Abstract
Individuals who suppress their emotions experience less positive emotions, worse relationships, and a reduced quality of life whereas those who tend to reappraise show an opposite pattern. Despite this divergent pattern, few have asked how the use of these emotion-regulation strategies relates to reward responsivity. We predicted that elevated suppression would be associated with blunted reward responsivity, whereas reappraisal would be associated with elevated reward responsivity. To test this hypothesis, participants completed a measure of individual differences in emotion-regulation strategies, measures of self-reported reward responsivity, and then a reward time-estimation task (Kotani et al., 2003) while electroencephalography (EEG) was recorded. Results revealed that individual differences in cognitive reappraisal were unrelated to self-report measures of reward responsivity, whereas suppression was associated with blunted reward responsivity. At the neural level, reappraisal was associated with greater attention to the rewarding cues, as indexed by the P300 event-related potential (ERP) component, whereas suppression was related to blunted reward anticipation, as indexed by the stimulus-preceding negativity (SPN) ERP component. Suppression prospectively predicted worse psychological well-being 2.5 years later and blunted neural reward anticipation partially explained this association. Taken together with past research, these results suggest reappraisal tendencies may lead to better outcomes due, in part, to enhanced reward responsivity, whereas the negative consequences of suppression may be associated with blunted reward responsivity.
Keywords: Emotion-regulation, reward, event-related potential, psychological well-being
Introduction
The anatomy, physiology, and chemistry of the brain have evolved to incentivize adaptive behavior and thereby promote survival. We call these incentives to engage in adaptive behavior rewards. Primary rewards like food and sex directly promote survival via nourishment and opportunities to procreate respectively. Secondary rewards like money indirectly promote survival through the procurement of resources like food, shelter, and social status. Because of their adaptive value, rewards are also powerful elicitors of emotion. Seeking rewards breeds desire and determination. Obtaining rewards begets joy and elation. The ability to regulate responses to emotion elicitors, like rewards, is a hallmark of psychological health, well-being, and optimal functioning in humans. Despite the importance of both rewards and emotion-regulation to optimal functioning and well-being, the literature connecting reward responsivity and emotion-regulation is sparse. The current study sought to fill this void by examining the relationship between emotion-regulation processes, self-reported reward responsivity, reward-related brain activity, and well-being.
Emotions involve changes in physiology, subjective experience, and expressive behavior, which unfold over time (Gross, 2015; Mauss, Levenson, McCarter, Wilhelm & Gross, 2005). Emotion-regulation is the conscious and non-conscious processes individuals use to influence the intensity, variety, and duration of their emotions. As emotions unfold over time they can be regulated along many points on a temporal sequence ranging from early processes like cue evaluation to later processes like response modulation. Antecedent-focused emotion-regulation strategies modulate nascent emotional responses. In contrast, response-focused emotion-regulation strategies modulate fully formed, ongoing emotional responses. These are the central tenets of Gross’ process model of emotion regulation (Gross, 1998b; 2001).
The two most frequently studied emotion-regulation strategies within the framework of the process model of emotion-regulation (Gross, 1998b; 2001) are cognitive reappraisal and expressive suppression. Cognitive reappraisal is an antecedent-focused strategy that involves interpreting a potential emotion elicitor in way that heightens or lessens its emotional impact (Lazarus & Alfert, 1964; Gross & John, 2003). Expressive suppression is response-focused and a form of response modulation, which involves inhibiting or suppressing an emotional experience once it has been activated or solidified (Gross, 1998). Both experimental and individual differences approaches to exploring the consequences of emotional regulation consistently find that cognitive reappraisal is more effective than expressive suppression (Gross 2002; Gross & Thompson, 2007; Webb, Miles, & Sheeran, 2012; Gross, 2015). For example, experiments find that directing participants to reappraise their reactions to emotional stimuli (usually negative emotions) decreases the experience of negative emotion (Feinberg, Willer, Antonenko, & John, 2012; Gross, 1998a; Kross & Ayduk, 2011; Lieberman, Inagaki, Tabibnia, & Crockett, 2011; Ray, McRae, Ochsner, & Gross, 2010; Szasz, Szentagotai, & Hofmann, 2011; Wolgast, Lundh, & Viborg, 2011). In contrast, suppression decreases the experience of positive emotion (Brans, Koval, Verduyn, Lim, & Kuppens, 2013; Gross, 1998a; Gross & Levenson, 1993; 1997).
Numerous individual differences studies largely echo the experimental findings above: cognitive reappraisal tendencies are associated with a pattern of positive outcomes in life whereas suppression tendencies are associated with an opposite profile (Gross & John, 2003). Reappraisal tendencies, as compared to suppression tendencies, are associated with more daily positive affect and less daily negative affect (Brans et al., 2013; Nezleck & Kuppens, 2008), less psychopathology (Aldao, Nolen-Hoeksema, & Schweizer, 2010; Hu et al., 2014), and better physical health (Appleton & Kubzansky, 2014; Gianros et al., 2014). Perhaps as a consequence, reappraisal tendencies are associated with better psychological well-being as compared to suppression (John & Gross, 2004; McRae, Jacobs, Ray, John, & Gross, 2012).
Neuroscientific research largely corroborates these findings. Cognitive reappraisal tends to increase activation in cognitive control regions like the dorsolateral prefrontal cortex, and decreases activity in emotion-related brain regions like the amygdala (e.g., Goldin, McRae, Ramel, & Gross, 2008; Kanske, Heissler, Schonfelder, Bongers, & Wessa, 2012; Ochsner & Gross, 2008; Ochsner et al., 2004). For example, a meta-analysis of 48 studies by Buhle and colleagues (2014) revealed that directed reappraisal activated cognitive control brain regions and decreased bilateral amygdala activation (in studies that asked participants to down-regulate negative emotion). In contrast to cognitive reappraisal, the use of expressive suppression increased activity in emotion-related brain regions, such as the amygdala and insula (e.g., Goldin et al., 2008). Despite the rich literature on emotion-regulation, which has most frequently concerned itself with down-regulating negative emotions, few studies have considered how emotion-regulation influences positive or reward-related processes.
Reward Responsivity and Emotion-regulation
Several experimental studies using functional neuroimaging have examined the effects of reappraisal on reward responsivity. These studies find that when individuals use reappraisal to think about potential rewards in a less emotional manner they show dampened activity in reward-related brain regions (Delgado, Gillis, & Phelps, 2008; Staudinger, Erk, Abler, & Walter, 2009; Staudinger, Erk, & Walter, 2011). From an individual differences perspective, research reports that elevated self-reported reward responsivity is associated with elevated tendencies toward reappraisal (Dennis et al; 2007; Taubitz, Pedersen, & Larson, 2015) and reduced tendencies toward suppression (Taubitz et al., 2015). Wellborn et al. (2009) found reappraisal tendencies were associated with greater volume whereas suppression tendencies were associated with smaller volume in reward-relevant brain regions (e.g., the ventromedial prefrontal cortex). These studies suggest that the reappraisal tendencies may be associated with greater reward responsivity whereas suppression tendencies may be associated with blunted reward responsivity.
While these imaging studies suggest that the reappraisal and suppression tendencies modulate reward-related brain regions, numerous studies over the past decade have shown that reward-responsivity is composed of multiple distinct psychological processes that dynamically unfold over time. Broadly construed, reward responsivity includes two neurobiologically distinct stages: reward-anticipation and reward-outcome (Berridge & Robinson, 2003; Knutson et. al., 2001; Salamone & Correa, 2012; Breiter et al., 2001; McClure et al., 2003). As reflected below, reward processing involves neurocognitive processes unfolding rapidly and in close temporal proximity (Glazer, Kelley, Pornpattananangkul, Mittal, & Nusslock, 2018), making it difficult to experimentally isolate reward-anticipation and reward-outcome processes.
Electroencephalogram (EEG) techniques that utilize event-related potentials (ERPs) are ideal for decomposing the time-course of neural activation at each stage of reward processing because they measure activity on the order of milliseconds (Luck, 2005; Luck & Kappenman 2012). For these reasons, an ERP paradigm is ideal for examining how emotion-regulation tendencies influences multiple different sub stages of reward responsivity during both reward-anticipation and reward-outcome processing.
Reward-anticipation is the approach-motivated wanting or desire for rewards. Anticipation can be further decomposed into cue-evaluation (i.e., monitoring the environment for potential rewards) and outcome-anticipation (i.e., waiting for feedback after a motoric response). Below we describe reward relevant ERPs observed during the cue-evaluation and outcome-anticipation phases. Cue evaluation is the earliest sub stage of reward-anticipation. During cue evaluation, a P300 is typically elicited. The cue-P300 is a positive ERP peaking at approximately 300–600 ms following a cued stimulus. This component reflects stimulus categorization processes related to context updating in working memory (Donchin & Coles, 1998; Johnson & Donchin, 1980; see Polich, 2007 for review) and is reliably enhanced for salient stimuli (Polich & Kok, 1995), notably reward cues (Goldstein et al., 2006; Hughes et al., 2013). In support of reward modulation, the cue-P300 for incentive (vs. neutral) cues has been found to covary with activation in the ventral striatum (Pfabigan et al., 2014). As reviewed below, upregulating one’s response to reward cues increases P300-like activity (Langeslag & van Strien, 2013).
Outcome anticipation is the last sub stage of reward anticipation. During this sub stage, anticipatory resources are mobilized immediately prior to the feedback stimulus. Outcome-anticipation is most commonly captured by an ERP component known as the stimulus-preceding negativity (SPN), a broad index of anticipatory attention frequently measured in a 200 ms intervals directly prior to feedback onset. In support of reward modulation, numerous studies report that the SPN is elevated prior to rewarding (versus non-rewarding) feedback (for a review see Hackley, Valle-Inclán, Masaki, & Hebert, 2014).
Reward outcome processing is the liking or savoring of obtained rewards. Reward outcome processing subsumes early (i.e., reward-evaluation) and late (i.e., salience and emotional impact of the feedback stimulus) processes. The reward positivity (RewP) is the earliest ERP component differentiating gains from losses. The RewP is a positive deflection following gains peaking approximately 250–350 ms following a feedback stimulus signaling an outcome has gone better than expected (Miltner, Braun, & Coles., 1997; Holroyd, Pakzad-Vaezi, & Krigolson, 2008; Proudfit, 2015; Angus, Kemkes, Schutter, & Harmon-Jones, 2015; Threadgill & Gable, 2016). A recent ERP study by Sai, Wang, Ward, Ku, and Sang (2015) observed that reappraisal tendencies, measured via the cognitive reappraisal subscale of Gross and John’s (2003) emotion regulation questionnaire, were associated with a larger RewP in response to monetary gains in a gambling task.
Later processes in the reward-outcome stage include the Feedack-P300 and the late positive potential (LPP). The Feedback-P300 occurs 300–600 ms following feedback and involves attention-driven categorization of salient outcome-related information, such as context updating, and subsequently integrating the contents of working memory to maximize future rewards (Sutton et al., 1965; Donchin, 1981; see Polich, 2007 for review). In support of reward-modulation, research reports that the feedback-P300 is increased following rewarding feedback (San Martín, 2012). Other studies suggest that the Feedback P300 may be modulated by emotion regulation. To illustrate this point, consider the study above by Sai and colleagues that found elevated reappraisal tendencies associated with a larger RewP. Their RewP time window overlapped substantially with the Feedback-P300 (Holyrod, Nieuwenhuis, Yeung, & Cohen, 2003), suggesting that reappraisal tendencies may also modulate the Feedback-P300 in addition to the RewP.
The next stage in outcome processing is extended affective processing and is reflected by the late-positive potential (LPP), a positive-going ERP component elicited approximately 500–1000 ms following an affective stimulus, such as a rewarding outcome, reflecting extended affective processing of the stimulus. A recent study found that upregulating one’s response to potential rewards enhanced the LPP following incentive cues (Langeslag & van Strien, 2013). However, the authors quantitated the LPP as 300–500 ms after cue onset, which is more consistent with the P300 ERP component (see Polich, 2007). This suggests that emotion-regulation processes, particularly the ability to flexibly modulate one’s emotional responses, influence early cue evaluation processing. A number of studies also suggest that the LPP is sensitive to emotion-regulation strategies, particularly cognitive reappraisal. For example, several studies find that reappraising negative stimuli in more neutral terms reduces the LPP (Hajcak and Nieuwenhuis, 2006; Moser, Hajcak, Bukay, and Simons, 2006; Moser, Krompinger, Dietz, and Simons 2009; Moser et al., 2010; Thiruchselvam, Blechert, Sheppes, Rydstrom, & Gross, 2011). However, very few studies have investigated the LPP during reward processing, although a handful of studies report the LPP is more positive following losses over gains (Donaldson, Oumeziane, Hélie, and Foti., 2016; Meadows, Gable, Lohse, and Miller, 2016; Pornpattananangkul & Nusslock, 2015). Taken together, this literature suggests that an ERP paradigm is ideal for examining how emotion-regulation tendencies influence multiple different sub stages of reward responsivity during both reward processing because it allows us to isolate ERPs associated with rapidly unfolding, often overlapping, neurocognitive processes.
Present Study
The first objective was to examine the relationship between individual differences in self-reported emotion-regulation strategies (i.e., reappraisal and suppression) and self-reported reward responsivity. Based on previous research, we predicted that elevated self-reported reward responsivity would be associated with elevated tendencies toward reappraisal (Dennis et al; 2007; Taubitz, et al., 2015) and reduced tendencies toward suppression (Taubitz, et al., 2015).
The second objective was to examine the relationship between individual differences in self-reported emotion-regulation strategies and neural indices of reward responsivity during reward-anticipation and reward outcome processing using an established time estimation task (Pornpattananangkul and Nusslock, 2015). We selected the time estimation task for two reasons. First, it allows us to separate reward-processing into different temporal stages (Glazer et al., 2018; Pornpattananangkul & Nusslock, 2015; 2016). Second, the time-estimation task was the first reward task used to demonstrate the FRN/RewP (Milner 1997) and is commonly used to elicit the SPN (Damen and Brunia, 1994). Anticipatory ERPs focused on early cue evaluation (indexed by the P300) and feedback anticipation (indexed by the Stimulus-Preceding Negativity; SPN). The SPN is a negative slow-wave that grows with anticipation of an impending feedback stimulus. Both the P300 following reward cues and the SPN prior to reward feedback are typically enhanced relative to neutral conditions where no reward is possible (Kotani, Hiraku, Suda, & Aihara, 2001; 2003). The process model of emotion-regulation (Gross, 1998b; 2001) suggests that reappraisal exerts its influence early in the emotion generation process. Accordingly, we predicted that reappraisal tendencies would influence early reward anticipation processes and thus enhance cue evaluation to incentive cues (i.e., elevated cue-P300) In contrast, suppression involves the tendency to dampen ongoing emotional experience. Accordingly, we predicted that suppression would reduce later processes during the anticipatory period (i.e., a reduced SPN).
Outcome processing focused on early reward-evaluation (i.e., the RewP), feedback cue-evaluation (i.e., the feedback P300), and extended affective-outcome processing (i.e., the LPP). Based on previous research by Sai et al. (2015), we predicted that reappraisal would be associated with an elevated RewP whereas suppression would be unrelated to the RewP. As the time window in Sai et al. had overlap between the RewP and feedback P300, we made similar predictions with the feedback P300. Consistent with previous experimental research showing that reappraisal modulates the LPP (Langeslag & van Strien, 2013), we predicted that reappraisal tendencies would be associated with a larger LPP for good (vs. bad) performance.
The third objective was to examine the extent to which reappraisal and suppression tendencies prospectively predicted psychological well-being over a two-and-a half year follow-up period. Generally, cognitive reappraisal is more effective than suppression (Webb, Miles, & Sheeran, 2012) and is associated with a robust pattern of positive outcomes in life, whereas suppression is associated with an opposite profile (Gross & John, 2003). Thus, we predict that reappraisal would be associated with better, and suppression with worse, well-being. Relatedly, we also examined the extent to which both self-reported reward responsivity explained or accounted for any associations between emotion-regulation tendencies and prospective wellbeing. To facilitate these analyses, participants were asked to complete a measure of psychological well-being approximately two-and-a half years after their laboratory visit.
Method
Participants.
Fifty-eight right-handed, native English speakers (29 female) reported individually to a study described as an investigation of how people process words, sentences, and pictures. All participants were received $20 for their participation. Participant ages ranged from 18 to 33 years old (M = 22.12, SD = 3.85). Eight participants were excluded for excessively noisy EEG data (see Pornpattananangkul & Nusslock, 2015). Six participants did not complete the self-report measures (including critical measures of emotion-regulation tendencies and reward sensitivity). After these exclusions, 44 participants (26 female) remained for analysis. This study was approved by the Northwestern University Internal Review Board.
Time-Estimation Task.
The current study utilized a reward time-estimation task from Pornpattananangkul and Nusslock (2015). This task instructed participants to place their right index finger on the space bar and press it 3.5 s after a cue went off the screen. There were two types of cues: incentive ($) and no-incentive (0) cues. Incentive trials began with an incentive cue, and participants were informed they could earn 20 cents for accurate estimates, and receive no money for inaccurate estimates. The no-incentive trials began with a no-incentive cue, and participants were informed they would receive no money irrespective of their performance on these trials. We employed no-incentive trials (as opposed to punishment trials) because no-incentive trials serve as a control stimulus to estimate non-reward-related factors that may influence ERPs during the time-estimation task (Luck, 2005). The incentive and no-incentive cues were presented on a gray background for 400 milliseconds. Good performance was defined as trials where a button-press occurred within the correct time-window, whereas bad performance was defined as a button-press occurring beyond the time window. Following previous studies (e.g., Kotani et al., 2003), the time-window was adapted to control for variance in time-estimation ability among participants. The time-window initially was 500 ms, centered at 3.5 s in the first practice trial. On the next trial, the time-window was shortened (or lengthened) by 20 ms if the response in the current trial was (or was not) within the time-window. This method controls for accuracy at about 50%.
Two seconds following the button-press, two lines of feedback text were presented to the participant in the middle of the screen for 1000 ms. Depending on their performance, participants saw one of the following on the top line: “=“ for a response within the correct time-window, “<2” for an extremely fast response (less than 2 s), “<3.5” for a response slower than 2 s but not within the time-window, “>5” for an extremely slow response (slower than 5 s), and “>3.5” for a response faster than 5 s but not within the time-window. Thus, “=“ indicated good performance, while others indicated bad performance. The bottom line indicated whether participants won money for that response and included the following: “$” indicated the participant won money (20 cents) for that trial, and “0” indicated the participant did not win money for that trial. Thus, for incentive trials, participants would see “$” for good performance, and see “0” for bad performance. For no-incentive trials, participants would see “0” regardless of whether their performance was good or bad. Trials were terminated with a random ITI of 1000–1150 ms. Participants were told to do their best across both incentive and no-incentive trials. To incentivize participants’ continued attention on both incentive and no-incentive trials, they were told they would receive no earnings if they saw “<2” or “>5” feedback more than 15 times. This ensured that they avoided extreme responses.
Procedure.
Following consent, participants first completed the emotion-regulation questionnaire (ERQ; Gross & John, 2003), the behavioral activation/inhibition sensitivity scale (BIS/BAS; Carver & White, 1994), and the sensitivity to punishment and sensitivity to reward questionnaire (SPSRQ; Torrubia, Avila, Molto, & Caseras, 2001).
ERQ.
The ERQ is a common and well-validated measure of individual differences in reappraisal and suppression emotion-regulation tendencies (see Gross and John, 2003). Participants responded to ERQ items using a scale from 1 (strongly disagree) to 7 (strongly agree). The reappraisal scale includes 6 items which tap into the tendency to think about potential emotion elicitors in ways which heighten or lessen their impact, for example “When I want to feel more positive (negative) emotion, I change what I’m thinking about.” By contrast, the suppression scale includes 4 items which tap into the tendency to try and change ongoing affective experiences, for example “When I am feeling positive (negative) emotions, I am careful not to express them.” In the current study, the average score on the reappraisal scale was M = 4.71 (SD = 1.12, α = 0.88) and the average score on the suppression scale was M = 3.99 (SD =1.24, α = 0.79).
BIS/BAS.
The BIS/BAS scales are a well-validated measure of behavioral inhibition and behavioral activation system sensitivities (see Carver and White, 1994). Participants responded to BIS/BAS items using a scale from 1 (very false for me) to 4 (Very true for me). The BAS scale included 13 items assessing desire for reward, positive responses to real or anticipated reward, and persistence in pursuing desired reward. Sample items include “I go out of my way to get things I want,” and “I often act on the spur of the moment.” In the current study, the average score on the BAS scale was M = 3.08 (SD = 0.43, α = 0.83). The seven-item BIS scale assessed threat sensitivity (“Criticism or scolding hurts me quite a bit”). The average score on the BIS scale was M = 2.90 (SD = 0.37, α = 0.75).
SPSRQ.
The SPSRQ is a well-validated measure of reward and punishment sensitivities (see Torrubia et al., 2001). The SPSRQ contains 48 questions which participants endorse using a binary Yes/No response scale. The 24-item sensitivity to reward scale contains items probing responses to specific rewards like money (“Does the good prospect of obtaining money motivate you strongly to do some things?”), sex (“Do you often take the opportunity to pick up people you find attractive?”), and power (“does the possibility of social advancement, move you to action even if this involves not playing fair?”). The average total affirmative responses on the sensitivity to reward scale was M = 12.18 (SD = 3.92, α = 0.71). The 24-item sensitivity to punishment scale contains items which reflect neuroticism (Are you often afraid of new or unexpected situations?”), introversion (“Are you a shy person?”), and risk aversion (“Do you often refrain from doing something because of your fear of being praised?”). The average total affirmative responses on the sensitivity to punishment scale was M = 11.80 (SD = 5.43, α = 0.85).
Next, EEG electrodes were applied. To familiarize participants with the span of a 3.5-s duration, participants first had the opportunity to listen to two beeps 3.5-s apart as many times as they desired. Participants were then given instructions regarding the incentive/no-incentive blocks and corresponding cues and feedback. Prior to beginning the 30 practice trials in the incentive/no-incentive blocks, participants were tested on their comprehension of the cues and feedback. The incentive/no-incentive blocks consisted of six blocks of 36 trials. Each of these blocks involved a randomized distribution of incentive and no-incentive trials with a 50/50 split across trial types (i.e., 18 incentive and 18 no-incentive trials). Blocks were separated by breaks of participant-determined length, which included reminders of earnings and cue meanings.
Electrophysiological Recording and Data Reduction
Continuous EEG data, with a sampling rate at 500 Hz (DC to 100 Hz on-line, Neuroscan Inc.) were collected from inside an electromagnetic shielded booth. Sixty-four Ag/AgCl scalp electrodes were used with four additional electrodes placed around the eyes. An online reference was recorded from the left mastoid and re-referenced offline to the average of both mastoids. Impedances were kept below 5 kΩ and 10 kΩ for scalp and ocular electrodes, respectively. Large muscular artifacts were removed via visual inspection during offline analyses. Next, independent-component analysis was used to isolate and remove blink and saccade ocular artifacts and additional muscle activity (Makeig, Bell, Jung, & Sejnowski, 1996). EEG was offline bandpass-filtered at .01–30 Hz. Epochs containing artifacts (±75 μV) were rejected.
Event-Related Potential Quantification.
The cue-P300 was epoched from −100 to 1000 ms time-locked to cue onset and was baseline corrected using a pre-stimulus (−100 to 0 ms) time-window. Mean-amplitude was used for cue-P300 measurement (between 300 and 600 ms at Pz). The SPN was epoched from −2000 to 3000 ms relative to the button-press with a −2000 to −1700 ms baseline. A linear detrend algorithm removed slow-wave artifacts (Baker et al., 2012; Goldstein et al., 2006) and a low-pass filter of 10 Hz was applied (Brunia, van Boxtel, & Böcker, 2011). After averaging, the SPN was quantified as the mean amplitude from 1000 to 2000 ms (i.e., 1000 ms time-window prior to Feedback onset) at FCz. Feedback-related ERPs were epoched from −100 to 1000 ms time-locked to feedback onset and was baseline corrected using a pre-stimulus (−100 to 0 ms) time-window. The RewP was quantified using a difference wave approach (Luck, 2012). First, the difference between good and bad performance trials was calculated collapsing across incentive/no incentive trials. Next, we identified the maximum peak of the difference wave at electrode site FCz using visual inspection (i.e., 266 ms). To quantify the RewP, we calculated the mean amplitude at ± 50 ms around the peak (i.e., 216–316 ms). The feedback-P300 was defined as the mean amplitude between 250 and 450 ms at Pz. Finally, the LPP was measured as the mean amplitude between 500 and 1000 ms at CPz after feedback onset.
To examine the relationships between emotion-regulation strategies, reward anticipation, and reward outcome ERPs, we calculated a difference score for the cue-P300 and the SPN between incentive and no-incentive trials (Δcue-P300 and ΔSPN). The cue-ΔP300 was quantified as the difference between the P300 to incentive and no-incentive cues such that larger values reflect a larger cue-P300 for incentive compared to no-incentive cues. The ΔSPN was quantified as the difference between the SPN to incentive cue and no-incentive cues at FCz. As the SPN is a negative deflection, a more negative SPN difference score reflects a larger SPN to incentive compared to no-incentive cues.
For outcome ERPs we first assessed differences in the RewP, feedback-P300, and LPP as a function of feedback (good vs. bad) and incentive (incentive vs. no incentive). We used these analyses to guide our created of difference waves for outcome ERPs. As is evident in the results section below, the RewP was sensitive to performance (good versus bad) on incentive trials but not on no-incentive trials. As a result, we quantified the ΔRewP as the difference between the RewP to good minus bad performance (on incentive trials only) such that larger scores reflect greater (more positive) RewP to good compared to bad performance on incentive trials.
Based on simple main effects noted below, we quantified the Feedback-ΔP300 as the difference between the Feedback P300 to good minus bad performance on incentive trials only. Finally, as noted in the results section, the LPP was sensitive to both incentive and performance independently. Thus, we created two ΔLPPs variables. First, ΔLPP-Incentive is the difference between incentives and no-incentives trials (collapsing across performance) such that higher values indicate greater LPPs following incentives. Second, ΔLPP-Performance is the difference between good and bad performance (collapsing across incentive) such that larger scores reflect greater LPPs to good performance. Using ΔERP difference scores helps isolate incentiveevaluation from performance-evaluation by controlling for non-specific and non-reward-related factors that may modulate ERP amplitude (Luck, 2005).
Longitudinal Follow-up
To assess the longitudinal relationship between emotion-regulation, reward responsivity, and psychological well-being, we invited the 44 participants with complete data at baseline to complete the Comprehensive Inventory of Thriving (CIT; Su, Tay, & Diener, 2014) several years after baseline assessment (M = 31.43 months, SD = 2.33 months). Of the 44 participants we invited, 27 participants (61.3 %) agreed to complete follow-up assessments.
CIT.
The CIT measures a broad range of 18 psychological well-being constructs. Each of these 18 well-being facets includes 3 items, resulting in a 54-item measure. Participants responded to CIT items using a scale from 1 (strongly disagree) to 5 (strongly agree). We did not have any apriori hypotheses about specific facets of psychological well-being, thus we utilized a 54-item composite score. The average CIT score was M = 3.85 (SD = 0.48, α = .89).
Results
Data analysis strategy
The current study had three objectives. The first was to examine how emotion regulation tendencies relate to self-reported reward responsivity as measured by the BAS and the SPSRQ Reward Responsivity scale. We conducted separate regression models regressing BAS and SPSRQ Reward Responsivity onto reappraisal tendencies, suppression tendencies, and their interaction. Based on the recommendations of Aiken & West (1991), both reappraisal and suppression tendencies were mean centered prior to creating the interaction term in order to create a meaningful, interpretable zero value. Significant interactions were further evaluated at ± 1 SD from the mean score of suppression and reappraisal (Aiken & West, 1991).
The second objective was to examine the relationship between individual differences in self-reported emotion-regulation strategies and neural indices of reward responsivity during reward anticipation and reward outcome processing. We first used ANOVAs to examine the effects of incentives on anticipatory ERPs and then to examine the effects of both incentives and performance on feedback ERPs. We followed up significant main and interactive effects of incentives and feedback by creating ΔERP difference scores to control for non-specific and non-reward-related factors that may modulate ERP amplitude (Luck, 2005). We then ran separate regression models regressing each ΔERP onto reappraisal tendencies, suppression tendencies, and their interaction. Again, significant interactions were further evaluated at ± 1 SD from the mean score of suppression and reappraisal based on the recommendations of Aiken & West (1991).
Our third objective was to examine the extent to which reappraisal and suppression tendencies prospectively predicted psychological well-being. In line with this objective, we conducted a parallel mediation analysis using Hayes (2013) process macro to examine whether the association between elevated suppression tendencies and worse prospective well-being was accounted for by any of the self-reported or ERP measures of reward responsivity. As indirect effects included in mediation models are the product of the paths that constitute the effect (i.e., predictor × mediator, mediator × outcome), we only included mediators which were related to either predictor (i.e., reappraisal, suppression) or the outcome. We used this data driven approach to reduce the number of mediators included in the model.
Self-report measures of reward responsivity
Behavioral Activation System (BAS).
We regressed BAS scores onto suppression (centered), reappraisal (centered), and their interaction. There was no main effect of either suppression, b = − 0.57, SE = .67, t (40) = − 0.86, p = .40, or reappraisal, b = 0.67, SE = .73, t (40) = 0.93, p = .36. There was a significant suppression × reappraisal interaction, b = 1.56, SE = .63, t (41) = 2.47, p = .02. We next examined this significant interaction at ± 1 SD from the mean score of both suppression and reappraisal. This analysis indicated that among participants high in suppression tendencies (+1 SD), being low in reappraisal tendencies (−1 SD) was associated with reduced BAS scores compared to being high in reappraisal tendencies (+1 SD), t (40) = 2.59, p = .02. There was no effect of reappraisal tendencies for those with low level of suppression tendencies (−1 SD), t (40) = − 1.25, p =.26 (Figure 1A).
Figure 1.
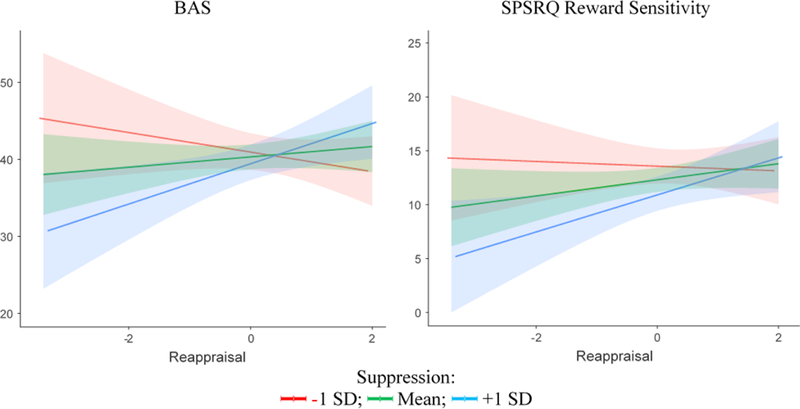
Simple slopes reflecting reappraisal-BAS relationships (A) and reappraisal-SPSRQ Reward Responsivity relationships (B) at 1 SD below the mean (red), at the mean (green), and 1 SD above the mean (blue) suppression score. Note: Shaded regions reflect 95% C.I. around simple slopes.
SPSRQ Reward Responsivity.
We regressed SPSRQ reward responsivity onto suppression (centered), reappraisal (centered), and their interaction. There was a significant main effect of suppression such that that participants reporting higher emotion suppression tendencies reported less SPSRQ reward responsivity, b = −1.04, SE = .46, t (40) = − 2.23, p = .03 There was no main effect of reappraisal, b = 0.75, SE = .50, t (40) = 1.50, p = .14. The suppression × reappraisal interaction was approaching significance, b = 0.78, SE = .44, t (40) = 1.78, p = .08. On an exploratory basis, we examined this interaction at ± 1 SD from the mean score of suppression and reappraisal. This analysis indicated that among participants high in suppression tendencies (+1 SD), being low in reappraisal tendencies (−1 SD) was associated with reduced SPSRQ reward responsivity scores compared to being high in reappraisal tendencies (+1 SD), t (41) = 2.40, p =.02. There was no effect of reappraisal tendencies for those with low level of suppression tendencies (−1 SD), t (41) = −0.28, p = .78 (Figure 1B).
Time estimation task
Behavioral Results.
Participants estimated 3.5 seconds equally well on both reward (M = 3.50 s, SD = 0.16) and no reward trials (M = 3.52 s, SD = 0.18), t (41) = − 0.90, p = .37. This indicates that our adaptation procedure worked well.
Reward Cue Evaluation ERPs.
Replicating past research (e.g., Pornpattananangkul & Nusslock, 2015), incentive cues elicited a significantly larger P300 compared to no-incentive cues, F (1, 43) = 18.58, p < .001, partial ɳ2 = .30 (see Table 1). The relationship between emotion-regulation strategies and attention to incentive cues was assessed via the cue-ΔP300 (See Table 3 for bivariate correlations between self-report questionnaires and ΔERPs). The cueΔP300 was quantified as the difference between the P300 to incentive and no-incentive cues such that larger values reflect a larger cue-P300 for incentive cues compared to no-incentive cues. We regressed the cue-ΔP300 onto suppression (centered), reappraisal (centered), and their interaction. As illustrated in Figures 2 and 3, there was a main effect of reappraisal such that elevated reappraisal was associated with an elevated cue-ΔP300 (i.e., higher cue P300 for incentive than no-incentive cues), b = .74, SE = .36, t (40) = 2.08, p = .041. There was no main effect of suppression on ΔP300, b = −.27, SE = .33, t (40) = −0.81, p = .42. The suppression × reappraisal interaction was not significant, b = −.32, SE = .31, t (40) = −1.03, p = .31.
Table 1.
Descriptive statistics for reward-anticipation ERPs
| ERP | Incentive Cue M (SD) |
No-Incentive Cue M (SD) |
|---|---|---|
| Cue-P300 | 4.58 (4.67) | 2.81 (3.52) |
| SPN | −3.69 (2.71) | −1.81 (2.62) |
Table 3.
Bivariate correlations between self-report questionnaires and ΔERPs.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Reappraisal | — | −.11 | .16 | −.05 | .26† | −.02 | −.13 | .31* | −.17 | .17 | .14 | .08 | .12 |
| 2. Suppression | −.11 | — | −.06 | −.10 | −.29† | .11 | −.49** | −.20 | .38* | −.11 | .01 | .08 | −.09 |
| 3. BAS | .16 | −.06 | — | −.06 | .56*** | −.19 | .09 | −.16 | −.11 | .29† | .06 | .007 | .12 |
| 4. BIS | −.05 | −.10 | −.06 | — | .25 | .65*** | −.12 | −.09 | −.01 | .08 | .13 | .04 | .04 |
| 5. SR | .26† | −.29† | .56*** | .25 | — | .04 | .008 | −.01 | −.38* | .18 | .06 | .09 | .12 |
| 6. SP | −.02 | .11 | −.19 | .65*** | .04 | — | −.25 | −.10 | .10 | .22 | .30† | .11 | .09 |
| 7. CIT | −.13 | −.49** | .09 | −.12 | .008 | −.25 | — | .07 | .20 | −.13 | −.10 | −.06 | .19 |
| 8. ΔCue-P300 | .31* | −.20 | −.16 | −.09 | −.01 | −.10 | .07 | — | −.26† | .07 | .10 | .18 | −.13 |
| 9. ΔSPN | −.17 | .38* | −.11 | −.01 | −.38* | .10 | .20 | −.26† | — | −.20 | .10 | −.24 | −.03 |
| 10. ΔRewP | .17 | −.11 | .29† | .08 | .18 | .22 | −.13 | .07 | −.20 | — | .53*** | .10 | .22 |
| 11. ΔFB-P300 | .14 | .01 | .06 | .13 | .06 | .30† | −.10 | .10 | .10 | .53*** | — | .07 | .55*** |
| 12. ΔLPP-Incentive | .08 | .08 | .007 | .04 | .09 | .11 | −.06 | .18 | −.24 | .10 | .07 | — | .02 |
| 13. ΔLPP-Performance | .12 | −.09 | .12 | .04 | .12 | .09 | .19 | −.13 | −.03 | .22 | .55*** | .02 | — |
Note:
p<.001
p<.01
p<.05
p <.10.
BAS = behavioral approach system; BIS = behavioral inhibition system sensitivity; SR = sensitivity to reward; SP = sensitivity to punishment. CIT = Comprehensive Inventory of Thriving.
Figure 2.
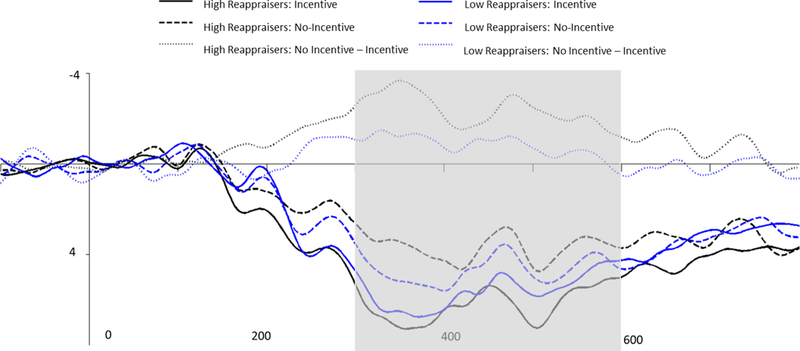
ERP waveforms depicting the cue P300 for incentive (noted with solid lines) and no-incentive cued trials (noted with dashed lines), as well as the difference score between incentive and no-incentive trials measured in microvolts (μV) at Pz. ERP waveforms and differences scores are depicted separately for high reappraisers (noted in black) and low reappraisers (noted in blue). Note: The time window used to measure the cue P300 is indicated in gray. Participants were classified as high or low reappraisers based upon a median split.
Figure 3.
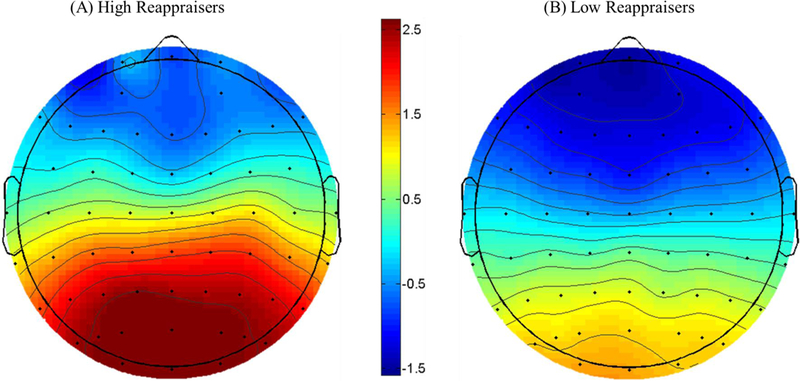
Scalp topography of the cue P300 depicting the difference between incentive and no-incentive cued trials for high reappraisers (A) and low reappraisers (B) from 300–600 ms following the presentation of the cue measured in microvolts (μV) at Pz. Note: Participants were classified as high or low reappraisers based upon a median split.
Reward Outcome Anticipation ERPs.
Replicating past research (e.g., Pornpattananangkul & Nusslock, 2015), incentive cues elicited a significantly larger SPN compared to no-incentive cues, F (1, 43) = 57.98, p < .001, partial ɳ2 = .55 (see Table 1). The relationship between emotion-regulation strategies and reward anticipation was assessed using the ΔSPN, which was quantified as the difference between the SPN to incentive cues and no incentive cue. Given that the SPN is a negative deflection, a more negative SPN difference score reflects a larger SPN to incentive compared to no-incentive cues. We regressed the ΔSPN onto suppression (centered), reappraisal (centered), and their interaction. As illustrated in Figures 4 and 5, there was a significant main effect of suppression such that participants reporting elevated emotion suppression had a blunted (less negative) SPN, b = .50, SE = .20, t (40) = 2.53, p = .022. There was no main effect of reappraisal, b = −.18, SE = .21, t (40) = −0.85, p = .40. The suppression × reappraisal interaction was also non-significant, b = −.06, SE = .19, t (40) = −0.31, p = .76.
Figure 4.
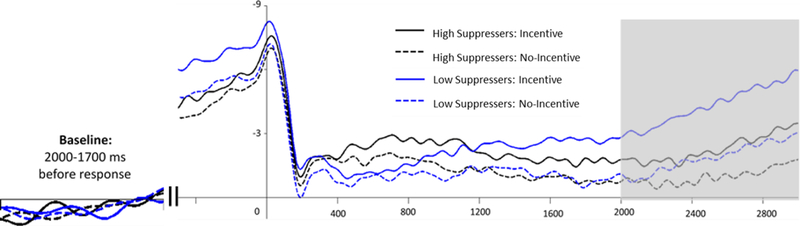
ERP waveforms depicting the SPN for incentive (noted with solid lines) and no-incentive cued trials (noted with dashed lines) measured in microvolts (μV) at FCz. ERP waveforms and differences scores are depicted separately for high suppressers (noted in black) and low suppressers (noted in blue). Note: The time window used to measure the cue SPN is indicated in gray. Baseline is shown to the left of the figure. Participants were classified as high or low suppressers based upon a median split.
Figure 5.
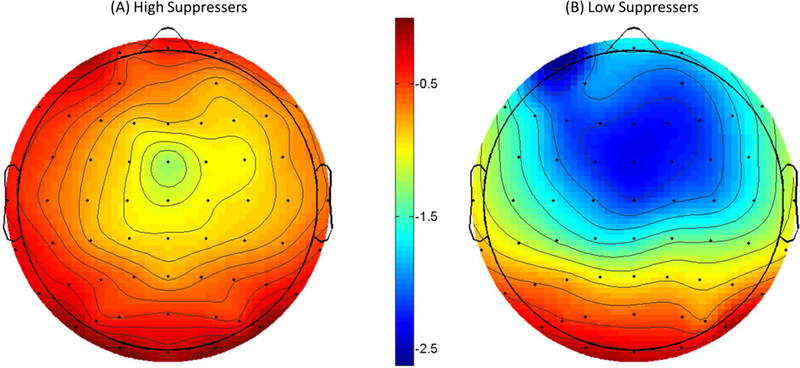
Scalp topography of the SPN depicting the difference between incentive cued and no-incentive cued trials for high suppressers (A) and low suppressers (B) for 1000 ms time-window prior to feedback onset, measured in microvolts (μV) at FCz. Note: Participants were classified as high or low suppressers based upon a median split.
Early Reward Evaluation ERPs.
We next examined the effect of performance and incentives on the RewP using a 2 (Performance: good vs. bad) × 2 (Incentive: incentive vs. no-incentive) within-subjects ANOVA. Replicating past research (e.g., Pornpattananangkul & Nusslock, 2015), good performance elicited a larger RewP compared to bad performance, F (1, 43) = 7.66, p = .008, partial ɳ2 = .15. Incentives elicited a larger RewP compared to no-incentive, F (1, 43) = 72.14, p < .001, partial ɳ2 = .63. These main effects were qualified by a significant Performance × Incentive interaction, F (1, 43) = 9.13, p = .004, partial ɳ2 = .18. We followed this interaction by examining the simple main effects of performance separately for incentive and no-incentive trials. On incentive trials, good performance elicited a larger RewP than bad performance, F (1, 43) = 18.95, p < .001, partial ɳ2 = .31. On no-incentive trials, there was no effect of performance on the RewP, F (1, 43) = 0.77, p = .39, partial ɳ2 = .02. See Figure 6.
Figure 6.
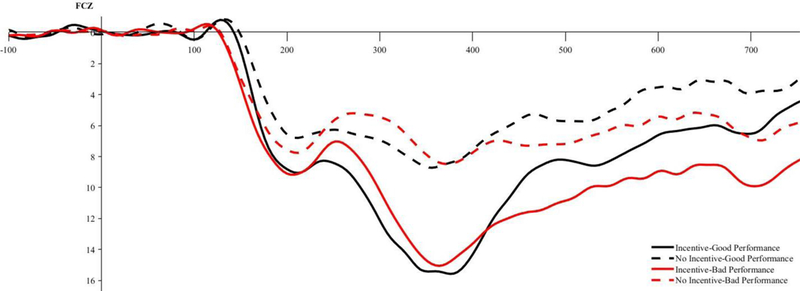
ERP waveforms depicting the RewP for incentive (noted with solid lines) and no-incentive cued trials (noted with dashed lines) measured in microvolts (μV) at FCz. ERP waveforms and differences scores are depicted separately for good performance (noted in black) and bad performance (noted in red). Note: We quantified the RewP by calculating the mean amplitude at ± 50 ms around the peak (i.e., 216–316 ms).
We used the ΔRewP to examine the relationship between the RewP and emotion-regulation strategies. Based on the simple main effects above, we quantified the ΔRewP as the difference between the RewP to good minus bad performance on incentive trials only. We regressed ΔRewP onto suppression (centered), reappraisal (centered), and their interaction. The ΔRewP was not related to suppression [b = −.18, SE = .31, t (40) = − 0.58, p = .58], reappraisal [b = .34, SE = .33, t (40) = 1.01, p = .32], or their interaction [b = .03, SE = .29, t (40) = 0.11, p = .91].
Feedback Cue Evaluation ERPs.
We next examined the effect of feedback and reward on the feedback P300 using a 2 (Performance: good vs. bad) × 2 (Incentive: incentive vs. no-incentive) within-subjects ANOVA. Good performance elicited a larger feedback P300 magnitude compared to bad performance, F (1, 43) = 1.93, p = .17, partial ɳ2 = .04. Replicating past research (e.g., Pornpattananangkul & Nusslock, 2015), incentive trials elicited a larger feedback P300 compared to no-incentive trials, F (1, 43) = 95.99, p <.001 partial ɳ2 = .69. We observed a non-significant trend for the Performance × Incentive interaction on the feedback P300, F (1, 43) = 2.93, p = .09, partial ɳ2 = .06. See Figure 7. Simple main effects revealed that on incentive trials, good performance elicited a significantly larger feedback P300 compared to bad performance, F (1, 43) = 4.41, p = .04. On incentive trials, there was no difference between good and bad performance on the feedback P300, F (1, 43) = 0.14, p = .71 (see Table 2). Based on these simple main effects, we quantified the feedback-ΔP300 as the difference between the feedback P300 for good and bad performance on incentive trials only. First, we regressed the feedback-ΔP300 onto suppression (centered), reappraisal (centered), and their interaction. The feedback-ΔP300 was not related to suppression [b = −.05, SE = .36, t (40) = −0.12, p = .90], reappraisal [b = .32, SE = .39, t (40) = 0.82, p = .42], or their interaction [b = .45, t (40) = 1.29, p = .20].
Figure 7.
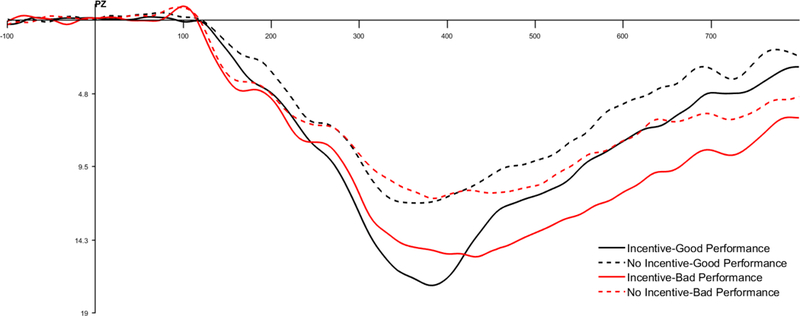
ERP waveforms depicting the Feedback-P300 for incentive (noted with solid lines) and no-incentive cued trials (noted with dashed lines) measured in microvolts (μV) at Pz. ERP waveforms and differences scores are depicted separately for good performance (noted in black) and bad performance (noted in red). Note: We quantified the Feedback-P300 was defined as the mean amplitude between 250 and 450 ms at Pz.
Table 2.
Descriptive statistics for reward-outcome ERPs
| Incentive/ Good Performance M (SD) |
Incentive/ Bad Performance M (SD) |
No-Incentive/ Good Performance M (SD) |
No-Incentive/ Bad Performance M (SD) |
|
|---|---|---|---|---|
| RewP | 10.11 (5.61) | 8.55(6.17) | 6.54 (4.67) | 6.15 (4.89) |
| FB-P300 | 13.97 (5.49) | 13.07 (6.19) | 10.10 (4.90) | 9.93 (5.25) |
| LPP | 6.46 (3.79) | 8.02 (4.10) | 4.61 (3.41) | 5.90 (3.72) |
Extended Affective Outcome Processing ERPs.
We next examined the effects of feedback and reward on the LPP using a 2 (Performance: good vs. bad) × 2 (Incentive: incentive vs. no-incentive) within-subjects ANOVA. Replicating past research (e.g., Pornpattananangkul & Nusslock, 2015), good performance elicited a larger LPP magnitude compared to bad performance, F (1, 43) = 41.47, p < .001, partial ɳ2 = .49, and incentive trials elicited a larger LPP compared to no-incentive trials, F (1, 43) = 79.87, p <.001 partial ɳ2 = .65. There was no Performance × Incentive interaction on the LPP, F (1, 43) = 0.05, p = .83, partial ɳ2 = .001 (see Table 2).
We used the ΔLPP-Performance and ΔLPP-Incentive assessed the relationship between reward outcome neural activity and emotion-regulation strategies. The ΔLPP-Performance was quantified as the difference between the LPP to good minus bad performance (collapsing across incentive) with larger values reflecting a larger LPP to good performance. The ΔLPP-Incentive was quantified as the difference between the LPP to incentive minus no-incentive trials (collapsing across Performance) with larger values reflecting a larger LPP to incentive trials. First, we regressed the ΔLPP-Performance onto suppression (centered), reappraisal (centered), and their interaction. The ΔLPP-Performance was not related to suppression [b = −.27, SE = .31, t (40) = − 0.89, p = .38], reappraisal [b = .20, SE = .33, t (40) = 0.60, p = .56], or their interaction [b = .47, SE = .29, t (40) = 1.61, p = .12]. We next regressed the ΔLPP-incentive onto suppression (centered), reappraisal (centered), and their interaction. The ΔLPP-incentive was also not related to suppression [b = −.06, SE = .22, t (40) = 0.29, p = .78], reappraisal [b = .12, SE = .23, t (40) = 0.50, p = .62], or their interaction [b = .21, SE = .20, t (40) = 1.06, p = .30].
Longitudinal Follow-up
The objective of the longitudinal analyses was to test whether any of the emotion-regulation and reward responsivity measures (both self-report and neural) that were significantly related to each other at the laboratory session prospectively related to psychological well-being over a two and a half follow-up period. As noted, 27 of the 44 participants completed the CIT measure of psychological well-being at follow-up. Participants who did, and did not, complete the CIT at follow-up did not differ from each other on any of the following variables showing significant associations at the baseline laboratory assessment: reappraisal [t (42) = − 0.42, p = .68, d = .13], suppression [t (42) = −0.46, p = .65, d = 0.15], BAS [t (42) = 0.25, p = .80, d = 0.08], SPSRQ reward responsivity [t (42) = 0.07, p = .94, d = 0.02], the cue-ΔP300 [t (42) = −0.19, p = .85, d = 0.06], or the ΔSPN [t (42) = − 0.61, p = .55, d = 0.18].
We first examined how self-reported reappraisal, suppression, BAS, SPSRQ reward responsivity, the cue-ΔP300, and the ΔSPN at the baseline laboratory session related to psychological well-being 2.5 years later. Although none of these variables were significantly related to well-being at the bivariate level (see Table 3), we selected these variables as mediators because they were significantly associated with one or more of our predictors. We limited mediation analyses to variables significantly associated with one or more of our predictors to constrain the number of potential mediators tested. Elevated suppression tendencies were associated with worse prospective psychological well-being, r (25) = −.49, p = 0.01. No other significant relationships were observed, ps > .30.
We next examined whether reward responsivity mediates the association between emotion-regulation and future well-being. This analysis focused on suppression and not reappraisal because we did not observe a significant association between reappraisal and wellbeing. Indirect effects are the product of the paths that constitute the effect (i.e., predictor × mediator, mediator × outcome). As a result, we sought to include mediators which were related to either predictor or the outcome. As we note above, no significant associations were observed between well-being and any of the other study variables. In the preceding section, we report significant associations between emotion regulation tendencies and the cue-P300, SPN, and selfreported measures of reward responsivity. Thus, the cue-P300, SPN, and self-reported measures of reward responsivity were included as mediators in the subsequent mediation analysis.
We conducted a parallel mediation analysis using Hayes (2013) process macro to examine whether the association between elevated suppression tendencies and worse prospective well-being was accounted for by BAS, SPSRQ reward responsivity, the cue-ΔP300, or the ΔSPN. Echoing the association above, we first observed a direct effect of suppression at the baseline laboratory assessment on future psychological well-being, b = −.22, SE = .07, t = −3.48, p = .002, 95% CI [−.36, −.09]. The indirect effects of BAS (b = −003, SE = .03, 95% CI [−.08, .03]), SPSRQ reward responsivity (b = .005, SE = .02, 95% CI [−.03, .08]), and the cue-ΔP300 (b = .002, SE = .03, 95% CI [−.05, .06]) were not significant as their confidence intervals included zero. There was a significant indirect effect of suppression on well-being through the ΔSPN, b = .05, SE = .04, 95% CI [.006, .193] as the confidence interval did not include zero. This suggests that suppressors experience worse well-being due in part to reduced reward outcome anticipation.
Discussion
The current study was the first to systematically examine the relationship between individual differences in emotion-regulation and both self-report and neural measures of reward responsivity. In particular, we examined (a) the relationship between emotion regulation tendencies (as measured by the ERQ) and self-reported reward responsivity as measured by the BAS and the SPSRQ reward responsivity subscale, (b) the relationship between emotion regulation tendencies and ERP components in the context of reward anticipation (cue-P300, SPN) and reward outcome (RewP, feedback P300, LPP), and (c) the extent to which selfreported and neural measures of reward responsivity mediate the relationship between emotion regulation tendencies and well-being 2.5 years later.
At the self-report level, we observed that elevated emotion suppression was associated with blunted reward responsivity among those low in reappraisal. These results are consistent with research linking reappraisal and suppression tendencies to self-reported BAS activation and reward responsivity (Dennis, 2007; Taubitz et al 2015). At the neural level, we examined the relationship between both reward-anticipation and reward-outcome neural activity and emotion-regulation tendencies. Regarding reward-anticipation neural activity, we report that enhanced attention to the incentive cue, as reflected in an elevated cue-P300, was associated with greater reappraisal tendencies. Insofar as the cue-P300 indexes salience processing, this finding suggests that individuals likely to engage in cognitive reappraisal allocate more attention to rewarding cues in their environment. On the other hand, greater suppression tendencies were associated with reduced reward-related neural activity during the feedback anticipation period, as reflected in a blunted SPN. Contrary to predictions, we did not observe significant relationships between emotion-regulation tendencies and ERPs during the reward-outcome phase (i.e., RewP, feedback-P300, LPP). Finally, elevated suppression, but not reappraisal, tendencies were associated with decreased psychological well-being at a two-and-a-half-year follow-up assessment. Decreased neural reward-anticipation (i.e., SPN) mediated this longitudinal relationship between suppression tendencies and psychological well-being.
Implications for the process model of emotion-regulation.
The results of the current study have implications for the process model of emotion-regulation. The process model of emotion-regulation (Gross, 1998b; 2001) theorizes that attempts to modulate emotions occur along a temporal sequence. Antecedent-focused emotion-regulation strategies (e.g., cognitive reappraisal) aim to modulate emotional responses before they solidify. As a result, these strategies are effective and operate at the earliest stages of emotion processing. Indeed, cue evaluation is among the earliest stages of emotion processing which is precisely where we observe a relationship between cognitive reappraisal and reward-related neural activity. Later, response-focused, emotion-regulation strategies like emotion suppression are aimed at modulating emotional responses after they have been initiated. In the current study, we can consider response initiation as the participants’ button-press on the time estimation task. From that perspective, shortly after response initiation, suppression tendencies begin to exert their effect in the form of blunted reward outcome anticipation (i.e., attenuated SPN). The finding that reappraisal affects early anticipatory neural activity and suppression affects later activity is consistent with the temporal proposition of the process model of emotion-regulation, which proposes that the effects of cognitive reappraisal attend to occur at earlier temporal stages than expressive suppression.
Emotion-regulation tendencies did not relate to reward outcome neural activity.
Contrary to prediction, we observed no relationship between emotion-regulation strategies (reappraisal, suppression) and reward-related neural activity during the outcome period (i.e., RewP, LPP). This null effect may have been observed because it is easier to modulate emotions and impulses at early rather than late temporal stages (Duckworth, Gendler, & Gross, 2016). The snowball metaphor is an easy way to conceptualize the temporal dynamics of emotion-regulation’s influence on emotion processing (e.g., Mennin & Fresco, 2009). This metaphor suggests that as a snowball rolls downhill, it grows larger, harder, and more difficult to stop or redirect. Likewise, as emotions unfold over time they grow, gain momentum, and can be more difficult to modulate. As a result, more effort may be required to alter emotional responses at later (vs. earlier) temporal stages. At later stages (e.g., reward outcome processing), conscious explicit emotion-regulation may be required to enhance or attenuate outcome processing. Future studies should examine the differential impact of automatic and conscious emotional regulation processes on reward-anticipation and reward-outcome processing.
Despite the theorizing above, one past study has observed a relationship between emotion-regulation tendencies and reward outcome processing. Recall that Sai et al. (2015) observed that elevated reappraisal tendencies were associated with an elevated RewP, whereas expressive suppression was unrelated to the RewP. Although our finding that suppression was unrelated to the RewP is consistent with their results, we observed no relationship between reappraisal and the RewP. Three considerations may explain these differences. First, concerns about power and sample size. It is widely understood that a study with low statistical power has both a lower chance of detecting a true effect and a lower chance that a statistically significant result reflects a true effect (Button et al., 2013). The sample size in the current study (N = 44) was more than twice that of Sai and colleagues (N = 20). Thus, one possibility is that the study by Sai and colleagues was simply underpowered and thus their statistically significant effect may not reflect a true effect. By more than doubling their sample size, the current study had greater statistical power to detect an association between reappraisal and the RewP. By observing a null association between reappraisal and the RewP, we suggest that the reappraisal-RewP link is not as strong reported by Sai and colleagues. Thus, we provide a more accurate estimate of the association between reappraisal and the RewP.
Second, in addition to statistical power, task differences between the two studies may explain why our results diverged from Sai et al. (2015). In the current study, we used a time-estimation task where feedback provides two forms of information: behavioral performance and monetary reinforcement. By contrast, the Sai et al. (2015) study used a gambling task with randomly administered feedback (see Foti and Hajcak, 2009). In such a task, winning may reflect a binary evaluation response (i.e., good vs. bad). Finally, the time window employed by Sai and colleagues measured the RewP 280–380 ms after feedback onset. This overlaps with the feedback P300, which begins at 300 milliseconds. Thus, their results appear to confound the RewP with the feedback-P300. Due to these task differences, the RewP across these two studies may index different aspects of reward outcome processing. If indeed these RewPs are measuring different processes, it is not surprising that they do not relate reappraisal and suppression in the same way. In summary, a multitude of reasons may explain the divergence between our findings and those of Sai and colleagues. Future studies should seek to clarify the extent to which emotion regulation tendencies relate to the RewP.
The absence of an effect for the LPP is not surprising due to mixed results in the emotion-regulation literature. Although many studies do find a relationship between emotion-regulation tendencies and the LPP (for a review see Hajcak, McNamara, & Olvet, 2010), other studies find no (or mixed) relationships between these processes (e.g., Moser et al., 2006; Bernat, Cadwallader, Seo, Vizueta, & Patrick, 2011; Murata, Moser, & Kitayama, 2012). Much of this previous work has used passive image viewing paradigms rather than active tasks, such as the time-estimation task. Additionally, the current study also assessed the LPP in the context of reward. These task differences may explain, at least in part, why we did not observe a relationship between emotion-regulation and the LPP. Additionally, only one study directly examined reward processing and the LPP, finding that reappraisal instructions did modulate the LPP (Langeslag & van Strien, 2013). The current study took an individual differences approach to the study of emotion-regulation rather than an experimental one and observed null relationships between an individual differences measure of emotion-regulation strategies and the LPP. Individual differences and experimental manipulations routinely do not always reflect the same processes. Whereas individual differences may reflect more implicit or automatic processes, experimental findings may reflect conscious and effortful processing. Finally, the time window in Langeslag & van Strien confounded the LPP and feedback P300. Additionally, as Hajcak et al. (2010) note, there is variability across studies in how the LPP is quantified. In line with previous work, we used a wide time window (500–1000 ms) in the present study after feedback presentation (e.g., Foti & Hajcak, 2008). In summary, the absence of a relationship between emotion-regulation and the LPP may be due a combination of task differences (e.g., active reward vs. passive picture viewing), methodological differences (e.g., experimental vs. individual differences), or differences in ERP quantification. Future research should address these inconsistencies to better understand the relationship between emotion-regulation strategies and reward outcome neural activity.
Emotion-regulation tendencies, reward responsivity, and psychological well-being
This is the first study to report that reward-related neural activity (i.e., reward-outcome anticipation SPN) plays a role in the relationship between emotion-regulation strategies and future psychological well-being. Many studies report that reappraisal and suppression tendencies are associated with diverging patterns of well-being (John & Gross, 2004; Haga, Kraft, & Corby 2009; McRae, Jacobs, Ray, John, & Gross, 2012). The current study offers one potential mechanism for these findings: blunted reward anticipation among suppressors. To the extent that suppression represents a maladaptive emotion-regulation strategy, developing skills aimed at reducing the reliance on this strategy may in turn increase reward anticipation and ultimately lead to improved well-being. Towards that end, reward-outcome anticipation neural activity (SPN) may prove to be a biomarker of emotion-regulation success. However, this assertion is speculative and of course there are many mechanisms which may be driving links between suppression and well-being. The results of the current study simply suggest that further experimental and longitudinal work is needed to address the relationships between emotion-regulation, reward processing, and well-being. A limitation of the mediational analyses reported in the present paper is the lack of a baseline measurement of psychological well-being. Thus, it is difficult to determine the extent to which changes in reward-outcome anticipation neural activity (SPN) account for the relationship between suppression tendencies and psychological wellbeing. Future research should continue to explore the relationship between emotion-regulation, reward-related neural activity, and well-being in a more comprehensive manner.
Conclusion
The present study suggests that two common emotion-regulation tendencies differentially relate to self-report and neural indices of reward responsivity. At the self-report level, elevated emotion suppression was associated with blunted reward responsivity among those low in reappraisal. At the neural level, elevated reappraisal tendencies were associated with greater reward cue salience (cue-P300), whereas elevated suppression tendencies were associated with blunted reward outcome anticipation (SPN). Further, blunted reward outcome anticipation, as assessed by the SPN, mediated the relationship between suppression tendencies at baseline and psychological well-being measured two and a half years later. Taken together with past research, these results highlight the importance of reward responsivity for both the short-term and long-term consequences of emotion-regulation.
Highlights.
Systematically examined the relationship between individual differences in emotion-regulation and reward responsivity.
High emotion suppression was associated with blunted self-reported reward responsivity among those low in reappraisal.
Enhanced neural early attention to reward cues (i.e., cue-P300) was associated with greater reappraisal tendencies.
Reduced neural reward outcome anticipation (i.e., the SPN) was associated with greater suppression tendencies.
Decreased neural reward-anticipation (SPN) mediated this longitudinal relationship between suppression tendencies and psychological well-being 2.5 years later.
Acknowledgements:
Preparation of this manuscript was supported by National Institute of Health (NIH) grant T32 NS047987 to NJK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Depression is known to be associated with reward processing ERPs. Thus we re-ran this analysis controlling for depression scores (as indexed by the Beck Depression Inventory). Results revealed that the association between reappraisal and the cue-P300 remained significant when controlling for depression, b = .78, SE = .36, t (39) = 2.14, p = .04.
The effect of suppression on the SPN remained significant when controlling for depression, b = .50, t (39) = 2.50, p = .02.
Contributor Information
Nicholas J. Kelley, Northwestern University
James E. Glazer, Northwestern University
Narun Pornpattananangkul, National Institute of Mental Health.
Robin Nusslock, Northwestern University.
References
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions Sage. [Google Scholar]
- Aldao A, Nolen-Hoeksema S, & Schweizer S (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30, 217–237. [DOI] [PubMed] [Google Scholar]
- Angus D, Kemkes K, Schutter D, & Harmon-Jones E (2015). Anger is associated with reward-related electrocortical activity: Evidence from the reward-related positivity. Psychophysiology, 52, 1271–1280. [DOI] [PubMed] [Google Scholar]
- Appleton AA, & Kubzansky LD (2014). Emotion regulation and cardiovascular disease risk. In Gross JJ (Ed.), Handbook of emotion regulation (2nd ed., pp. 596–612). New York, NY: Guilford. [Google Scholar]
- Baker KS, Piriyapunyaporn T, & Cunnington R (2012). Neural activity in readiness for incidental and explicitly timed actions. Neuropsychologia, 50, 715–722. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Cadwallader M, Seo D, Vizueta N, & Patrick CJ (2011). Effects of instructed emotion regulation on valence, arousal, and attentional measures of affective processing. Developmental Neuropsychology, 36, 493–518. [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (2003). Parsing reward. Trends in Neurosciences, 26, 507–513. [DOI] [PubMed] [Google Scholar]
- Brans K, Koval P, Verduyn P, Lim YL, & Kuppens P (2013). The regulation of negative and positive affect in daily life. Emotion, 13, 926–939. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, & Shizgal P (2001). Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron, 30, 619–639. [DOI] [PubMed] [Google Scholar]
- Brunia CHM, van Boxtel GJM, & Böcker KBE (2011). Negative slow waves as indices of anticipation: The bereitschaftspotential, the contingent negative variation, and the stimulus-preceding negativity. In Luck SJ & Kappenman ES (Eds). The Oxford handbook of event-related potential components (pp. 189–207). Oxford: Oxford University Press. [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, & Ochsner KN (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, & Munafò MR (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365–376. [DOI] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67, 319–333. [Google Scholar]
- Cohen MX, Elger CE, & Ranganath C (2007). Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage, 35(2), 968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen EJ, & Brunia CH (1994). Is a stimulus conveying task-relevant information a sufficient condition to elicit a stimulus preceding negativity?. Psychophysiology, 31, 129–139. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, & Phelps EA (2008). Regulating the expectation of reward via cognitive strategies. Nature Neuroscience, 11, 880–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA (2007). Interactions between emotion regulation strategies and affective style: Implications for trait anxiety versus depressed mood. Motivation and Emotion, 31, 200–207. [Google Scholar]
- Donaldson KR, Oumeziane BA, Hélie S, & Foti D (2016). The temporal dynamics of reversal learning: P3 amplitude predicts valence-specific behavioral adjustment. Physiology & Behavior, 161, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E, & Coles MG (1998). Context updating and the P300. Behavioral and Brain Sciences, 21, 152–154. [Google Scholar]
- Duckworth AL, Gendler TS, & Gross JJ (2016). Situational strategies for self control. Perspectives on Psychological Science, 11, 35–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M, Willer R, Antonenko O, & John OP (2012). Liberating reason from the passions. Psychological Science, 23, 788–795. [DOI] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2008). Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience, 20, 977–988. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DCH, Schirda BL, Jennings JR, Sheu LK, … & Manuck SB (2014). An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biological Psychiatry, 75, 738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer JE, Kelley NJ Pornpattananangkul N, Mittal VA, & Nusslock R (in press). Beyond the FRN: Broadening the Time-Course of EEG and ERP Components Implicated in Reward-Processing. International Journal of Psychophysiology [DOI] [PubMed]
- Goldin PR, McRae K, Ramel W, & Gross JJ (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry, 63, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Cottone LA, Jia Z, Maloney T, Volkow ND, & Squires NK (2006). The effect of graded monetary reward on cognitive event-related potentials and behavior in young healthy adults. International Journal of Psychophysiology, 62, 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (1998a). Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74, 224–237. [DOI] [PubMed] [Google Scholar]
- Gross JJ (1998b). The emerging field of emotion regulation: An integrative review. Review of General Psychology, 2, 271–299. [Google Scholar]
- Gross JJ (2001). Emotion regulation in adulthood: Timing is everything. Current Directions in Psychological Science, 10, 214–219. [Google Scholar]
- Gross JJ (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology, 39, 281–291. [DOI] [PubMed] [Google Scholar]
- Gross JJ (2015). Emotion regulation: Current status and future prospects. Psychological Inquiry, 26, 1–26. [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85, 348–362. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & Levenson RW (1993). Emotional suppression: Physiology, self–report, and expressive behavior. Journal of Personality and Social Psychology, 64, 970–986. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & Levenson RW (1997). Hiding feelings: The acute effects of inhibiting positive and negative emotions. Journal of Abnormal Psychology, 106, 95–103. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & Thompson RA (2007). Emotion regulation: Conceptual foundations. In Gross JJ (Ed.), Handbook of emotion regulation (pp. 3–24). New York: Guilford Press. [Google Scholar]
- Hackley SA, Valle-Inclán F, Masaki H, & Hebert K (2014). Stimulus-preceding negativity (SPN) and attention to rewards. Cognitive Electrophysiology of Attention: Signals of the mind, 216–225.
- Hajcak G, MacNamara A, & Olvet DM (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology, 35, 129–155. [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Nieuwenhuis S (2006). Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective, & Behavioral Neuroscience, 6, 291–297. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, & Cohen JD (2003). Errors in reward prediction are reflected in the event-related brain potential. Neuroreport, 14, 2481–2484. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, & Krigolson OE (2008). The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology, 45, 688–697. [DOI] [PubMed] [Google Scholar]
- Hu T, Zhang D, Wang J, Mistry R, Ran G, & Wang X (2014). Relation between emotion regulation and mental health: a meta-analysis review. Psychological Reports, 114, 341–362. [DOI] [PubMed] [Google Scholar]
- John OP, & Gross JJ (2004). Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. Journal of Personality, 72, 1301–1334. [DOI] [PubMed] [Google Scholar]
- Johnson R, & Donchin E (1980). P300 and stimulus categorization: Two plus one is not so different from one plus one. Psychophysiology, 17, 167–178. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, & Wessa M (2012). Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage, 61, 686–693. [DOI] [PubMed] [Google Scholar]
- Kotani Y, Hiraku S, Suda K, & Aihara Y (2001). Effect of positive and negative emotion on stimulus-preceding negativity prior to feedback stimuli. Psychophysiology, 38, 873–878. [DOI] [PubMed] [Google Scholar]
- Kotani Y, Kishida S, Hiraku S, Suda K, Ishii M, & Aihara Y (2003). Effects of information and reward on stimulus-preceding negativity prior to feedback stimuli. Psychophysiology, 40, 818–826. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, & Hommer D (2001). Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport, 12, 3683–3687. [DOI] [PubMed] [Google Scholar]
- Kross E, & Ayduk O (2011). Making meaning out of negative experiences by self– distancing. Current Directions in Psychological Science, 20, 187–191. [Google Scholar]
- Langeslag SJ, & van Strien JW (2013). Up-regulation of emotional responses to reward-predicting stimuli: an ERP study. Biological Psychology, 94, 228–233. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, & Alfert E (1964). Short-circuiting of threat by experimentally altering cognitive appraisal. The Journal of Abnormal and Social Psychology, 69, 195–205. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Inagaki TK, Tabibnia G, & Crockett MJ (2011). Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion, 11, 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2005). Ten simple rules for designing ERP experiments. In Handy TC (Ed.), Event-related potentials: A methods handbook (pp. 17–32). Cambridge, MA: MIT Press. [Google Scholar]
- Luck SJ, & Kappenman ES (2012). ERP components and selective attention. In Luck SJ & Kappenman ES (Eds.). The Oxford handbook of event-related potential components (pp. 295–328). Oxford: Oxford University Press. [Google Scholar]
- Makeig S, Bell AJ, Jung TP, & Sejnowski TJ (1996). Independent component analysis of electroencephalographic data. In Advances in neural information processing systems (pp. 145–151).
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, & Gross JJ (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion, 5, 175–190. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, & Montague PR (2003). Temporal prediction errors in a passive learning task activate human striatum. Neuron, 38(2), 339–346. [DOI] [PubMed] [Google Scholar]
- McRae K, Jacobs SE, Ray RD, John OP, & Gross JJ (2012). Individual differences in reappraisal ability: Links to reappraisal frequency, well-being, and cognitive control. Journal of Research in Personality, 46, 2–7. [Google Scholar]
- Meadows CC, Gable PA, Lohse KR, & Miller MW (2016). The effects of reward magnitude on reward processing: An averaged and single trial event-related potential study. Biological Psychology, 118, 154–160. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Fresco DM Emotion regulation as an integrative framework for understanding and treating psychopathology. In: Kring AM & Sloan DS (Eds). Emotion regulation and psychopathology Guilford Press; New York, NY: 2009. pp. 356–379. [Google Scholar]
- Miltner WH, Braun CH, & Coles MG (1997). Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience, 9, 788–798. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, & Simons RF (2006). Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology, 43, 292–296. [DOI] [PubMed] [Google Scholar]
- Moser JS, Krompinger JW, Dietz J, & Simons RF (2009). Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology, 46, 17–27. [DOI] [PubMed] [Google Scholar]
- Moser JS, Most SB, & Simons RF (2010). Increasing negative emotions by reappraisal enhances subsequent cognitive control: a combined behavioral and electrophysiological study. Cognitive, Affective, & Behavioral Neuroscience, 10, 195–207. [DOI] [PubMed] [Google Scholar]
- Murata A, Moser JS, & Kitayama S (2012). Culture shapes electrocortical responses during emotion suppression. Social Cognitive and Affective Neuroscience, 8, 595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezlek JB, & Kuppens P (2008). Regulating positive and negative emotions in daily life. Journal of Personality, 76, 561–579. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, & Gross JJ (2004). For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage, 23, 483–499. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, & Gross JJ (2008). Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science, 17, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfabigan DM, Seidel EM, Sladky R, Hahn A, Paul K, Grahl A, … & Windischberger C (2014). P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: An EEG and fMRI experiment. NeuroImage, 96, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J (2007). Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology, 118, 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, & Kok A (1995). Cognitive and biological determinants of P300: an integrative review. Biological Psychology, 41, 103–146. [DOI] [PubMed] [Google Scholar]
- Pornpattananangkul N, & Nusslock R (2015). Motivated to win: Relationship between anticipatory and outcome reward-related neural activity. Brain & Cognition, 100, 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornpattananangkul N, & Nusslock R (2016). Willing to wait: Elevated reward-processing EEG activity associated with a greater preference for larger-but-delayed rewards. Neuropsychologia, 91, 141–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52, 449–459. [DOI] [PubMed] [Google Scholar]
- Ray RD, McRae K, Ochsner KN, & Gross JJ (2010). Cognitive reappraisal of negative affect: Converging evidence from EMG and self–report. Emotion, 10, 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai L, Wang S, Ward A, Ku Y, & Sang B (2015). Individual differences in the habitual use of cognitive reappraisal predict the reward-related processing. Frontiers in Psychology, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, & Correa M (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron, 76, 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Abler B, & Walter H (2009). Cognitive reappraisal modulates expected value and prediction error encoding in the ventral striatum. Neuroimage, 47, 713–721. [DOI] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, & Walter H (2011). Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cerebral Cortex, 21, 2578–2588. [DOI] [PubMed] [Google Scholar]
- Su R, Tay L, & Diener E (2014). The development and validation of the Comprehensive Inventory of Thriving (CIT) and the Brief Inventory of Thriving (BIT). Applied Psychology: Health and Well-Being, 6, 251–279. [DOI] [PubMed] [Google Scholar]
- Szasz PL, Szentagotai A, & Hofmann SG (2011). The effect of emotion regulation strategies on anger. Behaviour Research and Therapy, 49, 114–119. [DOI] [PubMed] [Google Scholar]
- Taubitz LE, Pedersen WS, & Larson CL (2015). BAS Reward Responsiveness: A unique predictor of positive psychological functioning. Personality and Individual Differences, 80, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchselvam R, Blechert J, Sheppes G, Rydstrom A, & Gross JJ (2011). The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biological Psychology, 87, 84–92. [DOI] [PubMed] [Google Scholar]
- Threadgill AH, & Gable PA (2016). Approach-motivated pregoal states enhance the reward positivity. Psychophysiology, 53, 733–738. [DOI] [PubMed] [Google Scholar]
- Torrubia R, Avila C, Moltó J, & Caseras X (2001). The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences, 31, 837–862. [Google Scholar]
- Webb TL, Miles E, & Sheeran P (2012). Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin, 138, 775–808. [DOI] [PubMed] [Google Scholar]
- Wellborn BL, Papademetris X, Reis DL, Rajeevan N, Bloise SM, & Gray JR (2009). Variation in orbitofrontal cortex volume: relation to sex, emotion regulation and affect. Social Cognitive and Affective Neuroscience, 4, 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgast M, Lundh LG, & Viborg G (2011). Cognitive reappraisal and acceptance: An experimental comparison of two emotion regulation strategies. Behaviour Research and Therapy, 49, 858–866. [DOI] [PubMed] [Google Scholar]
- Yeung N, & Sanfey AG (2004). Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience, 24(28), 6258–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]


