Abstract
A modular, cost-effective route to a library of branched fluorous tags with two short, biocompatible, fluorinated chains (C6F13) is reported. These branched fluorous tags provide high fluorous content without the use of long-chain linear perfluorocarbons (e.g. perfluorooctanoic acid), which have rising health concerns due to their bioaccumulation. By attaching these tags to a porphyrin, it is demonstrated that high solubility can be achieved in fluorous solvents that are readily cleared from mammals. This work enhances the biocompatibility of perfluorocarbon nanoemulsions for photodynamic therapy.
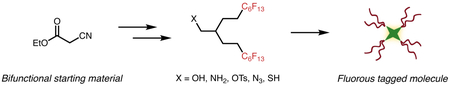
Graphical Abstract
Perfluorocarbons, molecules where all the hydrogen atoms are replaced with fluorine atoms, are an unnatural class of compounds with distinct characteristics due to the size and electronegativity of fluorine.1 The inert nature and non-polarizability of perfluorinated materials have been widely realized through the success of poly(tetrafluoroethylene) as a non-stick coating.2,3 The high gas content of perfluorocarbons, coupled with their low boiling points and metabolic stability, were exploited for oxygen delivery using perfluorocarbon nanoemulsions in the 1980s.4 The synthetic chemistry community was introduced to the orthogonality of perfluorocarbons in 1994 as a means to efficiently recycle catalysts.5 This work coined the term “fluorous” and initiated the field of fluorous phase chemistry where perfluorinated tags were appended to organic compounds to facilitate purification by fluorous extraction. The use of fluorous tags has since been extended to biological applications to streamline proteomics,6 display bioactive molecules on microarrays,7–9 improve transport into cells,10,11 and encapsulate chromophores inside perfluorocarbon nanoemulsions.12,13
Given the broad applications of perfluorinated compounds, the molecular requirements for obtaining fluorous phase solubility have been extensively explored. Strategies to solubilize compounds in perfluorocarbon include increasing the weight percent fluorine (wt% F, ideally to above 60%) through the addition of fluorous tags.14 Initially, C8F17 tags were determined to have the appropriate balance of synthetic ease and fluorous character.15 Flowever, concerns regarding the environmental persistence of long-chain perfluorinated compounds (C7 or greater, e.g. perfluorooctanoic acid) prevent the use of C8F17 chains as fluorous solubilizing groups.16,17 For the full potential of the fluorous phase to be realized, methods to render molecules soluble in perfluorocarbon with C6F13 fluorous tags or shorter are necessary.
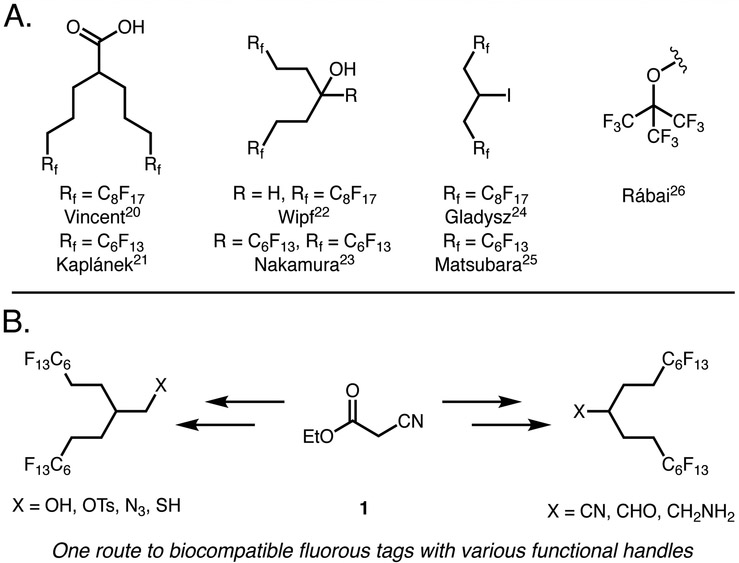
Branched tags are a promising approach to employ short fluorous segments, yet still obtain high wt% F. Furthermore, branched alkyl chains have been shown to improve the solubility of planar aromatic compounds in organic solution,18,19 indicating that branched tags may impart superior fluorous solubility at lower wt% F. Previously reported strategies to access branched C6F13 tags include malonate alkylation20,21 Grignard addition22,23 and sequential iodo-ene/elimination reactions24,25 (Figure 1A). Perfluorinated tert-butyl groups have also been investigated as biocompatible fluorous tags through the addition of perfluoro-tert-butoxide.26,27 Collectively, these approaches have validated the use of short fluorous segments to impart solubility in perfluorocarbons; yet, there remains no tag that can easily be appended to compounds with a variety of different chemistries.
Figure 1.
Approaches to branched fluorous tags. (A) Existing approaches. (B) Our approach from ethyl cyanoacetate 1 leading to multiple functional handles in minimal steps.
Here, we describe the preparation of branched C6F13 fluorous tags from ethyl cyanoacetate 1. The minimal steric hindrance of 1 facilitates alkylation with readily accessible (perfluorohexyl)ethyl iodide (<$ 1/g),28 as opposed to the expensive ($36/g)29 and difficult to prepare (perfluorohexyl)propyl iodide30 that is necessary for similar strategies21 Additionally, the use of ethyl cyanoacetate allows direct access to two distinct chemical functionalities, which can be easily converted to an array of functional groups for tagging compounds of interest (Figure 1B).
To showcase the general utility of these tags, we prepared a highly fluorous-soluble porphyrin. We compared the porphyrin containing branched C6F13 chains to one containing linear C8F17 chains. Leveraging the absorbance and emission of porphyrins, we determined that the branched fluorous tags resulted in an increased fluorous partition coefficient, solubility in an array of perfluorocarbons, and superior retention in droplets of fluorous solvent (e.g. perfluorocarbon nanoemulsions). Efficient encapsulation of porphyrins in perfluorocarbon nanoemulsions is of particular interest for applications in photomedicine. The combination of enhanced solubility imparted by branched fluorous tags and the removal of persistent fluorinated chains increases the biocompatibility of perfluorocarbon (PFC) nanoemulsions for photodynamic therapy.
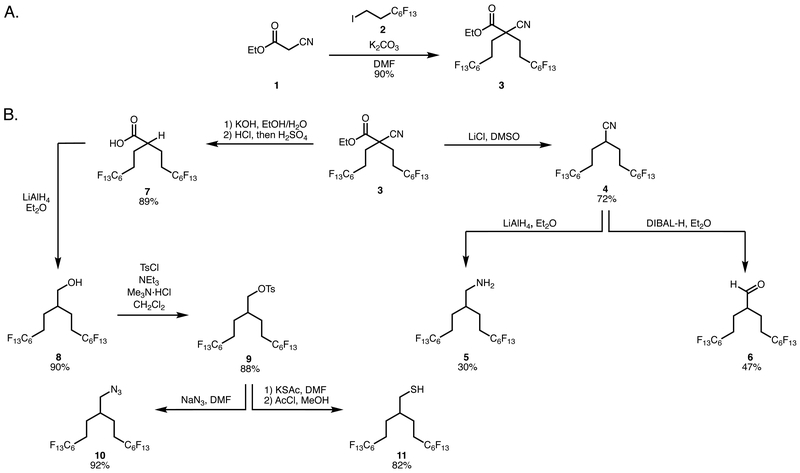
In efforts to develop a general strategy for the preparation of biocompatible branched fluorous tags, we looked to employ readily available starting materials that could be converted to multiple functional handles in a few, simple steps. We envisioned that alkylation chemistry would be the most efficient to install two fluorous chains in one pot. We targeted (perfluorohexyl)ethyl iodide 2 as the fluorous starting material due to its low cost and high wt% F. Previously, malonate esters have been employed for direct addition of two fluorous chains via alkylation; however, (perfluoroalkyl)propyl iodides were necessary (Figure 1A). Our attempts to doubly alkylate malonate esters with (perfluorohexyl)ethyl iodide resulted in monoaddition at mild temperatures and elimination at elevated temperatures (Scheme S1 and Table S1). Looking to increase the reactivity of the nucleophile, we found that ethyl cyanoacetate 1 could be successfully dialkylated with 2 to provide 3 in 90% yield, with less than 1% of fluoride elimination observed (Scheme 1A).31
Scheme 1.
(A) Synthesis of modular building block 3. (B) Synthesis ofa library of branched fluorous tags from 3.
The ethyl cyanoacetate provided increased reactivity as well as two separate functional groups that could be transformed into branched fluorous tags with different functional handles (Scheme 1B). In one step from 3, we could access nitrile tag 4 through Krapcho decarboxylation. The nitrile could be reduced to the primary amine 5 or aldehyde 6, demonstrating the immediate modularity of this approach. Concurrently, a sequential saponification and decyanation provided branched carboxylic acid tag 7 in 89% yield. The acid can be readily reduced to the corresponding alcohol 8. From primary alcohol 8, we prepared tosylate 9, which can be displaced with azide or thioacetate to give 10 and 11, respectively. Tags 4, 6,10, and 11 represent popular chemical handles for click chemistries32,33 facilitating the simple installation of fluorous content.
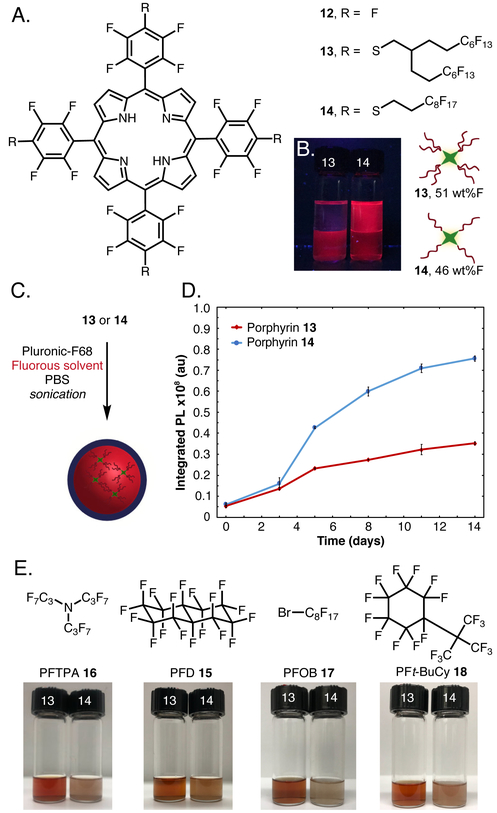
To evaluate the ability of the branched fluorous tags to solubilize organic compounds in perfluorocarbon solvents, we performed a nucleophilic aromatic substitution reaction with 11 and 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin 12 to yield 13 with 51 wt% F.34 We compared 13 to previously reported 14,35 which contained linear C8F17 tags and 46 wt% F (Figure 2B). We determined the fluorous partition coefficient (P)14,36 of 13 and 14 by subjecting each to a 1:1 mixture of perfluoro(methylcyclohexane) (C7F14):toluene (PhMe) and quantifying the amount in each layer by UV-Vis spectroscopy.37 Branched fluorous porphyrin 13 had P = 24 (96:4 in C7F14PhMe) whereas porphyrin 14 has a P = 1.7 (63:37 in C7F14:PhMe). This striking difference suggests that branching and increased wt% F are acting synergistically to enhance fluorous solubility. Another avenue to assay the fluorous character of the tagged molecules is to evaluate their tendency to remain in the core of PFC nanoemulsions (Figure 2C) as the aqueous suspension of droplets is continually partitioned against 1-octanol.12 Previously, we have shown that 14 displays some leaching into the 1-octanol.13 When we performed the identical assay with 13, we found that 50% less porphyrin escaped the droplets (Figure 2D), corroborating the partition coefficient results and demonstrating the efficacy of the branched fluorous tags.
Figure 2.
(A) Branched 13 and linear 14 fluorous porphyrins prepared from 12. (B) Fluorous partition coefficient in toluene (upper phase) and perfluoro(methylcyclohexane) (lower phase). (C) Preparation of PFC nanoemulsions containing branched 13 and linear 14 fluorous porphyrin. Both porphyrins were dissolved in separate 7:3 mixtures of PFD 15:PFTPA 16 (10 vol%) and combined with Pluronic F-68 (2.8 wt%) in phosphate buffered saline. Sonication (35% amp, 90 s, 0 °C) of the mixture provided PFC nanoemulsions. (D) Leaching of porphyrin 13 (red, diamond) or 14 (blue, circle) from the fluorous interior of PFC nanoemulsions into 1-octanol over time. Error bars represent the standard deviation of 3 replicates. (E) Structures of fluorous solvents previously employed as the core of PFC nanoemulsions and solubility of porphyrins 13 and 14 in each solvent.
The ability for branched fluorous porphyrin 13 to remain inside perfluorocarbon nanoemulsions in the presence of aqueous and lipophilic media is of particular interest for applications in photodynamic therapy. Photodynamic therapy is a clinically approved treatment for skin, esophageal, and lung cancers.38–40 This procedure requires three components: a photosensitizer, molecular oxygen, and light. We recently reported that enhanced photodynamic therapy could be achieved when perfluorocarbon nanoemulsions containing 14, acting as a photosensitizer, were irradiated with light.13 These nanoemulsions were composed of a mixture of 7:3 perfluorodecalin 15:perfluorotripropylamine 16, analogous to the previously FDA-approved formulation for oxygen delivery. Thus, with this system we are able to deliver both oxygen and photosensitizer simultaneously.
Despite the advantageous dual delivery, concerns regarding clearance of both the C8F17 chains16 and perfluorotripropylamine (PFTPA, 16)41,42 limit the translation of these materials to higher organisms. Looking to overcome both these limitations, we evaluated the solubility of 13 compared to 14 in the readily cleared solvents of perfluorodecalin (PFD, 15), perfluorooctyl bromide (PFOB, 17), and perfluoro(tert-butylcyclohexane) (PFt-BuCy, 18).43 We found that 13 was more soluble than 14 in all cases (Figure 2E), facilitating the preparation of emulsions for photodynamic therapy from these solvents.
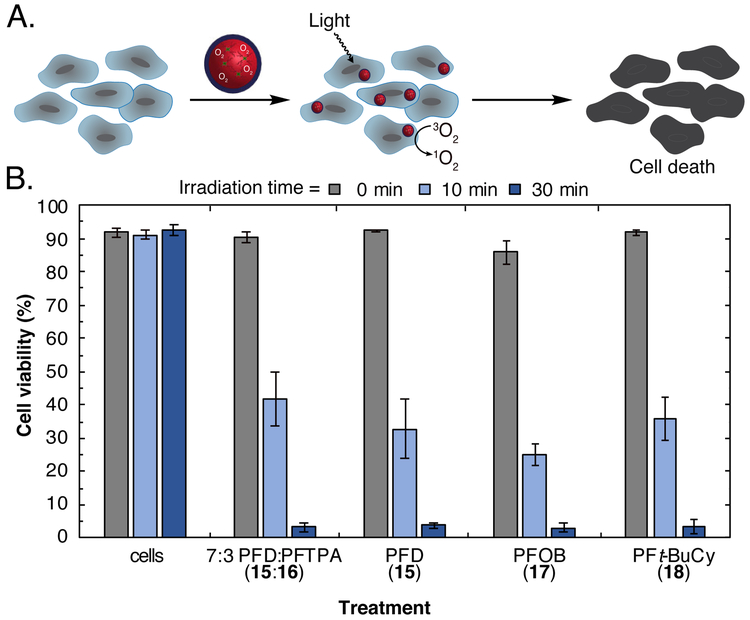
Finally, we performed photodynamic therapy with more biocompatible perfluorocarbon nanoemulsions. We prepared droplets of PFD 15, PFOB 17, PFt-BuCy 18, and 7:3 PFD 15:PFTPA 16 (20 wt% fluorous solvent) containing 13 stabilized by Pluronic F-68 (2.8 wt%) in phosphate buffered saline (PBS) (Scheme S2). As a control, we also prepared droplets without 13 (Figure S1 and S2) and droplets containing 14 for efficiency comparison (Figure S3). All emulsions were 160–190 nm in size with polydispersities of 0.06-0.07 (Figure S1). We incubated each of the emulsions with A375 melanoma cells for 3 hours, at which point, excess emulsions were washed away and the cells underwent light treatment for 0, 10, or 30 min. The degree of cell death was quantified by immediate treatment with propidium iodide and analysis by flow cytometry. We found that all emulsions containing branched porphyrin 13 displayed no dark toxicity and equivalent levels of cell death, greatly expanding the fluorous solvents that can be employed for photodynamic therapy with perfluorocarbon nanoemulsions. Ultimately, we envision that optimized versions of these nanomaterials can be systemically administered and accumulate at disease sites for light mediated therapies.
In summary, we have developed a route to a library of biocompatible branched fluorous tags with two C6F13 chains. These tags are derived from ethyl cyanoacetate 1 and (perfluorohexyl)ethyl iodide 2 to provide modular building block 3 with two functional handles and high fluorous content. We converted 3 to eight branched fluorous tags with distinct functionalities, including azides, aldehydes, and thiols for standard click chemistries. We employed the thiol tag for nucleophilic aromatic substitution to prepare fluorous porphyrin 13. We demonstrate that 13 is more soluble in fluorous solvent than its linear counterpart 14, facilitating the incorporation of 13 into stable perfluorocarbon nanoemulsions. The high solubility of 13 in readily cleared, volatile fluorous solvents allowed for photodynamic therapy to be carried out with PFC nanoemulsions composed of clinically relevant fluorous solvents. Looking forward, the simple, modular synthesis of branched fluorous tags from readily available starting materials will provide the community with biocompatible methods to impart fluorous content to molecules and materials, allowing the unique properties of perfluorocarbons to continue to be exploited.
Supplementary Material
Figure 3.
(A) Photodynamic therapy with perfluorocarbon nanoemulsions containing 13 (0.5 mM). Cells were incubated with PFC emulsions containing 13, washed via centrifugation, and irradiated (420 nm, 8.5 mW/cm2) for 0 min (grey), 10 min (light blue), or 30 min (dark blue). (B) Flow cytometry analysis after light treatment. After incubation, washing, and light treatment, cells were stained with propidium iodide and analyzed by flow cytometry to determine the degree of cell death. Dead cells were characterized as exhibiting fluorescence >102 (Figure S4). Error bars represent the standard deviation of 3 replicate samples.
ACKNOWLEDGMENT
M.A.M. is supported by the Chemistry-Biology Interface Training Program (5T32GM008496). This work was supported by shared instrumentation grants from the NSF (CHE-1048804) and the NIH (1S10OD016387-01). Further funding was provided by UCLA startup funds and ACS-PRF 57379-DNI4.
Footnotes
REFERENCES
- (1).Gladysz JA; Curran DP; Horváth IT Handbook of Fluorous Chemistry; Wiley-VHC: Weinheim, 2004. [Google Scholar]
- (2).Vincent JM Recent Advances of Fluorous Chemistry in Material Sciences. Chem. Commun. 2012, 48; 11382–11391. [DOI] [PubMed] [Google Scholar]
- (3).Ameduri B; Boutevin B Well-Architectured Fluoropolymers: Synthesis, Properties and Applications; Elsevier: Oxford, 2004. [Google Scholar]
- (4).Riess JG Understanding the Fundamentals of Perfluorocarbons and Perfluorocarbon Emulsions Relevant to in Vivo Oxygen Delivery. Artif. Cells. Blood Substit. Immobil. Biotechnol 2005, 33;47–63. [DOI] [PubMed] [Google Scholar]
- (5).Horvath IT; Rabai J Facile Catalyst Separation Without Water: Fluorous Biphase Hydroformylation of Olefins. Science 1994,266;72–75. [DOI] [PubMed] [Google Scholar]
- (6).Brittain SM; Ficarro SB; Brock A; Peters EC Enrichment and Analysis of Peptide Subsets Using Fluorous Affinity Tags and Mass Spectrometry. Nat. Biotechnol. 2005,23;463–468. [DOI] [PubMed] [Google Scholar]
- (7).Ko KS Jaipuri FA; Pohl NL Fluorous-Based Carbohydrate Microarrays. J. Am. Chem. Soc 2005, 127; 13162–13163. [DOI] [PubMed] [Google Scholar]
- (8).Vegas AJ; Bradner JE; Tang W; McPherson OM; Greenberg EF; Koehler AN; Schreiber SL Fluorous-Based Small-Molecule Microarrays for the Discovery of Histone Deacetylase Inhibitors. Angew. Chem. 2007, 46; 7960–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Flynn GE; Withers JM; Macias G; Sperling JR; Henry SL; Cooper JM; Burley GA; Clark AW Reversible DNA Micro-Patterning Using the Fluorous Effect. Chem. Commun. 2017; 53; 3094–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Dafik L; Kalsani V; Leung AKL; Kumar K Fluorinated Lipid Constructs Permit Facile Passage of Molecular Cargo into Living Cells. J. Am. Chem. Soc 2009, 131; 12091–12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Zamora CY; Dafik L; Kumar K Chemical Biology Using Fluorinated Building Blocks In Supramolecular Chemistry: From Molecules to Nanomaterials; John Wiley & Sons: Chichester, 2012. [Google Scholar]
- (12).Sletten EM; Swager TM Fluorofluorophores: Fluorescent Fluorous Chemical Tools Spanning the Visible Spectrum.J. Am. Chem. Soc 2014, 136; 13574–13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Day RA; Estabrook DA; Logan JK; Sletten EM Fluorous Photosensitizers Enhance Photodynamic Therapy with Perfluorocarbon Nanoemulsions. Chem. Commun 2017, 53;13043–13046. [DOI] [PubMed] [Google Scholar]
- (14).Kiss LE; Kovesdi I; Rabai J An Improved Design of Fluorophilic Molecules: Prediction of the Ln P Fluorous Partition Coefficient, Fluorophilicity, Using 3D QSAR Descriptors and Neural Networks. J. Fluorine Chem 2001, 108; 95–109. [Google Scholar]
- (15).Rocaboy C; Hampel F; Gladysz JA Syntheses and Reactivities of Disubstituted and Trisubstituted Fluorous Pyridines with High Fluorous Phase Affinities: Solid State, Liquid Crystal, and Ionic Liquid-Phase Properties. J. Org. Chem. 2002, 67; 6863–6870. [DOI] [PubMed] [Google Scholar]
- (16).Krafft MP; Riess JG Per- and Polyfluorinated Substances (PFASs): Environmental Challenges. Curr. Opin. Colloid Interface Sci 2015, 20; 192–212. [Google Scholar]
- (17).Krafft MP; Riess JG Selected Physicochemical Aspects of Poly- and Perfluoroalkylated Substances Relevant to Performance, Environment and Sustainability-Part One. Chemosphere 2015; 129; 4–19. [DOI] [PubMed] [Google Scholar]
- (18).Langhals H; Ismael R; Yu O Persistent Fluorescence of Perylene Dyes by Steric Inhibition of Aggregation. Tetrahedron 2000; 56; 5435–5441. [Google Scholar]
- (19).Thamyongkit P; Speckbacher M; Diers JR; Kee HL; Kirmaier C; Holten D; Bocian DF; Lindsey JS Swallowtail Porphyrins: Synthesis, Characterization and Incorporation into Porphyrin Dyads. J. Org. Chem. 2004, 69; 3700–3710. [DOI] [PubMed] [Google Scholar]
- (20).Loiseau J; Fouquet E; Fish RH; Vincent JM; Verlhac JB A New Carboxylic Acid with Branched Ponytails for the Solubilization of Mn(II) and Co(II) Ions in Perfluorocarbons.J. Fluorine Chem 2001, 108; 195–197. [Google Scholar]
- (21).Kaplanek R; Briza T; Havlik M; Dolensky B; Kejik Z; Martasek P; Kral V Branched Polyfluorinated Triflate-An Easily Available Polyfluoroalkylating Agent. J. Fluorine Chem. 2006,127; 386. [Google Scholar]
- (22).Wipf P; Reeves JT Synthesis and Applications of a Highly Fluorous Alkoxy Ethyl Ether Protective Group. Tetrahedron Lett. 1999, 40; 5139–5142. [Google Scholar]
- (23).Nakamura Y; Takeuchi S; Okumura K; Ohgo Y Enantioselective Addition of Diethylzinc to Aldehydes Catalyzed by Fluorous β-Aminoalcohols. Tetrahedron 2001,57 5565–5571. [Google Scholar]
- (24).Wende M; Seidel F; Gladysz JA Synthesis and Properties of Fluorous Arenes and Triaryl Phosphorus Compounds with Branched Fluoroalkyl Moieties (“Split Pony Tails”). J. Fluorine Chem 2003, 124; 45–54. [Google Scholar]
- (25).Matsubara S; Mitani M; Utimoto K A Facile Preparation of 1-Perflouroalkylalkenes and Alkynes. Palladium Catalyzed Reaction of Perfluoroalkyl Iodides with Organotin Compounds. Tetrahedron Lett. 1987, 28; 5857–5860. [Google Scholar]
- (26).Szabo D; Mohl J; Balint AM; Bodor A; Rabai J Novel Generation Ponytails in Fluorous Chemistry: Syntheses of Primary, Secondary, and Tertiary (Nonafluoro-tert-butyloxy)Ethyl Amines. J Fluorine Chem. 2006, 127; 1496–1504. [Google Scholar]
- (27).Zhao X; Ng WY; Lau KC; Collis AEC; Horvath IT Generation of (Nonafluoro-tert-butoxy)Methyl Ponytails for Enhanced Fluorous Partition of Aromatics and Heterocycles. Phys. Chem. Chem. Phys. 2012, 14; 3909–3914. [DOI] [PubMed] [Google Scholar]
- (28).Oakwood Chemicals, Accessed 9-03-18, 75g/$100.
- (29).Manchester Organics, Accessed 9-07-18, 25 g/$900.
- (30).In our hands, conversion of (perfluorohexyl)propyl alcohol to the corresponding iodide provided low yields and inefficient conversion.
- (31).We found one report of the alkylation of cyanoacetate with (perfluorohexyl)ethyl iodide in the patent literature: Read, R. Fluorous Acetylation, Int. Patent Appl. PCT/AU03/00218, 2003.
- (32).Kolb HC; Finn MG; Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem 2001, 40; 2004–2021. [DOI] [PubMed] [Google Scholar]
- (33).Hoyle CE; Lowe AB; Bowman CN Thiol-Click Chemistry: A Multifaceted Toolbox for Small Molecule and Polymer Synthesis. Chem. Soc. Rev. 2010, 39; 1355–1387. [DOI] [PubMed] [Google Scholar]
- (34).Highly fluorous porphyrins have been synthesized, but their fluorous partition coefficients have not been determined. Tuxen J; Eibenberger S; Gerlich S; Arndt M; Mayor M Highly Fluorous Porphyrins as Model Compounds for Molecule Interferometry. Eur. J. Org. Chem 2011, 4823–4833. [Google Scholar]
- (35).Gerlich S; Eibenberger S; Tomandl M; Nimmrichter S; Hornberger K; Fagan PJ; Tuxen J; Mayor M; Arndt M Quantum Interference of Large Organic Molecules. Nat. Commun 2011, 2; 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Horvath IT Fluorous Biphase Chemistry. Acc. Chem. Res. 1998, 31; 641–650. [Google Scholar]
- (37).Fluorous partition coefficients can be obtained by UV-Vis spectroscopy. El Bakkari M; McClenaghan N; Vincent JM The Pyridyl-Tag Strategy Applied to the Hydrocarbon/Perfluorocarbon Phase-Switching of a Porphyrin and a Fullerene. J. Am. Chem. Soc 2002, 124; 12942–12943. [DOI] [PubMed] [Google Scholar]
- (38).Lovell JF; Liu TWB; Chen J; Zheng G Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010,110; 2839–2857. [DOI] [PubMed] [Google Scholar]
- (39).Protti S; Albini A; Viswanathan R; Greer A Targeting Photochemical Scalpels or Lancets in the Photodynamic Therapy Field - The Photochemist’s Role. Photochem. Photobiol. 2017, 93; 1139–1153. [DOI] [PubMed] [Google Scholar]
- (40).van Straten D; Mashayekhi V; de Bruijn HS; Oliveira S; Robinson DJ; van Straten D; Mashayekhi V; de Bruijn HS; Oliveira S; Robinson DJ Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9; 19–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Flaim SF Pharmacokinetics and Side Effects of Perfluorocarbon-Based Blood Substitutes. Artif. Cels Blood Substit. Biotechnol 1994, 22; 1043–1054. [DOI] [PubMed] [Google Scholar]
- (42).Lowe KC Perfluorochemical Respiratory Gas Carriers: Applications in Medicine and Biotechnology. Sci. Prog. 1997, 80; 169–193. [PubMed] [Google Scholar]
- (43).Castro CI; Briceno JC Perfluorocarbon-Based Oxygen Carriers: Review of Products and Trials. Artif. Organs 2010,34, 622–634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.