwent staging para-aortic lymphadenectomy (median of 4 lymph nodes removed among the 64 lymphadenectomy patients with lymph node number data available, range 1–25). The proportion of patients who received staging pelvic and para-aortic lymphadenectomy remained largely unchanged when limited to patients who were known to have carcinosarcoma on preoperative biopsy. On post-operative histologic assessment, a similar number of patients had a majority (≥50%) epithelial versus sarcomatous component (48% vs. 52%). Fifty-seven patients (43%) had a heterologous component present, and of these, 31 patients (54% of those with heterologous components) had a rhabdomyosarcoma component. Only seven patients included in this study had had next generation sequencing for mutational analysis using a 50 gene AmpliSeq sequencing panel (CMS50; Life Technologies). Interestingly, all seven patients who had next generation sequencing of their tumor performed were found to have more than one mutation present, and six of the seven patients had a mutation in TP53. Other mutations that occurred concurrently with TP53 included FBXW7 (n=2), CDKN2A (one patient), KRAS (n=2), and PIK3CA (n=1). The patient without a TP53 mutation had mutations in PTEN and SMAD4.
Table 1:
Demographic, clinicopathologic, treatment, and recurrence data
| Characteristic | Uterine carcinosarcoma patients (n=140) |
|---|---|
| Clinical | |
| Median age, years (range) | 67 (36–91) |
| Median BMI, kg/m2 (range) | 30 (17–73) |
| Race | |
| White | 100 (71.4%) |
| Black | 35 (25.0%) |
| Asian | 2 (1.4%) |
| Other or unknown | 3 (2.1%) |
| Prior Treatment History | |
| Prior Pelvic Radiation | 1 (0.7%) |
| Prior Chemotherapy | 7 (5.3%) |
| Prior Tamoxifen Use | 13 (9.3%) |
| Pathology | |
| FIGO Stage | |
| IA | 94 (67.1%) |
| IB | 30 (21.4%) |
| II | 16 (11.4%) |
| Deep myometrial invasion (≥ 50%) (n=126) | 36 (29%) |
| Median tumor size, cm (range) | 6.0 (0.3–21.0) |
| Lymphovascular space invasion present (n=137) | 55 (40.2%) |
| Heterologous component present (n=132) | 57 (43.2%) |
| Majority sarcoma component (≥ 50%) (n = 67) | 35 (52.2%) |
| Rhabdomyosarcoma component present (n=128) | 31 (24.2%) |
| Adjuvant Treatment (n=140) | |
| Summary of adjuvant treatment approach | |
| None | 52 (37.1%) |
| VBT only | 20 (14.3%) |
| Whole pelvic radiation therapy (with or without VBT) | 22 (15.7%) |
| VBT with chemotherapy | 21 (15.0%) |
| Whole pelvic radiation therapy with chemotherapy | 12 (9.3%) |
| Chemotherapy alone | 13 (9.3%) |
| Recurrence (n=137) | |
| Recurrence during follow-up | 70 (51.1%) |
| Treatment for recurrence (n=70) | |
| No treatment | 14 (20.0%) |
| Chemotherapy | 32 (45.7%) |
| Radiation | 10 (14.3%) |
| Chemo and radiation | 5 (7.1%) |
| Surgery alone | 2 (2.8%) |
| Surgery with chemotherapy or radiation | 6 (8.6%) |
| Hormones alone | 1 (1.4%) |
VBT = vaginal brachytherapy
Adjuvant treatment data was available for 140 patients as outlined in Table 1. Fifty-two patients (37%) did not receive any adjuvant treatment. Patients receiving adjuvant treatment included: cuff brachytherapy alone (14%); whole pelvic radiation with or without concurrent chemotherapy (16%); cuff brachytherapy with adjuvant chemotherapy (15%); whole pelvic radiation with adjuvant chemotherapy (9%).
One hundred thirty nine patients had any contact after surgery and were therefore included in the overall survival analysis. Of the 137 patients with recurrence information available, 70 (51%) had recurrence of disease. Of the 70 patients who had recurrence of disease, 49 (70%) had received some form of adjuvant therapy after their initial surgery. Of the 38 patients with biopsy results available, 24 patients (63%) had an epithelial recurrence, 4 (11%) had a sarcomatous recurrence, and 10 (26%) had a mixed histology recurrence. Six of the 14 patients with a sarcomatous or mixed histology recurrence had a rhabdomyosarcoma component in their initial tumor. Treatment for recurrence of disease varied, with 59% of patients who recurred receiving chemotherapy and 26% receiving radiation with or without sensitizing chemotherapy. Few patients underwent surgery for disease recurrence (11%) or received hormonal therapy (4%). Most of the chemotherapy regimens administered for recurrence of disease were epithelial regimens (83%).
The median follow-up time for the cohort was 39.1 months (range 95% CI 2.9–297.4). The median overall survival was 48.0 months (95% CI 32.7–80.9). On the unadjusted analysis, increasing age at diagnosis (HR 1.05, 95% CI 1.02–1.07, p < 0.001), higher FIGO stage (stage IB: HR 1.76, 95% CI 1.08–2.87, p = 0.02; Stage II: HR 2.50, 95% CI 1.39–4.53, p = 0.002), and the presence of a rhabdomyosarcoma component (HR 1.82, 95% CI 1.14–2.93, p = 0.01) were all associated with worse overall survival (Table 2). A rhabdomyosarcoma element was not associated with worse RFS. For recurrence-free survival, only older age at diagnosis (HR 1.03, 95% CI 1.01–1.05, p = 0.006) and increasing FIGO stage (stage IB: HR 1.92, 95% CI 1.21–3.06, p = 0.006; stage II: HR 2.19, 95% CI 1.24–3.87, p = 0.007) were associated with worse outcomes (Table 2). Of note, when we limited the analyses to only the patients who had pelvic and/or para-aortic lymph node assessment, these overall and recurrence-free survival relationships remained unchanged. Furthermore, there were no statistically significant differences in overall or recurrence-free survival for patients who had had lymph node assessment performed compared with those that had not. Therefore, for the remainder of our survival analyses we included all patients with survival information available.
Table 2:
Survival analyses. A: Unadjusted overall survival and recurrence-free survival for the entire cohort (n=139); B: Unadjusted overall survival in the subset of patients who survived at least five years (n = 53)
| A: | ||||
|---|---|---|---|---|
| Characteristic | Overall survival | Recurrence-free survival | ||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age at diagnosis | 1.05 (1.02–1.07) | < 0.001 | 1.03 (1.01–1.05) | 0.006 |
| Tumor size (cm) | 1.06 (0.98–1.16) | 0.16 | 1.05 (0.97–1.13) | 0.22 |
| FIGO Stage | ||||
| IA | Ref | Ref | Ref | Ref |
| IB | 1.76 (1.08–2.87) | 0.02 | 1.92 (1.21–3.06) | |
| II | 2.50 (1.39–4.53) | 0.002 | 2.19 (1.24–3.87) | 0.007 |
| Black race (Ref: any other race) | 1.16 (0.75–1.81) | 0.50 | 0.96 (0.62–1.49) | 0.86 |
| LVSI present | 1.44 (0.95–2.18) | 0.08 | 1.24 (0.84–1.85) | 0.28 |
| Heterologous component present | 1.18 (0.78–1.79) | 0.44 | 1.07 (0.71–1.61) | 0.75 |
| Rhabdomyosarcoma component present | 1.82 (1.14–2.93) | 0.01 | 1.53 (0.97–2.41) | 0.07 |
| Sarcoma component ≥ 50% | 1.29 (0.71–2.34) | 0.41 | 1.51 (0.84–2.71) | 0.17 |
| Adjuvant therapy | ||||
| None | Ref | Ref | Ref | Ref |
| VBT only | 1.12 (0.61–2.05) | 0.71 | 1.21 (0.66–2.22) | 0.53 |
| Pelvic radiation only | 1.19 (0.66–2.11) | 0.56 | 1.25 (0.70–2.22) | 0.45 |
| VBT with chemotherapy | 0.78 (0.37–1.64) | 0.52 | 0.85 (0.42–1.72) | 0.65 |
| Pelvic RT with adjuvant chemotherapy | 1.01 (0.42–2.41) | 0.99 | 0.93 (0.41–2.09) | 0.86 |
| Adjuvant chemotherapy only | 0.55 (0.55–2.38) | 0.71 | 1.32 (0.68–2.58) | 0.42 |
| B: | ||
|---|---|---|
| Characteristic | Overall survival | |
| HR (95% CI) | p-value | |
| Age at diagnosis | 1.08 (1.03–1.13) | 0.001 |
| Tumor size (cm) | 1.00 (0.81–1.22) | 0.97 |
| FIGO Stage | ||
| IA | Ref | |
| IB | 2.02 (0.72–5.67) | 0.18 |
| II | 5.53 (1.53–20.0) | 0.009 |
| Black race (Ref: any other race) | 1.48 (0.65–3.35) | 0.35 |
| LVSI present | 1.32 (0.57–3.07) | 0.52 |
| Heterologous component present | 1.45 (0.64–3.31) | 0.37 |
| Rhabdomyosarcoma component present | 1.73 (0.49–6.09) | 0.39 |
| Sarcoma component ≥ 50% | 0.89 (0.22–3.64) | 0.87 |
| Adjuvant therapy | ||
| None | Ref | |
| VBT only | 1.94 (0.55) | 0.30 |
| Pelvic RT only | 0.70 (0.15–3.34) | 0.66 |
| VBT with chemotherapy | 4.41 (0.77–25.26) | 0.10 |
| Pelvic RT with chemotherapy | 4.58 (0.91–23.04) | 0.07 |
| Adjuvant chemo only | 1.51 (0.32–7.01) | 0.60 |
LVSI=lymphovascular space invasion
VBT=Vagina cuff brachytherapy
VBT = vaginal brachytherapy
LVSI = lymphovascular space invasion
On multivariable analysis of overall survival, older age at diagnosis (HR 1.05, 95% CI 1.03–1.08, p < 0.001), stage II disease (HR 3.04, 95% CI 1.51–6.13, p = 0.002), and the presence of a rhabdomyosarcoma component (HR 1.66, 95% CI 1.02–2.70, p = 0.04) were all associated with worse outcomes (Table 3). For recurrence-free survival, only higher stage at diagnosis (stage IB: HR 1.95, 95% 1.11–3.41, p=0.02; stage II disease: HR 2.86, 95% CI 1.46–5.60, p = 0.002) was associated with worse outcomes in a multivariable model. The presence of a rhabdomyosarcoma component was not statistically significant (HR 1.50, 95% CI 0.94–2.40, p = 0.09).
Table 3:
Multivariable analysis for recurrence-free survival and overall survival
| Survival | Characteristic | HR | 95% UB | 95% LB | p-value |
|---|---|---|---|---|---|
| OS | Age at diagnosis (in years) | 1.05 | 1.03 | 1.08 | <0.001 |
| FIGO stage (ref: IA) | |||||
| IB | 1.64 | 0.91 | 2.95 | 0.100 | |
| II | 3.04 | 1.51 | 6.13 | 0.002 | |
| LVSI | 1.10 | 0.66 | 1.81 | 0.722 | |
| Rhabdomyosarcoma component | 1.66 | 1.02 | 2.70 | 0.041 | |
| RFS | Age at diagnosis (in years) | 1.03 | 1.01 | 1.05 | 0.011 |
| FIGO stage (ref: IA) | |||||
| IB | 1.95 | 1.11 | 3.41 | 0.019 | |
| II | 2.86 | 1.46 | 5.60 | 0.002 | |
| LVSI | 0.86 | 0..53 | 1.39 | 0.532 | |
| Rhabdomyosarcoma component | 1.50 | 0.94 | 2.40 | 0.090 | |
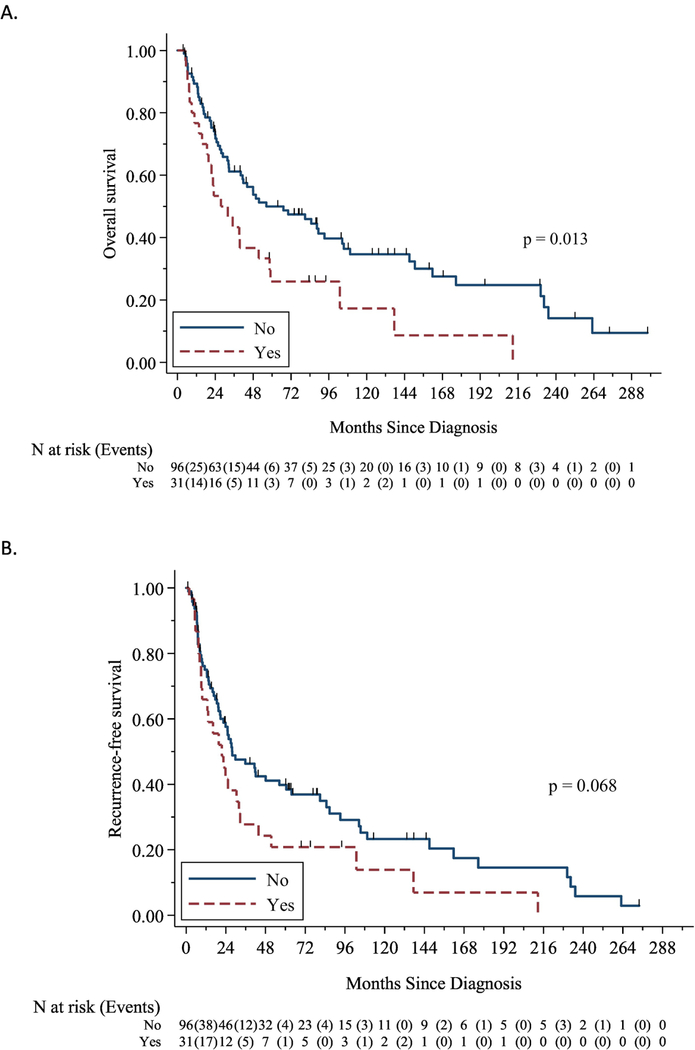
Because of the relationship between overall survival and a rhabdomyosarcoma component (Figure 1), we further evaluated those patients whose tumors contained this element. A comparison of patients who had tumors with or without rhabdomyosarcoma elements are outlined in Table 4. Tumors containing a rhabdomyosarcoma component were larger, and were more likely to have predominantly sarcomatous histology. There were no differences in other demographic, clinical, or histologic characteristics. When we included tumor size in the original multivariable analyses for OS and RFS, there were no changes to the relationship between a rhabdomyosarcoma component and each of the survival outcomes; additionally, tumor size was not significantly associated with survival. There were also no differences in approaches to adjuvant therapy for patients with or without a rhabdomyosarcoma component. Most patients with a rhabdomyosarcoma component who ultimately recurred received some combination of radiation and/or chemotherapy for their disease recurrence. Of the 10 patients with rhabdomyosarcomatous elements who had biopsy information available for recurrence, four had an epithelial recurrence, three had a sarcomatous recurrence, and three had a mixed recurrence.
Figure 1:
Overall survival (A) and recurrence-free survival (B) Kaplan Meier curves stratified around the presence or absence of a rhabdomyosarcoma element.
Table 4:
Demographic and clinicopathologic data stratified by the presence or absence of a rhabdomyosarcoma component
| No rhabdomyosarcoma component present (n=97) |
Rhabdomyosarcoma component present (n=31) |
p-value | |
|---|---|---|---|
| Median age, years (range) | 66 (36–91) | 70 (51–87) | 0.06 |
| Median BMI, kg/m2 (range) | 29.1 (17.3–73.3) | 30.4 (21.3–62.8) | 0.45 |
| Race | 0.24 | ||
| White | 70 (72.2%) | 21 (65.6%) | |
| Black | 24 (24.7%) | 9 (29.0%) | |
| Asian | 0 (0%) | 1 (3.2%) | |
| Other or unknown | 3 (3.1%) | 0 (0%) | |
| Prior Treatment History | |||
| Prior Pelvic Radiation | 1 (1.1%) | 0 (0%) | 1.00 |
| Prior Chemotherapy | 4 (4.4%) | 2 (6.5%) | 0.65 |
| Prior Tamoxifen Use | 8 (44.4%) | 4 (50%) | 1.00 |
| FIGO Stage | 0.85 | ||
| IA | 69 (71.1%) | 21 (67.7%) | |
| IB | 17 (17.5%) | 6 (19.3%) | |
| II | 11 (11.3%) | 4 (12.9%) | |
| Deep myometrial invasion (≥ 50%) | 24 (26.7%) | 6 (22.2%) | 0.80 |
| Median tumor size, cm (range) (n=77) | 6.0 (0.3–14.0) | 9.0 (4.0–21.0) | 0.003 |
| Lymphovascular space invasion present (n=127) | 39 (40.6%) | 13 (41.9%) | 0.90 |
| Majority sarcoma component (≥ 50%) (n=67) | 15 (34.1%) | 20 (87.0%) | < 0.001 |
| Overall adjuvant treatment approach | 0.85 | ||
| None | 35 (36.1%) | 13 (41.9%) | |
| Cuff brachytherapy | 15 (15.5%) | 5 (16.1%) | |
| Whole pelvic radiation therapy (with or without VBT) | 16 (16.5%) | 3 (9.7%) | |
| VBT with adjuvant chemotherapy | 15 (15.5%) | 4 (12.9%) | |
| Pelvic RT with adjuvant chemotherapy | 7 (7.2%) | 4 (12.9%) | |
| Chemotherapy alone | 9 (9.3%) | 2 (6.5%) | |
Lastly, we examined the subset of 53 patients from our cohort who experienced long-term survival (Table 2B). 38% of these women were dispositioned to surveillance after their initial surgery, and the remaining 62% received some form of adjuvant therapy. There was no significant difference in adjuvant therapy approach between the women who experienced long-term survival and those that did not. Additionally, none of the women who survived at least 5 years had next-generation sequencing of their tumors performed. Among women who survived at least five years, only age at diagnosis (HR 1.08, 95% CI 1.03–1.13, p = 0.001) and FIGO stage II disease (HR 5.53, 95% CI 1.53–19.98, p=0.009) were associated with an increased risk of death beyond the five-year landmark. Differences in other clinical and histologic characteristics were not associated with the resultant long-term survivorship.
DISCUSSION
Our data show that even women with early stage uterine carcinosarcomas have poor 5-year survival. Interestingly, the majority of our patients did not have other histologic attributes typically associated with aggressive disease, including lymphovascular space invasion or deep myometrial invasion. When we evaluated the subset patients who survived five years, only increasing age at diagnosis and stage II disease were associated with worse overall survival beyond 5 years.
Our data demonstrate a worse clinical prognosis in patients with a higher stage of disease as well as in patients with increasing age at diagnosis, which is common across many tumor types. However, we also found that the presence of a rhabdomyosarcoma component was associated with worse overall survival on multivariable analysis when controlling for those other prognostic factors.
Although the number of women with tumors containing rhabdomyosarcoma elements was relatively small, our data suggest that patients with uterine carcinosarcomas containing a rhabdomyosarcoma component tend to have tumors that are larger at diagnosis than uterine carcinosarcoma patients without this histologic finding. Interestingly, the presence of a rhabdomyosarcoma element was not associated with higher risk histologic characteristics, such as lymphovascular space invasion, deep myometrial invasion, or increasing FIGO stage. Additionally, although the majority of tumors with a rhabdomyosarcoma component were predominantly sarcomatous in histology, there were a small subset that were majority epithelial. This suggests that even tumors with a relatively small sarcomatous element could contain a rhabdomyosarcoma component, which might still have implications on survival outcomes. Finally, based on the distribution of adjuvant therapy in this cohort, the presence of a rhabdomyosarcoma component did not alter the adjuvant treatment strategy used. This was likely due to the current practice of using FIGO stage as the driving force for treatment decision-making. Unfortunately, even within the subset of patients who received chemotherapy, due to the small number of these patients overall we are unable to infer whether an epithelial or sarcoma-based regimen might be more effective in this population.
The presence of a heterologous sarcomatous component has been previously shown to be associated with worse outcomes [7, 13–15]. In the most recent study by Ferguson et al, 42 uterine carcinosarcoma patients found to have early stage disease were evaluated [7]. Twenty-two of the patients had tumors with heterologous elements. The authors found that those patients whose tumors had heterologous elements had significantly worse disease-free survival and overall survival. The authors did not compare the impact of this or other specific heterologous elements on survival outcomes, possibly due to the relatively small number of patients with rhabdomyoblastic differentiation. Our data therefore builds on that research. While our findings did have some differences (e.g., 54% of tumors with heterologous elements had a rhabdomyosarcoma component compared with only 22% in the other study), the idea that heterologous components are associated with worse survival outcomes is reflected in both populations. Interestingly, our data did not show a difference in survival outcomes when heterologous elements were analyzed as a combined group. However, the fact that a specific subtype of heterologous elements was linked with worse overall survival similarly supports a clinical approach which takes heterologous elements into consideration during counseling and treatment planning. It may be beneficial for future, larger studies to investigate the impact of specific elements, as combining all heterologous elements into one group may be oversimplified and therefore may fail to identify clinically important subsets of patients.
Due to the limited nature of data available for uterine carcinosarcoma, treatment decisions are currently based on FIGO stage at diagnosis. Although this was also shown to be an important prognostic marker among our patient population, these data suggest that other histologic findings may be important as well. Furthermore, our data suggest that at our institution the distribution of adjuvant treatment strategies used for uterine carcinosarcoma with a rhabdomyosarcoma element reflects the overall distribution of treatment approaches for the entire group of uterine carcinosarcoma patients. Thus, it is unlikely that a skewed adjuvant treatment approach is responsible for this discrepancy in clinical outcomes. It is possible, therefore, that including the presence of a rhabdomyosarcoma element in the treatment decision-making algorithm may impact survival outcomes for these patients.
In general, the ideal treatment for early stage carcinosarcoma patients remains uncertain. In a recent multi-institutional review, early stage carcinosarcoma patients who received no adjuvant therapy had significantly worse OS than those that received adjuvant therapy [16]. Furthermore, patients who received multimodal adjuvant therapy did better than those who received chemotherapy alone. However, they did not find any difference in survival outcomes between chemotherapy and radiation therapy alone. Even among patients who receive chemotherapy, however, the best regimen for uterine carcinosarcoma patients has yet to be established. Two Gynecologic Oncology Group (GOG) studies have evaluated different chemotherapy regimens in advanced/recurrent uterine carcinosarcoma. Both studies demonstrated a benefit in combination therapy over single agent ifosfamide, although survival outcomes even in the combination group remained poor [17, 18]. Another GOG study evaluated the tolerability of the combination of ifosfamide and cisplatin specifically in Stage I and II uterine carcinosarcoma patients [17]. However, this study did not have a control arm, and thus efficacy of this regimen in early stage patients could not be evaluated. Taken altogether, these data underscore the uncertainty surrounding adjuvant therapy in this patient population is most appropriate.
In our cohort, 37% of patients did not receive any adjuvant therapy. Our data suggest that uterine carcinosarcoma patients with a rhabdomyosarcoma component might benefit from some form of adjuvant therapy. Although our population was not large enough to determine which approach might be most effective, conceptually we question whether the addition of chemotherapy with a sarcoma-tailored regimen might have some benefit in these patients with a more aggressive sarcomatous component. The addition of radiation therapy should certainly also be considered, and most patients in our study (54%) did receive some form of radiation. Additionally, although the number of patients with mutational assessment was small, the high number of mutations present in the few that were studied suggests that further next-generation sequencing assessment may be beneficial. If common actionable mutations are identified in this relatively rare cancer type, this might ultimately allow for the use of novel targeted therapies. Future research addressing the question of treatment approach is certainly warranted.
The main strength of this study is the large number of patients with a rare, early stage tumor. Other strengths of this study include the extent of the clinical information available for each patient, and specifically details about the adjuvant treatment strategies that were used. Additionally, specialized gynecologic pathologists at our tertiary referral center evaluated all tumors in the cohort, which decreases the likelihood of misdiagnosis. This study also has several weaknesses. The population size did not allow for adequate power to evaluate the impact of treatment approach on survival outcomes, and did not allow for meaningful subanalyses to be conducted in some of the subgroups of interest. Additionally, due to the large time span over which data were collected, there may have been differences in management strategies over time. Approximately 33% of our patients were treated between 1989 and 1999, 44% were treated from 2000 to 2009, and 24% were treated after 2010. Patients after 2000 were significantly more likely to have been treated with a combination of chemotherapy and radiation therapy. However, this variation did allow for broader comparisons of a variety of treatment strategies in our patients, which may be useful in this group of patients whose optimal treatment strategy still remains unclear. We also acknowledge that patient and pathology information was not available for a proportion of the cohort (e.g., 6% of the cohort did not have information regarding whether the carcinosarcoma had heterologous elements, and an additional 3% did not comment on whether or not a rhabdomyosarcoma component was present). Therefore, we cannot be certain that the missing patients would not have imposed a bias on our results. However, although not all tumors with heterologous elements included information regarding the presence of absence of a rhabdomyosarcoma element, the 128 patients with this information available still represent the largest cohort of carcinosarcoma patients in which this specific heterologous element was investigated to date. Finally, we did not require full surgical staging for all of the patients included in this study, and thus it is possible that there were patients with microscopic metastatic disease who were included. However, we repeated our survival analyses limiting only to the subgroups of patients who had full staging and found no changes to the survival analyses. In addition, incomplete surgical staging is a real world consideration that is encountered in clinical practice, and thus this paper addresses a practical group of patients by including those without complete staging into our evaluation of early stage patients. Although our institution is a tertiary referral center, it is likely reflective of other tertiary referral centers across the country and our findings will be relevant to many carcinosarcoma patients.
In conclusion, early stage carcinosarcoma of the uterus is an aggressive and heterogeneous histologic subtype of endometrial cancer. Overall survival appears to be worse for patients who are older at diagnosis, have advancing stage, and for those with a rhabdomyosarcoma element present. Although our understanding of this disease is improving, more research is needed to better delineate prognostic and predictive biomarkers for this tumor type in order to help guide treatment approaches. Specifically, our data suggest that future research should focus on the relevance of various histologic elements within this relatively heterogeneous type of tumor. Although clinical trials are sorely needed in order to most effectively compare therapies, prospective studies are difficult due to the rare nature of this tumor type. Future research will likely require multi-institutional and collaborative approaches to better understand this important gynecologic malignancy.
Highlights:
-
-
Most early stage uterine carcinosarcoma patients receive adjuvant treatment with radiation and/or chemotherapy
-
-
Recurrence-free and overall survival for uterine carcinosarcoma patients is poor
-
-
Advancing age, stage, and the presence of a rhabdomyosarcoma component were associated with worse overall survival
Acknowledgments
Funding sources:
Supported in part by the NIH/NCI Training Grant (T32CA101642) (KCK); MD Anderson Cancer Center Support Grant (P30CA016672) SPORE in Uterine Cancer (NIH 2P50CA098258–06), (SNW, PTS, KHL), Andrew Sabin Family Fellowship (SNW)
SNW: AstraZeneca (grants and personal fees), Takeda (personal fees), Tesaro (grants), TRM Oncology (personal fees), ACI Clinical/Xenetic Biosciences (personal fees), Syndax/Watermark (personal fees), Clovis Oncology (personal fees), Genentech (personal fees), Bayer (grants), COTI (grants), Novartis (grants), BioAscend (personal fees).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No other authors report any disclosures.
REFERENCES
- [1].Boruta DM 2nd, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecologic oncology 2009;115(1):142–53. [DOI] [PubMed] [Google Scholar]
- [2].del Carmen MG, Birrer M, Schorge JO. Uterine papillary serous cancer: a review of the literature. Gynecologic oncology 2012;127(3):651–61. [DOI] [PubMed] [Google Scholar]
- [3].Amant F, Cadron I, Fuso L, Berteloot P, de Jonge E, Jacomen G, et al. Endometrial carcinosarcomas have a different prognosis and pattern of spread compared to high-risk epithelial endometrial cancer. Gynecologic oncology 2005;98(2):274–80. [DOI] [PubMed] [Google Scholar]
- [4].Olawaiye AB, Boruta DM 2nd. Management of women with clear cell endometrial cancer: a Society of Gynecologic Oncology (SGO) review. Gynecologic oncology 2009;113(2):277–83. [DOI] [PubMed] [Google Scholar]
- [5].de Jong RA, Nijman HW, Wijbrandi TF, Reyners AK, Boezen HM, Hollema H. Molecular markers and clinical behavior of uterine carcinosarcomas: focus on the epithelial tumor component. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2011;24(10):1368–79. [DOI] [PubMed] [Google Scholar]
- [6].Bansal N, Herzog TJ, Seshan VE, Schiff PB, Burke WM, Cohen CJ, et al. Uterine carcinosarcomas and grade 3 endometrioid cancers: evidence for distinct tumor behavior. Obstetrics and gynecology 2008;112(1):64–70. [DOI] [PubMed] [Google Scholar]
- [7].Ferguson SE, Tornos C, Hummer A, Barakat RR, Soslow RA. Prognostic features of surgical stage I uterine carcinosarcoma. The American journal of surgical pathology 2007;31(11):1653–61. [DOI] [PubMed] [Google Scholar]
- [8].Hosh M, Antar S, Nazzal A, Warda M, Gibreel A, Refky B. Uterine Sarcoma: Analysis of 13,089 Cases Based on Surveillance, Epidemiology, and End Results Database. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2016;26(6):1098–104. [DOI] [PubMed] [Google Scholar]
- [9].Callister M, Ramondetta LM, Jhingran A, Burke TW, Eifel PJ. Malignant mixed Mullerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome. International journal of radiation oncology, biology, physics 2004;58(3):786–96. [DOI] [PubMed] [Google Scholar]
- [10].Gonzalez Bosquet J, Terstriep SA, Cliby WA, Brown-Jones M, Kaur JS, Podratz KC, et al. The impact of multi-modal therapy on survival for uterine carcinosarcomas. Gynecologic oncology 2010;116(3):419–23. [DOI] [PubMed] [Google Scholar]
- [11].Network NCC. Uterine Neoplasms (Version 1.2017) [updated November 21, 2016. Available from: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
- [12].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Silverberg SG, Major FJ, Blessing JA, Fetter B, Askin FB, Liao SY, et al. Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus. A Gynecologic Oncology Group pathologic study of 203 cases. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists 1990;9(1):1–19. [DOI] [PubMed] [Google Scholar]
- [14].Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer 1993;71(4 Suppl):1702–9. [DOI] [PubMed] [Google Scholar]
- [15].Iwasa Y, Haga H, Konishi I, Kobashi Y, Higuchi K, Katsuyama E, et al. Prognostic factors in uterine carcinosarcoma: a clinicopathologic study of 25 patients. Cancer 1998;82(3):512–9. [PubMed] [Google Scholar]
- [16].Dickson EL, Vogel RI, Gehrig PA, Pierce S, Havrilesky L, Secord AA, et al. A multi- institutional study of outcomes in stage I-III uterine carcinosarcoma. Gynecologic oncology 2015;139(2):275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sutton G, Kauderer J, Carson LF, Lentz SS, Whitney CW, Gallion H. Adjuvant ifosfamide and cisplatin in patients with completely resected stage I or II carcinosarcomas (mixed mesodermal tumors) of the uterus: a Gynecologic Oncology Group study. Gynecologic oncology 2005;96(3):630–4. [DOI] [PubMed] [Google Scholar]
- [18].Homesley HD, Filiaci V, Markman M, Bitterman P, Eaton L, Kilgore LC, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2007;25(5):526–31 [DOI] [PubMed] [Google Scholar]